Abstract
Sensory and autonomic neurons of the peripheral nervous system (PNS) play a critical role in regulating the immune system during tissue inflammation and host defense. Recent studies have identified the molecular mechanisms underlying the bidirectional communication between the nervous system and the immune system. Here, we highlight the studies that demonstrate the importance of the neuro-immune interactions in health and disease. Nociceptor sensory neurons detect immune mediators to produce pain, and release neuropeptides that act on the immune system to regulate inflammation. In parallel, neural reflex circuits including the vagus nerve-based inflammatory reflex are physiological regulators of inflammatory responses and cytokine production. In transplantation, neuro-immune communication could significantly impact the processes of host-pathogen defense, organ rejection, and wound healing. Emerging approaches to target the PNS such as bioelectronics could be useful in improving the outcome of transplantation. Therefore, understanding how the nervous system shapes the immune response could have important therapeutic ramifications for transplantation medicine.
1. Introduction
The mammalian peripheral nervous system (PNS) coordinates the function and physiology of major organ systems and barrier tissues. It is increasingly clear that the PNS also plays a critical role in communicating with and regulating the function of the immune system. Sensory neurons innervate joints, skin, muscles and the visceral organs, transmitting information about thermal, mechanical, inflammatory, and noxious stimuli to brain, and are an important component of the peripheral nervous system (1). Catecholamines and acetylcholine released by sympathetic and vagus nerve fibers are important mediators of many physiological functions including heart rate, urinary output, and gut motility. In addition, neurotransmitters and neuropeptides interacting with cognate receptors expressed on immune cells including neutrophils, macrophages, dendritic cells, T cells, B cells, and innate lymphoid cells regulate inflammation and cytokine production (2, 3). Recent studies have advanced our understanding of peripheral neural pathways that regulate immune responses in autoimmune and inflammatory conditions including arthritis, colitis, asthma, endotoxemia and sepsis (2–4). Understanding this neuro-immune crosstalk in preclinical models has important implications for the treatment of disease.
Here we highlight studies elucidating bidirectional neuro-immune communication in health and disease. During inflammation, nociceptor sensory neurons respond to immune cells and their molecular mediators to signal pain; these neurons then release neuropeptides that actively regulates immune cell function (Figure 1). In addition, neural reflex pathways mediated by the afferent and efferent fibers in the vagus nerve, modulate innate and adaptive immune responses in different visceral organ systems (Figure 2). Given the role of neural reflex pathways in modulating immune responses in distal organs, it is likely that the immune status of donor tissue during transplantation could be controlled by neural pathways. The majority of donor organs are harvested from deceased donors, and are associated with a number of immunological abnormalities. Furthermore, in transplantation recipients, surgical or pharmacological perturbations may significantly alter PNS function. The resulting changes in neuro-immune signaling could affect the outcome of wound healing and inflammation. Targeting these neuro-immune pathways using vagus nerve stimulation or pharmacological approaches may improve the outcome of transplantation.
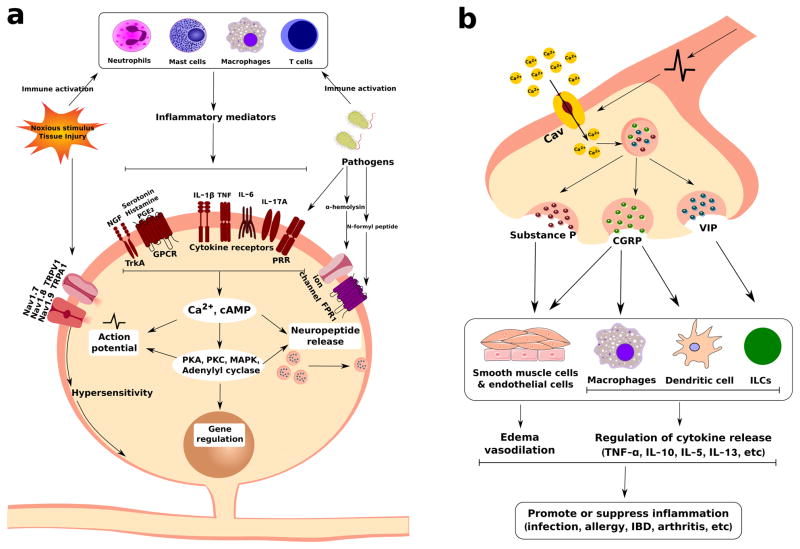
Figure 1. Nociceptor Sensory Neurons Crosstalk with Immune Cells in Pain and Inflammation.
