Abstract
Background
Recent changes in deceased donor organ allocation for livers (Share-35) and kidneys (KAS) have resulted in broader sharing of organs and increased cold ischemia time (CIT). Broader organ sharing however is not the only cause of increased CIT.
Methods
This was a retrospective registry study of CIT in same-hospital liver transplants (SHLT, n=4,347) and kidney transplants (SHKT, n=9,707) between 2004 and 2014.
Results
In SHLT, median (IQR) CIT was 5.0 (3.5–6.5) hours versus 6.6 (5.1–8.4) hours in other-hospital LT. DCD donors, donor biopsy, male recipient, recipient obesity, and previous transplant were associated with increased CIT. MELD at transplant of 29+ or status 1a was associated with decreased CIT. SHLT CIT varied by OPO and transplant-center (p<0.01), with center median CIT ranging from 2.0 – 7.8 hours across 118 centers. In SHKT, CIT was 13.0 (8.5–19.0) hours versus 16.5 (11.3–22.6) hours in other-hospital KT. Overweight donors, DCD donors, right-kidney, donor biopsy, recipient obesity, use of mechanical perfusion, additional KT procedures on the same day, and transplant center annual volume were associated with increased CIT. Older donor age, ECD donors, and underweight recipients were associated with decreased CIT. SHKT CIT varied by OPO and transplant-center (p<0.001), with center median CIT ranging from 3.3 – 29 hours across 206 centers. Transplant centers with longer SHKT also had longer SHLT (p=0.01).
Conclusion
Same-hospital transplants already have a significant amount of CIT, even without transporting the organ to another hospital.
INTRODUCTION
Cold ischemia time (CIT) is an undesirable, yet inevitable risk factor in deceased donor transplantation. Irish et al previously demonstrated the association between prolonged CIT and delayed graft function (DGF) after kidney transplantation 1. Other authors have reported associations between prolonged CIT and poor outcomes after transplantation. These include decreased graft survival in deceased donor kidney transplant (DDKT) recipients 2–7, primary non-function, early allograft dysfunction 8, and increased recurrence of HCC 9 among deceased donor liver transplant (DDLT) recipients.
Recent changes in organ allocation for deceased donor livers (Share-35) and kidneys (KAS) in the United States have increased the distances that organs may be transported, as candidates and donors are often geographically separated 10,11. This trend may be further exacerbated with redistricting efforts aimed at reducing geographic disparities 12. CIT in particular has come under increased scrutiny with the shift in allocation practices that may increase CIT in organs that are transported further distances.
Increased transport distance is not the only cause of prolonged CIT. There are many logistical concerns that occur within the transplant and donor centers well before the organ is ready for transport. In an English study of logistical factors influencing CIT in the UK, Shresta et al report a variety of factors influencing CIT 13. These include time for organ allocation, cross-matching, allograft biopsies, recipient-related issues such as transportation or medical comorbidity, or donor hospital issues such as operating room (OR) and surgeon availability. It is possible that these factors have a more profound impact on CIT in comparison to organ transport 14.
In the context of broader organ sharing, we sought to quantify in-hospital CIT resulting from factors other than organ transport. In this national retrospective study of CIT in the US, we studied DDLTs and DDKTs, where the donor and recipient operations occurred at the same hospital. We examined pairs of kidneys from the same donor where one kidney was transplanted at the same hospital as recovery and one was transported to a different hospital for transplant. We also aimed to describe heterogeneity in same-hospital CIT between transplant centers and OPOs, and whether centers with long CIT in LT also had long CIT in KT.
METHODS
Study Population
We investigated 4,347 deceased donor liver transplant recipients, and 9,707 deceased donor kidney transplant recipients who underwent transplantation at the same hospital where their organ was recovered between June 1, 2004 and May 31, 2014. We excluded split/partial liver transplants, liver transplants that were missing CIT (n=231) and those with CIT greater than 16 hours (n=35) as these were likely errors. We excluded en-bloc or sequential kidney transplants, kidney transplants that were missing CIT (n=675) and those with CIT greater than 48 hours (n=68).
Data Source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, waitlisted candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Identifying Same-Hospital Transplants
Same-hospital liver transplants (SHLT) and kidney transplants (SHKT) were identified by matching the recipient transplant center with the donor provider hospital using the OPO identifier, provider number, and provider type. Other-hospital liver transplants (OHLT) and kidney transplants (OHKT) were identified as transplants where the donor hospital differed from the recipient transplant center. We identified a subpopulation of donors with both a SHKT and OHKT for a subgroup analysis of paired kidneys and their CIT. Differences in demographics between same- and other-hospital transplants were tested using Chi-Squared, Student’s t-tests, and Wilcox Rank Sum statistics.
Cold Ischemia Time and Time Ratios
CIT was studied using an accelerated failure time (AFT) model. AFT models are an alternative to the more familiar proportional hazards (PH) models like Cox. AFT models provide time-ratios (TR) instead of hazard ratios. A time-ratio can be interpreted as a percentage increase (TR>1.0) or decrease (TR<1.0) to CIT. For example, if obesity is associated with CIT with a TR of 1.06, CIT is increased by 6% with obesity.
