Abstract
30% of kidney transplant recipients are readmitted in the first month post-transplant. Those with donor-specific antibody requiring desensitization and incompatible live donor kidney transplantation (ILDKT) constitute a unique subpopulation that might be at higher readmission risk. Drawing on a 22-center cohort, 379 ILDKTs with Medicare primary insurance were matched to compatible transplant matched controls and to waitlist-only matched controls on panel reactive antibody, age, blood group, renal replacement time, prior kidney transplantation, race, gender, diabetes, and transplant date/waitlisting date. Readmission risk was determined using multilevel, mixed-effects Poisson regression. In the first month, ILDKTs had a 1.28-fold higher readmission risk than compatible controls (95%CI: 1.13–1.46; P<0.001). Risk peaked at 6–12 months (RR 1.67; 95%CI: 1.49–1.87; P<0.001), attenuating by 24–36 months (RR 1.24; 95%CI: 1.10–1.40; P<0.001). ILDKTs had a 5.86-fold higher readmission risk (95%CI: 4.96–6.92; P<0.001) in the first month compared to waitlist-only controls. At 12–24 (RR 0.85; 95%CI: 0.77–0.95; P=0.002) and 24–36 months (RR 0.74; 95% CI: 0.66–0.84; P<0.001), ILDKTs had a lower risk than waitlist-only controls. These findings of ILDKTs having a higher readmission risk than compatible controls, but a lower readmission risk after the first year than waitlist-only controls should be considered in regulatory/payment schemas and planning clinical care.
INTRODUCTION
In an effort to reduce health care expenditures, the Affordable Care Act mandates that the Centers for Medicare & Medicaid Services (CMS) reduce payments to hospitals with excess readmissions (1). Significant legislative and lobbying efforts have been made to exempt transplants from these penalties, as transplant recipients are a population at high risk of readmission (2–4). Indeed, approximately 30% of kidney transplant recipients will be readmitted in the first month following discharge after the transplant (5).
When considering readmission risk, one might worry in particular about recipients with donor-specific antibody (DSA) requiring desensitization and subsequent incompatible live donor kidney transplantation (ILDKT). Desensitization increases the magnitude of immunosuppression early after transplantation (plasmapheresis) and this effect may extend beyond the first month (anti-CD20), possibly increasing the incidence of post transplant infections requiring readmission, though the data are conflicting (6–8). DSA certainly places these patients at higher risk of rejection, and up to half of such patients will develop antibody-mediated rejection in the first year post-transplant, also likely requiring readmission (9). Also, sensitized patients have longer transplant waiting times and years of renal replacement, which would be predicted to result in a greater burden of co-morbidity and higher rates of readmission related to these co-morbid conditions. While understanding and quantifying readmission risk in this population would be critically important for both patient care planning as well as regulatory policy, no data currently exist.
We hypothesized that ILDKT recipients would be at higher risk for readmission than the general compatible live donor kidney transplant population and at lower risk than patients remaining on the kidney transplant waitlist. The former comparison is important within the current regulatory framework that judges transplant center outcomes without accounting for the distinctions between compatible and ILDKT recipients. The latter comparison is important from a hospital-level and larger healthcare policy point of view. We linked data from a 22-center study of ILDKT recipients ((10, 11), and from the national dialysis registry, to Medicare claims data to ascertain readmission (5, 12–14).
METHODS
Study Population
The study population was drawn from a 22-center cohort of patients known to be ILDKT recipients and has been previously described (11, 15). Briefly, participating transplant centers provided the unique transplant recipient identification number and antibody strength of patients undergoing desensitization and ILDKT from 1999–2011. Data provided by transplant centers were linked to the Scientific Registry of Transplant Recipients (SRTR) for ascertainment of patient demographic characteristics and reliable ascertainment of death for censoring. The SRTR includes information on all donors, waitlisted transplant candidates and transplant recipients in the United States provided by members of the Organ Procurement and Transplantation Network (OPTN), and has been well-described elsewhere (16). These ILDKT patients were then linked to Medicare claims data within the United States Renal Data System (USRDS) to ascertain hospital readmissions.
Compatible Kidney Transplant Matched Controls
Compatible kidney transplant matched controls were drawn from a pool of 17,163 adult, ABO-compatible, live donor, kidney-only transplant recipients with Medicare insurance at least 60 days prior to transplant and for the year following transplant in a ratio of 5 matched controls per ILDKT recipient. These patients were drawn from the same centers as ILDKT recipients except in cases when an insufficient number of matches were identified.
Transplant Waitlist-Only Matched Controls
The waitlist-only matched controls were drawn from a pool of active 90,831 unique kidney transplant candidate registrants with Medicare insurance at least 60 days prior to their waitlist date.