(a) Nociceptor neurons are activated by immune cells and their molecular mediators. Pathogens, tissue injury, and other inflammatory insults trigger a immune response. These immune cells including neutrophils, mast cells, macrophages and T cells release molecular mediators including pro-inflammatory cytokines, nerve growth factor (NGF), prostaglandin E2 (PGE2), serotonin and histamine. Nociceptor sensory neurons express receptors for these immune-derived mediators, including cytokine receptors, G-protein coupled receptors, and tyrosine kinase receptor type 1 (TrkA). Sensory neurons also express pathogen recognition receptors including FPR1, and toll-like receptors (TLRs). The bacterial toxin α-hemolysin also forms pores in neuronal membranes to directly activate neurons. Upon ligand binding, generation of second messenger signaling through cAMP or Ca2+ can lead to intracellular phosphorylation cascades that produce changes in the gating properties of TRPV1, TRPA1 cation channels and the voltage-gated sodium channels Nav1.7, Nav1.8, and Nav1.9. These ion channels are critical for pain production, and their sensitization during inflammation leads to increased action potential generation, neuronal plasticity, and transcriptional changes in nociceptor neurons that results in pain. (b) Nociceptor neurons play an important role in regulating immune cell function by communication with them through neuropeptides. In peripheral nerve terminals, neuropeptides including substance P, calcitonin gene-related peptide (CGRP), and vasoactive intestinal peptide (VIP) are stored in dense-core vesicles. During inflammation, generation of action potentials, local axonal reflexes, and Ca2+ entry into these terminals leads to SNARE-mediated vesicle release of these neuropeptides. These neuropeptides then regulate tissue inflammation by activating receptors present on smooth muscle cells, endothelial cells, macrophages, dendritic cells and innate lymphoid cells (ILCs). These neuro-immune interactions mediate host-pathogen responses, asthma, inflammatory bowel disease, arthritis, and other inflammatory disease conditions.
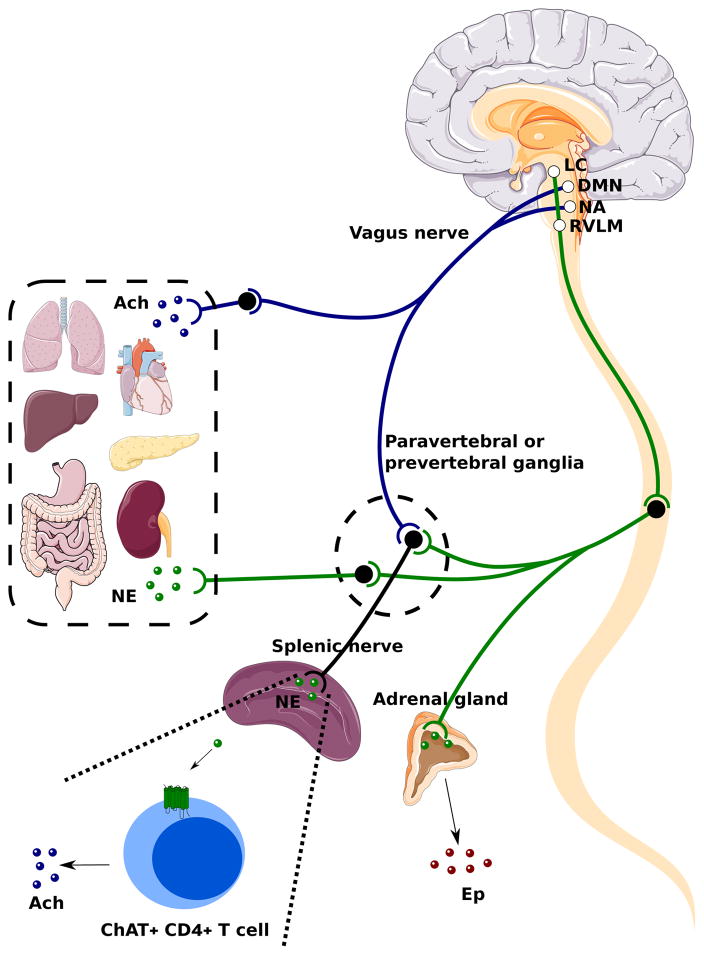
Figure 2. Vagal Efferent Anatomy and the Cholinergic Anti-inflammatory Reflex.
