Abstract
Introduction
Severe burns result in prolonged hypermetabolism and skeletal muscle catabolism. Rehabilitative exercise training (RET) programs improved muscle mass and strength in severely burned children. The combination of RET with β-blockade or testosterone analogues showed improved exercise-induced benefits on body composition and muscle function. However, the effect of RET combined with multiple drug therapy on muscle mass, strength, cardiorespiratory fitness and protein turnover are unknown. In this placebo-controlled randomized trial, we hypothesize that RET combined with oxandrolone and propranolol (Oxprop) will improve muscle mass and function and protein turnover in severely burned children compared to burned children undergoing the same RET with a placebo.
Methods
We studied 42 severely burned children (7 – 17 years) with severe burns over 30% of the total body surface area. Patients were randomized to placebo (22 control) or to Oxprop (20) and began drug administration within 96 hours of admission. All patients began RET at hospital discharge as part of their standardized care. Muscle strength (N·m), power (W), VO2peak, body composition, and protein fractional synthetic (FSR) and breakdown (FBR) rates were measured pre- (PRE) and post-RET (POST).
Results
Muscle strength and power, lean body mass and VO2peak increased with RET in both groups (p<0.01). The increase in strength and power was significantly greater in Oxprop vs. control (p<0.01), and strength and power was greater in Oxprop over control POST RET (p<0.05). FSR was significantly higher in Oxprop than control post RET (p<0.01), resulting in improved protein net balance POST RET (p<0.05).
Conclusion
RET improves body composition, muscle function and cardiorespiratory fitness in children recovering from severe burns. Oxprop therapy augments RET-mediated improvements in muscle strength, power, and protein turnover.
Keywords: Oxandrolone, propranolol, burns, pediatric, rehabilitation, exercise
Introduction
Severe burns encompassing over 30% of the total body surface area (TBSA) result in acute critical illness associated with profound metabolic dysregulation (1–3). Increased proteolysis compounded by prolonged bed rest results in muscle cachexia and loss of function (4–6). Altered body composition and reduced function can persist for several years after burn injury (7). Indeed, difficulty walking, running, feeling of weakness and fatigue have been reported in burn patients as much as 17 years post injury (8), underscoring the importance of finding therapeutic strategies that restore muscle mass and function in survivors of severe burn injury.
A structured rehabilitative exercise therapy (RET) program has been shown to be a safe and effective approach to rehabilitate burned patients (9). Those who participated in chronic resistive and aerobic exercise improved skeletal muscle strength, increased lean body mass, and improved joint range of motion (10, 11). Exercise has also been shown to improve pulmonary function (12) and cardiorespiratory performance (13) in severely burned children. The combination of RET with long-term drug therapy has also been studied as a strategy to hasten rehabilitation of massively burned individuals. Oxandrolone is a testosterone analog that promotes lean body mass accretion and shorten the hospitalization period in severely burned individuals (14–16). Interestingly, when combined with RET, oxandrolone therapy increased lean body mass (LBM) to a greater degree than in patients who exercised but did not take oxandrolone (17). The β-adrenergic receptor blocker propranolol has been shown to be effective in reducing tachycardia and resting metabolic rate in burn patients (18–21). Further, propranolol also promotes the restoration of LBM in burned individuals by improving protein synthesis efficiency in the acute period post injury, resulting in an improved protein net balance (19, 22). Furthermore, patients who were treated with propranolol during a RET program had a greater improvement in cardiorespiratory fitness than those who did not take propranolol (23).
Collectively, treatment with either oxandrolone or propranolol during an outpatient RET program has shown beneficial effects superior to RET alone in terms of restoring body muscle mass and function in severely burned children. Oxandrolone treatment combined with exercise promoted lean body mass accretion above that of exercise alone, but did not effect on cardiorespiratory function (17). Conversely, propranolol treatment combined with exercise showed improved cardiorespiratory function above that of exercise alone, but had no beneficial effects on muscle strength (23). The impact of the combined use of oxandrolone and propranolol with a hospital-based outpatient RET program on muscle mass and function in severely burned children remains unknown. We hypothesize that combined use oxandrolone and propranolol will augment the beneficial effects of outpatient RET on lean body mass, strength, and aerobic fitness in severely burned children. To further progress burn care, the purpose of this study is to determine the effects of oxandrolone and propranolol (Oxprop) treatment during a 6-week RET program in severely burned children.
Methods and materials
Patients
This study was approved by the Institutional Review Board at the University of Texas Medical Branch and registered with clinicaltrials.gov (NCT00675714). Written informed consent was obtained from the parents or legal guardians of severely burned children (7 – 17 years old) with severe burns over 30% of the TBSA who were admitted to the Shriners Hospitals for Children – Galveston between 2013 to 2016. All patients received standard acute burn care that included nutritional support guided by the patients’ injury characteristics and resting energy expenditure, early wound excision and skin grafting, as well as comprehensive antibiotics and analgesic therapy. Patients who consented to this study were randomized into a control group, receiving the standard of care and a placebo, or the Oxprop group; where they received the standard of care and oxandrolone and propranolol. Administration of Oxprop drugs began within 96 hours after admission and continued through the course of their hospitalization period. A metabolic study to determine protein turnover and DEXA scan were conducted within four days of the discharge date. At hospital discharge, all patients were enrolled into a 6-week in-hospital RET program. Prior to commencing this RET program, all patients underwent baseline (PRE) testing for strength (peak torque) and aerobic capacity (VO2peak). In our hospital, RET has been a standard component of the out-patient rehabilitation of severely burned children over the age of 7. The control group participated in the standard 6-week RET program, while the Oxprop group participated in the 6-week RET program and continued administration of oxandrolone and propranolol. At the completion of the 6-week RET program, the same metabolic study, DEXA scan, and strength and aerobic capacity tests were repeated within five days to determine post-exercise measurements (POST).
Drug administration
Oxandrolone was administered at 0.1 mg·kg−1 (BTG Pharmaceuticals, Iselin, NJ) twice a day during the patients’ entire stay in hospital and throughout the exercise training program. Propranolol was administered at a dose of 0.33 mg·kg−1 of body weight every four hours (1.98 mg·kg−1·day−1). This dose was adjusted to achieve an approximate 20% decrease from admission heart rate for each patient. Propranolol and oxandrolone administration began within 96 hours post-admission and continued through the acute hospitalization period and outpatient exercise program.
