Abstract
Background
Plasma levels of lactate and succinate are predictors of mortality in critically injured patients in military and civilian settings. In relative terms, these metabolic derangements have been recapitulated in rodent, swine and non-human primate models of severe hemorrhage. However, no direct absolute quantitative comparison has been evaluated across these species.
Methods
Ultra-high pressure liquid chromatography-mass spectrometry with stable isotope standards was used to determine absolute concentrations of baseline and post-shock levels of lactate and succinate in rats, pigs, macaques, and injured patients.
Results
Baseline levels of lactate and succinate were most comparable to humans in macaques, followed by pigs and rats. Baseline levels of lactate in pigs and baseline and post-shock levels of lactate and succinate in rats were significantly higher than those measured in macaques and humans. Post-shock levels of lactate and succinate in pigs and macaques, respectively, were directly comparable to measurements in critically injured patients.
Conclusions
Acknowledging the caveats associated with the variable degrees of shock in the clinical cohort, our data indicates that larger mammals represent a better model than rodents when investigating metabolic derangements secondary to severe hemorrhage.
Keywords: metabolomics, trauma, lactate, succinate, biomarker, shock
Introduction
Recent evidence of the poor correlation between genomic responses in mouse models of inflammatory disease and human data1 fueled the debate about the translational clinical relevance of results obtained in animal models. Similarly, reassuring evidence from randomized clinical trials on the influence of the age of blood on transfusion outcomes mitigated the concerns raised by observations in animal models.2 It is thus easy to understand how concerns about the translatability of observations in animal models into clinical practice may have been recently questioned in other biomedical fields, such as the field of critical care medicine. Limitations and caveats on the use of animal models in critical care medicine have been recently reviewed.3,4 Despite such limitations, paraphrasing George Box’s famous quote, it is reasonable to wonder that, if “all – animal - models are wrong”, how wrong does the model have to be not to be useful?
Rodents,5 swine,6 and more recently, non-human primates7 have been employed extensively as models of hemorrhagic shock, with the goal to improve our understanding of the molecular mechanisms underlying the sequelae to the most preventable cause of death in civilian and military trauma. Recently, we8 and others9 identified plasma succinate as a metabolic marker of hypoperfusion and predictor of mortality in civilian and military critically injured patients. This observation expanded on the well-established role of metabolic acidosis and circulating levels of lactate as a predictor of mortality in trauma and a marker of occult shock.10,11
Based on observations in animal models of ischemia/reperfusion injury,12 we proposed that hemorrhagic hypoxemia increases circulating levels of lactate and citric acid metabolites such as succinate5 due to oxygen deprivation driving uncoupling of the electron transport chain. In animal models, plasma levels of citric acid cycle metabolites begin to accumulate within 5 minutes of hemorrhagic shock.5 These observations are consistent in all tested animal models and clinical samples,5,6,13 as gleaned by relative quantitative values. However, to the best of our knowledge, no direct comparison of these models to absolute quantitative measurements of clinically relevant markers lactate and succinate in clinical cohorts has been performed. Here we performed such comparison by exploiting a quantitative ultra-high pressure liquid chromatography-mass spectrometry (UHPLC-MS) approach, using stable isotope labeled internal standards for lactate and succinate. The underlying hypothesis is that evolutionary divergence of mammals from rodents to swine, non-human primates and humans would influence the systemic metabolic response to severe hemorrhage, making genetically less related mammals a less reliable model for human metabolic derangements14 in shock. Comparison of baseline and post-shock values for plasma lactate and succinate were compared to inter-species measurements in rats, pigs, macaques and clinical samples.
Materials and Methods
The study was approved by the University of Colorado and Denver Health Ethic Committees and the Combined Multi-Institutional Internal Review Board (COMIRB protocol#: 12-1349) and University of Colorado International Animal Care and Use Committee #90814. The experiments on non-human primates were approved by the Institutional Animal Care and Use Committee (IACUC) at the 711th Human Performance Wing, Joint Base San Antonio-Fort Sam Houston, and conducted in accordance with the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council, National Academy Press, 2011. All procedures were performed in facilities accredited by the Association for Assessment and Accreditation for Laboratory Animal Care International (AAALAC).
