Abstract
Objectives
The aim of this study was to identify whether the three main primary progressive aphasia (PPA) variants would show differential profiles on measures of visuospatial cognition. We hypothesized that the logopenic variant would have the most difficulty across tasks requiring visuospatial and visual memory abilities.
Methods
PPA patients (n = 156), diagnosed using current criteria, and controls were tested on a battery of tests tapping different aspects of visuospatial cognition. We compared the groups on an overall visuospatial factor; construction, immediate recall, delayed recall, and executive functioning composites; and on individual tests. Cross-sectional and longitudinal comparisons were made, adjusted for disease severity, age, and education.
Results
The logopenic variant had significantly lower scores on the visuospatial factor and the most impaired scores on all composites. The nonfluent variant had significant difficulty on all visuospatial composites except the delayed recall, which differentiated them from the logopenic variant. In contrast, the semantic variants performed poorly only on delayed recall of visual information. The logopenic and nonfluent variants showed decline in figure copying performance over time, whereas in the semantic variant, this skill was remarkably preserved.
Conclusions
This extensive examination of performance on visuospatial tasks in the PPA variants solidifies some previous findings, for example, delayed recall of visual stimuli adds value in differential diagnosis between logopenic variant PPA and nonfluent variant PPA variants, and illuminates the possibility of common mechanisms that underlie both linguistic and non-linguistic deficits in the variants. Furthermore, this is the first study that has investigated visuospatial functioning over time in the PPA variants.
Keywords: Frontotemporal dementia, Alzheimer disease, Language, Neuropsychological tests, Mental processes, Spatial processing
INTRODUCTION
Primary progressive aphasia (PPA) is a clinical syndrome characterized by progressive decline in speech or language abilities over time and occurs due to neurodegenerative disease (Mesulam & Weintraub, 1992). Three main variants of PPA are recognized: a semantic variant (svPPA), a nonfluent/agrammatic variant (nfvPPA), and a logopenic (“word poverty”) variant (lvPPA) (Gorno-Tempini et al., 2004, 2011). Each of the variants has specifically defined clinical features, distinct atrophy patterns, and a likelihood of pathological subtype (Gorno-Tempini et al., 2011).
The semantic variant is characterized by fluent speech and a loss of semantic knowledge, including but not limited to word meaning, is associated with predominantly left anterior temporal lobe atrophy, and pathologically with frontotemporal lobar degeneration (FTLD), most often caused by FTLD TAR DNA Binding Protein, Type C (TDP-C). NfvPPA is recognized by apraxia of speech, impairments in articulation and grammar, left fronto-insular atrophy, and is most often associated with FTLD Tau and/or TDP pathology. LvPPA is unique in that it is most often associated with Alzheimer’s disease (AD) pathology and biomarkers (Leyton, Britton, Hodges, Halliday, & Kril, 2016; Spinelli et al., 2017) and is now considered a variant of early-age-of-onset AD (Dubois et al., 2014). LvPPA is identified by word finding pauses and impairments in phonological short-term memory (Henry et al., 2014) and atrophy that extends along the posterior portion of the left superior/middle temporal gyri into the inferior parietal lobule (supramarginal and angular gyri).
Since language difficulties can confound both task performance and comprehension of instructions on many neuropsychological measures, broader examination of cognitive domains other than language in PPA could allow researchers and clinicians to better understand the full spectrum of cognitive impairment in PPA and how it changes with disease progression, and potentially improve differential diagnosis. Even though exclusion criteria for PPA include initial and functionally significant impairments in visuospatial processing and visual memory, that is, by definition patients with PPA do not complain of difficulties with visuospatial functioning at presentation, many patients with PPA present with low scores on formal testing on tasks that are largely thought to be visuospatial in nature and can develop functional difficulties in this cognitive domain as the disease progresses.
There is limited research examining cognitive abilities other than language in PPA (Butts et al., 2015; Foxe et al., 2016; Foxe, Irish, Hodges, & Piguet, 2013; Ramanan et al., 2016), which confines interpretation of performance discrepancies in cognitive domains to subjective analysis. Our goal in this study was to explicitly study an understudied area of cognition in PPA to better describe and understand the cognitive profile of the different PPA variants as well as provide insights into the types of non-language tests that can help with differential diagnosis.
Among the three PPA variants, the most difficult differential diagnosis based solely on speech and language tasks is between nfvPPA and lvPPA, as both variants have difficulties in speech output and relatively intact semantic knowledge. Additionally, the short-term phonological memory deficits associated with the lvPPA variant can complicate assessment of grammar. Therefore, non-language domains, such as visuospatial tasks, may help to reveal other important differences between the lvPPA and nfvPPA variants that might aid in differential diagnosis. Because the lvPPA variant is known to have an atrophy pattern that begins in the temporal-parietal junction, includes medial temporal atrophy and longitudinally progresses in the parietal lobes bilaterally (Rohrer et al., 2013), whereas the parietal lobes are spared in nfvPPA, tasks that associate with parietal lobe functioning, such as visuospatial tasks, may be particularly helpful in understanding longitudinal clinical presentations in the PPA variants, especially when the aphasia is severe and difficult to assess.
We sought to investigate visuospatial abilities of all three PPA variants, using tasks distributed across visuospatial cognitive domains. Specifically, we report on an exploratory factor analysis of all our visuospatial data, four separate a priori composites of visuospatial functioning (construction, immediate recall, delayed recall, and executive functioning), specific performance on 11 individual neuropsychological measures, and change over time. We hypothesized that the lvPPA variant would have the most difficulty with visuospatial tasks compared to the other PPA variants and controls given that the lvPPA variant has more atrophy in regions typically associated with visuospatial functioning (in particular, the parietal lobes) than the other variants. Over time, we expected that the lvPPA group would have a more significant decline in performance on visuospatial tasks.