Rehabilitative Exercise Training
All subjects participated in a training program that was individualized to each patient in terms of frequency, intensity, duration, and mode of exercise. The training program included both resistive and aerobic components (9, 13, 24, 25). All exercise sessions were supervised by an exercise specialist and followed the standards and guidelines set forth by the American College of Sports Medicine (ACSM) and the American Academy of Pediatrics (26, 27). All patients in this study participated in a 6-weeks rehabilitation exercise program that began within a week of hospital discharge.
Resistive exercise
Eight different resistive exercises were used: bench press, squats, shoulder press, leg press, biceps curl, leg curl, triceps extension, and calf raises. Training apparatus were modified to accommodate the injury characteristics of each patient. All exercises were performed on resistance machines or utilized free-weights. Subjects performed three sets of upper and lower body exercises with a two-minute rest interval between each set. Resistive exercise sessions were performed on non-consecutive days. No other organized strength training types of activities were permitted outside of the supervised session. However, there were no limitations on their normal daily activities. Subjects were familiarized with the exercise equipment and taught proper techniques during the first week. The intensity for the first week was set to target a maximal effort at 15 – 20 repetitions per set. Intensity increased in their second week to achieve maximal effort at 8 – 12 repetitions per set, which continued for the remaining sessions. Adjustments in the load were made as applicable when the subject could consistently exceed 12 repetitions per set.
Aerobic exercise
Aerobic exercise was performed on a treadmill, rowing machine or cycle ergometer for approximately 20 – 45 minutes at least thrice weekly. The intensity was set at 60 – 75% of their heart rate reserve (HRR), which was determined during a modified Bruce treadmill test (28, 29). Each session started with a five-minute warm-up and subjects were asked for their rate of perceived exertion (RPE) on a 10-point scale at 10, 15, 20, and 25 minutes into the exercise session. Heart rate was monitored during the session using an Imara HRM wrist monitor (Nike, Beaverton, OR). The exercise intensity was adjusted according to the exercise heart rate and RPE.
Body composition
LBM was quantified by Dual Emission X-Ray Absorptiometry (DEXA); images were analyzed with QDR 4500A software (Hologics, Waltham, MA). This method has a 2 – 3% margin of error for human body composition (30). Whole-body scans were performed per the manufacturer’s instructions.
Assessment of resting energy expenditure
Resting energy expenditure (REE) of burned patients were determined by indirect calorimetry (Sensor Medics Vmax 29, Yorba Linda, CA). REE was calculated from whole body oxygen consumption and carbon dioxide production rates using the Weir equation (31). This measured value was compared to the predicted REE (% predicted) which was calculated using the Harris-Benedict equation (32). This is a standard method used by our group for estimating the degree of hypermetabolism in burned children.
Assessment of muscle protein turnover
Skeletal muscle protein synthesis and breakdown were measured utilizing stable isotope tracers of phenylalanine (L-[ring-13C6] phenylalanine (99% enriched), and L-[15N] phenylalanine (99% enriched)) (Cambridge Isotopes, Cambridge, MA) (33, 34). Briefly, following a baseline blood draw, bolus injections of stable isotope tracers were injected at time 0 and 30 minutes of the protocol. Blood draws were taken periodically for one hour (33). Skeletal muscle biopsies were taken from the m. vastus lateralis at 10 and 60 minutes following the injection of the first isotope bolus. Muscle biopsies samples were snap frozen in liquid nitrogen and stored at −80°C for future analysis. Blood samples were centrifuged and plasma stored at −80°C for future analysis. Isotope enrichments were determined by gas chromatography mass spectrometry (GCMS). Skeletal muscle fractional synthesis (FSR) and breakdown (FBR) rates were calculated by the precursor-product method (33, 34).
Assessment of cardiovascular exercise capacity
Cardiovascular exercise capacity (VO2peak) test was performed utilizing a treadmill test (modified Bruce Protocol (28, 29)). The rates of O2 uptake (VO2), CO2 production (VCO2), and minute ventilation (VE) were measured using the Medgraphic CardiO2 Combined O2/ECG Exercise System (St. Paul, Mn) (11). Inspired and expired gas, flow, and volume were measured continuously through a hose attached to a face-mask. The treadmill speed was set at 1.7 mph at the start of the test with a 0% grade elevation. Afterwards, the speed and elevation increased every three minutes. Subjects were constantly encouraged to give their maximal effort. The test ended once volitional fatigue was achieved, or the subject had an unwillingness to exercise further. However, the peak effort was based on an exercise heart rate above 190 bpm, respiratory exchange ratio greater than 1.10, unsteady gait, or a plateau in VO2.
Assessment of muscle function
Muscle strength test were performed using a Biodex dynamometer (Shirley, NY). The isokinetic test was performed on the dominant leg and tested at an angular velocity of 150°·s−1. Patients were seated and stabilized with straps across the mid-thigh, pelvis, and trunk following the guidelines of the Biodex Multi-Joint System 3 Testing and Rehabilitation System User’s Guide. The test administrator first demonstrated the test, followed by an explanation to the subject, followed by one practice set where the subject can be familiar with the movement without any load. The subject was asked to perform 10 maximal voluntary muscle contractions at full knee flexion and extension without rest between each repetition. A two-minute rest period was given and the same test was performed for a second time. Peak torque (Newton meter, N·m) and average power (Watts, W) was calculated using the Biodex Software System. The highest value of the two trials was recorded.
Statistical analysis
All data are presented as group means ± SEM unless stated otherwise. D’Agostino-Pearson omnibus normality test was conducted on each group data set. Differences between group means were analyzed by unpaired t-tests and differences within group means were analyzed by paired t-tests. Non-parametric analyses were conducted between and within groups on non-normal datasets. Statistical significance was reached when p≤0.05. Statistical analyses were performed using Graphpad Prism version 7 (GraphPad Software, La Jolla, CA).
Results
We consented 47 patients into the study (n=25 control, and n=22 Oxprop). Five patients were lost due to non-compliance issues (n=3 control, and n=2 Oxprop). We studied 42 severely burned pediatric patients (n=22 controls, and n=20 Oxprop) PRE and POST RET. There were sixteen males and six females in the placebo control group with sixteen males and four females the Oxprop group. Age (11 ± 3 vs. 12 ± 4 y, p>0.05), burn severity (47 ± 12 vs. 44 ± 10% TBSA, p>0.05), and the total number of days they exercised (25 ± 5 vs. 27 ± 3 days, p>0.05) were similar between control and Oxprop, respectively. No adverse events were reported as a result of this study.
