Abstract
The long chain n-3 polyunsaturated fatty acids (LC-PUFA) eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) in fish oil have immunomodulatory properties. B cells are a poorly studied target of EPA/DHA in humans. Therefore, in this pilot study, we tested how n-3 LC-PUFAs influence B cell responses of obese humans. Obese men and women were assigned to consume four 1 gram capsules per day of olive oil (OO, n=12), fish oil (FO, n=12) concentrate, or high DHA-FO concentrate (n=10) for 12 weeks in a parallel design. Relative to baseline, FO (n=9) lowered the percentage of circulating memory and plasma B cells whereas the other supplements had no effect. There were no post-intervention differences between the three supplements. Next, ex vivo B cell cytokines were assayed after stimulation of Toll-like receptors (TLRs) and/or the B cell receptor (BCR) to determine if the effects of n-3 LC-PUFAs were pathway-dependent. B cell IL-10 and TNFα secretion were respectively increased with high DHA-FO (n=10), relative to baseline, with respective TLR9 and TLR9+BCR stimulation. OO (n=12) and FO (n=12) had no influence on B cell cytokines compared to baseline and there was no differences in post-intervention cytokine levels between treatment groups. Finally, ex vivo antibody levels were assayed with FO (n=7) after TLR9+BCR stimulation. Compared to baseline, FO lowered IgM but not IgG levels accompanied by select modifications to the plasma lipidome. Altogether, the results suggest n-3 LC-PUFAs could modulate B cell activity in humans, which will require further testing in a larger cohort.
Keywords: Fish oil, B cells, cytokines, antibody levels, lipidomics, polyunsaturated fatty acids, Toll-like receptors, B cell receptor
1.0 Introduction
The long chain n-3 polyunsaturated fatty acids (LC-PUFAs) eicosapentaenoic (EPA, 20:5) and docosahexaenoic acid (DHA, 22:6) are consumed in low levels in the Western Diet [1, 2]. There is evidence that increasing the consumption of these fatty acids has potential health benefits for a range of inflammatory and autoimmune diseases [3]. Animal studies across model systems show that n-3 LC-PUFAs robustly improve inflammatory outcomes and aid in the resolution of inflammation [3–5]. However, studies in humans have generally provided mixed results about the effectiveness of n-3 LC-PUFAs for innate or adaptive immunity [3]. One major limitation in developing n-3 LC-PUFAs for clinical applications related to immunity is that their cellular targets and underlying mechanisms are not well delineated, particularly within the human population [6].
B cells are not well studied in response to n-3 LC-PUFA intervention at the human level. B cells are associated with antibody production but also play a role in cytokine secretion and antigen presentation to T cells. A series of rodent studies show that n-3 LC-PUFAs, administered as fish oil or as purified ethyl esters, enhance B cell cytokine secretion and/or antibody production in lean and obese mice [7–13]. Furthermore, rodent studies suggest that DHA is more effective than EPA in enhancing B cell cytokine secretion and antibody production, presumably through a lipid raft mediated mechanism [9–12]. The enhancement in B cell activity with n-3 LC-PUFAs is of biological relevance given that a range of metabolic diseases are associated with impaired B cell responses. To exemplify, subjects recently diagnosed with diabetes have diminished production of B cell cytokines [14]. Similarly, obese mice and humans, compared to lean controls, display lower antibody production in parallel with chronic inflammation upon influenza infection or vaccination [13, 15–18]. Thus, n-3 LC-PUFAs may have potential health applications for select clinical populations that have impaired B cell activity.
The overall goal of this study was to determine if administration of supplements containing n-3 LC PUFAs to obese subjects would influence select B cell responses. The primary objectives of this double-blind study were to determine if administration of one of two fish oil (FO) supplements or olive oil (OO) in a parallel design would change the frequency of circulating B cell subsets relative other immune cell populations and ex vivo B cell cytokine secretion after stimulation with agonists targeting TLR1/2, TLR9, BCR+TLR9. Cytokine secretion was assessed in response to stimulation of the B cell receptor (BCR) and Toll-like receptors (TLRs) to determine if the biochemical effects of n-3 PUFAs were mechanistically pathway-dependent. Finally, ex vivo B cell antibody production was assayed with one of the FO supplements upon BCR+TLR9 stimulation accompanied by a plasma lipidomic analysis. The rationale for the lipidomic analyses was to determine if known lipid meditators such as lipoxin A4 that modulate antibody production were modified by the FO supplement [19–22].
2.0 Methods
2.1 Subjects and inclusion/exclusion criteria
Obese men and women with a body mass index (BMI; kg/m2) >30 were recruited from the general population (Table 1). Approval for the study was obtained by the East Carolina University Institutional Review Board. The recruitment strategy was the following. First, potential participants completed a phone screen for initial eligibility based on age, body weight, absence of pregnancy, and low consumption of fatty fish and/or fish oil supplements. After passing the initial phone screen, written informed consent was obtained before participation and each patient received signed approval from a physician. Exclusion criteria for males and females were the following: blood clotting, taking aspirin, taking more than one fish meal per week, consuming short chain or long chain n-3 PUFA supplements in the last 3 months prior to enrollment, history of autoimmune diseases, allergies to fish or shellfish, atrial fibrillation or flutter, high LDL cholesterol, liver dysfunction, problems with blood clotting, underactive thyroid, taking estrogen, or thiazide diuretics. In addition, those females that were pregnant, breastfeeding, or lactating were also excluded.
Table 1. Patient Characteristics.
Subjects consumed olive oil (OO), FO, or high DHA-FO supplements for 12 weeks.