METHOD
Participants/Recruitment
Individuals with PPA were recruited and diagnosed through UCSF’s Frontotemporal Dementia Program Project Grant and Alzheimer’s Disease Research Center. A diagnosis of PPA, determined using the consensus criteria established in 2011 (Gorno-Tempini et al., 2011), and fluency in English were necessary for inclusion in the study. Baseline assessments from 156 participants were included (34 with logopenic variant, 74 with semantic variant, and 48 with nonfluent/agrammatic variant). Exclusion criteria for the PPA group consisted of a score below 10 on the Mini-Mental State Exam (MMSE) or Clinical Dementia Rating (CDR) greater than 2.
Seventy-nine control participants were recruited through the UCSF Hillblom Study on Healthy Aging and selected based on equivalent testing procedures to our PPA group. Exclusion criteria for controls were a history of major illness, including psychiatric illness, and a score below 27 on the MMSE or CDR greater than 0.5. Table 1 outlines the demographic characteristics of the participants. All participants provided informed written consent; the study was approved by the UCSF Committee on Human Research and conducted in accordance with the Helsinki Declaration.
Table 1.
Demographics and language scores
| lvPPA n = 34 | nfvPPA n = 48 | svPPA n = 74 | Controls n = 79 | Significance | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (mean [SD]) | 62.74 [8.18]c | 67.71 [6.97]b,d | 63.66 [6.95]c | 64.60 [8.08] | .011 |
| Gender (n; % female) | 19; 55.88 | 33; 68.75 | 33; 44.59 | 40; 50.63 | .067 |
| Handedness (n; % non-right) | 5; 15.15 | 5; 8.51 | 11; 14.86 | 10; 12.99 | .441 |
| Education (mean [SD]) | 16.55 [3.25] | 16.16 [3.37] | 16.50 [2.80] | 17.47 [2.08] | .049 |
| CDR (n; %) | .001 | ||||
| 0 | 1; 3.03a | 9; 20.45a,d | 2; 2.82a,c | 54; 87.10 | |
| 0.5 | 24; 72.73 | 29; 65.91 | 43; 60.56 | 8; 12.90 | |
| 1 | 6; 18.18 | 6; 13.64 | 19; 26.76 | 0; 0 | |
| 2 | 2; 6.06 | 0; 0 | 7; 9.86 | 0; 0 | |
| MMSE (mean [SD]) | 19.29 [7.65]a,c | 24.49 [5.66]a,b | 22.15 [7.25]a | 29.25 [0.99] | .001 |
| Language measures | |||||
| Boston Naming Test 15 items; mean [SD]) | 8.44 [4.58]a,c,d | 11.85 [3.11]a,b,d | 4.59 [3.62]a–c | 14.59 [0.69] | <.001 |
| Peabody Picture Vocabulary Test (16 items; mean [SD]) | 13.36 [2.23]a,d | 14.13 [2.32]d | 8.05 [4.12]a–c | 15.65 [0.64] | <.001 |
| WAB Repetition (100 items; mean [SD]) | 70.55 [16.39]d | 80.81 [19.79] | 87.79 [13.57]b | – | <.001 |
| WAB Sequential Commands (80 items; mean [SD]) | 66.43 [15.97] | 65.04 [14.66] | 72.26 [12.87] | – | .134 |
| WAB Fluency Rating (max = 10; mean [SD]) | 8.29 [1.9] | 6.7 [2.51]d | 9.17 [0.75]c | – | <.001 |
| AOS Severity Rating (max = 7; mean [SD]) | 0.46 [1.13]c | 2.22 [2.43]b,d | 0 [0]c | – | <.001 |
| Dysarthria Severity Rating (max = 7; mean [SD]) | 0 [0]c | 1.96 [2.7]b,d | 0 [0]c | – | <.001 |
| Syntax Comprehension (median [range]) | 4 [1–5]a,d | 4 [0–5]a | 5 [1–5]b | 5 [4–5] | <.001 |
| Digit Span Forward Length (median [range]) | 4 [2–7]a,d | 5 [2–8]a,d | 6 [2–9]a–c | 7 [3–9] | <.001 |
| Digit Span Backward Length (median [range]) | 3 [0–6]a,d | 3 [0–7]a,d | 5 [0–8]a–c | 6 [4–8] | <.001 |
| Stroop Color Naming (mean [SD]) | 47.7 [17.27]a,d | 43.40 [14.99]a,d | 65.70 [20.91]a–c | 88.27 [18.48] | <.001 |
| Letter Fluency (D words) (mean [SD) | 6.81 [4.67]a | 5.83 [4.90]a | 6.98 [4.40]a | 16.33 [4.90] | <.001 |
| Animal Fluency (mean [SD) | 8.31 [5.14]a,c | 10.63 [6.58]a,d | 7.61 [4.94]a,c | 23.67 [5.67] | <.001 |
vs controls.
vs lvPPA.
vs nfvPPA.
vs svPPA.
WAB = Western Aphasia Battery; AOS = Apraxia of Speech.
Demographic variables were compared using analysis of variance with Bonferroni post hoc tests. There were significant group differences in age at baseline, education, and CDR and MMSE scores (Table 1). The nfvPPA group was significantly older than the other PPA groups, but not controls. Although there was a significant group difference in amount of education, follow-up testing did not reveal specific between group significant differences after controlling for multiple comparisons. Control participants had significantly higher MMSE and significantly lower CDR scores than the PPA sample. Regarding MMSE scores, participants with lvPPA had significantly lower MMSE scores than those with nfvPPA (p = .001) and participants with svPPA had similar MMSE scores to both the lvPPA and nfvPPA groups (p’s > .1). On the CDR, participants with nfvPPA had significantly lower totals than the svPPA group (p = .007) and marginally lower scores than the lvPPA group (p = .06); the lvPPA group had similar CDR scores to the svPPA groups (p > .6). Groups had similar distributions of gender and handedness.
