Abstract
Background
Increasingly it is being appreciated that B cells have broad roles beyond the humoral response, and are able to contribute to and regulate inflammation. The specific role of B cells in the pathogenesis of early allograft inflammation remains unclear.
Methods
To address this question, we generated B cell deficient Lewis rats via CRISPR technology. In a full mismatch transplant model, kidneys from Brown Norway donors were transplanted into B cell deficient Lewis recipients (B−/−) or wild type Lewis recipients. T cell mediated rejection was attenuated with cyclosporine.
Results
Renal inflammation was reduced at 1 week after transplant (Banff scores for interstitial inflammation, microvascular inflammation, glomerulitis, and C4d) in allografts from B−/− recipients. The reduction in interstitial inflammation was predominantly due to a decline in graft infiltrating macrophages. Intragraft T cell numbers remained unchanged. In addition, B cell deficiency was associated with increased T regulatory cells and reduced splenic T follicular helper cells at baseline; and significantly increased intragraft and splenic IL-10 mRNA levels after transplant. In vitro, B−/− and wild type splenic T cells produced similar levels of IFN-γ in response to T cell specific activation.
Conclusions
B cell deficiency in this model produced an anti-inflammatory phenotype with a shift towards regulatory T cell populations, production of anti-inflammatory cytokines (IL-10), and a reduction in allograft inflammation. These findings define a role for B cells to influence the cell populations and mediators involved in the pathogenesis of early allograft inflammation.
INTRODUCTION
Although we have made great gains in the understanding and treatment of allograft inflammation and acute rejection, it is also clear there are gaps in our understanding of key immunologic mechanisms involved. Furthermore, our current immunosuppressive regimen does not effectively target all inflammatory cells (macrophages, plasma cells) or immune responses (complement system). While therapeutics targeted to these inflammatory cells and immune systems are now available, they typically do not comprise the backbone of standard immunosuppressive therapy in transplantation. Traditionally, induction therapy is directed at T cells to reduce acute cellular rejection; whether this approach translates into a long-term benefit of increasing allograft survival remains unclear.
While the idea that B cells have functions beyond the humoral response is gaining recognition, their specific role in the pathogenesis of early allograft inflammation and acute rejection remains unclear. Several clinical studies of acute cellular rejection demonstrate patient biopsies with graft infiltrating B cells (CD20+) correlate with a higher incidence of steroid resistant rejection and reduced graft survival compared to patients lacking CD20+ cell infiltrates.1–3 Others, however, found no difference in steroid resistance or graft loss at 1 year in patients with acute cellular rejection based on the presence or absence of CD20+ cell infiltrates.4,5 In a randomized clinical trial of patients diagnosed with acute rejection and graft-infiltrating B cells, anti-B cell therapy with rituximab was associated with improved graft function and rejection score on biopsy at 6 months but without effect on donor specific antibody (DSA).6 In contrast, another randomized clinical trial of a single dose of rituximab at induction showed no effect on steroid resistance or on graft survival at 4 years.7 Clinically, B cells have been identified in patients with acute rejection; however, trials with anti-B cell therapy have provided conflicting results.
In order to elucidate the role of B cells in allograft rejection, numerous methods to manipulate B cells and antibodies have been used in both mouse and rat studies. A genetic model of immunoglobulin deficient mice in a cardiac rejection model demonstrated reduced acute rejection and prolonged survival.8 Another cardiac rejection model in severe combined immunodeficiency mice (SCID, lacking B and T cells) showed recipients failed to develop vasculopathy of rejection.9 In a full mismatch mouse kidney transplant model, B cell depletion by treatment with an anti-CD19 antibody reduced pathologic lesions of interstitial inflammation, tubulitis, and tubular atrophy at 21 days, which translated into reduced mortality in the treated recipients at 100 days.10 Others have used a genetic B cell deficient rat in a model of cardiac rejection, in which the heavy chain of IgM was targeted. Since membrane immunoglobulin expression is mandatory for normal B cell maturation, this genetic modification results in a very early block of B cell production. The immunoglobulin heavy chain deficient rats failed to develop hyperacute allograft rejection in a sensitized cardiac transplant model.11 However, there is limited information in the literature detailing renal allograft and lymphoid tissue pathology in these models.
Despite some advantages to conducting experimental studies in genetically modified mice, there are significant limitations to mouse kidney transplant experiments. Limitations of mouse kidney transplant experiments include the relative ease of inducing tolerance, resistance of many mouse strains to glomerulosclerosis and immune-mediated injury, and the weaker complement system in the mouse.12,13 These limitations, plus the technical surgical challenges in performing kidney transplants in mice, make the rat model more reproducible and clinically relevant.14
We sought to examine the specific role of B cells in early allograft inflammation in a fully mismatched rat kidney transplant model using a genetically modified recipient with complete B cell deficiency. Kidney allografts were assessed for graft infiltrating inflammatory cells and regulatory phenotypes.
MATERIALS AND METHODS
Development of B cell deficient rat strain
B cell deficient Lewis rats (B−/−) were generated via CRISPR/Cas9 technology by targeting Igh6, the rat gene ortholog of IgM. More specifically, the N-terminal portion of the Igh6 constant domain (CH1) was selected. This region is highly conserved among human, mouse, and rat IgM. Therefore, a deletion was generated at this location as it was ensured to reside within the well-characterized constant region of IgM. Cas9 mRNA and guide RNA were microinjected into the cytoplasm of Lewis embryos and the embryos were transplanted into recipient females. A founder rat carrying the 8 base pair deletion was selected to generate a breeding colony. DNA from progeny ear punches were sequenced and genotyped.
