Abstract
Background
Targeted temperature management (TTM) with therapeutic hypothermia is an integral component of post-arrest care for survivors. However, recent randomized controlled trials (RCTs) have failed to demonstrate the benefit of TTM on clinical outcomes. We sought to determine if the pooled data from available RCTs supports the use of pre-hospital and/or in-hospital TTM after cardiac arrest.
Methods
A comprehensive search of SCOPUS from 1966 till November 2016 was performed using predefined criteria. Therapeutic hypothermia was defined as any strategy that aimed to cool post cardiac arrest survivors to a temperature ≤ 34 Celsius degrees. Normothermia was temperature of ≥ 36 Celsius degrees. We compared mortality and neurological outcomes in patients by categorizing the studies into two groups: 1) hypothermia versus normothermia and 2) pre-hospital hypothermia versus in-hospital hypothermia using standard meta-analytic methods. A random effects modeling was utilized to estimate comparative risk ratios (RR) and 95% confidence intervals (CIs).
Results
The hypothermia and normothermia strategies were compared in five RCTs with 1389 patients whereas pre-hospital hypothermia and in-hospital hypothermia were compared in 6 RCTs with 3393 patients. We observed no difference in mortality (RR; 0.88, 95% CI: 0.73–1.05) or neurological outcomes (RR; 1.26, 95% CI: 0.92–1.72) between the hypothermia and normothermia strategies. Similarly, no difference was observed in mortality (RR; 1.00, 95% CI: 0.97–1.03) or neurological outcome (RR; 0.96, 95% CI: 0.85–1.08) between the pre-hospital hypothermia vs. in-hospital hypothermia strategies.
Conclusions
Our results suggest that TTM with therapeutic hypothermia may not improve mortality or neurological outcomes in post-arrest survivors. Employing therapeutic hypothermia as a standard of care strategy of post-arrest care in survivors may need to be reevaluated.
Keywords: Cardiac arrest, meta-analysis, therapeutic hypothermia
Introduction
In recent years, therapeutic hypothermia and targeted temperature management (TTM) have been increasingly used in the post-resuscitation care of patients that have suffered cardiac arrest. Many mechanisms have been thought to confer benefit in therapeutic hypothermia after cardiac arrest. These mechanisms are thought to affect all three levels of injury after cardiac arrest: ischemic injury, immediate reperfusion injury, and delayed reperfusion injury.1 Almost a decade ago, two large randomized controlled trials (RCTs) in humans demonstrated improvement in mortality and neurologic outcomes with therapeutic hypothermia use after cardiac arrest.2,3
On the basis of such evidence, the 2010 American Heart Association guidelines recommended the usage of therapeutic hypothermia as a grade IB recommendation.4 Conflicting data then began to surface regarding the use of therapeutic hypothermia. Multiple investigations were published showing little impact between targeted temperature management (TTM) with the normothermia, pre-hospital hypothermia, and in-hospital hypothermia strategies on neurologic and mortality outcomes.5,6 This led to an alteration in the recommendation for the use of therapeutic hypothermia, with the 2015 American Heart Association guidelines7 for post-cardiac arrest care instead suggesting that TTM be used rather than strictly outlining a therapeutic hypothermia strategy. The grading of the recommendation was also altered to a class IB level B-R recommendation for out-of-hospital pulseless ventricular tachycardia and ventricular fibrillation cardiac arrest. Furthermore, it was changed to a class IB level C-EO recommendation (consensus of expert opinion based on clinical experience) for out-of-hospital pulseless electrical activity and asystole cardiac arrests and in-hospital cardiac arrests.7 This was also followed by the recommendation against pre-hospital intravenous cooled fluid infusion.
Due to the conflicting data, we sought to evaluate the effect that TTM had through normothermia, pre-hospital hypothermia, and in-hospital hypothermia on in-hospital mortality and neurologic outcomes after cardiac arrest through systematic review and meta-analytic comparisons of the RCTs comparing the three TTM strategies.
Methods
We searched SCOPUS from 1966 until November 2016 for English language RCTs detailing the use of TTM after cardiac arrest in adult patients. The SCOPUS databases indexes the full Medline database as well as Biobase, Embase, Fluidex, Geobase, and the World Textile Index. Three authors (P.A., N.S.B., and R.K.) used a pre-specified list of terms to locate studies. The full search strategy is detailed in Supplemental Section 1. We also reviewed reference lists from original manuscripts and published systematic reviews and meta-analyses to identify trials that were not listed in the original database search. After review of abstracts, full-text manuscripts were retrieved for review.
All English language RCTs evaluating the use of TTM in adults were eligible for inclusion. All combinations of cardiac rhythms (asystole, pulseless electrical activity, ventricular fibrillation, and pulseless ventricular tachycardia), TTM strategies (pre-hospital hypothermia, in-hospital hypothermia, and normothermia), and target temperatures were included. Hypothermia was defined as being 34 degrees Celsius or less. Normothermia was defined as being 36 degrees Celsius or more. Foreign language studies were excluded unless a full-text English translation of the study was available.
