Abstract
Objective
(1) To examine language performance in the context of cognitive abilities in young children who are deaf or hard-of-hearing; and (2) to identify factors associated with having a language underperformance, defined as a gap between the language standard score and nonverbal IQ (NVIQ) standard score.
Methods
Children 6-82 months of age with bilateral hearing loss were enrolled. Language performance was defined as a ratio of language skills relative to cognitive abilities with language underperformance defined as a ratio of language score to NVIQ<0.85.
Results
Among 149 children, approximately half had hearing loss that was clinically classified as mild or moderate and over one-third received a cochlear implant. Participants had a mean NVIQ in the average range (95.4 (20.3)). Receptive language scores were significantly lower than their NVIQ by 10.6 points (p<.0001). Among children with NVIQs 80-100, 62.5% had receptive scores <85 and 50% had a language underperformance (ratio <0.85). Among children with NIVQs >100, 21.1% had receptive scores <85 with 42% having a language underperformance. Children with language underperformance (n=61, 41.5%) were more likely to have more severe levels of hearing loss, lower socioeconomic status, and be nonwhite.
Conclusion
Many children early identified with hearing loss continue to demonstrate language underperformance, defined using their cognitive potential. Language deficits have a cascading effect on social functioning in children who are deaf or hard-of-hearing. This study highlights the need to understand a child's cognitive potential to adequately address language needs in existing intervention models.
Key terms: hearing loss, child development, language outcomes, predictors
Introduction
Hearing loss is one of the most common birth conditions. Approximately 2 to 3 per 1000 infants are identified at birth each year with a hearing loss which can impact development.1 The implementation of Universal Newborn Hearing Screening in the United States introduced early hearing detection and intervention (EHDI) recommendations, decreasing the age at which infants and toddlers are identified with a hearing loss and receive subsequent intervention. As a result, we have seen marked improvements in language acquisition for children who are deaf or hard-of-hearing (DHH).2,3 The increased opportunities to develop appropriate language skills are particularly pertinent for children who are DHH. Hearing loss can influence all aspects of a child's language acquisition, particularly if the primary communication strategy is oral communication.4 Language deficits, even if seemingly minor, have a cascading effect on social and communication functioning in children who are DHH.5-7 Additionally, these language deficits often worsen rather than improve through the school years, irrespective of hearing loss severity.8,9
Through the timely provision of early intervention services, infants and toddlers who are DHH have improved language abilities.2,10 While this is encouraging, many children who are DHH have average to low average language levels, despite their cognitive potential.2,6,11,12 For children with higher cognitive abilities (such as above average nonverbal intelligence), we expect that their capability to develop language could be in the above average range. Thus, scores in the average to low average range for children with higher cognitive abilities could be considered an “underperformance.” Therefore, we sought to better understand language performance of children who are DHH in the context of nonverbal abilities so that we could identify children who were not performing to their full cognitive potential, albeit average language scores. Thus, the main objectives of the current study were (1) to examine language performance in the context of cognitive abilities in a sample of young children, 6 months through 6 years of age, who are DHH; and (2) to identify factors that are associated with having a language underperformance, which we defined as a gap between the language standard score and the nonverbal IQ standard score. We hypothesized that a proportion of children who are DHH will be identified with a language underperformance, as defined by language levels relative to cognitive levels. We further hypothesized that there would be identifiable factors independently associated with language underperformance.
Participants
This study stems from a prospective cohort study of language and functional outcomes, with enrollment occurring in two phases. Phase 1 occurred between 2011 and 2014 and enrolled children between the ages of 3 and 6 years who had bilateral hearing loss of any degree (mild to profound). Children who were unable to complete standardized testing (including those with vision impairments), children who had a known syndrome or disorder that specifically affected language (e.g., Williams Syndrome, autism spectrum disorder), and children whose primary language was not English were excluded from the overall study. Phase 2 began in 2014 and enrolled children between 0 and 3 years of age using the same inclusion/exclusion criteria. We have previously reported on the first 65 children enrolled in the Phase 1 part of our study. This previous work focused on the impact of language on functional communication outcomes among children who are DHH.6
Parents of potentially eligible study participants were mailed a letter which included a brief study description. A follow-up phone call occurred approximately 2 weeks later to determine interest in the study. Parents could also actively contact study personnel through advertisements posted throughout the medical center (e.g., audiology clinic, otolaryngology clinic) and early intervention systems. The study was approved by the Institutional Review Board of a free-standing pediatric academic institution and written informed consent was obtained from all caregivers at the initial study visit.
Audibility
Objective information about the degree of hearing loss and the level of aided-hearing (via hearing aid or cochlear implant) was obtained from audiograms in audiologic charts. Data from audiograms closest to the study visit were used. For the purpose of this study, severity of hearing loss was classified using the clinical classification as it was reported on the audiogram (mild, moderate, moderate-to-severe, severe, severe-to-profound, profound). The clinical classification included the slope of the audiogram coupled with the decibel level across the different frequencies tested in the audiologic assessment. The classification assigned to the better hearing ear was used in the analysis. In addition, a four-frequency pure tone average (PTA) was calculated for both ears with the PTA of the better hearing ear used in the analysis. Effectiveness of amplification or cochlear implant was determined based on aided thresholds for hearing aid and cochlear implant recipients. Aided speech reception/speech awareness thresholds (SRT/SAT), which are measures of an individual's threshold for hearing speech, were collected from audiograms. These aided thresholds were a measure of access to sound or audibility. For three individuals who had no assistive hearing devices, the unaided PTA was used as the measure of audibility.
