Abstract
Preliminary evidence suggests that postoperative cognitive dysfunction (POCD) is common following lung transplantation. The impact of POCD on clinical outcomes has yet to be studied. The association between POCD and longer term survival was therefore examined in a pilot study of post-transplant survivors. Forty-nine participants from a prior randomized clinical trial underwent a neurocognitive assessment battery pre-transplant and 6-months following transplantation, including assessments of Executive Function (Trail Making Test, Stroop, Digit Span), Processing Speed (Ruff 2&7 Test, Digit Symbol Substitution Test), and Verbal Memory (Verbal Paired Associates, Logical Memory, Animal Naming, and Controlled Oral Word Association Test). Over a 13-year follow-up, 33 participants (67%) died. Greater neurocognition was associated with longer survival (HR = 0.49 [0.25, 0.96], P = .039), and this association was strongest on tests assessing Processing Speed (HR = 0.58 [0.36, 0.95], P = .03) and Executive Function (HR = 0.52 [0.28, 0.97], P = .040). In addition, unadjusted analyses suggested an association between greater Memory performance and lower risk of CLAD (HR = 0.54 [0.29, 1.00], P = .050). Declines in Executive Function tended to be predictive of worse survival. These preliminary findings suggest that postoperative neurocognition is predictive of subsequent mortality among lung transplant recipients. Further research is needed to confirm these findings in a larger sample and to examine mechanisms responsible for this relationship.
Introduction
Lung transplantation continues to be the only remaining option for many individuals with advanced pulmonary disease.1 Following the implementation of the lung allocation score in 2005, lung recipients have become increasingly older and sicker,2 with a median age of transplant approaching 60 years old.3 Despite changes in recipient characteristics and perioperative management strategies, post-transplant survival has remained constant at a median of 6-years, more than 50% lower than other solid organ transplant groups,4 and few medical characteristics have proven prognostic of long-term risk.5
Emerging research suggests that postoperative cognitive dysfunction (POCD) is common following lung transplantation, occurring in more than half of recipients.6–8 POCD may have important clinical implications, as poorer neurocognitive function has been associated with worse quality of life, greater risk of medication non-adherence9,10 and poorer clinical outcomes in other patient populations.11–14 However, to our knowledge, no studies have examined the association between POCD and clinical outcomes among lung transplant recipients. We therefore examined the association between POCD and mortality among participants from previously published randomized trial of coping skills training.15
Methods
The present analyses were based on data collected as part of the Investigational Study of Psychological Intervention in Recipients of Lung Transplant (INSPIRE) trial.15 INSPIRE was a randomized, telephone-based coping skills intervention for lung transplant candidates intended to improve quality of life and potentially increase survival. Participants were enrolled from Duke University Medical Center (DUMC) and Washington University School of Medicine in St. Louis (WUSM). The study was approved by the DUMC (IRB #9150) and WUSM (IRB #00-0861) Institutional Review Boards. However, neurocognitive test data were obtained only at DUMC. As previously reported,15 individuals were enrolled in the INSPIRE trial between September 2000 and August 2004. Primary results showed that the coping skills intervention significantly improved quality of life relative to health education controls, but did not result in improved survival.
Clinical and Demographic Characteristics
Demographic data was self-reported by participants at the time of their neurocognitive testing battery. Data on forced expiratory volume (FEV1), number of rejection episodes, and other medical data were obtained from participants’ medical records.
Neurobehavioral Assessments
Participants completed a battery of neurocognitive tests approximately six months following transplantation (mean = 6 months [SD = 0.14 months]). Assessments included measures of Processing Speed, Executive Function, and Verbal Memory. including Trail Making Tests A and B,16 the Stroop test,17 the Ruff 2&7 Test,18 the Wechsler Adult Intelligence Scale (WAIS) Digit Symbol Substitution Test (DSST),19 the WAIS Digit Span Test (DST),19 the Wechsler Memory Scale (WMS) Verbal Paired Associates and Logical Memory subtests,20 the Controlled Oral Word Association Test (COWA),21 and the Animal Naming Test.22 These tests have been widely advocated for this psychometric properties23,24 and sensitivity to subtle deficits in medical populations, particularly for frontal-lobe mediated functioning.25
The Stroop test17 consists of three timed sections: word, color, and color-word. In each section, participants are presented with a page consisting of five columns, each with 20 items for a total of 100 items per page. The score within each section is the total number of words read aloud in 45 seconds. The Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised19 is a measure of complex processing speed in which participants are asked to draw symbols that match one of 10 digits copied from a key. The Trail Making Test16 consists of two separate sections (A and B) in which participants connect circles in increasing order as quickly as possible. In section A, participants connect circles identified by the numbers 1 through 25, whereas section B requires participants to connect 25 circles identified by either a number or a letter, in alternating sequence (i.e. 1-A-2-B-3-C, and so on). The Ruff 2 and 7 Selective Attention Test18 requires participants to visually search for and identify the digits 2 and 7 when randomly embedded within 20 alternating sets of letter and digit distracters. The Digit Span subtest from the Wechsler Adult Intelligence Scale—Revised,19 requires participants to repeat progressively longer series of numbers immediately after they are read aloud by the examiner. In the forward subtest, participants repeat the numbers exactly as they are read. In the backward subtest, participants repeat numbers in reverse sequence.