Rodent samples
Samples were collected at baseline or at 1h 30min through shock from Sprague/Dawley male rats (n=16) undergoing laparotomy and severe hemorrhage to MAP <30 mmHg, as described previously.5 Briefly, animals weighing 407–515g (Harlan Labs, Indianapolis, IN) were anesthetized with 50 mg/kg Pentobarbital sodium via intraperitoneal injection at the beginning of the model and re-dosed intravenously (1mg/kg) as necessary during the procedure. 1% lidocaine injection was administered at all incision sites for analgesia. A tracheostomy was performed and a tracheostomy tube was placed. The right femoral artery and vein were cannulated, and femoral artery catheters were connected to Pro-Paq devices (Protocol Systems, Beaverton, OR) to monitor heart rate and mean arterial pressure (MAP). Nonlethal hemorrhagic shock was induced via controlled hemorrhage from the femoral artery to a MAP of 28–30 mmHg.
Swine samples
Samples were collected at baseline or at 1h 30min through shock from male outbreed swine (n=10) undergoing hemorrhage to MAP = 25 mmHg, as described previously.6 Male outbreed swine (50–55 kg) underwent anesthesia with ketamine (20mg/kg) (VETone, Boise, ID, USA) and acepromazine (0.2mg/kg) (VETone, Boise, ID, USA) and xylazine (2mg/kg) (Akorn, Decatur, IL, USA), then were maintained on general anesthesia using isoflurane (0.5–2%) in room air, by mask. Animals underwent tracheostomy, femoral artery cannulation to measure blood pressure and induce hemorrhage. After tracheostomy, the animals were placed on mechanical ventilation to maintain O2 saturation > 90%.
Non-human primate samples
Rhesus Macaques utilized in this study were housed in compliance with the Secretary of the Navy Instruction (SECNAVINST) 3900.38C regulations. Male Rhesus Macaques (n=16) weighing 11 +/−1kg underwent intubation followed by femoral artery cannulation to measure blood pressure, as described previously.7,15 NHPs were sedated with Telazol (3.0mg/kg; Fort Dodge Animal Health, Overland Park, KS, USA), pre-medicated with an analgesic (Buprenex 0.03mg/kg; Reckitt Benkiser, Slough, Berkshire, UK). Airway intubation was achieved with placement of a pediatric, size 4–5.5mm, endotracheal (ET) tube (Rusch-Teleflex, Research Triangle Park, NC, USA), and placed on a Dräger Apollo Anesthesia Workstation (Draeger Medical Inc., Telford, PA, USA) with volume-controlled respiration (10mL/kg) at 12–15 breaths per minute, FiO2 of 21–25% and isoflurane (1.0–2.0%) inhalational anesthesia. Hemorrhage was initiated by opening a stopcock in-line with the left arterial catheter allowing free-flow of blood until the mean arterial pressure (MAP) reached 20mmHg. This moment marked the beginning of the shock period, additional blood was withdrawn as needed to maintain a MAP of 20–24 mmHg until animals no longer exhibited cardiovascular compensatory responsiveness to maintain or elevate MAP (i.e. decompensation). Decompensation was defined as a spontaneous decline in an NHP’s MAP to a value 75% of the MAP average over the first 60min of shock. Samples were collected at baseline or at 1h 30min through shock.
Human samples
Human plasma samples were collected from severely injured patients enrolled at the Denver Health Medical Center, a level I Trauma Center in Denver, Colorado (inclusion criteria: age ≥ 18; acutely injured; SBP<70mmHg at admission; exclusion criteria: visibly or verbally reported pregnant women, known prisoners, unsalvageable injuries – defined as asystolic or cardiopulmonary resuscitation prior to randomization; known objection to blood products; patient with opt-out bracelet, necklace or wallet card; family member present at the scene objects to patient’s enrollment in research). Samples were collected from 25 male patients showing base deficit >7.5. For this analysis the earliest time point available for each one of those patients was used, with samples being collected either in the field (<30 min from traumatic injury), at the arrival in the emergency department (ED) in any case <2h from shock.
