Abstract
Introduction
Recent studies suggested that muscle mass and muscle strength may independently or synergistically affect aging-related health outcomes in older adults; however, prospective data on mortality in the general population are sparse.
Methods
We aimed to prospectively examine individual and joint associations of low muscle mass and low muscle strength with all-cause mortality in a nationally representative sample. This study included 4,449 participants aged 50 years and older from the National Health and Nutrition Examination Survey (NHANES) 1999–2002 with public-use 2011 linked mortality files. Weighted multivariable logistic regression models were adjusted for age, sex, race, BMI, smoking, alcohol use, education, leisure-time physical activity (LTPA), sedentary time, and comorbid diseases.
Results
Overall, the prevalence of low muscle mass was 23.1% defined by appendicular lean mass (ALM) and 17.0% defined by ALM/BMI, and the prevalence of low muscle strength was 19.4%. In the joint analyses, all-cause mortality was significantly higher among individuals with low muscle strength, whether they had low muscle mass (OR 2.03, 95% CI, 1.27–3.24 for ALM; OR 2.53, 95% CI, 1.64–3.88 for ALM/BMI) or not (OR 2.66, 95% CI 1.53–4.62 for ALM; OR 2.17, 95% CI 1.29–3.64 for ALM/BMI). In addition, the significant associations between low muscle strength and all-cause mortality persisted across different levels of metabolic syndrome (MetS), sedentary time, and LTPA.
Conclusions
Low muscle strength was independently associated with elevated risk of all-cause mortality, regardless of muscle mass, MetS, sedentary time, or LTPA among US older adults, indicating the importance of muscle strength in predicting aging-related health outcomes in older adults. Key words: Muscle Mass; Muscle Strength; Mortality; Sarcopenia; Aging
Introduction
Since 1989, when Rosenberg originally proposed the term sarcopenia, defined as an age-related decline in muscle mass among the elderly (1), sarcopenia has been increasingly recognized as a common, complex, and costly state of impaired health status in older individuals. It is well accepted that if sarcopenia progresses beyond the lower threshold of muscle functional capacity, it will eventually lead to disability, frailty, and mortality (2–5). As two integral components of muscular function, muscle mass and muscle strength are intimately linked. However, there is no universal consensus on optimal assessment methods and diagnostic criteria for sarcopenia in older adults. In particular, it remains controversial how muscle mass should be considered in combination with muscle strength/weakness and/or physical performance in the assessment of sarcopenia.
Recent evidence from longitudinal studies indicates that age-related decline in muscle strength was not proportionally related to muscle mass loss (6). Further, some studies have shown that low muscle strength was strongly associated with poor health status and mortality than low muscle mass (7–10), especially among elderly persons with chronic medical conditions (11, 12). The Foundation for the National Institutes of Health Sarcopenia Project (FNIH) proposed cutoff values for clinically relevant low muscle mass and grip strength based on their associations with incident mobility impairment (13), but there is a lack of prospective data on other health-related outcomes, including mortality. Also, though recommended by the FNIH, grip strength has not widely been accepted as a surrogate of overall muscle strength (14). Some studies suggested that participants with low knee extensor strength performed worse in physical performance tests than in tests of grip strength (15, 16). It is unclear whether or to what extent low muscle mass and lower-extremity muscle strength were independently or jointly associated with health outcomes in older and elderly adults.
In addition, LTPA and sedentary time are important lifestyle behaviors that are highly correlated with adiposity and muscle mass and/or function (17–19). Metabolic syndrome, a constellation of metabolic abnormalities including visceral obesity, glucose intolerance, hypertension, and dyslipidemia, may explain the relationship between muscle mass/strength and obesity-associated outcomes (20). However, few studies have specifically examined the effects of the interrelationship with muscle mass and muscle strength on health outcomes.
In a nationally representative sample of US adults aged 50 years and older from the NHANES, we aimed to 1) compare basic characteristics between participants with and without low muscle mass, and between those with and without low muscle strength; 2) calculate the prevalence of low muscle mass and low muscle strength and their combination subgroups stratified by sex and race, respectively; 3) prospectively examine individual or joint associations between low muscle mass/strength and all-cause mortality; and 4) additionally examine the effects of the joint associations between low muscle mass/strength and MetS, sedentary time, LTPA on all-cause mortality.
Method and materials
Study population
The data were from the National Health and Nutrition Examination Survey (NHANES), a nationally representative, complex, and multistage probability survey of the US civilian non-institutionalized population. All data, study design, and procedures, including questionnaires, results of examinations, and findings of laboratory components, are publically available online(21). The National Centers for Health Statistics Ethics Review Board approved the study protocol, and each participant provided written informed consent. Participants in NHANES were interviewed in their homes and underwent a standardized physical examination in a mobile examination center. Self-reported information on demographics, socioeconomic status, health conditions, and health behaviors were obtained during the interview. The examination component consisted of medical, dental, and physiological measurements, as well as laboratory tests administered by highly trained medical personnel.
The two cycle surveys (NHANES 1999–2000 and 2001–2002) were combined for this analysis. A total of 4,449 participants were included and satisfied using the following criteria: 50 years of age and older who underwent physical examination, were not pregnant, and had measurements of body composition and muscle strength, and laboratory results.
Mortality data
Public-use Linked Mortality Files are available for continuous NHANES 1999–2002. These files provide mortality data from the date of survey enrollment through December 31, 2011. Mortality source and cause of death were determined using death certificates. Cause of death followed the 9th revision International Statistical Classification of Disease, Injuries, and Cause of Death guidelines.
Demographic and comorbidities
Age, race (non-Hispanic white, non-Hispanic black, Hispanic and others), sex, and education were obtained from self reports. Smoking was categorized as never smokers and smokers. Never smokers were those who reported smoking fewer than 100 cigarettes during their lifetime (22). Alcohol users were those who had at least 12 drinks in the last 12 months (23). We used information on leisure-time physical activity (LTPA) over the past 30 days, which was obtained during the interview at home. LTPA was categorized into no LTPA, less than the recommended goal (>0 and <750 MET/week), and exceeding the recommended goal (≥ 750 MET/week)(24). Sedentary time (time watching TV or video or using a computer) per average day over the last 30 days was asked in the household interview. The cut-points were >2 hours per day for TV, >1 hour per day for computer use, and >3 hours for screen time (25).
