Abstract
A common complication of diabetes is distal symmetric polyneuropathy, which causes symptoms such as numbness, tingling and pain due to sensory axon degeneration. The mechanisms leading to degeneration remain incompletely understood but reactive oxygen species (ROS) that are formed by lipid oxidation are thought to contribute to this condition. In this study, we reassessed the role of ROS in glucose-induced sensory axon degeneration and analyzed candidate downstream targets, which could represent druggable targets. Using a larval zebrafish model for in vivo imaging, we show that glucose treatment induced metabolic changes that resemble a diabetic phenotype. Moreover, we find that glucose treatment induced sensory axon degeneration, which was mediated by ROS formation in the skin, and ROS-dependent MMP-13 activation. Glucose-induced sensory axon degeneration was prevented upon pharmacological inhibition of ROS with the antioxidant, N-acetylcysteine, and upon pharmacological MMP-13 inhibition. We further show that the MMP-13 dependent mechanism of axon degeneration is conserved in mammals. Neuropathy was reversed upon injection of MMP-13 inhibitor into obese mice on a high-fat/high sugar diet. Our findings demonstrate that zebrafish can serve as a model to study glucose-induced neurotoxicity and help identify mechanisms that may be beneficial in the treatment of diabetic neuropathy.
Keywords: hyperglycemia, glucose, diabetes, diabetic neuropathy, peripheral neuropathy, cutaneous sensory axons, zebrafish, axon degeneration, nerve endings, degeneration, sensory, epithelium, NF-κB, ROS, reactive oxygen species, H2O2, hydrogen peroxide, oxidative stress, MMP-13, matrix-metalloproteinase, mice, high-fat diet, epidermis
1. Introduction
Diabetes mellitus afflicts ~28 Million people in the United States and over 300 Million worldwide, a number that is expected to double in the coming decades 1, 2. Diabetes and pre-diabetes are associated with a number of complications, one of which is diabetic peripheral neuropathy. Approximately 50–60% of diabetic patients suffer from neuropathy throughout the course of the disease. The most prevalent type is distal symmetric neuropathy, which affects sensory fibers in the distal extremities, leading to symptoms such as numbness, pain and paresthesia (tingling). Symptoms often initiate in the large toe and progressively move toward more proximal regions 3. The incidence of neuropathy is accelerated under poor glycemic control 4, and often is already prevalent in pre-diabetic patients 5. While the etiology is incompletely understood, findings suggest that oxidative stress and lack of oxygenation underlies this condition. Another contributing factor is the loss of axon regenerative capacity. Regeneration is initially present in diabetic patients following an injury 6, however, progression of the disease reduces axonal regenerative capacity and shifts the balance toward degeneration without the prospect of recovery. So far, evidence suggests that the causes of diabetic neuropathy are multifactorial and hence there are no effective treatments besides pain management 4. Identifying druggable targets to treat diabetic neuropathy would greatly improve the lives of those affected.
Hyperglycemia promotes the production and accumulation of oxidative non-enzymatic end products that can cause tissue damage. These include advanced glycation end products (AGEs), their receptor (RAGE), as well as activating ligands. AGEs can stimulate the release of free radicals, which are thought to promote the development of peripheral neuropathy through glucose autoxidation, changes in the tissue concentrations of low molecular weight antioxidants like glutathione and vitamins A, C and E, or the inability to activate intracellular defense systems that reduce free radicals like catalase and superoxide dismutase (SOD) 4. Besides nervous system-specific oxidation leading to neuronal damage 7, it is possible that additional cell types are involved in neuropathy formation. For instance, we previously demonstrated that the chemotherapeutic agent, paclitaxel, induces oxidative stress in zebrafish keratinocytes, which induces sensory axon degeneration through activation of MMP-13 8. Increased glucose oxidation has also been detected in human epidermal keratinocytes and rodent wounds and is thought to impair wound healing 9. Given that MMP-13 is expressed in wound keratinocytes and stimulated by the small ROS, hydrogen peroxide (H2O2) 10, we hypothesized that H2O2 formation and MMP-13 activation due to increased glucose oxidation in keratinocytes might underlie diabetic neuropathy.
To test this, we have established a larval zebrafish model in which we induced hyperglycemia using glucose treatment. We find that glucose-treated zebrafish show reduced insulin receptor expression, display increased H2O2 levels in the skin and a progressive loss of sensory axons in the caudal fin. Glucose-treated fish moreover upregulate mmp13 expression in a ROS-dependent manner. Strikingly, pharmacological inhibition of ROS or MMP-13 rescued glucose-induced neurotoxicity in zebrafish and diabetic mice. Thus, our findings demonstrate the conservation of neuropathy mechanisms and we provide a new candidate for the treatment of this condition in humans.
2. Materials and Methods
2.1 Zebrafish husbandry
Zebrafish (Nacre, Tuebingen, Tg(isl2b:GFP) 11, Tg(ins:Eco.NfsB-mCherry) 12, and Tg(NF-κB:GFP)) were used. All fish were raised and bred according to NIH guidelines and handled in strict accordance with good animal practices as approved by the appropriate committee (MDI Biological Laboratory animal core IACUC numbers 13-20 and 17-04). Fish were kept on a 14:10hr light/dark cycle at 28.5°C. Embryos and larvae were maintained in Ringers solution (pH 7) throughout the procedures. To minimize suffering, animals were anesthetized in 1:1000 2-Phenoxyethanol, followed by euthanasia using a 1:500 dilution of 2-Phenoxyethanol.
2.2 Chemical treatments
Starting at 2dpf, zebrafish were kept in groups of 3–5 larvae/well in 24-well plates with daily exchanges of solutions to prevent microbial growth. Groups of untreated, 40mM glucose (β-D-glucose, Caymen Chemical), 40mM glucose + 0.05mg/L N-acetyl-L-cysteine (NAC, Caymen Chemical), or 40mM glucose + 10μM DB04760 (MMP-13 inhibitor) (sc-205756, Santa Cruz Biotechnology) were compared. For additional H2O2 detection, larvae were incubated in 4μM pentafluorobenzene sulfonyl-fluorescein (Caymen Chemical) 1–2hrs prior to imaging. All reagents were kept as stock solutions in vehicle and diluted prior to usage. For imaging, larvae were anesthetized in a 1:1000 dilution of 2-Phenoxyethanol. For qPCR, NAC was diluted to 0.1mg/L in Ringers solution and added into the larval media 3hrs prior to RNA isolation.