OPO, Transplant-Center, and Patient Level Associations
We studied whether CIT varied based on OPO, transplant center, or donor and recipient characteristics. We used multilevel (mixed effects) regression to estimate both OPO and center level effects and account for center clustering. We evaluated the effect of competition in DSAs by comparing single-center and multi-center OPOs. We adjusted for the following donor and recipient characteristics: donor and recipient age, ethnicity, BMI, previous (kidney or liver) transplant, and year of transplant. We also adjusted for performing transplant operation on weekend and performing additional KT procedures on same day. We adjusted for organ-specific transplant volume as a center-level characteristic. The LT model was also adjusted for MELD at transplant and receipt of MELD exception points. The KT model was adjusted for laterality (left versus right kidney), donor biopsy, and mechanical reperfusion.
Center-level correlation of long cold ischemia time
To determine whether hospitals with long CIT in SHLT also had long CIT in SHKT, we correlated the hospital’s average CIT for SHLT with the hospital’s average CIT for SHKT using a univariate linear regression. Hospitals in the regression were weighted by the hospital’s total liver transplant volume.
Statistical Analysis
Statistical analysis was performed using Stata 14.2 (College Station, Texas). Figures were prepared using R 3.1 (the R Foundation for Statistical Computing, Vienna, Austria). For all analyses, p < 0.05 was considered statistically significant. Multilevel AFT regressions were done using the mestreg command in Stata with Gamma (KT) and Log-logistic (LT) distributions.
RESULTS
Study Population
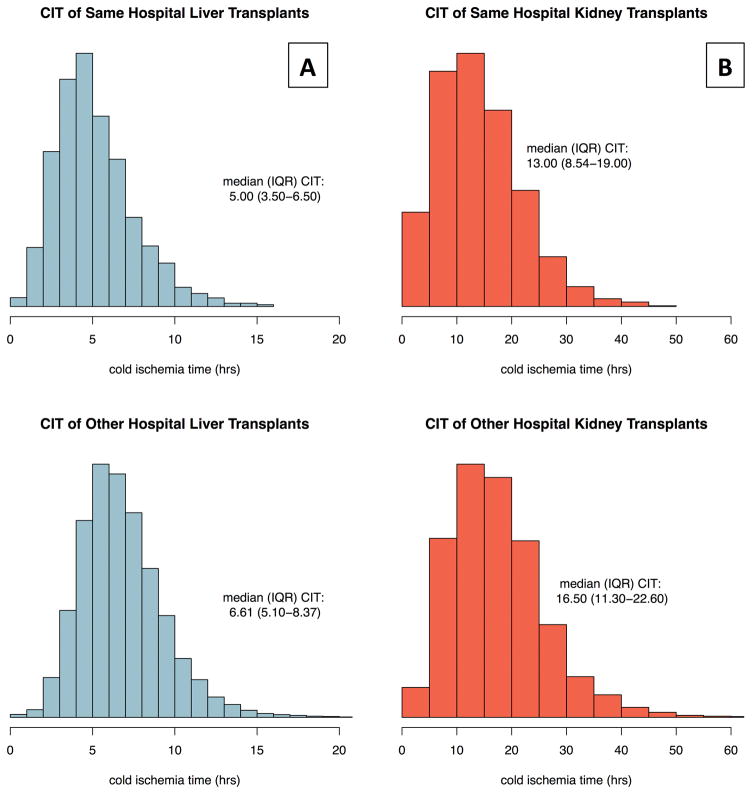
Out of 55,629 liver transplants, 4,347 (7.8%) occurred at the same hospital as organ recovery (Figure 1a). SHLT were performed at 118 transplant centers in all 52 OPOs with LT programs. Out of 96,641 kidney transplants, 9,707 (10%) were SHKT (Figure 1b). SHKT were performed at 206 transplant centers in all 58 OPOs with KT programs. A significantly lower proportion of LT were performed in the same hospital (7.8%) than KT (10%) (p<0.001). Donor and recipient characteristics of SHLT and SHKT are summarized in tables 1a and 1b.
Figure 1. Figure 1a/b: Cold Ischemia Time of Liver and Kidney Transplants.
Same-hospital transplants are when the transplant and recovery procedures occur in the same center. Other-hospital transplants are when the transplant and recovery procedures occur at difference centers.
Table 1a.