Child and Family Characteristics
All caregivers received a questionnaire at the initial study visit eliciting information regarding the child's primary strategies for communication; types, frequencies, and location of therapies attended; school attendance; insurance status; educational level of mother and father; and number of siblings in the home. Additional clinical information abstracted from the medical record (if available) included previous developmental assessment and information specific to hearing loss (i.e., age of identification, age of amplification, age at amplification, degree of hearing loss).
Assessments
Per the study protocol, all children received a language assessment and a neurocognitive assessment at the study visit. Parents filled out a questionnaire that collected information regarding demographics, schooling, and children's current therapy attendance. The Preschool Language Scales – 5th edition (PLS-5)13 was administered by a speech-language pathologist experienced with children who are deaf/hard-of-hearing to assess language skills. The PLS-5 includes Auditory Comprehension (for receptive language) and Expressive Communication subscales for children birth to 7 years, 11 months. Both subscales provide standard scores (mean of 100, standard deviation of 15), age equivalents and percentiles. The PLS-5 was selected to allow for a comprehensive language assessment for the range of age and cognitive abilities of participating children. The Leiter International Performance Scale-Revised (Leiter-R)14 was administered to children ≥24 months of age by a psychologist with experience in assessing young children who are deaf/hard-of-hearing. The Leiter-R is a nonverbal cognitive assessment and provides a standardized intelligence quotient (IQ) with a mean of 100 and standard deviation of 15. For the purpose of this analysis, the Leiter-R Brief IQ was used as the measure of nonverbal cognitive abilities. Children <24 months received the Mullen Scale of Early Learning15 which includes the Visual Reception domain. Because the Visual Reception domain capitalizes on nonverbal cognitive problem-solving skills, it was used as a measure of nonverbal cognitive abilities. The Visual Reception domain score is a t-score (mean of 50, standard deviation of 10), from which a standard score (mean of 100, standard deviation of 15) was calculated for the purpose of the analysis (to report one single nonverbal IQ or NVIQ). The Visual Reception domain has been shown to have predictive validity of later nonverbal cognitive abilities as measured on the Leiter-R.16
Language underperformance (primary outcome)
Because the aim of this study was to understand language in the context of cognitive or developmental abilities, we defined language performance as a ratio of language skills relative to cognitive abilities. We defined language performance in this way for the analysis for two reasons. First, a child with lower cognitive abilities would be expected to have lower language performance, making the interpretation of an absolute language score difficult in the context of varying cognitive abilities. Second, although a child's language score may indicate average language abilities as compared to same-age peers, the score does not indicate whether a child is meeting his/her individual capacity for language. To create this ratio, we divided the receptive standard score on the PLS-5 with the NVIQ [receptive standard score ÷ NVIQ]. Ratios close to 1 indicate receptive language levels that are commensurate with cognitive abilities or cognitive level. Language underperformance was defined as a ratio <0.85. A child with a ratio <0.85 would be considered to have language performance at 85% of the cognitive performance according to the NVIQ. We used a cutoff of 0.85 to define language underperformance for several reasons: 1) We previously reported the impact of a ratio cutoff of 0.80 on functional communication,6 but we felt clinically the original cutoff was too restrictive, possibly missing children; 2) From a clinical perspective, a ratio of 0.85 represents a degree of difference that is similar to the concept of 1 standard deviation below a population mean (mean=100, standard deviation=15), which is a degree of variation often associated with a clinically meaningful impact.
Statistical Analysis
Data analysis was conducted using SAS® version 9.3. Pearson correlations were used to assess the associations between the language ratio, nonverbal IQ, hearing-related factors, and demographic factors. Principle components analysis was used to create an index for socioeconomic status (SES) using the variables of maternal education level, income and insurance status.17 This index score was used in the regression analysis to control for possible confounding related to SES status. Higher SES scores indicate higher levels of SES. For the purpose of the analysis, NVIQ was analyzed both as a continuous variable and as a categorical variable (<80, 80-100, >100). The SES index score was also analyzed as a continuous variable and a categorical variable in quartiles.
General linear models were constructed to understand the independent and collective effects of predictors on language, as measured by the ratio (as a continuous variable). The nonverbal IQ was entered into the model first. Other covariates were selected into the model according to what was considered important for language development either clinically or through previous literature (e.g., age of hearing loss identification, age at study entry, degree of hearing loss, audibility, SES status). Additional covariates were included in the model if they were deemed to be correlated with the outcome in unadjusted analyses. Covariates remained in the final multivariable regression model if they were significant at the p≤0.05 level. The mean language ratios and mean language standard scores, after adjusting for confounders were compared across the NVIQ categories. Least square means with 95% confidence intervals were produced from the models and were adjusted for multiple comparisons using the Tukey-Kramer method. Models were assessed for influential outliers (through model diagnostic statistics) and a sensitivity analysis was performed to determine the effect of removing potentially influential observations from the overall model. Multiple imputation was used to address missing data for child or family characteristics. Rates of missing data were highest for information regarding aided thresholds (8.7% of observations, n=13).
Finally, to understand factors such as age, degree of hearing loss, and SES status that are associated with having a language underperformance (having a ratio<0.85) Pearson's χ2 tests and Student's t tests were used. Multiple logistic regression was used to determine which factors were independently associated with the outcome. Degree of hearing loss and SES status were included as categorical variables. Results were reported as adjusted odds ratios (OR) with 95% confidence intervals (CI).