Matching Algorithm
Matching was performed using a previously described iterative expanding radius matching algorithm (9, 17–21) with the following restrictions: those ILDKT recipients with a peak panel reactive antibody (PRA) of 100, 98–99, and 95–97 could only be matched to controls with PRA of 100, 98–99, and 95–97, respectively. ILDKT recipients with PRA of 85–94, 65–84, and 1–64 were matched to controls with PRA ±2, ±5, and ±10, respectively. ILDKT recipients with PRA of 0 were matched only to controls with PRA of 0. Preemptive transplant recipients with no prior kidney transplant were matched to eligible controls with up to 3 months of renal replacement therapy. ILDKT recipients with >0 renal replacement time were matched within a 1 year radius, but were not matched to controls without any prior renal replacement time. ILDKT patients were matched to controls on age, blood group, number of previous kidney transplants, race, gender, diabetes status, percent of renal replacement time with a functioning kidney transplant ±10%, date of addition to the waitlist ±30 days of the transplant date of the ILDKT recipient, and transplant center.
If an insufficient number of controls could be identified for an IDKT recipient, the radius was expanded in the following order until 5 matches were found: expand allowable age difference 1 year at a time up to 5 years, ignore blood group differences, expand allowable difference in the number of previous kidney transplants 1 at a time until necessary to ignore differences, further expand allowable age difference 1 year at a time up to 10 years, expand allowable difference in percent renal replacement time with a functioning kidney transplant 5% at a time up to 60%, further expand allowable age difference 1 year at a time up to 15 years, expand allowable renal replacement time difference 1 year at a time up to 4 years, further expand allowable age difference 1 year at a time up to 35 years, further expand renal replacement time 1 year at a time up to 10 years, ignore race, gender, and then diabetes status differences, expand allowable difference between transplant date for ILDKT patients and date of waitlisting for controls 1 month at a time up to 60 months. Every time the secular difference radius was expanded, we reset all other radii to their initial (restrictive) settings and searched again for matches with the new secular difference radius.
Readmissions
Readmission was defined as admission to any acute care hospital, based on Medicare claims, after discharge from the index kidney transplant hospitalization. For transplant waitlist-only matched controls, “readmission” was considered as any hospitalization to an acute care hospital that occurred from the day of matching to an ILDKT recipient onward. In a separate analysis, readmission rates of ILDKT recipients that died were compared to their matched controls, as were readmission rates of ILDKT recipients who experienced graft loss.
Primary Diagnosis for Readmission
The primary diagnosis, as recorded using the International Classification of Disease--Ninth Revision (ICD-9) diagnosis code in the USRDS, for each readmission was tallied for each patient group and across pre-specified time periods.
Statistical Analysis
Between-group characteristics were compared using Pearson’s chi-square test for categorical variables and Somers’ D rank statistic test for continuous variables to account for clustering (22–24). The incidence of readmission at various time points was determined within the first three years following transplant (or matching in the case of waitlist-only matched controls). Multilevel Poisson regression analysis was performed to estimate the risk of readmission, with bootstrapping performed to estimate 95% CIs. Poisson regression was also performed to identify predictors of readmission for ILDKT recipients. Comparisons to each of the two matched control cohorts were performed separately. A two-tailed p-value of <0.05 was statistically significant.
RESULTS
Study Population
The 379 eligible ILDKT recipients from 18 centers were matched to 1,895 compatible kidney transplant recipients and 1,895 waitlist-only recipients. There was no significant difference in age between ILDKT recipients and compatible transplant matched controls (43.8 versus 44.9; P=0.14), though waitlist-only matched controls were slightly older than ILDKT recipients (46.0; P=0.003) (Table 1). Compared to ILDKT recipients, there was a higher proportion of black recipients in the compatible transplant matched controls, though there was no difference compared to waitlist-only matched controls (18.2%, 24.3%, and 18.6%; P=0.01, P=0.88). ILDKT recipients had an average of 9.0 years of renal replacement therapy, compared to 8.0 years for compatible transplant matched controls (P=0.033) and 9.1 years for waitlist-only matched controls (P=0.50). There was no difference in percentage of renal replacement time with a functioning allograft between ILDKT recipients and compatible transplant matched controls (22.5% versus 21.0%; P=0.22), though waitlist-only matched controls had a lower percentage (18.7%; P=0.03). There were no differences across the three groups in terms of female sex (64.1%, 59.2%, and 64.1%; P=0.08, p=0.99), median peak PRA (78, 80, and 80; P=0.98, p=0.99), diabetes status (22.2%, 23.1%, and 22.2%; P=0.71, P=0.99), and number of prior transplants (50.6%, 54.7%, and 55.5% with no prior transplant; P=0.3, p=0.14). The mean duration of the index hospitalization for ILDKT recipients was 13.8 days (SD 16.2) versus 7.7 (SD 8.9) for compatible transplant recipients (P<0.001).
Table 1.
