Abstract
The majority of estrogen-based hormone therapies are administered in combination with a progestogen, such as Levonorgestrel (Levo). Individually, the estrogen 17β-estradiol (E2) and Levo can improve cognition in preclinical models. However, although these hormones are often given together clinically, the impact of the E2 + Levo combination on cognitive function has yet to be methodically examined. Thus, we investigated E2 + Levo treatment on a cognitive battery in middle-aged, ovariectomized rats. When administered alone, E2 and Levo treatments each enhanced spatial working memory relative to vehicle treatment, while the E2 + Levo combination impaired high working memory load performance relative to E2-Only, and Levo-Only. There were no effects on spatial reference memory. MAPK/ERK pathway activation, which is involved in memory formation and estrogen-induced memory effects, was evaluated in five brain regions implicated in learning and memory. A distinct relationship was seen in the E2-only treatment group between MAPK/ERK pathway activation in the frontal cortex and working memory performance. Collectively, the results indicate that the differential neurocognitive effects of combination versus sole treatments are vital considerations as we move forward as a field to develop novel, and to understand currently-used, exogenous hormone regimens across the lifespan.
Keywords: estrogen, progesterone, memory, working memory, MAPK/ERK, hormone therapy
1. Introduction
Menopause, defined as the cessation of menses for at least one year, is marked by a reduction in levels of ovarian hormones, including estrogens and progesterone. This decrease in circulating levels of ovarian hormones can lead to the onset of several undesired physiological symptoms, including hot flashes, vaginal atrophy, and osteoporosis (Al-Safi and Santoro, 2014; NAMS, 2015). In women, several domains of memory performance, as well as focus and concentration, are also sensitive to changes in ovarian hormone levels, and have been associated with menopausal status (Maki, 2012). The presence and severity of these symptoms vary amongst women, and these symptoms can greatly impact a woman’s quality of life; as a result, some women choose to take hormone therapy to ameliorate their symptoms. Thus, it is imperative to acquire a thorough understanding of how alterations in levels of ovarian hormones, and exogenously administered hormones, impact issues associated with menopause, such as changes in memory. Moreover, elucidating the roles of ovarian hormone loss and hormone therapies on the brain and its functions could lead to novel hormone therapy options that are tailored to alleviating specific symptoms associated with menopause.
17β-estradiol (E2) is the most potent, naturally circulating estrogen in mammals, and it is commonly used as the estrogenic component in hormone therapy for menopause. As early as the 1950s, studies have suggested a beneficial role of estrogens in cognitive and related molecular processes of the central nervous system (e.g., Bimonte and Denenberg, 1999; Caldwell and Watson, 1952; Komnenich et al., 2013; Matsumoto et al., 1985; Singh et al., 1995; Woolley and McEwen, 1993). Today, there are an extensive array of studies aimed at understanding the effects of E2 on learning and memory in humans as well as in animal models (for reviews: Frick, 2015; Koebele and Bimonte-Nelson, 2015; Korol and Pisani, 2015; Maki, 2012; Mennenga and Bimonte-Nelson, 2013; Sherwin, 2006). The ovariectomy (Ovx) model in rodents, whereby the primary source for circulating ovarian hormones, the ovaries, are surgically removed, provides a low circulating ovarian hormone profile or a ‘blank hormonal slate’. Although some ovarian hormones (e.g., E2 and progesterone) can also be synthesized in the brain (Kretz et al., 2004; Micevych and Sinchak, 2008; Tuscher et al., 2016), the Ovx rodent model can be employed to study the cognitive effects of exogenously administered hormone regimens that aim to achieve a specific circulating hormone profile. In Ovx rats, E2 treatment enhanced cognitive performance on a multitude of learning and memory behavioral paradigms, such as the radial-arm maze (Bimonte and Denenberg, 1999; Daniel et al., 2006, 1997; Fader et al., 1999; Gibbs and Johnson, 2008; Luine et al., 1998; Rodgers et al., 2010), Morris water maze (MWM; Bimonte-Nelson et al., 2006; El-Bakri et al., 2004; Feng et al., 2004; Kiss et al., 2012; Lowry et al., 2010; McLaughlin et al., 2008; Talboom et al., 2008), delayed match-to-position T-maze (Gibbs, 2007, 2002, 2000, 1999; Gibbs et al., 2004), and object placement (Conrad et al., 2012; Frye et al., 2007; Luine et al., 2003; McLaughlin et al., 2008).
In the brain, E2 can modulate the MAPK/ERK pathway, which is involved in the formation of different memory types (Atkins et al., 1998; Blum et al., 1999; Schafe et al., 2000). When the MAPK/ERK signaling pathway is activated, the signal travels from a cell receptor (e.g., estrogen receptors) to the nucleus DNA via a sequence of proteins, including the activated extracellular signal-regulated kinase 1 and 2 (Erk1/2) (Ciccarelli and Giustetto, 2014; Koebele and Bimonte-Nelson, 2017; Witty et al., 2012). Research indicates that, in the dorsal hippocampus, MAPK/ERK activation is essential for long-term memory formation, as well as for E2-induced beneficial effects on memory consolidation (Blum et al., 1999; Fan et al., 2010; Fernandez et al., 2008; Harburger et al., 2009). Indeed, there is increased expression of activated Erk2 in the dorsal hippocampus following E2 treatment (Fernandez et al., 2008; Harburger et al., 2009; Witty et al., 2013). Blocking this E2-induced increase in Erk2 activation attenuated the beneficial cognitive effects of E2 on the object recognition task (Fan et al., 2010; Fernandez et al., 2008). E2 treatment can also increase the expression of neurotrophins that are associated with learning and memory, including brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and neurotrophin 3 (NT-3), in regions of the brain that are involved in cognitive function (i.e. entorhinal cortex and hippocampus) (Bimonte-Nelson et al., 2004; Kiss et al., 2012; Zhou et al., 2005). Neurotrophins and the MAPK/ERK pathway have been implicated in learning and memory as well as neuroplasticity (Bechara et al., 2014; Gooney et al., 2002; McGauran et al., 2008). Taken together, these studies highlight the significant role of E2 in cognitive function that is mediated through its role in several defined pathways associated with neuroplasticity and memory.
For women with an intact uterus, estrogen-based hormone therapies must also include a progestogen component to offset the increased risk for developing endometrial hyperplasia and cancer following exposure to unopposed estrogens (NAMS, 2012). Progestogens are a class of steroid hormones, and include natural progesterone and progestins (synthetic progestogens), which bind to the progesterone receptor. There is preclinical evidence that some progestogens can also offset the cognitive benefits of E2 (Bimonte-Nelson et al., 2006; Harburger et al., 2009, 2007; Lowry et al., 2010). For instance, studies testing combination hormone therapies have shown that the addition of progesterone to E2 treatment reversed the enhancing cognitive effects of E2 on the spatial reference memory MWM task in Ovx rodents (Bimonte-Nelson et al., 2006; Harburger et al., 2007; Lowry et al., 2010). There is also preclinical evidence that the addition of progesterone can attenuate E2-induced changes in several neuromolecular mechanisms in the brain that are essential for cognitive function. For example, in the entorhinal cortex, the E2-induced increases in BDNF, NGF, and NT-3 levels were obviated with the addition of progesterone (Bimonte-Nelson et al., 2004). In the dorsal hippocampus, progesterone in combination with E2 treatment attenuated the E2-induced increase in activated Erk2 expression (Harburger et al., 2009). These findings indicate that the addition of a progesterone component in hormone therapy to oppose undesired E2 stimulation in the periphery may not be cognitively beneficial, and that it can attenuate associated E2-induced benefits.
Since progesterone has low systemic bioavailability with oral and transdermal delivery, synthetic progestogens are often used for both contraceptive and hormone therapy purposes (Du et al., 2013; Kuhl, 2005; Pickar et al., 2015). Medroxyprogesterone acetate (MPA) is a synthetic progestogen that is commonly prescribed for birth control (Depo-Provera), as well as used in combination with an estrogen for menopausal hormone therapy. Our laboratory found that exogenous treatment with MPA alone in female rats impaired cognitive function (Braden et al., 2017, 2011, 2010). Furthermore, a study testing the effects of a tonic MPA and E2 hormone combination showed that subcutaneous MPA (via a pellet) plus oral E2 (via drinking water) treatment resulted in impaired learning on the MWM in middle-aged Ovx rats compared to chronic E2, chronic E2 plus progesterone, or cyclic E2 (Lowry et al., 2010). Additionally, subcutaneous tonic administration of progesterone or of MPA in adult Ovx rats blocked the neuroprotective effects of E2 following excitotoxic lesion with kainate (Rosario et al., 2006). These studies further indicate that the role of E2 on the brain and cognitive function can be altered by the addition of a progestogen, and the magnitude of this effect may be governed by the type of progestogen administered (e.g., progesterone versus MPA).
Research done thus far supports the hypothesis that MPA has detrimental effects on cognition, alone and in combination with estrogen; there are other FDA-approved progestogens that satisfy the uterus opposing effects that have not been cognitively profiled. An important goal to aid women’s health is to find a progestogen that will accomplish uterine protection while not imposing negative cognitive effects. Levonorgestrel (Levo) is a synthetic progestogen utilized in multiple contraceptives, such as intrauterine devices (i.e. Mirena) and emergency contraception (i.e. Plan B), as well as in combination with estrogens in oral birth control pills such as Lutera, Aviane, Seasonique, and Seasonale. In menopausal hormone therapy, Levo is combined with E2 in the transdermal patch, Climara Pro. The contraceptive efficacy of Levo, and of combination estrogen plus Levo, hormone formulations are well established. However, research has only just begun to address the potential effects of Levo on cognitive performance, and only one preclinical study has evaluated such impact in the context of aging. Our laboratory has demonstrated that daily subcutaneous administration of 0.6 μg Levo, a dose that is equivalent to the clinically available Climara Pro patch when accounting for body weight, enhanced working memory performance on the water radial-arm maze (WRAM) relative to administration of the vehicle control in middle-aged, Ovx rats (Braden et al., 2017). This is especially exciting, as this is the first progestogen given chronically shown to benefit memory in a preclinical model of menopause. However, whether these benefits will hold when given in combination with an estrogen is yet to be determined. This question is critically important given the strong clinical use of combined regimens for menopausal hormone therapy.