Body composition
All body mass and composition measures are shown in Table 1. Total body mass increased from PRE to POST in control (38.4 ± 2.0 vs. 40.5 ± 2.2 kg, p<0.01) and Oxprop (44.6 ± 2.9 vs. 47.3 ± 3.2 kg, p< 0.01) groups. Lean mass also increased from PRE to POST in control (26.6 ± 1.7 vs. 28.4 ± 1.7 kg, p<0.0 1) and Oxprop (30.0 ± 1.9 vs. 31.9 ± 2.0 kg, p<0.01) groups. Similarly, BMI increased from PRE to POST in control (18.0 ± 0.5 vs. 19.1 ± 0.5 kg·m2, p<0.01) and Oxprop (19.2 ± 0.7 vs. 21.0 ± 0.8 kg·m2, p<0.05) groups. Also, BMI in Oxprop was greater than control PRE (18.0 ± 0.5 vs. 19.2 ± 0.7 kg·m2, p=0.01) and POST RET (19.1 ± 0.5 vs. 21.0 ± 0.8 kg·m2, p<0.05). We determined the LBM Index (LBMI) by calculating the total amount of lean mass (kg) divided by height (m2). LBMI significantly improved from PRE to POST in control (12.3 ± 0.3 vs. 13.2 ± 0.3 kg·m2, p<0.01) and Oxprop (13.7 ± 0.4 vs. 14.3 ± 0.4 kg·m2, p<0.01) treated children. Additionally, LBMI in Oxprop was significantly greater than control at PRE (12.3 ± 0.3 vs. 13.7 ± 0.4 kg·m2, p<0.05) and POST RET (13.2 ± 0.3 vs. 14.3 ± 0.4 kg·m2, p=0.05). Lean mass as a percentage of total body mass was similar at PRE and POST time-points in both groups. The magnitude of change in total body mass, LBM, BMI, or LBMI were also not different between control and Oxprop groups.
Table 1.
DEXA analysis
| Control (n=22) | Oxprop (n=20) | |||
|---|---|---|---|---|
|
| ||||
| PRE | POST | PRE | POST | |
| Total body mass (kg) | 38.4 ± 2.0 | 40.5 ± 2.2** | 44.6 ± 2.9 | 47.3 ± 3.2** |
| Lean mass (kg) | 26.6 ± 1.7 | 28.4 ± 1.7** | 30.0 ± 1.9 | 31.9 ± 2.0** |
| % Lean mass | 69 ± 2 | 70 ± 1 | 69 ± 2 | 69 ± 1 |
| BMI | 18.0 ± 0.5 | 19.1 ± 0.5** | 19.2 ± 0.7+ | 21.0 ± 0.8**+ |
| LBMI | 12.3 ± 0.3 | 13.2 ± 0.3** | 13.7 ± 0.4+ | 14.3 ± 0.4**+ |
Values are mean ± SE. BMI, Body mass index; LBMI, Lean body mass index.
p<0.01 vs. PRE,
p≤0.05 vs. control.
Resting energy expenditure
We calculated the degree of hypermetabolism in patients as a ratio of calculated REE measured by respiratory gas exchange to predicted REE estimated by the Harris-Benedict equation. REE differed significantly from PRE to POST RET in control (148 ± 6 vs. 122 ± 7 % predicted, p<0.01) and in Oxprop treated patients (121 ± 6 vs. 102 ± 3 % predicted, p<0.05) (Fig. 1A). Furthermore, Oxprop treated patients had a significantly lower REE than the control group at both PRE (148 ± 6 vs. 121 ± 6 % predicted, p<0.01) and POST (122 ± 7 vs. 102 ± 3% predicted, p=0.01) time-points. The change in REE from PRE to POST was not different between groups (Fig. 1B).
Figure 1.
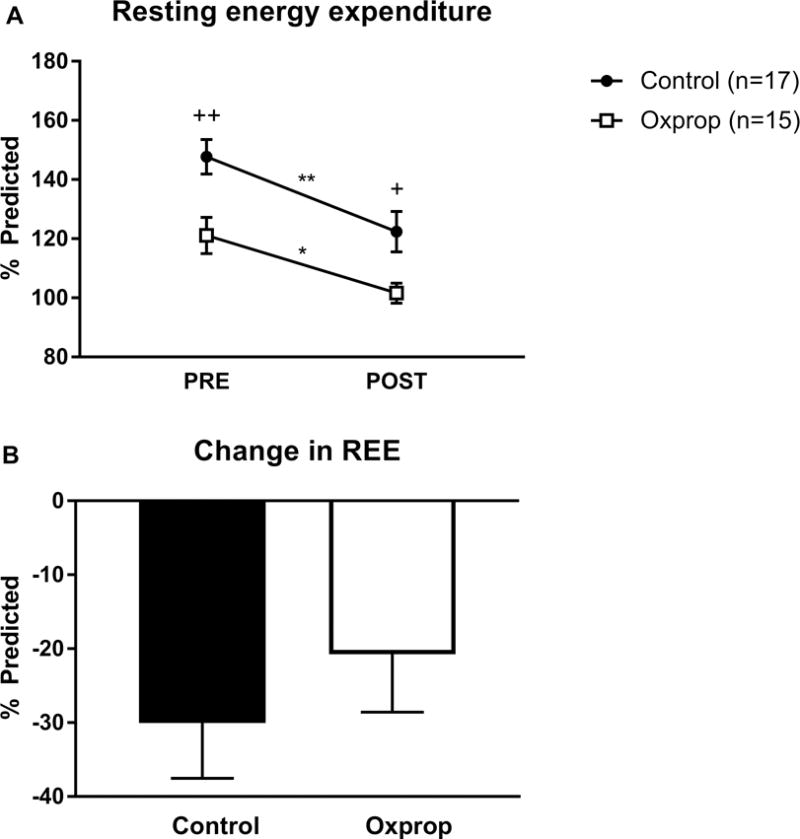
Resting energy expenditure in control and Oxprop treated children with severe burns PRE and POST exercise training (A). Resting energy expenditure (REE) was measured by indirect calorimetry and compared to the predicted value by Harris-Benedict equation. Change in REE (B) was determined by substracting POST REE from PRE. *p<0.05 vs. PRE, **p<0.01 vs. PRE, +p<0.05 vs. control, ++p<0.01 vs control.