Baseline characteristics of incompatible live donor kidney transplant recipients (ILDKT), compatible transplant matched controls, and waitlist-only matched controls.
| Matched Controls | P-Value | |||||
|---|---|---|---|---|---|---|
| ILDKT (n=379) | Compatible Transplant Matched Controls (n=1895) |
Waitlist-Only Matched Controls (n=1895) |
ILDKT vs Compatible Transplant Matched Controls |
ILDKT vs Waitlist-Only Matched Controls |
||
| Mean Age at Transplant (SD) | 43.8 (13.1) | 44.9 (13.1) | 46.0 (12.3) | 0.14 | 0.003 | |
| Female Sex | 64.1% | 59.2% | 64.1% | 0.08 | 0.99 | |
| Black Race | 18.2% | 24.3% | 18.6% | 0.01 | 0.88 | |
| Number of Previous Transplants | 0.30 | 0.14 | ||||
| 0 | 50.6% | 54.7% | 55.5% | |||
| 1 | 40.9% | 38.6% | 38.8% | |||
| 2 | 7.4% | 6.2% | 5.1% | |||
| ≥3 | 1.1% | 0.5% | 0.6% | |||
| Median PPRA (IQR) | 78 (32–98) | 80 (32–98) | 80 (33–98) | 0.98 | 0.99 | |
| Diabetes Mellitus | 22.2% | 23.1% | 22.2% | 0.71 | 0.99 | |
| Mean Years of Renal Replacement Time (SD) | 9.0 (7.3) | 8.0 (6.6) | 9.1 (7.0) | 0.033 | 0.50 | |
| Mean % Renal Replacement Time with a functioning graft (SD) | 22.5 (30.9) | 21.0 (30.0) | 18.7 (26.5) | 0.22 | 0.038 | |
Readmission Incidence and Cumulative Incidence
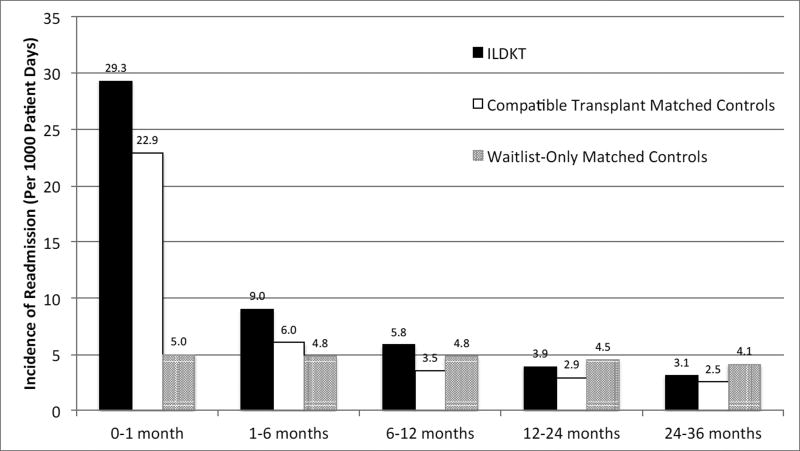
Within the first month of discharge from the hospital after kidney transplant, ILDKT recipients had an incidence of hospital readmission of 29.3 per 1,000 patient days, compared to 22.9 and 5.0 for compatible transplant matched controls and transplant waitlist-only matched controls (Figure 1). From 1–6 months, the incidence of hospital readmission for ILDKT, compatible transplant matched controls, and waitlist-only matched controls was 9.0, 6.0, and 4.8 readmissions per 1,000 patient days. From 6–12 months, the incidence was 5.8, 3.5, and 4.8 readmissions per 1,000 patient days. From 12–24 months, the incidence was 3.9, 2.9, and 4.5 readmissions per 1,000 patient days. From 24–36 months, the incidence of hospital readmission was 3.1, 2.5, and 4.1 readmissions per 1,000 patient days. The cumulative incidence of hospital readmission at 1 month, 6 months, 12 months, 24 months, and 36 months was 56.0%, 87.5%, 96.0%, 99.0%, and 99.7% for ILDKT patients, 47.4%, 77.2%, 88.5%, 96.0%, and 98.4% for compatible transplant matched controls, and 13.1%, 55.5%, 82.6%, 96.7%, and 99.3% for waitlist-only matched controls (Table 2). In the first month, 1–6 months, 6–12 months, 12–24 months, and 24–36 months, ILDKT recipients spent 17.9%, 7.3%, 4.5%, 2.9%, and 1.9% of the days at risk readmitted to the hospital, compared to 12.6%, 4.2%, 2.4%, 1.9%, and 1.7% for compatible transplant matched controls, and 2.8%, 3.5%, 3.5%, 3.5%, and 3.2% for waitlist-only matched controls (Table 3).
Figure 1.
Incidence of readmission per 1000 patient days of incompatible live donor kidney transplant (ILDKT) recipients, compatible transplant matched controls, and waitlist-only matched controls.
Table 2.
Cumulative incidence of readmission for incompatible live donor kidney transplant recipients (ILDKT), compatible transplant matched controls, and waitlist-only matched controls at 1, 6, 12, 24, and 36 months.
| Time Post-Kidney Transplant Discharge/Matching |
Cumulative Incidence of Readmission for ILDKT Recipients |
Cumulative Incidence of Readmission for Compatible Transplant Matched Controls |
Cumulative Incidence of Readmission for Waitlist- Only Matched Controls |
|---|---|---|---|
| 1 month | 56.0% | 47.4% | 13.1% |
| 6 months | 87.5% | 77.2% | 55.5% |
| 12 months | 96.0% | 88.5% | 82.6% |
| 24 months | 99.0% | 96.0% | 96.7% |
| 36 months | 99.7% | 98.4% | 99.3% |
Table 3.