Liver Transplant Deceased Donor and Recipient Characteristics
| Same-Hospital Liver Transplants |
Other-Hospital Liver Transplants |
|
|---|---|---|
| Transplants, n* | 4347 | 51282 |
| Cold Ischemia Time hours, median (IQR) | 5.0 (3.5–6.5) | 6.6 (5.1–8.3) |
| Donor Characteristics: | ||
| Age in years, mean (SD) | 39 (23–52) | 41 (24–54) |
| Female, n (%) | 1700 (39) | 21008 (41) |
| Ethnicity, n (%) | ||
| White | 2857 (66) | 33758 (66) |
| Black | 921 (21) | 9085 (18) |
| Hispanic | 492 (11) | 6792 (13) |
| Other | 77 (1.8) | 1647 (3.2) |
| BMI, n (%) | ||
| Underweight | 157 (3.6) | 1781 (3.5) |
| Normal | 1583 (36) | 18641 (36) |
| Overweight | 1412 (32) | 16194 (32) |
| Obese | 1195 (27) | 14666 (29) |
| DCD, n (%) | 304 (7.0) | 2323 (4.5) |
| Biopsy, n (%) | 1372 (32) | 17065 (33) |
| Recipient Characteristics: | ||
| Age at transplant, mean (SD) | 55 (48–61) | 55 (47–60) |
| Female, n (%) | 1412 (32) | 17192 (34) |
| Ethnicity, n (%) | ||
| Caucasian | 3320 (76) | 35770 (70) |
| African American | 422 (9.7) | 5437 (11) |
| Hispanic | 442 (10) | 7058 (14) |
| Other | 163 (3.8) | 3017 (5.9) |
| BMI, n (%) | ||
| underweight | 182 (4.3) | 2573 (5.0) |
| normal | 1218 (28) | 14353 (28) |
| Overweight | 147 (33) | 16415 (32) |
| obese | 1497 (34) | 17941 (35) |
| Received HCC exception points, n(%) | 965 (22) | 12545 (25) |
| MELD at transplant, n (%) | ||
| 6–14 | 125 (2.9) | 1880 (3.7) |
| 15–21 | 1080 (25) | 8742 (17) |
| 22–28 | 2023 (47) | 20506 (41) |
| 29–34 | 509 (12) | 8381 (17) |
| 35+ | 468 (11) | 8123 (16) |
| Status 1a/b | 100 (2.3) | 2791 (5.5) |
| Previous LT, n (%) | 253 (5.8) | 3645 (7.1) |
| >1 transplants on same day, n (%) | 290 (6.7) | 4158 (8.1) |
Livers with CIT >16hrs excluded (n=35)
Table 1b.
Kidney Transplant Deceased Donor and Recipient Characteristics
| Same-Hospital Kidney Transplants | Other-Hospital Kidney Transplants | |
|---|---|---|
| Transplants, n* | 9707 | 86934 |
| Cold Ischemia Time, median (IQR) | 13.0 (8.53–19.0) | 16.4 (11.3–22.4) |
| Donor Characteristics: | ||
| Age, mean (SD) | 39 (23–51) | 40 (24–51) |
| Female, n (%) | 3835 (40) | 34386 (40) |
| Ethnicity, n (%) | ||
| White | 6880 (71) | 59468 (68) |
| Black | 1523 (16) | 11989 (14) |
| Hispanic | 1069 (11) | 12582 (14) |
| Other | 235 (2.4) | 2895 (3.3) |
| BMI, n (%) | ||
| underweight | 386 (4) | 3256 (3.8) |
| Normal | 3405 (35) | 31042 (36) |
| Overweight | 3154 (32) | 27255 (31) |
| obese | 2762 (28) | 25381 (29) |
| DCD, n (%) | 1842 (19) | 10115 (12) |
| ECD, n (%) | 1487 (15) | 14004 (16) |
| Right Kidney, n (%) | 4954 (51) | 45353 (52) |
| Biopsy, n (%) | 3923 (40) | 36156 (41) |
| Recipient Characteristics: | ||
| Age at transplant, mean (SD) | 52 (41–61) | 53 (42–62) |
| Female, n (%) | 3711 (38) | 34153 (39) |
| Ethnicity, n (%) | ||
| White | 4745 (49) | 39448 (45) |
| Black | 3266 (34) | 27012 (31) |
| Hispanic | 1034 (11) | 13774 (16) |
| Other | 662 (6.8) | 6700 (7.7) |
| BMI, n (%) | ||
| underweight | 306 (3.2) | 3157 (3.6) |
| Normal | 2666 (27) | 24882 (29) |
| Overweight | 2938 (30) | 27091 (31) |
| obese | 3797 (39) | 31804 (37) |
| Previous KT, n (%) | 1075 (11) | 11027 (13) |
| Pre-Transplant Biopsy, n (%) | 1617 (22) | 12196 (17) |
| Ice and Mechanical perfusion, n (%) | ||
| Received and kept on ice | 6218 (64) | 62356 (72) |
| Received on ice, put on pump | 1120 (12) | 6993 (8.1) |
| Received on pump, put on ice | 636 (6.6) | 5065 (5.8) |
| Received and kept on pump | 1721 (18) | 12380 (14) |
Kidneys with CIT >48hrs excluded (n=68)
Cold Ischemia Time for Same-Hospital Liver and Kidney Transplants
The distribution of CIT for both LT and KT was right-skewed regardless of recovery location (Figure 1). After excluding livers with CIT >16hrs, the median (IQR) CIT for SHLT was 5.0 (3.5 – 6.5) hours (Figure 1a), versus 6.6 (5.1–8.4) hours for OHLT. After excluding kidneys with CIT >48hrs, the median (IQR) CIT for SHKT was 13.0 (8.5 – 19.0) hours (Figure 1b), versus 16.5 (11.3 – 22.6) hours of CIT in OHKT. CIT was lower in same-hospital transplant versus other-hospital transplants in both LT and KT (p<0.001). In SHLT, the shortest (5th percentile) CIT was 2 hours and the longest (95th percentile) was 10 hours. In SHKT, the shortest (5th percentile) CIT was 4 hours and the longest (95th percentile) was 28 hours.