Hypertension was defined as blood pressure ≥ 130/85 mmHg or treatment for hypertension; in this study, cardiovascular diseases (CVD) included congestive heart failure, coronary heart disease (angina pectoris, heart attack), stroke, and hypertension. Self-report history was obtained for medical comorbidities by asking the question, “Has a doctor or other health professional ever told you that you had (disease state): congestive heart failure, coronary heart disease (angina pectoris, heart attack), stroke, cancer or malignancy, diabetes mellitus.” A participant was defined as having self-reported chronic obstructive pulmonary disease (COPD) if the participant reported having been told that he or she had chronic bronchitis and still had it or if he or she reported that he or she had emphysema. A chronic kidney disease (CKD) was defined as an eGFR of <60 mL/minute/1.73 m2, according to the definition proposed by the National Kidney Foundation - Kidney Disease Outcomes Quality Initiative work group and the Kidney Disease: Improving Global Outcomes(26, 27).
Muscle mass
Body composition measures were assessed by certified radiology technologists using a DEXA QDR-4500 Hologic scanner (Hologic, Inc., Bedford, MA). Subjects taller than 192.5 cm or weighing >136.4kg were excluded from the assessment. The densitometer uses an X-ray source to scan participants in supine position on the tabletop with their feet in a neutral position and hands flat by their side. Two NHANES cycles performed similar operation procedures. Low muscle mass was defined using the Foundation for the National Institutes of Health Sarcopenia Project (FNIH) proposed definitions: appendicular lean mass (ALM) and ALM divided by BMI. The cutoffs were <19.75 kg and <0.789 for men, and <15.02 kg and <0.512 for women (13).
Muscle strength
Muscle strength is the ability to exert a maximal amount of force against a resistance (28). Isokinetic strength testing was completed on survey participants aged 50 years and older. A Kin Com MP dynamometer (Chattanooga Group, Inc, Chattanooga, TN) was used to evaluate knee extensor strength, which in turn was used to quantify muscle strength.
Metabolic syndrome
The five individual metabolic syndrome (MetS) components were assessed from the examination data or laboratory data in NHANES 1999–2002. The MetS was defined according to the National Cholesterol Education Program’s Adult Treatment Panel III report (NCEP/ATPIII) (29). An individual was diagnosed as having MetS if at least three of the following five criteria are satisfied: 1) Waist circumference ≥102 cm in men or ≥88 cm in women; 2) Triglycerides ≥150 mg/dL; 3) High-density lipid cholesterol < 40 mg/dL in men or < 50 mg/dL in women; 4) Blood pressure ≥ 130/85 mmHg or treatment for hypertension; 5) Fasting blood glucose ≥100 mg/dL or treatment for diabetes.
Statistical analysis
According to guidelines of the NHANES on data analysis and procedure (21), statistical analyses should take into account the survey’s complex sampling design to ensure unbiased estimation. Specifically, all the analyses used weights and the primary sampling unit and strata supplied by the NHANES. All continuous variables were presented as means ± standard errors, and categorical variables were presented as percentages. A t-test compared means between continuous variables, and a chi-square test compared categorical variables at baseline between participants with and without low muscle mass.
We calculated the quartiles of the measurements of knee extensor strength and designated the lowest 25% as low muscle strength in men and women, and compared baseline characteristics between participants with and without low muscle strength. The prevalence of low muscle mass, low muscle strength, and low muscle mass with or without low muscle strength was stratified by sex and race and compared by weighted estimates from chi-square tests.
Multivariable logistic regression models with the calculation of odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess the individual and joint association of low muscle mass and low muscle strength with all-cause mortality. Separate analyses were performed for each definition of low muscle mass (ALM and ALM/BMI). All multivariable logistic regression models were adjusted for demographic characteristics, including age, sex, race, BMI, smoking, alcohol use, education, sedentary time, LTPA, CVD, diabetes, cancer, COPD, and CKD. In the joint association, participants were stratified by low muscle mass and low muscle strength, and four subgroups were created. The subgroup with normal muscle mass and muscle strength was considered the reference group when ORs for the three other subgroups were calculated. We also used multivariable logistic regression models to explore the effects of the joint associations of MetS (yes vs. no), sedentary time (< 3 hours vs. ≥3 hours), and LTPA (0, <750, and ≥750 MET/week) with low muscle mass and low muscle strength on all-cause mortality. To test for effect modification in each joint analysis, we examined the p-value for the interaction term which was added in the multivariable logistic regression models. All analyses were conducted using SAS, version 9.4 (SAS Institute, Inc). Two-sided tests and a 5% significance level were used in the analysis.
Results
1. Baseline characteristics stratified by low muscle mass and low muscle strength
A total of 4,449 US adults aged ≥50 and older were included. The prevalence of low muscle mass was 23.1% as defined by ALM and 17.0% as defined by ALM/BMI; the prevalence of low muscle strength was 19.4%. Basic participant characteristics stratified by low muscle mass and low muscle strength are shown in Table 1. Participants with low muscle mass were much older, had lower LTPA, and had lower knee extensor peak force than those without low muscle mass. Participants with low muscle mass (adjusted by BMI) were more likely to have cardiovascular diseases and diabetes, higher BMI and fat mass, systolic blood pressure, serum concentration of total triglycerides, fasting glucose, fasting insulin, and HOMA-IR, and lower diastolic blood pressure and serum concentrations of high-density lipoprotein than those with normal muscle mass. Similarly, participants with low muscle strength were also older and had lower LTPA than those without low muscle strength. Anthropometric measures except fat mass were significantly lower in those with low muscle strength than those with normal muscle strength. Participants with low muscle strength had higher systolic blood pressure, lower diastolic blood pressure, higher serum concentrations of low-density lipoprotein, and higher likelihood of CVD, diabetes, cancer, COPD, and CKD compared to those without low muscle strength.
Table 1.