The current study examined the effect of E2 + Levo hormone combination treatment on cognitive function, with interpretations relative to vehicle control treatment, as well as relative to E2 alone and Levo alone treatments. The intent was to study these regimens in the context of older age and ovarian hormone loss. Thus, treatments were administered to middle-aged Ovx rats. The 0.6 μg Levo dose was carefully chosen based on prior work from our laboratory that showed enhancing cognitive effects of Levo alone at this dose (Braden et al., 2017); we were interested in examining whether a cognitively enhancing Levo dose when given alone would also enhance cognitive function when given in combination with E2. First, cognitive function was assessed using a battery of behavioral tasks to test spatial learning and memory using the WRAM (working and reference memory) and the MWM (reference memory), including a control behavioral task (visible platform). Second, following behavioral testing, activated Erk1 and Erk2 levels in brain regions that are involved in cognitive function were evaluated, including the frontal cortex, dorsal hippocampus, CA1/CA2 ventral hippocampus, entorhinal cortex, and perirhinal cortex. Given that the E2 and Levo regimens tested here were based on our prior effects benefitting cognition, we hypothesized that E2 alone and Levo alone treatments would yield favorable cognitive effects. Because Levo is often used in combination with estrogens in clinical formulations, and because Levo is the first progestogen we have shown to initiate beneficial cognitive effects when given alone, we sought to determine whether its beneficial effects would hold when given combined with estrogen. Thus, here, we ask: in a surgical menopause model, will the combination of two cognitively-enhancing hormones result in strengthened beneficial effects, or in null or attenuating effects, on learning and memory performance?
2. Methods
2.1 Animals
Forty middle-aged, 11-month old, Fischer-344 CDF virgin female rats from the National Institute on Aging, Harlan Laboratories (Indianapolis, IN) were used based on prior publications (Braden et al., 2017; Chisholm and Juraska, 2012; Rodgers et al., 2010). Rats were pair housed on a 12-hour light/dark cycle, and food (Teklad global 18% protein rodent diet, Envigo) and water were ad libitum. All procedures were done with approval from the Arizona State University IACUC and followed the standards set by the National Institutes of Health.
2.2 Ovariectomy (Ovx)
All rats underwent Ovx surgery from the dorsolateral aspect under acute isoflurane inhalation anesthesia. Each rat was administered a single subcutaneous injection of Rimadyl (5 mg/kg) for pain. After dorsolateral incisions were made in the skin and peritoneum, a ligature was applied to the tip of each uterine horn and each ovary was removed. The muscle and skin were sutured (Coated VICRYL Suture, Ethicon) and rats were subcutaneously administered saline (2 ml) to prevent dehydration.
2.3 Treatment Administration
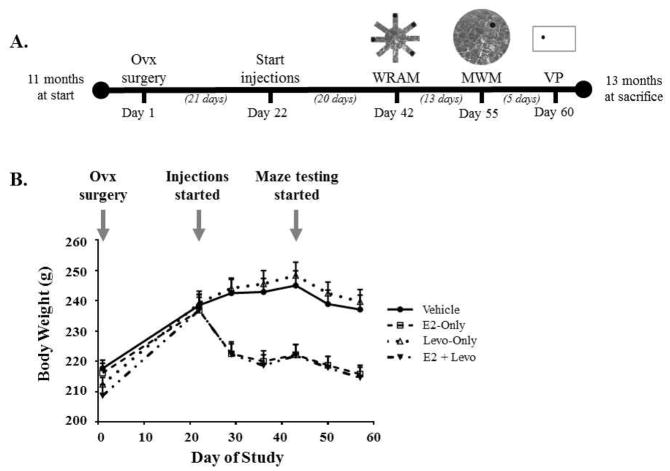
Treatment administration started 21 days after Ovx surgery, and continued until the end of the study (see Figure 1A for the study timeline). Rats were randomly assigned to receive either a daily subcutaneous injection of sesame oil as control (Vehicle, n = 10) or a hormone injection of 3 μg E2 (E2-Only, n = 10), 0.6 μg Levo (Levo-Only, n = 9), or a combination of 3 μg E2 and 0.6 μg Levo (E2 + Levo, n = 10), in 0.1 ml of sesame oil. All treatment injections were done between 7:00–8:00 am; behavioral tasks were initiated half an hour after the last treatment injection, and treatment groups were counterbalanced throughout the day to account for timing differences between treatment injection and behavioral testing. One animal from the Levo-Only group was excluded from all analyses due to premature death, the cause of which was not related to experimental conditions. The dose for Levo-Only treatment was based on published work from our laboratory where a daily subcutaneous injection of 0.6 μg of Levo enhanced performance on the WRAM in middle-aged Ovx rats (Braden et al., 2017). After 20 days of treatment, all animals started testing on a battery of behavioral tasks to assess cognitive performance. The timing of treatment initiation and duration prior to behavior testing was methodically decided based on prior studies from our laboratory that have shown effects of hormone treatment on cognitive performance (Bimonte-Nelson et al., 2006; Engler-Chiurazzi et al., 2011; Mennenga et al., 2015b; Talboom et al., 2008). Throughout the span of the study, body weights were measured weekly to evaluate the expected increase in body weight as a result of Ovx, followed by the expected decrease in body weight for animals that received an estrogen-containing treatment (Figure 1B; Geary et al., 1994; McLaughlin et al., 2008; Mennenga and Bimonte-Nelson, 2013).
Figure 1. Study timeline and trajectory of rat body weight throughout the span of the study.
(A) Study timeline depicts time periods between Ovx, start of treatment injections, and order of behavioral testing. (B) Changes in body weight accurately represent expected fluctuations as a result of Ovx, type of treatment, and maze testing.
2.4 Water Radial-arm Maze (WRAM)
The first behavioral task was the win-shift WRAM used to examine spatial working and reference memory (Bimonte-Nelson, 2015a; Bimonte and Denenberg, 1999; Braden et al., 2017; Mennenga et al., 2015c; Mennenga and Bimonte-Nelson, 2015). WRAM testing started on day 21 of treatment administration and lasted for 13 days. The maze was an 8 arm apparatus, and 4 out of the 8 arms contained hidden platforms. Each arm’s dimensions were 38.1 cm × 12.7 cm, and platforms were 10 cm in diameter. The maze was filled with water made opaque with nontoxic black paint that was kept between 18–20°C for the duration of testing, and spatial cues were set up around the room to aid in spatial navigation. The room was 248 cm × 243 cm in size.
Each subject was randomly assigned a set of platform locations, which were kept fixed for the duration of testing for a subject. For each trial, subjects were dropped off in the start arm and allowed a maximum of 3 min to find a platform; once on the platform, subjects remained there for 15s before being placed in a heated testing cage for a 30s inter-trial interval (ITI). A trial was completed when a platform was found. During the ITI, the just-located platform was removed from the maze, and the maze water was cleaned of debris with a fishnet. At the end of the 30s ITI, the next trial was started. There were a total of 4 trials per day (one trial per platform). There was an increase in working memory load across trials as the memory system was increasingly taxed with the removal of each additional found platform. On the last day of testing, a 6-hour delay between trials 2 and 3 was implemented to examine delayed memory retention.
Performance on the WRAM for each subject was determined by scoring the orthogonal measures of working and reference memory. Briefly, the first entry into a non-platformed arm within a day was defined as a reference memory (RM) error. A re-entry into a RM (non-platformed) arm within the same day was defined as a working memory incorrect (WMI) error. An entry into a previously platformed arm within a day was defined as a working memory correct (WMC) error.
2.5 Morris Water Maze (MWM)
The day after WRAM testing was completed, all animals began testing on the MWM to evaluate spatial reference memory performance (Bimonte-Nelson, 2015b; Talboom et al., 2014, 2008). The MWM apparatus was a circular tub, 188 cm in diameter, filled with water (18–20°C) that was made opaque with nontoxic paint. One platform, 10 cm in diameter, was submerged in the northeast (NE) quadrant of the tub. The location of the platform remained constant across all trials and days of testing. There were abundant spatial cues set up around the room to aid in spatial navigation. The room was 348 cm × 337 cm in size. Each animal received 4 trials per day for each of the 5 testing days. The subject was dropped off at one of four starting locations (north, south, east, or west) at the start of each trial and was allowed 60s to locate the platform. The order of the drop off starting locations was randomized across days, but kept constant for all animals across 4 trials within a day. Once the platform was found, the subject remained on the platform for 15s and was then placed back into a heated cage for a 5–8 min ITI. If the platform was not found within the allotted 60s trial time, the subject was led to the platform and remained there for 15s before being placed back into a heated cage for a 5–8 min ITI. Each rat’s swim path was recorded using the Ethovision tracking system (Noldus Instruments, Wageningen, The Netherlands), and total swim distance to the platform was analyzed. On the last day of testing, a probe trial was administered as an additional 5th trial to test for spatial localization. For the probe trial, the platform was removed and the subject was allowed to swim for a total of 60s after being dropped off from the furthest drop off location (west) in relation to where the platform was located (NE quadrant).