Visceral organs in the thoracic and abdominal cavity including the lungs, heart, liver, stomach, gut, pancreas and kidneys are innervated by parasympathetic and sympathetic nerves. The efferent portion of the vagus nerve, which is the preganglionic parasympathetic fiber, originates from the dorsal motor nucleus (DMN) and nucleus ambiguous (NA) in the medulla oblongata and project to postganglionic fibers in proximity with or within visceral organs. Both the preganglionic and postganglionic vagal fibers are cholinergic. Postganglionic vagal fibers release acetylcholine (Ach) into viscerl organs to regulate smooth muscle activity, gut motility, and gland secretion. In the spinal cord, sympathetic neurons received descending projects from the locus coeruleus (LC) and the rostroventrolateral medulla (RMLM) in the brainstem. The sympathetic preganglionic fibers interact with postganglionic fibers within the paravertebral and prevertebral ganglia. Postganglionic fibers from paravertebral and prevertebral ganglia innervate visceral organs in the thorax (lungs, heart) and the abdominal cavity (liver, stomach, gut, kidneys, pancreas) respectively, and release norepinephrine (NE). Sympathetic preganglionic fibers also innervate the adrenal medulla directly and stimulate the secretion of epinephrine (EP) from chromaffin cells. Both vagal and sympathetic preganglionic fibers give projections to the splenic nerve within the celiac and superior mesenteric ganglion. In a cholinergic anti-inflammatory reflex, NE released by the splenic nerve acts on choline acetyltransferase (ChAT)+ CD4+ T cells through β2-adrenergic receptors. These T cells release Ach to regulate macrophage production of TNF-α and other cytokines.
2.1. Sensory Neuron-Immune Interactions in Pain and Inflammation
Pain (Dolor) was defined as one of the four cardinal signs of inflammation by Celsus in 20 A.D. Pain is mediated by nociceptor sensory neurons, which detect noxious/harmful stimuli to protect organisms from danger. Nociceptor neurons are pseudo-unipolar; their cell bodies reside within the Dorsal Root Ganglia (DRG), while their axon is split into one peripheral branch, which innervates the barrier tissues including the skin, lungs, gut, and one central branch, which innervates the spinal cord to transmit noxious signals to the central nervous system (CNS) to be processed as pain. The nerve terminals of nociceptors are equipped with an array of molecular sensors, which detect heat, cold, protons, ATP, as well as receptors for immune mediators including cytokines, lipid mediators, and growth factors.
Immune cells play a critical role in mediating pain during inflammation. Pain hypersensitivity occurs due to an increased responsiveness of nociceptor neurons to thermal and mechanical stimuli. Several types of tissue-resident and infiltrating immune cells act in concert to release cytokines and other mediators to sensitize nociceptor neurons (Figure 1A). Upon degranulation, mast cells release TNF, IL-6, IL-1β, serotonin (5-HT), histamine, and nerve growth factor (NGF) which act on pain fibers (5, 6). After nerve injury, mast cells infiltrate the spinal cord and thalamus to sensitize CNS pain circuits (6). Tissue-resident macrophages and circulating monocytes drive pain associated with incisional wound injury and sciatic nerve injury (7, 8). Microglia, the resident innate immune cells of the CNS, also critically mediate neuropathic pain (9–12). P38/ERK is activated in microglia following nerve injury, leading to their release of prostaglandins and IL-1β within the spinal cord (9, 12). Inflammatory neutrophils contribute to pain by release of neutrophil elastase, which activates protease activated receptor-2 (PAR2) on neurons (13). T cells also contribute to chronic pain by infiltrating the DRG and release of leukocyte elastase (14). The immune contribution to pain may be sexually dimorphic: a recent study showed that T cells were necessary for chronic neuropathic pain in female mice, whereas microglia but not T cells drove chronic pain sensitization in male mice (11).
To respond to the immune system, nociceptor neurons express a wide array of receptors for cytokines, lipids, and growth factors (Figure 1A). For example, nociceptors express receptors for the proinflammatory cytokines IL-1β, TNF, IL-6, and IL-17A, which play roles in pain sensitization in rheumatoid arthritis and osteoarthritis. Nociceptors express G-protein coupled receptors that respond to mast cell-derived histamine and serotonin (5-HT). Prostaglandin E2 (PGE2) is also a critical mediator of pain by targeting EP1-EP4 on neurons. Non-steroidal anti-inflammatory drugs (NSAIDs) act to inhibit cyclooxygenase mediated production of PGE2. Nociceptors also express TrkA, which recognizes nerve growth factor (NGF) released by macrophages and mast cells during inflammation. In addition to responding to immune-derived mediators, nociceptor neurons are also able to respond to pathogens and their molecular mediators including N-formyl peptides, the toxin α-hemolysin, and TLR ligands (15, 16) (Figure 1A). Neuronal signaling through cytokine and growth factor receptors leads to calcium influx, cAMP induction, as well as kinase signaling pathways through PKC, PLCγ and MAP Kinases, which converge to induce changes in neuronal membrane properties and the gating of nociceptive ion channels (Figure 1A). Nav1.7, Nav1.8, and Nav1.9 are voltage-gated sodium channels that play a critical role in generation of action potentials in nociceptors; congenital mutations in these channels are linked to human insensitivity to pain. Transient Receptor Potential (TRP) channels including TRPV1, TRPA1, TRPV4, mediate the sensation of noxious thermal and mechanical stimuli (1, 17). Immune-mediated signaling within nociceptor neurons lead to phosphorylation of TRP channels and voltage-gated sodium channels to decrease their threshold for activation, thus potentiating pain hypersensitivity. Therefore, nociceptor neurons are exquisitely tuned to respond to the immune system to produce pain.