We intended to compare mortality and neurological outcomes in patients by categorizing the studies into two groups: 1) hypothermia versus normothermia and 2) pre-hospital hypothermia versus in-hospital hypothermia. The pre-hospital hypothermia versus in-hospital comparison was done to evaluate whether the timing of hypothermia affected outcomes and to alleviate any concerns about the delay in institution of hypothermia as a cause of null results in the primary comparison (therapeutic hypothermia versus normothermia). These comparisons were decided upon in an a priori fashion as the RCTs comparing the two approaches have conflicting results. The primary outcome measure was in-hospital all-cause mortality following cardiac arrest. The secondary outcome measure was the cerebral performance category (CPC) after TTM.8 The CPC scale is a five point scale graded from 1–5, where the outcome of neurologic disability after significant damage is graded into one of the following five categories: good recovery (CPC 1), moderate disability (CPC 2), severe disability (CPC 3), persistent vegetative state (CPC 4), or death (CPC 5).8 Within this scale, good neurologic outcome was defined as CPC categories 1–2 and poor neurologic outcomes were defined as CPC categories 3–5 for meta-analyses. Discharge to rehabilitation facilities was classed as CPC 2 and discharge to a nursing home was classified as CPC 3. A CPC score of two or less was considered a favorable neurological outcome.2 Where there were multiple studies reporting outcomes for the same cohort, we chose the study with the longest and most complete follow-up.
Four authors (P.A., N.S.B., R.K., G.A.) searched the titles and abstracts of all studies. Multiple authors (G.A., N.S.B., and R.K.) then carried out data extraction. Data were extracted to elucidate baseline characteristics of patients and outcome measures in all of the included studies. All inconsistencies during data extraction were resolved by mutual consensus so that a unanimous decision was made.
Quality assessment of the included studies was carried out according to the Jadad scale for RCTs.9 The Jadad scale evaluates the quality of RCTs through assessment of study randomization, blinding, and description of withdrawals and dropouts. Two authors (N.S.B. and R.K.) independently carried out quality assessment of all of the included RCTs using the Jadad scale.9
The systematic review and meta-analyses were reported based on the recommendations outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.10
Statistical Analysis
Statistical analyses were carried out by Comprehensive Meta-Analysis version 2.2.046 (Biostat, Englewood, NJ, U.S.A.) and STATA version 14.1 (StataCorp LP, College Station, TX, U.S.A.). To estimate summary effects, a random effects model using the DerSimonian & Laird method was used in our investigation. The Mantel-Haenszel model was then used to estimate the heterogeneity for the meta-analyses.11,12 Heterogeneity was estimated using the I2 statistic proposed by Higgins and Thompson.12 Summary treatment effects and results were presented as risk ratios (RR) with 95% confidence intervals (CI). Funnel plots were generated to outline the publication bias. We used log RR as the X-axis variable to exhibit estimated treatment effect for the included studies and plotted standard error as the Y-axis variable to provide a measure of sample size.13 Publication bias was assessed using Egger’s regression intercept.14 Egger’s regression test is a simple linear regression to detect asymmetry of the funnel plot on the logarithm scale of the risk ratio.14 The one-sided Egger’s test was used as the two-sided test may produce a false publication bias or inconsistency in the tail.15,16 Based on the estimated standard normal deviate (treatment effect size divided by standard error) and the precision (the inverse of standard error) of the included studies, an Egger’s regression line is generated. If there is symmetry in the funnel plot, the intercept should be nonsignificant. In other words, the intercept value should be zero or near zero, and deviation of intercept from zero suggests publication bias. However, the power of Egger’s regression test is proportionally increase with number of studies.14,16 A limited number of studies may have low power to detect publication bias. In case of significant heterogeneity in primary outcome among either comparison, meta-regression analyses were conducted in posthoc fashion using a mixed-effects (unrestricted maximal likelihood) meta-regression model to explore the reasons for the heterogeneity. The variables used for meta-regression were age, bystander cardiopulmonary resuscitation (CPR), presenting rhythm, and duration of return to spontaneous circulation (ROSC) on treatment effects comparisons between Hypothermia and Normothermia trials. Power calculations were performed assuming a 20% reduction in mortality and adverse neurological outcome as a clinically important treatment effect for the hypothermia versus normothermia comparison.5 Conversely, for the pre-hospital hypothermia versus in-hospital hypothermia comparison, a 15% reduction mortality and adverse neurological outcome was considered clinically important.17 These calculations were based on the assumptions that the number of patients and the outcome rate in the control group are equal to those in our meta-analysis. A two-sample proportions Pearson's chi-squared test was used to compute power.
Results
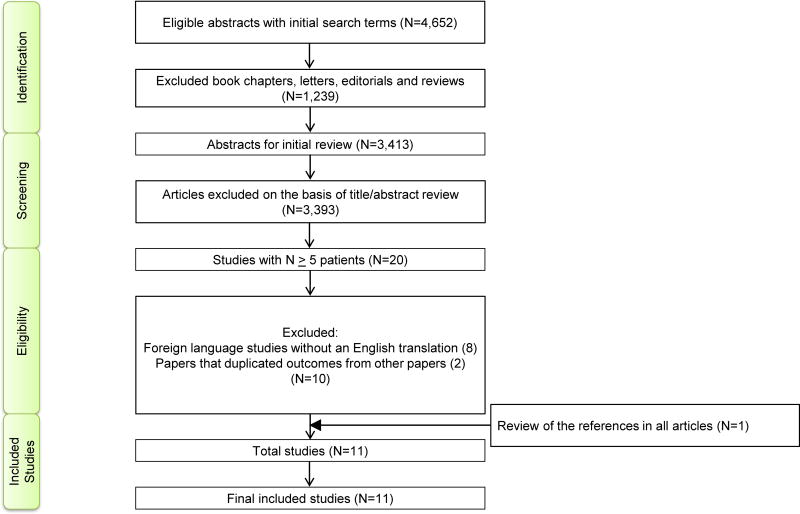
Eleven RCTs (Figure 1) with 4,782 patients were eligible for analyses (Table 1).2,3,5,18–25 The results of this systematic review and meta-analyses are presented as per the PRISMA extension statement (Supplemental Table 1).10 All included studies were graded as good to excellent based on the Jadad scale (Supplemental Table 2).
Figure 1.
Flow Diagram for Study Selection.
Table 1.