Results
One hundred fifty-two children had enrolled; 3 participants were excluded from the analysis due to a subsequent diagnosis of autism spectrum disorder that was suspected at the time of the study visit. These 3 children were subsequently evaluated and clinically diagnosed through interdisciplinary developmental evaluations. The mean (SD) age at the baseline visit for the 149 participants included in this analysis was 47.7 (19) months; 79 (53.7%) were <48 months of age. Approximately half of the children had hearing loss that was clinically classified as either mild or moderate, while over one third had received at least one cochlear implant. The etiology of hearing loss was unknown for 42% (n=62) participants. Syndromes associated with the etiology of hearing loss included Ushers syndrome (n=4), Towne-Brocks (n=2), Waardenburg (n=2), Sticklers (n=1), Branchio-Oto-Renal (n=1), CHARGE (n=1), Pendred (n=1), VATER with aural atresia (n=1). Table 1 includes a description of the characteristics of the study participants. No caregiver of the study participants was DHH.
Table 1. Participant characteristics.
| Characteristics | N=149 |
|---|---|
| Mean Age at study in months | 47.2 (18.9) Range: 6-82 months |
|
| |
| Mean Age at HL identification in months | 10.3 (12.3); Median 4 |
|
| |
| Male | 85 (57.1%) |
|
| |
| Race | |
| Caucasian | 120 (80.5%) |
| African American | 23 (15.4%) |
| Asian | 3 (2%) |
| Other | 3 (2%) |
|
| |
| Hispanic | 6 (4%) |
|
| |
| Born premature | 29 (19.5%) |
| Etiology of hearing loss | |
| Unknown | 61 (40.9%) |
| Inner ear malformations | 18 (12.1%) |
| Prematurity related | 17 (11.4%) |
| Genetic | 15 (10.1%) |
| Syndrome-related | 13 (8.7%) |
| Infection (in-utero) | 9 (6.0%) |
| Other | 16 (10.7%) |
|
| |
| Degree of hearing loss | |
| Mild | 20 (13.4%) |
| Moderate | 62 (41.6%) |
| Severe | 28 (18.8%) |
|
| |
| Profound | 39 (26.2%) |
|
| |
| Received a cochlear implant | 54 (36.7%) |
|
| |
| Age at cochlear implantation surgery | 26.3 (14.8) |
|
| |
| Duration with implant in months | 26.3 (16.9); Median 24 |
|
| |
| Mother's education | |
| Some HS | 2 (1.3%) |
| HS/GED | 23 (15.4%) |
| Some college | 37 (24.8%) |
| College graduate | 46 (30.9%) |
| Post college | 40 (26.9%) |
| Unknown | 1 (0.7%) |
|
| |
| Father's education | |
| <8th grade | 2 (1.3%) |
| Some HS | 10 (6.7%) |
| HS/GED | 28 (18.8%) |
| Some college | 25 (16.8%) |
| College graduate | 47 (31.5%) |
| Post college | 27 (18.1%) |
| Unknown/NA | 10 (6.7%) |
|
| |
| Insurance coverage | |
| Private Insurance | 73 (49%) |
| Public health insurance only | 51 (34.2%) |
| Combination of private and public | 25 (16.8%) |
|
| |
| Income at or below poverty level | 24 (16.1%) |
|
| |
| Socioeconomic status* | |
|
| |
| 1 (Lowest) | 38 (25.5%) |
| 2 | 33 (22.2%) |
| 3 | 26 (17.5%) |
| 4 (highest) | 48 (32.2%) |
| unknown | 4 (2.7%) |
|
| |
| Nonverbal IQ | 95.5 (20.2) |
|
| |
| NVIQ Categories | |
| <80 | 31 (20.8%) |
| 80-100 | 40 (26.9%) |
| >100 | 78 (52.4%) |
|
| |
| Language receptive standard score | 85 (18.8) |
|
| |
| Language expressive standard score | 85 (18.9) |
|
| |
| Language receptive score <85 | 71 (47.7%) |
|
| |
| Ratio of receptive language to NVIQ | 0.89 (0.22) |
|
| |
| Ratio <0.85 | 61 (41%) |
Quartiles of SES index using mother's education, father's education and income level. Lower quartiles indicate lower SES status.
Language performance in children who are deaf or hard-of-hearing
Participants had a mean NVIQ in the average range (95.4 (20.3)). The NVIQ was derived from the Mullen Scales in 34 participants. Receptive language scores were significantly lower than children's NVIQ by 10.6 points (p<.0001). There was no difference between the receptive standard score and expressive standard score (p=0.99) (see Table 1). Receptive language standard scores were not associated with age at the study visit (r=-0.10; p=0.23), age of hearing loss identification (r=0.03; p=0.75), or the duration a child may have had either a hearing aid or cochlear implant (r=0.09; p=0.32).
In order to understand language performance in the context of a child's cognitive abilities, we focused on the ratio of the receptive standard score to the NVIQ. The mean ratio for all participants was 0.89 (0.22); 61 participants (41.5%) had a ratio <0.85 indicating the presence of language underperformance. Sixteen participants (10.7%) had a ratio >115. According to results from the correlation analyses, the ratio was decreased slightly with increasing age (r=-0.17, p=0.03), increasing NVIQ (r=-0.31; p=0.0001), increasing unaided PTA (r=-0.36; p<.0001), increasing aided thresholds (r=-0.29; p=0.0008), and decreasing SES (r=0.26; p=0.002).