| Parameters | OO | FO | High DHA-FO |
|---|---|---|---|
| Age (mean and range) | 42.50 (30–55) | 45.33 (31–56) | 39.10 (29–55) |
| BMI (mean and range) | 39.39 (30.19–65.57) | 40.15 (29.17–52.67) | 35.72 (30.96–41.33) |
| Sex n (%) | |||
| Female | 9 (75.00%) | 6 (50.00%) | 3 (30.00%) |
| Male | 3 (25.00%) | 6 (50.00%) | 7 (70.00%) |
| Race n (%) | |||
| Caucasian | 10 (83.33%) | 10 (83.33%) | 6 (60.00%) |
| African American | 2 (16.67%) | 1 (8.33%) | 3 (30.00%) |
| Hispanic | 0 (0%) | 1 (8.33%) | 0 (0%) |
| Asian | 0 (0%) | 0 (0%) | 1 (10.00%) |
| Medications n (%) | |||
| Biguanide | 1 (8.33%) | 2 (20.00%) | |
| Antidepressant | 1 (8.33%) | 1 (8.33%) | 2 (20.00%) |
| SSRI | 1 (8.33%) | 1 (8.33%) | 1 (10.00%) |
| Statin | 2 (16.67%) | ||
| Insulin | 2 (20.00%) | ||
| ACE Inhibitor | 2 (16.67%) | 2 (16.67%) | 2 (20.00%) |
| Benzodiazepine | 1 (8.33%) | 2 (20.00%) | |
| GABA Analog | 1 (10.00%) | ||
| Xanthine Oxidase Reducer | 1 (8.33%) | ||
| Calcium Channel Blocker | 1 (8.33%) | 1 (10.00%) | |
| Antihyperglycemic | |||
| Antiepileptic | 1 (8.33%) | ||
| Proton Pump Inhibitor | 2 (16.67%) | ||
| Antihistamine | 2 (20.00%) | ||
| Beta blocker | 1 (8.33%) | ||
| Diuretic | 1 (8.33%) | 1 (10.00%) | |
| Birth Control | 1 (8.33%) | 1 (10.00%) | |
A total of 36 subjects were enrolled with 12 subjects randomly assigned per treatment group. Subjects were age-matched between the three supplement groups described below. One subject withdrew from the study due to mild discomfort from burping. Another subject started the use of an anti-inflammatory drug during the study and was therefore removed.
2.2 Intervention and study design
Enrolled participants were assigned to receive four identical 1-gram capsules per day of either olive oil (OO), concentrated fish oil (FO) (~400mg EPA/300mg DHA ethyl esters per capsule), or a high DHA concentrated FO (~100mg EPA/500mg DHA ethyl esters per capsule). The OO supplement provided 2.8g of oleic acid per day, the FO concentrate supplement provided 1.7g of EPA and 1.2g of DHA per day, and the high DHA concentrated FO provided 0.5g EPA and 2.0g DHA per day. The minor differences in dosing arose from the need to maintain the concentration of total oil constant at 4 grams between the three arms of the study. The FO concentrate, the high DHA-FO concentrate, and olive oil were encapsulated and provided by AlaskOmega®, Organic Technologies (Coshocton, Ohio). The olive oil was not extra virgin olive oil. The fatty acid analyses of the three dietary supplements is provided in Table 2.
Table 2. Fatty acid profile of dietary supplements.
Obese subjects were administered olive oil (OO), FO, or high DHA-FO supplements for 12 weeks. Fatty acids (mg/g) were analyzed with gas chromatography.
| Fatty Acid | OO | FO | High DHA-FO |
|---|---|---|---|
| 14:0 | 0.2 | 0.0 | 0.0 |
| 16:0 | 114.2 | 0.0 | 0.0 |
| 16:1 | 8.0 | 0.0 | 0.0 |
| 18:0 | 26.0 | 0.5 | 0.0 |
| 18:1c | 704.2 | 1.9 | 0.0 |
| 18:1t | 23.0 | 0.6 | 0.0 |
| 18:2(n-6) | 91.7 | 0.4 | 0.0 |
| 18:3(n-3) | 6.9 | 0.4 | 0.0 |
| 18:4 | 0.0 | 3.6 | 0.0 |
| 20:0 | 4.8 | 0.2 | 0.0 |
| 20:1 | 0.2 | 1.5 | 1.0 |
| 20:1 | 3.7 | 0.7 | 0.5 |
| 20:2 | 0.0 | 0.2 | 0.2 |
| 20:3 | 0.0 | 0.5 | 0.3 |
| 20:3 | 0.0 | 0.2 | 0.0 |
| 20:4(n-3) | 0.0 | 4.4 | 4.6 |
| 20:4(n-6) | 0.0 | 9.6 | 1.1 |
| 20:5(n-3) | 0.3 | 416.0 | 121.6 |
| 22:0 | 1.8 | 0.4 | 1.0 |
| 22:1 | 0.0 | 2.6 | 4.2 |
| 22:1 | 0.0 | 1.4 | 0.6 |
| 22:3(n-3) | 0.0 | 22.3 | 32.2 |
| 22:4 | 0.2 | 10.4 | 11.8 |
| 22:5(n-3) | 0.5 | 18.7 | 66.6 |
| 22:6(n-3) | 0.6 | 299.7 | 500.5 |
| 24:0 | 0.8 | 9.0 | 15.2 |
| 24:1 | 0.0 | 12.4 | 2.2 |
Supplements were provided for 12 weeks and blood was obtained prior to and after intervention. Subjects were instructed to consume 2 capsules with breakfast and 2 with dinner on a daily basis. The study was double-blinded and all samples were collected and stored with a subject number. Results were unblinded after all analyses were completed.
Subjects completed surveys at the initial and final blood draws to assess work behavior as a factor that can influence stress and food questionnaires to confirm that n-3 PUFA intake was low [23, 24]. An additional survey was also administered to account for potential differences in physical activity [25]. Subjects were provided 6 weeks of supplements after which they were brought in to receive another round of supplementation for 6 weeks. Compliance was assessed based on pill count, as provided by the subjects at each visit, and was measured to be 100%. Compliance was also confirmed by measuring EPA and DHA levels in circulation. This pilot study was not registered at clinicaltrials.gov since the study did not have any clinical outcomes.
2.3 Peripheral blood mononuclear cell (PBMC) analyses
Subjects were fasted overnight before obtaining blood. Peripheral blood taken in vacutainer tubes (Franklin Lakes, New Jersey) was diluted 1:1 in PBS followed by separation of PBMCs using Ficoll Paque (GE Healthcare, Washington, NC) gradient centrifugation. The following subsets were analyzed using a BDLSRII flow cytometer: CD45+CD3+CD4+ (CD4 helper T cells), CD45+CD3+CD8+ (CD8 cytotoxic T cells), CD45+CD3−CD14+ (monocytes), CD45+CD14−CD19+ (B cells) [13]. All fluorophore antibody markers were obtained from Biolegend (San Diego, CA) or Miltenyi Biotech (San Diego CA) and consisted of: CD45 (PE), CD3 (Pacific Blue), CD4 (FITC), CD8 (PE-Cy5), CD14 (FITC), and CD19 (APC).
2.4 B cell purification and analyses of subsets
B cells were isolated from PBMCs using a B cell isolation kit II (Miltenyi Biotec) with a resulting purity of >99%. Given that the number of B cells were limited, B cell subsets were analyzed from a fraction of the total number of subjects, as indicated in the results. The following subsets were analyzed using a BDLSRII flow cytometer: CD19+CD27+IgD+ (memory B cells), CD19+CD27−IgD+ (naïve B cells), and CD19+ CD38+ CD27+IgD− (plasma cells). All fluorophore conjugated antibodies were obtained from Biolegend or Miltenyi Biotech and consisted of: CD19 (APC), CD27(Pacific Blue), CD38 (FITC) and IgD (PE-Cy7).