Baseline characteristics of low muscle mass (LMM) and low muscle strength (LMS) in US adults aged 50 years and older in the NHANES 1999–2002
| LMM | Non-LMM | P | LMM | Non-LMM | P | LMS | Non-LMS | P | |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| ALM | ALM/BMI | ||||||||
| Age | 68.7 (0.5) | 62.4 (0.2) | < 0.001 | 67.8 (0.5) | 62.7 (0.2) | < 0.001 | 71.5 (0.5) | 60.8 (0.2) | < 0.001 |
| Gender | |||||||||
| Male | 319 (21.8) | 1797 (52.6) | < 0.001 | 503 (51.9) | 1559 (44.5) | < 0.001 | 386 (42.2) | 1154 (48.2) | 0.028 |
| Female | 779 (78.2) | 1377 (47.4) | 418 (48.1) | 1683 (55.5) | 376 (57.8) | 1126 (51.8) | |||
| Race | |||||||||
| Non-Hispanic whites | 616 (77.7) | 1754 (79.0) | < 0.001 | 463 (77.6) | 1837 (79.0) | < 0.001 | 454 (78.8) | 1304 (80.9) | 0.569 |
| Non-Hispanic blacks | 63 (3.1) | 670 (10.1) | 38 (2.3) | 684 (9.9) | 123 (8.2) | 380 (7.7) | |||
| Hispanics | 376 (13.0) | 676 (7.9) | 393 (15.2) | 634 (7.7) | 163 (8.9) | 537 (8.4) | |||
| Other | 43 (6.2) | 74 (2.9) | 27 (4.9) | 87 (3.5) | 22 (4.2) | 59 (3.0) | |||
| Education | |||||||||
| Less than high school | 522 (33.2) | 1197 (25.5) | < 0.001 | 498 (38.3) | 1164 (24.4) | < 0.001 | 347 (36.2) | 760 (21.4) | < 0.001 |
| High school diploma (including GED) | 246 (28.7) | 694 (24.8) | 190 (27.9) | 726 (25.3) | 168 (25.4) | 524 (25.6) | |||
| More than high school | 323 (38.1) | 1279 (49.7) | 230 (33.7) | 1345 (50.3) | 243 (38.4) | 995 (53.0) | |||
| Smoking | |||||||||
| Smoked at least 100 cigarettes in life | 530 (48.9) | 1745 (56.1) | 0.001 | 492 (53.7) | 1725 (54.5) | 0.727 | 390 (50.6) | 1256 (56.1) | 0.012 |
| Alcohol use | |||||||||
| At least 12 alcohol drinks per year | 17 (2.0) | 88 (3.4) | 0.033 | 12 (1.3) | 92 (3.5) | 0.008 | 17 (2.6) | 66 (3.5) | 0.303 |
| LTPA(leisure time physical activity) | |||||||||
| No leisure time physical activity | 692 (56.2) | 1625 (44.0) | < 0.001 | 604 (61.0) | 1628 (43.2) | < 0.001 | 476 (58.2) | 1037 (38.7) | < 0.001 |
| 0–750 MET/week | 217 (21.8) | 697 (24.7) | 181 (23.8) | 720 (24.4) | 144 (22.8) | 551 (26.5) | |||
| ≥750 MET/week | 189 (22.0) | 852 (31.2) | 136 (15.2) | 894 (32.4) | 142 (19.0) | 692 (34.8) | |||
| Sedentary time (screen time) | |||||||||
| <3hours/day | 557 (50.0) | 1584 (51.3) | 0.538 | 422 (40.8) | 1672 (53.3) | < 0.001 | 346 (41.5) | 1210 (55.6) | < 0.001 |
| ≥3hours/day | 536 (50.0) | 1585 (48.7) | 496 (59.2) | 1563 (46.7) | 413 (58.5) | 1067 (44.4) | |||
| Anthropometric measures | |||||||||
| Weight (kg) | 60.1 (0.4) | 85.5 (0.4) | < 0.001 | 80.3 (0.7) | 79.6 (0.5) | 0.397 | 71.3 (0.9) | 81.3 (0.5) | < 0.001 |
| BMI (kg/m2) | 24.0 (0.2) | 29.9 (0.2) | < 0.001 | 31.2 (0.3) | 28.0 (0.2) | < 0.001 | 26.2 (0.3) | 28.7 (0.2) | < 0.001 |
| Waist circumference (cm) | 87.2 (0.4) | 103.1 (0.4) | < 0.001 | 105.2 (0.7) | 98.3 (0.4) | < 0.001 | 96 (0.7) | 99.5 (0.4) | < 0.001 |
| Body fat (%) | 37.2 (0.2) | 36.3 (0.2) | 0.006 | 40.4 (0.3) | 35.6 (0.2) | < 0.001 | 36.5 (0.3) | 36 (0.2) | 0.207 |
| Fat mass (kg) | 22.8 (0.2) | 31.6 (0.3) | < 0.001 | 33.0 (0.38) | 28.9 (0.3) | < 0.001 | 26.6 (0.4) | 29.7 (0.3) | < 0.001 |
| Appendicular lean mass (%) | 24.0 (0.1) | 26.3 (0.1) | < 0.001 | 23.2 (0.1) | 26.3 (0.1) | < 0.001 | 25.1 (0.1) | 26.2 (0.1) | < 0.001 |
| Appendicular lean mass (kg) | 14.4 (0.1) | 22.5 (0.1) | < 0.001 | 18.8 (0.2) | 21.1 (0.1) | < 0.001 | 18.0 (0.3) | 21.5 (0.1) | < 0.001 |
| Muscular strength | |||||||||
| Knee extensor peak force (N/m) | 259.4 (4.2) | 392.0 (3.9) | < 0.001 | 321.8 (6.4) | 371.4 (2.8) | < 0.001 | 212.9 (3.1) | 398.2 (2.8) | < 0.001 |
| Metabolic risk factors | |||||||||
| Systolic blood pressure (mmHg) | 139.0 (0.9) | 132.1 (0.6) | < 0.001 | 138.1 (1.6) | 132.7 (0.5) | 0.001 | 138.7 (1.5) | 131.2 (0.6) | < 0.001 |
| Diabolic blood pressure (mmHg) | 69.3 (0.7) | 72.8 (0.4) | < 0.001 | 70.1 (0.7) | 72.5 (0.4) | 0.001 | 68 (0.9) | 73.9 (0.4) | < 0.001 |
| Cholesterol (mg/dl) | 218.8 (1.8) | 212.5 (1.2) | 0.009 | 213.7 (2.1) | 214.3 (0.9) | 0.8 | 212.6 (1.7) | 215.9 (1.1) | 0.089 |