Resting heart rate
Resting heart rate (RHR) was reduced from PRE to POST in control (120 ± 3 vs. 110 ± 3 bpm, p<0.05) and Oxprop (102 ± 3 vs. ± 92 ± 4 bpm, p<0.05) groups. Additionally, RHR was significantly lower in the Oxprop group compared to control at both the PRE (120 ± 3 vs. 102 ± 3 bpm, p<0.01) and POST (110 ± 2 vs. 92 ± 4 bpm, p<0.001) time-points (Fig. 2A). However, the magnitude of change from PRE to POST were not significantly different between groups (−9 ± 3 vs. −10 ± 4 bpm, p>0.05) (Fig. 2B).
Figure 2.
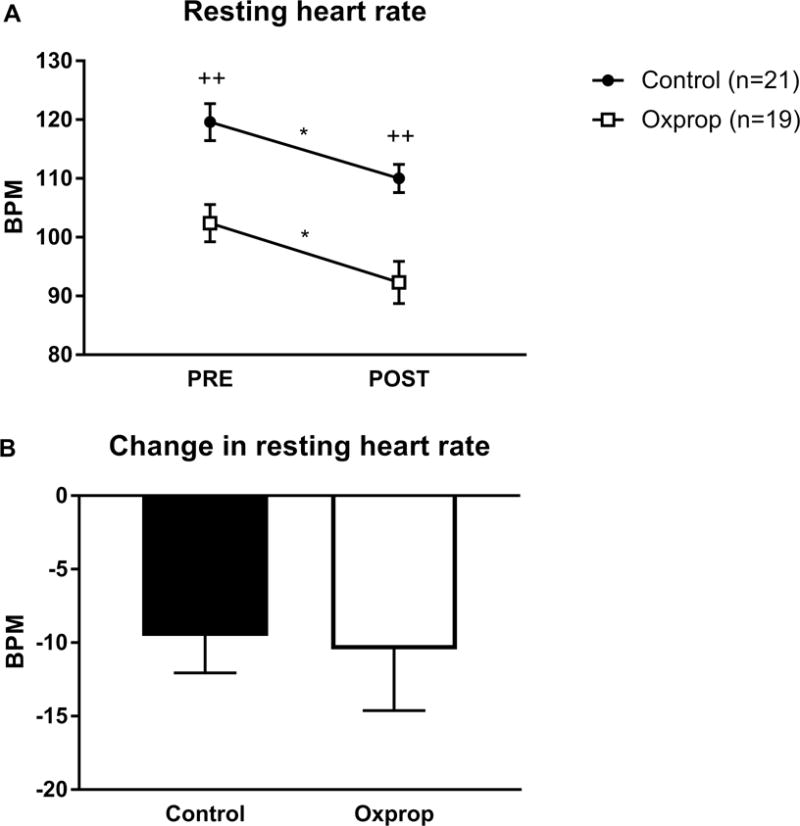
Resting heart rate in children with severe burns in control and Oxprop PRE and POST exercise (A). The change in resting heart rate was determined by the difference seen POST minus PRE (B). Bpm, beats per minute. *p<0.05 vs. PRE; ++p<0.01 vs. control.
Muscle protein turnover
FSR did not significantly change from PRE to POST in control (0.17 ± 0.03 vs. 0.07 ± 0.01 %·h−1, p>0.05) or Oxprop treated patients (0.09 ± 0.01 vs. 0.11 ± 0.09 %·h−1, p>0.05). FSR was not significantly different in control vs. Oxprop at PRE (0.17 ± 0.03 vs. 0.09 ± 0.01 %·h−1, respectively, p>0.05). However, FSR was significantly higher in the Oxprop vs. control at POST (0.11 ± 0.09 vs. 0.07 ± 0.01 %·h−1, respectively, p<0.01) (Fig. 3A). Control patients showed improvement in FBR from PRE to POST (0.35 ± 0.06 vs. 0.19 ± 0.04 %·h−1, p = 0.05). FBR was not significantly changed in Oxprop from PRE to POST (0.14 ± 0.01 vs. 0.11 ± 0.02 %·h−1, p>0.05). However, FBR was significantly lower at PRE in Oxprop vs control (0.14 ± 0.01 vs 0.35 ± 0.06 %·h−1, respectively, p<0.01) (Fig 3B). FBR tended to be lower in Oxprop vs control at POST (0.11 ± 0.02 vs 0.19 ± 0.04 %·h−1, respectively, p=0.06), but it was not statistically significant. Protein net balance was determined by subtracting FBR from FSR. No improvement was found from PRE to POST in control (−0.18 ± 0.06 vs. −0.12 ± 0.04 %·h−1, p>0.05) or Oxprop (−0.05 ± 0.02 vs. 0.01 ± 0.03 %·h−1, p>0.05). Protein net balance was less negative in Oxprop group compared to the control group at the PRE (−0.05 ± 0.02 vs. −0.18 ± 0.06 %·h−1, respectively, p<0.05) and POST RET (0.01 ± 0.03 vs. −0.12 ± 0.04 %·h−1, respectively, p<0.05) time-points (Fig 3C).
Figure 3.
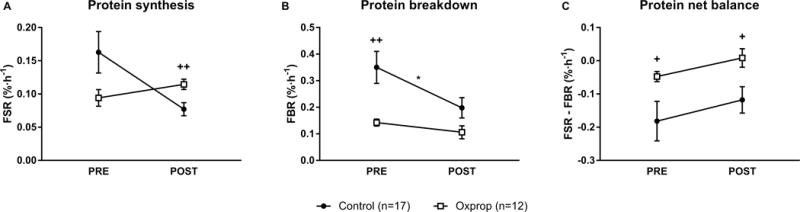
Skeletal muscle protein turnover in control and Oxprop treated children with severe burns PRE and POST 6-weeks outpatient exercise training. Protein synthesis (A), breakdown (B), and net balance (C) were measured utilizing stable isotope tracers of phenylalanine. FSR, fractional synthesis rate; FBR, fractional breakdown rate. *p≤0.05 vs. PRE, +p≤0.05 vs. control, ++p<0.01 vs. control.
Cardiorespiratory fitness
VO2peak significantly increased from PRE to POST in control (23.9 ± 1.6 vs. 29.8 ± 1.4 ml·kg−1·min−1, p<0.001) and Oxprop (24.3 ± 1.6 vs 31.0 ± 1.7 ml·kg−1·min−1, p<0.001) groups. No significant differences were found in the change of VO2peak (6.3 ± 1.2 vs. 7.1 ± 1.2 ml·kg−1·min−1, p>0.05), and no differences in absolute values were found between groups at PRE (23.9 ± 1.6 vs. 24.3 ± 1.6 ml·kg−1·min−1, p>0.05) and POST (29.8 ± 1.4 vs 31.0 ± 1.7 ml·kg−1·min−1, p>0.05) time-points.