2.2. Nociceptor Sensory Neuron Regulation of the Immune Response
Nociceptor neurons also play an important role modulating the function of immune cells (Figure 1B). The neuropeptides substance P (SP), calcitonin gene-related peptide (CGRP), and vasoactive intestinal peptide (VIP) are small, positively charged peptides stored within dense-core vesicles at nociceptor nerve terminals. During pain production, local axonal reflexes and calcium influx into peripheral nerve terminals leads to SNARE-mediated vesicular fusion and release of neuropeptides which have potent effects on the vasculature and immune system, a process termed “neurogenic inflammation”. SP and CGRP act on smooth muscle cells and endothelial cells to produce edema and vasodilation, respectively.
Recent work has shown that SP, CGRP, and VIP have potent effects on the immune response by binding their cognate receptors on macrophages, dendritic cells, T cells and innate lymphoid cells to regulate their function (Figure 1B). Nociceptor neurons play an important role in host defense against pathogens. In skin infection by the bacterial pathogen Staphylococcus aureus, nociceptor neurons suppressed the infiltration of myeloid immune cells into infected skin and draining lymph node immune cell recruitment (16). By contrast, in skin infections caused by the fungal pathogen Candida albicans, nociceptors drove dendritic cell activation and expression of IL-23 to mediate host protection (18). The differential effects observed may be due to the type of nociceptor neurons and immune cells involved. For example, the epidermal and dermal layers are innervated by many types of sensory fibers, including peptidergic and nonpeptidergic nociceptors. Each of these nociceptor subsets could be interacting differentially with distinct innate or adaptive immune cells to mediate either immune activation or suppression depending on the type of pathogenic stimulus. Given the importance of preventing infections during and after organ transplantation, the role of nociceptor neurons and modulation of pain could aberrantly affect the outcome of infection.
Nociceptor neurons also play direct roles in driving inflammatory diseases of the respiratory tract, skin, and gut. In mouse models of asthma, nociceptor neurons drive bronchoconstriction, airway hyperreactivity, and type 2 immune cell responses (19–21). Neurons have been found to release VIP in the lungs, which acts on type 2 innate lymphoid cells (ILC2) to recruit downstream T cell and eosinophil responses to drive antigen-specific allergic responses (19). Nociceptor neurons have also been found to drive inflammation in a mouse model of psoriasis; nociceptors interact with Dendritic cells to induce their production of IL-23, that in turn drives IL-17A production and skin pathology (22). In mouse models of colitis, the nociceptive ion channel TRPA1 and neuropeptide SP drive cytokine production and tissue inflammation (23). By contrast, the cold-sensing ion channel TRPM8 and the nociceptive neuropeptide CGRP downmodulates colitis-associated inflammation (24). Therefore, nociceptor neurons actively communicate with immune cells through neuropeptides to play potent roles in host defense and inflammation.