Baseline Characteristics of Included Studies
| Study (Reference) (IH or PH/NT) |
Presenting Rhythm |
Method of Cooling |
Duration of Cooling |
Time That Cooling Was Commenced |
Target Temperature (Degrees Celsius) |
Follow-Up Period |
ROSC (Time in Minutes) (TTM/NT) |
Age (Years) (TTM/NT) |
Male (%) (TTM/NT) |
Patients Receiving Bystander CPR (%) (IH or PH/NT) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bernard et al 20022 (N=43 PH/34 NT) | VF | Ice packs | 12 hours | After ROSC | 33° | Till discharge | 27/25 | 67/65 | 58/79 | 49/71 |
| Holzer et al 20023 (N=137 IH/138 NT) | VF/Pulseless VT | External cooling device | 24 hours | After ROSC | 32–34° | 6 months | 21/22 | 59/59 | 77/75 | 49/43 |
| Hachimi-Idrissi et al 200518 (N=30 IH/31 NT) | Asystole/PEA and VF/Pulseless VT | Cooling helmet | Up to 24 hours | After ROSC | 33° | 6 months | 29/28 | 67/69 | 77/68 | 30/26 |
| Kämäräinen et al 200923 (N=19 IH/18 NT) | Asystole/PEA and VF/Pulseless VT | Coooled intravenous fluid infusion | - | After ROSC | 33° | Till discharge | 23/22 | 59/63 | 95/94 | 58/22 |
| Nielsen et al 20135 (N=473 IH/466 NT) | Asystole/PEA and VF/Pulseless VT | Ice packs and cooled intravenous fluid infusion | 28 hours | After ROSC | 33° | 8.5 months | 25/25 | 64/64 | 83/79 | 73/73 |
| Study (PH/IH) |
Presenting Rhythm |
Method of Cooling |
Duration of Cooling |
Time That Cooling Was Commenced |
Target Temperature (Degrees Celsius) |
Follow-Up Period |
ROSC (Time in Minutes) (PH/IH) |
Age (Years) (PH/IH) |
Male (%) (PH/IH) |
Patients Receiving Bystander CPR (%) (PH/IH) |
|---|---|---|---|---|---|---|---|---|---|---|
| Castrén et al 201019 (N=93/101) | Asystole/PEA and VF/Pulseless VT | Transnasal evaporative cooling then systemic cooling | - | Intra-arrest | 34° | 7 days | 32/30 | 66/64 | 67/79 | 33/46 |
| Bernard et al 201020 (N=118/116) | VF | Cooled intravenous fluid infusion | 24 hours | After ROSC | 33° | Till discharge | 26/26 | 63/63 | 83/86 | 69/67 |
| Bernard et al 201221 (N=82/81) | Asystole/PEA | Surface cooling using machines, blankets, and ice packs; cooled intravenous fluid infusion | 24 hours | After ROSC | 32–34° | Till discharge | 29/29 | 64/61 | 57/48 | 36/31 |
| Debaty et al 201425 (N=123/122) | Asystole/PEA and VF/Pulseless VT | Cooled intravenous fluid infusion and external cooling | 24 hours | Intra-arrest | 32–34° | 12 months | 27/30 | 66/69 | 72/71 | 50/52 |
| Maynard et al 201524 (N=688/671) | Asystole/PEA and VF | Cooled intravenous fluid infusion | Up to 24 hours | After ROSC | 34° | 12 months for mortality outcomes and three months for neurological outcomes | 27/26 | 66/65 | 64/63 | 57/59 |
| Bernard et al 201622 (N=618/580) | Asystole/PEA and VF/Pulseless VT | Cooled intravenous fluid infusion | 24 hours | After ROSC | 33° | Till discharge | 23/20 | 65/64 | 75/74 | 66/67 |
Footnote: CPR=Cardiopulmonary Resuscitation, IH=In-Hospital Hypothermia, N=Number, NT=Normothermia, PEA=Pulseless Electrical Activity, PH=Pre-Hospital Hypothermia, ROSC=Return of Spontaneous Circulation, TTM=Targeted Temperature Management, VF=Ventricular Fibrillation, VT=Ventricular Tachycardia.
There was variation in the individual characteristics of the trials. The mean/median age of the patients in the pre-hospital hypothermia, in-hospital hypothermia and normothermia arms ranged from 63–67, 61–67, and 59–69 years of age, respectively. Mean/median ROSC time in the pre-hospital hypothermia, in-hospital hypothermia, and normothermia arms ranged from 26–32, 21–30, and 22–28 minutes, respectively. All combinations of presenting rhythms during out-of-hospital cardiac arrest were studied. There was a predominance of male patients in nearly all study arms (Table 1).