The Animal Naming Test22 requires participants to generate the names of as many animals as possible in 60 seconds. The Controlled Oral Word Association (COWA) test 21 assesses phonemic fluency, requiring participants to generate as many different words as possible that begin with a particular letter, excluding proper nouns and suffix variations. Three letters are used in separate, 60-second sections. The Logical Memory subtest of the Wechsler Memory Scale-Revised20 requires participants to learn and recall two paragraph-length stories after they are read aloud by the examiner. In the Verbal Paired Associates subtest,20 participants are verbally presented with a set of eight word pairs, half of which are semantically related (e.g. baby-cries) and half of which are unrelated (e.g. pen-grocery). Participants are then cued with one word from each pair and asked to produce the other word.
Post-Transplant Survival and Chronic Lung Allograft Dysfunction (CLAD)
DUMC medical records were reviewed to confirm participant’s date of transplantation, survival status, and date of death. If no date of death was found in a patient’s medical record, a Social Security Death Index search was also conducted. Patients who did not die were censored at the time of last contact with medical staff based on DUMC electronic medical records as of June 1, 2016. Pulmonary function testing was performed on all patients using American Thoracic Society guidelines. Participants were considered to have CLAD if they experienced an FEV1 loss, defined by current FEV1/FEV1Best less than 0.8, where FEV1Best was the average of the two FEV1 measurements that paired with the two best post-transplant FEV1 in the absence of other clinical factors that could affect FEV1 decline, as per the ISHLT guidelines.
Statistical Analyses
Neurocognitive predictors of post-transplant survival were examined using separate Cox proportional hazards models (SAS 9.4 and R 3.4.1). In order to control for potential background and medical factors that would also influence mortality, we included native disease (cystic fibrosis [CF] vs. non-CF), FEV1, total numbers of rejection episodes during the first six months following transplant, and years of education. Because native disease and age are confounded, we chose to model native disease instead of age as a covariate. In addition, because our model had limited power due to a small sample size, we chose to model native disease as CF vs. non-CF in order to account for both native disease differences and age differences in the most parsimonious manner. Results were unchanged if both age and native disease were included as independent predictors.
Neurocognitive function subtests were combined in order to minimize the number of statistical tests in the present analysis. Principal axis factor analysis was used to combine the information from the 13 individual neurocognitive tests into three domains: Processing Speed, Executive Function, and Verbal Memory. A scree test was used to determine the total number of factors retained for analysis. A minimum loading of 0.50 was required and a Promax rotation was used. Based on the observed factor structure, unit-weighted composite scores were created by standardizing individual test scores and then summing all subtests within a given domain. Consistent with our prior work, unit-weighting was accomplished by creating a within-study z-score for each subtest, which were then averaged within domains. Our Processing Speed composite included the DSST, TMT-A, Stroop Word, Stroop Color, the Ruff 2&7 test. Our Executive Function composite included the DST forwards and backwards, TMT-A, TMT-B, and the Stroop Color-Word subtest. Our Verbal Memory composite included the VPA, Logical Memory test, COWA, and the Animal Naming Test. These composites were then used as the predictors of interest in separate proportional hazards regression analyses due to their co-linearity. In addition, in order to minimize the potential impact of our factor groupings on our mortality analyses, we also examined the association between an overall composite of neurocognitive functioning comprised of all subtests. All continuous predictors, including our neurocognitive predictors, were scaled using the interquartile range, which allows for the comparison of individuals at the 75th and 25th percentile of a given predictor variable. In a final set of exploratory analyses, we examined differences in survival between individuals whose neurocognition remained stable or improved from pre-transplant assessments (demographically corrected t-score mean change ≥ 0) compared to those whose performance declined (demographically corrected t-score mean change < 0). Because there is no standardized definition for neurocognitive decline,26–28 these analyses are presented only to characterize the magnitude of association between worsening neurocognition and subsequent mortality.