2.3 Quantitative PCR and RNA stability
Total RNA was purified using a Trizol/Chloroform/Glycogen (ThermoFisher) extraction. Superscript III (Invitrogen) was used for reverse transcription using an equal mix of poly-dT and random hexamer oligonucleotides. Gene expression was normalized to zebrafish S18 RNA and analyzed using the comparative CT Livak method (Livak KJ, Schmittgen TD, 2001) with Brilliant II SYBR® Green qPCR Master Mix (Agilent).
2.4 Transient injections of plasmid DNA
Approximately 40–60pL plasmid diluted in deionized water supplemented with Phenol-red and 100–200pL Tol2 transposase mRNA was injected into early zygotes. Embryos were raised in Ringers solution at 28.5°C.
2.5 Live imaging
Larvae were mounted in 1.2% low-melt agarose as described in 13. Ten to twenty larvae per session were imaged on a FV1000 (Olympus) confocal microscope with motorized stage (20x objective, 0.75NA) using 3μm sections. Stacks were projected into single images and processed in Photoshop. For axon quantifications, fluorescent branches were detected and quantified using the semi-automated Filament function in Imaris 8.1.2 (Bitplane, Switzerland).
2.6 Mouse MMP-13 inhibitor treatments
C57BL6/J male mice at 12–14wks were given a high-fat and high-sugar diet (Research Diets D12450B) for 16wks to induce diabetic neuropathy, according to 14. Hyperglycemia was confirmed with a glucose tolerance test. Briefly, mice were fasted overnight and a glucose bolus injected i.v., followed by measuring glucose levels every 30min for 120min using tail vein blood. Subsequently, animals were randomized and 50% i.p.-injected with either vehicle (N=4) or MMP-13 inhibitor (Tocris CL-82198 hydrochloride, 5mg/kg; N=4) every other day at 11AM for 14d, according to 15. Von Frey testing was used to assess tactile sensitivity in the hind paw pad of mice, as a measure of neuropathy, according to 16, 17. Briefly, 1 day after the last injection, mice were placed on a perforated plastic grid platform in individual compartments and allowed to acclimate with no stimulus for at least 20 minutes. After acclimation, each mouse was subjected to sensory touch testing using 5 Semmes-Weinstein monofilaments of varying strength (4.56, 4.31, 4.08, 3.61, 2.36) each corresponding to a specific target force (4, 2, 1, 0.4, 0.02 – grams of force, respectively). In order of decreasing severity, the tip of each monofilament was placed through perforations on the grid platform at a right angle to the mid-plantar surface of the hind paw; slight pressure was applied until the mouse showed a response or the monofilament bent. Mouse response was categorized as either positive (immediate removal of paw), neutral (delayed paw removal), or negative (no reaction to monofilament stimulus). This was repeated 5 times with a 5-minute no stimulus interval. Total number of categorized retractions was recorded for each filament size and quantified.
2.7 Statistical analyses
Statistical comparisons were made using Prism7 (GraphPad). Unpaired Student’s t-test with a 95% confidence interval was used to compare the means of two unmatched groups, assuming that the values followed a Gaussian distribution. For multiple comparison tests of three or more groups, one-way ANOVA at an alpha=0.05 (95% confidence interval) and Tukey’s multiple comparisons post-test was used. Significance is denoted with asterisks: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. The standard error of the mean (s.e.m.) is shown, reflecting multiple biological replicates.
3. Results
3.1 Glucose treatment induces neurotoxicity
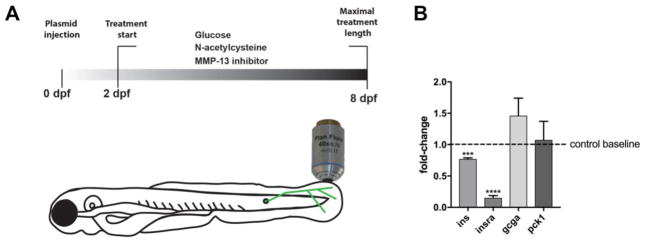
We used glucose supplementation in the media to induce hyperglycemia in larval zebrafish, based on previous reports 18. To validate this method, we incubated larval fish for 6d (2–8dpf) in glucose (Fig. 1a) and subsequently performed qPCR to assess the effects on glucose metabolism. This analysis showed that insulin and insulin receptor levels were reduced, as expected. Glucagon expression was increased, indicative of increased gluconeogenesis. The expression levels however varied substantially between fish (Fig. 1b). PCK1 overexpression in rodents has been associated with the development of hyperglycemia 19, 20, but we did not observe a change in pck1 expression in our zebrafish model.
Figure 1. Glucose treatment influences glucose metabolism in larval zebrafish.
(a) Treatment scheme of larval zebrafish. Treatments with glucose were performed between 2 and 8dpf for lengths indicated in the text. Larvae either remained untreated or 40mM glucose was added to the media, followed by confocal imaging of GFP-expressing sensory axons innervating the caudal fin. Additional treatments were performed with N-acetylcysteine and MMP-13 inhibitor, DB04760.
(b) Quantitative PCR shows downregulation of ins and insra in animals treated with 40mM glucose for 6 days compared to untreated controls (dotted line depicts control baseline levels; pools of ~20–30 larvae were used per group in 2 biological replicates). ins and insra expression is significantly reduced but gcga and pck1 expression does not significantly change.