3.1. Neural reflex regulation of inflammation
Advances in the immunological and neurophysiological techniques have facilitated the studies of neural reflex circuits that regulate inflammatory responses. A typical neural reflex circuit is comprised of sensory neurons that transmit information about peripheral changes to the interneurons in the CNS, and motor neurons which in turn relay efferent signals to the peripheral tissues. The inflammatory reflex is the prototypical neural circuit comprised of afferent and efferent signals in the vagus nerve that regulate the output of the innate immune system (2, 25, 26). Within the immune system, lymphoid organs including thymus, lymph nodes, liver and spleen are richly innervated by a peripheral neural network that transmits information to and from the CNS in response to infection or sterile injury, and modulate inflammatory responses. Peripheral inflammatory signals play an important role in regulating host defense responses orchestrated by the CNS. Pioneering work by the Watkins group in the 1990s have mapped how the intact vagus nerve is required for a fever response to low doses of intraperitoneal IL-1β, suggesting that vagus sensory fibers are important to detect and transmit the information about peripheral immune responses to CNS (27, 28). Afferent vagus nerve signals are also implicated in mediating the sickness syndrome, associated with febrile response, social withdrawal, lethargy and other symptoms, in response to peripheral inflammatory challenges (29, 30). Vagal sensory neurons are activated in response to infectious challenges and inflammatory mediators via pattern recognition receptors or cytokine receptors expressed on these sensory neurons or on the chemosensory cells in the associated vagal paraganglia (30–32). Furthermore, in addition to inducing afferent vagus nerve activity, peripheral increases in IL-1β induce increased splenic nerve activity (33–35). Together, these studies opened up an important question of whether inflammatory mediators lead to an activation of a selective neural ensemble within the vagus nerve. Using electrophysiogical recordings, a recent study demonstrated that inflammatory cytokines (IL-1β and TNF), induce discriminating patterns of afferent vagus nerve activity or “neurograms” (36). This cytokine-specific activity is absent in animals with genetic ablation of the IL-1β or TNF receptors. Furthermore, selective vagus transection distal to the recording electrodes ablates the signals, indicating that majority of the signals are associated with afferent vagus fibers (36). These studies indicate that afferent vagus nerve fibers mediates the sensory arc of the inflammatory reflex, where it senses perturbations in peripheral immune homeostasis and transmits the information to the CNS in a mediator-specific manner.
The discovery of the motor arc of the inflammatory reflex occurred during studies focused on delineating the anti-inflammatory mechanisms of CNI-1493, a tetravalent guanylhydrazone. CNI-1493 (Cytokine Network Incorporation-1493) was originally described as an inhibitor of macrophage activation, carrageenan-induced inflammation and endotoxin-induced lethality (37). CNI-1493 suppressed cytokine release by inhibiting phosphorylation of p38 mitogen activated protein kinase (37–39). During the course of studying the activity of CNI1493 in cerebral ischemia, it was observed that intracerebral administration of low quantities of CNI-1493 not only inhibited inflammatory responses in the brain but also in peripheral organs including spleen, liver and heart (40). This was a surprising result as the quantities administered in the brain were less than required to suppress macrophages in vitro. By selectively cutting the vagus nerve, it was then possible to isolate signals from the brain to the periphery, and show that an intact vagus nerve was required for cytokine inhibition in the periphery. These results indicated that signals descending from the brain stem, and transmitted in the vagus nerve regulated inflammatory responses in the visceral organs (Figure 2). Efferent signals traveling in the vagus nerve terminate in the celiac ganglia (41, 42), and activate adrenergic splenic nerve, which originate from the celiac ganglia. Norepinephrine released by splenic neurons induces acetylcholine release by a small subset of splenic T cells that express choline acetyltransferase (ChAT), a rate-limiting enzyme in the acetylcholine biosynthesis (43, 44). Nude mice deficient in T cells fail to respond to suppressive effects of the vagus nerve stimulation, and adoptive transfer of ChAT-expressing T cells into nude mice restores the anti-inflammatory effect, indicating that ChAT-expressing T cells are necessary for the integrity of the inflammatory reflex (44). Acetylcholine released by these splenic T cells under vagus nerve control binds to α7 nicotinic acetylcholine receptor (α7nAChR) on macrophages (44, 45) and suppresses cytokine production via activation of a JAK2/STAT3 pathway, suppression of NF-kB nuclear translocation, and attenuation of inflammasome activation (2, 46–48). Subsequent studies have established the anti-inflammatory and disease-alleviating function of vagus nerve signaling in a number of preclinical disease models including endotoxemia (42), sepsis (49), arthritis (50), pancreatitis (51), ischemia reperfusion and injury (52, 53), inflammatory bowel disease (54), post-operative ileus (55), hemorrhagic shock (47), and autoimmune myocarditis (56).
These preclinical studies have paved the way to clinical exploration of neuromodulation modalities using bioelectronic devices. Bioelectronic medicine is an emerging field that focuses on targeting neural networks that regulate molecular mechanisms of disease, and using bioelectronic devices to modulate these neural networks as a treatment strategy. Two recent clinical studies demonstrated efficacy of electrical stimulation of the inflammatory reflex in attenuating disease severity in inflammatory and autoimmune diseases (57, 58). Rheumatoid arthritis is a disabling chronic autoimmune disease characterized by synovial inflammation and painful swollen joints. Stimulation of the inflammatory reflex using implanted bioelectronic devices in rheumatoid arthritis patients inhibited cytokine production and attenuated disease severity (58). Withdrawal of the vagus nerve stimulation exacerbated the disease, and reinstating the stimulation treatment restored the protective effect. Another clinical study reported significant benefit of vagus nerve stimulation using implanted devices in Crohn’s disease patients (57). Crohn’s disease is a debilitating chronic inflammatory disease of the bowel, and affects mainly young adults. Chronic vagus nerve stimulation for six months offered significant clinical, biological and endoscopic remission in five out of seven patients. These beneficial effects were associated with restoration of vagus nerve activity as assessed by heart rate variability (57).