2.6 Visible Platform
To confirm their capability to perform the procedural components of a water-escape task, animals were tested on the visible platform task the day after completion of MWM testing (Bimonte-Nelson, 2015a; Mennenga et al., 2015a, 2015c). The visible platform task was composed of a rectangular tub, 100 cm × 60 cm, filled with clear water kept at 18–20°C; a black platform, 10 cm in diameter, remained 4 cm above the water surface throughout testing. A curtain was used to block obvious spatial cues located in the area distal from the maze. The room was 168 cm × 155 cm in size. There were a total of 6 trials per animal for the one day of testing, with a 90s maximum trial time. Once the platform was found, subjects were given 15s on the platform and then placed back into a heated cage (ITI was 5–8 min). The drop off location remained constant throughout testing, and the platform location was varied semi-randomly between three distinct locations.
2.7 Blood Serum Analysis
The day after visible platform testing, animals were euthanized with isoflurane, starting at the regular testing time and in the same order in which they were tested. Blood was collected via cardiocentesis, allowed to clot at 4°C (Vacutainer 367986, Becton Dickinson and Company, Franklin Lakes, NJ, USA), and centrifuged at 3000 rpm for 20 min at 4°C to obtain blood serum. Serum was stored at −20°C until analysis. Circulating E2 and estrone levels were determined by radioimmunoassay at the Core Endocrinology Laboratory of the Pennsylvania State University, College of Medicine. Specifically, E2 and estrone levels were measured in duplicate using a double antibody liquid-phase radioimmunoassay (Beckman Coulter, Brea, CA) as previously reported (Engler-Chiurazzi et al., 2012; Koebele et al., 2017; Mennenga et al., 2015c). For the E2 assay, E2-specific antibodies were used with 125I-labeled E2 as the tracer. The E2 assay had a functional sensitivity of 4 pg/ml. Inter-assay coefficients of variation at a mean level of 6 pg/ml E2 averaged 8%, and intra-assay coefficients of variation averaged 6%. For the estrone assay, estrone-specific antibodies were used with 125I-labeled estrone as the tracer. The estrone assay had a functional sensitivity of 16 pg/ml. Inter-assay coefficients of variation for estrone at a mean level of 90 pg/ml averaged 11%, and intra-assay coefficients of variation averaged 8%.
2.8 Uterine Horn Weights
It is known that uterine horn weight is impacted by the presence of ovarian hormones (e.g., Engler-Chiurazzi et al., 2012; Mennenga et al., 2015b; Westerlind et al., 1998). To confirm complete Ovx as well as E2 exposure, and to assess whether the addition of Levo impacted the expected E2-induced increases, uterine horns were inspected and removed at sacrifice, trimmed of visible fat, and weighed (wet weight).
2.9 Brain Dissection
Immediately following cardiocentesis, the brain was rapidly dissected. The Rat Brain Atlas (Paxinos and Watson, 1998) was used as a reference for plate designations. The frontal cortex was taken from the dorsal aspect of the brain (plates 5–14). The brain was then cut across the coronal plane to gain access to the dorsal hippocampus (plates 33–35), and the CA1/CA2 ventral hippocampus, entorhinal cortex, and perirhinal cortex (plates 39–42). Brain regions were frozen and stored at −70°C until western blot analyses.
2.10 Western Blots
Activated Erk1/2 expression levels in the frontal cortex, dorsal hippocampus, CA1/CA2 ventral hippocampus, entorhinal cortex, and perirhinal cortex (all left hemisphere) were analyzed using western blots (Orr et al., 2012). Samples were suspended in 1:50 w/v RIPA buffer (150mm NaCl, 1% Triton X-100, 0.1% SDS, 0.5% Na deoxycholate, 50 mm Tris, protease inhibitor (cat# 5892791001, Millipore-Sigma), and phosphatase inhibitor (cat# 524625, Millipore-Sigma)), homogenized with a probe sonicator (Ultrasonic Processor, Cole Parmer, IL, USA), and centrifuged at 10,000 rpm for 10 min at 4°C. The BCA protein assay (ThermoFisher Scientific, Pittsburgh, PA, USA) was used to determine protein concentration. Brain homogenates were run on 4–12% NuPAGE Bis-Tris gel using the SureLock mini-cell (Invitrogen, Carlsbad, CA, USA) and blotted to an Immobilon PVDF membrane. All samples were loaded at the same protein concentration per brain region, and all gels were counterbalanced by treatment group, with a total of three gels run per brain region. The western blot was blocked in 10% non-fat milk for 1 hour, and incubated overnight in anti-phospho p44/p42 Erk1/2 primary antibody (1:2000, Cell Signaling) at 4°C. The blot was then incubated with anti-rabbit HRP (1:2000, Cell Signaling) for 1 hour at room temperature, and visualized using chemiluminescence (LumiGlo and Peroxide, Cell Signaling) in a film developer (Konica SRX-101A Film Processor, Tokyo, Japan). After imaging, the blot was stripped in 0.2M NaOH and re-probed for anti-total p44/p42 Erk1/2 (1:1000, Cell Signaling). Densitometry was performed using ImageJ software. Activated Erk1/2 levels were expressed as phosphorylated Erk1/2 expression normalized to total Erk1/2 expression.
2.11 Statistical Analyses
Behavioral measures obtained from each maze were analyzed separately, using two-tailed tests unless otherwise specified. Alpha was set at p < 0.05 for all statistical analyses, and Fisher’s PLSD tests were used for post hoc analyses.
To evaluate overall WRAM learning across all days, an omnibus repeated measures ANOVA was run to examine the Day main effect (days 2–12) for each of the three error types (WMC, WMI, and RM errors). Next, based on prior publications (Bimonte-Nelson et al., 2015; Braden et al., 2017), WRAM testing days were blocked into three blocks: Days 2–5 (Block 1), Days 6–9 (Block 2), and Days 10–12 (Block 3). Each block was analyzed separately using repeated measures ANOVA for each of the three error types, as done previously (Bimonte-Nelson et al., 2015; Braden et al., 2017). To determine the effects of hormone treatment on WRAM performance, the independent variable was Treatment, and the repeated measures were Trials nested within Days. In the case of a significant Trial x Treatment interaction, Trials 3 and 4 were analyzed separately to test the higher memory load trials, with Treatment as the independent variable and Days as the repeated measures. For the WRAM delay, each treatment group was evaluated separately for WMC, WMI, and RM errors using one-tailed repeated measures ANOVA since the delay period typically impairs performance on the WRAM, as shown in prior findings (Braden et al., 2015; Hiroi et al., 2016). Specifically, to analyze performance following the 6-hour delay, the post-delay trials, Trials 3 and 4 on Day 13 (delay performance) were averaged and compared to the average of the baseline trials, Trials 3 and 4 on Day 12 (baseline performance), as done previously (Camp et al., 2012; Engler Chuirazzi et al., 2011; Mennenga et al., 2015; 2014).
MWM Total Swim Distance data were analyzed using repeated measures ANOVA. The independent variable was Treatment, and the repeated measures were Trials nested within Days. For the probe trial, repeated measures ANOVA was used to compare Percent Swim Distance in the NE quadrant (the previously platformed quadrant) to the quadrant that was located diagonally opposite to the NE quadrant (SW, southwest) to confirm spatial localization of the platform location (Bimonte-Nelson, 2015b; Bimonte-Nelson et al., 2015).
For the visible platform analysis, Time to Platform was analyzed using repeated measures ANOVA. The independent variable was Treatment and the repeated measures were Trials (6 trials).
For blood serum levels of E2 (pg/ml) and estrone (pg/ml), and uterine horn weights (g), a one-way ANOVA was used with Treatment as the independent variable. A one-way ANOVA was also used to analyze activated Erk1 and Erk2 expression in each brain region, with Treatment as the independent variable and activated Erk expression (phosphorylated Erk normalized to total Erk) as the dependent variable. To examine relationships between activated Erk expression and cognitive performance, Pearson r correlations were run between activated Erk1 and activated Erk2 expression in each brain region and WMC, WMI, and RM error measures for Block 1 of WRAM, the block of testing where main behavioral effects were seen. To account for multiple correlations, a false discovery rate (FDR) threshold of 0.1 was used; both uncorrected (P) and FDR-corrected (Q) statistics are reported (Benjamini and Hochberg, 1995).