Student’s t-test was used for comparisons of each group to the untreated control group. The standard error of the mean is shown for all bars. ****p<0.001, p****<0.0001, ns= not significant
Abbreviations: dpf: days post fertilization, ins: insulin, insra: insulin receptor, gcga: glucagon a, pck1: phosphoenolpyruvate carboxykinase 1
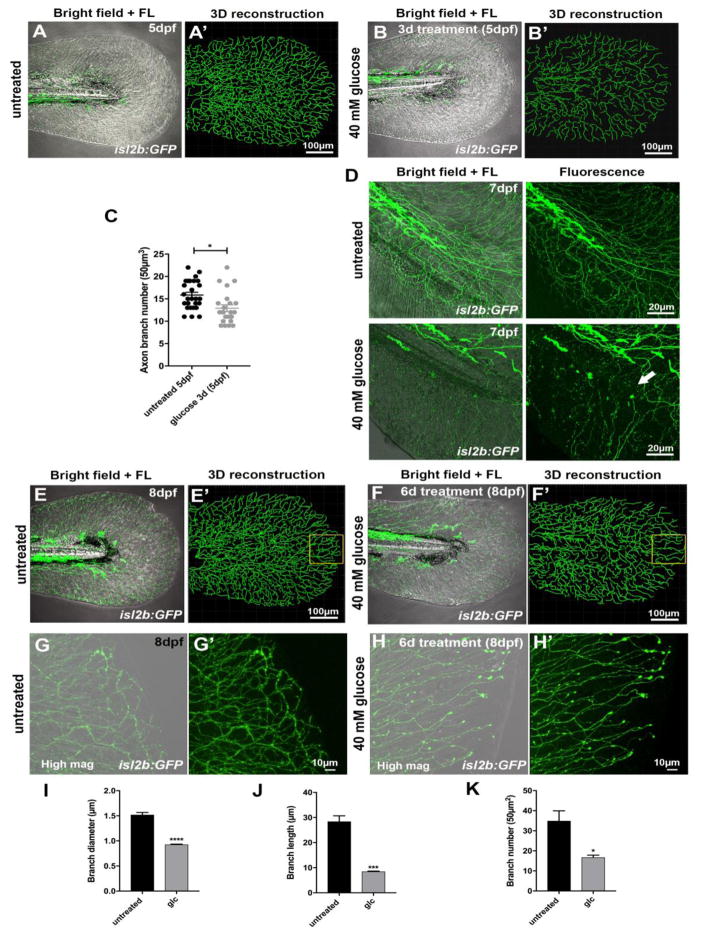
To analyze whether glucose treatment damages sensory axons, we supplemented the water of Tg(isl2b:GFP) 11 animals in which somatosensory axons are fluorescently labeled, with 40mM glucose (between 2–8dpf) and subsequently analyzed axon degeneration in the caudal fin. Since the sensory axon network in this transgenic line is very dense, we used semi-automated analyses and 3D reconstruction to analyze axon branch numbers. About 40–50% of animals developed significant axon loss after 3 days of treatment (5dpf)(Fig. 2a–c). After 5 days (7dpf), axon debris was present in some areas of the fin, which we did not observe in untreated controls (Fig. 2d). After 6 days of glucose treatment, most animals showed a significant decrease in axon branch diameter, number, and length when compared with age-matched untreated control animals (Fig. 2e–k). This demonstrates that glucose treatment induces sensory neurotoxicity in larval zebrafish.
Figure 2. Glucose treatment induces neurotoxicity.
(a, a′) Caudal fin of a transgenic Tg(isl2b:GFP) zebrafish larva at 5dpf (a, merged bright field and fluorescence images) and 3D reconstruction of GFP-labeled sensory axons using Imaris (Bitplane) software (a′).
(b, b′) Caudal fin of an isl2b:GFP transgenic fish at 5dpf (b, merged bright field and fluorescence images) treated with 40mM glucose for 3 days. 3D reconstruction (b′) of GFP-labeled sensory axons shows reduced axon branch numbers compared with untreated controls (a′) at 5dpf.
(c) Quantification of (a, b) shows a significant reduction in axon branch number following glucose treatment compared with untreated controls (n=3 biological replicates, ≥5 animals each).
(d) Caudal fin of Tg(isl2b:GFP) zebrafish larvae at 7dpf either untreated (upper panels) or after treatment with glucose (lower panels) for 5 days. Sensory axon debris is visible after glucose treatment (arrow).
(e, f) Caudal fin of untreated Tg(isl2b:GFP) larva (e, e′) and larva treated with 40mM glucose for 6 days (2–8dpf) (f, f′). Left panels show merged bright field images. Right panels in each figure show 3D reconstructions of GFP-labeled sensory axons. The yellow box depicts decreased axon branches, which are most evident in the distal caudal fin following glucose treatment.
(g, h) High magnification images of sensory axons in the distal caudal fin of untreated control
(g, g′) and glucose-treated fish (h, h′), the latter showing fewer axon branches.
(i–k) Quantification of axon branch diameter (i), branch length (j), and branch number (k) in a 50μm2 region of the medial distal caudal fin (yellow box in e′, f′) compared between untreated control and glucose-treated fish at 8dpf.
Student’s t-test was used for comparing the treatment groups to the respective untreated control group. The standard error of the mean is shown in each graph. *p<0.05, ***p<0.001, p****<0.0001
Abbreviations: d: days, dpf: days post fertilization, FL: fluorescence, glc: glucose, mag: magnification
3.2 NF-κB activation in glucose-treated fish
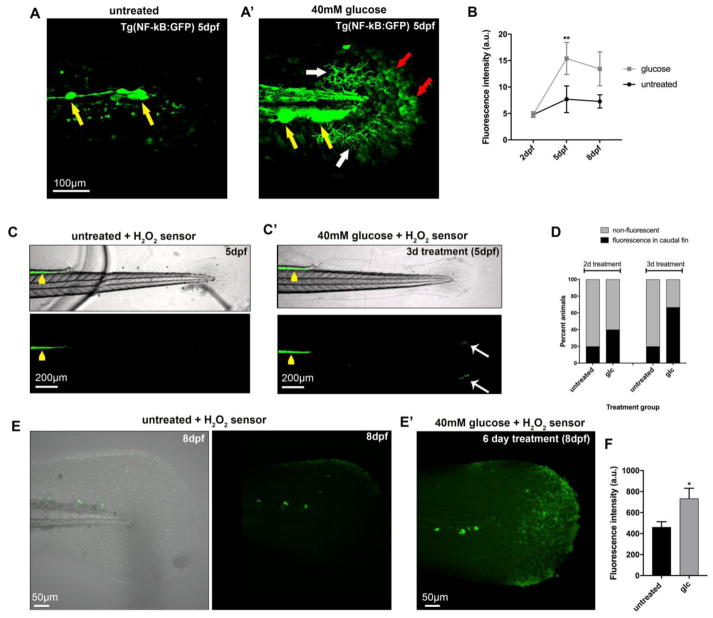
The NF-κB stress response pathway was originally identified in B-lymphocytes but has subsequently been described in various other cell types, including keratinocytes. This pathway has also a role in the pathogenesis of diabetic neuropathy 21 where activation of NF-κB through RAGE leads to pro-inflammatory cytokine release that has been implicated in the establishment of diabetic polyneuropathies 22. We therefore assessed NF-κB signaling in response to glucose treatment using a reporter line in which GFP expression is activated upon NF-κB binding to specific promoter elements 23. Treatment of larval fish with glucose for 3 days significantly increased GFP expression within keratinocytes and mesenchymal cells of the caudal fin (Fig. 3a, b), suggesting that glucose-dependent activation of NF-κB is also stimulated in zebrafish, similar to mammals. The conservation of known molecular components induced by glucose treatment further supports the idea that zebrafish can serve as a model to study glucose-induced neurotoxicity.