There were also variations in the resuscitation and cooling protocols of the trials. Percentage of bystander CPR varied from 26–73% amongst all study arms. The pre-hospital and in-hospital hypothermia arms of the trials had target temperatures ranging from 32–34 degrees Celsius. The duration of cooling in the pre-hospital hypothermia and in-hospital hypothermia arms ranged from 12–28 hours. Multiple methods of cooling were employed, such as cooling via a helmet, ice packs, and intravenous infusion of cooled fluids (Table 1). These interventions were studied in two groups: 1) the hypothermia versus normothermia comparison (studies=5 and patients=1389)2,3,5,18,23 and 2) the pre-hospital hypothermia versus in-hospital hypothermia comparison (studies=6, patients=3393).19–22,24,25
Hypothermia versus Normothermia
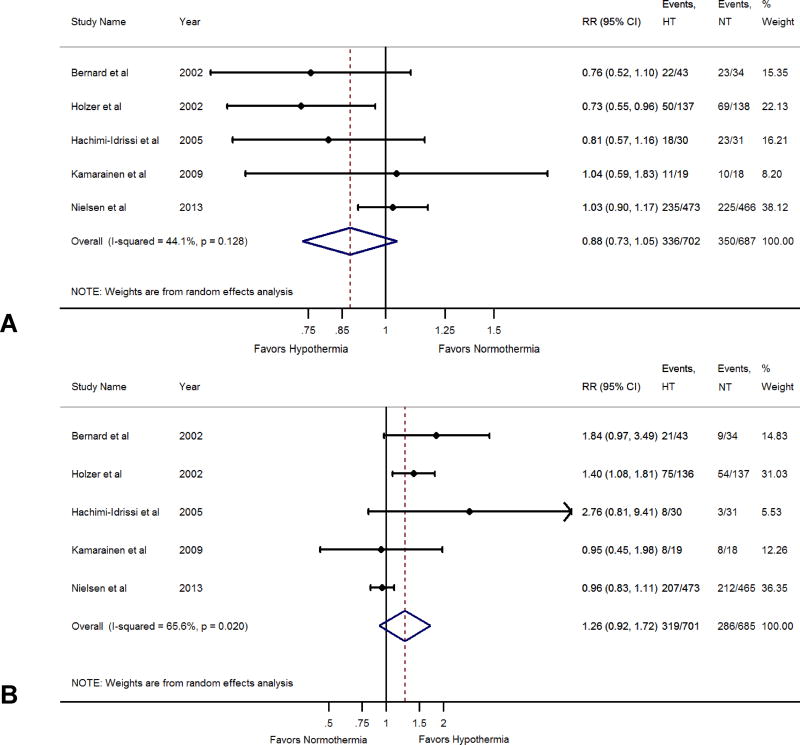
Among the five RCTs which compared the hypothermia and normothermia strategies,2,3,5,18,23 702 patients received hypothermia as part of post-arrest care whereas 687 received normothermia. We observed no difference in all-cause mortality rates in the hypothermia versus normothermia comparison (RR; 0.88, 95% CI: 0.73–1.05) (Figure 2, Panel A). Similarly, we did not observe any difference in the rates of favorable neurologic outcome in the hypothermia versus normothermia comparison (RR; 1.26, 95% CI: 0.92–1.72) (Figure 2, Panel B).
Figure 2.
Panel A: Forest Plot Comparing All-Cause Mortality Between Hypothermia and Normothermia.
Panel B: Forest Plot Comparing Favorable Neurological Outcome Between Hypothermia and Normothermia.
The blue diamond depicts the point estimate and the 95% confidence interval. The red dotted lines represent a random effects generated overall estimate. This was generated with a random effects model using the method of DerSimonian & Laird, with the estimate of heterogeneity calculated from the Mantel-Haenszel model. Data are presented with risk ratios and 95% confidence intervals.
Pre-Hospital Hypothermia versus In-Hospital Hypothermia
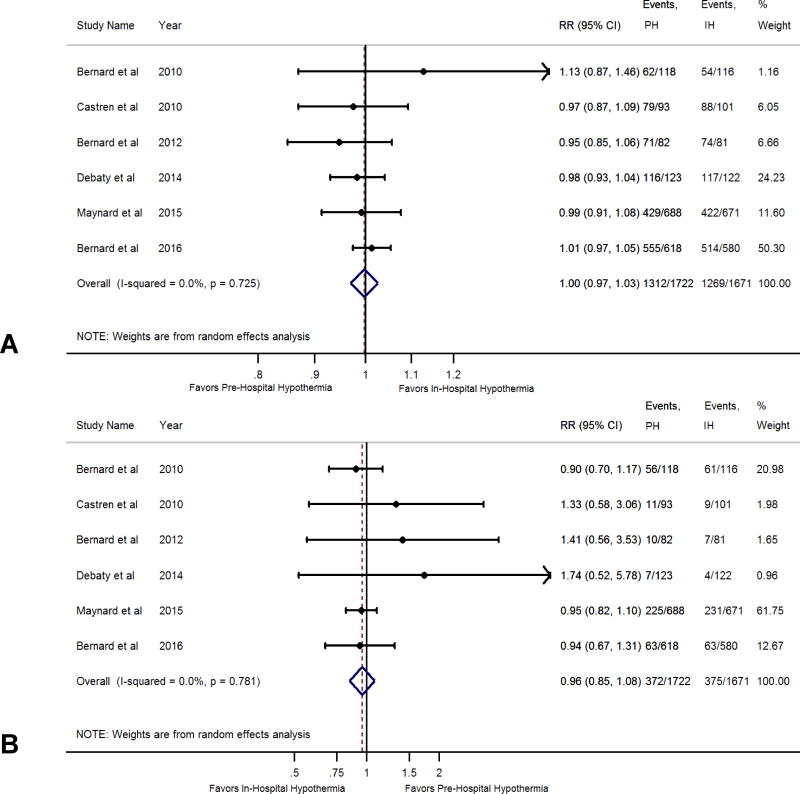
Among the six RCTs which compared the pre-hospital hypothermia and in-hospital hypothermia strategies,19–22,24,25 1722 patients received pre-hospital hypothermia as part of post arrest care whereas 1671 received in-hospital hypothermia. We observed no difference in all-cause mortality rates in the pre-hospital hypothermia versus in-hospital hypothermia comparison (RR; 1.00, 95% CI: 0.97–1.03) (Figure 3, Panel A). Similarly we did not observe any difference in the rates of favorable neurologic outcome in the pre-hospital hypothermia versus in-hospital hypothermia comparison (RR 0.96, 95% CI 0.85–1.08) (Figure 3, Panel B).
Figure 3.
Panel A: Forest Plot Comparing All-Cause Mortality Between Pre-Hospital Hypothermia and In-Hospital Hypothermia.