| High density lipoprotein (mg/dl) | 61.8 (1.0) | 50.6 (0.4) | < 0.001 | 51.1 (0.7) | 53.4 (0.5) | 0.003 | 55.4 (1.3) | 52.8 (0.6) | 0.073 |
| Low density lipoprotein (mg/dl) | 127.5 (2.0) | 129.6 (1.3) | 0.357 | 127.0 (2.0) | 130.0 (1.2) | 0.212 | 124.9 (2.4) | 132.2 (1.7) | 0.018 |
| Total triglycerides (mg/dl) | 145.7 (3.9) | 172.0 (7.1) | 0.001 | 186.5 (22.5) | 161.8 (6.0) | 0.31 | 165.9 (8.6) | 165.7 (8.5) | 0.986 |
| Fasting glucose (mg/dl) | 103.7 (1.6) | 110.4 (1.3) | 0.002 | 114.3 (2.8) | 107.7 (1.3) | 0.049 | 109.6 (2.0) | 107.2 (1.4) | 0.331 |
| Fasting insulin (μU/ml) | 9.2 (0.3) | 13.6 (0.3) | < 0.001 | 15.2 (1.3) | 12.0 (0.3) | 0.032 | 11.7 (1.1) | 12.5 (0.4) | 0.546 |
| HOMA-IR (mg/dl×μU/ml) | 43.9 (1.5) | 70.7 (2.6) | < 0.001 | 82.2 (9.6) | 60.7 (2.1) | 0.047 | 62.2 (8.7) | 62.2 (2.5) | 0.998 |
| Metabolic syndrome | 166 (28.0) | 705 (50.0) | < 0.001 | 245 (56.1) | 604 (42.3) | < 0.001 | 139 (39.1) | 488 (44.8) | 0.138 |
| Comorbid conditions | |||||||||
| Cardiovascular diseases | 601 (52.3) | 1892 (55.9) | 0.126 | 582 (65.7) | 1826 (52.3) | < 0.001 | 453 (61.3) | 1222 (49.3) | < 0.001 |
| Diabetes | 148 (10.6) | 545 (13.0) | 0.006 | 184 (18.1) | 482 (11.1) | < 0.001 | 135 (15.8) | 283 (9.0) | < 0.001 |
| Cancer or malignancy | 185 (18.1) | 468 (15.4) | 0.109 | 139 (16.8) | 491 (15.7) | 0.581 | 145 (21.6) | 301 (13.4) | < 0.001 |
| Chronic Obstructive Pulmonary Disease | 135 (14.2) | 250 (8.6) | < 0.001 | 99 (12.4) | 275 (9.4) | 0.049 | 83 (13.2) | 159 (7.6) | < 0.001 |
| Chronic Kidney Disease | 378 (37.2) | 974 (30.7) | 0.005 | 273 (35.3) | 1012 (30.7) | 0.064 | 298 (42.7) | 602 (26.8) | < 0.001 |
All values are mean (SE) for continuous variables and counts (weighted percentages) for categorical variables.
A P value represents a t test for continuous variables or a Chi-Square test for categorical variables.
2. Prevalence of low muscle mass and low muscle strength
The prevalence of low muscle mass, low muscle strength, and their four combined subgroups differed significantly by sex and race (Figure 1). The prevalence of low muscle mass (defined by ALM) was significantly lower among men than among women (11.1% vs. 33.1%), while the prevalence of low muscle mass (defined by ALM/BMI) was higher in men than in women (19.3% vs. 15.1%). When the prevalence of four combined subgroups was compared between men and women, the trends were not consistent between two definitions of low muscle mass (ALM vs. ALM/BMI) (Figure 1A and 1B). The prevalence of low muscle mass was much higher in Hispanics (33.0% for ALM, and 28.9% for ALM/BMI) and other races (38.6% for ALM, and 22.6% for ALM/BMI) than in non-Hispanic whites (22.8% for ALM, 16.8% for ALM/BMI) or blacks (8.4% for ALM, 4.6% for ALM/BMI). The prevalence of four combined subgroups among races was similar between two definitions of low muscle mass (ALM vs. ALM/BMI). The prevalence of low muscle mass with or without low muscle strength was much higher in Hispanics and other races than in non-Hispanic whites and blacks (Figure 1C and 1D).
Figure 1.
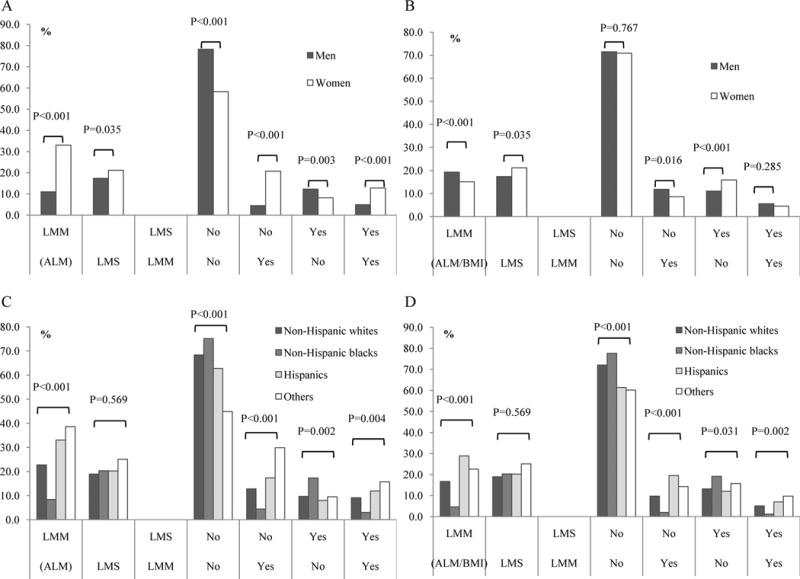
Prevalence of low muscle mass (LMM) and low muscle strength (LMS) in US adults aged 50 years and older in the NHANES 1999–2002.
A. Prevalence of LMM (ALM) and LMS in single and combination by gender; B. Prevalence of LMM (ALM/BMI) and LMS in single and combination by gender; C. Prevalence of LMM (ALM) and LMS in single and combination by race; D. Prevalence of LMM (ALM/BMI) and LMS in single and combination by race.
3. Associations of low muscle mass and low muscle strength with all-cause mortality
The results of the associations of low muscle mass or low muscle strength with all-cause mortality are presented in Figure 2A. In the fully adjusted models (Model 3), Low muscle strength was strongly associated with all-cause mortality (OR 2.34, 95% CI 1.71–3.20). But low muscle mass was not significantly associated with increased risk of all-cause mortality (OR 1.40, 95% CI 0.91–2.14), unless low muscle mass adjusted by BMI (OR 1.47, 95% CI 1.08–2.00).