Figure 3. Increased oxidative stress and NF-κB activity in the caudal fin after glucose treatment.
(a, a′) Control (a) and glucose-treated (a′) transgenic Tg(NF-κB:GFP) larvae at 5dpf display fluorescence in the lateral line neuromasts (yellow arrows). Additional fluorescence can be observed after 3 days of glucose treatment in mesenchymal cells (white arrows, a′) and keratinocytes (red arrows, a′).
(B) Fluorescence intensity measurements in keratinocytes of the distal caudal fin in untreated and glucose-treated (40mM) Tg(NF-κB:GFP) fish at 2dpf (before treatment), 5dpf (3-day treatment), and 8dpf (6-day treatment) reveals increased NF-κB activity in the glucose-treated group (n=5 animals per group using identical imaging settings).
(c, c′) Zebrafish larvae at 5dpf treated with the chemical H2O2-selective sensor pentafluorobenzene sulfonyl-fluorescein 2hr prior to imaging show increased oxidation (green fluorescence) in the intestinal tract (yellow arrowheads). The glucose-treated larva (c′) displays additional fluorescence in the distal caudal fin (white arrows), indicative of skin injury (compare with bright field image).
(d) Quantification of the percentage of animals with fluorescence in the distal fin as shown in (c, c′) reveals that more animals fluoresce following glucose treatment (5 animals per group imaged with identical settings).
(e, e′) Shown is the caudal fin of an untreated (e) and glucose-treated (e′) zebrafish larva at 8dpf in which H2O2 is detected with pentafluorobenzene sulfonyl-fluorescein. Increased H2O2 levels are detected after 6 days of glucose treatment.
(f) Quantification of H2O2 fluorescence as shown in (e) reveals increased levels in the glucose-treated group compared with untreated control animals (6 animals per group imaged under identical conditions).
One-Way ANOVA with Tukey’s multiple comparisons test (b) and Student’s t-test (f) was used to compare the treatment groups with the respective untreated control group. Standard errors of the mean are shown. *p<0.05, **p<0.01
Abbreviations: a.u.: arbitrary units, dpf: days post fertilization, glc: glucose
3.3 Glucose treatment increases reactive oxygen species formation in the skin
Increased blood glucose levels lead to oxidation and generation of ROS, which can induce cellular damage and are thought to contribute to diabetic neuropathy 24. To test whether glucose also induces ROS/H2O2 in zebrafish, which may contribute to axon degeneration, we treated larvae with 40mM glucose for either 2 or 3 days (4 and 5dpf, respectively) and detected H2O2 with the selective chemical sensor 25, pentafluorobenzene sulfonyl-fluorescein. This sensor is normally non-fluorescent but fluoresces upon perhydrolysis of the sulfonyl linkage by H2O2. Following treatment, we detected oxidation in defined regions of the distal caudal fin in a subset of animals (40–60%)(Fig. 3c, d). These H2O2-positive regions were primarily present at the fin edges and likely caused by injuries. We previously showed that the distal fin is especially prone to injury formation 8, which could be related to decreased tissue thickness and/or increased exposure to mechanical stress of this region. Prolonged treatment with glucose for up to 6 days, in contrast, induced widespread H2O2 production in the caudal fin, which was not observed in untreated animals (Fig. 3e, f). These findings indicate that glucose treatment induces oxidative stress formation and injuries in regions that are prone to mechanical stress, such as the distal fin, similar as seen after paclitaxel treatment 8.
3.4 ROS and MMP-13 inhibition improves glucose-induced neurotoxicity
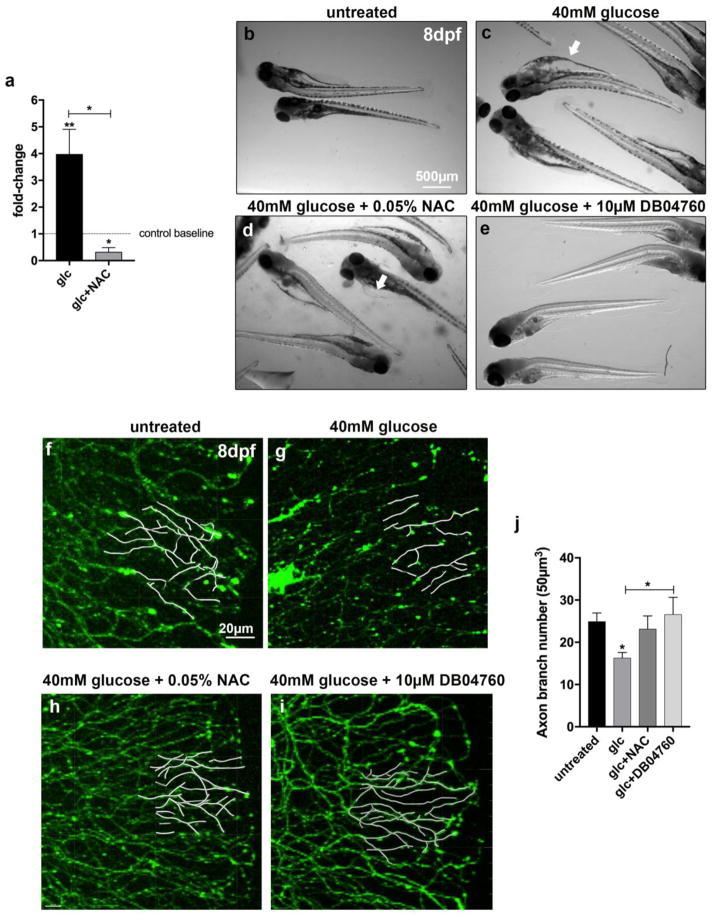
Glucose-dependent oxidative stress formation has been detected in mammalian keratinocytes 9, 26, and we previously showed in zebrafish that treatment with exogenous H2O2 induces mmp13 expression 10. We therefore asked whether also glucose-induced endogenous ROS/H2O2 induces mmp13 expression, and whether increased MMP-13 activity could underlie sensory axon degeneration, as it does in paclitaxel-treated animals 8. Quantification via qPCR following 6 days of glucose treatment (2–8dpf) showed that mmp13a expression, the zebrafish homolog of mammalian MMP13 (Fig. 4a), was significantly upregulated when compared with untreated controls. Co-administration of NAC, in contrast, led to mmp13a downregulation (Fig. 4a), suggesting that mmp13a expression is regulated by glucose-dependent H2O2 formation. Next, we pharmacologically inhibited ROS/H2O2 with N-acetylcysteine (NAC) and MMP-13 inhibitor (DB04760) in the presence of glucose over the course of 6 days (2–8dpf) to assess its effects on axons. First, we determined whether the inhibitors are toxic to zebrafish larvae in the presence of glucose. While treatment with glucose alone promoted the formation of cardiac edema and general unhealthiness of larval fish compared with untreated controls (Fig. 4b, c), NAC co-administration rescued these defects to some extent (Fig. 4d). Strikingly, co-treatment of larvae with glucose and DB04760 fully rescued the glucose-induced phenotypes (Fig. 5e). To further determine the effects of these inhibitors on sensory axons, we analyzed transgenic Tg(isl2b:GFP) animals in which sensory axons are fluorescently labeled. Unlike glucose treatment, which significantly reduced the number of sensory axon branches quantified in the distal caudal fin, NAC and DB04760 prevented glucose-induced neurotoxicity (Fig. 4f–j). Thus, ROS/H2O2 and MMP-13 appear to be downstream effectors of glucose and play a role in sensory axon degeneration.