Panel B: Forest Plot Comparing Favorable Neurological Outcome Between Pre-Hospital Hypothermia and In-Hospital Hypothermia.
The blue diamond depicts the point estimate and the 95% confidence interval. The red dotted lines represent a random effects generated overall estimate. This was generated with a random effects model using the method of DerSimonian & Laird, with the estimate of heterogeneity calculated from the Mantel-Haenszel model. Data are presented with risk ratios and 95% confidence intervals.
Meta-Regression to Explore Heterogeneity in All-Cause Mortality Across Trials
We observed a substantial heterogeneity in treatment effect for mortality across trials comparing hypothermia and normothermia (I2 =44%) (Figure 2, Panel A) No heterogeneity was seen in trials comparing pre-hospital hypothermia and in-hospital hypothermia (I2=0%) (Figure 3, Panel A).
We conducted a posthoc analysis to see if age, bystander CPR, presenting rhythm, and time to ROSC would explain this heterogeneity. In studies comparing normothermia and hypothermia, we observed a significant trend towards a favorable effect of hypothermia on all-cause mortality with decreasing proportion of patients undergoing bystander CPR (p=0.04). The other characteristics were not related to treatment effect of mortality (p>0.05) (Table 2).
Table 2.
Meta-Regression to Assess the Effect of Predictors of Mortality (Hypothermia versus Normothermia)
| Variable | Range | β-estimate (95% CI) Hypothermia versus Normothermia |
p-value |
|---|---|---|---|
| Mean Trial Age, years | 59–67 | 0.01 (−0.044, 0.068) | 0.671 |
| Mean Trial Time to ROSC, minutes | 21–29 | 0.01 (−0.060, 0.300) | 0.764 |
| % Trial Bystander CPR | 28–73 | 0.007 (0.0001, 0.014) | 0.046 |
| % shockable Rhythm | 46–100 | −0.002 (−0.012, 0.007) | 0.602 |
The interpretation is valid in between the specified range of variables. β-estimate> 0 indicates RR>1. Meta-regression analyses were conducted using a mixed-effects meta-regression (unrestricted maximal likelihood) model.
Footnote: CPR=Cardiopulmonary Resuscitation, ROSC=Return of Spontaneous Circulation.
Publication Bias
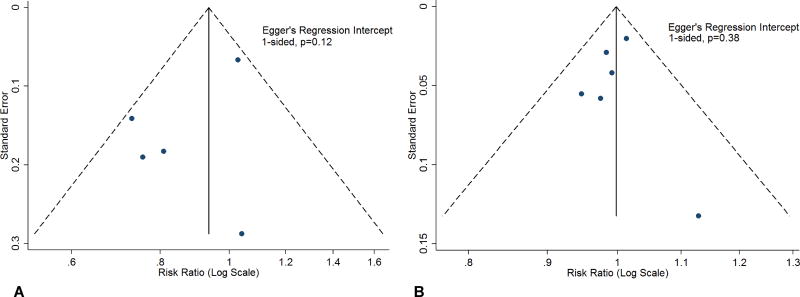
Publication bias was assessed using the Egger’s regression intercept for the primary outcome for the normothermia versus hypothermia and pre-hospital hypothermia versus in-hospital hypothermia comparisons. We did not observe any significant publication bias (Figure 4, Panel A and B).
Figure 4.
Panel A: Depiction of Publication Bias For the All-Cause Mortality Comparison Between Hypothermia and Normothermia.
Panel B: Depiction of Publication Bias For The All-Cause Mortality Comparison Between Pre-Hospital Hypothermia and In-Hospital Hypothermia.
Blue circles represent available studies. The dashed lines indicated the triangular region with pseudo 95% confidence interval. The Egger’s regression intercept for funnel plot was non-significant (Panel A, 1-sided, p=0.12 and Panel B, 1-sided, p=0.38).
CI: Confidence interval; HT: Hypothermia; IH: In-hospital hypothermia; NH: Normothermia; PH: Pre-hospital hypothermia; RR: Risk ratio.
Power calculation
We observed that >90% power would be achieved for mortality comparisons for both the hypothermia versus normothermia and pre-hospital hypothermia versus in-hospital hypothermia comparisons under this premise. For favorable neurological outcome, the hypothermia versus normothermia comparison would achieve a power of > 80% whereas the pre-hospital hypothermia versus in-hospital hypothermia comparison would achieve a power of 62%. The pre-hospital hypothermia versus in-hospital hypothermia comparison may be affected by a future trial as the power to detect a clinically important treatment effect in neurological outcome was low (Supplemental Table 3).
Discussion
Our systematic review and meta-analyses explore and compare the mortality and neurologic outcomes in cardiac arrest patients undergoing in-hospital hypothermia, pre-hospital hypothermia, and the normothermia TTM strategies. Despite heterogeneity in the duration of cooling, target temperatures, and presenting rhythms, we found that there was no difference in mortality or neurologic outcomes when comparing these strategies.