Follow-up assessments were available on 83 participants (17 with logopenic variant, 43 with semantic variant, and 23 with nonfluent/agrammatic variant). The mean visit gap (14.64 ± 6.4 months) did not differ by diagnosis, p > .20 (see Table 4). At follow-up, there was a significant group difference in age; the nfvPPA group was significantly older than the svPPA group. There were no significant group differences at follow-up in education, gender, or handedness.
Table 4.
Neuropsychological test scores change from time 1 to time 2
| Multiple time-point data | lvPPA n = 17 | nfvPPA n = 23 | svPPA n = 43 | Significance |
|---|---|---|---|---|
| Age at baseline (mean [SD]) | 63.65 [9.54] | 68.70 [8.34]d | 63.23 [6.35]c | .0205 |
| Gender (n; % female) | 8; 47.06 | 17; 73.91 | 17; 39.53 | .063 |
| Handedness (n; % non-right) | 4; 23.53 | 1; 4.55 | 5; 13.96 | .558 |
| Education (mean [SD]) | 15.94 [3.31] | 16.30 [3.08] | 16.67 [2.94] | .473 |
| Change from time 1 to time 2 in: | ||||
| Time gap (years) | 1.24 [0.66] | 1.17 [0.49] | 1.23 [0.53] | .907 |
| CDR Total (median [IQR]) | 0.25 [0.00–0.50] | 0.00 [0.00–0.25] | 0.00 [0.00–0.50] | .513 |
| MMSE (mean [SD]) | −4.94 [5.53] | −3.73 [5.40] | −3.31 [4.17] | .545 |
| Visual-Motor Construction | ||||
| Abbreviated Beery VMI (mean [SD]) | −4.79 [3.51]c,d | −1.33 [2.33]b | −0.32 [2.47]b | ≤.0016 |
| Benson Copy (mean [SD]) | −1.63 [2.58]d | −1.94 [2.93]d | 0.17 [1.25]b,c | .0168 |
| Block Design (mean [SD]) | −3.8 [7.20] | −5.80 [7.65] | −2.03 [9.30] | .278 |
| Visual-Spatial Immediate Recall | ||||
| Spatial Span Forward Length (median [IQR]) | −1.00 [−1.00–0.00] | 0.00 [−1.00–0.00] | 0.00 [−1.00–0.00] | .474 |
| Visual Reproduction I (mean [SD]) | −8.08 [31.40] | −11.30 [24.02] | −4.29 [16.40] | .519 |
| Visual-Spatial Working Memory | ||||
| Spatial Span Backward Length (median [IQR]) | −1.00 [−1.00–0.00] | 0.00 [−2.00–1.00] | 0.00 [−1.00–1.00] | .110 |
| Visual-Spatial Localization | ||||
| VOSP Number Location (median [IQR]) | 0.00 [−1.00–1.00] | 0.00 [−1.00–1.00] | 0.00 [−1.00–0.00] | .625 |
| Visual-Spatial Long-Term Recall/Recognition | ||||
| Visual Reproduction II (mean [SD]) | −7.25 [12.38] | −5.20 [28.05] | −2.51 [17.46] | .761 |
| Benson Recall (mean [SD]) | −2.50 [2.68] | −0.75 [3.62] | −1.24 [5.10] | .485 |
| Visual-Spatial Switching & Fluency | ||||
| Modified Trails B (mean [SD]) | 10.08 [38.99] | 7.00 [21.37] | −0.83 [29.08] | .455 |
| Modified Trails B Correct Lines (median [IQR]) | 0.00 [−5.50–2.00] | 0.00 [−1.00–0.00] | 0.00 [0.00] | .932 |
| Design Fluency (mean [SD]) | −0.27 [2.28] | −1.82 [2.48] | −1.33 [3.17] | .370 |
vs lvPPA.
vs nfvPPA.
vs svPPA.
n.s. = not significant; IQR = interquartile range.
Neuropsychological Assessment
Neuropsychological testing was administered by research staff or neuropsychology fellows who were trained and supervised by neuropsychologists. Nurses performed the CDR assessment. Neuropsychological testing covered screening of global cognition, processing speed, immediate recall, working memory, visual memory, visuospatial abilities, and executive functioning. In particular, we used an abbreviated Beery VMI to assess visuomotor integration (copying of geometric forms of increasing difficulty), Benson figure copy and recall to examine visuomotor figure construction and visual figure delayed recall (Kramer et al., 2003; Possin, Laluz, Alcantar, Miller, & Kramer, 2011), an abbreviated VOSP Number Location as a measure of visuospatial localization (Warrington & James, 1991), WAIS Block Design for visuospatial construction and Spatial Span Forward and Backward for visual attention and working memory, WMS Visual Reproduction I and II for visuomotor figure construction and delayed recall (Wechsler, 1997), a modified and abbreviated trails B type test (visuomotor sequencing that alternates between numbers and days of the week) to evaluate timed visuospatial sequencing and switching (Kramer et al., 2003), and DKEFS Design Fluency filled dots for figural fluency (Delis, Kaplan, & Kramer, 2001).
Language measures were also used to confirm PPA diagnoses. We used the fluency rating, sequential commands, and repetition from the Western Aphasia Battery (Kertesz, 1982); an apraxia of speech and a dysarthria severity rating (Wertz & Rosenbek, 1991); and abbreviated versions of the Boston Naming Test and Peabody Picture Vocabulary Test, syntax comprehension, digit span length forward and backward, rapid color naming, category fluency (animals), and phomenic fluency (“D” words) from our neuropsychological screen (Kramer et al., 2003).