Figure 4. Inhibition of ROS and MMP-13 prevents glucose-induced toxicity.
(a) Quantitative PCR comparing mmp13a expression in larval fish treated for 6 days either with glucose (40mM) or glucose (40mM) plus the antioxidant N-acetyl-L-cysteine (NAC, 0.1mg/L) shows that mmp13a expression is significantly upregulated after glucose treatment and downregulated when NAC is co-administered. Asterisks depict comparisons of each group to the control group (control baseline) and brackets indicate comparisons between groups.
(b–e) General toxicity assessment at 8dpf in larval fish either untreated (b) or treated for 6 days with glucose (40mM) (c), glucose plus NAC (0.05mg/L) (d), or glucose plus MMP-13 inhibitor, DB04760 (10μM) (e). Glucose treatment induces cardiac edema (arrow in c), which is partially rescued upon co-administration of NAC (d), and fully rescued upon co-administration of the MMP-13 inhibitor, DB04760 (e).
(f–i) Partial tracings of sensory axons in a 50μm2 area in the distal caudal fin of zebrafish larvae at 8dpf, superimposed on fluorescence images. Larvae are shown untreated (f), and treated for 6 days with either glucose (g), glucose plus NAC (h), or glucose plus DB04760. Glucose induces a loss of axons, which is rescued upon co-administration of NAC or MMP-13 inhibitor.
(j) Quantification of (f–i).
Student’s t-test (a) and One-Way ANOVA with Tukey’s multiple comparisons test (j) was used to compare the treatment groups to the respective untreated control group. Brackets indicate alternative comparisons. The standard error of the mean is shown. *p<0.05, **p<0.01
Abbreviations: Glc: glucose, NAC: N-acetyl-L-cysteine
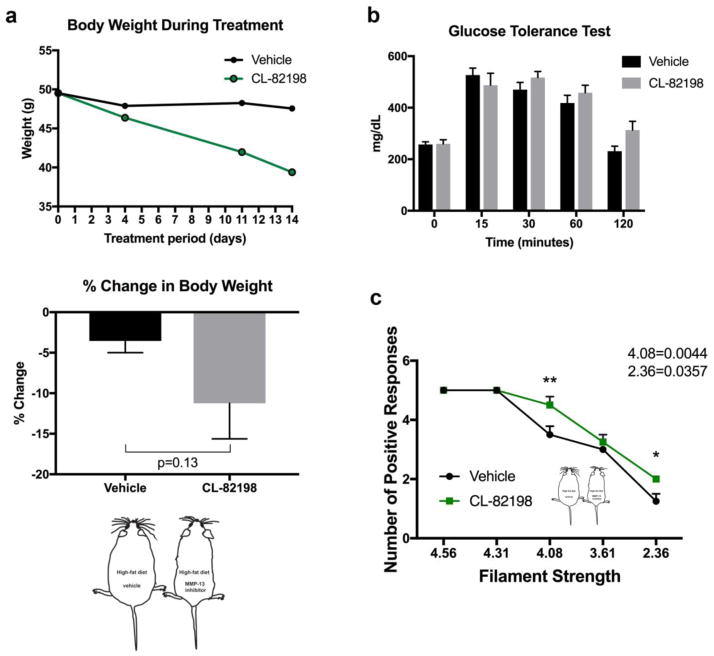
Figure 5. MMP-13 inhibition rescues neuropathy in mice.
(a) Median weight of mice on a high-fat/high-sugar diet over the 14-day treatment period with either vehicle or MMP-13 inhibitor (CL-82198) shows a reduction in body weight (upper graph, n=4 animals per group). The mean body weight of mice on a high fat/high sugar diet following the last injection is slightly but not significantly decreased at the end of the treatment period with vehicle or MMP-13 inhibitor (CL-82198) (lower graph).
(b) Glucose levels measured every 30 minutes following overnight fasting and intravenous injection of a glucose bolus shows that vehicle control mice and mice injected with CL-82198 lack glucose clearance capacity.
(c) Von Frey assessment after injections with either vehicle or CL-82198 for 14 days shows that CL-82198 administration significantly rescues the reduced tactile sensitivity (touch response) for the 4.08 and 2.36 filaments following a high fat/high sugar diet.