2.5 B cell stimulation and proliferation
Purified human B cells were cultured in RPMI 1640 with 5% FBS, 2mM L-glutamine, 50μM 2-betamercaptoethanol, 10mM HEPES, and 50μg/ml gentamicin at a concentration of 2.9–3.1 × 106 cells/ml. B cells were stimulated with: 1) CpG oligodeoxynucleotides (ODN) 2395 (a TLR9 agonist; Hycult Biotech, Plymouth Meeting, PA) and 1μg/ml plus BCR stimulation using rabbit anti-human IgM Ab fragment (Jackson ImmunoResearch, West Baltimore Pike, PA) at a concentration of 2μg/ml; 2) PAM3CSK4 (a TLR1/2 agonist; Invivogen, San Diego, CA) at a concentration of 10μg/ml; 3) CpG-ODN targeting TLR9 at a concentration of 10μg/ml. B cells were plated in round bottom inert grade 96-well plates (Thermo Fisher Scientific, Waltham, MA) and cultured in duplicate for 2 days and the supernatant was collected. Supernatant IL-6, IL-10, and TNFα concentrations were measured using Luminex Assay kits (Thermo Fisher Scientific) following the manufacturer’s instructions. IgM and IgG levels were assayed with ELISAs (Abcam, USA) in response to BCR+TLR9 stimulation on day 3 post-activation. B cell proliferation relied on a CyQUANT cell proliferation assay kit (Eugene, OR) per the manufacturer’s instructions.
2.6 Fatty acid analysis
Fatty acid analyses were completed at the University of Stirling, UK, according to the method of Bell et al [26]. Transmethylation followed by gas chromatography was used to establish pre- and post-fatty acid levels in the blood and to analyze the fatty acid composition of the supplements.
2.7 Lipidomics with mass spectrometry
All standards and internal standards used for LC/MS/MS analysis of arachidonic acid, docosahexaenoic acid and linoleic acid derived lipid mediators were purchased from Cayman Chemical (Ann Arbor, Michigan, USA). All HPLC solvents and extraction solvents were HPLC grade or better.
Plasma samples were pretreated for solid phase extraction. Briefly, proteins were precipitated from 100μl of plasma or blood by adding 400μl of ice cold methanol and 10μl of the internal standard solution (10pg/μl each of 5(S)-HETE-d8, 8-iso-PGF2a-d4, 9(S)-HODE-d4, LTB4-d4, LTD4-d5, LTE4-d5, PGE2-d4, PGF2a-d9 and RvD2-d5 in ethanol) in a 1.5ml microfuge tube, followed by vortexing and then incubating on ice for 15 minutes. The samples were then centrifuged for 10 minutes at 4C at 14,000 RPM. The supernatant was transferred to a new microfuge tube and an additional 100μl of ice cold methanol was added to the tube and the pellet was resuspended. The samples were then placed in a microcentrifuge tube for 10 minutes at 4C at 14,000 RPM and the supernantant was removed and combined with the first supernatant. The sample was then dried in a vacuum centrifuge until dry. The sample was then immediately reconstituted in 1.0ml of 90:10 water:methanol before purification by solid phase extraction (SPE).
Lipid mediators were isolated using Strata-X 33um 30mg/1ml SPE columns (Phenomenex, Torrance, CA) on a Biotage positive pressure SPE manifold (Biotage, Charlotte, NC). SPE columns were pre-washed with 2 ml of MeOH followed by 2 ml of water. After applying the entire 1 ml of reconstituted sample, the columns were washed with 1ml of 10% MeOH, and the lipid mediators were then eluted sequentially with 1 ml of methyl formate followed by 1 ml of methanol directly into a reduced surface activity/maximum recovery glass autosampler vial (MicroSolv Technology Corp. Leland, NC). The sample was dried after each solvent elution with a steady stream of nitrogen directly on the SPE manifold. The sample was then immediately reconstituted with 20μl of ethanol and analyzed immediately or stored at −70C until analysis for no more than 1 week. Mass spectrometry was conducted as recently described [13].
2.8 Analyses
Data are presented as the mean ± SEM. Data were analyzed with GraphPad Prism version 5.0b. A food frequency questionnaire was analyzed for the total number of kcal consumed from fat, carbohydrates, and protein per month [23]. The Workaholism Analysis Questionnaire (WAQ) consisted of 29 items in which respondents were asked to select from a five-point Likert scale, ranging from 1 (strongly disagree) to 5 (strongly agree). The measure has been psychometrically tested on a heterogeneous working population and has demonstrated strong reliability and validity [24]. The WAQ demonstrates a strong internal consistency of α = 0.93. Physical activity was assessed with the International Physical Activity Questionnaire (IPAQ) and the resting metabolic rate (MET)-minutes were calculated as described [25]. MET-minutes are equivalent to kilocalories for a 60kg individual [25].
Analyses were conducted between pre- and post-intervention. All the reported results were analyzed for normalized distributions with a D’Agostino & Pearson omnibus normality test. Ex vivo cytokine measurements and antibody levels displayed non-parametric distributions and were analyzed with a Wilcoxon signed t tests. Immune cell frequencies and B cell proliferations displayed parametric distributions and were analyzed with paired two tailed t-tests. Post-intervention results for all data sets were also analyzed between the three supplement groups using either a one-way ANOVA followed by a post-hoc Bonferroni t test or Kruskal-Wallis followed by a Dunn’s multiple comparison test. P values less than 0.05 were considered significant.
3.0 Results
3.1 Food intake, work behavior, and physical activity
Food intake was assessed based on a questionnaire to ensure that individuals were not consuming high levels of n-3 PUFAs. The survey showed no change in consumption of food products containing n-3 PUFAs and total kcal from carbohydrates, fats, and proteins were the same pre- and post-intervention (Table 3). We also assessed if work behavior, which can indicate stress, was modified between pre- and post-intervention (Suppl. Table 1). Workaholism was identified using respondents overall self-reported scores, with larger scores indicating higher levels of workaholism. The results showed no differences in work behavior with all of the supplements when comparing pre- and post-intervention. Physical activity was also surveyed and there were no differences in activity for all three supplement groups when comparing pre- and post-intervention (Suppl. Table 2). We also compared post-intervention values for food intake (Table 3), work behavior (Suppl. Table 1), and physical activity (Suppl. Table 2) and found no differences between individuals consuming OO (n=12), FO (n=12) and the high DHA-FO (n=10).
Table 3. Food intake for subjects consuming OO, FO, or high DHA-FO supplements.