Statistical Analyses
Factor and composite scores for a priori domains (total visuospatial, visuospatial construction, immediate recall, delayed recall, and visuospatial executive) were created based on a priori hypotheses about different cognitive domains within the visual-spatial province. Our a priori hypotheses for composite domains stemmed from previous literature that suggested distinct syndromic performances on visuospatial tasks, for example, studies that have reported that the lvPPA variant has difficulties with immediate and delayed recall of visual material (Foxe et al., 2013; Ramanan et al., 2016), and conversely, that the svPPA variant may have somewhat preserved visuospatial abilities particularly with regard to figure copying, attention, and speed (Butts, Machulda, Duffy, Strand, Whitwell, & Josephs, 2015; Viskontas, 2011) (see Table 3.).
Table 3.
Neuropsychological test factors, composites, and scores
| lvPPA n = 34 |
nfvPPA n = 48 |
svPPA n = 74 |
Controls n = 79 |
Significance | |
|---|---|---|---|---|---|
| Visual-Spatial Factor | −0.908 [0.81]a,c,d | −0.285 [0.97]a,b | −0.030 [0.79]b | 0.777 [0.55] | <0.001 |
| Visual-Motor Construction Composite | −0.43 [0.74]a,d | −0.20 [0.81]d | 0.22 [0.48]b,c | 0.40 [0.49] | <0.001 |
| Abbreviated Beery VMI (mean [SD]) | 10.55 [4.37]a,d | 11.02 [4.80]d | 13.68 [2.32]b,c | 13.84 [2.08] | <0.001 |
| Benson Copy (mean [SD]) | 13.09 [4.91]a,d | 14.74 [2.01] | 15.45 [1.19]a,b | 15.72 [1.25] | <0.001 |
| Block Design (mean [SD]) | 19.03 [11.09]a,c,d | 24.81 [13.27]a,b,d | 31.85 [12.26]b,c | 41.74 [11.38] | <0.001 |
| Visual-Spatial Immediate Recall Composite | −0.75 [0.88]a,d | −0.33 [0.77]a,d | 0.06 [0.62]b,c | 0.53 [0.42] | <0.001 |
| Spatial Span Forward Length (median[IQR]) | 4 [3.00–5.00]a,d | 4 [4.00–5.00]a,d | 6 [4.00–6.00]b,c | 6 [5.00–6.00] | <0.001 |
| Visual Reproduction I (mean [SD]) | 44.28 [22.05]a,c,d | 63.10 [21.65]b | 59.69 [20.28]b | 84.36 [12.65] | <0.001 |
| Spatial Span Backward Length (median [IQR]) | 4 [3.00–4.00]a,d | 4 [3.00–5.00]d | 5 [4.00–6.00]b,c | 5 [4.00–6.00 | <0.001 |
| Visual-spatial Localization | |||||
| VOSP Number Location (median [IQR]) | 9 [7.00–10.00] | 9 [8.00–10.00] | 10 [9.00–10.00] | 10 [9.00–10.00] | 0.006 |
| Visual-Spatial Delayed Recall Composite | −0.63 [0.54]a,c | 0.06 [0.68]b | −0.59 [0.80]a | 0.85 [0.52] | <0.001 |
| Visual Reproduction II (mean [SD]) | 14.17 [15.89]a,c | 33.65 [25.53]a,b | 17.36 [22.36]a | 64.95 [20.38] | <0.001 |
| Benson Recall (mean [SD]) | 6.55 [3.50]a,c | 10.05 [3.31]a,b,d | 6.84 [4.58]a,c | 12.68 [2.55] | <0.001 |
| Visual-Spatial Executive Composite | −0.53 [0.52]a,d | −0.44 [0.74]a,d | 0.08 [0.60]b,c | 0.65 [0.55] | <0.001 |
| Modified Trails B (mean [SD]) | 88.30 [36.26]a,d | 77.28 [37.67]a,d | 53.79 [32.60]b,c | 27.20 [14.19] | <0.001 |
| Modified Trails B Correct Lines (median [IQR]) | 10 [3.00–14.00]a,d | 14 [8.00–14.00]d | 14 [14.00]b,c | 14 [14.00] | <0.001 |
| Design Fluency (mean [SD]) | 5.9 [3.62]a | 6.15 [3.17]a | 7.5 [3.33] | 11.17 [3.17] | <0.001 |
vs controls.
vs lvPPA.
vs nfvPPA.
: vs svPPA.
n.s. = not significant; IQR = interquartile range.
Composite scores were created by averaging Z-scores from multiple tests that have similar neuropsychological features, for example, delayed recall, for each subject. Specifically, we divided the neuropsychological data into visuospatial subdomains of construction, immediate recall, delayed recall, and executive. The construction composite included performance on the abbreviated Beery VMI, Benson figure copy, and the WAIS Block Design. The immediate recall composite was based on scores on WMS Visual Reproduction I and WAIS Spatial Span Forward and Backward. The delayed recall composite was based on scores on WMS Visual Reproduction II and Benson recall. The visual executive composite included Spatial Span Backward, a ratio of modified trails number of correct lines divided by the completion time, and Design Fluency correct designs. Each subject’s score on each test was standardized based on the overall sample, and then mean Z-scores were computed for each subject for each composite set. We report the composite scores as group averages of these Z-scores.
The visuospatial factor score was created using factor analysis on baseline data. Specifically, we used the principal-factor method, Kaiser cutoff of eigenvalues greater than 1, and promax ^ 3 oblique rotation because cognitive variables likely correlate. Regression-based score generation (Osborne & Costello, 2009) was used in the factor analysis. Initially, we analyzed a visual-spatial factor that included all visuospatial neuropsychological variables (Table 2). However, the initial Benson Figure copy factor loading was 0.22, which is less than a 0.3 cutoff (regression coefficient based on sample size), so that item was removed from the factor. The eigenvalue for the final visual-spatial factor was 4.88 and this factor explained 87.29 percent of the variance in the data. Subject to variable ratio was greater than 10:1 (only complete datasets were included in the factor generation (total N = 117 based on controls = 34, lvPPA = 20, nfvPPA = 25, svPPA = 38). Participants with complete datasets did not differ from participants without complete datasets on age, handedness, gender, or education (all p’s > .59). However, there were significantly fewer participants with complete datasets who had CDR scores of 2 (n = 1 compared to n = 8; p = .034). Participants with complete datasets also had higher MMSE scores than participants without complete datasets (diff = 3.89; p < .001).