Results
Sample Characteristics
Eighty-seven participants from Duke were alive six months following transplantation, among whom 49 completed cognitive testing (Table 1). Participants who completed testing did not appear to differ appreciably from the remainder of the cohort on any relevant demographic or medical characteristics, including native disease (P = .506), age (P = .912), gender (P = .686), years of education (P = .869), type of transplant (P = .164), number of rejection episodes (P = .661), duration of transplant hospitalization (P = .426), pre-transplant functional capacity (P = .178), or 6-month FEV1 (P = .900).
Table 1.
Demographic and background characteristics of the study sample (n = 49).
| Variable | |
|---|---|
|
| |
| Native Disease | |
| COPD | 19 (39%) |
| Cystic Fibrosis | 12 (24%) |
| Pulmonary Fibrosis | 11 (22%) |
| Other | 7 (14%) |
|
| |
| Age | 49.6 (12.9) |
|
| |
| Gender, % Male | 21 (43%) |
|
| |
| FEV1 at 6 Months | 0.63 (0.91) |
|
| |
| Rejection episodes during first 6 months, # | |
| 0 | 28 (57%) |
| 1 | 14 (29%) |
| ≥ 2 | 7 (14%) |
|
| |
| Years of Education | 13.6 (2.7) |
|
| |
| INSPIRE Coping Skills Training Treatment Group | 18 (37%) |
|
| |
| Cognitive Test Performance | |
|
| |
| Stroop Word | 94.8 (16.0) |
|
| |
| Stroop Color | 69.0 (12.7) |
|
| |
| Stroop Color-Word | 33.7 (10.1) |
|
| |
| Trail Making Part A, secs | 31.2 (13.6) |
|
| |
| Trail Making Part B, secs | 73.1 (31.3) |
|
| |
| Digit Span Forwards | 8.4 (2.2) |
|
| |
| Digit Span Backwards | 6.4 (1.8) |
|
| |
| Digit Symbol Substitution Test | 53.9 (11.8) |
|
| |
| Ruff 2 & 7 Test | 239.5 (45.5) |
|
| |
| Logical Memory | 27.3 (6.3) |
|
| |
| Verbal Paired Associates | 17.9 (3.9) |
|
| |
| Animal Naming Test | 18.7 (5.4) |
|
| |
| Controlled Oral Word Association Test | 33.9 (9.2) |
|
| |
| Post-Pre Δ | |
|
| |
| Stroop Word | 3.2 (6.2) |
|
| |
| Stroop Color | 1.4 (6.2) |
|
| |
| Stroop Color-Word | −0.7 (8.0) |
|
| |
| Trail Making Part A, secs | 0.3 (11.6) |
|
| |
| Trail Making Part B, secs | 6.0 (37.2) |
|
| |
| Digit Span Forwards | −0.4 (2.1) |
|
| |
| Digit Span Backwards | 0 (2.3) |
|
| |
| Digit Symbol Substitution Test | 1.5 (7.8) |
|
| |
| Ruff 2 & 7 Test | 3.1 (33.8) |
|
| |
| Logical Memory | 5.2 (6.3) |
|
| |
| Verbal Paired Associates | 1.1 (4.1) |
|
| |
| Animal Naming Test | −0.1 (5.5) |
|
| |
| Controlled Oral Word Association Test | −0.2 (9.0) |
Neurocognitive Test Performance
As shown in Table 2, demographically-corrected levels of neurocognitive test performance revealed that 10 individuals (20%) exhibited at least one impairment on the test battery at their six-month assesment, although performance across the cohort was generally in the Average range (i.e. T-scores between 43 and 57). Principal axis factor analysis revealed three underlying factors within our test battery: Processing Speed, Executive Function, and Verbal Memory (Table 3). Unit-weighted composite measures were subsequently created for each cognitive factor, which were used as the predictors of interest in our survival models described below.
Table 2.