Muscle strength
Absolute muscle strength significantly improved from PRE to POST in control (35.9 ± 3.7 vs. 50.3 ± 4.1 N·m, p<0.001) and Oxprop (46.3 ± 5.8 vs. 69.8 ± 7.1 N·m, p<0.001) groups (Fig 4A). No significant differences in strength were seen between control and Oxprop at PRE (35.9 ± 3.7 vs. 46.3 ± 5.8 N·m, p>0.05), but strength was significantly greater in Oxprop than control POST RET (50.3 ± 4.1 vs. 69.8 ± 7.1 N·m, p<0.05). The change from PRE to POST was significantly greater in Oxprop vs control (22.3 ± 2.0 vs. 14.4 ± 1.5 N·m, respectively, p<0.01) (Fig 4B). Similar results were found when absolute strength was normalized to body weight. Relative muscle strength improved from PRE to POST in both control (0.91 ± 0.06 vs. 1.22 ± 0.04 N·m·kg−1, p<0.001) and Oxprop (0.99 ± 0.08 vs. 1.41 ± 0.08 N·m·kg−1, p<0.001). No differences in relative or absolute strength were found between groups at PRE (p>0.05), but Oxprop treated patients had higher relative strength than control POST RET (1.41 ± 0.08 vs. 1.22 ± 0.04 N·m·kg−1, respectively, p< .05) (Fig 4C). The improvement in relative strength with RET was significantly greater in Oxprop compared to control (0.31 ± 0.03 vs. 0.40 ± 0.03 N·m·kg−1, p<0.05) (Fig. 4D).
Figure 4.
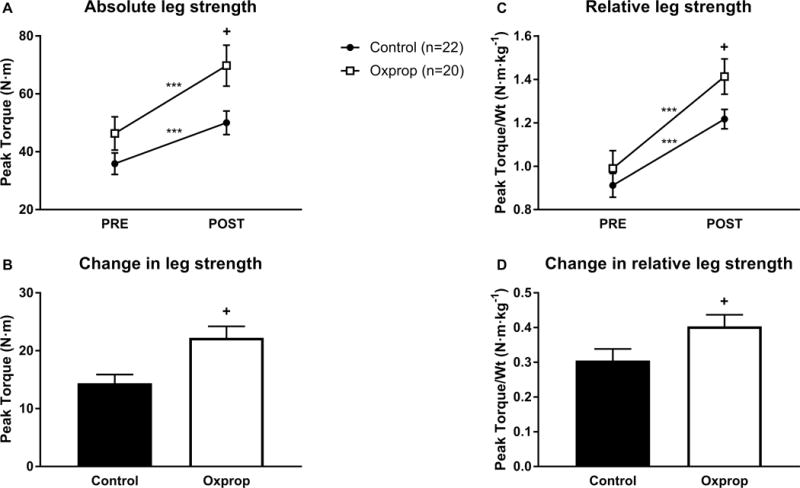
Muscle strength in control and Oxprop treated patients PRE and POST 6-weeks exercise training by absolute strength (A) or relative strength (C). The change in absolute strength (B) and relative strength (D) was calculated by subtracting PRE from POST measures. ***p<0.001 vs. PRE; +p<0.05 vs. control.
Muscle power
Average muscle power significantly improved from PRE to POST in control (44.9 ± 5.9 vs. 61.6 ± 5.5 W, p<0.001) and Oxprop (58.1 ± 7.6 vs. 89.8 ± 10.3 W, p<0.001) (Fig. 5A). There were no significant differences in average power at PRE between control and Oxprop (44.9 ± 5.9 vs. 58.1 ± 7.6 W, respectively, p>0.05). However, power was significantly greater in Oxprop vs. control POST RET (89.8 ± 10.3 vs. 61.6 ± 5.5 W, respectively, p<0.05). The magnitude of change was significantly greater in Oxprop vs. control (16.7 ± 2.1 vs. 30.8 ± 2.7 W, p<0.01) (Fig. 5B).
Figure 5.
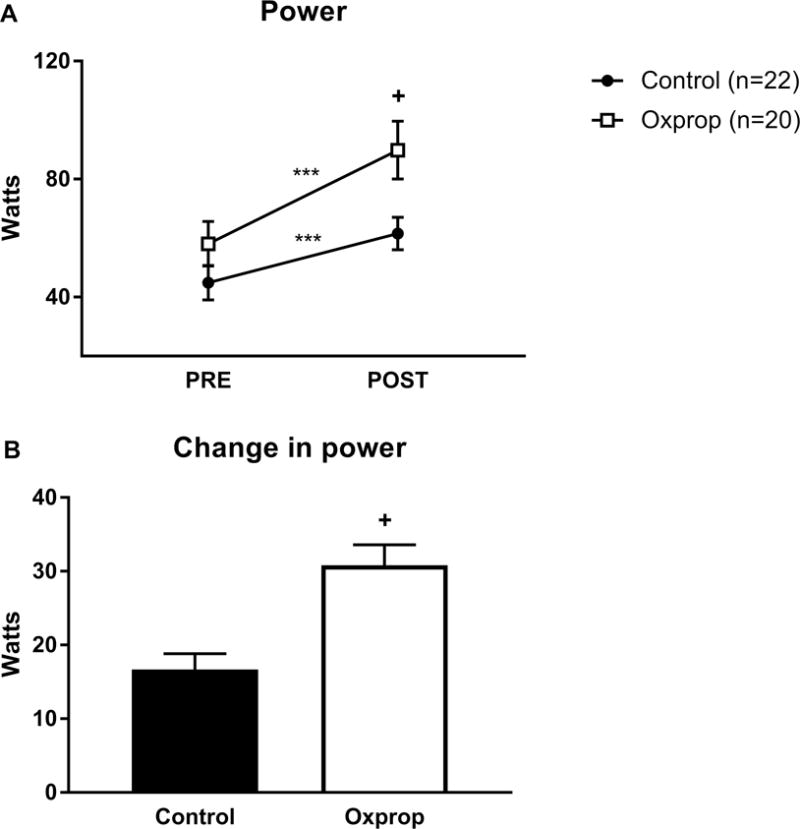
Muscle power in control and Oxprop treated children with severe burns PRE and POST 6-weeks exercise training program (A). Change in strength was determined by subtracting PRE from POST measures (B). ***p < 0.001 vs PRE; +p < 0.01 vs control.