3.2. Neural Reflex Regulation of Transplantation
In transplantation medicine, inflammatory reactions in the graft have a pivotal influence on acute as well as long-term function of the transplanted graft. The inflammatory responses in the donor organs are principally mediated by ischemia/reperfusion injury in the donor organ, infection, and rejection episodes in the recipient. Currently, majority of the organs for transplantation are acquired from brain-dead donors; however, the presence of brain death is an important risk factor that dictates the graft function. As the demand for organ transplantation continues to grow, a major challenge in the transplantation medicine is to increase graft acceptance and function in chronic condition. Using pharmacological interventions, Yeboah and colleagues (59) have demonstrated that cholinergic agonists attenuate renal ischemia and reperfusion injury in experimental models. In a series of studies using preclinical models of acute and chronic renal transplantation, Hoeger and colleagues (60, 61) noted that vagus nerve stimulation in brain dead donors improves both acute and long-term graft function in the recipients after transplantation. These studies suggest that activation of the inflammatory reflex via vagus nerve stimulation in brain dead donors improves ischemia reperfusion mediated renal graft injury, and paves a way to improve graft function in the recipient. A very recent study provided an important mechanistic insight into the mechanisms underlying the protective effects of vagus nerve stimulation in attenuating ischemia and reperfusion injury (53). Ischemia reperfusion injury is a critical determinant of the graft function in transplantation. Vagus nerve stimulation prior to renal ischemia and reperfusion significantly attenuated acute kidney injury and decreased plasma TNF. Genetic ablation of α7nAChR abrogated this protective effect. Furthermore, splenectomy abolishes the effects of vagus nerve stimulation that can be restored by adoptive transfer of splenocytes obtained from vagus nerve stimulated animals. These observations indicate that α7nAChR-positive splenocytes play an important role in mediating vagus nerve induced attenuation of kidney injury during ischemia reperfusion. Together, these results suggest that bioelectronic devices for modulation of neural circuitries can be an efficient alternative of drug treatments in the transplantation medicine.
4. Implications for Transplantation Medicine
Neuro-immune interactions could play an important role in clinical settings of transplantation. Organ transplantation and surgery could lead to changes in the peripheral neural circuits that disrupts or aberrantly activates sensory or autonomic neuron communication with immune cells. Pharmacological interventions could also directly affect these neuro-immune interactions. For example, opioids, NSAIDs and other analgesics are often given to treat pain during surgery and for post-surgical care. Given the role of nociceptor neurons in modulating innate and adaptive immune cell function, alterations in pain signaling could adversely affect normal inflammatory processes required for wound healing. Similarly, pharmacological agonists or antagonists of sympathetic and parasympathetic neurons could affect immune responses and transplant rejection.
Targeted modulation of neuro-immune interactions to treat inflammation could be an exciting way to improve inflammatory outcomes during and after transplantation. Vagus nerve stimulation, currently applied in treatment of rheumatoid arthritis and inflammatory bowel disease, could be an interesting approach to modulate the immune response in organ transplantation. Implantable bioelectronics devices that work on specific branches of the vagus nerve could further target this immunomodulation to distinct organ systems. In addition to electrical stimulation of the inflammatory reflex, pharmacological interventions, such as α7nAChR or β2 adrenoreceptor agonists to activate the inflammatory reflex have been explored in preclinical settings. Future advances in the field of neuro-immunology in genetic targeting, electronic stimulation of neural circuits, and pharmacological modulation of the communication between the nervous and immune system could lead to new approaches to modulate the immune response and improve the outcomes of transplantation.
Acknowledgments
We acknowledge generous funding from the national institutes of health (NIH) grant NIH/NIAID 1P01AI102852-01A1 (S.S.C.), NIH/NCCIH DP2AT009499 (I.M.C.) and Kaneb Fellow Award (I.M.C.).