Cell phenotyping
Single cell suspensions of splenocytes, bone marrow, and lymph node cells were stained for B and T cells and analyzed by flow cytometry (BD LSR II at the UWCCC Flow Cytometry Laboratory) using FlowJo software (TreeStar) as previously described.15 B cell populations were defined as: transitional B cells IgD+ CD45R+ CD24+(hi) CD38+(hi); splenic marginal zone B cells CD45R+ HIS57+ CD45RA+; and lymph node naïve B cells (IgD+ CD45R+ CD27−). Antibodies used for B cell stains included: anti-IgD (clone MARD-3, BioRad); anti-CD45R (clone HIS24, eBioscience); anti-CD24 (clone REA405, Miltenyi Biotec); anti-IgM (clone G53-238, BD Pharmingen); anti-CD3 (clone 1F4, BD Horizon); anti-CD27 (clone LG.3A10, BD Horizon); and anti-CD38 (clone 14.27, BioLegend). Antibodies for marginal zone B cell staining included: HIS57 (BD Pharmingen) and anti-CD45RA (clone Ox33, BD Pharmingen). Antibodies for T cell staining included: anti-CD8 (cloneOX-8, BioLegend); anti-CD45R (clone HIS24, eBioscience); anti-CD4 (clone W3/25, Biolegend); anti-CD3 (clone 1F4, BioLegend); anti-CD25 (clone OX-39, BioLegend); anti-FoxP3 (clone 150D, BioLegend); anti-CD278 (clone C398.4A, BioLegend); and anti-CXCR5 (clone EPR8837, Abcam). Surface Ig staining was detected using: anti-IgG (Jackson ImmunoResearch); anti-IgM (clone G53-238, BD Pharmingen); and anti-IgD (clone MARD-3, BioRad).
ELISA, ELISPOT, and Biochemical assays
Serum levels of IgM and IgG were determined by ELISA according to manufacturers’ instructions (Affymetrix, Inc.). Blood urea nitrogen (BUN) and serum creatinine measurements were determined by automated analysis (VetTest Analyzer Technology).
For IgM and IgG ELISPOT assay, splenoctyes were incubated overnight on a plate coated with capture antibody (anti-IgM, or anti-IgG antibody). Plates were washed and incubated with biotinylated anti-IgM or anti-IgG antibody. Then plates were washed and incubated with streptavidin-alkaline phosphatase conjugate. The plates were developed with BCIP/NBT substrate (MabTech) until distinct spots appeared. Color development was stopped by extensively washing with water. After drying, spots were quantified through a dissecting microscope.
For IFN-γ ELISPOT assay, splenocytes were incubated overnight at 37° C with media or the stimulant Concanavalin A (final concentration 10 μg/mL, Vector Labs). After incubation, plates were washed and IFN-γ biotinylated antibody was added (R&D Systems). After incubation with streptavidin-alkaline phosphatase conjugate, plates were developed with BCIP/NBT substrate. Once spots formed, development was stopped by flooding plates with water and allowed to dry. Spots were quantified by plate reader (AID).
Rat kidney transplant model
All Brown Norway and Lewis rats (Harlan Laboratories) were housed under pathogen free conditions in the animal care facility of the William S. Middleton VA Hospital (Madison, WI). All procedures were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. Full mismatch allogeneic kidney transplants were performed with Brown Norway kidney donors to B−/− Lewis recipients (N=7) or Brown Norway donors to wild type Lewis recipients (WT, N=6). To avoid graft loss from severe T cell mediated acute rejection, all recipients were given cyclosporine (10 mg/kg/day) via intraperitoneal injection until harvest at 7 days. See In-Depth Methods (Supplemental Digital Content) for a complete description of transplant microsurgery.
Histology
Kidney and splenic tissues were fixed in formalin, embedded in paraffin, cut into 5 μm sections, stained with hematoxylin and eosin or periodic acid-Schiff stain, and evaluated by light microscopy. Histologic analysis was scored by a transplant pathologist blinded to study groups (Weixiong Zhong, MD, PhD) according to Banff 2013 criteria.16 High-powered images were obtained on an Olympus BX51 microscope with an Olympus KP70 camera and software (Olympus America Inc.).
Immunohistochemistry, immunofluorescence microscopy, and immunoblotting
Immunoperoxidase, immunofluorescence stain, and Western blot were performed as previously described.17,18 The following antibodies were used: polyclonal anti-C4d (American Research Products); polyclonal anti-CD3 (Abcam); anti-CD68 (clone ED1, Biorad); anti-PAX5 (clone A-11, Santa Cruz); and anti-GAPDH (clone 6C5, Abcam).
Reverse transcriptase PCR analysis
Total RNA was extracted from frozen kidney tissue with Trizol (Life Technologies) and purified using the RNAeasy kit (Qiagen). cDNA was generated with SuperScript IV first strand synthesis system (Invitrogen). TaqMan gene expression assays (Applied Biosystems) were run on a 7500 Reverse Transcriptase PCR System (Applied Biosystems). CT, standard deviation, and ΔΔ CT (compared to GAPDH control) were used to calculate fold changes.
Statistical analyses
Data were analyzed using GraphPad Prism software. Student’s t-test or Mann-Whitney rank sum test were used to compare parametric and nonparametric continuous data. Chi-squared or Fisher’s exact tests were used to compare categorical data. Correlations were determined by Pearson correlation test. A P-value less than 0.05 was considered significant.
RESULTS
Characterization of B cell deficient rat strain
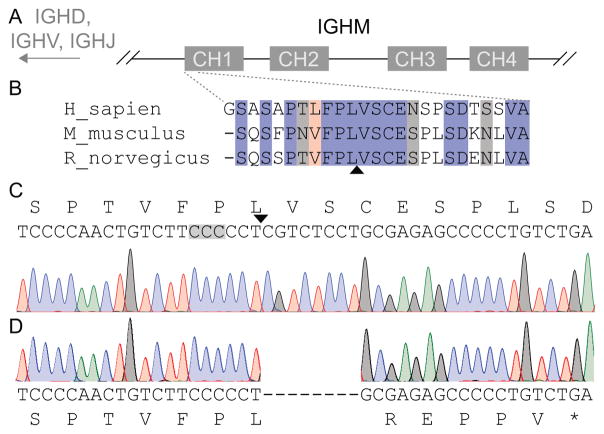
CRISPR/Cas9 targeting technology was used to generate B cell deficient Lewis rats. A targeted deletion of the Igh6 constant region (CH1) was performed (Figure 1A). This region is upstream of the gene sequences for the variable regions and is highly conserved among human, mouse, and rat (Figure 1B). The Cas9 target sequence and cut site is shown in Figure 1C. Characterization of the recovered nonsense allele demonstrated an 8 base-pair deletion resulting in an early truncation of IgM (Figure 1D).
Figure 1. Generation of B cell deficient rats by CRISPR/Cas9 targeting of the rat Igh6 gene.