Figure 1 (online) illustrates the relationship of the language ratio with the receptive language score by NVIQ categories (<80, 80-100, >100). Nearly all children with NVIQ<80 had receptive scores <85, though only 29% of children in this NVIQ category had language that would be considered as an underperformance (ratio <0.85). Among children with NVIQs 80-100, 62.5% had receptive scores <85 and 50% had a language underperformance (ratio <0.85). Among children with NIVQs >100, 21.1% had receptive scores <85 but as many as 42% had a language underperformance.
Figure 1.
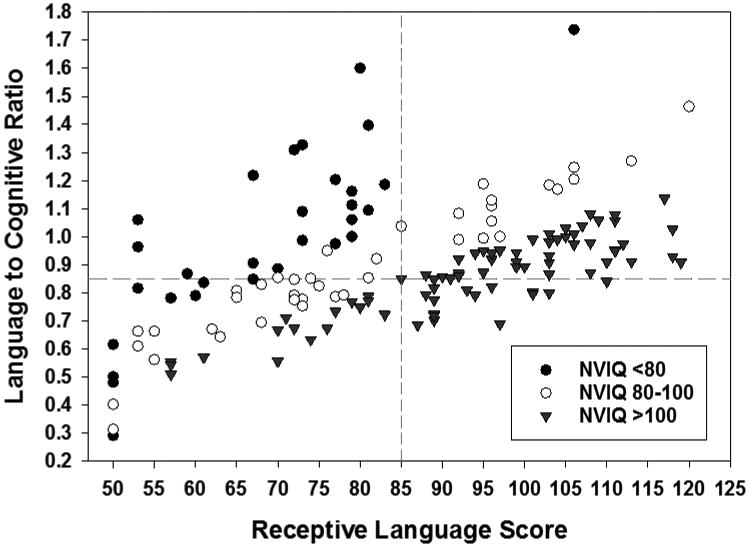
Scatter plot, by nonverbal IQ (NVIQ) categories of the receptive standard language score on the Preschool Language Scale-5th edition on the x-axis with the ratio of the language score to the nonverbal IQ on the y-axis. The vertical dashed line (at 85 on the x-axis) represents 1 standard deviation below the population mean of 100. The horizontal dashed line (at 0.85 on the y-axis) represents the cutpoint used to define language underperformance. Lower ratio values indicate a lower language scores relative to nonverbal IQ score.
Multiple linear regression of factors associated with language performance
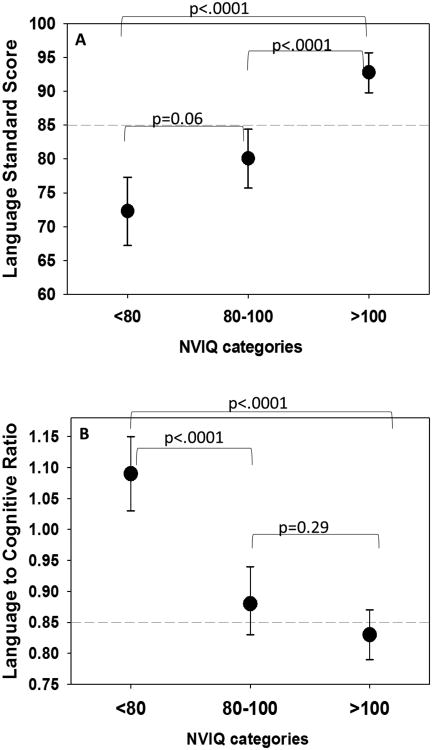
General linear models were constructed to understand factors related to language underperformance. Factors associated with higher ratios included decreasing NVIQ, increasing SES index scores, better hearing thresholds, and younger age at time of study (Table 2). These factors accounted for 50% of the variance (R2) seen in the language ratio. Two observations were identified as influential statistical outliers through model diagnostics (i.e., Cook's D). These two participants both had NVIQ in the 50's and had very low ratios (0.29 and 0.48). Although the parameter estimates remained consistent with the inclusion or exclusion of the outliers, including the 2 outliers reduced the R2 to 0.38. Figure 2 illustrates adjusted mean values (reported from LS means of the models) of both the receptive language standard score and the ratio (language performance). Models were adjusted for the same factors listed in Table 2. Although children in the lowest NVIQ category had the lowest language scores, they had on average the highest ratios and had significantly higher ratios than children in the other 2 NVIQ categories. Results based on a multiple imputation model were nearly identical; for simplicity, only the non-imputed results are presented.
Table 2. Factors associated with increase in the language ratio (language relative to cognitive abilities): results from multivariable general linear model.
| Variable | Parameter estimate | SE | p-value |
|---|---|---|---|
| NVIQ | -0.54 | 0.07 | <.0001 |
| SES Index score | 1.99 | 0.46 | <.0001 |
| Unaided PTA | -0.20 | 0.04 | <.0001 |
| Aided thresholds | -0.27 | 0.09 | 0.005 |
| Age at time of study | -0.23 | 0.10 | 0.02 |
| Duration with HA/CI* | 0.21 | 0.10 | 0.03 |
Time child has had either a hearing aid (HA) or cochlear implant (CI). If child had no device, then age was used.