Readmission burden for incompatible live donor kidney transplant (ILDKT) recipients, compatible transplant matched controls, and waitlist-only matched controls.
| Time Post-Kidney Transplant Discharge/ Matching |
Number of Readmissions |
Readmission Days |
Non-Readmission Days |
Percent of Days Readmitted |
Mean Readmission Duration (SD) |
P-Value* |
|---|---|---|---|---|---|---|
| ILDKT (n=379) | ||||||
| 1 month | 294 | 1,951 | 8,930 | 17.9 | 5.1 (6.9) | -- |
| 1–6 months | 452 | 3,781 | 48,358 | 7.3 | 10.0 (18.2) | -- |
| 6–12 months | 393 | 3,107 | 65,885 | 4.5 | 8.5 (18.7) | -- |
| 12–24 months | 451 | 3,368 | 114,024 | 2.9 | 10.0 (21.3) | -- |
| 24–36 months | 324 | 2,010 | 103,132 | 1.9 | 6.5 (13.6) | -- |
| Compatible Transplant Matched Controls (n=1895) | ||||||
| 1 month | 1,172 | 6,811 | 47,176 | 12.6 | 3.6 (5.6) | <0.001 |
| 1–6 months | 1,524 | 10,923 | 249,173 | 4.2 | 5.8 (11.8) | <0.001 |
| 6–12 months | 1,218 | 8,487 | 341,865 | 2.4 | 4.7 (12.1) | <0.001 |
| 12–24 months | 1,745 | 11,881 | 598,120 | 1.9 | 6.8 (15.6) | 0.01 |
| 24–36 months | 1,369 | 8,965 | 533,573 | 1.7 | 5.6 (13.1) | 0.01 |
| Waitlist-Only Matched Controls (n=1895) | ||||||
| 1 month | 263 | 1,501 | 51,447 | 2.8 | 0.8 (2.9) | <0.001 |
| 1–6 months | 1,212 | 8,910 | 248,890 | 3.5 | 4.8 (11.3) | <0.001 |
| 6–12 months | 1,603 | 12,072 | 328,210 | 3.5 | 6.7 (15.0) | 0.053 |
| 12–24 months | 2,493 | 19,306 | 538,417 | 3.5 | 11.6 (22.7) | 0.3 |
| 24–36 months | 1,876 | 14,475 | 443,927 | 3.2 | 10.4 (20.1) | 0.003 |
P-value refers to the comparison with ILDKT for the corresponding time period.
SD – standard deviation
Relative Risk of Readmission versus Compatible Transplants Matched Controls
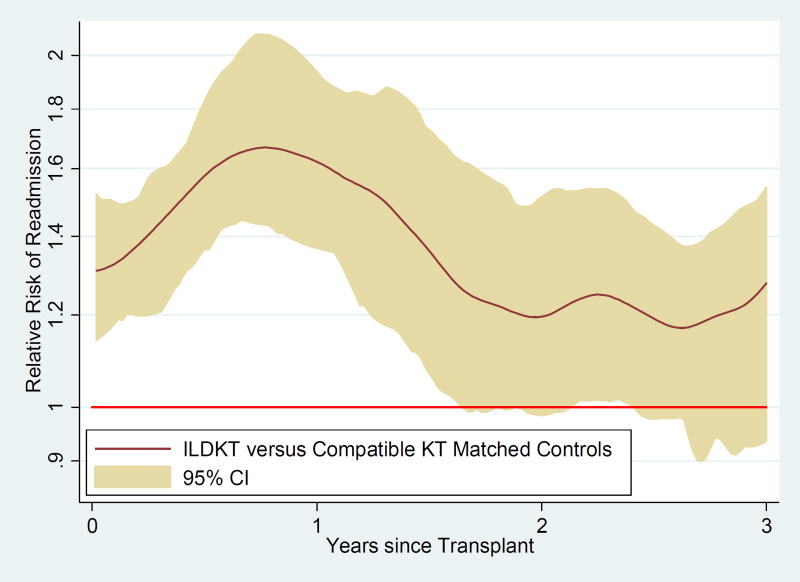
For the entire duration of follow-up, the relative risk of readmission for ILDKT recipients was higher than for compatible transplant matched controls: 0–1 month (RR 1.28; 95% CI: 1.13–1.46; P<0.001), 1–6 months (RR 1.51; 95% CI: 1.36–1.68; P<0.001), 6–12 months (RR 1.67; 95% CI: 1.49–1.87; P<0.001), 12–24 months (RR 1.37; 95% CI: 1.23–1.52; P<0.001), and 24–36 months (RR 1.24; 95% CI: 1.10–1.40; P<0.001) (Table 4.; Figure 2). A sensitivity analysis comparing the relative risk of readmission of ILDKT recipients and compatible transplant matched controls after controlling for the statistically significantly different variables in Table 1 showed point estimates, confidence intervals, and P-values that were virtually identical (data not shown).
Table 4.