There are likely several mechanistic explanations for our findings. Therapeutic hypothermia and TTM as a whole have been under investigation for well over a decade. Along with the release of a number of high-profile RCTs, consensus guidelines have moved towards protocol-driven post-cardiac arrest care. The emergence of therapeutic hypothermia strategies may have led to the institution of formal protocols for post-cardiac arrest care where they were previously lacking. There are some data to suggest improvement in mortality after implementation of such protocols and this is similar to trends seen elsewhere in medicine, such as in the treatment of sepsis. Hence we postulate that making post-cardiac arrest care more standardized may well have itself improved the outcomes of post-cardiac arrest patients, thereby neutralizing some of the effects conferred by therapeutic hypothermia strategies. Additionally, the physiologic effect of cooling on cardiac function remains unclear. There are conflicting data on the topic, with some reports to suggest that in transplant patients there is less damage to the donor heart in the absence of profound hypothermia and therefore improvement in cardiac function.26 Conversely, Shao et al27 reported benefit after starting a therapeutic hypothermia at lower temperatures in an animal model by potentially reducing cardiac myocyte death through generation of nitric oxide. However, this appears to itself conflict with clinical data by Bernard et al in the form of the recently published RINSE trial.22 These conflicting data raise important questions about the physiologic implications of hypothermia on cardiac function and the timing of institution of hypothermia. We hoped to address the latter question through our investigation. More importantly, it suggests that the mechanisms are largely unclear to us and that manipulating this delicate physiology may induce harm. This is particularly important when a significant proportion of deaths after use of therapeutic hypothermia are due to a cardiac cause. Finally, we note that there is a vast difference in the percentage of patients receiving bystander CPR prior to initiation of therapeutic hypothermia in the trials. This ranged from 26 to 73% in the included trials.5,18 As early and good quality CPR is very clearly linked to survival after cardiopulmonary arrest,28,29 we postulate that the large variation in percentage of patients receiving CPR may have been a confounding factor in the mortality and neurologic outcome results attributed to therapeutic hypothermia protocols.
Our findings can also be used to draw interesting conclusions when compared to the existing literature base. There appears to be a significant interest in TTM and this has led to the publication of numerous meta-analyses and a Cochrane review that attempt to summarize the literature. To the best of our knowledge, our investigation remains the first to use meta-analyses to compare mortality and neurologic outcomes between the pre-hospital hypothermia, in-hospital hypothermia, and normothermia strategies. Most of the other investigations are limited by sole comparisons between the pre-hospital hypothermia and normothermia strategies.30–34 Of these published meta-analyses, one investigation included only non-shockable rhythms35 and another primarily included the results of cohort studies.31 We also note the trend in the meta-analyses published after 2014 to largely suggest that therapeutic hypothermia does not confer a benefit with regards to neurologic outcome or mortality. This may be affected by the inclusion of the investigation authored by Nielsen et al,5 which was the largest trial to suggest this. We note that similar results were reflected in the recently published RINSE trial authored by Bernard et al.22
There are also important clinical implications of our findings. Induction of therapeutic hypothermia and care of therapeutic hypothermia is associated with a significant cost to modern health care system. This has been estimated to be somewhere between $100,000-$160,000 per hospitalization per patient treated.36 In the modern era, this economic concern cannot be discarded if the proven outcome remains unclear. Therapeutic hypothermia is also associated with significant risks during the rewarming period. If patients are able to achieve equally good neurologic and mortality outcomes without the use of the hypothermia strategy, then clinicians may well be able to ameliorate some of these risks through avoidance of therapeutic hypothermia. We also note that there are further trials aiming to examine the future of therapeutic hypothermia.37 The HYPERION trial37 is a multi-center randomized controlled superiority trial that aims to compare neurologic status and mortality outcomes in patients with cardiac arrest due to a non-shockable rhythm. This trial aims to compare patients treated with a TTM strategy maintaining a temperature between 32.5–33.5 to patients treated with a TTM strategy maintaining a temperature between 36.5–37.5 degrees Celsius.37 We hope that this will yield information as to whether it is therapeutic hypothermia or simply a TTM normothermic strategy with avoidance of fever that confers neurologic or mortality benefit.
Kämäräinen et al23 note differences in the rates of bystander cardiopulmonary resuscitation in the two groups as well as minor differences in the end tidal carbon dioxide measurements at the time of hospital admission. Hence we evaluated the effect of rates of bystander CPR on treatment effect using metaregression. Both our meta-analysis and recent evidence suggests that conventional bystander CPR confers a clear mortality benefit over both no compression CPR and no bystander CPR.38 Such evidence highlights the need to create prediction models to identify who may benefit from hypothermia, such as patients who have not received bystander CPR. Hypothermia has been demonstrated in animal models to reduce ischemia-reperfusion injury, suppress ischemia-induced inflammatory cytokine surge, reduce free radicals, protect blood brain barrier integrity and resultant brain edema/intracranial hypertension, improve brain glucose metabolism, reduce convulsive activity and increased expression of immediate early genes which may prevent stress injury.39–43 The important distinction between animal and human studies is that the former are conducted in a tightly controlled environment with rapid and effective institution of mild hypothermia. On the other hand, human studies often have heterogeneity in the aforementioned. There are also key differences in the “bundle of care” in post arrest settings across human studies. This may also reduce the efficacy of hypothermia in humans. Hence future studies should aim to differentiate the effectiveness of hypothermia from other standard of care treatments that are instituted in post arrest settings in order to identify individuals who may benefit the most from hypothermia.
We recognize that our analyses also have limitations. Amalgamation of data in the form of meta-analyses has well-recognized limitations.13 Furthermore, we acknowledge significant differences in the cooling methods, cooling protocols, and even the rewarming protocols of the included trials (Table 1). This remains a topic of great discussion and we hope that future studies will help to derive the optimal protocols for these characteristics. Moreover, in the contemporary trials, therapeutic hypothermia was compared to the standard of care. This was poorly defined given that most of these trials were multi-center trials, some of which took place over multiple countries. We acknowledge that the standard of care likely became more protocol-driven, and therefore more homogenous, as the trials integrated newer consensus guidelines due to the development of an evidence base for post-resuscitation care. However, we feel that this may be a more accurate depiction of modern-day care and this consistency in care provision should be heralded as an important advance in the care of post-cardiac arrest patients.
In conclusion, targeted temperature management after cardiac arrest is an integral part of post-resuscitation care. Our analyses showed that the normothermic TTM strategy and the pre-hospital and in-hospital hypothermia TTM strategies produced comparable mortality and neurologic outcomes. More RCTs are required to determine the ideal timing, protocol, and patient population that would benefit from these interventions.