Subjects completed a food survey before and after intervention with OO (n=12), FO (n=12), or high DHA-FO (n=10) supplements. Values are average ± SEM.
| Parameters | OO | FO | High DHA-FO | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre | Post | Pre | Post | Pre | Post | |
|
|
||||||
| Calories (daily total, kcal) | 649.87±82.03 | 590.43±102.66 | 574.89±110.07 | 557.08±79.38 | 730±104.13 | 728.10±91.21 |
| Fats (daily total, g) | 251.74±40.92 | 215.87±37.48 | 214.82±47.48 | 204.3±30.39 | 330±69.74 | 303.57±49.25 |
| Omega 3 | 0.56±0.10 | 0.46±0.10 | 0.43±0.13 | 0.59±0.16 | 0.46±0.13 | 0.85±0.39 |
| Omega 6 | 4.69±0.62 | 3.61±0.62 | 3.97±1.28 | 3.74±0.98 | 6.75±2.13 | 5.51±1.41 |
| Carbohydrates (daily total, g) | 60.36±10.85 | 62.92±12.92 | 58.98±12.46 | 60.20±10.92 | 60.16±7.74 | 58.75±11.87 |
| Protein (daily total, g) | 42.85±5.51 | 35.38±5.61 | 34.98±5.44 | 31.64±4.72 | 45.41±6.72 | 51.77±7.61 |
3.2. BMI and blood pressure
BMI was not influenced by any of the interventions (Fig. 1A) when comparing pre-treatment with post-intervention values or between treatment groups after the intervention. Relative to baseline, systolic and diastolic blood pressure were unaffected by OO (n=12) or high DHA-FO (n=10) (Fig. 1B). The only exception was FO (n=12), which lowered systolic, but not diastolic, blood pressure by ~5% when comparing post-intervention to pre-intervention (Fig. 1B). There were no differences in systolic or diastolic blood pressure between the three different supplement groups after treatment (Fig. 1B).
Figure 1. FO lowers systolic blood pressure relative to the baseline.
(A) BMI and (B) blood pressure values for subjects consuming OO (n=12), FO (n=12), or the high DHA-FO (n=10) supplements for 12 weeks. Data are average ± SEM. Asterisk indicates statistical significance between pre- and post-intervention (*p<0.05). There was no statistical differences in BMI or blood pressure between groups post-intervention.
3.3 FO and high DHA-FO boosts circulating levels of EPA and DHA
Fatty acid analyses were conducted to ensure elevated levels of circulating n-3 LC-PUFAs upon FO supplementation. OO intervention (n=12) had no effect on n-3 LC-PUFA levels relative to baseline (Table 4). FO increased 20:5 and 22:6 levels respectively by 2.7 and 2.5-fold relative to baseline. The high DHA-FO supplement increased 20:5, 22:5(n-3), and 22:6 levels respectively by 1.3, 1.4, and 1.8-fold relative to baseline (Table 4). The n-6 PUFAs 18:2, 20:4, and 22:5 levels were also analyzed in blood. OO had no effect on n-6 PUFA levels (Table 4). The FO supplement had no effect on 18:2 levels and lowered 22:5(n-6) by 0.7-fold relative to the baseline. High DHA-FO had no effect on 18:2 and tended to lower the levels of 20:4 (p=0.07) relative to baseline. 22:5(n-6) levels were lowered by 0.7-fold with the high DHA-FO supplement compared to baseline (Table 4).
Table 4.
Blood fatty acid profile for subjects consuming OO, FO, or high DHA-FO supplements.
| OO | FO | High DHA-FO | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| PUFA | Pre | Post | Pre | Post | Pre | Post | |
| n-3 (%) | 20:5 | 0.347±0.032 | 0.369±0.032 | 0.353±0.038 | 0.973±0.138*† | 0.347±0.033 | 1.159±0.200*† |
| 22:5 | 1.093±0.054 | 1.071±0.056 | 1.063±0.064 | 1.081±0.057 | 0.962±0.066 | 1.323±0.098*† | |
| 22:6 | 1.891±0.122 | 1.880±0.134 | 1.707±0.098 | 3.846±0.387*† | 1.814±0.146 | 3.085±0.235*† | |
|
|
|||||||
| n-6 (%) | 18:2 | 19.602±0.784 | 20.612±0.955 | 21.073±0.702 | 21.157±0.542 | 19.935±0.823 | 19.217±0.737 |
| 20:4 | 11.947±0.508 | 11.757±0.543 | 10.291±0.514 | 9.650±0.464† | 10.522±0.490 | 9.555±0.585† | |
| 22:5 | 0.414±0.032 | 0.410±0.034 | 0.337±0.020 | 0.241±0.024*† | 0.401±0.022 | 0.264±0.029*† | |
Blood was collected before and after consumption of OO (n=12), FO (n=12), and high DHA-FO (n=10) supplements. Samples were analyzed with gas chromatography. Values are average ± SEM,
p<0.05 relative to baseline,
p<0.05 relative to OO.
Post-intervention values were also analyzed between the three supplement groups (Table 4). 20:5(n-3) and 22:6(n-3) levels were increased by both FO supplements by ~1.6 to 3.1-fold relative to the OO group. High DHA-FO also increased 22:5(n-3) levels by 1.2-fold compared to OO. 20:4(n-6) was decreased by 1.2-fold by FO and high DHA-FO when compared to OO post-intervention. 22:5(n-6) levels were also decreased by FO and high DHA-FO by 1.6–1.7-fold compared to OO (Table 4).
3.4 The percentage and frequency of differing circulating immune cells are maintained after intervention with both FO supplements
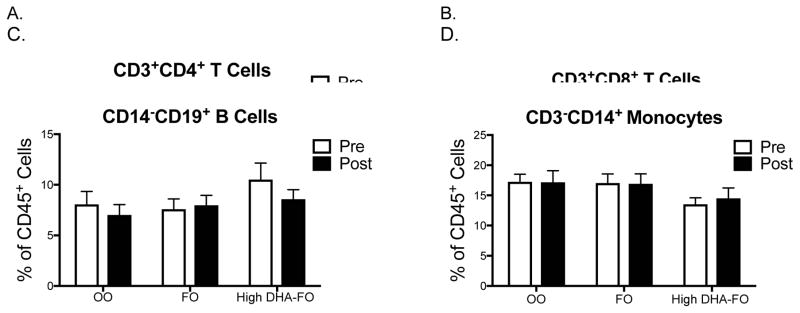
PBMCs were analyzed for the percentage of differing immune cell populations. The flow cytometry gating strategy for PBMC analyses is depicted in Suppl. Fig. 1. The percentage of CD3−CD14+ monocytes, helper CD3+CD4+ T, cytotoxic CD3+CD8+ T cells, and CD14−CD19+ B cells were assessed in the CD45+ PBMC fraction (Fig. 2A–D). The percentage of helper T, cytotoxic T, B cells, and monocytes did not change between pre- and post-intervention for the OO (n=12), FO (n=12) and high DHA-FO (n=10) groups. The frequency of these immune cell populations also did not change between pre- and post-treatment (data not shown). There were no post-treatment differences in the percentage of immune cell populations between the OO and the two FO groups (Fig. 2A–D).