Sample extraction and UHPLC-MS quantitation of succinate and lactate
Whole blood samples were collected in sodium citrate tubes, centrifuged at 1,000g for 15min at 4°C and 12,600g for 6min at 4°C to sort plasma from blood components, prior to storage at −80°C. Plasma (10 μl) was extracted in presence of 13C1-lactate and 1 μM of 13C4-succinate as internal standards.5,16 Metabolomics analyses were performed by ultra-high performance liquid chromatography coupled with high resolution mass spectrometry (Vanquish-Q Exactive – Thermo Fisher, Bremen, Germany), as reported.5,16 Absolute quantitation was determined by integrating peak areas of the light and heavy isotopologues for lactate and succinate (matched, in each sample tested), according to the formula:
Absolute levels of lactate were determined by first subtracting the naturally occurring 13C1-lactate (3.3% abundance of the parent 12C-lactate) from the observed 13C1-lactate signal.
Description and analytical validation of the method has been described recently.16 Statistical analyses (including, as appropriate, paired and unpaired T-test or ANOVA of lactate and succinate measurements) were performed through GraphPad Prism 5.0.
Results
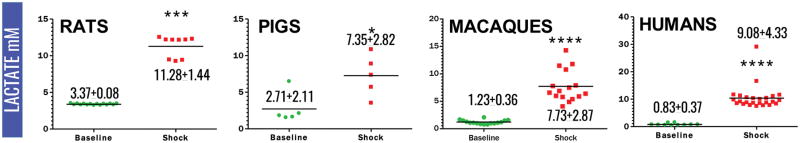
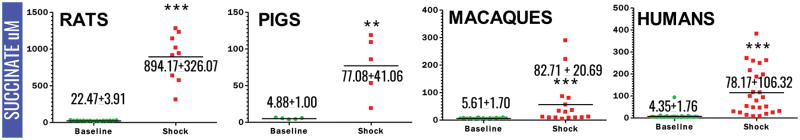
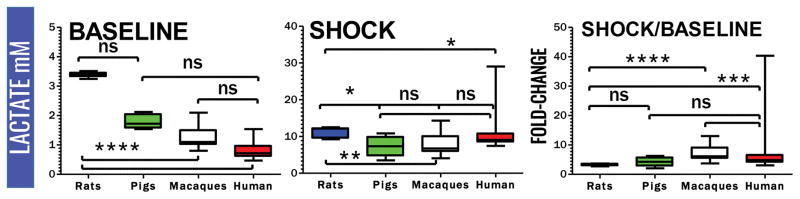
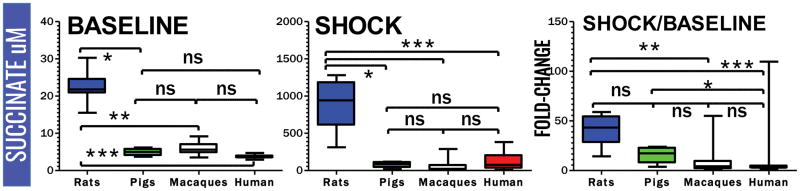
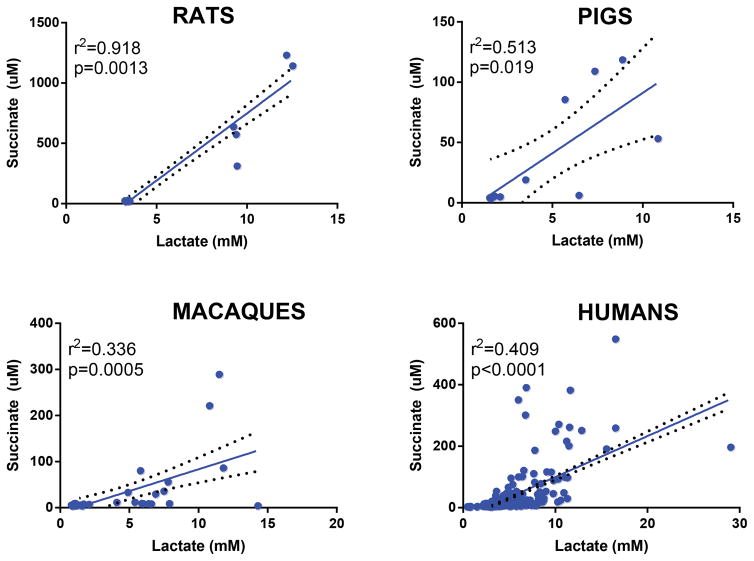
Plasma lactate and succinate levels were measured in rats, pigs, macaques and clinical patients by UHPLC-MS (means ± standard deviations are graphed in the dot-plots in Figure 1 and 2). Baseline levels of lactate differed significantly (p<0.001) in rats compared to pigs, macaques and humans (Figure 1). Though not reaching significance, baseline plasma lactate levels in pigs were almost twofold higher in pigs than in macaques and humans (Figure 3). Post-shock levels of lactate were not significantly different between pigs, macaques and humans. On the other hand, post-shock rats had the highest increases in plasma lactate (Figure 3), in a model designed to obtain comparable final MAP (<30 mmHg) to hemorrhaged pigs or macaques. Similarly, basal and post-shock levels of succinate in rats were significantly higher than those measured in the other groups (Figure 4). Post-shock levels of succinate in pigs were significantly lower than humans (Figure 4), while no significant differences were observed between basal or post-shock succinate levels in macaques and humans. Shock/baseline ratios for plasma lactate and succinate were comparable in pigs, macaques and humans, while statistically different in rats (Figure 3 and 4). Post-shock levels of plasma succinate and lactate were positively and significantly correlated in all tested groups (Figure 5). Exponential increases in plasma succinate were observed in rats, macaques and humans with lactate levels >5 mM (Figure 5).