Level of impairment by neurocognitive subtest. Results are based on comparison of each participant’s performance to demographically corrected normative data, based on age, education, and gender.
| Variable | T-score | ≥ Average | Low Average | Borderline | Impaired |
|---|---|---|---|---|---|
| Ruff 2 & 7 Test, Digits | 44.7 (9.8) | 28 (57%) | 11 (22%) | 8 (16%) | 2 (4%) |
| Ruff 2 & 7 Test, Letters | 46.3 (8.8) | 31 (63%) | 11 (23%) | 7 (14%) | 0 (0%) |
| Stroop Interference | 45.7 (7.8) | 34 (69%) | 8 (16%) | 4 (8%) | 3 (6%) |
| Trail Making Part A, secs | 49.1 (9.7) | 36 (73%) | 7 (14%) | 5 (10%) | 1 (2%) |
| Trail Making Part B, secs | 51.4 (10.3) | 42 (86%) | 4 (8%) | 2 (4%) | 1 (2%) |
| Digit Span | 46.2 (7.6) | 32 (65%) | 11 (22%) | 6 (12%) | 0 (0%) |
| Logical Memory | 55.3 (9.3) | 46 (94%) | 2 (4%) | 1 (2%) | 0 (0%) |
| Verbal Paired Associates | 51.0 (4.2) | 48 (98%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Animal Naming Test | 44.7 (8.8) | 32 (65%) | 6 (12%) | 6 (12%) | 5 (10%) |
| Controlled Oral Word Association Test | 42.4 (7.4) | 24 (49%) | 16 (33%) | 8 (16%) | 1 (2%) |
Table 3.
Neurocognitive factor structure following Promax rotation.
| Variable | Processing Speed | Executive Function | Verbal Memory |
|---|---|---|---|
| Digit Symbol Substitution Test | 92* | −22 | −4 |
| Ruff 2 & 7 Test | 81* | −21 | 15 |
| Stroop Word | 75* | 27 | −12 |
| Stroop Color | 61* | 32 | 4 |
| Trail Making Part A, secs | −53* | −53* | −17 |
| Trail Making Part B, secs | −10 | −50* | −33 |
| Stroop Color-Word | 41 | 52* | −15 |
| Digit Span Forwards | −2 | 83* | −8 |
| Digit Span Backwards | −16 | 82* | 8 |
| Logical Memory | −27 | 10 | 66* |
| Verbal Paired Associates | 17 | −15 | 62* |
| Animal Naming Test | 9 | 4 | 60* |
| Controlled Oral Word Association Test | 17 | −15 | 64* |
Neurocognition and Mortality
Over a 13-year follow-up, 33 participants (67%) died (median survival 6.9 years [IQR = 6.6]). Of the patients who died, the primary cause of death was graft failure (n = 25 [76%]), although 3 patients also died from malignancies, 2 died from cardiovascular events and 3 died from unknown causes. In addition, 20 individuals (41%) developed CLAD.
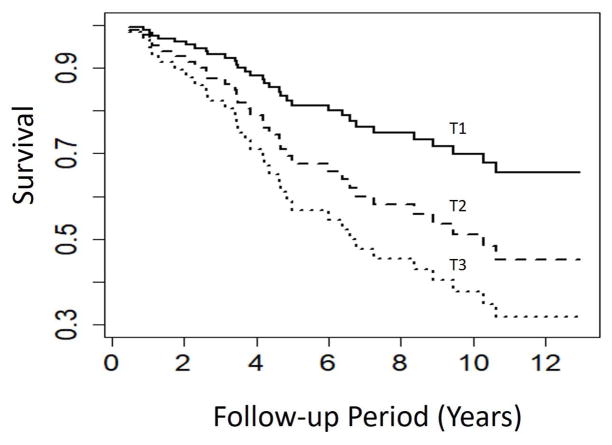
Examination of neurocognitive predictors revealed that greater performance across all subtests was associated with longer survival (HR = 0.49 [0.25, 0.96], P = .039) (Figure 1). Examination of specific domains revealed that greater Processing Speed (HR = 0.58 [0.36, 0.95], P = .030) and Executive Function (HR = 0.52 [0.28, 0.97], P = .040) were associated with longer survival (Table 4; Figure 1), with a similar, non-significant trend observed for greater memory performance (HR = 0.65 [0.38, 1.11], P = .115). Explanatory, ancillary analyses of individual subtests revealed that the strongest associations between neurocognitive performance and subsequent survival were observed on the Digit Span Backwards (HR = 0.48 [0.28, 0.82]), Stroop Color (HR = 0.49 [0.28, 0.87]) and Word (HR = 0.57 [0.34, 0.95]) tests, and the COWA (HR = 0.65 [0.40, 1.04]) (Table 5). In order to characterize the magnitude of mortality risk based on clinically informative cognitive performance levels, we also compared individuals based on their demographically-corrected performance levels (e.g. compared to age, education, and gender-matched normative data). Results revealed that performance differences of one IQR on the Digit Span (16th [Low Average] vs. 62nd [Average] percentile) were associated with 23% lower 6-year survival levels (32% vs. 55%). Similarly, one IQR difference on the COWA (10th [Low Average] vs. 34th [Average] percentile) were associated with 17% lower 6-year survival levels (31% vs. 48%).