A, The rat Igh6 gene structure contains 4 constant domains (CH1–CH4) within the constant region of the heavy chain of IgM (IGHM). The relative location of variable region gene segments (IGHD, IGHV, and IGHJ) are shown. B, The N-terminal 25 amino acids of the constant domain CH1 are highly conserved across human, mouse, and rat. Fully conserved amino acids are shown in blue. Strongly similar amino acid sequences (scoring > 0.5 in the Gonnet point accepted mutation (PAM) 250 matrix) and weakly similar amino acid sequences (scoring ≤ 0.5 in the Gonnet PAM 250 matrix) are highlighted in red and grey, respectively. The predicted Cas9 cleavage site (arrowhead) clearly lies within the constant domain of CH1. C, The rat wild type Igh6 nucleotide sequence is shown with the site of the PAM (grey highlight) and the cut site (arrowhead). D, Sanger trace demonstrates the recovered nonsense allele carries an 8 base pair deletion resulting in an early truncation of IgM.
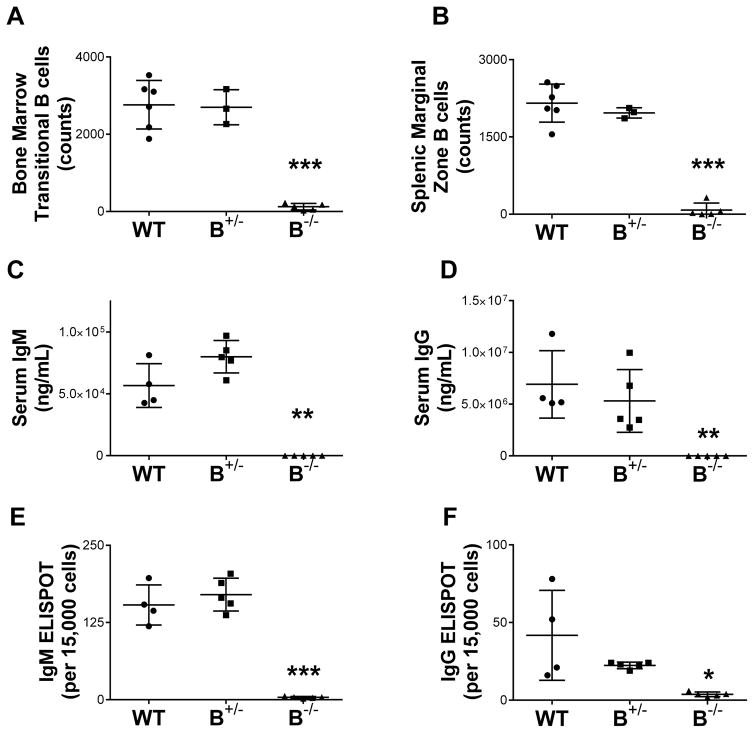
To confirm the phenotype of our B−/− strain, we examined B cell and immunoglobulin expression in bone marrow, secondary lymphoid organs, and circulating blood. Analysis of B cell populations in bone marrow and spleen by flow cytometry demonstrated the absence of B cell populations (Figure 2A and B, P < 0.0001 for B−/− compared to WT). Flow staining scheme for bone marrow transitional B cells: IgD+ CD45R+ CD24+(hi) CD38+(hi). Flow staining scheme for splenic marginal zone B cells: CD45R+ HIS57+ CD45RA+ Similar results were seen in lymph nodes and peripheral blood. Systemic levels of IgM and IgG were absent in the B−/− strain as determined by serum ELISA (Figure 2C and D, P < 0.001 for B−/− compared to WT). Wild type littermate controls and heterozygous rats (B+/−) demonstrated normal circulating levels of IgM and IgG. Lack of antibody production by B−/− was also confirmed by ELISPOT analysis of the production of IgM and IgG by splenocytes (Figure 2E and F. P < 0.0001 and P < 0.05, respectively, for B−/− compared to WT). This confirmed the complete B cell deficient phenotype and the absence of circulating antibodies in B−/− rats.
Figure 2. Immunophenotyping of B cell deficient rats demonstrated a lack of B cell populations and a lack of antibody production.
A and B, Flow cytometry demonstrated absence of B cells in lymphoid tissues in B−/− rats. Heterozygous (B+/−) and wild type (WT) rats demonstrated similar B cell numbers. Systemic levels of IgM (C) and IgG (D) were undetectable in B−/− rats as measured by ELISA. WT and heterozygous rats had similar levels of circulating IgM and IgG. Similarly, splenocytes did not produce IgM or IgG as determined by ELISPOT analysis (E and F, respectively). * P < 0.05, ** P < 0.001, *** P < 0.0001 for WT compared to B−/−.
B cell deficient recipients had similar kidney function to wild type recipients at 7 days following transplant
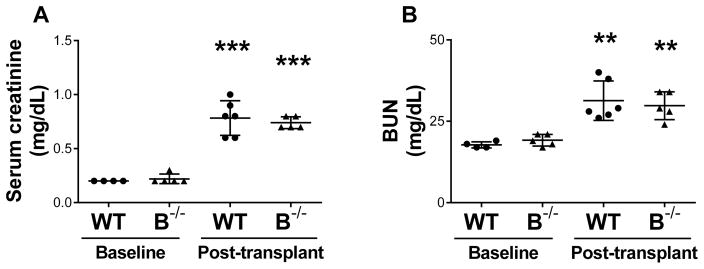
A complete mismatch transplant model was utilized with a Brown Norway kidney donor to Lewis recipient. Lewis recipients were either the B cell deficient strain (B−/−, N=5) or littermate wild type controls (WT, N=6). Baseline levels of serum creatinine and BUN were normal for WT and B−/−. At 7 days after transplant, both WT and B−/− recipients demonstrated elevated serum creatinine and BUN compared to baseline values (Figure 3). The posttransplant increase in creatinine may be related to ischemia-reperfusion or early inflammation.
Figure 3. B cell deficient recipients had similar kidney function to wild type recipients at 7 days following transplant.
Baseline levels of serum creatinine and BUN for the B−/− and WT rats were normal (A and B). At 7 days after kidney transplant, both B−/− and WT recipients had a significant increase in serum creatinine and BUN compared to baseline (A and B). ** P < 0.001, *** P < 0.0001 for baseline values compared to posttransplant values.