Relative risk of readmission for incompatible live donor kidney transplant (ILDKT) recipients versus compatible transplant matched controls and for ILDKT versus waitlist-only matched controls.
| Time Post-Kidney Transplant Discharge/Matching |
Relative Risk of Readmission—ILDKT vs Compatible Transplant Matched Controls |
P-value | Relative Risk of Readmission— ILDKT vs Waitlist-Only Matched Controls |
P-value |
|---|---|---|---|---|
| 1 month | 1.28 (1.13–1.46) | <0.001 | 5.86 (4.96–6.92) | <0.001 |
| 1–6 months | 1.51 (1.36–1.68) | <0.001 | 1.89 (1.69–2.10) | <0.001 |
| 6–12 months | 1.67 (1.49–1.87) | <0.001 | 1.22 (1.09–1.36) | <0.001 |
| 12–24 months | 1.37 (1.23–1.52) | <0.001 | 0.85 (0.77–0.95) | 0.002 |
| 24–36 months | 1.24 (1.10–1.40) | <0.001 | 0.74 (0.66–0.84) | <0.001 |
Figure 2.
Relative risk of readmission for incompatible live donor kidney transplant (ILDKT) recipients versus compatible kidney transplant (KT) matched controls.
Relative Risk of Readmission versus Waitlist-Only Matched Controls
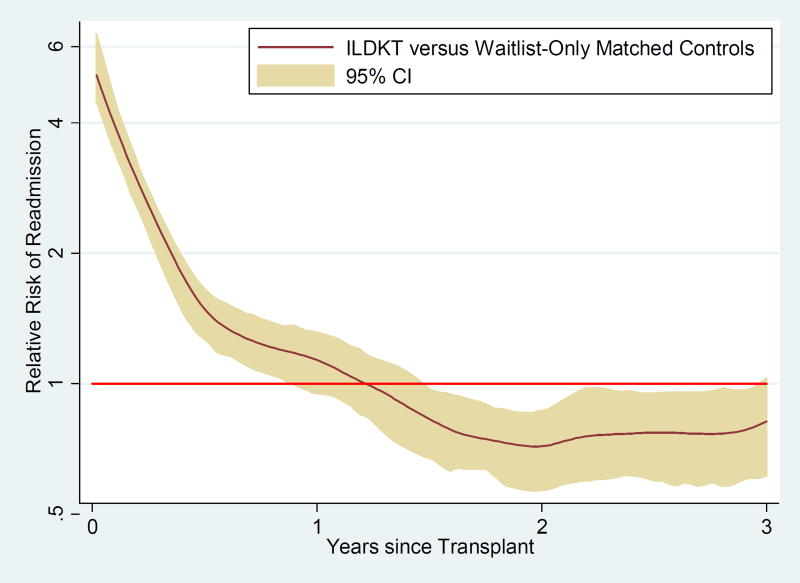
From 0–1 month, ILDKT recipients had a 5.86-fold (95% CI: 4.96–6.92; P<0.001) higher risk of readmission than waitlist-only matched controls. From 1–6 months, ILDKT recipients had a 1.89-fold (95% CI: 1.69–2.10; P<0.001) higher risk, and from 6–12 months, the risk was 1.22-fold higher (95% CI: 1.09–1.36; P<0.001). However, after the first year, the relative risk of readmission was lower for ILDKT recipients than for waitlist-only matched controls. From 12–24 months, the relative risk of readmission was 0.85-fold (95% CI: 0.77–0.95; P=0.002) lower for ILDKT recipients compared to waitlist-only matched controls. From 24–36 months, the relative risk of readmission was 0.74-fold (95% CI: 0.66–0.84; P<0.001) lower for ILDKT recipients compared to waitlist-only matched controls. A sensitivity analysis comparing the relative risk of readmission of ILDKT recipients and waitlist-only matched controls after controlling for the statistically significantly different variables in Table 1 showed point estimates, confidence intervals, and P-values that were virtually identical (data not shown).
Primary Diagnosis for Readmissions
At all time points and across all three groups, the most common primary diagnosis for readmission was “Complications peculiar to specific conditions” (Supplementary Figure 1). Following that, in the first month, 9.2%, 5.0%, and 3.7% of ILDKT readmissions were for procedural complications, fluid electrolyte/acid-base disorders, and urethra/urinary tract disorders, compared to 8.7%, 8.1%, and 3.9% of compatible transplant matched control readmissions for procedural complications, fluid electrolyte/acid-base disorders, and other abdomen/pelvis symptoms, and 8.8%, 4.0%, and 3.7% of waitlist-only matched control readmissions for hypertensive chronic kidney disease, septicemia, and heart failure. From months 1–6, 4.3%, 3.4%, and 3.4% of ILDKT readmissions were for diabetes mellitus, fluid electrolyte/acid-base disorders, and procedural complications, compared to 6.0%, 4.8%, and 4.8% of compatible transplant matched control readmissions for urethra/urinary tract disorders, fluid electrolyte/acid-base disorders, and procedural complications, and 5.5%, 4.9%, and 4.1% of waitlist-only matched control readmissions for fluid electrolyte/acid-base disorders, hypertensive chronic kidney disease, and heart failure. From months 6–12, 5.6%, 4.9%, and 3.8% of ILDKT readmissions were for urethra/urinary tract disorders, fluid electrolyte/acid-base disorders, and diabetes mellitus, compared to 5.1%, 4.8%, and 4.1% of compatible transplant matched control readmissions for septicemia, urethra/urinary tract disorders, and diabetes mellitus, and 6.5%, 6.0%, and 4.1% of waitlist-only matched control readmissions for hypertensive chronic kidney disease, fluid electrolyte/acid-base disorders, and heart failure. From months 12–24, 4.2%, 4.0%, and 3.6% of ILDKT readmissions were for urethra/urinary tract disorders, diabetes mellitus, and pneumonia, compared to 4.0%, 3.9%, and 3.5% of compatible transplant matched control readmissions for urethra/urinary tract disorders, pneumonia, and fluid electrolyte/acid-base disorders, and 5.2%, 4.7%, and 3.9% of waitlist-only matched control readmissions for hypertensive chronic kidney disease, fluid electrolyte/acid-base disorders, and septicemia. From months 24–36, 4.3%, 4.0%, and 4.0% of ILDKT readmissions were for septicemia, pneumonia, and fluid electrolyte/acid-base disorders, compared to 3.9%, 3.7%, and 3.6% of compatible transplant matched control readmissions for diseases of the pancreas, pneumonia, and septicemia, and 5.3%, 4.4%, and 4.2% of waitlist-only matched control readmissions for septicemia, heart failure, and hypertensive chronic kidney disease.