Table 2.
Factor loadings for the first, and only retained, factor.
| Variable | Initial Factor | Final Factor |
|---|---|---|
| Beery VMI | 0.555 | 0.543 |
| Block Design | 0.796 | 0.793 |
| Spatial Span Forward | 0.501 | 0.505 |
| Spatial Span Backward | 0.677 | 0.680 |
| Visual Reproduction I | 0.799 | 0.795 |
| Visual Reproduction II | 0.762 | 0.764 |
| Benson Copy | 0.223 | – |
| Benson Recall | 0.627 | 0.622 |
| VOSP Number Location | 0.386 | 0.383 |
| Modified Trails Time | −0.788 | −0.792 |
| Modified Trails # Correct | 0.642 | 0.648 |
| Design Fluency # Correct | 0.663 | 0.665 |
Note. Initial Factor loadings are given, as well as after removing unrelated variables (Final Factor).
Since the factor analysis included participants with lower disease severity, this likely makes our final factor scores more conservative, as we would expect that group differences by diagnostic group would be subtler among milder patients. Disease severity was included as a covariate in the statistical analyses. The one-factor model is the model that fit the data the best; the next highest factor had an eigenvalue of 0.76, which is below the Kaiser cutoff (for a scree plot see Supplementary Figure S1). To validate that this was in fact a visuospatial factor and not a general cognitive factor, we performed a sensitivity analysis. We ran the same factor analysis but added two language measures, abbreviated forms of confrontation naming and single word comprehension (Kramer et al., 2003). This analysis yielded a two-factor model, wherein the visuospatial data loaded onto the first factor with an eigenvalue of 5.37 and explained 68.43 percent of the variance while the two language measures loaded on a second factor that had an eigenvalue of 1.89 and explained 24.15 percent of the variance, which suggests that we identified a distinct visuospatial factor rather than a general cognitive factor.
In our analysis of change over time, there was not a significant difference among the PPA groups in time between visits, gender, handedness, or education. There were significant group differences in age at baseline.
Statistical analyses of demographics and neuropsychological scores between groups were performed using Stata version 14 or higher. Multivariate analysis of covariance was used to conduct omnibus significance testing; analysis of covariance and one-way analysis of variance were used to conduct follow-up tests. Non-parametric tests were conducted using Kruskal-Wallis and Dunn’s tests. Bonferroni correction was applied to all follow-up tests of group differences by number of group comparisons, such that findings were considered statistically significant at an alpha level < 0.008 (0.05 divided by six comparisons). Effect sizes were calculated based on Cohen’s d. Omnibus testing of group differences on all neuropsychological factors, composites, and specific tests included age at testing, education, and CDR total as covariates (Table 3). Follow-up tests were also adjusted for age, education, and CDR total differences because we were interested in differences due to visuospatial functioning, rather than absolute performance differences.
RESULTS
Cross-sectional Visuospatial Performance
There was a significant group difference in performance on the visual-spatial factor after controlling for age, education, and CDR scores (Figure 1). Controls had significantly higher scores on the visual-spatial factor than the nfvPPA (p = .04; d = 0.79) and lvPPA (p < .001; d = 1.91) groups, but similar scores to the svPPA group (p > .9). The svPPA group performed better than the lvPPA group (p < .001; d = 1.55) but did not differ from the nfvPPA group (p = .102). The nfvPPA group also had significantly better performance than the lvPPA group (p = .03; d = 0.79).
Fig. 1.
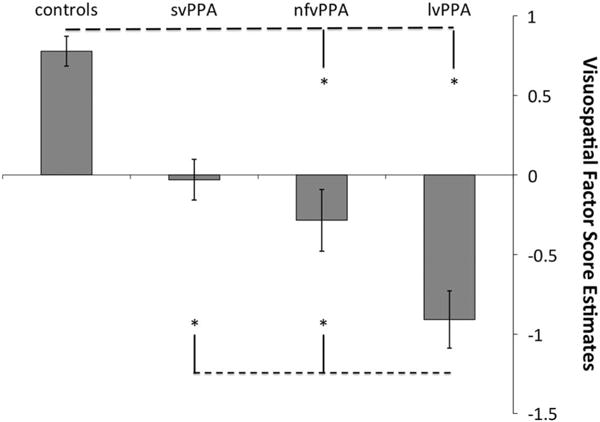
Visuospatial Factor score estimates by group, unadjusted. *indicates a significant difference p < .05 after Bonferroni correction for multiple comparisons and adjusted for differences in age, education, and CDR totals.
Group differences on the visuospatial composite scores and individual tests are reported in Table 3 and summarized here.
Visuospatial construction
There was a significant main effect of group on the visuomotor construction composite. Post hoc analyses revealed that controls performed significantly better than the lvPPA group (p = .001; d = 1.01) and marginally better than the nfvPPA group (p = .080; d = 0.56). The svPPA group performed significantly better than the nfvPPA (p = .002; d = 0.83) and lvPPA (p < .001; d = 1.33) groups, but similarly to controls (p > .9). LvPPA and nfvPPA groups were not significantly different (p = .713).
Visuospatial immediate recall
There was a significant main effect of group on the visual-spatial immediate recall composite. Controls performed significantly better than the nfvPPA (p = .001; d = 0.89) and lvPPA (p < .001; d = 1.76) groups, but similarly to the svPPA group (p > .9). The svPPA group also performed significantly better than the nfvPPA (p < .001; d = 0.79) and lvPPA (p < .001; d = 1.43) groups. The nfvPPA group was not significantly different from the lvPPA group (p = .141). There was a significant group effect on both visual-spatial measures of immediate recall (Spatial Span and Visual Reproduction I) (p’s < .001).