Interstitial inflammation was attenuated in allografts of B cell deficient recipients
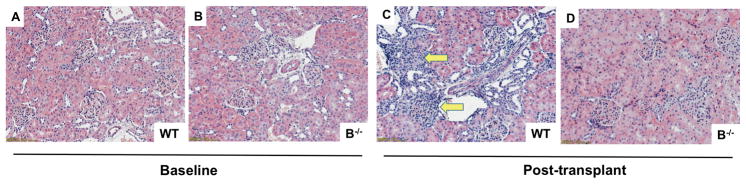
Kidney allografts demonstrated normal histology at baseline for both WT and B−/− rats (Figure 4A and B). Banff scores at baseline for both WT and B−/− were zero in all Banff measures. The allografts of WT recipients developed areas of interstitial inflammation and this was attenuated in the allografts of B−/− recipients (Figure 4C and D). Quantitative assessment of interstitial inflammation by Banff score demonstrated significantly reduced levels of interstitial inflammation in allografts of B−/− compared to WT recipients (Table 1, P=0.007). Glomerulitis (P=0.002), microvascular inflammation (P=0.006), and C4d staining (P=0.005) were also significantly reduced in B−/− compared to WT (Table 1).
Figure 4. Interstitial inflammation of the allograft is attenuated in B cell deficient recipients compared to wild type recipients at 7 days posttransplant.
H&E stained kidney allografts demonstrated normal histology at baseline in WT and B−/− rats (A and B). C, At 7 days after transplant, interstitial inflammation (arrows) was present in the allografts of WT recipients. D, The allografts of B−/− recipients had minimal interstitial inflammation.
Table 1.
Allografts of B cell deficient rats demonstrated reduced interstitial inflammation compared to allografts from wild type rats at 7 days posttransplant.
| Banff score | WT (N=6)
|
B−/− (N=5)
|
P-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| t | 0.2 | 0.4 | 0.4 | 0.5 | NS |
| i | 1.0 | 0.6 | 0.0 | 0.0 | 0.007 |
| g | 1.8 | 0.4 | 0.6 | 0.5 | 0.002 |
| ah | 0.0 | 0.0 | 0.0 | 0.0 | NS |
| v | 0.3 | 0.8 | 0.0 | 0.0 | NS |
| ptc | 1.3 | 0.8 | 0.6 | 0.5 | NS |
| cg | 0.0 | 0.0 | 0.2 | 0.4 | NS |
| ci | 0.0 | 0.0 | 0.0 | 0.0 | NS |
| ct | 0.0 | 0.0 | 0.0 | 0.0 | NS |
| cv | 0.0 | 0.0 | 0.0 | 0.0 | NS |
| mm | 0.0 | 0.0 | 0.00 | 0.0 | NS |
| mvi | 3.2 | 1.0 | 1.2 | 1.1 | 0.006 |
| C4d | 3.0 | 0.0 | 1.2 | 0.8 | 0.0005 |
Abbreviations: t tubulitis, i interstitial inflammation, g glomerulitis, ah arteriolar hyaline thickening, v intimal arteritis, ptc peritubular capillaritis, cg chronic glomerulopathy, ci interstitial fibrosis, ct tubular atrophy, cv vascular intimal thickening, mm mesangial matrix increase, mvi microvascular inflammation, SD standard deviation, NS not significant, WT wild type, B−/− B cell deficient
B cell deficient recipients had reduced intragraft macrophages, but similar levels of intragraft T cells compared to wild type recipients
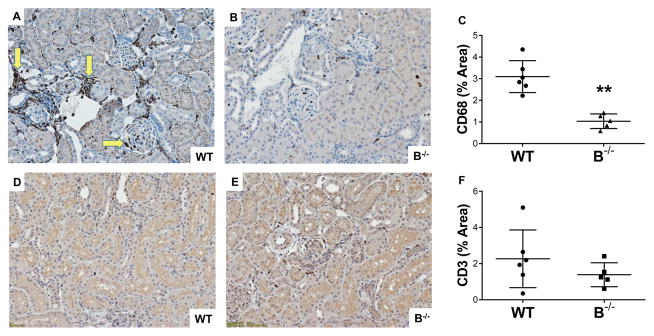
Characterization of the graft infiltrating cells was performed by immunoperoxidase stain for macrophages (CD68+), which showed the inflammatory cell infiltrate was predominantly due to graft infiltrating macrophages (Figure 5A and B). Quantification by densitometry demonstrated a significant reduction in intragraft macrophages in B−/− compared to WT recipients (Figure 5C, P < 0.001). Pearson correlation demonstrated an association between levels of intragraft macrophages and levels of C4d in the allograft (R2=0.52, P=0.01). Immunohistochemistry staining for T cells (CD3+) was also performed on allografts and revealed similar levels of T cells in WT and B−/− (Figure 5D, E, and F). Thus, the reduction in interstitial inflammation in B−/− appeared to be driven by a reduction in the numbers of graft infiltrating macrophages and not T cells.
Figure 5. B cell deficient animals demonstrated reduced interstitial inflammation due to a reduction in graft infiltrating macrophages at 7 days posttransplant.
A, Immunoperoxidase stain for macrophages (CD68+) showed areas of graft infiltrating macrophages in WT recipients (arrows). B, Allografts from B−/− recipients had low numbers of intragraft macrophages. C, There was a significant attenuation of graft infiltrating macrophages in B−/− compared to WT recipients as determined by quantitative densitometry. ** P < 0.001. D and E, Immunohistochemistry staining for T cells (CD3+) demonstrated similar levels of graft infiltrating T cells in both WT and B−/− recipients. F, Quantitative densitometry demonstrated similar levels of intragraft T cells.
B cell deficient recipients did not develop splenic germinal centers following kidney transplant
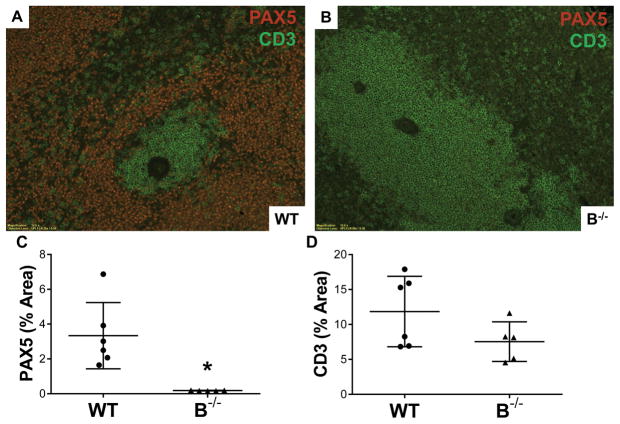
Germinal center formation was seen in WT recipients following transplantation. Immunofluorescent staining of splenic tissue was performed for T cells (CD3+, green) and B cells (PAX5+, red) (Figure 6A and B). In WT, staining showed germinal centers and periarteriolar T cells with a surrounding B cell zone (Figure 6A). Splenic tissue in B−/− demonstrated a loss of typical architecture and lacked germinal center formation (Figure 6B). Quantitative immunofluorescence confirmed absence of B cells in spleen B−/− (Figure 6C, P=0.005 for WT versus B−/−). Similarly, assessment for DSA demonstrated absence of DSA in B−/− recipients following transplant (Figure S1).