Figure 2. FO and high DHA-FO maintain the percentage of circulating immune cell populations in obese subjects.
Percentage of circulating (A) helper CD3+CD4+ T cells, (B) cytotoxic CD3+CD8+ T cells, (C) CD14−CD19+ B cells, and (D) CD3−CD14+ monocytes for subjects consuming OO (n=12), FO (n=12), or the high DHA-FO (n=10) supplements for 12 weeks. Data are average ± SEM.
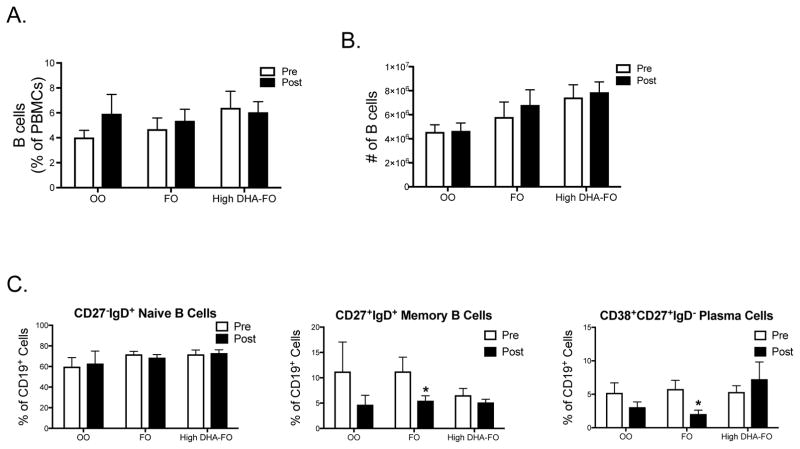
3.5 FO lowers the percentage of circulating B memory and plasma cells relative to baseline
The percentage and frequency of circulating B cells were also analyzed as the percentage of PBMCs (Fig. 3A) and the frequency of B cells isolated via negative selection (Fig. 3B). The OO (n=12), FO (n=12) and the high DHA-FO (n=10) had no effect on B cell percentage or frequency when analzyed relative to baseline for each treatment or between treatments after intervention.
Figure 3. FO lowers the percentage of CD27+IgD+ memory and CD38+CD27+IgD− plasma cells relative to the baseline.
(A) Percentage of B cells from the PBMC population and the (B) frequency of B cells enumerated using trypan blue exclusion after isolation with negative selection for subjects consuming OO (n=12), FO (n=12), or the high DHA-FO (n=10) supplements for 12 weeks. (C) Percentage of differing B cells subsets for subjects consuming OO (n=4), FO (n=9), or the high DHA-FO (n=9) for 12 weeks. Data are average ± SEM. There was no statistical differences between groups post-intervention. Asterisk indicates statistical significance between pre- and post-intervention (*p<0.05).
Circulating B cell subsets were also quantified and the flow cytometry gating strategy for these analyses is presented in Suppl. Fig 2. Due to a limited number of B cells available from each subject, the B cell subset analyses could only be conducted with some of the enrolled subjects. The OO treatment group (n=4) had no effect on the percentage of B cells subsets when comparing post-intervention values relative to the baseline (Fig. 3C). FO (n=9) lowered the percentage of CD19+CD27+IgD+ memory and CD19+CD38+CD27+IgD− plasma cells by ~ 2-fold with no effect on naïve B cells (Fig. 3C) relative to the baseline. The high DHA-FO supplement (n=9) also had no effect on the percentage of naïve, memory, and plasma B cells relative to baseline (Fig. 3C). There was no difference in the percentage of B cell subsets when comparing post-intervention results between OO (n=4), FO (n=9), and high DHA-FO (n=9) (Fig 3C).
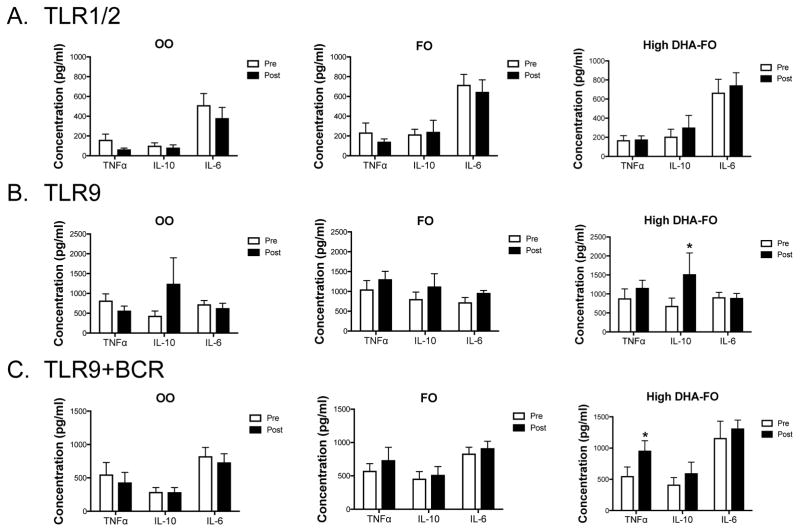
3.6 Ex vivo B cell cytokine secretion is selectively increased upon intervention with high DHA-FO relative to the baseline
Ex vivo B cell cytokine secretion was assayed after TLR1/2, TLR9, or TLR9/BCR activation (Fig. 4). Upon stimulation of TLR1/2, TNFα, IL-10, and IL-6 secretion were identical between pre- and post-intervention for all three supplements (Fig. 4A). Activation through TLR9 revealed no effect of OO (n=12) or FO (n=12) on TNFα, IL-10, and IL-6 levels when comparing pre- and post-intervention (Fig. 4B). Relative to the baseline, the high DHA-FO (n=10) supplement increased IL-10 section by 2.2-fold without an effect on TNFα or IL-6 upon TLR9 stimulation (Fig. 4B). Activation of B cells with TLR9+anti-BCR showed no effect of OO (n=12) or FO (n=12) on cytokine secretion when comparing pre- and post-treatment values (Fig. 4C). TNFα levels were elevated by 1.7-fold for the high DHA-FO group (n=10) relative to baseline (Fig. 4C). IL-10 and IL-6 levels were not influenced by high DHA-FO after TLR9+BCR stimulation. Analyses of all post-treatment cytokine values in response to TLR1/2, TLR9, TLR9+BCR stimulation revealed no differences between the OO group (n=12), FO (n=12), and high DHA-FO (n=10) (Fig. 4A–C).