Discussion
Severe burn injury results in an extreme pathophysiological stress response characterized by increased REE, elevated HR, and a marked loss of skeletal muscle mass and function (2–4, 6, 35). Severe muscle cachexia increases morbidity and mortality in burn survivors (36, 37). Individuals with severe burns have reduced functional capacity long after their skin wounds have healed (7). Therefore, it is imperative to develop new rehabilitation strategies that hasten the restoration of skeletal muscle mass and function following severe burns. Our study shows for the first time, that pharmacological treatment with the combination of oxandrolone and propranolol (Oxprop) augments the beneficial effects of outpatient exercise therapy in children recovering from severe burns. This suggests that long-term therapy with Oxprop and exercise may hold value in improving outcomes in severely burned children.
In line with our previous observations, propranolol and oxandrolone treatment blunt the hypermetabolic response to severe burns. Specifically, RHR and REE were significantly lower in the Oxprop group compared to control at PRE and POST exercise training. While the effect of Oxprop on HR was undoubtedly the result of β-blockade on the receptors on cardiomyocytes, the decrease in REE may be mediated by both propranolol and oxandrolone, since these drugs have both been shown to independently lower REE in severely burned children (18, 19, 38). We should note that the current study was not designed to test this hypothesis.
Our control and Oxprop treated groups showed significant improvement in body composition following RET. Furthermore, both groups exhibited significant improvement in functional performance in terms of absolute and relative strength, and average power production. The improvement seen in both groups after exercise supports the utility of RET in severely burned individuals. Although we did not have a non-exercising group to compare the data and account for the effect of time as patients convalesce, previous randomized studies suggest that improvements in muscle mass and strength in the first few months post burn injury are significantly augmented in patients who participate in an exercise program compared to those who do not (9, 13, 39).
Although beneficial effects in body composition were seen in both control and Oxprop after RET, changes in various parameters of body composition were largely not different between groups. However, BMI and LBMI were significantly higher in Oxprop than control at PRE and POST RET suggesting that Oxprop therapy may improve the maintenance of lean mass during their acute hospitalization period and through the duration of the study. However, we did not find significant differences in total or lean mass with the addition of Oxprop therapy with RET. In a previous study, oxandrolone and RET increased LBM in burned children to greater degree that in burned children receiving RET alone (17). In that particular study the placebo and non-exercise group showed no change in lean mass, the placebo and exercise group had 5.8% increase in lean mass, the oxandrolone with no exercise showed 5.4% increase in lean mass, and the oxandrolone with exercise showed 13.1% increase in lean mass (17). Both of our groups showed a 6.8% and 6.3% increase in control and Oxprop, respectively. The increase in lean mass of our subjects appear to be greater than the group who had no exercise or oxandrolone but similar to the oxandrolone only group and placebo with exercise groups of the study by Przkora et al. (17). However, that exercise program persisted for 12-weeks and was performed at six- to nine-months post-injury. Since that study protocol was double the length of this exercise training program employed in the current protocol, and was also performed at a later time frame post-injury, it may offer an explanation for the reason we did not find a greater increase in lean mass in our Oxprop group over control.
We observed time and drug effect on skeletal muscle protein turnover. There was a reduction in FSR in the control group, which is in agreement with the previous findings of Hardee et. al., where FSR significantly decreased following 12 weeks of exercise training (13). Conversely, our Oxprop group showed a significant increase in FSR following RET suggesting that it is Oxprop itself that promoted skeletal muscle synthesis in burn rehabilitation and not exercise. Skeletal muscle FBR was greater in the control versus Oxprop group prior to RET. FBR tended to decrease after RET in both groups, although not statistically significant. The trend in reduction of both FSR and FBR in our control group may indicate the reduction in overall protein turnover as a result of the gradual reduction in hypermetabolism and protein turnover associated with the long-term healing process (40). Perhaps of most importance, protein net balance tended to improve (i.e. become more positive) in both groups. Interestingly though, protein net balance was significantly greater at both time points in the Oxprop group, providing evidence of a nitrogen sparing effect of this treatment strategy in catabolic burned children.
The nitrogen sparing effect of Oxprop therapy appeared to be time dependent. Prior to RET, Oxprop had a pronounced impact on nitrogen balance by blunting the rates of muscle proteolysis (FBR). In contrast, POST RET, Oxprop arguably had a greater effect on improving nitrogen balance by increasing the FSR of new proteins. As we discussed previously, in the first few months post burn injury, the FSR of muscle proteins is likely influenced by the elevated FBR, which continuously saturates intracellular free pool of amino acids (33). However, as muscle FBR declines in the months following burn injury, this effect on muscle FSR is perhaps lost. This may offer an explanation as to why Oxprop exerts an anti-catabolic effect at the first study time-point (~ 20 days post injury) and an anabolic effect at the second time-point (~ 106 days post injury). Our protein turnover data suggests that the protein net balance in skeletal muscle is improved over time with RET and the addition of Oxprop therapy augments this improvement.
We did not find any differences in VO2peak in the Oxprop versus control group at PRE or POST RET; nor was the improvement over time different between groups. Previously, data suggest that while oxandrolone does not augment RET mediated improvements in VO2peak in burned children (17), administration of propranolol during RET resulted in a greater improvement in VO2peak in burned children (36% increase in VO2peak) when compared to children who underwent RET without propranolol (22% increase in VO2peak) (23). It was speculated that this effect may be attributed to the reduced capillary blood flow allowing greater gas exchange in exercising muscles (23). Again, the RET program in this study was 12-weeks, unlike the 6-week RET program employed in the current study. Further, in a recent study comparing six- vs. twelve- weeks of RET in burned children found that relative VO2peak in 12-weeks trained patients was significantly greater than patients who participated in 6-weeks exercise training (39). Thus, although we found an improvement in cardiorespiratory fitness in both control (24.7 % increase in VO2peak) and Oxprop (27.6% increase in VO2peak) treated patients in the current study, it is conceivable that the differences in RET program duration training explain the discrepancy between our current data and our previously published work (23). Perhaps a significant difference in the amount of VO2peak increase between our control and Oxprop group might be found if the exercise period was doubled to 12-weeks.