Student’s t-test and One-Way ANOVA with Bonferroni’s multiple comparisons test was used to compare the treatment groups to the respective control groups. *p<0.05, **p<0.01
3.5 MMP-13 inhibition improves diabetic neuropathy mice
To analyze whether the function of MMP-13 in diabetic neuropathy is conserved in mice, we fed mice with a high-fat/high-sugar diet, similar to 27. Groups of hyperglycemic mice were subsequently injected with either vehicle or the MMP-13 inhibitor, CL-82198 15. We previously demonstrated identical effects for DB04760 and CL-82198 in paclitaxel-induced neurotoxicity in zebrafish 8 and used CL-82198 in this study due to its common usage in other mouse models and effectiveness at 5mg/kg 8. Inhibition of MMP-13 resulted in a trend toward body weight reduction during the 14-day treatment period, which was however not significant (Fig. 5a). Glucose tolerance testing showed that the hyperglycemic state was preserved in both groups (Fig. 5b). Nevertheless, tactile sensitivity was significantly improved upon co-administration of the MMP-13 inhibitor, as measured using von Frey filaments (Fig. 5c). These findings suggest that MMP-13 inhibition reverses neuropathy symptoms but does not influence the diabetic state of animals. These findings are consistent with MMP-13 being expected to be a downstream target of glucose toxicity. Thus, inhibition of MMP-13 represents a potential treatment for neuropathy in diabetic patients.
4. Discussion
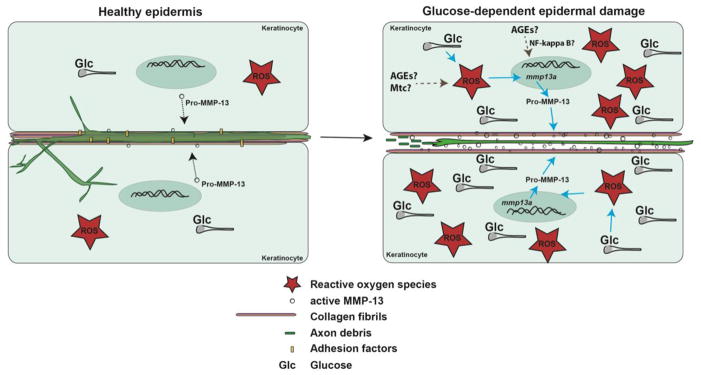
We developed a zebrafish model with which to study glucose-induced neurotoxicity in live animals using in vivo imaging. We show that glucose treatment induces sensory axon loss in the zebrafish caudal fin, similar to loss of axons in the distal extremities of diabetic patients. The neurotoxic effects of glucose can be explained by glucose-dependent increased ROS formation in the skin, which stimulates MMP-13 expression and renders the epidermis susceptible to mechanical stress (consistent with the formation of injuries in the distal caudal fin). Since unmyelinated axons are embedded in the matrix of the basal epidermis, damage to the matrix likely renders axons vulnerable to degeneration (Fig. 6). Given our finding that MMP-13 inhibition can prevent axon degeneration, this metalloproteinase may be a possible target in the treatment of diabetic neuropathy in humans. Additional studies are required to assess its precise functions in the stimulation of axon regeneration.
Figure 6. Model of glucose-induced neurotoxicity.
Somatosensory axons traverse the basement membrane and nerve endings are embedded in the matrix between epidermal keratinocytes. Under homeostatic conditions, low levels of ROS and glucose are present to maintain cellular functions and molecular flow between axons and keratinocytes. Under high glucose conditions, increased ROS formation (possibly emanating from mitochondria and involving AGEs) leads to upregulation of MMP-13 (possible involvement of AGEs and NF-κB), which promotes enhanced matrix remodeling and ECM degradation. This damages sensory axons and leads to their degeneration.
Abbreviations: Glc: glucose, ROS: reactive oxygen species, AGEs: Advanced glycation end products, Mtc: mitochondria
Overall our findings suggest that zebrafish are valuable for studies of glucose neurotoxicity since the larval fish are optically clear and thus amenable for in vivo imaging, which is powerful to study the progression of this condition in live animals. Moreover, zebrafish display similarities in insulin metabolism and the expression of insulin pathway genes 18. For instance, glucose treatment led to reduced insulin receptor expression, a hallmark of type 2 diabetes in humans, and also show an increase in NF-κB activity, as has been reported in mammals. Since zebrafish and diabetic mice both show improvements in neuropathy upon MMP-13 inhibitor administration, it suggests that the mechanisms of this diabetic complication are also conserved.
Diabetic neuropathy most commonly manifests as distal symmetrical neuropathy, which initiates in sensory fibers of the toes and progresses proximally toward the leg in a stocking-glove distribution 3. Severe cases also involve the upper extremities with similar progression from fingers toward the proximal arm. In humans, poor glycemic control and genetic predisposition are thought to underlie variations in the severity among individuals. In zebrafish, we also noticed differences among animals, even when derived from the same genetic background. Similar variations in the development of hyperglycemia have been observed in adult zebrafish treated with streptozotocin 28. A factor causing this variance in larval fish could be their dependence on the yolk until around 6–8dpf, which may lead to differences in glucose requirements. A more likely reason however could be the influence of mechanical stress in the development of axon degeneration, whereby increased MMP-13 activity renders the skin less resistant to mechanical stress given the role of this collagenase in the degradation of matrix molecules. This would most often manifest in areas of the body that are more susceptible to damage, such as the caudal fin edge in zebrafish and the palms and soles in humans. This idea is consistent with our observation that short-term treatment with glucose increased the prevalence of injuries in this region. We made similar observations in our paclitaxel model 8 and showed that inhibition of MMP-13 rescued injury-induced skin damage as well as axon degeneration in the caudal fin. The mechanical stress theory could also explain the variation in onset and severity among patients. Mechanical stress in hands and feet could induce subtle changes in the epidermal environment that ultimately manifest in axon degeneration and in more severe cases, it may lead to wound healing defects.
Glucose-dependent oxidation and ROS formation is a hallmark of diabetes and studies have linked this process to neuropathy. Oxidative stress has been found in peripheral nerves, DRG neurons, sympathetic neurons, and the vasculature surrounding the nerves. It is thought that increased oxidative stress in these tissues may contribute to reduced blood flow and nerve conduction velocities, as well as decreased neurotrophic support and signal transduction 29. Surprisingly, no study thus far has investigated whether glucose-dependent oxidation in the epidermis contributes to axon degeneration despite that basal epidermal keratinocytes establish direct interactions with unmyelinated nerve endings. In our previous studies, we found that MMP-13 inhibition prevented paclitaxel-induced sensory axon degeneration, comparable to our glucose studies presented here, suggesting that MMP-13 might also be underlying various other sensory neuropathies, which remains to be determined.