Figure 4. High DHA-FO enhances B cell IL-10 and TNFα secretion relative to baseline upon respective stimulation of TLR9 and TLR9+BCR.
B cell cytokine secretion in response to stimulation of (A) TLR1/2, (B) TLR9, and (C) TLR9+anti-BCR for subjects consuming OO (n=12), FO (n=12), or the high DHA-FO (n=10) supplements for 12 weeks. B cells were stimulated with PAM3CSK4 for TLR1/2, CpG-ODN for TLR9 and anti-BCR antibody for the BCR. Cytokine levels were measured after 48 hours of stimulation. Data are average ± SEM. There was no statistical differences between groups post-intervention. Asterisk indicates statistical significance between pre- and post-intervention (*p<0.05).
We determined if the increase in IL-10 and TNFα secretion by the high DHA-FO supplement was driven by enhanced B cell proliferation upon stimulation. Proliferation assays revealed no change between pre- and post-intervention values with all three supplements after activation of TLR1/2, TLR9, and TLR9+anti-BCR (data not shown).
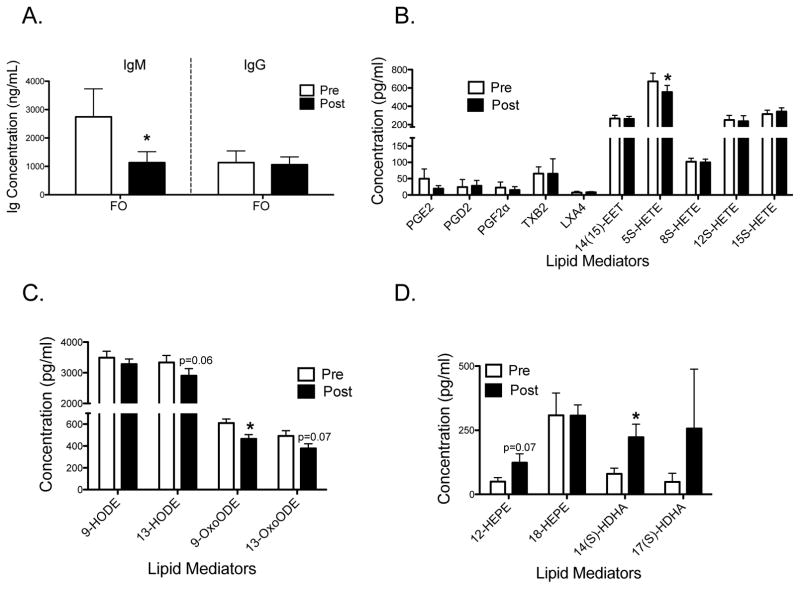
3.7 FO lowers IgM but not IgG levels relative to the baseline accompanied by changes in the circulating lipidome
We next determined if the FO supplement could influence ex vivo antibody production by B cells. Due to a limited supply of B cells, we only had enough samples to study the FO group (n=7). Analyses of supernatants at day 3 post-activation upon TLR9+BCR activation revealed that FO lowered secretion of IgM, but not IgG, by 2.4-fold relative to the baseline (Fig. 5A).
Figure 5. FO lowers IgM levels relative to baseline upon stimulation of TLR9+BCR accompanied by changes in the plasma lipidome.
(A) Ex vivo IgM and IgG levels upon stimulation of TLR9 + BCR. Antibody levels were measured 3 days post-stimulation. LC/MS analyses for (B–C) n-6 PUFA and (D) n-3 PUFA derived mediators. Subjects (n=7) consumed FO for 12 weeks. Data are average ± SEM. Asterisk indicates statistical significance between pre- and post-intervention (*p<0.05).
Mechanistically, lipid mediators synthesized from differing PUFAs regulate antibody production [27]. In particular, lipoxin A4 (LXA4) has been shown to decrease B cell antibody production [21]. Therefore, we conducted a plasma lipidomic analysis of the subjects consuming FO (n=7) to detect differences in select lipid mediators that could influence antibody production. Analyses of arachidonic acid derived lipid mediators showed that the FO supplement had no effect on LXA4 although 5S-HETE levels were lowered by 18% compared to baseline (Fig. 5B). The oxidative marker 9-OxoODE (Fig. 5C) was also lowered by 24% and 13-OxoODE had a tendency (p=0.07) to be lowered (Fig. 5C). Finally, the EPA-derived mediator 12-HEPE tended to be elevated (p=0.07) and the specialized pro-resolving lipid mediator precursor 14-HDHA was elevated by 2.8-fold compared to baseline (Fig. 5D).
4.0 Discussion
4.1 N-3 LC-PUFAs may have potential immunomodulatory effects on B cells
This study attempted to understand how n-3 LC-PUFAs influence B cell activity in obese humans. The rationale for focusing on obese individuals was based on data from our lab to show that B cell cytokine secretion and/or antibody production in obese mice can be modified through dietary inclusion of n-3 LC-PUFAs [9–12]. For instance, we have found that EPA and DHA ethyl esters, upon incorporation in an obesogenic diet, can boost B cell cytokines such as TNFα and IL-10 in response to TLR4 stimulation [10]. Furthermore, we have shown that DHA ethyl esters can improve influenza-specific antibody production in obese mice [13].
We speculate that the increase in B cell cytokine secretion with the high DHA-FO supplement relative to baseline could be of potential value for select populations. For instance, newly diagnosed diabetics have lowered B cell cytokine secretion and B cell TNFα secretion is diminished relative to controls in established type II diabetics [14, 28]. However, extensive additional work is needed in this area since our results with the high DHA-FO supplement only showed an effect relative to baseline. Furthermore, we need to study more B cell cytokines since some studies show that obesity can enhance B cell cytokine secretion [29]. We did not conduct an extensive time course with the ex vivo B cell studies, so it is difficult to conclude whether the effects of the high DHA-FO were specific to a time point or generalizable to other time points post-activation.
The results to show that ex vivo IgM levels could be lowered with FO, relative to the baseline, are generally consistent with studies on a few subjects by Virella et al to demonstrate that EPA can lower circulating immunoglobulins [30, 31]. In our case, we did not measure a change in ex vivo IgG but only IgM with FO. These results could be of value given our recent data to show that increasing BMI is associated with an elevation in ex vivo IgM but not IgG levels [13]. Again, more experiments are needed with a larger cohort to determine how differing FO supplements influence antibody production, particularly in the context of influenza infection which is a major health burden in the obese [15].