Readmission Rates of ILDKT Recipients that Died Compared to Matched Controls
During the study period, 122 ILDKT recipients died. In the first month, 1–6 months, 6–12 months, 12–24 months, and 24–36 months, the readmission rate per 1 person-year amongst the ILDKT recipients who died was 13.2, 5.1, 3.9, 2.5, and 1.6, compared to 8.3 (P<0.001), 2.2 (P<0.001), 1.4 (P<0.001), 0.9 (P<0.001), and 1.1 (P<0.001) for their compatible transplant matched controls, and 2.2 (P<0.001), 2.1 (P<0.001), 2.1 (P<0.001), 1.9 (P<0.001), and 1.8 (P=0.2) for their waitlist-only matched controls.
Readmission Rates of ILDKT Recipients with Graft Loss Compared to Matched Controls
During the study period, 134 ILDKT recipients experienced death-censored graft loss. In the first month, 1–6 months, 6–12 months, 12–24 months, and 24–36 months, the readmission rate per 1 person-year amongst the ILDKT recipients who experienced graft loss was 11.6, 3.7, 2.7, 1.8, and 1.9, compared to 8.3 (P=0.002), 2.2 (P<0.001), 1.3 (P<0.001), 1.1 (P<0.001), and 0.9 (P<0.001) for their compatible transplant matched controls, and 2.0 (P<0.001), 1.9 (P<0.001), 1.9 (P<0.001), 1.8 (P=0.7), and 1.7 (P=0.1) for their waitlist-only matched controls.
Predictors of Readmission Among ILDKT Recipients
Black race (incidence rate ratio [IRR] 1.17; 95% CI: 1.06–1.30); P<0.001), PRA (per 10 point increment; IRR 1.04; 95% CI: 1.03–1.05; P<0.001, and length of index hospitalization (per 5 day increment; IRR 1.08; 95% CI: 1.07–1.09; P<0.001) were all associated with an increased risk of readmission following ILDKT. Female sex trended toward significantly predicting readmission (IRR 1.09; 95% CI: 0.997–1.18; P=0.058). Age (per 10 year increment; IRR 0.99; 95% CI: 0.96–1.02; P=0.5), history of previous transplantation (IRR 1.00; 95% CI: 0.88–1.14; P=0.97), renal replacement time (per 5 year increment; IRR 1.01; 95% CI: 0.98–1.04; P=0.4), and percentage of renal replacement time with a functioning kidney transplant (per 10%; IRR 0.99; 95% CI: 0.97–1.01; P=0.2) did not predict hospital readmission. Amongst the 90.2% of the study population with Medicare primary insurance coverage for the 180 days leading up to transplant (or matching date for the dialysis group), every 1 hospital admission before transplant was associated with a 9% higher likelihood of post-transplant (or post-matching in the case of the dialysis group) hospital readmission (IRR 1.09; 95% CI: 1.06–1.12; P<0.001).
DISCUSSION
In this 22-center study, ILDKT was associated with a higher risk of hospital readmission than compatible transplant matched controls, a finding that held true even 3 years post-transplant. In the first month after discharge, ILDKT recipients had a 1.28-fold higher risk of readmission than compatible kidney transplant recipients; the risk peaked at 1.67-fold higher during the 6–12 month period post-index hospitalization discharge and then decreased to 1.24-fold higher by 24–36 months. It is important to point out that the choice for many of the ILDKT patients is not between and incompatible and compatible transplant, rather, it is between an incompatible transplant and remaining on the waitlist. Compared to waitlist-only matched controls, ILDKT recipients initially had a 5.86-fold higher risk of readmission in the first month. However, by 12–24 months, ILDKT had a significantly lower risk of readmission than waitlist-only matched controls (RR 0.85; 0.74 from 24–36 months).