Figure 1.
Neurocognitive test performance averaged across all subtests within all domains, including Processing Speed, Executive Function, and Verbal Memory. Data are presented in Tertiles, in which the composite z-score of neurocognitive test performance across all domains was partitioned into thirds based on performance within the sample.
Table 4.
Cox proportional hazards results from three separate models examining the association between neurocognition and graft survival. Continuous predictors are scaled using the interquartile range (IQR).
| Predictor | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| Processing Speed model | |||
| FEV-1 at 6-months | 0.87 | 0.59, 1.28 | .428 |
| Rejection Episodes | 0.76 | 0.46, 1.25 | .249 |
| CF vs. Non-CF | 0.46 | 0.14, 1.52 | .169 |
| Years of Education | 1.00 | 0.87, 1.15 | .976 |
| INSPIRE Group | 1.34 | 0.66, 2.72 | .426 |
| Processing Speed | 0.58 | 0.36, 0.95 | .030 |
| Executive Function model | |||
| FEV-1 at 6-months | 0.90 | 0.61, 1.32 | .581 |
| Rejection Episodes | 0.68 | 0.40, 1.15 | .152 |
| CF vs. Non-CF | 0.40 | 0.12, 1.31 | .129 |
| Years of Education | 1.07 | 0.91, 1.25 | .421 |
| INSPIRE Group | 1.44 | 0.69, 2.99 | .332 |
| Executive Function | 0.52 | 0.28, 0.97 | .040 |
| Verbal Memory model | |||
| FEV-1 at 6-months | 0.80 | 0.53, 1.20 | .275 |
| Rejection Episodes | 0.74 | 0.45, 1.22 | .237 |
| CF vs. Non-CF | 0.43 | 0.13, 1.39 | .157 |
| Years of Education | 1.01 | 0.88, 1.17 | .853 |
| INSPIRE Group | 1.17 | 0.57, 2.42 | .666 |
| Verbal Memory | 0.65 | 0.38, 1.11 | .115 |
Table 5.
Explanatory analyses of individual neurocognitive subtests from separate Cox proportional hazards models. Analyses included all participants but did not correct for multiple testing.
| Neurocognitive Subtest (IQR) | Domain | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|---|
| Digit Span Backwards (2) | Executive Function | 0.48 | 0.28, 0.82 | .007 |
| Stroop Color (17) | Processing Speed | 0.49 | 0.28, 0.87 | .015 |
| Stroop Word (20) | Processing Speed | 0.57 | 0.34, 0.95 | .032 |
| COWA (10) | Verbal Memory | 0.65 | 0.40, 1.04 | .069 |
| Trail Making Test Part A (12) | Processing Speed / Executive Function | 1.33 | 0.97, 1.82 | .075 |
| Logical Memory (9) | Verbal Memory | 0.68 | 0.43, 1.09 | .106 |
| Ruff 2 & 7 Letters (36) | Processing Speed | 0.62 | 0.34, 1.13 | .116 |
| Ruff 2 & 7 Digits (30) | Processing Speed | 0.68 | 0.38, 1.20 | .183 |
| Digit Span Forwards (3) | Executive Function | 0.76 | 0.43, 1.33 | .336 |
| Digit Symbol Substitution Test (19) | Processing Speed | 0.72 | 0.35, 1.50 | .380 |
| Verbal Paired Associates (6) | Verbal Memory | 0.79 | 0.45, 1.40 | .419 |
| Trail Making Test Part B (39) | Executive Function | 1.16 | 0.68, 1.98 | .598 |
| Stroop Color-Word (14) | Executive Function | 0.86 | 0.46, 1.59 | .622 |
| Animal Naming (5) | Verbal Memory | 1.02 | 0.72, 1.44 | .930 |
Examination of CLAD outcomes revealed that, in unadjusted analyses, greater memory performance was associated with lower CLAD risk (HR = 0.54 [0.29, 1.00], P = .050), with a weaker, non-significant relationship for executive functioning (HR = 0.61 [0.34, 1.11], P = .103). However, both the association between memory performance (HR = 0.61 [0.30, 1.21], P = .156) and executive functioning (HR = 0.67 [0.31, 1.47], P = .321) following adjustment for covariates. Processing Speed was unrelated to CLAD outcomes in either unadjusted (HR = 0.71 [0.38, 1.33], P = .285) or adjusted (HR = 0.82 [0.42, 1.62], P = .568) analyses.