Figure 2.
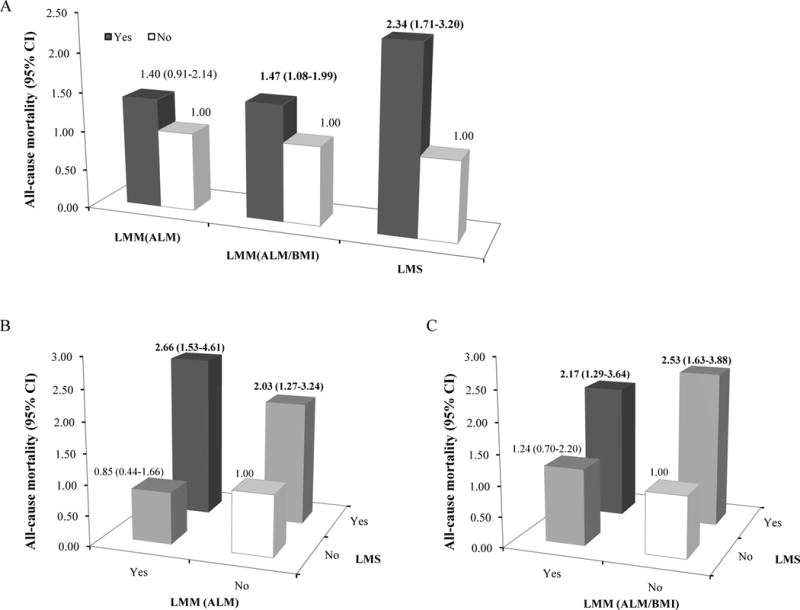
Associations of low muscle mass (LMM) and low muscle strength (LMS) with all-cause mortality.
A. Individual associations of LMM and LMS with all-cause mortality. B and C. Joint associations of LMM and LMS with all-cause mortality in US adults aged 50 years and older in the NHANES 1999–2002. The definitions of LMM were based on ALM (B) and ALM/BMI (C). Relative Risks (RR) of all-cause mortality were estimated from multivariable logistic regression models which were adjusted by age, gender, race, BMI, smoking, alcohol use, education, LTPA, sedentary time, CVD, diabetes, cancer, COPD, and CKD. CI: confidence interval. P for interaction = 0.235 in B, and 0.432 in C.
In the joint analyses in which those with normal muscle mass and muscle strength were the reference group, incidence of all-cause mortality was significantly higher among participants with low muscle strength but with normal muscle mass (OR 2.03, 95% CI 1.27–3.24 for ALM; OR 2.53, 95% CI 1.65–3.88 for ALM/BMI) and among those with low muscle strength and low muscle mass (OR 2.66, 95% CI 1.53–4.61 for ALM; OR 2.17, 95% CI 1.29–3.64 for ALM/BMI). However, the combination of low muscle mass with normal muscle strength was not significantly associated with all-cause mortality (OR 0.85, 95% CI 0.44–1.66, for ALM; OR 1.24, 95% CI 0.70–2.20 for ALM/BMI) (Figure 2 B and 2C). The interaction between low muscle strength and low muscle mass was not significant on all-cause mortality (P for interactions =0.19 if low muscle mass was defined by ALM and =0.40 if defined by ALM/BMI).
4. Joint associations of low muscle mass or low muscle strength with MetS, sedentary time, and LTPA
The results of the independent associations of MetS, sedentary time, or LTPA with all-cause mortality are presented in Figure 3A, 4A, and 5A, respectively. In the fully adjusted models, MetS and sedentary time were not significantly associated with all-cause mortality (OR 1.12, 95% CI 0.69–1.83 for MetS and OR 1.18, 95% CI 0.96–1.46 for sedentary time) whereas LTPA was significantly associated with increased risk of all-cause mortality (OR 1.84, 95% CI 1.44–2.35). The results of joint associations of low muscle mass or low muscle strength with MetS, sedentary time, and LTPA are shown in Figures 3–5, respectively. Among participants without MetS, those with low muscle mass had a significant risk of all-cause mortality (OR 2.67, 95% CI 1.35–5.27 for ALM and OR 2.18, 95% CI 1.17–4.07 for ALM/BMI) (Figure 3B and 3C).
Figure 3.
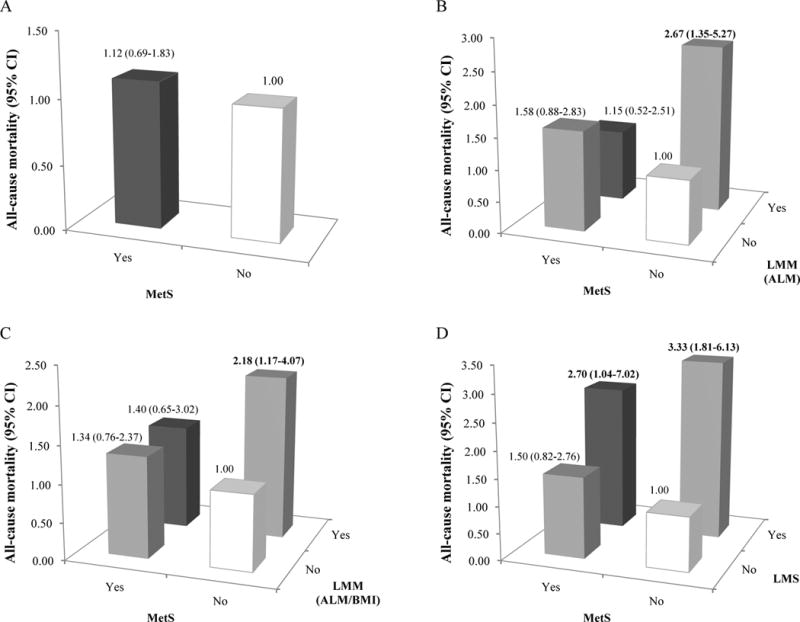
Joint associations of low muscle mass (LMM) and MetS, or low muscle strength (LMS) and MetS with all-cause mortality.
Individual association of MetS with all-cause mortality was shown in A. The definitions of LMM were based on ALM (B) and ALM/BMI (C) in the joint analyses of LMM and MetS; the joint associations of LMS and MetS with all-cause mortality were shown in D. Relative Risks (RR) of all-cause mortality were estimated from multivariable logistic regression models which were adjusted by age, gender, race, BMI, smoking, alcohol use, education, LTPA, sedentary time, CVD, diabetes, cancer, COPD, and CKD. P for interaction = 0.058 in A, 0.212 in B, and 0.317 in C.