Hospital readmissions, particularly 30-day readmissions, are increasingly scrutinized as a proxy for quality of care (25), with such metrics frequently reported publicly and used to determine reimbursements. ILDKT recipients in our study had a consistently higher risk of readmission than compatible transplant matched controls; this reality of desensitization may serve as a disincentive for transplant centers to undertake ILDKT, even in light of the substantial survival benefit seen with this treatment modality (11, 17). This only worsens the existing disincentive to care for these patients given a higher likelihood of flagging by CMS for further regulatory scrutiny (15).
Understanding readmission patterns is particularly important given the increased cost associated with ILDKT. We recently reported that ILDKT is associated with a 41% increase in cost compared to compatible live donor kidney transplant ($151,024 versus $106,636; P<0.001), highlighting the importance of paired kidney exchange. However, even under optimal circumstances, less than half of patients will find a compatible donor (26), and many of the ensuing matches are not compatible, but rather, less incompatible. Vo and colleagues reported that compared to dialysis, desensitization with rituximab and IVIg was associated with nearly $19,000 in savings by three years post-transplant. More recent kidney allocation system changes have given priority to sensitized patients on a sliding scale, leading to a bolus effect in which patients with PRA 98–100 have become more likely to get transplanted at the expense of sensitized patients with lower PRA values (27). While the long-term effects of these policy changes remain to be seen, these allocation system changes and kidney paired exchange may obviate the need for desensitization for some patients and reduce time on and cost of dialysis, but certainly not all patients. Desensitization, including in combination with paired kidney exchange, is likely to remain a major treatment modality for difficult-to-match patients.
Limitations include the restriction to Medicare beneficiaries, which was necessary for outcome ascertainment but reduced sample size and may somewhat limit generalizability. However, this is, to our knowledge, the largest study of readmissions in this challenging patient population, and our findings were statistically significant, indicating we had sufficient sample size to ask the questions we were asking. Additionally, there may be some residual confounding, particularly as some significant differences remained between the groups even after matching. However, most of those differences, while statistically significant, were not clinically meaningful. While we were able to identify the primary diagnosis codes for the readmissions, the data are limited in their granularity. Further study will be needed to understand better why these patients require readmission to the hospital.
In conclusion, ILDKT is perpetually associated with an increased risk of hospital readmission compared to compatible live donor kidney transplants. Compared to waitlist-only matched controls, however, the risk of readmission is only elevated in the first year. Beyond the first year post-transplant, ILDKT is associated with a lower risk of hospital readmission. Understanding the short-term pattern of readmissions in ILDKT patients may help inform efforts to prevent readmission and minimize poor outcomes in this patient population. The long-term pattern of readmissions following ILDKT, especially in relation to compatible kidney transplant controls and to waitlist-only controls, should inform payment and regulatory policies.
Supplementary Material
Figure S1: Primary readmission diagnosis for incompatible live donor kidney transplant (ILDKT) recipients, compatible transplant matched controls, and waitlist-only matched controls from A) 0–1 months, B) 1–6 months, C) 6–12 months, D) 12–24 months, and E) 24–36 months post-transplant/matching. NEC – Not elsewhere classified.
Figure 3.
Relative risk of readmission for incompatible live donor kidney transplant (ILDKT) recipients versus waitlist-only matched controls.
Acknowledgments
Some of the data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Some of the data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of interpretation of the U.S. Government.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01DK098431 and K24DK10182801 to DLS, F32DK093218 to BJO, and 3R01DK098431-02S1 to RAM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CMS
Centers for Medicare & Medicaid Studies
- DSA
Donor specific antibody
- ILDKT
Incompatible live donor kidney transplant
- OPTN
Organ Procurement and Transplantation Network
- PRA
Panel reactive antibody
- SRTR
Scientific Registry of Transplant Recipients
- USRDS
United States Renal Data System
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Patient Protection and Affordable Care Act, 42 U.S.C. § 18001. 2010 [Google Scholar]
- 2. [2016 November 1];American Society of Transplant Surgeons Legislative and Regulatory Update. 2014 Available from: https://asts.org/docs/default-source/legislative-and-regulatory-update/asts-legislative-and-regulatory-update-april-2014.pdf?sfvrsn=4.
- 3. [2016 November 1];Association of American Medical Colleges Washington Highlights. 2014 Available from: https://www.aamc.org/advocacy/washhigh/highlights2014/373138/031414rep.renacciintroduceslegislationtoamendthehospitalreadmiss.html.
- 4. [2016 November 1];American Society of Transplantation - AST endorses Capitol Hill legislation establishing beneficiary equity in the hospital readmission program. 2014 Available from: https://www.myast.org/news/ast-endorses-capitol-hill-legislation-establishing-beneficiary-equity-hospital-readmission.