Abbreviations
- PNS
Peripheral nervous system
- DRG
Dorsal root ganglia
- Ach
Acetylcholine
- NE
Norepinephrine
- CNS
Central nervous system
- SP
Substance P
- CGRP
calcitonin gene-related peptide
- VIP
Vasoactive intestinal peptide
- NGF
Nerve Growth Factor
- PGE2
Prostaglandin E2
- TRP
Transient Receptor Potential
- α7nAChR
α7 nicotinic acetylcholine receptor
- ChAT
choline acetyltransferase
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and Therapeutic Relevance of Neuro-immune Communication. Immunity. 2017;46(6):927–942. doi: 10.1016/j.immuni.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinho-Ribeiro FA, Verri WA, Jr, Chiu IM. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2016 doi: 10.1016/j.it.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue T, Tanaka S, Okusa MD. Neuroimmune Interactions in Inflammation and Acute Kidney Injury. Frontiers in immunology. 2017;8:945. doi: 10.3389/fimmu.2017.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinho-Ribeiro FA, Verri WA, Jr, Chiu IM. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017;38(1):5–19. doi: 10.1016/j.it.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heron A, Dubayle D. A focus on mast cells and pain. J Neuroimmunol. 2013;264(1–2):1–7. doi: 10.1016/j.jneuroim.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Ghasemlou N, Chiu IM, Julien JP, Woolf CJ. CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 2015;112(49):E6808–6817. doi: 10.1073/pnas.1501372112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100(13):7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23(10):4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi M, Konishi H, Sayo A, Takai T, Kiyama H. TREM2/DAP12 Signal Elicits Proinflammatory Response in Microglia and Exacerbates Neuropathic Pain. J Neurosci. 2016;36(43):11138–11150. doi: 10.1523/JNEUROSCI.1238-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, Alam A, Chen Q, MAE, Pal A, Eguchi S, et al. The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br J Anaesth. 2017;118(4):504–516. doi: 10.1093/bja/aex006. [DOI] [PubMed] [Google Scholar]
- 13.Zhao P, Lieu T, Barlow N, Sostegni S, Haerteis S, Korbmacher C, et al. Neutrophil Elastase Activates Protease-activated Receptor-2 (PAR2) and Transient Receptor Potential Vanilloid 4 (TRPV4) to Cause Inflammation and Pain. J Biol Chem. 2015;290(22):13875–13887. doi: 10.1074/jbc.M115.642736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vicuna L, Strochlic DE, Latremoliere A, Bali KK, Simonetti M, Husainie D, et al. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat Med. 2015;21(5):518–523. doi: 10.1038/nm.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28(2):131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501(7465):52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julius D. TRP channels and pain. Annual review of cell and developmental biology. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 18.Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity. 2015;43(3):515–526. doi: 10.1016/j.immuni.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talbot S, Abdulnour RE, Burkett PR, Lee S, Cronin SJ, Pascal MA, et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron. 2015;87(2):341–354. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A. 2014;111(31):11515–11520. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D’Amours M, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009;106(22):9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510(7503):157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engel MA, Leffler A, Niedermirtl F, Babes A, Zimmermann K, Filipovic MR, et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141(4):1346–1358. doi: 10.1053/j.gastro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandran R, Hyun E, Zhao L, Lapointe TK, Chapman K, Hirota CL, et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc Natl Acad Sci U S A. 2013;110(18):7476–7481. doi: 10.1073/pnas.1217431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavan SS, Tracey KJ. Essential Neuroscience in Immunology. J Immunol. 2017;198(9):3389–3397. doi: 10.4049/jimmunol.1601613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 27.Milligan ED, McGorry MM, Fleshner M, Gaykema RP, Goehler LE, Watkins LR, et al. Subdiaphragmatic vagotomy does not prevent fever following intracerebroventricular prostaglandin E2: further evidence for the importance of vagal afferents in immune-to-brain communication. Brain research. 1997;766(1–2):240–243. doi: 10.1016/s0006-8993(97)00705-1. [DOI] [PubMed] [Google Scholar]
- 28.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neuroscience letters. 1995;183(1–2):27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 29.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacology & therapeutics. 2011;130(2):226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Autonomic neuroscience : basic & clinical. 2000;85(1–3):49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 31.Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain, behavior, and immunity. 2005;19(4):334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Hosoi T, Okuma Y, Matsuda T, Nomura Y. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Autonomic neuroscience : basic & clinical. 2005;120(1–2):104–107. doi: 10.1016/j.autneu.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Niijima A. The afferent discharges from sensors for interleukin 1 beta in the hepatoportal system in the anesthetized rat. Journal of the autonomic nervous system. 1996;61(3):287–291. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- 34.Niijima A, Hori T, Aou S, Oomura Y. The effects of interleukin-1 beta on the activity of adrenal, splenic and renal sympathetic nerves in the rat. Journal of the autonomic nervous system. 1991;36(3):183–192. doi: 10.1016/0165-1838(91)90042-2. [DOI] [PubMed] [Google Scholar]