Neurocognitive Decline and Mortality
In order to examine the relationship between changes in neurocognition following transplantation and subsequent mortality, we conducted an additional set of analyses in which individuals were grouped into those whose cognitive performance either improved or remained stable compared with those individuals whose cognitive performance declined from baseline. Examination of cognitive changes demonstrated that 28 (57%) of individuals experienced a decline in Executive Function, 23 (47%) in Processing Speed, and 15 (31%) in Memory performance. Participants who exhibited a decline in Executive Functioning showed a trend towards greater mortality (HR = 2.14 [0.97, 4.73], P = .060) and risk of CLAD (HR = 2.22 [0.79, 6.20], P = .128). However, declines in other domains were not predictive of subsequent mortality or CLAD (Ps ≥ 0.15).
Discussion
Results from the present analyses suggest that poorer neurocognitive performance following lung transplantation is predictive of subsequent survival, after accounting for other medical and background characteristics. These findings extend previous findings suggesting that POCD is common among lung transplant recipients and may be associated with worse short-term outcomes by demonstrating that POCD may predict long-term risk following transplantation, similar to associations observed in other medical populations.29
Previous studies have suggested that POCD is common following lung transplantation, occurring in an estimated 50–67% of recipients, depending, in part, on the timing and modality of assessment.6–8 For example, we previously demonstrated that impairments are common within the first month following transplantation and may persist in some patients when assessed again approximately three months postoperatively.7 Impairments appear to occur most commonly on tests of executive function,6,7 which is thought to reflect the integrity of brain regions in the prefrontal cortex and subcortical connections.30 Interestingly, impairments in tests associated with frontal lobe functioning, including the Stroop, Digit Span, and COWA, were most predictive of subsequent mortality, consistent with prior studies.11–14 Although we previously reported that neurocognitive performance associated prior to transplant was associated with increased mortality,31 to our knowledge no studies have examined this association following transplantation, after which many individuals experience neurocognitive decline.
There are several plausible mechanisms by which neurocognition may be associated with clinical outcomes. Among solid organ transplant recipients, adherence to immunosuppression medications is critical to survival and has been strongly associated with mortality.32,33 Although no studies have examined the association between neurocognition and adherence in lung transplant recipients, numerous epidemiological studies in other health populations suggest that impairments in executive function and working memory are predictive of greater non-adherence.9,10 In addition, preliminary studies among patients undergoing cardiac surgery suggest that brain structures critical for intact executive functioning and working memory are often impacted following surgery,34,35 with inefficiencies in areas associated with default mode network functioning associating highly with behavioral performance.36 We have previously demonstrated that POCD likely results both from individual vulnerabilities37 as well as operative characteristics,38 suggesting that there may be multiple avenues from which interventions can be designed to protect neurocognition in an increasingly older patient population. Neuroimaging data could therefore provide important insights into both the nature (e.g. ischemic, metabolic, etc.) and functional significance of postoperative brain changes, given that both processes may be impacting post-transplant cognitive function.
Limitations
These preliminary findings must be viewed with caution. First, the study cohort was relatively small and larger prospective studies are needed in order to verify the observed associations. Second, neurocognitive performance was only collected at one time point following transplant. Nevertheless, our neurocognitive test battery was comprehensive and assessed multiple domains of functioning, which we consider a strength despite only one assessment time point. Third, future studies utilizing neuroimaging methods are needed to better characterize the mechanisms underlying POCD following transplant, as noted above. In addition, future studies more comprehensively assessing potential behavioral mechanisms are also important, as behavioral compliance characteristics (e.g. medication adherence and lifestyle practices) or additional physiological processes may also explain the observed associations.
Conclusions
In conclusion, our results suggest that poorer neurocognition 6-months following transplant is a marker of long-term risk and should be examined when possible among transplant recipients. If the present findings are replicated, it may indicate a need to identify and more closely manage individuals exhibiting neurocognitive impairment following transplant. In addition, prospective studies examining neuroimaging mechanisms of POCD are needed in order to identify risk factors and possible treatment targets to mitigate POCD risk.
Acknowledgments
This research was supported by Grant No. HL 065503 from the National Institutes of Health, Bethesda, Maryland.