Donor and Recipient Characteristics Associated with Liver Transplant Cold Ischemia Time
DCD donors were associated with an 8% increase in CIT (TR 1.031.081.13, p<0.01). Donor biopsy was associated with a 9% increase in CIT (TR 1.061.091.12, p<0.001). Male LT candidates had a 3% increase in CIT (TR 1.031.031.06, p=0.02). Obese recipients had a 5% increased CIT compared to normal BMI recipients (TR 1.021.051.09, p<0.001). Candidates who had a previous LT had an 11% increased CIT (TR 1.051.111.17, p<0.001). Conversely, candidates with MELD at transplant of 29–34, 35+, or status 1a/b had a decreased CIT by 4% (p=0.04), 9% (p<0.001), and 26% (p<0.001) respectively. Over time, CIT decreased by 3% per year(p<0.001) (Table 2a).
Table 2a.
Patient Factors Associated with Cold Ischemia Time in Same-Hospital Liver Transplantation
| Time Ratio (95% CI) | p-value | |
|---|---|---|
| Donor Age (centered at 40, per decade) | 1.00 (0.99 – 1.01) | >0.9 |
| Donor Male Gender | 0.99 (0.96 – 1.01) | 0.3 |
| Donor Race | ||
| White | REF | |
| Black | 0.99 (0.95 – 1.02) | 0.4 |
| Hispanic | 0.99 (0.95 – 1.03) | 0.7 |
| Other | 1.08 (0.99 – 1.18) | 0.1 |
| Donor BMI | ||
| Normal | REF | |
| Underweight | 1.03 (0.96 – 1.10) | 0.4 |
| Overweight | 1.01 (0.98 – 1.04) | 0.6 |
| Obese | 1.00 (0.97 – 1.03) | >0.9 |
| DCD | 1.08 (1.03 – 1.13) | <0.01 |
| Donor Biopsy | 1.09 (1.06 – 1.12) | <0.001 |
| Recipient Age (centered at 55, per decade) | 0.99 (0.98 – 1.00) | 0.1 |
| Recipient Male Gender | 1.03 (1.00 – 1.06) | 0.02 |
| Recipient Race | ||
| White | REF | |
| Black | 1.00 (0.96 – 1.04) | >0.9 |
| Hispanic | 0.97 (0.93 – 1.02) | 0.3 |
| Other | 0.96 (0.90 – 1.02) | 0.2 |
| Recipient BMI | ||
| Normal | REF | |
| Underweight | 0.96 (0.88 – 1.03) | 0.2 |
| Overweight | 1.02 (0.99 – 1.06) | 0.1 |
| Obese | 1.05 (1.02 – 1.09) | 0.001 |
| HCC points | 0.98 (0.95 – 1.02) | 0.3 |
| MELD (allocation) | ||
| 6–14 | 1.03 (0.95 – 1.11) | 0.5 |
| 15–21 | 0.99 (0.96 – 1.02) | 0.508 |
| 22–28 | REF | - |
| 29–34 | 0.96 (0.92 – 1.00) | 0.04 |
| 35+ | 0.91 (0.87 – 0.96) | <0.001 |
| status 1a | 0.74 (0.68 – 0.81) | <0.001 |
| Previous Liver Transplant | 1.11 (1.05 – 1.17) | <0.001 |
| Year of transplant (centered at 2014) | 0.97 (0.97 – 0.98) | <0.001 |
| Additional LT procedures on same day | 0.97 (0.92 – 1.01) | 0.2 |
| Transplant Center Annual Volume | 1.00 (1.00 – 1.00) | 0.1 |
| Weekend Transplant | 0.97 (0.95 – 1.01) | 0.3 |
| Single-center OPO (low competition) | 1.03 (0.90 – 1.18) | 0.5 |
Multilevel Accelerated Failure Time Model with OPO-level and Hospital-level variance (p<0.01).