4.2 Potential mechanisms of n-3 LC-PUFAs
Due to the limited number of B cells that could be obtained per individual, the current experiments did not address underlying molecular mechanisms but did tackle specificity of ligands that target differing signaling networks. The study used three different stimulation protocols that either targeted TLR1/2, TLR9, or TLR9+anti-BCR. We speculate that the effects of n-3 LC-PUFAs on B cell activation are likely dependent on which signaling pathway is being targeted with a given agonist. Signaling networks can be dependent on the molecular organization of lipid rafts, which are a major target of n-3 LC-PUFAs [3]. Thus, a raft driven mechanism could explain some potential changes in B cell responses [9, 11, 32, 33].
There are likely additional mechanisms by which EPA and DHA could modulate B cell cytokine secretion and/or antibody production. Most notably, specialized pro-resolving mediators synthesized from PUFAs can influence mouse and human B cell activity [20–22, 29]. For instance, 17-HDHA, synthesized from DHA, boosts IgM and IgG levels upon vaccination and lowers B cell IgE [19, 20]. In particular, lipoxin A4 synthesized from arachidonic acid decreases antibody production from memory B cells [21]. Our lipidomic analyses did not reveal a change in lipoxin A4. However, we did observe an increase in circulating 14-HDHA, which can boost antibody production in vivo upon influenza infection, and a trend in elevating 12-HEPE [13]. Thus, future studies will need to establish how changes in PUFA-derived mediators such as 12-HEPE or 14-HDHA can potentially influence antibody production upon TLR9+BCR stimulation. The results with FO supplement and antibody production will require a study with a larger cohort to validate.
4.3 Limitations of the study
There are several major limitations of this study that will need to be addressed in in the future. One major limitation of the study was that all of the observed changes were relative to baseline with the FO supplements. There were no post-intervention differences in B cell endpoints between OO and the two FO groups. This could have been due to several reasons, most notably gender and power. The OO group had a disproportionately larger proportion of females than males compared to the FO concentrate groups. There is emerging literature to show differences in lymphocyte function between females and males [34]. Therefore, any conclusions from this study will need to be confirmed with a larger cohort that would tightly control for gender. Supporting studies with human B cells are also needed to discriminate how B cell responses vary between sexes and the potential role of estrogen in influencing B cell cytokine secretion and antibody production in obesity.
This pilot study was not powered enough to dissect differences between males and females. Future measurements will require extensive power to detect differences not only between sexes but also include other variables such as BMI and race. In addition, we did not ask the enrolled subject to guess which treatment group they had been assigned to. Subsequent studies will need to ask the subjects this question and compare the results to the actual assignment in order to validate the blinding of the subjects.
An added limitation was that our fatty acid analyses were not conducted with B cells. We did verify that both n-3 LC-PUFA supplements increased the relative levels of EPA and DHA in whole blood. Surprisingly, EPA and DHA levels were statistically identical between the FO and the high DHA-FO groups with some minor differences between n-6 PUFA and DPA (22:5n-3) levels. This suggests that although EPA and DHA levels were the same in whole blood, there are likely differences in EPA and DHA levels between subcellular compartments or in differing metabolic depots such as phospholipids compared to neutral lipids. Therefore, a limitation of our study was that we relied on whole blood samples for n-3 LC-PUFA levels. In the future, B cell levels of n-3 LC-PUFAs will need to be assayed and perhaps compared to plasma phosphatidylcholine EPA and DHA levels, which is an excellent biomarker for n-3 LC-PUFA intervention [35]. These studies will inform how efficiently humans B cells take up n-3 LC-PUFAs relative to circulating levels of EPA and DHA.
There are several additional factors that will need to be controlled for in subsequent studies. One such factor is stress that could potentially boost the inflammatory cascade [36]. Although we conducted a work-life balance survey to determine if any of the measured inflammatory cytokines correlated with stress related to work behavior, we did not directly assess biological measures of stress. Another variable that could influence B cell responses is age [37]. The age was well controlled for in this study but it is conceivable that older individuals may have a different response to FO supplementation and this will need to be tested in the future.
We confirmed with a survey that the subjects enrolled in the study were not showing changes in physical activity that could also confound the data. However, subsequent experiments could go further by examining the type of physical activity and its influence on B cell function. It is essential to acknowledge that surveys, as used in this study, have their own limitations due to self-reporting. This was evident with our food intake survey, which revealed the total kcal consumed per day was far lower than one would expect for obese individuals. Thus, the subjects in this study were under-reporting their caloric intake.
A final limitation was that we only assessed the major circulating immune cell populations in response to the OO and the two FO supplements. We did not quantify dendritic cells or natural killer cells, which have been shown to be influenced by n-3 LC-PUFAs [38, 39]. In addition, we assayed only major B cell subsets and did not study the B1 population or other B cell subsets such as B10 cells, which is an area of future investigation [40].
4.4 Conclusions
The data demonstrate that B cell ex vivo cytokine secretion is enhanced upon dietary supplementation with a high DHA-FO supplement, relative to baseline, in obese subjects. The enhancement in cytokine secretion was ligand dependent for BCR/TLR signaling networks. Furthermore, the results show that FO decreased select circulating B cell subsets and levels of ex vivo IgM compared to baseline upon TLR9+BCR stimulation, accompanied by modifications to the plasma lipidome. However, extensive additional measurements are needed given that the effects of FO and high DHA-FO were only relative to baseline and additional variables, particularly sex, were not well controlled. Altogether, the findings from this pilot study suggest B cell activity may be sensitive to n-3 LC-PUFA intervention, warranting more in-depth experiments with a larger cohort.