Visuospatial localization
On a brief measure of visuospatial localization, there was a significant group difference.
Visuospatial delayed recall
A main effect of group was found on the visual-spatial delayed recall composite. Controls performed significantly better than the svPPA (p = .002; d = 0.70) and lvPPA (p < .001; d = 1.42) groups, but had a similar level of performance to the nfvPPA group (p > .9). The nfvPPA had significantly higher scores on the delayed recall composite than the lvPPA group (p = .008; d = 0.88) but not the svPPA group (p = .407). This was the only composite that differentiated the nfvPPA from the lvPPA group. SvPPA and lvPPA variants had similar scores on the delayed recall composite (p = .346). On each of the delayed recall measures, there were significant group differences.
Visuospatial executive functions
On the visual executive composite, there was a main effect of group (Fig. 2). Follow-up analyses revealed that controls performed significantly better than the nfvPPA (p < .001; d = 1.16) and lvPPA (p < .001; d = 1.49) groups. The svPPA group also performed significantly better than the nfvPPA (p < .001; d = 1.01) and lvPPA (p < .001; d = 1.31) groups, but similarly to controls (p > .9). The nfvPPA and lvPPA groups performed similarly (p > .9).
Fig. 2.
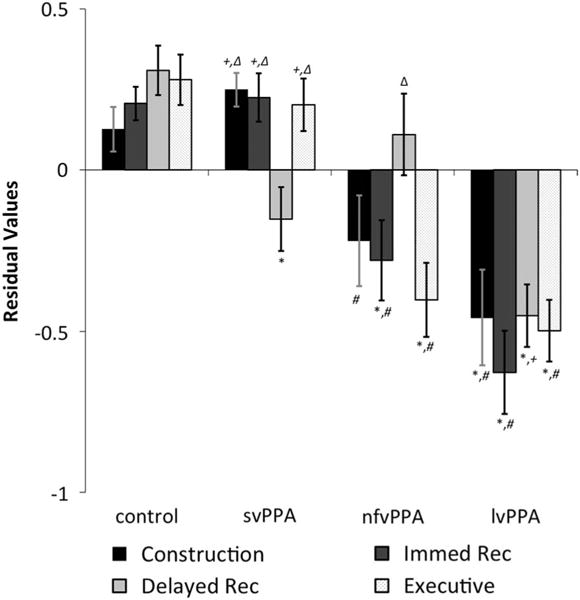
Visuospatial composite residual values after adjusting for differences in age, education, and CDR totals. Symbols indicate a significant difference at p < .05 after Bonferroni correction for multiple comparisons. *vs. controls, #vs. svPPA, + vs. nfvPPA, Δvs. lvPPA.
Visuospatial Performance Over Time
Declines in MMSE or CDR were not significant for any group, controlling for age at baseline differences. Furthermore, only two neuropsychological tests showed a significant group effect for change over time: Beery VMI (p < .002) and the Benson Figure Copy (p = .017) (Table 4.). Follow-up analyses demonstrated that the lvPPA group’s performance on the abbreviated Beery VMI declined by an average of 4.8 points in approximately a year and this amount of decline was significantly different from the other PPA groups (effect sizes: vs. nfvPPA = −1.16; and vs. svPPA = −1.47). There were no other significant differences between groups on change in performance on the abbreviated Beery VMI. On the Benson figure copy, the svPPA group showed a small improvement in performance of almost two tenths of a point and this small improvement was significantly different from the change seen in the other two PPA groups, both of which had a decline in performance by more than 1.5 points (effect sizes: vs. nfvPPA = 0.94; vs. lvPPA = 0.89).
DISCUSSION
Our study aimed to elucidate the performance of patients with PPA on visuospatial neuropsychological tasks using a large sample of PPA participants and an extensive neuropsychological battery. We identified that one visuospatial factor could account for most of the variance in the data. Based on this factor, the lvPPA group had significantly worse overall performance. The svPPA group performed better than the lvPPA group on every visuospatial composite except the delayed recall composite where they performed similarly. The nfvPPA group had significantly higher scores than the lvPPA group on the delayed recall composite and this was the only composite that differentiated these two variants.
When performance over time was examined, only the lvPPA group had a significantly greater decline than other groups on the Beery VMI. The svPPA group showed a small improvement in performance over time on the Benson figure copy whereas the other PPA groups showed a slight decline, which was a significant group difference.
Difficulties in visuospatial tests were present in the PPA sample at baseline even after controlling for disease severity, which suggests that visuospatial functioning is affected in this population even if it is less severely affected than language deficits and does not result in a functional impairment in everyday life. Our study investigated visuospatial cognition across several tasks and sub-domains and showed that the different variants have distinct difficulties on visuospatial tasks that mirror their difficulties in language sub-domains and are consistent with patterns of anatomical damage in each variant. However, this is not the first time visuospatial difficulties have been reported in this population.
A few studies have expanded the non-linguistic cognitive literature base in PPA. Foxe et al. (2013) found that patients with lvPPA performed as poorly as patients with classic Alzheimer’s disease (AD) on visuospatial short-term memory tasks. While the lvPPA patients performed worse on a verbal auditory (digit) span, they were similarly impaired on the spatial span. A subsequent analysis by Foxe et al. (2016) demonstrated that this dissociation in span performance also related to distinct cortical thinning patterns. Both Foxe studies only investigated the lvPPA variant of PPA, which left a question remaining about the performance of the other two PPA variants on these tasks. Our study showed that both the lvPPA and nfvPPA groups were impaired on visuospatial immediate recall tasks but that the svPPA group was not, a pattern similar to their language profiles and differential patterns of dorsal versus ventral atrophy, respectively (Henry, Wilson, Babiak, Mandelli, Beeson, Miller, & Gorno-Tempini, 2016).