Donor and Recipient Characteristics Associated with Kidney Transplant Cold Ischemia Time
Overweight donors were associated with an increased CIT by 3% (p=0.02) compared to normal BMI donors, DCD donors had a 9% increased CIT (p<0.001), donor right kidney was associated with a 5% increased CIT (p<0.001), and donor biopsy had a 13% increased CIT (p<0.001). Obese recipients were associated with an increased CIT by 3% (p=0.02). Transport that involved mechanical perfusion at some point was associated with a 30–41% increased CIT (p<0.001). Conversely, older donors were associated with a decreased CIT by 2% per decade of age (p<0.01), ECD donors had a 6% decreased CIT (p<0.01), underweight recipients had a decreased CIT by 7% (p=0.04). Over time, CIT decreased by 2% per year (p<0.001) (Table 2b). When evaluating paired kidneys from the same donor, one of which was a SHKT and the other an OHKT, CIT was 20% shorter in SHKTs (p<0.001).
Table 2b.
Patient Factors Associated with Cold Ischemia Time in Same-Hospital Kidney Transplants
| Time Ratio (95% CI) | p-value | |
|---|---|---|
| Donor Age (centered at 40, per decade) | 0.98 (0.98 – 1.00) | <0.01 |
| Donor male gender | 1.00 (0.98 – 1.03) | 0.7 |
| Donor Race | ||
| White | REF | |
| Black | 0.98 (0.95 – 1.01) | 0.3 |
| Hispanic | 1.00 (0.96 – 1.04) | >0.9 |
| Other | 1.06 (0.98 – 1.14) | 0.2 |
| Donor BMI | ||
| Normal | REF | |
| Underweight | 0.98 (0.92 – 1.04) | 0.5 |
| Overweight | 1.03 (1.00 – 1.06) | 0.02 |
| Obese | 1.02 (0.99 – 1.05) | 0.3 |
| DCD | 1.09 (1.05 – 1.12) | <0.001 |
| ECD | 0.94 (0.90 – 0.98) | <0.01 |
| Donor Right Kidney | 1.05 (1.03 – 1.07) | <0.001 |
| Donor Biopsy | 1.13 (1.09 – 1.16) | <0.001 |
| Recipient Age (centered at 50, per decade) | 1.01 (1.00 – 1.02) | 0.1 |
| Recipient male gender | 0.99 (0.97 – 1.01) | 0.3 |
| Recipient Race | ||
| White | REF | |
| Black | 1.01 (0.98 – 1.03) | 0.7 |
| Hispanic | 1.01 (0.97 – 1.06) | 0.5 |
| Other | 1.05 (1.00 – 1.10) | 0.06 |
| Recipient BMI | ||
| Normal | REF | |
| Underweight | 0.93 (0.87 – 1.00) | 0.04 |
| Overweight | 1.03 (1.00 – 1.06) | 0.09 |
| Obese | 1.03 (1.00 – 1.06) | 0.02 |
| Recipient Previous Transplant | 1.02 (0.98 – 1.06) | 0.3 |
| Pre-Transplant Biopsy | 1.00 (0.97 – 1.03) | >0.9 |
| Ice and Mechanical Perfusion | ||
| Received and kept on ice | REF | |
| Received on ice, put on pump | 1.41 (1.33 – 1.49) | <0.001 |
| Received on pump, put on ice | 1.30 (1.23 – 1.38) | <0.001 |
| Received and kept on pump | 1.40 (1.34 – 1.45) | <0.001 |
| Year of Transplant (centered on 2014) | 0.98 (0.98 – 0.99) | <0.001 |
| Additional KT procedures on same day | 1.03 (1.01 – 1.05) | <0.01 |
| Transplant Center Annual Volume | 1.00 (1.00 – 1.00) | 0.01 |
| Single-center OPO (low competition) | 0.95 (0.76 – 1.18) | 0.6 |
| Weekend Transplant | 0.98 (0.96 – 1.01) | 0.2 |
| Same Donor SHKT vs OHKT | 0.80 (0.79 – 0.81) | <0.001 |
Multilevel Accelerated Failure Time Model with OPO-level and Hospital-level variance (p<0.001).