Figure 4.
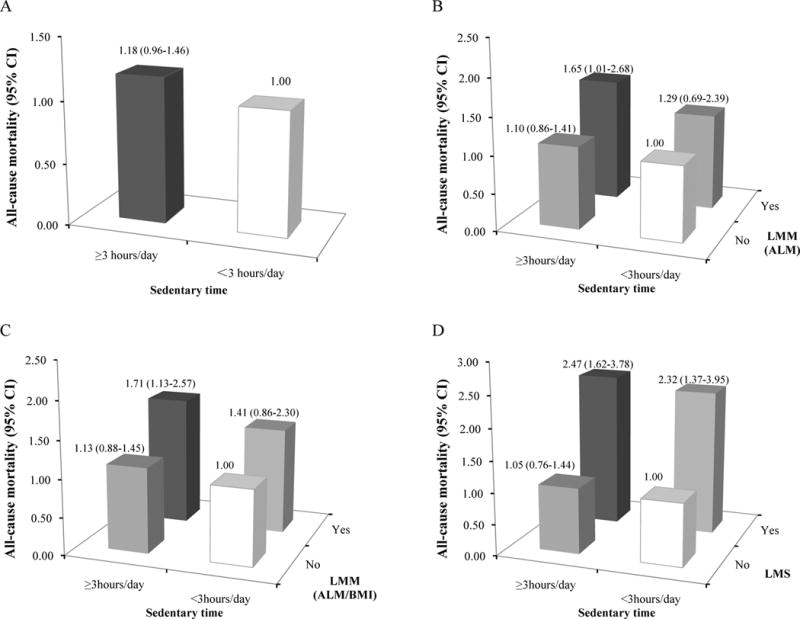
Joint associations of low muscle mass (LMM) and sedentary time, or low muscle strength (LMS) and sedentary time with all-cause.
Individual association of sedentary time with all-cause mortality was shown in A. The definitions of LMM were based on ALM (B) and ALM/BMI (C) in the joint analyses of LMM and sedentary time; the joint associations of LMS and sedentary time with all-cause mortality were shown in D. Relative Risks (RR) of all-cause mortality were estimated from multivariable logistic regression models which were adjusted by age, gender, race, BMI, smoking, alcohol use, education, LTPA, sedentary time, CVD, diabetes, cancer, COPD, and CKD. P for interaction = 0.651 in A, 0.808 in B, and 0.807 in C.
Figure 5.
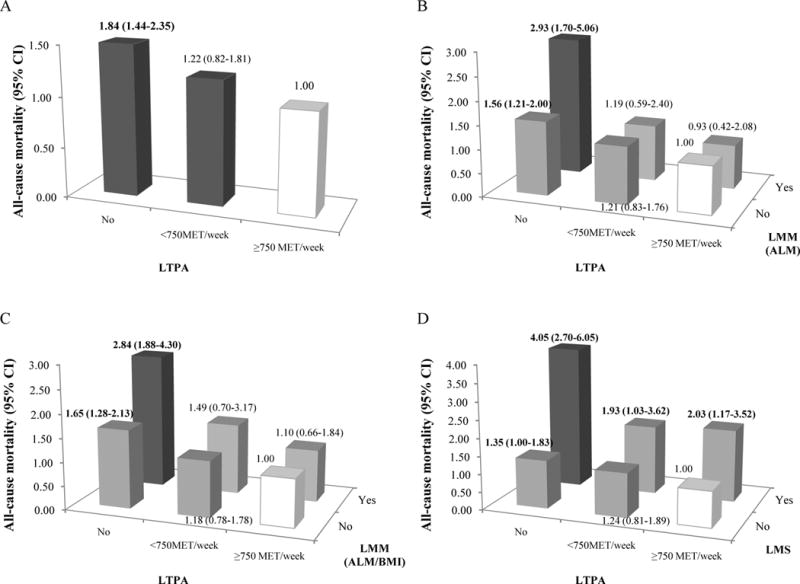
Joint associations of low muscle mass (LMM) and LTPA, or low muscle strength (LMS) and LTPA with all-cause mortality.
Individual associations of LTPA with all-cause mortality were shown in A. The definitions of LMM were based on ALM (B) and ALM/BMI (C) in the joint analyses of LMM and LTPA; the joint associations of LMS and LTPA with all-cause mortality were shown in D. Relative Risks (RR) of all-cause mortality were estimated from multivariable logistic regression models which were adjusted by age, gender, race, BMI, smoking, alcohol use, education, LTPA, sedentary time, CVD, diabetes, cancer, COPD, and CKD. P for interaction = 0.038 in A, 0.152 in B, and 0.187 in C.
The participants with low muscle mass and sedentary time (≥3 hours) had significant association (OR 1.65, 95% CI 1.01–2.68 for ALM and OR 1.71, 95% CI 1.13–2.57 for ALM/BMI) with all-cause mortality comparing to those with normal muscle mass and sedentary time (<3 hours) (Figure 4B and 4C). As participants with LTPA (≥ 750 MET/week) and normal muscle mass were the reference groups, those with low muscle mass and no LTPA had significant associations (OR 2.93, 95% CI 1.70–5.06 for ALM and OR 2.84, 95% CI 1.88–4.30 for ALM/BMI) (Figure 5B and 5C). In contrast, there was always significantly increased all-cause mortality among participants with low muscle strength, regardless of the presence or absence of MetS (Figure 3D), sedentary time (≥3 hours) (Figure 4D), and three levels of LTPA (Figure 5D). Interaction testing was statistically significant only between low muscle mass (defined by ALM) and LTPA (p=0.04) on all-cause mortality.
Discussion
In a nationally representative sample of 4,449 US adults aged 50 years and older from the NHANES surveys, our study found that low muscle strength was more strongly and significantly associated with all-cause mortality than low muscle mass. Furthermore, we found that individuals in the subgroup with low muscle mass had significantly increased risk for all-cause mortality only when they also had low muscle strength. Consistently, the significant associations between low muscle strength and increased risk of all-cause mortality persisted regardless of MetS, sedentary time, and LTPA, suggesting that muscle strength may be more important than muscle mass in predicting aging-related health outcomes in older and elderly adults.