3. Results
3.1 Water Radial-arm Maze (WRAM)
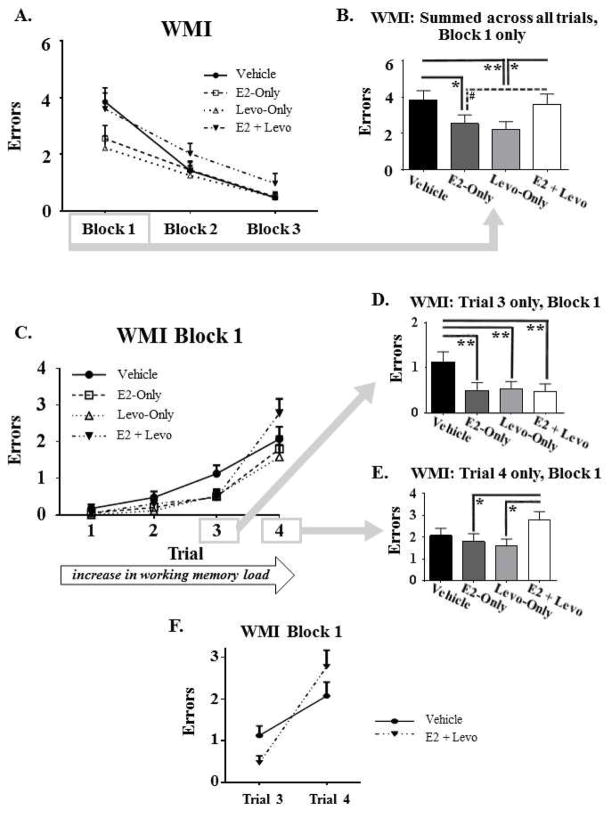
Spatial working and reference memory performance were measured using the WRAM. To analyze overall learning across all days of testing, there was a main effect of Day for each memory measure, whereby errors decreased for WMC [F(10,35) = 7.042, p < 0.0001], WMI [F(10,35) = 12.603, p < 0.0001; Figure 2A], and RM [F(10,35) = 8.176, p < 0.0001] across days 2–12 of testing, demonstrating learning of the WRAM task for all three measures of memory (data not shown). For each memory measure, there was no significant Treatment x Day interaction for Days 2–12, suggesting that groups did not differ in WRAM learning across the entire learning curve. In Block 1, there was a main effect of Treatment for WMI errors [F(3,35) = 3.597, p < 0.05; Figure 2B]. Post hoc analysis of this measurement collapsed across all trials revealed fewer errors in the E2-Only [p < 0.05] and Levo-Only [p < 0.01] treatment groups relative to the Vehicle control group, while there were no significant differences between the Vehicle and E2 + Levo groups. These results indicate that the individual hormone treatments, E2-Only and Levo-Only, enhanced WMI performance during task acquisition, while the combination treatment did not. Additionally, post hoc analyses for Block 1 for WMI revealed that the E2 + Levo group made more errors than the Levo-Only group [p < 0.05], demonstrating that the E2 + Levo hormone combination treatment impaired acquisition of the WMI measure relative to Levo-Only treatment; there was a marginal trend for the E2 + Levo group to make more errors than the E2-Only group [p < 0.1], suggesting that the E2 + Levo hormone combination treatment tended to impair acquisition of the WMI measure relative to E2-Only treatment. For Block 1, there was also a significant Treatment x Trial interaction [F(9,105) = 2.23, p < 0.05; Figure 2C] for WMI errors. Post hoc analyses showed that E2-Only [p < 0.01; Figure 2D], Levo-Only [Figure 2D], and E2 + Levo [p < 0.01; Figure 2D] treatment groups made fewer WMI errors compared to the Vehicle control group on Trial 3, the moderate working memory load trial. On Trial 4, the highest working memory load trial, post hoc analyses revealed that the E2 + Levo group made more WMI errors than the E2-Only [p < 0.05; Figure 2E] and Levo-Only [p < 0.05; Figure 2E] groups, signifying that the combination hormone treatment impaired the ability to handle a high working memory demand relative to the groups treated with either hormone alone. Due to these divergent cognitive effects of the E2 + Levo treatment across trials, a post hoc decision was made to test the interaction between the high working memory load trials, Trial 3 and 4, and E2 + Levo treatment group WMI performance relative to Vehicle control. This interaction was indeed significant [F(1,18) = 6.67, p < 0.05], indicating that the cognitive impact of the E2 + Levo hormone combination treatment is modulated by demand in working memory load (Figure 2F). The Treatment main effects for WMC or RM for Blocks 1, 2 and 3, and for WMI for Blocks 2 and 3, were not significant (data not shown).
Figure 2. Water radial-arm maze (WRAM) performance.
A) Learning curve for WMI errors, collapsed across trials for Blocks 1, 2, and 3. B) WMI Block 1 only, summed across trials, depicting a decrease in WMI errors for E2-Only and Levo-Only treatment groups relative to the Vehicle group. C) WMI errors across Trials 1–4 for Block 1 of testing. D) WMI errors on Trial 3 only, the moderate working memory load trial, for Block 1 of testing, illustrating that all hormone treatment groups made fewer WMI errors relative to the Vehicle group. E) WMI errors on Trial 4 only, the high working memory load trial, for Block 1 of testing, depicting that the E2+Levo group made more WMI errors relative to E2-Only and Levo-Only groups. F) Treatment x Trial interaction, representing WMI errors made on the moderate and high working memory load trials (Trials 3 and 4, respectively) and Treatment (Vehicle and E2+Levo) for Block 1 of testing. The impact of E2 + Levo treatment on WMI performance was dependent on working memory demand. All errors are expressed as mean ± SEM. #p < 0.1, *p < 0.05, **p < 0.01.
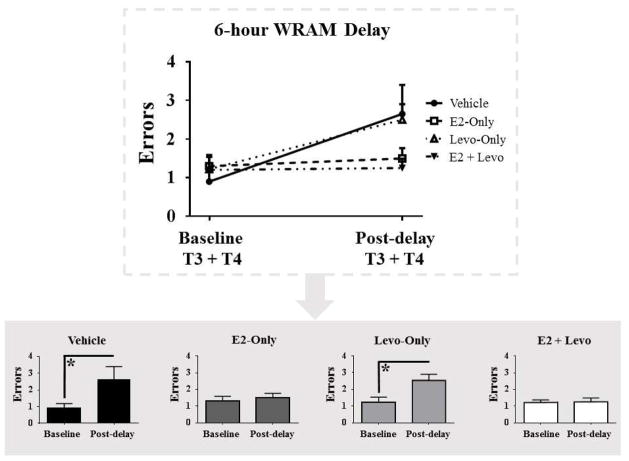
Following the 6-hour delay on Day 13, the Vehicle control group [F(1,9) = 4.509, p < 0.05] and Levo-Only group [F(1,8) = 0.5099, p < 0.05] made more WMC errors on the post delay trials relative to baseline trials, indicating forgetting across the delay period (Figure 3). E2-Only and E2 + Levo groups did not significantly differ in WMC errors for post delay trials compared to baseline trials (Figure 3), suggesting that these groups did not show significant forgetting across the delay period. No differences were seen for WMI and RM error measures for each treatment group on the post-delay trials relative to baseline trials (data not shown).
Figure 3. Water radial-arm maze (WRAM) performance following a 6-hour delay.
Top graph shows WMC errors made on baseline trials (T3+4 on Day 12 of WRAM) and post-delay trials (T3+4 on Day 13 of WRAM) for all treatment groups. Bottom graphs illustrate WMC errors made on baseline trials and post-delay trials split by treatment group. All errors are expressed as mean ± SEM. *p < 0.05
3.2 Morris Water Maze (MWM)
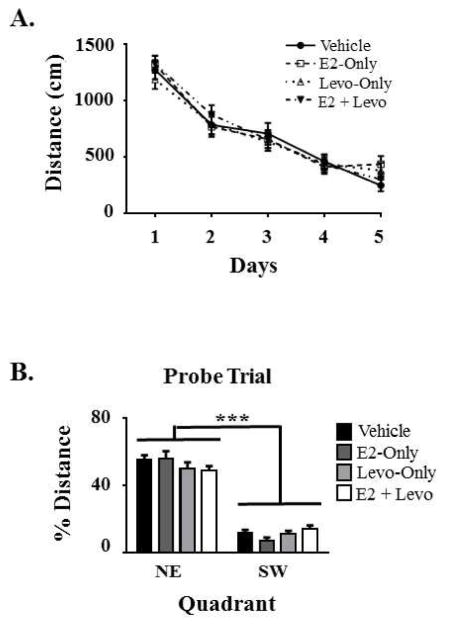
The MWM was used to evaluate spatial reference memory performance. There was a main effect of Day across all five days of testing, with Total Swim Distance scores decreasing across days [F(4,35) = 83.997, p < 0.0001; Figure 4A]. The lack of a significant Treatment x Day interaction indicates that the groups did not differ in learning trajectory across days. The Treatment main effect for swim distance to the platform was not significant. For the probe trial, there was a main effect of Quadrant, with a greater percent swim distance spent in the NE target quadrant, the previously-platformed quadrant, relative to the opposite, SW quadrant [F(1,35) = 246.174, p < 0.0001; Figure 4B], indicating spatial localization of the platform location. The lack of a significant Treatment x Quadrant interaction suggests that the groups did not differ in their pattern of spatial localization by the end of testing.
Figure 4. Morris water maze (MWM) performance.
A) Swim distance to the platform for the 5 days of testing, illustrating that all treatment groups learned the task as depicted by the decrease in swim distance across days. B) Percent swim distance in the northeast (NE) quadrant that previously contained the platform compared to the opposing southwest (SW) quadrant that never contained a platform on the probe trial, confirming spatial localization of the platform by animals from all treatment groups. All measurements are expressed as mean ± SEM. ***p < 0.0001
3.3 Visible Platform
Motor and visual competence to solve a water-escape maze task was evaluated using the visible platform test. There was a main effect of Trial [F(5,35) = 5.609, p < 0.0001], with an 8.6s average latency to the platform across trials (data not shown). During the last trial of testing, each animal reached the platform in under 18s, confirming the ability to complete a water-escape maze task. Neither the Treatment, nor the Treatment x Trial interaction, was significant, indicating that the groups did not differ in the ability to learn and perform the procedural components of a water-escape maze task.
3.4 Blood Serum Analysis
E2 and estrone levels in blood serum, collected at sacrifice, were analyzed. For E2 levels, there was a main effect of Treatment [F(3,34) = 10.691, p < 0.0001], with post hoc analyses showing higher E2 levels for the E2-Only [p < 0.0001] and E2 + Levo [p < 0.0001] groups compared to the Vehicle group, as well as higher E2 levels for the E2-Only [p < 0.0001] and E2 + Levo [p < 0.0001] groups compared to the Levo-Only group (Figure 5A). For estrone levels, there was a main effect of Treatment [F(3,35) = 25.014, p < 0.0001], with higher estrone levels for both E2-Only [p < 0.0001] and E2 + Levo [p < 0.0001] groups relative to the Vehicle group, as well as higher estrone levels for both E2-Only [p < 0.0001] and E2 + Levo [p < 0.0001] groups relative to the Levo-Only group (Figure 5B). These results verify systemic presence of E2 and estrone in the groups receiving exogenous E2 treatment.
Figure 5. Circulating E2 and estrone levels and uterine horn weights.