OPO and Transplant-Center Variation in Cold Ischemia Time
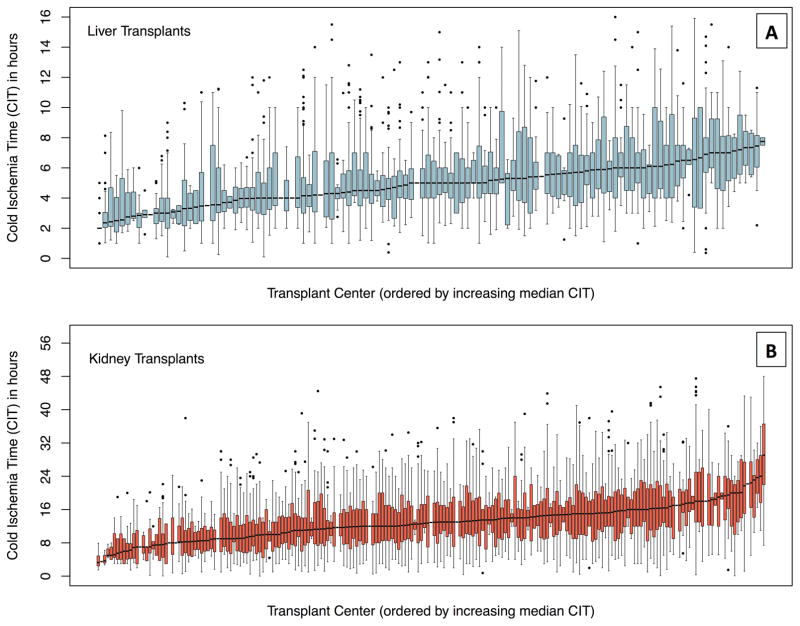
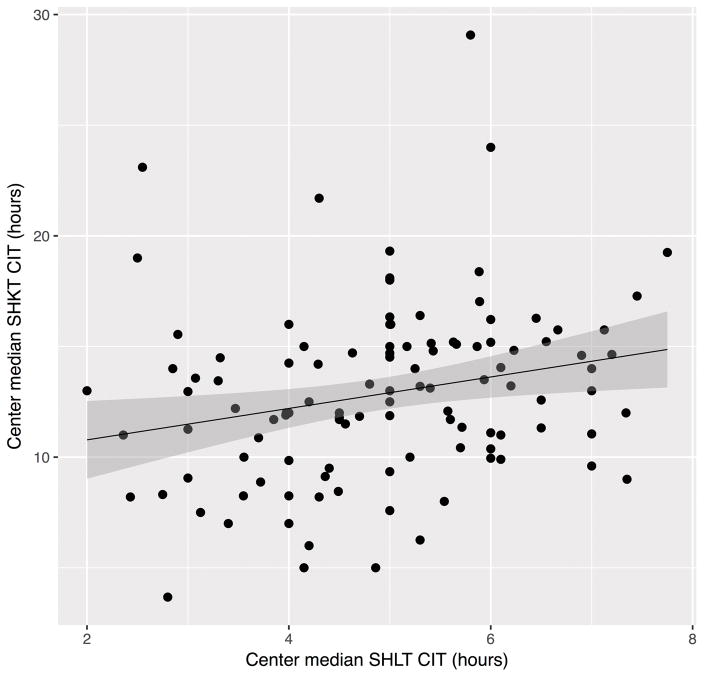
For both SHLT and SHKT, CIT varied between transplant centers and between OPOs (p<0.01). In SHLT, the median CIT for individual transplant centers ranged from 2 to 7.8 hours with an average center having a median of 4.9 hours of CIT (Figure 2a). In SHKT, the median CIT for individual transplant centers ranged from 3.3 to 29 hours with an average center having a median of 12.8 hours of CIT (Figure 2b). While we did not observe any associations with same-day or annual LT volumes at SHLT transplant centers (p=0.2, p=0.1 respectively), we found that each additional KT procedure on the same day as another SHKT increased CIT by 3% (p<0.01) and that increased annual KT volume was associated with an increased CIT by 1.2% per 10 transplants (p=0.01). Same-hospital transplants occurring on weekends did not have significantly different CIT than those occurring on weekdays (p=0.2). For SHLT, there was no significant difference in CIT between OPOs with a single center (low competition) and those with multiple centers (high competition) (p=0.5). Similarly, for SHKT, there was no significant difference in CIT between single center and multi-center OPOs (p=0.6) Furthermore, in transplant centers that perform both KT and LT, centers that had a high median CIT for SHLT also had a high median CIT for SHKT (Figure 3, p=0.01).
Figure 2. Figure 2a/b: Range of Cold Ischemia Time by Transplant Center for Same-Hospital Liver and Kidney Transplants.
The median for each center is shown as the dark line, IQR as grey bars, and outliers as points.
Figure 3. Median CIT of Centers that Perform both Liver and Kidney Transplants.
SHKT: Same-hospital Kidney Transplant, SHLT: Same-hospital Liver Transplant, CIT: Cold Ischemia Time. Each point represents a transplant center that performs both liver and kidney transplants. Centers that had a high median CIT for SHLT also had a high median CIT for SHKT (p=0.01).
DISCUSSION
In this retrospective study of CIT, we found that same-hospital LT and KT had a median of 5.0 and 13.0 hours of CIT respectively, compared to 6.6 and 16.4 hours in other-hospital CIT. If we consider the association between CIT and DGF reported by Irish et al 1, the median same-hospital KT would have increased odds of DGF by 69% associated with CIT, while the other-hospital KT would have increased odds of DGF by 93% compared to a KT with no CIT. The 95th percentiles of same-hospital CIT were 10hrs and 28hrs for LT and KT respectively. Our findings demonstrate that a major portion of CIT occurred in the hospital and not from transport alone.
For risk factors associated with CIT, we found that SHLT CIT was increased with DCD donors (8%), donor biopsy (9%), male recipients (3%), obese recipients (5%), and candidates with previous LT (11%), and that it was decreased in MELD 29+ and status 1 candidates (4–26%). In SHKT, we found that CIT was increased with overweight donors (3%), DCD donors (9%), donor right kidney (5%), donor biopsy (13%), obese recipients (3%), and any mechanical perfusion (41%). We found that SHKT CIT was decreased with donor age (2% per decade), ECD donors (6%), and in underweight recipients (7%).