Figure 1.
Plasma levels of lactate (mM) in rats, pigs, non-human primates and critically ill patients at baseline (green) and shock (red). Mean ± standard deviations are indicated. (* p <0.05; ** p <0.01; *** p<0.0001 T-test).
Figure 2.
Plasma levels of succinate (μM) in rats, pigs, non-human primates and critically ill patients at baseline (green) and shock (red). Mean ± standard deviations are indicated. (* p <0.05; ** p <0.01; *** p<0.0001 T-test).
Figure 3.
Comparative quantitation of baseline and post-shock plasma levels of lactate in rats, pigs, macaques and humans, and relative fold-changes (shock divided median of baseline values). (ns = not significant; * p <0.05; ** p <0.01; *** p<0.0001 ANOVA)
Figure 4.
Comparative quantitation of baseline and post-shock plasma levels of succinate in rats, pigs, macaques and humans, and relative fold-changes (shock divided median of baseline values). (ns = not significant; * p <0.05; ** p <0.01; *** p<0.0001 ANOVA)
Figure 5.
Correlations between baseline and post-shock plasma levels of succinate and lactate in rats, pigs, macaques and humans
Discussion
Following recent comprehensive reviews on animal models in trauma,3,4 in the present manuscript we provide the first trans-species comparative quantitative analysis of post-hemorrhagic shock levels of plasma lactate and succinate. Notably, initial levels of plasma lactate and succinate were significantly higher at baseline in rats in comparison to all the other tested animals and clinical patients. However, it is worth noting that baseline levels are affected by anesthesia, catheter insertion and, in rats more than in the other larger mammals investigated here, initial blood volume loss for sampling (as we previously reported5), all factors that may affect the comparability of results across species. Post-shock levels of succinate, as well as shock/baseline fold-changes for this metabolite were significantly different in rats and pigs in comparison to macaques and humans. This is relevant in the light of the recent appreciation of the role of succinate as a predictor of mortality at least comparable and potentially more specific than lactate in critically ill patients.8
Post-shock levels of circulating lactate and (where reported in the literature) succinate are comparable to previous observations in rats,17 pigs, 18 and macaques.19 However, >150 uM levels of post-shock succinate have been reported in a cohort of severely injured military patients,9 suggesting that the clinical cohort investigated here was not suffering from extreme tissue hypoxia.
The present study has several limitations. Varying degrees of shock were achieved across animals, despite targeting a final MAP consistently <30 mmHg. Similarly, clinical cohort consists of patients with varying degrees of bleeding as well as tissue injury and blood sampling was not perfectly matched to the animal experiments. In this view, it is worth noting that comparability of human data from patients with circulating lactate >6 mM highlighted similarities to rats and macaques in terms of simultaneous exponential increases of plasma succinate above 200 μM. Of note, in the animals and clinical setting post-shock circulating levels of lactate may also be confounded, respectively, by different anesthetics and extra loading (e.g. through the administration of lactated Ringer), reduced hepatic clearance and post-shock catecholamine signaling influencing pyruvate dehydrogenase activity and thus pyruvate shuttling to the Krebs cycle.20 Moreover, this analysis was gender-biased, in that all animals and patients were male.