(A) E2 blood serum levels were increased in the E2-Only and E2+Levo treatment groups compared to the Vehicle group and compared to the Levo-Only group. (B) Estrone blood serum levels were elevated in the E2-Only and E2 + Levo treatment groups relative to the Vehicle group and relative to the Levo-Only group. (C) Uterine horn weight increased for the two E2 treated groups (E2-Only, and E2 + Levo) relative to the Vehicle group and relative to the Levo-Only group. All measurements are expressed as mean ± SEM. ***p < 0.0001
3.5 Uterine Horn Weight
There was a main effect of Treatment [F(3,35) = 58.915, p < 0.0001] for uterine horn weights; higher weights were seen in the E2-Only [p < 0.0001] and E2 + Levo [p < 0.0001] groups compared to the Vehicle group, and higher weights were seen in the E2-Only [p < 0.0001] and E2 + Levo [p < 0.0001] groups compared to the Levo-only group, as expected with estrogen exposure (Figure 5C; Engler-Chiurazzi et al., 2012; Mennenga et al., 2015b; Westerlind et al., 1998). There was no significant difference in uterine horn weights between the E2-Only group and the E2 + Levo group, suggesting that the addition of the currently-utilized Levo regimen to the E2 treatment did not impact the E2-induced increase in uterine horn weight at the time of uterine data collection.
3.6 Brain Analysis
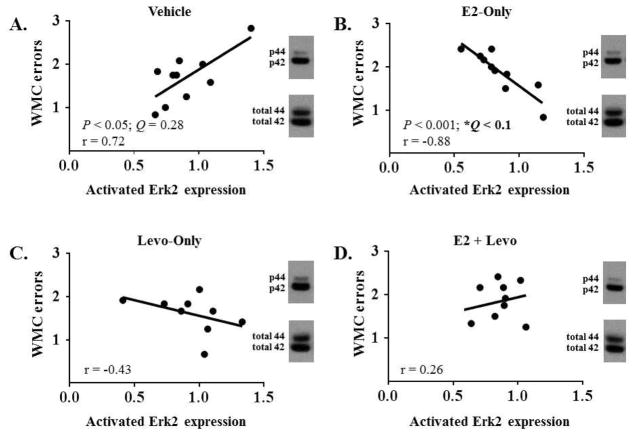
Western blots were performed to examine activated Erk1 and Erk2 expression in several regions of the brain that are indicated in learning and memory. There were no significant main effects of Treatment for activated Erk1 and Erk2 expression in the frontal cortex, dorsal hippocampus, CA1/CA2 ventral hippocampus, entorhinal cortex, or perirhinal cortex (data not shown). A correlation table summarizing the relationship between activated Erk1 and activated Erk2 expression in the frontal cortex and error measures for Block 1 of WRAM for each treatment group is presented in Table 1. After adjusting for multiple correlations using a set FDR threshold of 0.1, a relationship between cognitive performance and activated Erk2 expression was seen that was specific to animals treated with E2-Only, with higher levels of activated Erk2 associated with better performance. In particular, in the frontal cortex, there was a significant negative correlation between activated Erk2 expression and Block 1 WMC errors within the E2-Only group [r(19) = −0.88, P < 0.001, Q < 0.1; Figure 6B], suggesting that the E2-treated animals that tended to have higher levels of activated Erk2 in the frontal cortex tended to make fewer WMC errors. In contrast to the relationship seen with E2-Only treatment, there was no significant correlation between activated Erk2 expression in the frontal cortex and Block 1 WMC errors within the E2 + Levo treatment group [r(19) = 0.26, P = 0.47, Q = 0.87; Figure 6D], suggesting that the addition of Levo obviated the E2-induced association between working memory performance and activated Erk2 levels in the frontal cortex. There was also no significant correlation between activated Erk2 expression in the frontal cortex and Block 1 WMC errors within the Vehicle control group [r(19) = 0.72, P = 0.02, Q = 0.28; Figure 6A] and within the Levo-Only treatment group [r(18) = −0.43, P = 0.25, Q = 0.87; Figure 6C]. There was no significant relationship between cognitive performance and activated Erk1 expression, nor between cognitive performance and activated Erk2 expression, in the dorsal hippocampus, CA1/CA2 ventral hippocampus, entorhinal cortex, or perirhinal cortex (correlation tables not shown).
Table 1.
Correlation matrix showing Pearson r correlations, for each treatment group, between activated Erk1 and activated Erk2 expression in the frontal cortex and error measures for Block 1 of WRAM, the block of testing where main behavioral effects were seen. To account for multiple correlations, a false discovery rate (FDR) threshold of 0.1 was used; both uncorrected (P) and FDR-corrected (Q) statistics are reported (Benjamini and Hochberg, 1995). Significant correlations following FDR-correction are noted with a * and are in bold; significant correlations prior to FDR-correction are noted with a # and are italicized.
| Frontal Cortex | WMC errors | WMI errors | RM errors | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Protein | r | P | Q | r | P | Q | r | P | Q |
| Vehicle | Erk 1 | 0.6059 | 0.0634 | 0.8747 | 0.1273 | 0.7260 | 0.8909 | −0.6502 | 0.0418# | 0.8747 |
| Erk2 | 0.7242 | 0.0179# | 0.7160 | 0.2288 | 0.5249 | 0.8909 | −0.5830 | 0.0769 | 0.8909 | |
| E2-Only | Erk 1 | −0.4873 | 0.1531 | 0.8747 | 0.0781 | 0.8302 | 0.9224 | −0.1046 | 0.7736 | 0.9099 |
| Erk2 | −0.8803 | 0.0008# | 0.0960* | −0.3846 | 0.2725 | 0.8747 | −0.8079 | 0.0047# | 0.2820 | |
| Levo-Only | Erk 1 | −0.6021 | 0.0862 | 0.8747 | −0.5979 | 0.0890 | 0.8747 | −0.7244 | 0.0273# | 0.8190 |
| Erk2 | −0.4252 | 0.2538 | 0.8747 | −0.5054 | 0.1652 | 0.8747 | −0.3817 | 0.3107 | 0.8747 | |
| E2 + Levo | Erk 1 | 0.3383 | 0.3389 | 0.8747 | 0.3297 | 0.3522 | 0.8747 | 0.3823 | 0.2756 | 0.8747 |
| Erk2 | 0.2607 | 0.4670 | 0.8747 | 0.4711 | 0.1694 | 0.8747 | 0.1609 | 0.6570 | 0.9099 | |
Figure 6. Pearson r correlations between Block 1 WMC errors and activated Erk2 expression in the frontal cortex.
For each treatment group, a single sample was chosen to provide a representative image of the blots for phosphorylated Erk1 and Erk2 as well as total Erk1 and Erk2 in the frontal cortex. (A) Block 1 WMC errors did not correlate with activated Erk2 expression within the Vehicle group. (B) Block 1 WMC errors correlated with activated Erk2 expression within the E2-Only group. (C) Block 1 WMC errors did not correlate with activated Erk2 expression within the Levo-Only group. (D) Block 1 WMC errors did not correlate with activated Erk2 expression within the E2+Levo group. Of note, the false discovery rate-corrected statistics (Q) are reported here to account for multiple correlations. * Q < 0.1. Activated Erk2 expression is expressed as phosphorylated Erk2/total Erk2.
4. Discussion
The current study demonstrated that E2-Only and Levo-Only treatments enhanced working memory performance during acquisition of the WRAM, as measured by WMI errors collapsed across all trials, and that the combination of E2 + Levo attenuated these beneficial cognitive effects. In fact, at the highest demand working load (trial 4) during acquisition, the E2 + Levo combination impaired performance, as compared to the E2-Only and Levo-Only groups. Thus, even a progestin that we have shown here and in prior work (Braden et al., 2017) to enhance the ability to handle an increasing working memory load on the WRAM task when given alone, can reverse the beneficial effects of E2 at a high memory demand. Additionally, we showed that there was a distinct relationship between activated Erk2 expression in the frontal cortex and working memory performance within the E2-Only treatment group, which was mitigated by the addition of Levo to the E2 treatment. However, Levo did not uniformly attenuate the benefits of E2. At a working memory load that was less demanding, the addition of Levo did not reverse the cognitive benefits of E2 relative to vehicle treatment, as determined by trial 3 for WMI errors on the WRAM. Indeed, similar to each hormone treatment given alone, the hormone combination treatment benefitted this moderate working memory load trial performance as compared to the vehicle treatment.
Overall, we found that all hormone treatment groups were able to learn spatial working and reference memory tasks, as shown by their performance across all days on the WRAM (Days 1–12, collapsed across the 4 trials) and the MWM (Days 1–5, collapsed across the 4 trials). During the acquisition phase of the WRAM (Block 1 of testing), when rats were initially learning the rules of the task, the E2-Only and Levo-Only treatment groups made fewer WMI errors across all four trials compared to the vehicle group, indicating that E2-Only and Levo-Only enhanced working memory performance during the learning phase of the task relative to control. With reference to prior publications testing E2 (using various regimens, doses, and rat ages), in general, our results here are consistent with previously published findings suggesting enhanced cognitive performance with E2 only treatment (Bimonte and Denenberg, 1999; Daniel et al., 2006, 1997; Fader et al., 1999; Gibbs and Johnson, 2008; Luine et al., 1998; Rodgers et al., 2010). Our results are also consistent with previously published findings of enhanced cognitive performance with Levo only treatment (Braden et al., 2017; Simone et al., 2015). When evaluating all four trials combined, the combination of E2 + Levo obviated the working memory benefits of E2-Only and Levo-Only. There has been only one other published preclinical study testing an estrogen/Levo combination, whereby the synthetic estrogen ethinyl estradiol (EE) plus Levo was tested in a different model and behavior paradigm: the young ovary-intact rat tested in object memory (Simone et al., 2015). However, even with the important differences between our studies, similar results were reported, with the EE plus Levo hormone combination treatment attenuating EE-induced improvements in novel object memory and Levo-induced enhancements in visuospatial memory in ovary-intact young rats (Simone et al., 2015). Preclinical studies testing other combination hormone therapies have also shown that, in Ovx rodents, the addition of natural progesterone to E2 treatment can reverse the enhancing cognitive effects of E2 (Bimonte-Nelson et al., 2006; Harburger et al., 2007; Lowry et al., 2010). It has also been shown that the addition of MPA to an E2 treatment resulted in impaired learning on the MWM compared to E2 treatment alone (Lowry et al., 2010).