In the current study, RET alone resulted in a significant improvement in absolute and relative muscle strength, and average muscle power. Previous work has shown that propranolol (23) or oxandrolone (17) administered during RET did not further improve muscle function when compared to RET alone. In those previous studies, they found that after 12-weeks of exercise with oxandrolone muscle strength increased by 48.3% (17), whereas strength increased by 50% when exercise was combined with propranolol (23). In our study, we found that strength increased by 50.8% when the combination of oxandrolone and propranolol was utilized with exercise. Again, it should be noted that it is not an equal comparison with our current study and those referenced studies because they tested patients at approximately six- to nine-months post-burn using a 12-week RET program, whereas we tested our patients approximately one month post-burn using a 6-week RET program. However, our current data suggests that combined treatment with oxandrolone and propranolol (Oxprop) augments the improvement in muscle function brought about by exercise over the current standard of care. Further, muscle function was subsequently greater in the Oxprop group compared to control after RET, suggesting that functional capacity may be restored to a greater degree when Oxprop therapy is combined with exercise.
There are some limitations to our study. First, we did not have an oxandrolone alone and propranolol alone group. We previously determined the individual effects of oxandrolone with exercise (17) and propranolol with exercise (23). With the resources that were available for this study, it was only feasible to study the effects of combined use of Oxprop versus standard of care. Therefore, our approach in the current study was to compare a new treatment strategy (oxandrolone + propranolol + early exercise) versus the current standard of care (early exercise only) on clinical and functional outcomes. Secondly, our protein turnover analysis only provides a snapshot of the rates of protein synthesis and breakdown in the fasted state. Also, nutrition and physical activity were not directly monitored during the intervention. Changes in weight and body composition can be influenced by diet. Finally, physical activity outside of exercise sessions was not quantified in the current study. Despite these limitations, our data show that in free-living patients, the combined treatment of oxandrolone and propranolol blunts hypermetabolism and augments the beneficial effect of a 6-week RET program.
When undertaking this clinical trial, standard burn care at our institution did not include propranolol or oxandrolone therapy. Previously, clinical trials have shown that propranolol attenuates hypermetabolism in burn patients (19, 22), while improving aerobic exercise capacity in burned children when combined with exercise (23). Previous studies have also demonstrated an anabolic effect of oxandrolone on muscle mass following severe burns (16), as well as an additive effect in restoring muscle mass after burn injury when provided during a rehabilitative exercise regime (17). The novelty of this study was in demonstrating a beneficial effect of Oxprop therapy over standard of care in restoring body composition and function during a RET regime performed immediately after hospital discharge.
To summarize, in agreement with previous studies (13, 17, 23, 24), we show that and early outpatient exercise rehabilitation program improves body composition, muscular function, and cardiorespiratory fitness in burned children. For the first time, we show that the addition of Oxprop therapy with exercise further blunted hypermetabolism and improved skeletal muscle protein turnover in burned children. Further, in combination with a 6-week RET program, the addition of Oxprop treatment augmented RET-induced improvements in muscle mass and function in burned children. Therefore, combined long-term drug therapy with testosterone analogues and β-blockers with exercise may be of benefit in hastening the rehabilitation of severely burned individuals.
Acknowledgments
We would like to thank Clark Andersen for assisting us with the statistical analyses, Ileanna Gutierrez, Shauna Glover, and Angie Agudelo for their work with the children in the Wellness Center.
Source of Funding:
The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
This study was funded by the National Institutes of Health (P50 GM060338, R01 GM56687, R01 HD049471, R01 AR049877, P30 AG024832, T32 GM008256, NIDILRR 90DP0043-01-00), Shriners Hospitals for Children (84080, 84090, 71006, 71009, 85310).
T.C. was supported in part by an appointment to the Postgraduate Research Participation Program at the U.S. Army Institute of Surgical Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and USAMRMC.
A.S. was funded by the Greek State Scholarships Foundation and the European Program “Education and Lifelong Learning”, European Social Fund (ESF) NSRF 2007–2013.
Footnotes
Conflicts of Interest: The authors have no conflicts of interests to declare.
References
- 1.Williams FN, Herndon DN, Hawkins HK, et al. The leading causes of death after burn injury in a single pediatric burn center. Critical Care. 2009;13(6) doi: 10.1186/cc8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Annals of Surgery. 2008;248(3):387–400. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128(2):312–9. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 4.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Annals of Surgery. 2000;232(4):455–63. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrando AA, Lane HW, Stuart CA, DavisStreet J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. American Journal of Physiology-Endocrinology and Metabolism. 1996;270(4):E627–E33. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- 6.Alloju SM, Herndon DN, McEntire SJ, Suman OE. Assessment of muscle function in severely burned children. Burns. 2008;34(4):452–9. doi: 10.1016/j.burns.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 7.St-Pierre DMM, Choiniere M, Forget R, Garrel DR. Muscle strength in individuals with healed burns. Archives of Physical Medicine and Rehabilitation. 1998;79(2):155–61. doi: 10.1016/s0003-9993(98)90292-1. [DOI] [PubMed] [Google Scholar]
- 8.Holavanahalli RK, Helm PA, Kowalske KJ. Long-Term Outcomes in Patients Surviving Large Burns: The Musculoskeletal System. Journal of Burn Care & Research. 2016;37(4):243–54. doi: 10.1097/bcr.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 9.Porter C, Hardee JP, Herndon DN, Suman OE. The Role of Exercise in the Rehabilitation of Patients with Severe Burns. Exercise and Sport Sciences Reviews. 2015;43(1):34–40. doi: 10.1249/jes.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neugebauer CT, Serghiou M, Herndon DN, Suman OE. Effects of a 12-Week Rehabilitation Program With Music & Exercise Groups on Range of Motion in Young Children With Severe Burns. Journal of Burn Care & Research. 2008;29(6):939–48. doi: 10.1097/BCR.0b013e31818b9e0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. Journal of Applied Physiology. 2001;91(3):1168–75. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 12.Suman OE, Mlcak RP, Herndon DN. Effect of exercise training on pulmonary function in children with thermal injury. Journal of Burn Care & Rehabilitation. 2002;23(4):288–93. doi: 10.1097/01.bcr.0000020443.04389.6b. [DOI] [PubMed] [Google Scholar]