Also antioxidants represent potential treatments for diabetic neuropathy since they were found to be largely protective against various other diabetic complications. For instance, NAC protected streptozotocin-induced hyperglycemic rats from cardiomyocyte death 30. And clinical studies in which the antioxidants, vitamins C and E were administered orally in diabetic patients with microvascular disease found an overall improvement of various oxidative stress parameters and an improved ocular surface milieu 31. Improvements with antioxidants have been mostly observed when ingested from plant sources. Vitamins such as vitamin A, C, and E, as well α-lipoic acid, coenzyme Q10, several bioflavonoids, cofactors (folic acid, vitamins B1, B2, B6, B12), glutamine, glutathione, and antioxidant minerals (copper, zinc, selenium, and manganese) seem to be less effective when administered orally 32. Despite some of these positive outcomes, studies have also reported side effects of antioxidant treatments 4. Our results in zebrafish also suggest sub-optimal treatment benefits with NAC. Although NAC prevented axon degeneration, it rescued glucose-induced heart edema only in some animals. Because of the observed variability of antioxidants also in humans, no clinical recommendations have been established thus far. A major problem could be that H2O2 functions as a signaling molecule under homeostatic conditions, and hormetic concentrations of this ROS may be difficult to restore with antioxidant treatment. Known downstream targets of H2O2, such as MMP-13 may therefore be more effective as neuropathy treatment. MMP-13 inhibition with CL-82198 has been tested in various animal models and shows low toxicity and improvement of conditions such as osteoarthritis 15 and lung injury 33. Moreover, our studies in zebrafish suggest that treatment with the MMP-13 inhibitor, DB04760, also significantly improved the health of larval fish in the presence of high glucose. The low toxicity effects of MMP-13 inhibitors may be due to a redundancy of MMPs in various tissues. For instance, we showed that H2O2 upregulates both MMP-9 and 13 10, and blocking the activity of one MMP may be sufficient to restore skin homeostasis without interrupting processes that require some MMP activity.
Upstream regulators of H2O2 might also be potential targets. For instance, H2O2 formation in keratinocytes is dependent on the accumulation of advanced glycation end products (AGEs). Intriguingly, AGEs have been shown to activate NF-κB in vascular smooth muscle cells, leading to diabetic vascular disease 22. NF-κB expression by AGEs has also been observed in peripheral nerves of diabetic mice, where it has been suggested to be a relevant disease mechanism in diabetic polyneuropathies 22. Further studies are required to assess a potential link between AGEs, NF-κB and MMP-13 within keratinocytes. AGEs have been shown to promote MMP-13 expression in an NF-κB dependent manner in chondrocytes 34, a mechanism that could be conserved in the epidermis and promote diabetic neuropathy.
5. Conclusions
Diabetic neuropathy is non-reversible and patients suffer from various complications. Because treatments are unavailable it is imperative to define the underlying mechanisms. This study investigated the role of oxidative stress and MMP-13 in glucose-induced somatosensory axon degeneration and found that both play a role in this process. We previously reported that MMP-13 activity also underlies paclitaxel(chemotherapy)-induced peripheral neuropathy where it is upregulated specifically in the epidermis. The epidermis is innervated by unmyelinated sensory fibers, suggesting that perturbations in the interactions between nerve endings and epidermal keratinocytes stimulates axon degeneration. The involvement of MMP-13 in both neuropathies and its conservation in mice indicates that the mechanism may be conserved among sensory neuropathies and translate into humans. Therefore, selective MMP-13 inhibitor applications to the epidermis may be a valuable treatment option for diabetic neuropathy.
Highlights.
Diabetic neuropathy is non-reversible and patients suffer from various complications. This study investigated the role of oxidative stress and MMP-13 in glucose-induced somatosensory axon degeneration. Using zebrafishin vivo imaging, we show that glucose treatment induces epidermis-specific hydrogen peroxide formation and upregulation of the matrix-degrading enzyme, MMP-13. Antioxidant treatment was sufficient to prevent MMP-13 upregulation. Antioxidant treatment and MMP-13 inhibition furthermore prevented glucose-induced sensory axon degeneration. In mice fed on a high-fat/high sugar diet, MMP-13 inhibition also restored tactile sensitivity, suggesting the conservation of MMP-13 function in mammals.
Acknowledgments
We thank Chi-Bin Chien and John Rawls (Duke University) for providing transgenic lines and reagents. We thank Novartis for providing intellectual input into the establishment of the diabetic peripheral neuropathy model. We also thank the zebrafish facility staff for their support.