- 5.McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL. Early hospital readmission after kidney transplantation: patient and center-level associations. Am J Transplant. 2012;12(12):3283–3288. doi: 10.1111/j.1600-6143.2012.04285.x. [DOI] [PubMed] [Google Scholar]
- 6.Habicht A, Broker V, Blume C, Lorenzen J, Schiffer M, Richter N, et al. Increase of infectious complications in ABO-incompatible kidney transplant recipients--a single centre experience. Nephrol Dial Transplant. 2011;26(12):4124–4131. doi: 10.1093/ndt/gfr215. [DOI] [PubMed] [Google Scholar]
- 7.Kahwaji J, Sinha A, Toyoda M, Ge S, Reinsmoen N, Cao K, et al. Infectious complications in kidney-transplant recipients desensitized with rituximab and intravenous immunoglobulin. Clin J Am Soc Nephrol. 2011;6(12):2894–2900. doi: 10.2215/CJN.03710411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamar N, Milioto O, Puissant-Lubrano B, Esposito L, Pierre MC, Mohamed AO, et al. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant. 2010;10(1):89–98. doi: 10.1111/j.1600-6143.2009.02785.x. [DOI] [PubMed] [Google Scholar]
- 9.Orandi BJ, Chow EH, Hsu A, Gupta N, Van Arendonk KJ, Garonzik-Wang JM, et al. Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant. 2015;15(2):489–498. doi: 10.1111/ajt.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ, et al. Quantifying the Risk of Incompatible Kidney Transplantation: A Multicenter Study. Am J Transplant. 2014 doi: 10.1111/ajt.12786. [DOI] [PubMed] [Google Scholar]
- 11.Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R, et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. N Engl J Med. 2016;374(10):940–950. doi: 10.1056/NEJMoa1508380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King EA, Bowring MG, Massie AB, Kucirka LM, McAdams-DeMarco MA, Al-Ammary F, et al. Mortality and Graft Loss Attributable to Readmission following Kidney Transplantation: Immediate and Long-Term Risk. Transplantation. 2016 doi: 10.1097/TP.0000000000001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King EA, Kucirka LM, McAdams-DeMarco MA, Massie AB, Al Ammary F, Ahmed R, et al. Early Hospital Readmission After Simultaneous Pancreas-Kidney Transplantation: Patient and Center-Level Factors. Am J Transplant. 2016;16(2):541–549. doi: 10.1111/ajt.13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAdams-Demarco MA, Grams ME, King E, Desai NM, Segev DL. Sequelae of early hospital readmission after kidney transplantation. Am J Transplant. 2014;14(2):397–403. doi: 10.1111/ajt.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ, et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant. 2014;14(7):1573–1580. doi: 10.1111/ajt.12786. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson DM, Dykstra DM, Levine GN, Li S, Welch JC, Webb RL. Transplant data: sources, collection and research considerations, 2004. Am J Transplant. 2005;5(4 Pt 2):850–861. doi: 10.1111/j.1600-6135.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365(4):318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 18.Orandi BJ, Alachkar N, Kraus ES, Naqvi F, Lonze BE, Lees L, et al. Presentation and Outcomes of C4d-Negative Antibody-Mediated Rejection After Kidney Transplantation. Am J Transplant. 2015 doi: 10.1111/ajt.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579–586. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, Taranto SE, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303(10):959–966. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 21.Orandi BJLX, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R, Van Arendonk KJ, Stegall MD, Jordan SC, Oberholzer J, Dunn TB, Ratner LE, Kapur S, Pelletier RP, Roberts JP, Melcher ML, Singh P, Sudan DL, Posner MP, El-Amm JM, Shapiro R, Cooper M, Lipkowitz GS, Rees MA, Marsh CL, Sankari BR, Gerber DA, Nelson P, Wellen J, Bozorgzadeh A, Gaber AO, Montgomery RA, Segev DL. Survival Benefit in HLA-Incompatible Live Donor Kidney Transplantation. New Engl J Med. 2016 doi: 10.1056/NEJMoa1508380. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao JNK, Scott AJ. On Chi-Squared Tests for Multiway Contingency-Tables with Cell Proportions Estimated from Survey Data. Annals of Statistics. 1984;12(1):46–60. [Google Scholar]
- 23.Wilcox RR. A note on the Theil-Sen regression estimator when the regressor is random and the error term is heteroscedastic. Biometrical Journal. 1998;40(3):261–268. [Google Scholar]
- 24.Newson R. Parameters behind"nonparametric” statistics: Kendall’s tau, Somers’ D and median differences. Stata Journal. 2002;2(1):45–64. [Google Scholar]
- 25.Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505. doi: 10.1001/jama.2011.72. [DOI] [PubMed] [Google Scholar]
- 26.Segev DL, Gentry SE, Warren DS, Reeb B, Montgomery RA. Kidney paired donation and optimizing the use of live donor organs. JAMA. 2005;293(15):1883–1890. doi: 10.1001/jama.293.15.1883. [DOI] [PubMed] [Google Scholar]
- 27.Massie AB, Luo X, Lonze BE, Desai NM, Bingaman AW, Cooper M, et al. Early Changes in Kidney Distribution under the New Allocation System. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Primary readmission diagnosis for incompatible live donor kidney transplant (ILDKT) recipients, compatible transplant matched controls, and waitlist-only matched controls from A) 0–1 months, B) 1–6 months, C) 6–12 months, D) 12–24 months, and E) 24–36 months post-transplant/matching. NEC – Not elsewhere classified.