Supplementary Material
Acknowledgments
The research was supported by Organic Technologies (SRS, SD) and by NIH R01AT008375 (SRS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–9S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 2.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–62. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–84. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, et al. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. 2016;8:353ra111. doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaikh SR, Jolly CA, Chapkin RS. n-3 Polyunsaturated fatty acids exert immunomodulatory effects on lymphocytes by targeting plasma membrane molecular organization. Mol Aspects Med. 2012;33:46–54. doi: 10.1016/j.mam.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teague H, Fhaner CJ, Harris M, Duriancik DM, Reid GE, Shaikh SR. n-3 PUFAs enhance the frequency of murine B-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity. J Lipid Res. 2013;54:3130–8. doi: 10.1194/jlr.M042457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J Lipid Res. 2010;51:1284–97. doi: 10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockett BD, Teague H, Harris M, Melton M, Williams J, Wassall SR, et al. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J Lipid Res. 2012;53:674–85. doi: 10.1194/jlr.M021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teague H, Harris M, Fenton J, Lallemand P, Shewchuk BM, Shaikh SR. Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance B-cell activity in murine obesity. J Lipid Res. 2014;55:1420–33. doi: 10.1194/jlr.M049809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurzell EA, Teague H, Harris M, Clinthorne J, Shaikh SR, Fenton JI. DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. J Leukoc Biol. 2013;93:463–70. doi: 10.1189/jlb.0812394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurzell EA, Teague H, Duriancik D, Clinthorne J, Harris M, Shaikh SR, et al. Marine fish oils are not equivalent with respect to B-cell membrane organization and activation. J Nutr Biochem. 2015;26:369–77. doi: 10.1016/j.jnutbio.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosaraju R, Guesdon W, Crouch MJ, Teague HL, Sullivan EM, Karlsson EA, et al. B cell activity is impaired in human and mouse obesity and is responsive to an essential fatty acid upon murine influenza infection. J Immunol. 2017;198:4738–52. doi: 10.4049/jimmunol.1601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhumitha H, Mohan V, Kumar NP, Pradeepa R, Babu S, Aravindhan V. Impaired toll-like receptor signalling in peripheral B cells from newly diagnosed type-2 diabetic subjects. Cytokine. 2015;76:253–9. doi: 10.1016/j.cyto.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2012;36:1072–7. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paich HA, Sheridan PA, Handy J, Karlsson EA, Schultz-Cherry S, Hudgens MG, et al. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity (Silver Spring) 2013;21:2377–86. doi: 10.1002/oby.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milner JJ, Sheridan PA, Karlsson EA, Schultz-Cherry S, Shi Q, Beck MA. Diet-induced obese mice exhibit altered heterologous immunity during a secondary 2009 pandemic H1N1 infection. J Immunol. 2013;191:2474–85. doi: 10.4049/jimmunol.1202429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milner JJ, Rebeles J, Dhungana S, Stewart DA, Sumner SC, Meyers MH, et al. Obesity increases mortality and modulates the lung metabolome during pandemic H1N1 influenza virus infection in mice. J Immunol. 2015;194:4846–59. doi: 10.4049/jimmunol.1402295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim N, Ramon S, Thatcher TH, Woeller CF, Sime PJ, Phipps RP. Specialized proresolving mediators (SPMs) inhibit human B-cell IgE production. Eur J Immunol. 2016;46:81–91. doi: 10.1002/eji.201545673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, Serhan CN, et al. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? J Immunol. 2014;193:6031–40. doi: 10.4049/jimmunol.1302795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramon S, Bancos S, Serhan CN, Phipps RP. Lipoxin A(4) modulates adaptive immunity by decreasing memory B-cell responses via an ALX/FPR2-dependent mechanism. Eur J Immunol. 2014;44:357–69. doi: 10.1002/eji.201343316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J Immunol. 2012;189:1036–42. doi: 10.4049/jimmunol.1103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritter-Gooder PK, Lewis NM, Heidal KB, Eskridge KM. Validity and reliability of a quantitative food frequency questionnaire measuring n-3 fatty acid intakes in cardiac patients in the Midwest: a validation pilot study. J Am Diet Assoc. 2006;106:1251–5. doi: 10.1016/j.jada.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Aziz S, Uhrich B, Wuensch KL, Swords B. The Workaholism Analysis Questionnaire: Emphasizing work-life imbalance and addiction in the measurement of workaholism. JBAM. 2013;14:71–86. [Google Scholar]
- 25.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 26.Bell JG, Mackinlay EE, Dick JR, Younger I, Lands B, Gilhooly T. Using a fingertip whole blood sample for rapid fatty acid measurement: method validation and correlation with erythrocyte polar lipid compositions in UK subjects. Br J Nutr. 2011;106:1408–15. doi: 10.1017/S0007114511001978. [DOI] [PubMed] [Google Scholar]
- 27.Croasdell A, Duffney PF, Kim N, Lacy SH, Sime PJ, Phipps RP. PPARgamma and the innate immune system mediate the resolution of inflammation. PPAR Res. 2015;2015:549691. doi: 10.1155/2015/549691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jagannathan M, McDonnell M, Liang Y, Hasturk H, Hetzel J, Rubin D, et al. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia. 2010;53:1461–71. doi: 10.1007/s00125-010-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110:5133–8. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virella G, Fourspring K, Hyman B, Haskill-Stroud R, Long L, Virella I, et al. Immunosuppressive effects of fish oil in normal human volunteers: correlation with the in vitro effects of eicosapentanoic acid on human lymphocytes. Clin Immunol Immunopathol. 1991;61:161–76. doi: 10.1016/s0090-1229(05)80021-2. [DOI] [PubMed] [Google Scholar]
- 31.Virella G, Kilpatrick JM, Rugeles MT, Hyman B, Russell R. Depression of humoral responses and phagocytic functions in vivo and in vitro by fish oil and eicosapentanoic acid. Clin Immunol Immunopathol. 1989;52:257–70. doi: 10.1016/0090-1229(89)90177-3. [DOI] [PubMed] [Google Scholar]
- 32.Shaikh SR, Fessler MB, Gowdy KM. Role for phospholipid acyl chains and cholesterol in pulmonary infections and inflammation. J Leukoc Biol. 2016;100:985–97. doi: 10.1189/jlb.4VMR0316-103R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaikh SR, Kinnun JJ, Leng X, Williams JA, Wassall SR. How polyunsaturated fatty acids modify molecular organization in membranes: insight from NMR studies of model systems. Biochim Biophys Acta. 2015;1848:211–9. doi: 10.1016/j.bbamem.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–38. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 35.Browning LM, Walker CG, Mander AP, West AL, Madden J, Gambell JM, et al. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr. 2012;96:748–58. doi: 10.3945/ajcn.112.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almadi T, Cathers I, Chow CM. Associations among work-related stress, cortisol, inflammation, and metabolic syndrome. Psychophysiology. 2013;50:821–30. doi: 10.1111/psyp.12069. [DOI] [PubMed] [Google Scholar]
- 37.Pinti M, Appay V, Campisi J, Frasca D, Fulop T, Sauce D, et al. Aging of the immune system: Focus on inflammation and vaccination. Eur J Immunol. 2016;46:2286–301. doi: 10.1002/eji.201546178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teague H, Rockett BD, Harris M, Brown DA, Shaikh SR. Dendritic cell activation, phagocytosis and CD69 expression on cognate T cells are suppressed by n-3 long-chain polyunsaturated fatty acids. Immunology. 2013;139:386–94. doi: 10.1111/imm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaqoob P, Newsholme EA, Calder PC. Inhibition of natural killer cell activity by dietary lipids. Immunol Lett. 1994;41:241–7. doi: 10.1016/0165-2478(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 40.Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunol. 2015;194:1395–401. doi: 10.4049/jimmunol.1401329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.