Supplementary Material
Key Points.
-
-
Question: Does targeted temperature management affect outcomes after cardiopulmonary arrest?
-
-
Findings: Pooled evidence from available randomized control trials indicated that targeted temperature management did not improve all-cause mortality or neurological outcomes but variability among studies was high.
-
-
Meaning: The role of targeted temperature management in post-resuscitation care remains unclear.
Acknowledgments
Funding: This work was supported in part by the Walter B. Frommeyer Investigative Fellowship awarded to Dr. Pankaj Arora. Dr. Bajaj was supported by National Institutes of Health grant 5T32HL094301-07. Dr. Patel was supported by National Institutes of Health grant 1T32HL129948-01A1.
None.
Footnotes
Conflicts of interest: None. The authors declare that there are no conflicts of interest.
Individual Author Contributions:
Conceptualization: P.A., N.S.B.
Data curation: G.A., N.S.B., R.K., N.P.,
Formal analysis: N.S.B.
Funding acquisition: P.A.
Investigation: P.A., N.S.B, RK.
Methodology: G.A., P.A., N.S.B., L.B., R.D., R.K., N.P.
Project administration: N.S.B., R.K.
Resources: P.A.
Software: P.A., N.S.B.
Supervision: P.A., N.S.B.
Validation: N.S.B., R.K.
Visualization: R.K.
Writing – original draft: G.A., P.A., N.S.B., L.B., R.D., R.K., N.P.
Writing – review & editing: G.A., P.A., N.S.B., L.B., R.D., R.K., N.P.
Final approval of the project to be submitted: G.A., P.A., N.S.B., L.B., R.D., R.K., N.P.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: G.A., P.A., N.S.B., L.B., R.D., R.K., N.P.
References
- 1.Perman SM, Goyal M, Neumar RW, Topjian AA, Gaieski DF. Clinical applications of targeted temperature management. Chest. 2014;145(2):386–393. doi: 10.1378/chest.12-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 3.The Hypothermia After Cardiac Arrest Study Group. Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 4.Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: Post–Cardiac Arrest Care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted Temperature Management at 33°C versus 36°C after Cardiac Arrest. N Engl J Med. 2013;369(23):2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 6.Hunter BR, O'Donnell DP, Allgood KL, Seupaul RA. No benefit to prehospital initiation of therapeutic hypothermia in out-of-hospital cardiac arrest: A systematic review and meta-analysis. Acad Emerg Med. 2014;21(4):356–364. doi: 10.1111/acem.12342. [DOI] [PubMed] [Google Scholar]
- 7.Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post–Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 suppl 2):S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 9.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.Kalra R, Arora P, Morgan C, Hage FG, Iskandrian AE, Bajaj NS. Conducting and interpreting high-quality systematic reviews and meta-analyses. Journal of Nuclear Cardiology. 2017;24(2):471–481. doi: 10.1007/s12350-016-0598-9. [DOI] [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001;20(4):641–654. doi: 10.1002/sim.698. [DOI] [PubMed] [Google Scholar]
- 16.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. Jama. 2006;295(6):676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 17.Kim F, Nichol G, Maynard C, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. Jama. 2014;311(1):45–52. doi: 10.1001/jama.2013.282173. [DOI] [PubMed] [Google Scholar]
- 18.Hachimi-Idrissi S, Zizi M, Nguyen DN, et al. The evolution of serum astroglial S-100 beta protein in patients with cardiac arrest treated with mild hypothermia. Resuscitation. 2005;64(2):187–192. doi: 10.1016/j.resuscitation.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Castren M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness) Circulation. 2010;122(7):729–736. doi: 10.1161/CIRCULATIONAHA.109.931691. [DOI] [PubMed] [Google Scholar]
- 20.Bernard SA, Smith K, Cameron P, et al. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation. 2010;122(7):737–742. doi: 10.1161/CIRCULATIONAHA.109.906859. [DOI] [PubMed] [Google Scholar]
- 21.Bernard SA, Smith K, Cameron P, et al. Induction of prehospital therapeutic hypothermia after resuscitation from nonventricular fibrillation cardiac arrest*. Crit Care Med. 2012;40(3):747–753. doi: 10.1097/CCM.0b013e3182377038. [DOI] [PubMed] [Google Scholar]
- 22.Bernard SA, Smith K, Finn J, et al. Induction of Therapeutic Hypothermia During Out-of-Hospital Cardiac Arrest Using a Rapid Infusion of Cold Saline: The RINSE Trial (Rapid Infusion of Cold Normal Saline) Circulation. 2016;134(11):797–805. doi: 10.1161/CIRCULATIONAHA.116.021989. [DOI] [PubMed] [Google Scholar]
- 23.Kamarainen A, Virkkunen I, Tenhunen J, Yli-Hankala A, Silfvast T. Prehospital therapeutic hypothermia for comatose survivors of cardiac arrest: a randomized controlled trial. Acta Anaesthesiol Scand. 2009;53(7):900–907. doi: 10.1111/j.1399-6576.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 24.Maynard C, Longstreth WT, Jr, Nichol G, et al. Effect of prehospital induction of mild hypothermia on 3-month neurological status and 1-year survival among adults with cardiac arrest: long-term follow-up of a randomized, clinical trial. J Am Heart Assoc. 2015;4(3):e001693. doi: 10.1161/JAHA.114.001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debaty G, Maignan M, Savary D, et al. Impact of intra-arrest therapeutic hypothermia in outcomes of prehospital cardiac arrest: a randomized controlled trial. Intensive Care Med. 2014;40(12):1832–1842. doi: 10.1007/s00134-014-3519-x. [DOI] [PubMed] [Google Scholar]