Figure 6. B cell deficient animals lacked splenic germinal centers at 7 days posttransplant.
A, Immunofluorescent staining for both B cells (red, PAX5+) and T cells (green, CD3+) in splenic tissue demonstrated typical germinal center formation in WT. B, B−/− animals lacked B cells and germinal centers. C, Quantitative immunofluorescence demonstrated absence of B cells (PAX5+) in spleens from B−/− rats. * P = 0.005. D, Numbers of splenic T cells (CD3+) were similar between WT and B−/−.
B cell deficient rats had reduced splenic T follicular helper cells and increased T regulatory cells at baseline compared to wild type rats
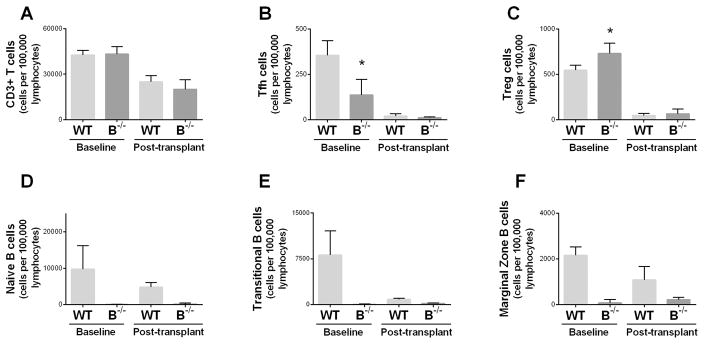
To assess if T cell populations were influenced by B cell deficiency, flow cytometry of splenocytes was performed. Flow cytometry confirmed similar levels of total splenic CD3+ T cells in WT and B−/− rats at baseline (Figure 7A). However, B−/− rats had reduced numbers of T follicular helper (Tfh) cells at baseline (Figure 7B) (CD3+CD4+CD278+CXCR5+) and a higher number of T regulatory (Treg) cells (Figure 7C) (CD3+CD4+CD25+FoxP3+) compared to WT at baseline. Transplanted animals were given cyclosporine from time of transplant until harvest, resulting in a reduction of splenic T cell populations at 7 days posttransplant compared to baseline levels. Splenic B cell populations were absent in the B−/− rats (Figure 7D, E, and F).
Figure 7. B cell deficient rats had reduced splenic T follicular helper cells and increased T regulatory cells at baseline.
A, Flow cytometry at baseline and at 7 days following transplant demonstrated similar levels of total CD3+ T cells in the spleens of WT and B−/− rats. B, Splenic T follicular helper (Tfh) cells were reduced in B−/− rats compared to WT at baseline. C, T regulatory (Treg) cells were increased in the spleens of B−/− rats at baseline compared to WT. D, E, and F, B−/− rats lacked splenic naïve, transitional, and marginal zone B cells. Y-axis for all panels represents absolute number of cells per 100,000 lymphocytes. * P < 0.05 for baseline WT compared to baseline B−/−.
T cell activation resulted in IFN-γ production from wild type and B cell deficient rats in vitro
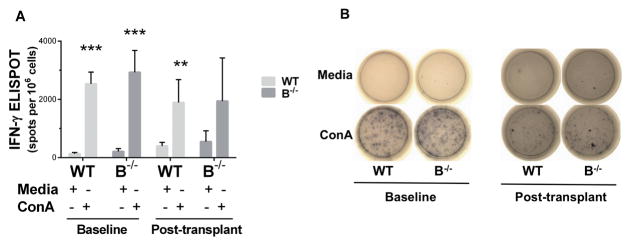
To assess T cell function in the setting of B cell deficiency, splenocytes were stimulated with the potent T cell activator Concanavalin A (ConA) and the production of IFN-γ was measured by ELISPOT in vitro. ConA stimulation resulted in a significant increase in IFN-γ production by T cells from WT and B−/− at baseline (P<0.0005) and from WT following transplant (P=0.001) (Figure 8). There was a trend towards increased IFN-γ production by T cells from B−/− posttransplant compared to B−/− baseline (P=0.07).
Figure 8. Splenocytes from wild type and B cell deficient rats produced IFN-γ in response to activation in vitro.
A, Low levels of IFN-γ producing cells were present in media treated splenocytes from wild type and B−/− rats. Stimulation with the T-cell activator Concanavalin A (ConA) resulted in a significant increase in IFN-γ producing cells compared to media treated controls. *** P < 0.0005, ** P = 0.001 for media treated compared to ConA treated. B, Representative wells of media treated (top row) and ConA treated (lower row) are shown for WT and B−/− splenocytes.
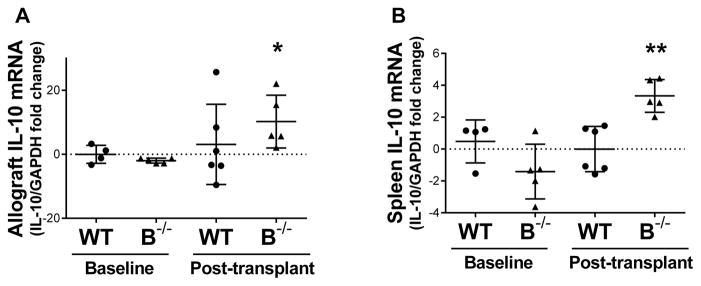
Transcriptional levels of IL-10 were increased in B cell deficient recipients following transplant
As IL-10 is known to be produced by monocytes, T cells, and B cells, the transcriptional expression of IL-10 was assessed. RT PCR demonstrated an increase in the transcriptional level of IL-10 in the allografts and spleens of B−/− recipients following transplant compared to baseline (Figure 9A and B) (P=0.01 and P<0.001, respectively).