Butts et al. (2015) studied all three PPA variants and found that there were significant group differences between the PPA variants on figure copying, some aspects of visual memory, and a visuospatial executive functioning task. In particular, the lvPPA variant had lower scores than the nfvPPA variant on visual memory, and lower scores than the svPPA variant on figure copying, visual immediate recall, and visual executive functioning. Many of our findings are similar to those reported by Butts et al. (2015), with the exception of delayed recall in the svPPA group. Butts et al. (2015) reported average visual recall in an svPPA sample; it is possible that sample size played a role in this difference and/or that this sample differed from ours in disease severity, length of disease, or degree of left versus right atrophy.
Classically, svPPA is associated with greatest atrophy in the left temporal lobe initially but volume loss in the right temporal lobe is nevertheless present, including the medial temporal lobe, and becomes more severe over time (Henry et al., 2014; Kumfor et al., 2016; Rohrer et al., 2008). Visuospatial memory impairments associated with right temporal lobe damage have been reported in the literature (Milner, Johnsrude, & Crane, 1997; Pigott & Milner, 1993). Therefore, there could be an anatomical basis for visuospatial delayed recall difficulties in svPPA. Further studies are needed to better understand when and how individuals with svPPA have difficulties with visuospatial delayed recall.
Ramanan et al. (2016) analyzed non-verbal episodic memory in PPA and found that, of a few different episodic memory tests, performance on delayed recall of the Rey Complex Figure was the most powerful discriminator between lvPPA and nfvPPA patients, with the lvPPA patients more impaired. Our result of impairment in delayed visual recall in the lvPPA group supports the similar finding by Ramanan et al. (2016) but extends the finding to include the domain of visual delayed recall; a finding that may relate to Alzheimer’s disease targeting medial temporal lobe structures (Ossenkoppele et al., 2015).
Overall, the lvPPA group had the lowest scores on the visuospatial factor, which was expected given that part of the clinical criteria for lvPPA includes parietal atrophy on structural MRI or hypometabolism on PET/SPECT. Future studies examining the neuroanatomical and neurometabolic correlates of visuospatial performance in the lvPPA group will help to clarify these associations.
One surprising outcome of this study is the degree of impairment the nfvPPA group evidenced on visuospatial tasks given that visuospatial processing is commonly thought of as a right parietal activity. One possible reason the nfvPPA displayed difficulty on these tasks is that several of the tasks rely on visuomotor abilities and nfvPPA has been associated with degradation of white matter pathways connecting the left inferior frontal gyrus (Broca’s area) to premotor and supplementary motor regions (Budisavljevic et al., 2017; Mandelli et al., 2014). In this sense, the deficits may relate more to motor planning and sequencing. However, further investigation is necessary to determine the underlying mechanism.
Of all the visuospatial variables we analyzed, decline in only two were found to have a main effect of group, raising the question of practice effects on several tests. Both tasks that showed decline were figure-copying tasks, and the lvPPA and nfvPPA groups showed the most decline. These tasks require integration of multiple cognitive abilities and poor performance can be due to damage within the dorsal frontoparietal network (Possin et al., 2011).
Strengths of the study include a large PPA sample, investigation of visuospatial functioning overall and within different sub-domains, and a multiple time point perspective. Limitations include a relatively small follow-up sample. Additionally, although we tried to control for disease severity, the CDR may provide a biased perspective on disease severity for some PPA groups more than others because of a greater emphasis placed on memory impairments compared with other functions such as speech intelligibility. Future studies should include more time points with larger longitudinal samples and specifically investigate earlier patterns of cognitive dysfunction with the PPA population as well as more specific models and evidence for how visuospatial functioning is distributed across the brain.
In conclusion, performance on visuospatial cognition highlights differential patterns of performance in the PPA variants, likely in relation to the underlying cognitive and anatomical deficits. The results provide important information for differential diagnosis within the context of PPA and for understanding cognition in PPA more broadly.
Supplementary Material
Acknowledgments
This work was funded by the National Institutes of Health (M.T., NINDS R01 NS050915), (M.T., NIDCD K24 DC015544), (B.M., NIA P50 AG03006), (B.M., NIA P50 AG023501), (B.M., NIA P01 AG019724); (M.T., State of California (DHS04-35516)); (B.M., Alzheimer’s Disease Center of California (03-75271 DHS/ADP/ARCC)); (J.K., Larry L. Hillblom Foundation); (B.M., John Douglas French Alzheimer’s Foundation); Koret Family Foundation; (B.M., Consortium for Frontotemporal Dementia Research); and (B.M., McBean Family Foundation). Disclosures; Dr. Possin receives research funding from Quest Diagnostics. Dr. Rabinovici receives research support from Avid Radiopharmaceuticals, GE Healthcare, and Piramal, and has received consulting fees or speaking honoraria from Roche, Eisai, Lundbeck, Piramal and Putnam. Dr. Kramer receives royalties from Pearson, Inc. for the California Verbal Learning Test. Dr. Bruce L. Miller receives grant support from the NIH/NIA and the Centers for Medicare & Medicaid Services (CMS) as grants for the Memory and Aging Center. As an additional disclosure, Dr. Miller serves as Medical Director for the John Douglas French Foundation; Scientific Director for the Tau Consortium; Director/Medical Advisory Board of the Larry L. Hillblom Foundation; Scientific Advisory Board Member for the National Institute for Health Research Cambridge Biomedical Research Centre and its subunit, the Biomedical Research Unit in Dementia (UK); and Board Member for the American Brain Foundation (ABF). Dr. Tempini has received personal compensation in an editorial capacity from Neuroimage Clinical. No other disclosures exist.