Accounting for differences in donor and recipient characteristics, we found that there was significant heterogeneity in CIT between OPOs as well as between transplant centers. This heterogeneity was exemplified in the range of center median CIT from 2.0 – 7.8 hours in SHLT, and 3.3 – 29 hours in SHKT. In SHKT but not in SHLT, we found that additional KT procedures being performed on the same day at a center would increase CIT, and that annual center transplant volume also increased CIT. We saw evidence that centers with long CIT in LT also had long CIT in KT. This association along with our findings that hospitals were capable of reducing CIT for LT candidates with greater perceived urgency (MELD 20+, Status 1A) suggests that transplant centers might be able to improve in-hospital efficiency to reduce CIT.
There were similarities as well as some notable differences in our findings compared to the English study by Shrestha et al regarding CIT in KT. Our reported median CIT was 13 hours for same-hospital KT, and 16.4 hours when transport was involved. These values are comparable to the overall mean CIT of 13.8 hours in the Shrestha study. Both studies found significant heterogeneity in CIT between centers. While their study considered virtual versus pre-transplant crossmatch policies at transplant centers, we did not ascertain whether these policies were in place for the transplant centers in the US and could not study their associations with CIT. In both studies, we observed increased CIT with transport (aka reallocated), and with pumping to kidney on ice. While Shrestha et al found that DCD donors were associated with reduced CIT, we found that DCD donors were associated with an increased CIT. These conflicting findings could be a result of differences in unmeasured confounding in either study, or by the allocation and geographic differences between the English and American systems.
Echoing this difference, we found same-hospital KTs to have 20% lower CIT than other-hospital KTs when comparing paired kidneys from the same donor. In prior work, our group created an extensively detailed model to estimate transport times for livers, and we found that estimated transport time comprised only 21% of CIT in liver transplant14. These 2 separate results lead us to conclude that 80% of CIT is due to non-transport factors. Reducing allocation delays or reducing hospital delays are the only avenues to decreasing this largest fraction of CIT.
Unfortunately, organ procurement organizations are not required to record the time of acceptance of an organ offer, so the length of allocation delays is unknowable. Either center-driven events, or allocation delays, or both, could contribute to longer CIT. We cannot separate CIT into that which is attributable to allocation delays vs center-driven delays. Our study did not find any difference in same-hospital CIT in liver or kidney comparing single to multi-center OPOs. Presumably, the greater complexity of allocation in multi-center OPOs would make allocation delays longer, so this counter-intuitive finding suggests that allocation delays might not be the primary driver of differences in same-hospital CIT. Further data from UNOS specifying time of final offer acceptance would allow for an improved understanding of CIT.
There are limitations of this study that warrant discussion. First, all registry studies are limited by self-reporting error, missing data, and reporting bias. The effect of missing CIT on inferences is likely minimal, as only 5–7% of CIT were missing. We did not describe mechanical perfusion among deceased donor liver transplants as this relatively new practice was not captured in the registry data. A second limitation in our design is that we did not account for changes in CIT due to secular trends or changes in allocation policy; however, we selected a study period that ended before the start of the new KAS in December 2014, and only captured a short period of Share-35 starting in June of 2013. The results of our study would mostly reflect a prepolicy state of allocation and might under-estimate the CIT that we would expect to see in other-hospital transplants under the current KAS and Share35 allocation systems.
We have shown that there is significant in-hospital CIT without transport. Furthermore, our findings suggest that motivated transplant centers were able to reduce CIT for candidates who urgently needed a transplant – those with MELD 35+ or Status 1A. A better understanding of what in-hospital factors are mechanistically related to long CIT may provide more effective avenues to reduce long CIT. Identifying modifiable factors that influence CIT is a necessary next step to reducing in-hospital CIT. Our findings are most relevant in the context of allocation policy where increased transportation from broader sharing would increase CIT for some recipients. The perceived detriments of longer CIT may be assuaged however by increasing in-hospital efficiency rather than fixating on transport time. While broader sharing may contribute additional CIT, much more CIT is being accumulated for other reasons.
Acknowledgments
This work was supported by a contract from the US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, HHSH250201000018C. The work was also supported by an American Recovery and Reinvestment Act grant from the National Institute of Diabetes Digestive and Kidney Diseases, RC1 1RC1DK086450–01, and by grant number R01DK111233 Reducing Geographic Disparities in Kidney and Liver Allocation. Dr. Segev is supported by grant number K24DK101828 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Sandra DiBrito is supported by grant number F32DK105600 (NIDDK).