Interestingly, results from this study showed that working memory demand influenced the direction of hormone treatment effects on cognitive performance specifically during the acquisition phase of the WRAM. On Trial 3, when the working memory load was moderate, the E2-Only, Levo-Only, and E2 + Levo treatment groups made fewer WMI errors than the vehicle group, suggesting that all hormone treatments enhanced performance relative to control treatment during this moderate demand trial. However, on Trial 4, when the working memory load was highest, the E2 + Levo treatment group made more WMI errors compared to E2-Only and Levo-Only treatment groups, revealing that this estrogen/progestin combination treatment impaired high demand working memory ability relative to each individual hormone treatment. This is additionally represented in Figure 2F, with effects clearly illustrated by the interaction between the moderate and the high working memory load trials (Trials 3 and 4) and Treatment (E2 + Levo group versus the vehicle group). Taken together, these results indicate that the hormones E2 and Levo impact cognitive function in a model of surgical menopause, with the direction of this mnemonic impact dependent on: 1) whether E2 and Levo are administered alone as an individual exogenous regimen, or together as a hormone combination exogenous regimen, and 2) cognitive demand. Each individual hormone regimen and the combined hormone regimen enhanced cognitive performance when the working memory demand was moderate; however, when the working memory demand was high, these two hormones in combination impaired performance compared to each hormone alone. These findings are in accordance with previous studies demonstrating distinct hormone effects across an increase in working memory demand, including with estrogens (Bimonte and Denenberg, 1999; Hiroi et al., 2016; Mennenga et al., 2015b), progestogens (Braden et al., 2017, 2015, 2011), and androgens (Camp et al., 2012; Mennenga et al., 2015c).
When a 6-hour delay period was implemented for the WRAM, neither of the E2-treated groups (E2-Only and E2 + Levo) differed in WMC errors between the post-delay trials and the baseline trials. This suggests that exogenous E2 treatment protected from delay-induced impairment in performance on working memory. Of note, the addition of Levo to E2 did not reverse the protective effects of E2-Only against delay-induced impairment in working memory performance. However, the vehicle and Levo-Only treatment groups exhibited impaired performance, where they made more WMC errors on the post-delay trials relative to their baseline performance. Together, these findings demonstrate a potential protective effect of E2 on cognitive function across the delay period that was not seen with the vehicle and the Levo-Only treatment, regardless of whether Levo is on board with the E2 treatment or not. These results are consistent with previous studies where E2 enhanced cognitive performance following the implementation of a delay period (Harburger et al., 2007; Talboom et al., 2008). For instance, one study trained aged Ovx mice on the MWM and then immediately administered vehicle, E2, or E2 plus a low or high dose of progesterone treatment (Harburger et al., 2007). Following a 24 hour delay period, results showed that the E2 treatment enhanced performance (Harburger et al., 2007). The addition of a low dose of progesterone did not alter the beneficial cognitive effects of E2 on post-delay performance, but the addition of a high dose of progesterone attenuated the beneficial cognitive effects of E2 on post-delay performance (Harburger et al., 2007). Another study from our laboratory also showed that E2 treatment had beneficial effects on overnight forgetting on the MWM task compared to vehicle control (Talboom et al., 2008). Collectively, these results reveal a protective role of E2 in cognitive function following the implementation of a delay period.
The hormone impact was working memory-specific in the current study. For the spatial reference memory MWM, all treatment groups decreased in swim distance to the platform across all days of testing. However, there were no treatment differences in the swim distance to the platform, suggesting similar spatial reference memory performance on this task. On the probe trial for MWM, all treatment groups swam a greater percent distance in the NE target quadrant, which previously contained the platform, compared to the opposing SW quadrant. Thus, results from the MWM task showed that all groups were able to effectively learn the spatial reference memory task and spatially localize to the platform location in a similar pattern. It is important to note that the MWM was administered following the WRAM. Some studies suggest that previous cognitive experience can impact learning and memory performance (Markowska, 2002; Talboom et al., 2014). Thus, prior learning experience may have affected learning and memory performance on the MWM in the present study, and may be in part contributing to the lack of significant treatment effects on the MWM. However, these data are consistent with the lack of a treatment effect for the spatial reference memory measure of the WRAM in the current study.
The visible platform task tests the ability of animals to effectively perform the procedural components of a water-escape maze task. There were no treatment differences and no treatment by trial interactions on the visible platform task, indicating that all groups had similar capabilities required to effectively complete a water-escape maze task. Therefore, our interpretations of treatment effects on cognitive performance are not impacted by the motor and visual capabilities of the animals.
In the current study, we confirmed E2 exposure by showing that E2 treatment elevated circulating levels of E2 and its metabolite, estrone, relative to vehicle treatment, and by demonstrating that uterine horn weight increased with E2 treatment as compared to vehicle treatment. In women, the addition of a progestogen is meant to offset the uterine stimulation induced by estrogen, and this has been seen in rat models (Armstrong, 1968; Creasy et al., 1992; Mennenga and Bimonte-Nelson, 2015). However, in the current study, the addition of Levo to E2 treatment did not significantly reduce E2-induced increase in uterine horn weight. This may be explained in part by the 5:1 E2 to Levo ratio used in the present study, which was based on the dose of Levo that has previously been shown to enhance spatial learning and memory when administered alone. Additional investigation is warranted to address whether decreasing the E2 to Levo ratio, including to the 3:1 ratio used in Climara Pro, can significantly reduce uterine horn stimulation.
In the frontal cortex, the present study found a relationship between working memory performance and activated Erk2 expression for the E2-Only group, where animals that tended to perform better on the WMC measure tended to have higher activated Erk2 expression. This suggests that there is a unique relationship between working memory performance and activated Erk2 expression in the presence of E2 in the frontal cortex, a region that is heavily involved in normal working memory function (Funahashi and Kubota, 1994). It is noteworthy that the beneficial cognitive effects of E2 in this study were specific to working memory, and here the activation of a signaling pathway implicated in cognitive function was linked particularly to E2-Only treatment and its effects in a region of the brain that plays a significant role in processing working memory information. In contrast to the relationship seen with E2-Only treatment, there was no relationship between cognitive performance as measured by WMC errors made and activated Erk2 expression within the E2 + Levo hormone combination treatment group, indicating that the addition of Levo obviated the E2-induced association between cognitive performance and activated Erk2 levels in the brain. Additionally, there was a relationship trend within the Vehicle group between activated Erk2 expression in the frontal cortex and WMC errors, whereby higher activated Erk2 levels correlated with higher WMC errors, indicating that E2-Only treatment may have reversed the relationship between activated Erk2 expression and working memory performance in the control group; this correlation was statistically significant before the correction, but it was not statistically significant after correcting for multiple correlations. Although the relationship between cognitive performance and activated Erk2 levels in the frontal cortex was specific to the WMC measure and the beneficial cognitive effects of E2-Only treatment were specific to the WMI measure, it is important to note that these measures of working memory performance are orthogonal and may be governed by different neurological pathways and brain regions. For example, a hippocampal lesion study found that a complete hippocampal lesion in male rats resulted in increased WMC errors compared to WMI errors when tested on an 8-arm radial arm maze, but no differences were seen between WMC errors and WMI errors in control rats and in rats with partial hippocampal lesions (Jarrard et al., 2012). Thus, the type of memory affected, and the directionality of the cognitive effect following hormone treatment, may be governed by the brain regions and neural pathways that are specific to the hormones examined. There were no treatment-induced differences in activated Erk1 and Erk2 expression in the dorsal hippocampus, CA1/CA2 ventral hippocampus, frontal cortex, entorhinal cortex, or perirhinal cortex. It is important to highlight that the treatment regimen in this study was a chronic and cyclic low dose injection, whereas studies that have shown E2-induced increase of activated Erk2 expression in the dorsal hippocampus are typically acute or injected at a higher or tonic dose (Fernandez et al., 2008; Harburger et al., 2009; Witty et al., 2013).
The growing preclinical and clinical evidence indicates great complexity in the cognitive effects of ovarian hormone loss and hormone therapy. This drives us toward opening new avenues of study to help us understand which factors play into this complexity in order to truly understand hormone-related impacts. Many publications indicate that ovarian hormone loss in women, and in rodents, is associated with changes in memory performance across multiple domains of memory, that subsequent E2 treatment can result in beneficial effects on cognitive function, and that the addition of a progestogen can attenuate these E2-induced cognitive benefits (for reviews: Frick, 2015; Koebele and Bimonte-Nelson, 2015; Korol and Pisani, 2015; Maki, 2012; Mennenga and Bimonte-Nelson, 2013; Sherwin, 2006). There are notable exceptions to these outcomes, as the extent and direction of hormone therapy effects are sensitive to a myriad of variables. The details and parameters of how such factors impact outcomes are just beginning to be understood (Koebele and Bimonte-Nelson, 2015, 2017; Korol and Pisani, 2015). For example, age is a critical factor affecting the efficacy of estrogens on cognitive and brain function, with diminished or lost efficacy as aging ensues (Bean et al., 2014; Foster et al., 2003; Gibbs, 2010; Koebele and Bimonte-Nelson, 2017; Maki, 2013; Mennenga and Bimonte-Nelson, 2013; Talboom et al., 2008). Thus, the hormone treatment effects observed in the present study in middle-aged rats may be more pronounced if tested in young rats, and may be attenuated in aged rats.