Footnotes
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617717000984
References
- Budisavljevic S, Dell’Acqua F, Djordjilovic V, Miotto D, Motta R, Castiello U. The role of the frontal aslant tract and premotor connections in visually guided hand movements. NeuroImage. 2017;146:419–428. doi: 10.1016/j.neuroimage.2016.10.051. [DOI] [PubMed] [Google Scholar]
- Butts AM, Machulda MM, Duffy JR, Strand EA, Whitwell JL, Josephs KA. Neuropsychological profiles differ among the three variants of Primary Progressive Aphasia. Journal of the International Neuropsychological Society. 2015;21(6):429–435. doi: 10.1017/S1355617715000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system (D-KEFS) San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, Bateman R. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. The Lancet Neurology. 2014;13(6):614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Foxe D, Leyton CE, Hodges JR, Burrell JR, Irish M, Piguet O. The neural correlates of auditory and visuospatial span in logopenic progressive aphasia and Alzheimer’s disease. Cortex. 2016;83:39–50. doi: 10.1016/j.cortex.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Foxe DG, Irish M, Hodges JR, Piguet O. Verbal and visuospatial span in logopenic progressive aphasia and Alzheimer’s disease. Journal of the International Neuropsychological Society. 2013;19(3):247–253. doi: 10.1017/S1355617712001269. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Wilson SM, Babiak MC, Mandelli ML, Beeson PM, Miller ZA, Gorno-Tempini ML. Phonological processing in primary progressive aphasia. Journal of Cognitive Neuroscience. 2016;28(2):210–222. doi: 10.1162/jocn_a_00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Wilson SM, Ogar JM, Sidhu MS, Rankin KP, Cattaruzza T, Seeley WW. Neuropsychological, behavioral, and anatomical evolution in right temporal variant frontotemporal dementia: A longitudinal and post-mortem single case analysis. Neurocase. 2014;20(1):100–109. doi: 10.1080/13554794.2012.732089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Western aphasia battery test manual. New York: Grune & Stratton; 1982. [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and Behavioral Neurology. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Kumfor F, Landin-Romero R, Devenney E, Hutchings R, Grasso R, Hodges JR, Piguet O. On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain. 2016;139:986–998. doi: 10.1093/brain/awv387. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Britton AK, Hodges JR, Halliday GM, Kril JJ. Distinctive pathological mechanisms involved in primary progressive aphasias. Neurobiology of Aging. 2016;38:82–92. doi: 10.1016/j.neurobiolaging.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Mandelli ML, Caverzasi E, Binney RJ, Henry ML, Lobach I, Block N, Henry RG. Frontal white matter tracts sustaining speech production in primary progressive aphasia. Journal of Neuroscience. 2014;34(29):9754–9767. doi: 10.1523/JNEUROSCI.3464-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S. Spectrum of primary progressive aphasia. Bailliere’s Clinical Neurology. 1992;1(3):583–609. [PubMed] [Google Scholar]
- Milner B, Johnsrude I, Crane J. Right medial temporal–lobe contribution to object–location memory. Philosophical Transactions of the Royal Society B: Biological Sciences. 1997;352(1360):1469–1474. doi: 10.1098/rstb.1997.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne JW, Costello AB. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Pan-Pacific Management Review. 2009;12(2):131–146. [Google Scholar]
- Ossenkoppele R, Cohn-Sheehy BI, La Joie R, Vogel JW, Moller C, Lehmann M, Rabinovici GD. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Human Brain Mapping. 2015;36(11):4421–4437. doi: 10.1002/hbm.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott S, Milner B. Memory for different aspects of complex visual scenes after unilateral temporal- or frontal-lobe resection. Neuropsychologia. 1993;31(1):1–15. doi: 10.1016/0028-3932(93)90076-c. [DOI] [PubMed] [Google Scholar]
- Possin KL, Laluz VR, Alcantar OZ, Miller BL, Kramer JH. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer’s disease and behavioral variant frontotemporal dementia. Neuropsychologia. 2011;49(1):43–48. doi: 10.1016/j.neuropsychologia.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan S, Flanagan E, Leyton CE, Villemagne VL, Rowe CC, Hodges JR, Hornberger M. Non-verbal episodic memory deficits in Primary Progressive Aphasias are highly predictive of underlying amyloid pathology. Journal of Alzheimer’s Disease. 2016;51(2):367–376. doi: 10.3233/JAD-150752. [DOI] [PubMed] [Google Scholar]
- Rohrer J, McNaught E, Foster J, Clegg S, Barnes J, Omar R, Fox N. Tracking progression in frontotemporal lobar degeneration serial MRI in semantic dementia. Neurology. 2008;71(18):1445–1451. doi: 10.1212/01.wnl.0000327889.13734.cd. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Caso F, Mahoney C, Henry M, Rosen HJ, Rabinovici G, Gorno-Tempini ML. Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain and Language. 2013;127(2):121–126. doi: 10.1016/j.bandl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli EG, Mandelli ML, Miller ZA, Santos‐Santos MA, Wilson SM, Agosta F, Meyer M. Typical and atypical pathology in primary progressive aphasia variants. Annals of Neurology. 2017;81:430–443. doi: 10.1002/ana.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas IV, Boxer AL, Fesenko J, Matlin A, Heuer HW, Mirsky J, Miller BL. Visual search patterns in semantic dementia show paradoxical facilitation of binding processes. Neuropsychologia. 2011;49(3):468–478. doi: 10.1016/j.neuropsychologia.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington E, James M. The Visual Object and Space Perception Battery. Suffolk, England: Thames Valley Test Company; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale–Third Edition and Wechsler Memory Scale–Third Edition technical manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wertz RT, Rosenbek JC. Apraxia of speech in adults: The disorder and its management. Norwich, UK: Singular Publishing Group; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