Author contributions: S.R. designed the zebrafish studies, analyzed the data and wrote the paper. A.L.W., P.A.S., K.L.B., E.A.B., J.K.B., J.L.M., and A.D.P. performed the zebrafish experiments and edited the manuscript. A.L.D., and M.B. performed the mouse experiments. K.L.T. provided experimental guidance for the mouse studies and edited the manuscript. The authors do not declare any conflicts of interest. Dr. Sandra Rieger is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Research reported in this publication was supported by a Diabetic Complications Consortium grant under grant number DCC Pilot & Feasibility project, 12GHSU179, and institutional Development Awards (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant numbers P20GM0103423 and P20GM104318. K.L.B. was recipient of an INBRE undergraduate student fellowship with grant number P20GM0103423.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119(5 Suppl 1):S10–16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aristidis Veves M, DSc, John M, Giurini D, Frank W, LoGerfo M. The Diabetic Foot: Medical and surgical management. Humana Press, Inc; 2002. [Google Scholar]
- 4.Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7(10):573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- 5.Lee CC, Perkins BA, Kayaniyil S, et al. Peripheral Neuropathy and Nerve Dysfunction in Individuals at High Risk for Type 2 Diabetes: The PROMISE Cohort. Diabetes Care. 2015;38(5):793–800. doi: 10.2337/dc14-2585. [DOI] [PubMed] [Google Scholar]
- 6.Apfel SC. Nerve regeneration in diabetic neuropathy. Diabetes Obes Metab. 1999;1(1):3–11. doi: 10.1046/j.1463-1326.1999.00006.x. [DOI] [PubMed] [Google Scholar]
- 7.Andrés RC, Helena BC, Juliana PP, Viviana AM, Margarita GB, Marisa CG. Diabetes-related neurological implications and pharmacogenomics. Curr Pharm Des. 2017 doi: 10.2174/1381612823666170317165350. [DOI] [PubMed] [Google Scholar]
- 8.Lisse TS, Middleton LJ, Pellegrini AD, et al. Paclitaxel-induced epithelial damage and ectopic MMP-13 expression promotes neurotoxicity in zebrafish. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1525096113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan CC, Wu CS, Huang SM, Wu IH, Chen GS. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: new insights into impaired diabetic wound healing. Diabetes. 2013;62(7):2530–2538. doi: 10.2337/db12-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisse TS, King BL, Rieger S. Comparative transcriptomic profiling of hydrogen peroxide signaling networks in zebrafish and human keratinocytes: Implications toward conservation, migration and wound healing. Sci Rep. 2016;6:20328. doi: 10.1038/srep20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittman AJ, Law MY, Chien CB. Pathfinding in a large vertebrate axon tract: isotypic interactions guide retinotectal axons at multiple choice points. Development. 2008;135(17):2865–2871. doi: 10.1242/dev.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curado S, Stainier DY, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc. 2008;3(6):948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisse TS, Brochu EA, Rieger S. Capturing Tissue Repair in Zebrafish Larvae with Time-lapse Brightfield Stereomicroscopy. J Vis Exp. 2015;(95) doi: 10.3791/52654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obrosova IG, Ilnytska O, Lyzogubov VV, et al. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56(10):2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- 15.Wang M, Sampson ER, Jin H, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15(1):R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biessels GJ, Bril V, Calcutt NA, et al. Phenotyping animal models of diabetic neuropathy: a consensus statement of the diabetic neuropathy study group of the EASD (Neurodiab) J Peripher Nerv Syst. 2014;19(2):77–87. doi: 10.1111/jns5.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guilford BL, Ryals JM, Wright DE. Phenotypic changes in diabetic neuropathy induced by a high-fat diet in diabetic C57BL/6 mice. Exp Diabetes Res. 2011;2011:848307. doi: 10.1155/2011/848307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elo B, Villano CM, Govorko D, White LA. Larval zebrafish as a model for glucose metabolism: expression of phosphoenolpyruvate carboxykinase as a marker for exposure to anti-diabetic compounds. J Mol Endocrinol. 2007;38(4):433–440. doi: 10.1677/JME-06-0037. [DOI] [PubMed] [Google Scholar]
- 19.Valera A, Pujol A, Pelegrin M, Bosch F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1994;91(19):9151–9154. doi: 10.1073/pnas.91.19.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Liu S, Ferguson S, et al. Phosphoenolpyruvate carboxykinase overexpression selectively attenuates insulin signaling and hepatic insulin sensitivity in transgenic mice. J Biol Chem. 2002;277(26):23301–23307. doi: 10.1074/jbc.M200964200. [DOI] [PubMed] [Google Scholar]
- 21.Negi G, Kumar A, Sharma SS. Nrf2 and NF-κB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr Neurovasc Res. 2011;8(4):294–304. doi: 10.2174/156720211798120972. [DOI] [PubMed] [Google Scholar]
- 22.Patel S, Santani D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol Rep. 2009;61(4):595–603. doi: 10.1016/s1734-1140(09)70111-2. [DOI] [PubMed] [Google Scholar]
- 23.Kanther M, Sun X, Muhlbauer M, et al. Microbial colonization induces dynamic temporal and spatial patterns of NF-kappaB activation in the zebrafish digestive tract. Gastroenterology. 2011;141(1):197–207. doi: 10.1053/j.gastro.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Román-Pintos LM, Villegas-Rivera G, Rodríguez-Carrizalez AD, Miranda-Díaz AG, Cardona-Muñoz EG. Diabetic Polyneuropathy in Type 2 Diabetes Mellitus: Inflammation, Oxidative Stress, and Mitochondrial Function. J Diabetes Res. 2016;2016:3425617. doi: 10.1155/2016/3425617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda H, Fukuyasu Y, Yoshida S, et al. Fluorescent probes for hydrogen peroxide based on a non-oxidative mechanism. Angew Chem Int Ed Engl. 2004;43(18):2389–2391. doi: 10.1002/anie.200452381. [DOI] [PubMed] [Google Scholar]
- 26.Negi G, Kumar A, Joshi RP, Sharma SS. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem Biophys Res Commun. 2011;408(1):1–5. doi: 10.1016/j.bbrc.2011.03.087. [DOI] [PubMed] [Google Scholar]
- 27.Russell JW, Berent-Spillson A, Vincent AM, Freimann CL, Sullivan KA, Feldman EL. Oxidative injury and neuropathy in diabetes and impaired glucose tolerance. Neurobiol Dis. 2008;30(3):420–429. doi: 10.1016/j.nbd.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen AS, Sarras MP, Intine RV. Limb regeneration is impaired in an adult zebrafish model of diabetes mellitus. Wound Repair Regen. 2010;18(5):532–542. doi: 10.1111/j.1524-475X.2010.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22(4):257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- 30.Fiordaliso F, Bianchi R, Staszewsky L, et al. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J Mol Cell Cardiol. 2004;37(5):959–968. doi: 10.1016/j.yjmcc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Peponis V, Papathanasiou M, Kapranou A, et al. Protective role of oral antioxidant supplementation in ocular surface of diabetic patients. Br J Ophthalmol. 2002;86(12):1369–1373. doi: 10.1136/bjo.86.12.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahimi-Madiseh M, Malekpour-Tehrani A, Bahmani M, Rafieian-Kopaei M. The research and development on the antioxidants in prevention of diabetic complications. Asian Pac J Trop Med. 2016;9(9):825–831. doi: 10.1016/j.apjtm.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Wohlauer M, Moore EE, Silliman CC, et al. Nebulized hypertonic saline attenuates acute lung injury following trauma and hemorrhagic shock via inhibition of matrix metalloproteinase-13. Crit Care Med. 2012;40(9):2647–2653. doi: 10.1097/CCM.0b013e3182592006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang HB, Zhang Y, Chen C, Li YQ, Ma C, Wang ZJ. Pioglitazone inhibits advanced glycation end product-induced matrix metalloproteinases and apoptosis by suppressing the activation of MAPK and NF-κB. Apoptosis. 2016;21(10):1082–1093. doi: 10.1007/s10495-016-1280-z. [DOI] [PubMed] [Google Scholar]