Clark and Manini initially proposed that the term “sarcopenia” be used to describe age-related reduction of muscle mass and the term “dynapenia” to describe age-related loss of muscle strength (6, 30). Over the past three decades, the scientific and medical communities have recognized that muscle dysfunction, as reflected by low muscle strength, is a debilitating and life-threatening condition in older adults (30). For example, age-related loss of muscle strength has been strongly associated with physical disability and mortality in older adults (10, 31, 32). In the present study, we provided confirmatory evidence that low knee extensor strength indicative of low muscle strength is independently and significantly associated with increased risk of all-cause mortality among US older adults regardless of muscle mass, MetS risk factors, sedentary time, or LTPA. Our findings in US older adults were consistent with previous studies indicating that muscle strength may be an more important independent risk factor for all-cause mortality compared with muscle mass among elderly Medicare enrolled adults (10). Our findings also support the notion that loss of muscle mass alone may not fully reflect muscular function and that muscle strength should be considered an important component in defining sarcopenia.
Longitudinal studies have shown that age-related decline in muscle strength is not proportionally related to loss of muscle mass (6). This may, at least in part, explain our observation that muscle strength is independently and significantly associated with health outcomes regardless of muscle mass. The mechanisms underlying age-related decline in muscle strength can be attributed to a combination of neural and muscular factors, such as a reduction in descending excitatory drive from supraspinal centers/cortical levels and/or suboptimal recruitment and rate coding of motor units, the loss in the intrinsic force-generating capacity of muscle, changes in actomyosin structure and function, and infiltration of adipocytes into muscle fibers (30).
Our study also indicated the potential clinical usefulness of a simple knee extensor strength-based definition for low muscle strength. First, knee extensor strength can be measured clinically with a simple, inexpensive, and reliable strain gauge, which is quick to administer for populations at risk. Compared with its counterparts, those with low knee extensor strength performed worse in physical performance tests, which include balance, reaction time, gait speed, and the Timed Up and Go (16, 33). Those with low knee extensor strength also had higher fall risk scores and were 43% more likely to fall at home in the year following assessment (16). In addition, the knee extensors are the key muscle group in performing sit-to-stand transfers (34), maintaining balance (35), and walking steadily (15).
Loss of muscle mass with aging may not be more useful than low muscle strength in predicting all-cause mortality. However, maintenance of muscle mass with advancing age is critical, because it serves as a metabolic reservoir that is needed to effectively withstand disease (36, 37). Recent studies have suggested that obesity might be closely associated with low muscle mass and contribute to an additive or synergistic risk for CVD derived from both sarcopenia and obesity (38, 39). The majority of obese persons who develop adverse health outcomes, including mortality, typically have a clustering of major and emerging risk factors, including insulin resistance, dyslipidemia, and elevated BP, as known as the MetS. We found that participants with MetS did not have significantly increased risk of mortality compared with those without MetS when they have normal muscle mass. Only participants with low muscle mass but without MetS had a significant increased risk of all-cause mortality.
Sedentary time and LTPA are highly correlated with muscular adiposity and/or muscle mass and function. Although a science advisory from the American Heart Association suggests that sedentary behavior could contribute to excess morbidity and mortality (18), there is insufficient prospective evidence to establish such an association between sedentary time and mortality (19). However, in less-active adults, replacing 1 h of sedentary time with activity of light or moderate-to-vigorous intensity was significantly associated with 18% and 42% lower mortality, respectively (40). We found that participants with a great deal of sedentary time had significant increased risk for all-cause mortality when they also had low muscle mass or low muscle strength. Lack of LTPA was significantly associated with all-cause mortality regardless of muscle strength. On the other hand, there were consistently significant associations between low muscle strength and all-cause mortality in participants with any LTPA, even LTPA≥750 MET/week. Although there was no statistically significant interaction between low muscle strength and LTPA, our data suggest that muscle strength and LTPA are independent predictors of all-cause mortality in older and elderly adults.
A major strength of this study is its prospective design based on a nationally representative sample of US older adults in the NHANES with a large number of deaths, which provides sufficient statistical power to support the generalizability of the findings. Our study also included objective measures of muscle mass and knee extensor strength. Furthermore, we examined not only the interrelationship of muscle mass and knee extensor strength but also their joint associations with physical activity, sedentary time, and MetS. However, there are several limitations that deserve consideration. First, the survey assesses only noninstitutionalized older adults, omitting nursing home residents or those with severe disabilities, who have higher degrees of low muscle mass and low muscle strength. Second, the appendicular muscle mass detected by DEXA may not accurately reflect overall muscle mass throughout the body. Third, only knee extensor strength was used to represent muscle strength because the NHANES lacked a complete set of muscle-strength tests. Fourth, sedentary time, leisure-time physical activity, and several medical comorbidities were assessed based on self-reported information, which may introduce measurement errors. Finally, we acknowledge that logistic regression modeling may not fit mortality data very well since it cannot account for time-to-event and censoring information. Additionally, the NHANES only provides a baseline assessment of muscle strength, which cannot allow us to model longitudinal strength decline.
In conclusion, our study based on a nationally representative sample of US older adults demonstrates that low knee extensor strength indicative of low muscle strength was independently and significantly associated with an increased risk of all-cause mortality regardless of muscle mass, MetS, sedentary time, and LTPA. Our findings indicate that low muscle strength may be a more meaningful component of a sarcopenia definition than loss of muscle mass in predicting health outcomes. Further investigation is warranted to validate different operational criteria for assessing muscle mass, muscle strength, and/or physical performance for defining sarcopenia in population studies in predicting various age-related health outcomes. Our findings imply that muscle strength appears more important than muscle mass are of considerable clinical interest as muscle strength can be improved (without increasing muscle mass) in older adults with resistance exercise.
Acknowledgments
The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the United States Centers for Disease Control and Prevention. The mortality data are from the National Center for Health Statistics. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation, and statement that results of the present study do not constitute endorsement by ACSM.
Funding Sources
Dr. Ran Li was supported by the State Scholarship Fund of China; Dr. Yiqing Song was supported by the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative Grant.