- 13.Hardee JP, Porter C, Sidossis LS, et al. Early Rehabilitative Exercise Training in the Recovery from Pediatric Burn. Medicine and Science in Sports and Exercise. 2014;46(9):1710–6. doi: 10.1249/mss.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf SE, Edelman LS, Kemalyan N, et al. Effects of oxandrolone on outcome measures in the severely burned: A multicenter prospective randomized double-blind trial. Journal of Burn Care & Research. 2006;27(2):131–9. doi: 10.1097/01.bcr.0000202620.55751.4f. [DOI] [PubMed] [Google Scholar]
- 15.Wolf SE, Thomas SJ, Dasu MR, et al. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Annals of Surgery. 2003;237(6):801–11. doi: 10.1097/00000658-200306000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Annals of Surgery. 2001;233(4):556–64. doi: 10.1097/00000658-200104000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Przkora R, Herndon DN, Suman OE. The effects of oxandrolone and exercise on muscle mass and function in children with severe burns. Pediatrics. 2007;119(1):E109–E16. doi: 10.1542/peds.2006-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baron PW, Barrow RE, Pierre EJ, Herndon DN. Prolonged use of propranolol safely decreases cardiac work in burned children. The Journal of burn care & rehabilitation. 1997;18(3):223–7. doi: 10.1097/00004630-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. New England Journal of Medicine. 2001;345(17):1223–9. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 20.Williams FN, Herndon DN, Kulp GA, Jeschke MG. Propranolol decreases cardiac work in a dose-dependent manner in severely burned children. Surgery. 2011;149(2):231–9. doi: 10.1016/j.surg.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flores O, Stockton K, Roberts JA, Muller MJ, Paratz JD. The efficacy and safety of adrenergic blockade after burn injury: A systematic review and meta-analysis. Journal of Trauma and Acute Care Surgery. 2016;80(1):146–55. doi: 10.1097/ta.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 22.Herndon DN, Rodriguez NA, Diaz EC, et al. Long-Term Propranolol Use in Severely Burned Pediatric Patients A Randomized Controlled Study. Annals of Surgery. 2012;256(3):402–11. doi: 10.1097/SLA.0b013e318265427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porro LJ, Al-Mousawi AM, Williams F, Herndon DN, Mlcak RP, Suman OE. Effects of Propranolol and Exercise Training in Children with Severe Burns. Journal of Pediatrics. 2013;162(4):799–+. doi: 10.1016/j.jpeds.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Mousawi AM, Williams FN, Mlcak RP, Jeschke MG, Herndon DN, Suman OE. Effects of Exercise Training on Resting Energy Expenditure and Lean Mass During Pediatric Burn Rehabilitation. Journal of Burn Care & Research. 2010;31(3):400–8. doi: 10.1097/BCR.0b013e3181db5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diego AM, Serghiou M, Padmanabha A, Porro LJ, Herndon DN, Suman OE. Exercise Training After Burn Injury: A Survey of Practice. Journal of Burn Care & Research. 2013;34(6):E311–E7. doi: 10.1097/BCR.0b013e3182839ae9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson MA, Goldberg B, Harris SS, et al. RISKS IN DISTANCE RUNNING FOR CHILDREN. Pediatrics. 1990;86(5):799–800. [PubMed] [Google Scholar]
- 27.Small EW, McCambridge TM, Benjamin HJ, et al. Strength training by children and adolescents. Pediatrics. 2008;121(4):835–40. doi: 10.1542/peds.2007-3790. [DOI] [PubMed] [Google Scholar]
- 28.Cumming GR, Everatt D, Hastman L. Bruce treadmill test in children: normal values in a clinic population. Am J Cardiol. 1978;41(1):69–75. doi: 10.1016/0002-9149(78)90134-0. Epub 1978/01/01. [DOI] [PubMed] [Google Scholar]
- 29.Bruce RA, McDonough JR. Stress testing in screening for cardiovascular disease. Bull N Y Acad Med. 1969;45(12):1288–305. Epub 1969/12/01. [PMC free article] [PubMed] [Google Scholar]
- 30.Haarbo J, Gotfredsen A, Hassager C, Christiansen C. Validation of body-composition by dual energy x-ray absorptiometry (DEXA) Clinical Physiology. 1991;11(4):331–41. doi: 10.1111/j.1475-097X.1991.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 31.Weir JBD. New methods for calculating metabolic rate with special reference to protein metabolism. Journal of Physiology-London. 1949;109(1–2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roza AM, Shizgal HM. The Harris-Benedict equation reevaluated - resting energy-requirements and the body cell mass. American Journal of Clinical Nutrition. 1984;40(1):168–82. doi: 10.1093/ajcn/40.1.168. [DOI] [PubMed] [Google Scholar]
- 33.Chao T, Herndon DN, Porter C, et al. SKELETAL MUSCLE PROTEIN BREAKDOWN REMAINS ELEVATED IN PEDIATRIC BURN SURVIVORS UP TO ONE-YEAR POST-INJURY. Shock. 2015;44(5):397–401. doi: 10.1097/shk.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang XJ, Chinkes DL, Wolfe RR. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. American Journal of Physiology-Endocrinology and Metabolism. 2002;283(4):E753–E64. doi: 10.1152/ajpendo.00053.2002. [DOI] [PubMed] [Google Scholar]
- 35.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-Term Persistance of the Pathophysiologic Response to Severe Burn Injury. Plos One. 2011;6(7) doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang DW, DeSanti L, Demling RH. Anticatabolic and anabolic strategies in critical illness: A review of current treatment modalities. Shock. 1998;10(3):155–60. doi: 10.1097/00024382-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe RR. The underappreciated role of muscle in health and disease. American Journal of Clinical Nutrition. 2006;84(3):475–82. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 38.Porro LJ, Herndon DN, Rodriguez NA, et al. Five-Year Outcomes after Oxandrolone Administration in Severely Burned Children: A Randomized Clinical Trial of Safety and Efficacy. Journal of the American College of Surgeons. 2012;214(4):489–502. doi: 10.1016/j.jamcollsurg.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clayton RP, Wurzer P, Andersen CR, Mlcak RP, Herndon DN, Suman OE. Effects of different duration exercise programs in children with severe burns. Burns. 2016 doi: 10.1016/j.burns.2016.11.004. Epub 2016/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz EC, Herndon DN, Porter C, Sidossis LS, Suman OE, Borsheim E. Effects of pharmacological interventions on muscle protein synthesis and breakdown in recovery from burns. Burns. 2014 doi: 10.1016/j.burns.2014.10.010. Epub 2014/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]