Figure 9. IL-10 transcript was increased in the allografts and spleens of B cell deficient recipients at 7 days posttransplant.
A, RT PCR demonstrated an increase in IL-10 transcript in the allografts of B−/− recipients posttransplant compared to baseline. B, RT PCR demonstrated an increase in splenic IL-10 at the transcriptional level in B−/− recipients following transplant compared to baseline. * P = 0.01, ** P < 0.001 for baseline values compared to posttransplant values.
DISCUSSION
We investigated the effect of complete B cell deficiency on allograft inflammation in a rat kidney transplant model. This study found attenuated renal inflammation in the allografts of B cell deficient recipients at 1 week following transplant as evidenced by reduced interstitial inflammation, glomerulitis, microvascular inflammation, and C4d deposition. The reduction in interstitial inflammation was mainly due to a decrease in graft infiltrating macrophages, as the levels of intragraft T cells were similar. B cell deficient rats displayed an increased number of splenic T regulatory cells (Treg) and decreased T follicular helper cells (Tfh) at baseline. After transplant, B cell deficient rats demonstrated increased levels of the anti-inflammatory cytokine IL-10 in the spleen and allograft. In aggregate, these findings suggest that cross talk between B cells, T cells, and macrophages is critical to the development of allograft inflammation. It also underscores the importance of studies targeting the regulatory role of the innate system in transplantation.
We found a reduction in interstitial inflammation in the allografts of B cell deficient recipients due to a reduction in intragraft macrophages. Interstitial inflammation due to intragraft macrophages has gained recent attention as it correlates with severity of rejection, development of interstitial fibrosis, and graft loss.19–23 A recent study demonstrated interstitial inflammation in early protocol biopsies due to CD68+ macrophages, but not CD3+ T cells or CD20+ B cells, was associated with eGFR decline at 4 years.21 Thus, intragraft macrophages appear to play a role in allograft pathology and have an important role in contributing to long-term allograft outcomes. Others have shown the majority of intragraft CD68-positive cells were also positive for HLA-DR.20 This finding provides a link between innate and adaptive immunity, with a mechanism for binding and presenting antigen by macrophages. Macrophages are routinely categorized in subsets as classically-activated, M1, or alternatively-activated, M2. However, limitations of the M1–M2 paradigm are increasingly being appreciated. The plasticity of macrophages in response to their microenvionment is substantial; with the ability to moderate Th1, Th2, inflammatory, fibrotic, and reparative responses.24 The lack of available reagents in rat models to characterize inflammatory cells continues to be an obstacle, but work is ongoing to better define macrophage phenotypes and to standardize experimental conditions to study macrophage biology.24 In clinical and preclinical studies, macrophages are emerging as having an important role in the inflammatory and reparative processes in ischemia-reperfusion and rejection.
We found significant reductions in many of the pathologic features often associated with acute antibody mediated rejection (AMR) in the allografts from B cell deficient recipients. Specifically, we observed decreased glomerulitis, microvascular inflammation, and C4d deposition. The time course for this model was only 1 week, at which point we would not expect to see a major contribution from an allospecific antibody response. Thus, the presence of early-onset pathologic features typically associated with AMR raises the possibility of immune injury from a nonallospecific antibody source. One such candidate for nonallospecific antibody mediated injury are natural antibodies. Natural antibodies exist in the circulation of healthy individuals and are produced without previous antigen exposure or immunization. In previous studies, we demonstrated a role for natural antibodies to cause renal injury via the complement system and promote glomerulosclerosis in murine models of glomerular disease.25,26 Others have demonstrated contributions from natural antibodies to complement activation and clearance of apoptotic cells.27–29 A role for natural antibodies in rejection is now being investigated clinically. Circulating natural antibodies have been identified in patients with acute rejection and were associated with graft loss.30–32 The pathologic role of nonallospecific antibodies in transplantation is an exciting line of research with future clinical implications.
B cell deficient rats displayed an anti-inflammatory phenotype in this model. At baseline, B cell deficient rats had increased splenic Treg and decreased Tfh cell populations. Splenic germinal center formation arises from bidirectional interaction between Tfh cells and B cells.33–36 Ultimately, the recognition of cognate alloantigen by Tfh and B cells within germinal centers results in the generation of memory B cells, long-lived plasma cells, and donor-specific antibody production.33,37 The clinical significance of Tfh cells kidney transplant patients has recently been reported. Patients with high levels of circulating Tfh cells also had high levels of preexisting DSA, indicating a role for Tfh cells in the anti-donor response and rejection.38 The role of B cells to modulate Tfh cells is of interest, as ways to alter Tfh cells in transplant as a potential therapeutic target are under study.39
Following transplant, we observed increased levels of the regulatory cytokine IL-10 in B cell deficient recipients, but similar IFN-γ production. IL-10 is known to be produced by monocytes, T cells, and B cells. Clinically, it has been shown that cytokine polarization with low anti-inflammatory cytokine (IL-10) production and increased pro-inflammatory cytokines (IFN-γ or TNF-α) is associated with graft dysfunction and poor graft outcomes.40–42 Additionally, studies have demonstrated the importance of IL-10 in tolerant renal transplant recipients. Tolerant recipients had increased levels of B cells producing IL-10 and had an enhanced ability to produce IL-10 compared to healthy controls.43 In sum, B cell deficiency in this model produced an anti-inflammatory phenotype with presence of regulatory cell populations and production of anti-inflammatory mediators.
Our study has several limitations. We used a genetic model of complete B cell deficiency and some of our results differ from what has been observed in clinical studies. Potential reasons for these differences may stem from difficulty in eliminating antibody producing plasma cells in clinical studies and the lack of reduction of DSA in patients treated with rituximab.6,44 B cell depletion by rituximab provides good systemic B cell depletion. However, its effect on B cell depletion in secondary lymphoid tissues, such as spleen and lymph nodes, is less effective.45,46 Our genetic model of complete B cell deficiency differs in this regard, as no B cell populations were seen in circulation or lymphoid tissues. Future studies are needed in this model examine the effect of complete B cell deficiency in chronic rejection. However, the current model allowed us to study the role of B cells in acute rejection without interference by repopulation of B cells or alterations in B cell mediators or cytokines.
The current study demonstrates B cells play a key role in exacerbating allograft inflammation and macrophage infiltration. B cell deficiency was associated with an anti-inflammatory phenotype with a shift towards populations of Treg cells, a reduction in Tfh cells, and production of anti-inflammatory cytokines (IL-10). Further studies are needed to better understand the regulatory role of each arm of the immune system to improve preventative and therapeutic strategies for allograft injury.