Abbreviations
- CF
cystic fibrosis
- COPD
chronic obstructive pulmonary disease
- DUMC
Duke University Medical Center
- FEV1
forced expiratory volume in one second
- HR
hazard ratio
- INSPIRE
Investigational Study of Psychological Intervention in Recipients of Lung Transplant
- IPF
idiopathic pulmonary fibrosis
- IQR
interquartile range
- Q
quartile
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-first Adult Lung and Heart-Lung Transplant Report-2014; Focus Theme: Retransplantation. J Heart Lung Transpl. 2014;33(10):1009–1024. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Singer LG, Chowdhury NA, Faughnan ME, et al. Effects of Recipient Age and Diagnosis on Health-related Quality-of-Life Benefit of Lung Transplantation. Am J Resp Crit Care. 2015;192(8):965–973. doi: 10.1164/rccm.201501-0126OC. [DOI] [PubMed] [Google Scholar]
- 3.Kniepeiss D, Wagner D, Pienaar S, et al. Solid organ transplantation: technical progress meets human dignity: a review of the literature considering elderly patients’ health related quality of life following transplantation. Ageing Res Rev. 2012;11(1):181–187. doi: 10.1016/j.arr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Vock DM, Durheim MT, Tsuang WM, et al. Survival Benefit of Lung Transplantation in the Modern Era of Lung Allocation. Ann Am Thorac Soc. 2017;14(2):172–181. doi: 10.1513/AnnalsATS.201606-507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Resp Crit Care. 2005;171(11):1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman BM, Blumenthal JA, Carney RC, et al. Changes in neurocognitive functioning following lung transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(9):2519–2525. doi: 10.1111/j.1600-6143.2012.04072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith PJ, Rivelli S, Waters A, et al. Neurocognitive changes after lung transplantation. Ann Am Thorac Soc. 2014;11(10):1520–1527. doi: 10.1513/AnnalsATS.201406-232OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen DG, Christie JD, Anderson BJ, et al. Cognitive Function, Mental Health, and Health-related Quality of Life after Lung Transplantation. Ann Am Thorac Soc. 2014;11(4):522–530. doi: 10.1513/AnnalsATS.201311-388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell NL, Boustani MA, Skopelja EN, Gao S, Unverzagt FW, Murray MD. Medication adherence in older adults with cognitive impairment: a systematic evidence-based review. Am J Geriatr Pharmacother. 2012;10(3):165–177. doi: 10.1016/j.amjopharm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Stilley CS, Bender CM, Dunbar-Jacob J, Sereika S, Ryan CM. The impact of cognitive function on medication management: three studies. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2010;29(1):50–55. doi: 10.1037/a0016940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pressler SJ, Kim J, Riley P, Ronis DL, Gradus-Pizlo I. Memory dysfunction, psychomotor slowing, and decreased executive function predict mortality in patients with heart failure and low ejection fraction. J Card Fail. 2010;16(9):750–760. doi: 10.1016/j.cardfail.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amirian E, Baxter J, Grigsby J, Curran-Everett D, Hokanson JE, Bryant LL. Executive function (capacity for behavioral self-regulation) and decline predicted mortality in a longitudinal study in Southern Colorado. Journal of clinical epidemiology. 2010;63(3):307–314. doi: 10.1016/j.jclinepi.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62(10):1134–1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavery LL, Dodge HH, Snitz B, Ganguli M. Cognitive decline and mortality in a community-based cohort: the Monongahela Valley Independent Elders Survey. J Am Geriatr Soc. 2009;57(1):94–100. doi: 10.1111/j.1532-5415.2008.02052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumenthal JA, Babyak MA, Keefe FJ, et al. Telephone-based coping skills training for patients awaiting lung transplantation. J Consult Clin Psychol. 2006;74(3):535–544. doi: 10.1037/0022-006X.74.3.535. [DOI] [PubMed] [Google Scholar]
- 16.Reitan RM. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Tucson, AZ: Reitan Neuropsychological Laboratories, Inc; 1979. [Google Scholar]
- 17.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychiat. 1935;18:643–662. [Google Scholar]