R2=0.50
Figure 2.
Adjusted mean language standard scores (Panel A) and language ratio values (Panel B) by nonverbal IQ (NVIQ) categories. Adjusted least square means reported from general linear models after controlling for SES, unaided PTA, aided thresholds, age, and duration with device. P-values reported were adjusted for multiple comparisons using Tukey-Kramer method. Horizontal dashed lines indicate 1 standard deviation below the population mean (Panel A) and cut off for language underperformance (Panel B).
Logistic regression of factors associated with language underperformance
Children with language underperformance (n=61, 41.5%) were more likely to have more severe levels of hearing loss, have a cochlear implant, have lower SES index scores, and be nonwhite (Table 3). Results of the multiple logistic regression showed that as NVIQ increases the odds of language underperformance increases (OR 1.2 for every 5 point increment in IQ). Table 4 lists the OR with 95% confidence intervals for the multiple logistic regression model. How well a child was aided, an indication of access to sound, was significantly associated with the odds of language underperformance; higher aided thresholds (worse access to sound) were associated with increased odds of language underperformance. Children with the lowest SES index scores (lowest quartiles vs. higher quartiles) and children who were nonwhite (vs. white) were more likely to have language underperformance (OR 5.1 and 3.3 respectively).
Table 3. Characteristic differences between those with and without language underperformance.
| Language underperformance N=61 | Commensurate language N=88 | p-value | |
|---|---|---|---|
| Receptive language to NVIQ ratio | 0.70 (12.9) | 1.03 (0.17) | --- |
|
| |||
| Age at study in months | 50.2 (17.9) | 45.1 (19.5) | 0.10 |
|
| |||
| Sex– Male | 33 (54.1%) | 52 (59.1%) | 0.54 |
|
| |||
| Race | |||
| Caucasian | 43 (72.9%) | 74 (86.1%) | |
| African American | 12 (20.3%) | 10 (11.6%) | 0.049* |
| Asian | 3 (5.1%) | 0 | |
| Other | 1 (1.7%) | 2 (2.3%) | |
|
| |||
| Premature | 8 (13.1%) | 21 (23.9%) | 0.14 |
|
| |||
| Mother education college or greater | 27 (44.3%) | 59 (67.1%) | 0.006 |
|
| |||
| Public insurance only | 31 (50.8%) | 20 (22.7%) | 0.0004 |
|
| |||
| Socioeconomic status* | |||
| 1 (Lowest) | 23 (38.3%) | 15 (17.7%) | |
| 2 | 13 (21.7%) | 20 (23.5%) | 0.006 |
| 3 | 13 (21.7%) | 13 (15.3%) | |
| 4 (highest) | 11 (18.3%) | 37 (43.5%) | |
| unknown | 1 | 3 | |
| Age at HL identification in months | 10.0 (12.4) Median 2.5 | 10.4 (12.4) Median 5.0 | 0.28* |
|
| |||
| Degree of hearing loss | |||
| Mild | 4 (6.6%) | 16 (18.2%) | |
| Moderate | 18 (29.5%) | 44 (50%) | 0.0008 |
| Severe | 14 (23%) | 14 (15.9%) | |
| Profound | 25 (41%) | 14 (15.9%) | |
|
| |||
| Has cochlear implant | 32 (52.5%) | 22 (25%) | 0.0006 |
|
| |||
| Age at cochlear implant | 29.1 (16.8) | 22.0 (10.1) | 0.06 |
|
| |||
| Duration with implant | 28.0 (14.5) | 23.7 (20.1) | 0.37 |
|
| |||
| NVIQ | 99.4 (20.4) | 92.8 (19.7) | 0.048 |
|
| |||
| Language | |||
|
| |||
| Receptive standard score | 72.9 (15.9) | 93.4 (16.0) | <.0001 |
|
| |||
| Expressive standard score | 73.1 (16.6) | 93.3 (15.8) | <.0001 |
|
| |||
| Currently enrolled in speech-language therapy | 49 (80.3%) | 60 (70.6%) | 0.18 |
p-value from Wilcoxon Rank Sum Test
Table 4. Multiple logistic regression of language underperformance.
| Odds Ratio | 95% CI | |
|---|---|---|
| NVIQ (every 5 unit increase) | 1.2 | 1.09, 1.41 |
|
| ||
| HL Severity | ||
| Profound | 11.4 | 2.0, 65.3 |
| Severe | 3.3 | 0.54, 20.0 |
| Moderate | 1.8 | 0.37, 8.87 |
| Mild | Ref | |
|
| ||
| Socioeconomic status* | ||
| Lowest quartile | 5.1 | 1.67, 15.83 |
| Upper 3 quartiles | Ref | |
|
| ||
| Aided thresholds (every 1 dB increase) | 1.04 | 1.004, 1.08 |
|
| ||
| Nonwhite | 3.3 | 1.07, 10.44 |
|
| ||
| Age at study visit (every 1 month increase) | 1.02 | 0.99, 1.04 |
|
| ||
| AUC | 0.83 | |
Based on the SES index score
Because there was a significant difference in the percentage of children who received a cochlear implant between those with and without language underperformance (52.5% vs. 25.6%), we also tested a model that included whether a child has an implant (instead of the hearing loss severity categories). Results were similar to those of the original model with hearing loss severity categories. Children with a cochlear implant were more likely to have language underperformance (OR 4.27, 95% CI 1.68, 10.86). The length of time a child had either a cochlear implant or hearing aid was included in the model as a possible confounder, though this factor was not statistically significant (p=0.96).