Supplementary Material
Acknowledgments
Funding: This project was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427, and the KL2 training Award (KL2TR000428). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
We would like to thank the technical assistance of Isabelle Renteria, Lucy Ptak, Sibel Aydemir, Brittney Boldt, Kathy Krentz, and the UW-Madison Transgenic Animal Facility. We acknowledge the Medical College of Wisconsin Rat Resource Center for their assistance with generation of the genetic rat strain. We thank Dana Clark, MA and Laura Hogan, PhD for their editorial assistance. We acknowledge the support of the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ah
arteriolar hyaline thickening
- B−/−
B cell deficient
- BUN
blood urea nitrogen
- cg
chronic glomerulopathy
- ci
interstitial fibrosis
- ct
tubular atrophy
- cv
vascular intimal thickening
- CRISPR
clustered regularly interspaced short palindromic repeats
- ConA
Concanavalin A
- DSA
donor specific antibody
- ELISA
enzyme-linked immunosorbent assay
- g
glomerulitis
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- i
interstitial inflammation
- IFN
interferon
- IL
interleukin
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- mm
mesangial matrix increase
- mvi
microvascular inflammation
- NS
not significant
- ptc
peritubular capillaritis
- RT PCR
reverse transcription polymerase chain reaction
- SD
standard deviation
- Tfh
T follicular helper cell
- Treg
T regulatory cell
- t
tubulitis
- v
intimal arteritis
- WT
wild type
Footnotes
Authorship statement:
Sarah E. Panzer: concept, study design, performance of research, data analysis, writing of paper
Nancy A. Wilson: performance of research, data analysis, writing of paper
Bret M. Verhoven: performance of research, data analysis, writing of paper
Ding Xiang: performance of research, review of manuscript
C. Dustin Rubinstein: performance of research, data analysis, writing of paper
Robert R. Redfield: data analysis, review of manuscript
Weixiong Zhong: performance of research, review of manuscript
Shannon R. Reese: study design, performance of research, data analysis, writing of paper
Disclosure: The authors declare no conflict of interest.
References
- 1.Hippen BE, DeMattos A, Cook WJ, Kew CE, 2nd, Gaston RS. Association of CD20+ infiltrates with poorer clinical outcomes in acute cellular rejection of renal allografts. Am J Transplant. 2005;5(9):2248–2252. doi: 10.1111/j.1600-6143.2005.01009.x. [DOI] [PubMed] [Google Scholar]
- 2.Hwang HS, Song JH, Hyoung BJ, et al. Clinical impacts of CD38+ B cells on acute cellular rejection with CD20+ B cells in renal allograft. Transplantation. 2010;89(12):1489–1495. doi: 10.1097/TP.0b013e3181dd35b8. [DOI] [PubMed] [Google Scholar]
- 3.Zarkhin V, Kambham N, Li L, et al. Characterization of intra-graft B cells during renal allograft rejection. Kidney Int. 2008;74(5):664–673. doi: 10.1038/ki.2008.249. [DOI] [PubMed] [Google Scholar]
- 4.Kayler LK, Lakkis FG, Morgan C, et al. Acute cellular rejection with CD20-positive lymphoid clusters in kidney transplant patients following lymphocyte depletion. Am J Transplant. 2007;7(4):949–954. doi: 10.1111/j.1600-6143.2007.01737.x. [DOI] [PubMed] [Google Scholar]
- 5.Scheepstra C, Bemelman FJ, van der Loos C, et al. B cells in cluster or in a scattered pattern do not correlate with clinical outcome of renal allograft rejection. Transplantation. 2008;86(6):772–778. doi: 10.1097/TP.0b013e3181860a74. [DOI] [PubMed] [Google Scholar]
- 6.Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. Am J Transplant. 2008;8(12):2607–2617. doi: 10.1111/j.1600-6143.2008.02411.x. [DOI] [PubMed] [Google Scholar]
- 7.van den Hoogen MW, Steenbergen EJ, Baas MC, Florquin S, Hilbrands LB. Absence of Intragraft B Cells in Rejection Biopsies After Rituximab Induction Therapy: Consequences for Clinical Outcome. Transplant Direct. 2017;3(4):e143. doi: 10.1097/TXD.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasowska BA, Qian Z, Cangello DL, et al. Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation. 2001;71(6):727–736. doi: 10.1097/00007890-200103270-00007. [DOI] [PubMed] [Google Scholar]
- 9.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. II. Importance of humoral immunity. J Immunol. 1994;152(10):5135–5141. [PubMed] [Google Scholar]
- 10.DiLillo DJ, Griffiths R, Seshan SV, et al. B lymphocytes differentially influence acute and chronic allograft rejection in mice. J Immunol. 2011;186(4):2643–2654. doi: 10.4049/jimmunol.1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menoret S, Iscache AL, Tesson L, et al. Characterization of immunoglobulin heavy chain knockout rats. Eur J Immunol. 2010;40(10):2932–2941. doi: 10.1002/eji.201040939. [DOI] [PubMed] [Google Scholar]
- 12.Nishino T, Sasaki N, Nagasaki K, Ahmad Z, Agui T. Genetic background strongly influences the severity of glomerulosclerosis in mice. J Vet Med Sci. 2010;72(10):1313–1318. doi: 10.1292/jvms.10-0144. [DOI] [PubMed] [Google Scholar]
- 13.Bergman I, Basse PH, Barmada MA, Griffin JA, Cheung NK. Comparison of in vitro antibody-targeted cytotoxicity using mouse, rat and human effectors. Cancer Immunol Immunother. 2000;49(4–5):259–266. doi: 10.1007/s002620000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang LJ, Reese S, Djamali A. Contributing factors to complications and surgical success in mouse kidney transplantation. Int Braz J Urol. 2012;38(3):395–403. doi: 10.1590/s1677-55382012000300013. discussions 403–394. [DOI] [PubMed] [Google Scholar]