- 26.White CW, Ambrose E, Müller A, et al. Avoidance of Profound Hypothermia During Initial Reperfusion Improves the Functional Recovery of Hearts Donated After Circulatory Death. Am J Transplant. 2016;16(3):773–782. doi: 10.1111/ajt.13574. [DOI] [PubMed] [Google Scholar]
- 27.Shao ZH, Chang WT, Chan KC, et al. Hypothermia-induced cardioprotection using extended ischemia and early reperfusion cooling. Am J Physiol Heart Circ Physiol. 2007;292(4):H1995–2003. doi: 10.1152/ajpheart.01312.2005. [DOI] [PubMed] [Google Scholar]
- 28.Stiell IG, Brown SP, Christenson J, et al. What is the role of chest compression depth during out-of-hospital cardiac arrest resuscitation? Crit Care Med. 2012;40(4):1192–1198. doi: 10.1097/CCM.0b013e31823bc8bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abella BS, Sandbo N, Vassilatos P, et al. Chest Compression Rates During Cardiopulmonary Resuscitation Are Suboptimal. A Prospective Study During In-Hospital Cardiac Arrest. 2005;111(4):428–434. doi: 10.1161/01.CIR.0000153811.84257.59. [DOI] [PubMed] [Google Scholar]
- 30.Yu T, Longhini F, Wu R, Yao W, Lu W, Jin X. The role of the induction of mild hypothermia in adult patient outcomes after cardiac arrest: Systematic review and meta-analysis of randomized controlled studies. J Int Med Res. 2015;43(4):471–482. doi: 10.1177/0300060515576010. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XW, Xie JF, Chen JX, et al. The effect of mild induced hypothermia on outcomes of patients after cardiac arrest: a systematic review and meta-analysis of randomised controlled trials. Crit Care. 2015;19:417. doi: 10.1186/s13054-015-1133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharjee S, Baidya DK, Maitra S. Therapeutic hypothermia after cardiac arrest is not associated with favorable neurological outcome: a meta-analysis. J Clin Anesth. 2016;33:225–232. doi: 10.1016/j.jclinane.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Mahmoud A, Elgendy IY, Bavry AA. Use of Targeted Temperature Management After Out-of-hospital Cardiac Arrest: A Meta-Analysis of Randomized Controlled Trials. Am J Med. 2016;129(5) doi: 10.1016/j.amjmed.2015.11.004. 522-527.e522. [DOI] [PubMed] [Google Scholar]
- 34.Villablanca PA, Makkiya M, Einsenberg E, et al. Mild therapeutic hypothermia in patients resuscitated from out-of-hospital cardiac arrest: A meta-analysis of randomized controlled trials. Ann Card Anaesth. 2016;19(1):4–14. doi: 10.4103/0971-9784.173013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YM, Yim HW, Jeong SH, Klem ML, Callaway CW. Does therapeutic hypothermia benefit adult cardiac arrest patients presenting with non-shockable initial rhythms?: A systematic review and meta-analysis of randomized and non-randomized studies. Resuscitation. 2012;83(2):188–196. doi: 10.1016/j.resuscitation.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 36.Gajarski RJ, Smitko K, Despres R, Meden J, Hutton DW. Cost-effectiveness analysis of alternative cooling strategies following cardiac arrest. Springerplus. 2015;4:427. doi: 10.1186/s40064-015-1199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lascarrou JB, Meziani F, Le Gouge A, et al. Therapeutic hypothermia after nonshockable cardiac arrest: The HYPERION multicenter, randomized, controlled, assessor-blinded, superiority trial. Scand J Trauma Resusc Emerg Med. 2015;(1):23–26. doi: 10.1186/s13049-015-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuda T, Ohashi-Fukuda N, Kobayashi H, et al. Conventional Versus Compression-Only Versus No Bystander Cardiopulmonary Resuscitation for Pediatric Out-of-Hospital Cardiac Arrest. Circulation. 2016 doi: 10.1161/CIRCULATIONAHA.116.023831. [DOI] [PubMed] [Google Scholar]
- 39.Fischer S, Renz D, Wiesnet M, Schaper W, Karliczek GF. Hypothermia abolishes hypoxia-induced hyperpermeability in brain microvessel endothelial cells. Brain Res Mol Brain Res. 1999;74(1–2):135–144. doi: 10.1016/s0169-328x(99)00272-7. [DOI] [PubMed] [Google Scholar]
- 40.Siesjo BK, Bengtsson F, Grampp W, Theander S. Calcium, excitotoxins, and neuronal death in the brain. Ann N Y Acad Sci. 1989;568:234–251. doi: 10.1111/j.1749-6632.1989.tb12513.x. [DOI] [PubMed] [Google Scholar]
- 41.Povlishock JT, Buki A, Koiziumi H, Stone J, Okonkwo DO. Initiating mechanisms involved in the pathobiology of traumatically induced axonal injury and interventions targeted at blunting their progression. Acta Neurochir Suppl. 1999;73:15–20. doi: 10.1007/978-3-7091-6391-7_3. [DOI] [PubMed] [Google Scholar]
- 42.Xu L, Yenari MA, Steinberg GK, Giffard RG. Mild hypothermia reduces apoptosis of mouse neurons in vitro early in the cascade. J Cereb Blood Flow Metab. 2002;22(1):21–28. doi: 10.1097/00004647-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Globus MY, Busto R, Lin B, Schnippering H, Ginsberg MD. Detection of free radical activity during transient global ischemia and recirculation: effects of intraischemic brain temperature modulation. J Neurochem. 1995;65(3):1250–1256. doi: 10.1046/j.1471-4159.1995.65031250.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.