- 18.Ruff RM, Neiman H, Allen CC. The Ruff 2 and 7 Selective Attention Test: A neuropsychological application. Percept Mot Skills. 1992;75:1311–1319. doi: 10.2466/pms.1992.75.3f.1311. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. The Wechsler Adult Intelligence Scale Revised. 1981. [Google Scholar]
- 20.Wechsler D. WMS-III Technical Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 21.Spreen O, Strauss E. A compendium of neuropsychological tests. New York: Oxford University Press; 1991. [Google Scholar]
- 22.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. Vol. 4. Oxford, New York, Auckland, Bangkok, Buenos Aires, Cape Town, Chenna, Dar es Sallam, Delhi, Hong Kong, Istanbul, Karachi, Kolkata, Kuala Lumpur, Madrid, Melbourne, Mexico City, Mumbai, Nairobi, Sao Paulo, Shanghai, Taipei, Tokyo, Toronto: Oxford University Press; 2004. [Google Scholar]
- 23.Attix DK, Story TJ, Chelune GJ, et al. The prediction of change: normative neuropsychological trajectories. Clin Neuropsychol. 2009;23(1):21–38. doi: 10.1080/13854040801945078. [DOI] [PubMed] [Google Scholar]
- 24.Messinis L, Kosmidis MH, Tsakona I, Georgiou V, Aretouli E, Papathanasopoulos P. Ruff 2 and 7 Selective Attention Test: normative data, discriminant validity and test-retest reliability in Greek adults. Arch Clin Neuropsychol. 2007;22(6):773–785. doi: 10.1016/j.acn.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 26.Mahanna EP, Blumenthal JA, White WD, et al. Defining neuropsychological dysfunction after coronary artery bypass grafting. Ann Thorac Surg. 1996;61(5):1342–1347. doi: 10.1016/0003-4975(95)01095-5. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph JL, Schreiber KA, Culley DJ, et al. Measurement of post-operative cognitive dysfunction after cardiac surgery: a systematic review. Acta anaesthesiologica Scandinavica. 2010;54(6):663–677. doi: 10.1111/j.1399-6576.2010.02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubens FD, Boodhwani M, Nathan H. Interpreting studies of cognitive function following cardiac surgery: a guide for surgical teams. Perfusion. 2007;22(3):185–192. doi: 10.1177/0267659107080943. [DOI] [PubMed] [Google Scholar]
- 29.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, Grp I. Long-term Consequences of Postoperative Cognitive Dysfunction. Anesthesiology. 2009;110(3):548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 30.Alosco ML, Gunstad J, Jerskey BA, et al. The adverse effects of reduced cerebral perfusion on cognition and brain structure in older adults with cardiovascular disease. Brain Behav. 2013;3(6):626–636. doi: 10.1002/brb3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith PJ, Blumenthal JA, Carney RM, et al. Neurobehavioral functioning and survival following lung transplantation. Chest. 2014;145(3):604–611. doi: 10.1378/chest.12-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77(5):769–776. doi: 10.1097/01.tp.0000110408.83054.88. [DOI] [PubMed] [Google Scholar]
- 33.Castleberry AW, Bishawi M, Worni M, et al. Medication Nonadherence After Lung Transplantation in Adult Recipients. Annals of thoracic medicine. 2017;102(1):274–280. doi: 10.1016/j.athoracsur.2016.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bendszus M, Stoll G. Silent cerebral ischaemia: hidden fingerprints of invasive medical procedures. Lancet Neurol. 2006;5(4):364–372. doi: 10.1016/S1474-4422(06)70412-4. [DOI] [PubMed] [Google Scholar]
- 35.Bendszus M, Reents W, Franke D, et al. Brain damage after coronary artery bypass grafting. Arch Neurol. 2002;59(7):1090–1095. doi: 10.1001/archneur.59.7.1090. [DOI] [PubMed] [Google Scholar]
- 36.Browndyke JN, Berger M, Harshbarger TB, et al. Resting-State Functional Connectivity and Cognition After Major Cardiac Surgery in Older Adults without Preoperative Cognitive Impairment: Preliminary Findings. J Am Geriatr Soc. 2017;65(1):e6–e12. doi: 10.1111/jgs.14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith PJ, Rivelli SK, Waters AM, et al. Delirium affects length of hospital stay after lung transplantation. J Crit Care. 2015;30(1):126–129. doi: 10.1016/j.jcrc.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith PJ, Blumenthal JA, Hoffman BM, et al. Reduced Cerebral Perfusion Pressure during Lung Transplant Surgery Is Associated with Risk, Duration, and Severity of Postoperative Delirium. Ann Am Thorac Soc. 2016;13(2):180–187. doi: 10.1513/AnnalsATS.201507-454OC. [DOI] [PMC free article] [PubMed] [Google Scholar]