- 35.Niijima A, Hori T, Katafuchi T, Ichijo T. The effect of interleukin-1 beta on the efferent activity of the vagus nerve to the thymus. Journal of the autonomic nervous system. 1995;54(2):137–144. doi: 10.1016/0165-1838(95)00003-g. [DOI] [PubMed] [Google Scholar]
- 36.Steinberg BE, Silverman HA, Robbiati S, Gunasekaran MK, Tsaava T, Battinelli E, et al. Cytokine-specific neurograms in the sensory vagus nerve. Biolectronic Medicine. 2016;(3):7–17. [PMC free article] [PubMed] [Google Scholar]
- 37.Bianchi M, Ulrich P, Bloom O, Meistrell M, 3rd, Zimmerman GA, Schmidtmayerova H, et al. An inhibitor of macrophage arginine transport and nitric oxide production (CNI-1493) prevents acute inflammation and endotoxin lethality. Molecular medicine. 1995;1(3):254–266. [PMC free article] [PubMed] [Google Scholar]
- 38.Bianchi M, Bloom O, Raabe T, Cohen PS, Chesney J, Sherry B, et al. Suppression of proinflammatory cytokines in monocytes by a tetravalent guanylhydrazone. The Journal of experimental medicine. 1996;183(3):927–936. doi: 10.1084/jem.183.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tracey KJ. Suppression of TNF and other proinflammatory cytokines by the tetravalent guanylhydrazone CNI-1493. Progress in clinical and biological research. 1998;397:335–343. [PubMed] [Google Scholar]
- 40.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. The Journal of experimental medicine. 2002;195(6):781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microscopy research and technique. 1996;35(1):80–86. doi: 10.1002/(SICI)1097-0029(19960901)35:1<80::AID-JEMT7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 42.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 43.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105(31):11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334(6052):98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 46.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nature immunology. 2005;6(8):844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 47.Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, et al. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107(8):1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 48.Lu B, Kwan K, Levine YA, Olofsson PS, Yang H, Li J, et al. alpha7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Molecular medicine. 2014;20:350–358. doi: 10.2119/molmed.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF, et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. The Journal of infectious diseases. 2005;191(12):2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 50.Levine YA, Koopman FA, Faltys M, Caravaca A, Bendele A, Zitnik R, et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS One. 2014;9(8):e104530. doi: 10.1371/journal.pone.0104530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130(6):1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 52.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, et al. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. Journal of vascular surgery. 2002;36(6):1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 53.Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, Huang L, et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through alpha7nAChR+ splenocytes. J Clin Invest. 2016;126(5):1939–1952. doi: 10.1172/JCI83658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131(4):1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Lubbers T, Buurman W, Luyer M. Controlling postoperative ileus by vagal activation. World journal of gastroenterology. 2010;16(14):1683–1687. doi: 10.3748/wjg.v16.i14.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leib C, Goser S, Luthje D, Ottl R, Tretter T, Lasitschka F, et al. Role of the cholinergic antiinflammatory pathway in murine autoimmune myocarditis. Circulation research. 2011;109(2):130–140. doi: 10.1161/CIRCRESAHA.111.245563. [DOI] [PubMed] [Google Scholar]
- 57.Bonaz B, Sinniger V, Hoffmann D, Clarencon D, Mathieu N, Dantzer C, et al. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2016;28(6):948–953. doi: 10.1111/nmo.12792. [DOI] [PubMed] [Google Scholar]
- 58.Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;113(29):8284–8289. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeboah MM, Xue X, Duan B, Ochani M, Tracey KJ, Susin M, et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney international. 2008;74(1):62–69. doi: 10.1038/ki.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoeger S, Bergstraesser C, Selhorst J, Fontana J, Birck R, Waldherr R, et al. Modulation of brain dead induced inflammation by vagus nerve stimulation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(3):477–489. doi: 10.1111/j.1600-6143.2009.02951.x. [DOI] [PubMed] [Google Scholar]
- 61.Hoeger S, Fontana J, Jarczyk J, Selhorst J, Waldherr R, Kramer BK, et al. Vagal stimulation in brain dead donor rats decreases chronic allograft nephropathy in recipients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(3):544–549. doi: 10.1093/ndt/gft451. [DOI] [PubMed] [Google Scholar]