ABBREVIATIONS
- CIT
Cold Ischemia Time
- DGF
Delayed Graft Function
- DDKT
Deceased Donor Kidney Transplant
- HCC
Hepatocellular Carcinoma
- DDLT
Deceased Donor Liver Transplant
- KAS
Kidney Allocation System
- OR
Operating Room
- OPO
Organ Procurement Organization
- LT
Liver Transplant
- KT
Kidney Transplant
- SRTR
Scientific Registry of Transplant Recipients
- OPTN
Organ Procurement and Transplantation Network
- HRSA
Health Resources and Services Administration
- SHLT
Same-Hospital Liver Transplant
- SHKT
Same-Hospital Kidney Transplant
- OHLT
Other-Hospital Liver Transplant
- OHKT
Other-Hospital Kidney Transplant
- AFT
Accelerated Failure Time
- PH
Proportional Hazards
- TR
Time Ratio
- BMI
Body Mass Index
- MELD
Model for End-Stage Liver Disease
- IQR
Inter-Quartile Range
- DCD
Donation after Cardiac Death
- ECD
extended criteria donors
Footnotes
DISCLOSURES: The authors of this paper do not have any conflicts of interest to disclose.
AUTHORSHIP
Eric KH Chow: participated in data analysis and writing the paper, echow8@jhmi.edu
Sandra DiBrito: participated in writing the paper, dibrito@jhmi.edu
Xun Luo: participated in data analysis, xluo9@jhu.edu
Corey Wickliffe: participated in preparing figures, corey@jhmi.edu
Allan B Massie: participated in statistical methods, amassie1@jhmi.edu
Jayme E Locke: participated in writing the paper, jlocke@uabmc.edu
Sommer E Gentry: participated in writing the paper and research design, gentry@usna.edu
Jacqueline Garonzik-Wang: participated in writing the paper, jgaronz1@jhmi.edu
Dorry L Segev: participated in research design, dorry@jhmi.edu
References
- 1.Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010;10(10):2279–2286. doi: 10.1111/j.1600-6143.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- 2.Quiroga I, McShane P, Koo DD, et al. Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol Dial Transplant. 2006;21(6):1689–1696. doi: 10.1093/ndt/gfl042. [DOI] [PubMed] [Google Scholar]
- 3.Bryan CF, Luger AM, Martinez J, et al. Cold ischemia time: an independent predictor of increased HLA class I antibody production after rejection of a primary cadaveric renal allograft. Transplantation. 2001;71(7):875–879. doi: 10.1097/00007890-200104150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Lee CM, Carter JT, Alfrey EJ, Ascher NL, Roberts JP, Freise CE. Prolonged cold ischemia time obviates the benefits of 0 HLA mismatches in renal transplantation. Arch Surg. 2000;135(9):1016–1019. doi: 10.1001/archsurg.135.9.1016. discussion 1019–1020. [DOI] [PubMed] [Google Scholar]
- 5.Salahudeen AK, Haider N, May W. Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int. 2004;65(2):713–718. doi: 10.1111/j.1523-1755.2004.00416.x. [DOI] [PubMed] [Google Scholar]
- 6.Debout A, Foucher Y, Trebern-Launay K, et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2014 doi: 10.1038/ki.2014.304. [DOI] [PubMed] [Google Scholar]
- 7.Mikhalski D, Wissing KM, Ghisdal L, et al. Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression. Transplantation. 2008;85(7 Suppl):S3–9. doi: 10.1097/TP.0b013e318169c29e. [DOI] [PubMed] [Google Scholar]
- 8.Lee DD, Singh A, Burns JM, Perry DK, Nguyen JH, Taner CB. Early allograft dysfunction in liver transplantation using donation after cardiac death donors results in inferior survival. Liver Transpl. 2014 doi: 10.1002/lt.23985. [DOI] [PubMed] [Google Scholar]
- 9.Nagai S, Yoshida A, Facciuto M, et al. Ischemia time impacts recurrence of hepatocellular carcinoma following liver transplantation. Hepatology. 2014 doi: 10.1002/hep.27358. [DOI] [PubMed] [Google Scholar]
- 10.Massie AB, Chow EK, Wickliffe CE, et al. Early changes in liver distribution following implementation of Share 35. Am J Transplant. 2015;15(3):659–667. doi: 10.1111/ajt.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massie AB, Luo X, Lonze BE, et al. Early Changes in Kidney Distribution under the New Allocation System. Journal of the American Society of Nephrology : JASN. 2016;27(8):2495–2501. doi: 10.1681/ASN.2015080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentry SE, Massie AB, Cheek SW, et al. Addressing geographic disparities in liver transplantation through redistricting. Am J Transplant. 2013;13(8):2052–2058. doi: 10.1111/ajt.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrestha S, Bradbury L, Boal M, et al. Logistical Factors Influencing Cold Ischemia Times in Deceased Donor Kidney Transplants. Transplantation. 2016;100(2):422–428. doi: 10.1097/TP.0000000000000844. [DOI] [PubMed] [Google Scholar]
- 14.Gentry SE, Chow EK, Wickliffe CE, Massie AB, Leighton T, Segev DL. Impact of broader sharing on the transport time for deceased donor livers. Liver Transpl. 2014;20(10):1237–1243. doi: 10.1002/lt.23942. [DOI] [PMC free article] [PubMed] [Google Scholar]