Discussion
This study assessed language performance as receptive language scores measured using the PLS-5 relative to nonverbal cognitive levels measured by standardized nonverbal cognitive assessments. We found that among children with bilateral hearing loss, the mean standard receptive language score was 85, which is considered within 1 standard deviation from the population mean. The NVIQ of this study sample was 95; a score that was significantly higher than the language scores. A high percentage of children (41%) had a significant disparity between their language scores and nonverbal cognitive scores, which we defined as a language underperformance. Additionally, children with language underperformance were more likely to have higher NVIQ, even though children with higher NVIQ had higher mean language scores.
Our study findings regarding continued language delays in children who are DHH are consistent with some of the recent studies that have assessed language development among children with bilateral hearing loss. Nittrouer reported that despite the advents of early intervention and advanced technology, deficits in language acquisition remained in children who were DHH who had received a cochlear implant.18 Tomblin et. al. provided evidence supporting the continued effect of hearing loss on language development in children with mild to severe hearing loss.19 Although the risks of language delays were mitigated for many by the use of hearing aids, a consequential number of children continued to demonstrate poor language skills (standard scores below 85) despite normal nonverbal cognitive abilities. Even children who had the “best case scenario” of early identification, early amplification, and high quality linguistic exposure appeared to develop language skills that were within the average to low-average range on standardized language measures.19 Ching summarized evidence from the Longitudinal Outcomes of Children with Hearing Impairment (LOCHI) study regarding spoken language outcomes and the effectiveness of early intervention. Although great strides have been made regarding early language outcomes for children who are DHH, the results of the LOCHI study support the need for continued monitoring of language after the intervention period.20 In addition, despite positive effects of early intervention, children who were DHH continued to exhibit deficits in phonological awareness by 5 years of age which could affect development of literacy skills later in life. Our study extends previous research by considering language in the context of cognitive abilities, which may help identify subgroups of children who may be overlooked in terms of language intervention needs.
Factors associated with language underperformance in our study sample included degree of hearing loss, audibility (according to aided thresholds), and SES levels (using a combination of maternal education level and income). These factors are consistent with the literature on language development among children who are deaf or hard-of-hearing. We found a significant effect of unaided PTA and severity classification (mild to profound) on the ratio of language relative to cognitive abilities as a continuous variable, as well as a significant effect on the odds of having a language underperformance. We also found a significant effect of audibility (aided SAT/SRT), which is a marker, though imperfect, of the functional hearing status of the child. Multiple studies have found associations with measures of audibility and language development among children who are DHH.19,21 In contrast to other studies, we had a mix of hearing aid and cochlear implant users. We did not see a significant association between the age at which a child received the device and outcomes. However, we did not have a measure of consistency with wearing the device, which could contribute to language outcomes.19 We did not find an association of language underperformance with hearing loss etiology. While the small number of children within any specific etiology category made it difficult to statistically assess potential associations, it is probable that any association between etiology and language underperformance would be significantly confounded by cognitive abilities. Unfortunately, we did not have the age at which a child received early intervention services or the duration of these services. Because there is evidence supporting the effects of early intervention on language development in children who are DHH, it is important to understand the role early intervention plays in improving language development in children who are DHH and in ensuring children develop language commensurate to their cognitive abilities.
The association between SES and language in our sample was not entirely surprising. Socio-economic status has been shown to be associated with vocabulary and language in children who are DHH, though the literature is not consistent regarding specific factors.21,22 Environmental influences are important for solid language development,23 with the quality of linguistic input and family involvement being important factors for language acquisition in children who are DHH.2,21,24 Measuring the quality and quantity of family involvement was beyond the scope of the current study, though, family involvement should be considered in future study designs.
Nonverbal intelligence is a significant predictor of language functioning among DHH children.25,26 Similar to previous studies, we found that higher nonverbal IQ was significantly associated with higher language standard scores. However, our findings indicated that NVIQ was inversely related to the ratio of language to cognitive scores. Because language skills in children should be appropriate for their developmental (or cognitive) level,27 we believe this finding can be interpreted as children with higher cognitive abilities are not achieving their language potential. For example, a child with a NVIQ >100 should have similar language standard scores (potentially 5-10 point difference in standard scores between language and NVIQ).In our study, children in the lowest NVIQ category did not appear to display the same discrepancies between language and cognitive levels. Since many of the children in our study sample did not have a standardized cognitive assessment in their record prior to enrollment, it is likely that the true developmental levels of participants were unknown to therapists working on language development. Children with unknown lower cognitive abilities could have been “pushed” regarding language development, and thus were able to achieve their cognitive potential. Once language levels were deemed as average or within “normal limits”, efforts to achieve higher scores may have been relaxed, which would disproportionately affect children with higher cognitive abilities. Although cognitive referencing is not supported by the American Speech-Language-Hearing Association to determine eligibility for services,28 knowledge about developmental and cognitive abilities could assist clinicians and families with expectations and goal setting. With no developmental framework from which to set language specific goals, children who are DHH will continue to fall short regarding their potential language development.