- 15.Huang G, Wilson NA, Reese SR, Jacobson LM, Zhong W, Djamali A. Characterization of transfusion-elicited acute antibody-mediated rejection in a rat model of kidney transplantation. Am J Transplant. 2014;14(5):1061–1072. doi: 10.1111/ajt.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 17.Vidyasagar A, Reese S, Acun Z, Hullett D, Djamali A. HSP27 is involved in the pathogenesis of kidney tubulointerstitial fibrosis. Am J Physiol Renal Physiol. 2008;295(3):F707–716. doi: 10.1152/ajprenal.90240.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djamali A, Vidyasagar A, Adulla M, Hullett D, Reese S. Nox-2 is a modulator of fibrogenesis in kidney allografts. Am J Transplant. 2009;9(1):74–82. doi: 10.1111/j.1600-6143.2008.02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girlanda R, Kleiner DE, Duan Z, et al. Monocyte infiltration and kidney allograft dysfunction during acute rejection. Am J Transplant. 2008;8(3):600–607. doi: 10.1111/j.1600-6143.2007.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergler T, Jung B, Bourier F, et al. Infiltration of Macrophages Correlates with Severity of Allograft Rejection and Outcome in Human Kidney Transplantation. PLoS One. 2016;11(6):e0156900. doi: 10.1371/journal.pone.0156900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brasen JH, Khalifa A, Schmitz J, et al. Macrophage density in early surveillance biopsies predicts future renal transplant function. Kidney Int. 2017 doi: 10.1016/j.kint.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Copin MC, Noel C, Hazzan M, et al. Diagnostic and predictive value of an immunohistochemical profile in asymptomatic acute rejection of renal allografts. Transpl Immunol. 1995;3(3):229–239. doi: 10.1016/0966-3274(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 23.Toki D, Zhang W, Hor KL, et al. The role of macrophages in the development of human renal allograft fibrosis in the first year after transplantation. Am J Transplant. 2014;14(9):2126–2136. doi: 10.1111/ajt.12803. [DOI] [PubMed] [Google Scholar]
- 24.Hutchinson JA. Macrophages in transplantation. Transplantation. 2015;99(5):898–899. doi: 10.1097/TP.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 25.Strassheim D, Renner B, Panzer S, et al. IgM contributes to glomerular injury in FSGS. J Am Soc Nephrol. 2013;24(3):393–406. doi: 10.1681/ASN.2012020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panzer SE, Laskowski J, Renner B, et al. IgM exacerbates glomerular disease progression in complement-induced glomerulopathy. Kidney Int. 2015;88(3):528–537. doi: 10.1038/ki.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleming SD, Shea-Donohue T, Guthridge JM, et al. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J Immunol. 2002;169(4):2126–2133. doi: 10.4049/jimmunol.169.4.2126. [DOI] [PubMed] [Google Scholar]
- 28.Haas MS, Alicot EM, Schuerpf F, et al. Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc Res. 2010;87(4):618–627. doi: 10.1093/cvr/cvq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182(10):6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porcheray F, Fraser JW, Gao B, et al. Polyreactive antibodies developing amidst humoral rejection of human kidney grafts bind apoptotic cells and activate complement. Am J Transplant. 2013;13(10):2590–2600. doi: 10.1111/ajt.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao B, Moore C, Porcheray F, et al. Pretransplant IgG reactivity to apoptotic cells correlates with late kidney allograft loss. Am J Transplant. 2014;14(7):1581–1591. doi: 10.1111/ajt.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.See SB, Clerkin KJ, Kennel PJ, et al. Ventricular assist device elicits serum natural IgG that correlates with the development of primary graft dysfunction following heart transplantation. J Heart Lung Transplant. 2017;36(8):862–870. doi: 10.1016/j.healun.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwun J, Manook M, Page E, Burghuber C, Hong J, Knechtle SJ. Crosstalk Between T and B Cells in the Germinal Center After Transplantation. Transplantation. 2017;101(4):704–712. doi: 10.1097/TP.0000000000001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nurieva RI, Chung Y, Martinez GJ, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumjohann D, Preite S, Reboldi A, et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38(3):596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Chong AS, Sciammas R. Memory B cells in transplantation. Transplantation. 2015;99(1):21–28. doi: 10.1097/TP.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Graav GN, Dieterich M, Hesselink DA, et al. Follicular T helper cells and humoral reactivity in kidney transplant patients. Clin Exp Immunol. 2015;180(2):329–340. doi: 10.1111/cei.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walters GD, Vinuesa CG. T Follicular Helper Cells in Transplantation. Transplantation. 2016;100(8):1650–1655. doi: 10.1097/TP.0000000000001217. [DOI] [PubMed] [Google Scholar]
- 40.Cherukuri A, Rothstein DM, Clark B, et al. Immunologic human renal allograft injury associates with an altered IL-10/TNF-alpha expression ratio in regulatory B cells. J Am Soc Nephrol. 2014;25(7):1575–1585. doi: 10.1681/ASN.2013080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiu KY, McLaughlin L, Rebollo-Mesa I, et al. B-lymphocytes support and regulate indirect T-cell alloreactivity in individual patients with chronic antibody-mediated rejection. Kidney Int. 2015;88(3):560–568. doi: 10.1038/ki.2015.100. [DOI] [PubMed] [Google Scholar]
- 42.Shiu KY, McLaughlin L, Rebollo-Mesa I, et al. Graft dysfunction in chronic antibody-mediated rejection correlates with B-cell-dependent indirect antidonor alloresponses and autocrine regulation of interferon-gamma production by Th1 cells. Kidney Int. 2017;91(2):477–492. doi: 10.1016/j.kint.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nova-Lamperti E, Chana P, Mobillo P, et al. Increased CD40 Ligation and Reduced BCR Signalling Leads to Higher IL-10 Production in B Cells From Tolerant Kidney Transplant Patients. Transplantation. 2017;101(3):541–547. doi: 10.1097/TP.0000000000001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamburova EG, Koenen HJ, van den Hoogen MW, Baas MC, Joosten I, Hilbrands LB. Longitudinal analysis of T and B cell phenotype and function in renal transplant recipients with or without rituximab induction therapy. PLoS One. 2014;9(11):e112658. doi: 10.1371/journal.pone.0112658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos EJ, Pollinger HS, Stegall MD, Gloor JM, Dogan A, Grande JP. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant. 2007;7(2):402–407. doi: 10.1111/j.1600-6143.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 46.Kamburova EG, Koenen HJ, Borgman KJ, ten Berge IJ, Joosten I, Hilbrands LB. A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. Am J Transplant. 2013;13(6):1503–1511. doi: 10.1111/ajt.12220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.