Several large-scale clinical studies have set out with the common goal to further understand the cognitive impact of menopause and hormone therapies taken by women (Gleason et al., 2015; Greendale et al., 2011; Karlamangla et al., 2017; Rapp et al., 2003; Shumaker et al., 2003). Only one human study thus far has specifically evaluated the impact estrogen and Levo have on cognitive function. In this study, the estradiol valerate plus Levo oral hormone therapy, Kilomonorm, was assessed; after two months, Kilomonorm improved concentration, speed of cognitive function, and short-term memory in peri- and post- menopausal women that either had received a hysterectomy (n = 6), ovariectomy (n = 14), hysterectomy plus ovariectomy (n = 6), or no surgical manipulations (n = 52) (Rudolph et al., 2000). In the current study, we showed that the cognitive effects of the estrogen plus Levo treatment in surgically menopausal rats are contingent on the level of cognitive demand and memory type evaluated. Thus, systematically designed studies of clinically-relevant hormone formulations and their effects in animal models of menopause are critical; information gained from such studies can inform future human study designs as steps are taken to further understand the complex interactions between hormones and various memory domains.
5. Conclusion
To our knowledge, this study is the first to examine the effect of an E2 + Levo hormone combination treatment, as well as E2-Only and Levo-Only treatments, on cognitive function in a preclinical model of surgical menopause. Hormone therapy, which can contain only estrogens, only progestogens, or an estrogen plus progestogen combination, is used to decrease the onset and severity of undesired changes associated with menopause. Thus, it is critical to not only examine the individual effects of hormones on symptoms associated with menopause, including the impact on learning and memory, but also how the combination of both an estrogen and a progestogen impacts these symptoms. Our results showed that E2 and Levo each have beneficial effects on spatial working memory when administered separately, replicating our prior studies (Bimonte-Nelson et al., 2006; Bimonte and Denenberg, 1999; Braden et al., 2017; Talboom et al., 2008). However, the E2 + Levo hormone combination impaired spatial working memory relative to either of the hormones alone when the spatial working memory task was highly taxing. Furthermore, a relationship between activated Erk2 levels in the frontal cortex and spatial working memory following E2-Only treatment was observed. These findings are significant as they highlight opposing effects of an estrogen and progestogen hormone combination treatment, and raise further questions regarding which underlying neurobiological mechanisms are responsible for the individual cognitive enhancements of E2-Only and Levo-Only, and for the negating effects when the two hormones are administered together. This study illustrates that although an estrogen and a progestogen can be cognitively beneficial when administered alone, the clinically used combination of the same estrogen and progestogen does not necessarily result in added benefits, and can in fact yield impairments. Indeed, in the context of hormone therapy, one plus one does not always equal two.
17β-estradiol (E2) and Levonorgestrel (Levo) are exogenous hormones given alone and in combination to women
The cognitive effects of E2 and Levo were tested in middle-aged, ovariectomized rats
E2 alone and Levo alone improved spatial working memory performance
The E2+Levo combination attenuated the cognitive effects of E2 and of Levo as memory load increased
While E2 and Levo alone improved memory, the E2+Levo combination did not maintain these effects
Acknowledgments
This work was supported by the National Institutes of Aging [grant number AG028084]; state of Arizona, and Arizona Department of Health Services (ADHS 14-052688). The authors would also like to acknowledge the Barrow Neurological Institute and the ASU-BNI Interdepartmental Neuroscience Program. Alesia Prakapenka was funded by the NSF Graduate Research Fellowship. We thank Dr. Laurence Demers and the Core Endocrinology Laboratory of the Pennsylvania State University, College of Medicine for performing the hormone assays.
Footnotes
Conflicts of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Safi ZA, Santoro N. Menopausal hormone therapy and menopausal symptoms. Fertil Steril. 2014;101:905–915. doi: 10.1016/j.fertnstert.2014.02.032. [DOI] [PubMed] [Google Scholar]
- Armstrong DT. Hormonal control of uterine lumen fluid retension in the rat. Am J Physiol. 1968;214:764–771. doi: 10.1152/ajplegacy.1968.214.4.764. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Bean LA, Ianov L, Foster TC. Estrogen receptors, the hippocampus, and memory. Neuroscientist. 2014;20:534–545. doi: 10.1177/1073858413519865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara RG, Lyne R, Kelly ÁM. BDNF-stimulated intracellular signalling mechanisms underlie exercise-induced improvement in spatial memory in the male Wistar rat. Behav Neurosci. 2014;275:297–306. doi: 10.1016/j.bbr.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- Bimonte-Nelson HA. The water radial-arm maze: Four out of eight arms platformed protocol for rodents. In: Bimonte-Nelson HA, editor. The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition. Humana Press; 2015a. pp. 411–419. [Google Scholar]
- Bimonte-Nelson HA. The Morris maze protocol for rodents. In: Bimonte-Nelson HA, editor. The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition. Humana Press; 2015b. pp. 441–449. [Google Scholar]
- Bimonte-Nelson HA. The visible platform task for rodents. In: Bimonte-Nelson HA, editor. The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition. Humana Press; 2015c. pp. 451–454. [Google Scholar]
- Bimonte-Nelson HA, Daniel JM, Koebele SV. The mazes. In: Bimonte-Nelson HA, editor. The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition. Humana Press; 2015. pp. 37–72. [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Nelson ME, Granholm AE. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15:2659–63. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–44. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden BB, Andrews MG, Acosta JI, Mennenga SE, Lavery C, Bimonte-Nelson HA. A comparison of progestins within three classes: differential effects on learning and memory in the aging surgically menopausal rat. Behav Brain Res. 2017;322:258–268. doi: 10.1016/j.bbr.2016.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden BB, Garcia AN, Mennenga SE, Prokai L, Villa SR, Acosta JI, Lefort N, Simart AR, Bimonte-Nelson HA. Cognitive-impairing effects of medroxyprogesterone acetate in the rat: independent and interactive effects across time. Psychopharmacology (Berl) 2011;218:405–418. doi: 10.1007/s00213-011-2322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden BB, Kingston ML, Whitton E, Lavery C, Tsang CWS, Bimonte-Nelson HA. The GABAA antagonist bicuculline attenuates progesterone-induced memory impairments in middle-aged ovariectomized rats. Front Aging Neurosci. 2015;7:1–8. doi: 10.3389/fnagi.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, Scheldrup MR, Bowman BL, Bimonte-Nelson HA. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem. 2010;93:444–453. doi: 10.1016/j.nlm.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, Watson RI. An evaluation of psychologic effects of sex hormone administration in aged women. J Gerentology. 1952;7:228–244. doi: 10.1093/geronj/7.2.228. [DOI] [PubMed] [Google Scholar]
- Camp BW, Gerson JE, Tsang CWS, Villa SR, Acosta JI, Blair Braden B, Hoffman AN, Conrad CD, Bimonte-Nelson HA. High serum androstenedione levels correlate with impaired memory in the surgically menopausal rat: A replication and new findings. Eur J Neurosci. 2012;36:3086–3095. doi: 10.1111/j.1460-9568.2012.08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm NC, Juraska JM. Long term replacement of estrogen in combination with medroxyprogesterone acetate improves acquisition of an alternation task in middle aged female rats. Behav Neurosci. 2012;126:128–136. doi: 10.1037/a0026461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli A, Giustetto M. Role of ERK signaling in activity-dependent modifications of histone proteins. Neuropharmacology. 2014;80:34–44. doi: 10.1016/j.neuropharm.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Huynh TN, El-Ashmawy M, Sparks M. Chronic stress and a cyclic regimen of estradiol administration separately facilitate spatial memory: Relationship with CA1 spine density and dendritic complexity. Behav Neurosci. 2012;126:142–156. doi: 10.1037/a0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasy GW, Kafrissen ME, Upmalis D. Review of the endometrial effects of estrogens and progestins. Obs Gynecol Surv. 1992;47:654–678. doi: 10.1097/00006254-199209000-00026. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Du J, Sanchez P, Kim L, Azen C. Percutaneous progesterone delivery via cream or gel application in postmenopausal women: A randomized cross-over study of progesterone levels in. Menopause. 2013;20:1–7. doi: 10.1097/gme.0b013e31828d39a2. [DOI] [PubMed] [Google Scholar]
- El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, Adem A. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: Relationship to Morris water maze performance. J Cell Mol Med. 2004;8:537–544. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, Huentelman MJ, Bimonte-Nelson HA. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol Aging. 2011;32:680–697. doi: 10.1016/j.neurobiolaging.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi EB, Talboom JS, Braden BB, Tsang CWS, Mennenga S, Andrews M, Demers LM, Bimonte-Nelson HA. Continuous estrone treatment impairs spatial memory and does not impact number of basal forebrain cholinergic neurons in the surgically menopausal middle-aged rat. Horm Behav. 2012;62:1–9. doi: 10.1016/j.yhbeh.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader AJ, Johnson PEM, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacol Biochem Behav. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Cheng Y, Zhang J. Long-term effects of melatonin or 17β-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J Pineal Res. 2004;37:198–206. doi: 10.1111/j.1600-079X.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Lauren L, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal Erk activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015;74:4–18. doi: 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Kubota K. Working memory and prefrontal cortex. Neurosci Res. 1994;21:1–11. doi: 10.1016/0168-0102(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav. 1994;56:281–289. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52:352–359. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42:245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–33. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason CE, Dowling NM, Wharton W, Manson JAE, Miller VM, Atwood CS, Brinton EA, Cedars MI, Lobo RA, Merriam GR, Neal-Perry G, Santoro NF, Taylor HS, Black DM, Budoff MJ, Hodis HN, Naftolin F, Harman SM, Asthana S. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12:1–25. doi: 10.1371/journal.pmed.1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooney M, Shaw K, Kelly A, O’Mara SM, Lynch MA. Long-term potentiation and spatial learning are associated with increased phosphorylation of TrkB and extracellular signal-regulated kinase (ERK) in the dentate gyrus: Evidence for a role for brain-derived neurotrophic factor. Behav Neurosci. 2002;116:455–463. doi: 10.1037//0735-7044.116.3.455. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Derby CA, Maki PM. Perimenopause and cognition. Obstet Gynecol Clin North Am. 2011;38:519–535. doi: 10.1016/j.ogc.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28:602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Saadi A, Frick KM. Dose-dependent effects of post-training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience. 2009;160:6–12. doi: 10.1016/j.neuroscience.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi R, Weyrich G, Koebele SV, Mennenga SE, Talboom JS, Hewitt LT, Lavery CN, Mendoza P, Jordan A, Bimonte-Nelson HA. Benefits of hormone therapy estrogens depend on estrogen type: 17β-estradiol and conjugated equine estrogens have differential effects on cognitive, anxiety-like, and depressive-like behaviors and increase tryptophan hydroxylase-2 mRNA levels in dorsal raphe nucleus subregions. Front Neurosci. 2016;10:517. doi: 10.3389/fnins.2016.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE, Luu LP, Davidson TL. A study of hippocampal structure-function relations along the septo-temporal axis. Hippocampus. 2012;22:680–692. doi: 10.1002/hipo.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Lachman ME, Han W, Huang M, Greendale GA. Evidence for cognitive aging in midlife women: Study of Women’s Health Across the Nation. PLoS One. 2017;12:1–13. doi: 10.1371/journal.pone.0169008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss Á, Delattre AM, Pereira SIR, Carolino RG, Szawka RE, Anselmo-Franci JA, Zanata SM, Ferraz AC. 17β-Estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav Brain Res. 2012;227:100–108. doi: 10.1016/j.bbr.2011.10.047. [DOI] [PubMed] [Google Scholar]
- Koebele SV, Bimonte-Nelson HA. Trajectories and phenotypes with estrogen exposures across the lifespan: What does Goldilocks have to do with it? Horm Behav. 2015;74:86–104. doi: 10.1016/j.yhbeh.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebele SV, Bimonte-Nelson HA. The endocrine-brain-aging triad where many paths meet: Female reproductive hormone changes at midlife and their influence on circuits important for learning and memory. Exp Gerontol. 2017;94:14–23. doi: 10.1016/j.exger.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebele SV, Mennenga SE, Hiroi R, Quihuis AM, Hewitt LT, Poisson ML, George C, Mayer LP, Dyer CA, Aiken LS, Demers LM, Carson C, Bimonte-Nelson HA. Cognitive changes across the menopause transition: A longitudinal evaluation of the impact of age and ovarian status on spatial memory. Horm Behav. 2017;87:96–114. doi: 10.1016/j.yhbeh.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komnenich P, Lane DM, Dickey RP, Stone SC. Gonadal hormones and cognitive performance. Physiol Psychol. 1978;6:115–120. [Google Scholar]
- Korol DL, Pisani SL. Estrogens and cognition: Friends or foes? An evaluation of the opposing effects of estrogens on learning and memory. Horm Behav. 2015;74:105–115. doi: 10.1016/j.yhbeh.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8:3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- Lowry NC, Pardon LP, Yates MA, Juraska JM. Effects of long-term treatment with 17 β-estradiol and medroxyprogesterone acetate on water maze performance in middle aged female rats. Horm Behav. 2010;58:200–207. doi: 10.1016/j.yhbeh.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–62. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Maki PM. The critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20:695–709. doi: 10.1097/GME.0b013e3182960cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM. Minireview: Effects of different HT formulations on cognition. Endocrinology. 2012;153:3564–3570. doi: 10.1210/en.2012-1175. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Protective effect of practice on cognition during aging: Implications for predictive characteristics of performance and efficacy of practice. Neurobiol Learn Mem. 2002;78:294–320. doi: 10.1006/nlme.2002.4064. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Osanai M. Estrogen stimulates neuronal plasticity in the deafferented hypothalamic arcuate nucleus in aged female rats. Neurosci Res. 1985;2:412–418. doi: 10.1016/0168-0102(85)90052-5. [DOI] [PubMed] [Google Scholar]
- McGauran AT, Moore JB, Madsen D, Barry D, O’Dea S, Mahon BP, Commins S. A possible role for protein synthesis, extracellular signal-regulated kinase, and brain-derived neurotrophic factor in long-term spatial memory retention in the water maze. Behav Neurosci. 2008;122:805–815. doi: 10.1037/0735-7044.122.4.805. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson HA, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Horm Behav. 2008;54:386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennenga SE, Bimonte-Nelson HA. The importance of incorporating both sexes and embracing hormonal diversity when conducting rodent behavioral assays. In: Bimonte-Nelson HA, editor. The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition. Humana Press; 2015. pp. 299–321. [Google Scholar]
- Mennenga SE, Bimonte-Nelson HA. Translational cognitive endocrinology: Designing rodent experiments with the goal to ultimately enhance cognitive health in women. Brain Res. 2013;1514:50–62. doi: 10.1016/j.brainres.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennenga SE, Gerson JE, Dunckley T, Bimonte-Nelson HA. Harmine treatment enhances short-term memory in old rats: Dissociation of cognition and the ability to perform the procedural requirements of maze testing. Physiol Behav. 2015a;138:260–265. doi: 10.1016/j.physbeh.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennenga SE, Gerson JE, Koebele SV, Kingston ML, Tsang CWS, Engler-Chiurazzi EB, Baxter LC, Bimonte-Nelson HA. Understanding the cognitive impact of the contraceptive estrogen ethinyl estradiol: Tonic and cyclic administration impairs memory, and performance correlates with basal forebrain cholinergic system integrity. Psychoneuroendocrinology. 2015b;54:1–13. doi: 10.1016/j.psyneuen.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennenga SE, Koebele SV, Mousa AA, Alderete TJ, Tsang CWS, Acosta JI, Camp BW, Demers LM, Bimonte-Nelson HA. Pharmacological blockade of the aromatase enzyme, but not the androgen receptor, reverses androstenedione-induced cognitive impairments in young surgically menopausal rats. Steroids. 2015c;99:16–25. doi: 10.1016/j.steroids.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008;290:44–50. doi: 10.1016/j.mce.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAMS. The North American Menopause Society (NAMS): A Clinician’s Guide. 5 2015. [Google Scholar]
- NAMS. The 2012 hormone therapy position statement of The North American Menopause Society (NAMS) Menopause J North Am Menopause Soc. 2012;19:257–271. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr PT, Rubin AJ, Fan L, Kent BA, Frick KM. The progesterone-induced enhancement of object recognition memory consolidation involves activation of the extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) pathways in the dorsal hippocampus. Horm Behav. 2012;61:487–495. doi: 10.1016/j.yhbeh.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pickar JH, Bon C, Amadio JM, Mirkin S, Bernick B. Pharmacokinetics of the first combination 17β-estradiol/progesterone capsule in clinical development for menopausal hormone therapy. Menopause. 2015;22:1308–131. doi: 10.1097/GME.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass MGS, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women. J Am Med Assoc. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151:1194–1203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Ramsden M, Pike CJ. Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res. 2006;1099:206–210. doi: 10.1016/j.brainres.2006.03.127. [DOI] [PubMed] [Google Scholar]
- Rudolph I, Zimmermann T, Kaminski K, Jandova K, Borovsky B, Ahrendt HJ, Golbs S. Changes in psychic and somatic well-being and cognitive capabilities of peri- and postmenopausal women after the use of a hormone replacement drug containing estradiol valerate and levonorgestrel. Methods Find Exp Clin Pharmacol. 2000;22:51–56. doi: 10.1358/mf.2000.22.1.795841. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD, Morley Kotchen J, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. J Am Med Assoc. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Simone J, Bogue EA, Bhatti DL, Day LE, Farr NA, Grossman AM, Holmes PV. Ethinyl estradiol and levonorgestrel alter cognition and anxiety in rats concurrent with a decrease in tyrosine hydroxylase expression in the locus coeruleus and brain-derived neurotrophic factor expression in the hippocampus. Psychoneuroendocrinology. 2015;62:265–278. doi: 10.1016/j.psyneuen.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Talboom JS, West SG, Engler-Chiurazzi EB, Enders CK, Crain I, Bimonte-Nelson HA. Learning to remember: Cognitive training-induced attenuation of age-related memory decline depends on sex and cognitive demand, and can transfer to untrained cognitive domains. Neurobiol Aging. 2014;35:2791–2802. doi: 10.1016/j.neurobiolaging.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, Frick KM. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm Behav. 2016;83:60–67. doi: 10.1016/j.yhbeh.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlind KC, Gibson KJ, Malone P, Evans GL, Turner RT. Differential effects of estrogen metabolites on bone and reproductive tissues of ovariectomized rats. J Bone Min Res. 1998;13:1023–1031. doi: 10.1359/jbmr.1998.13.6.1023. [DOI] [PubMed] [Google Scholar]
- Witty CF, Foster TC, Semple-Rowland SL, Daniel JM. Increasing hippocampal estrogen receptor alpha levels via viral vectors increases MAP kinase activation and enhances memory in aging rats in the absence of ovarian estrogens. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty CF, Gardella LP, Perez MC, Daniel JM. Short-term estradiol administration in aging ovariectomized rats provides lasting benefits for memory and the hippocampus: A role for insulin-like growth factor-I. Neuroendocrinology. 2013;154:842–852. doi: 10.1210/en.2012-1698. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of BDNF and CREB expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]