Long term effects of impaired language and social functioning can impact academic outcomes, educational choices, and long term vocation.29,30 Thus, the language underperformance identified in this study can have downstream effects on other areas of child development. Meinzen-Derr et.al.6 found that children who had a language underperformance, or language gap, had significantly lower social and communication functioning than children with language commensurate with their cognitive abilities. The investigators also found that children who had higher NVIQ but lower language had social function scores similar to children with mild intellectual disabilities. An important finding from that study was that the impact on lower social functioning occurred even when language was in the average range (albeit lower than what would be expected for NVIQ).
This study had several limitations to address. It is possible that families were motivated to enroll their children in this study because they were concerned about poor language development, which would bias our study sample. The goal of this work was not to report the prevalence of low or poor language, but to understand potential factors associated with having low language relative to a child's capacity for language. This study is reporting on cross-sectional data (one time point), so we do not know whether persistent language underperformance impacts the development of social skills or functional communication over time. We did not have information regarding the frequency and duration of otitis media with effusion. We collected information regarding school placement, however this information was strictly by parent report and was not fully accurate as parents would often state their child was not in school if the visit occurred during the summer months. We did not ask about individualized education programs. Children who had a nonverbal cognitive assessment that resulted in a low NVIQ may present later with a nonverbal learning disability (particularly if their language is significantly higher than the NVIQ), which would make the interpretation of the NVIQ measure less indicative of general cognitive abilities. We cannot truly diagnose a nonverbal learning disability because a) we did not assess verbal IQ and b) our sample of children are still quite young. This small subgroup of children would have had language scores that were significantly higher than their NVIQ. We did not power our study for the ability to stratify our analysis by age groups or by device status (i.e. cochlear implants). However, the purpose of this study was to evaluate language in the context of cognitive abilities (as opposed to an isolated score) across all ages and levels of ability. Future studies should consider whether our findings hold true for subgroups. Finally, it is possible that the use of the PLS-5 for assessing language might not be considered as “challenging” as other available language assessments. A similar study using a more challenging language outcome measure that assesses discrete language skills might perhaps yield greater discrepancies between NVIQ and language. Thus, it is possible that what we are reporting is an under-representation of the true problem with language underperformance.
Despite these limitations, this study also had several strengths. The use of objective measures which were systematically applied allowed for comparisons across children regardless of age. The age range of the cohort allows for considerations about different needs across intervention periods (from early intervention to preschool age). We incorporated a nonverbal IQ assessment that is appropriate for children who are DHH and requires no language to administer. Thus, our measures of intelligence were not biased by potentially low language levels of some of the children. This study offers a perspective on language skill potential that is unique and personalized to each child. This level of understanding could encourage providers to set goals depending on each child's individual potential, which minimizes the tendency to put a ceiling on language targets. Although the cross-sectional nature of the data prevents us from understanding language growth or trajectories over time, we are following this cohort of children and will ultimately be able to assess how language performance relative to NVIQ changes with age.
Results from this study could have broader implications on the health care and educational systems. Many systems, including early intervention (Part C), educational systems, (Part B) and health insurance coverage use scores from standardized assessments to determine a child's eligibility for specific types of interventions (e.g. speech-language therapy). Our data suggest that using specific standard scores in isolation will leave many children who are DHH unsupported for clinically and educationally meaningful language needs. These aspects of care delivery prompt challenges in ameliorating the negative impact of language underperformance for young children who are DHH. More research is needed to better understand factors underlying language underperformance as well as interventions to close the gap between a child's language abilities and cognitive capabilities.
Conclusions
Over the years, Universal Newborn Hearing Screening has had success at improving the ages of identification of children born with a hearing loss as well as facilitating access to early intervention services. The subsequent goal of early intervention is to enhance language development, a goal that state Early Hearing Detection and Intervention programs have been achieving. However, this study highlights the need for continuing work in the field of hearing loss, particularly around language development. Children who are identified with hearing loss, even as early as in the newborn period, continue to demonstrate delayed language development and associated social interactions. A better understanding of a child's cognitive potential may shed some light into appropriate language goals and expectations so every child who is deaf or hard-of-hearing has the opportunity to reach his or her full potential.
Acknowledgments
This study was supported in part by the March of Dimes (#12FY14-178), Health Resources and Services Administration (R40MC21513), NIDILRR (#90IF0122), and the Center for Clinical and Translational Science and Training grant (NIH 8UL1-TR000077)
Footnotes
Author Disclosure Statement: The authors have no conflicts of interest relevant to this article to disclose. The funding agencies had no involvement in the study design, collection, analysis, and interpretation of data, writing of the report or decision to submit for publication.
References
- 1.Summary of 2013 National CDC EHDI Data: 2013 CDC EHDI Hearing Screening and Follow-up Survey. 2013 http://www.cdc.gov/ncbddd/hearingloss/2013-data/2013_ehdi_hsfs_summary_a.pdf.