Footnotes
Disclosures
None
References
- 1.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990S–1S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 2.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 3.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55(8):1216–23. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 4.Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr Res. 2015;35(12):1031–9. doi: 10.1016/j.nutres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68(9):1001–7. doi: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 6.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63(8):829–34. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 7.Katzmarzyk PT, Craig CL. Musculoskeletal fitness and risk of mortality. Med Sci Sports Exerc. 2002;34(5):740–4. doi: 10.1097/00005768-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:a439. doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artero EG, Lee DC, Ruiz JR, et al. A prospective study of muscular strength and all-cause mortality in men with hypertension. J Am Coll Cardiol. 2011;57(18):1831–7. doi: 10.1016/j.jacc.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–7. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 11.Warburton DE, Bredin SS. Reflections on Physical Activity and Health: What Should We Recommend? Can J Cardiol. 2016;32(4):495–504. doi: 10.1016/j.cjca.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Giacomantonio NB, Bredin SS, Foulds HJ, Warburton DE. A systematic review of the health benefits of exercise rehabilitation in persons living with atrial fibrillation. Can J Cardiol. 2013;29(4):483–91. doi: 10.1016/j.cjca.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–58. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki M, Kirimoto H, Inamura A, Yagi M, Omori Y, Yamada S. The relationship between knee extension strength and lower extremity functions in nursing home residents with dementia. Disabil Rehabil. 2012;34(3):202–9. doi: 10.3109/09638288.2011.593678. [DOI] [PubMed] [Google Scholar]
- 15.Tiedemann A, Sherrington C, Lord SR. Physiological and psychological predictors of walking speed in older community-dwelling people. Gerontology. 2005;51(6):390–5. doi: 10.1159/000088703. [DOI] [PubMed] [Google Scholar]
- 16.Menant JC, Weber F, Lo J, et al. Strength measures are better than muscle mass measures in predicting health-related outcomes in older people: time to abandon the term sarcopenia? Osteoporos Int [Internet] 2016 Jul 9; doi: 10.1007/s00198-016-3691-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27394415. [DOI] [PubMed]
- 17.Edwards MK, Loprinzi PD. All-cause mortality risk as a function of sedentary behavior, moderate-to-vigorous physical activity and cardiorespiratory fitness. Phys Sportsmed. 2016;44(3):223–30. doi: 10.1080/00913847.2016.1221751. [DOI] [PubMed] [Google Scholar]
- 18.Endorsed by The Obesity S. Young DR, Hivert MF, et al. Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory From the American Heart Association. Circulation. 2016;134(13):e262–79. doi: 10.1161/CIR.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 19.Evenson KR, Wen F, Herring AH. Associations of Accelerometry-Assessed and Self-Reported Physical Activity and Sedentary Behavior With All-Cause and Cardiovascular Mortality Among US Adults. Am J Epidemiol. 2016;184(9):621–32. doi: 10.1093/aje/kww070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buch A, Carmeli E, Boker LK, et al. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age–An overview. Exp Gerontol. 2016;76:25–32. doi: 10.1016/j.exger.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Centers for Disease Control and Prevention Web site [Internet] Atlanta(GA): Centers for Disease Control and Prevention; [cited 2016 July 30]. Available from: http://www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- 22.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Wei G, Jalili T, et al. The Associations of Plant Protein Intake With All-Cause Mortality in CKD. Am J Kidney Dis. 2016;67(3):423–30. doi: 10.1053/j.ajkd.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navaneethan SD, Kirwan JP, Arrigain S, Schold JD. Adiposity measures, lean body mass, physical activity and mortality: NHANES 1999–2004. BMC Nephrol. 2014;15:108. doi: 10.1186/1471-2369-15-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Healy GN, Clark BK, Winkler EA, Gardiner PA, Brown WJ, Matthews CE. Measurement of adults’ sedentary time in population-based studies. Am J Prev Med. 2011;41(2):216–27. doi: 10.1016/j.amepre.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67(6):2089–100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Plowman SA, Smith DL. Exercise physiology for health, fitness, and performance. 3rd. Philadelphia: Lippincott Williams & Wilkins; 2011. p. 726. [Google Scholar]
- 29.Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67(1):28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraschnewski JL, Sciamanna CN, Poger JM, et al. Is strength training associated with mortality benefits? A 15year cohort study of US older adults. Prev Med. 2016;87:121–7. doi: 10.1016/j.ypmed.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 32.Volaklis KA, Halle M, Meisinger C. Muscular strength as a strong predictor of mortality: A narrative review. Eur J Intern Med. 2015;26(5):303–10. doi: 10.1016/j.ejim.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Schoene D, Wu SM, Mikolaizak AS, et al. Discriminative ability and predictive validity of the timed up and go test in identifying older people who fall: systematic review and meta-analysis. J Am Geriatr Soc. 2013;61(2):202–8. doi: 10.1111/jgs.12106. [DOI] [PubMed] [Google Scholar]
- 34.Corrigan D, Bohannon RW. Relationship between knee extension force and stand-up performance in community-dwelling elderly women. Arch Phys Med Rehabil. 2001;82(12):1666–72. doi: 10.1053/apmr.2001.26811. [DOI] [PubMed] [Google Scholar]
- 35.Carty CP, Barrett RS, Cronin NJ, Lichtwark GA, Mills PM. Lower limb muscle weakness predicts use of a multiple- versus single-step strategy to recover from forward loss of balance in older adults. J Gerontol A Biol Sci Med Sci. 2012;67(11):1246–52. doi: 10.1093/gerona/gls149. [DOI] [PubMed] [Google Scholar]
- 36.Kim TN, Choi KM. The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease. J Cell Biochem. 2015;116(7):1171–8. doi: 10.1002/jcb.25077. [DOI] [PubMed] [Google Scholar]
- 37.Shintakuya R, Uemura K, Murakami Y, et al. Sarcopenia is closely associated with pancreatic exocrine insufficiency in patients with pancreatic disease. Pancreatology. 2017;17(1):70–5. doi: 10.1016/j.pan.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Srikanthan P, Horwich TB, Tseng CH. Relation of Muscle Mass and Fat Mass to Cardiovascular Disease Mortality. Am J Cardiol. 2016;117(8):1355–60. doi: 10.1016/j.amjcard.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 39.Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc. 2014;62(2):253–60. doi: 10.1111/jgs.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthews CE, Keadle SK, Troiano RP, et al. Accelerometer-measured dose-response for physical activity, sedentary time, and mortality in US adults. Am J Clin Nutr. 2016;104(5):1424–32. doi: 10.3945/ajcn.116.135129. [DOI] [PMC free article] [PubMed] [Google Scholar]