- 2.Moeller MP. Early intervention and language development in children who are deaf and hard of hearing. Pediatrics. 2000;106:E43. doi: 10.1542/peds.106.3.e43. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy CR, McCann DC, Campbell MJ, et al. Language ability after early detection of permanent childhood hearing impairment. N Engl J Med. 2006;354:2131–2141. doi: 10.1056/NEJMoa054915. [DOI] [PubMed] [Google Scholar]
- 4.Marschark M, Sapere P, Convertino CM, et al. Are deaf students' reading challenges really about reading? Am Ann Deaf. 2009;154:357–370. doi: 10.1353/aad.0.0111. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman MF, Quittner AL, Cejas I. Comparisons of social competence in young children with and without hearing loss: a dynamic systems framework. J Deaf Stud Deaf Educ. 2015;20:115–124. doi: 10.1093/deafed/enu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meinzen-Derr J, Wiley S, Grether S, et al. Functional communication of children who are deaf or hard-of-hearing. Journal of developmental and behavioral pediatrics : JDBP. 2014;35:197–206. doi: 10.1097/DBP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 7.Niparko JK, Tobey EA, Thal DJ, et al. Spoken language development in children following cochlear implantation. JAMA. 2010;303:1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geers AE. Predictors of reading skill development in children with early cochlear implantation. Ear Hear. 2003;24:59S–68S. doi: 10.1097/01.AUD.0000051690.43989.5D. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson J, McCann D, Watkin P, et al. The relationship between language development and behaviour problems in children with hearing loss. Journal of child psychology and psychiatry, and allied disciplines. 2010;51:77–83. doi: 10.1111/j.1469-7610.2009.02124.x. [DOI] [PubMed] [Google Scholar]
- 10.Vohr B, Jodoin-Krauzyk J, Tucker R, et al. Early language outcomes of early-identified infants with permanent hearing loss at 12 to 16 months of age. Pediatrics. 2008;122:535–544. doi: 10.1542/peds.2007-2028. [DOI] [PubMed] [Google Scholar]
- 11.Ching TY, Crowe K, Martin V, et al. Language development and everyday functioning of children with hearing loss assessed at 3 years of age. International journal of speech-language pathology. 2010;12:124–131. doi: 10.3109/17549500903577022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund E. Vocabulary Knowledge of Children With Cochlear Implants: A Meta-Analysis. J Deaf Stud Deaf Educ. 2016;21:107–121. doi: 10.1093/deafed/env060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scales, Fifth Edition (PLS-5) San Antonio, TX: Pearson; 2011. [Google Scholar]
- 14.Roid G, Miller L. Leiter International Performance Scale - Revised. Wood Dale, IL: Stoelting, Co; 1997. [Google Scholar]
- 15.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 16.Caudle SE, Katzenstein JM, Oghalai JS, et al. Nonverbal cognitive development in children with cochlear implants: relationship between the Mullen Scales of Early Learning and later performance on the Leiter International Performance Scales-Revised. Assessment. 2014;21:119–128. doi: 10.1177/1073191112437594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health policy and planning. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 18.Nittrouer S. Beyond Early Intervention: Supporting Children With CIs Through Elementary School. Otol Neurotol. 2016;37:e43–49. doi: 10.1097/MAO.0000000000000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomblin JB, Harrison M, Ambrose SE, et al. Language Outcomes in Young Children with Mild to Severe Hearing Loss. Ear Hear. 2015;36(1):76S–91S. doi: 10.1097/AUD.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ching TY. Is Early Intervention Effective in Improving Spoken Language Outcomes of Children With Congenital Hearing Loss? Am J Audiol. 2015;24:345–348. doi: 10.1044/2015_AJA-15-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrose SE, Walker EA, Unflat-Berry LM, et al. Quantity and Quality of Caregivers' Linguistic Input to 18-Month and 3-Year-Old Children Who Are Hard of Hearing. Ear Hear. 2015;36(1):48S–59S. doi: 10.1097/AUD.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geers AE, Strube MJ, Tobey EA, et al. Epilogue: factors contributing to long-term outcomes of cochlear implantation in early childhood. Ear Hear. 2011;32:84S–92S. doi: 10.1097/AUD.0b013e3181ffd5b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronfani L, Vecchi Brumatti L, Mariuz M, et al. The Complex Interaction between Home Environment, Socioeconomic Status, Maternal IQ and Early Child Neurocognitive Development: A Multivariate Analysis of Data Collected in a Newborn Cohort Study. PloS one. 2015;10:e0127052. doi: 10.1371/journal.pone.0127052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suskind DL, Graf E, Leffel KR, et al. Project ASPIRE: Spoken Language Intervention Curriculum for Parents of Low-socioeconomic Status and Their Deaf and Hard-of-Hearing Children. Otol Neurotol. 2016;37:e110–117. doi: 10.1097/MAO.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 25.Khan S, Edwards L, Langdon D. The cognition and behaviour of children with cochlear implants, children with hearing aids and their hearing peers: a comparison. Audiol Neurootol. 2005;10:117–126. doi: 10.1159/000083367. [DOI] [PubMed] [Google Scholar]
- 26.Cupples L, Ching TY, Button L, et al. Language and speech outcomes of children with hearing loss and additional disabilities: identifying the variables that influence performance at five years of age. Int J Audiol. 2016:1–12. doi: 10.1080/14992027.2016.1228127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleason JB. The development of language. 6th. Boston: Allyn and Bacon; 2005. [Google Scholar]
- 28.American Speech-Language-Hearing Association. Eligibility and Dismissal in Schools. http://www.asha.org/slp/schools/prof-consult/eligibility/
- 29.Justice LM, Bowles RP, Pence Turnbull KL, et al. School readiness among children with varying histories of language difficulties. Developmental psychology. 2009;45:460–476. doi: 10.1037/a0014324. [DOI] [PubMed] [Google Scholar]
- 30.Antia SD, Jones PB, Reed S, et al. Academic status and progress of deaf and hard-of-hearing students in general education classrooms. J Deaf Stud Deaf Educ. 2009;14:293–311. doi: 10.1093/deafed/enp009. [DOI] [PubMed] [Google Scholar]