In sum, from the present study it is evident that baseline levels of lactate and succinate in plasma become increasingly comparable across mammals the closer we got (in evolutionary terms) to humans (rodents<pigs<macaques<humans), as hypothesized. Shock/baseline ratios were comparable across all tested species for key metabolites such as lactate, suggesting that small animals may still represent a valuable model to test specific hypotheses in trauma. Still, despite confounders limiting the generalization of the present study, we suggest that larger mammals may represent a better model than smaller animals when investigating metabolic derangements secondary to severe hemorrhage.
Supplementary Material
Acknowledgments
Funding: AD received funds from the National Blood Foundation and the Boettcher Webb-Waring Biomedical Research Award – Early Career Grant. This study is supported by the US Army Medical Research Acquisition Act of the Department of Defense under Contract Award Number W81XWH1220028 and the National Institutes of Health (P50 GM049222, T32 GM008315, and UMHL120877), Navy Advanced Medical Development (G1501), and the Office of Naval Research (G1505). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. CDR Forest R. Sheppard is a military service member. This work was prepared as part of their official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Army, Department of Defense, nor the U.S. Government.
Footnotes
Conflict of interest: The Authors disclose no conflicts of interest relevant to the present study.
Authors’ contributions
AD, JAR, MJW performed metabolomics analysis. AD prepared figures and wrote the first draft and revision of the manuscript. ALS, EEM, HBM, AG, JC, GN, MF, LS, FRS contributed to sample collection. EEM, AB, FRS, CCS, KCH and AD designed and supervised the study. All the authors critically contributed to the finalization of the manuscript.
References
- 1.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci. 2013;110(9):3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimring JC, Spitalnik SL. Large retrospective effects, clear differences in animals, and multiple negative randomised controlled trials: this is exactly how it is supposed to work. Blood Transfus. 2017;15(2):104–6. doi: 10.2450/2017.0307-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pène F, Ait-Oufella H, Taccone FS, Monneret G, Sharshar T, Tamion F, Mira JP. Insights and limits of translational research in critical care medicine. Ann Intensive Care. 2015;5:8. doi: 10.1186/s13613-015-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valparaiso AP, Vicente DA, Bograd BA, Elster EA, Davis TA. Modeling acute traumatic injury. J Surg Res. 2015;194(1):220–32. doi: 10.1016/j.jss.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 5.D’Alessandro A, Moore HB, Moore EE, Wither M, Nemkov T, Gonzalez E, Slaughter A, Fragoso M, Hansen KC, Silliman CC, et al. Early hemorrhage triggers metabolic responses that build up during prolonged shock. Am J Physiol - Regul Integr Comp Physiol. 2015;308(12):R1034–44. doi: 10.1152/ajpregu.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clendenen N, Nunns GR, Moore EE, Reisz JA, Gonzalez E, Peltz E, Silliman CC, Fragoso M, Nemkov T, Wither MJ, et al. Hemorrhagic Shock and Tissue Injury Drive Distinct Plasma Metabolome Derangements in Swine. J Trauma Acute Care Surg. 2017 doi: 10.1097/TA.0000000000001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaub LJ, Moore HB, Cap AP, Glaser JJ, Moore EE, Sheppard FR. Nonhuman primate model of polytraumatic hemorrhagic shock recapitulates early platelet dysfunction observed following severe injury in humans. J Trauma Acute Care Surg. 2017;82(3):461–9. doi: 10.1097/TA.0000000000001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Alessandro A, Moore HB, Moore EE, Reisz JA, Wither MJ, Ghasabyan A, Chandler J, Silliman CC, Hansen KC, Banerjee A. Plasma succinate is a predictor of mortality in critically injured patients. J Trauma Acute Care Surg. 2017 doi: 10.1097/TA.0000000000001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lusczek ER, Muratore SL, Dubick MA, Beilman GJ. Assessment of key plasma metabolites in combat casualties. J Trauma Acute Care Surg. 2017;82(2):309–16. doi: 10.1097/TA.0000000000001277. [DOI] [PubMed] [Google Scholar]
- 10.Cuthbertson D. Post-shock metabolic response. Lancet. 1942;239(6189):433–7. [Google Scholar]
- 11.Husain FA, Martin MJ, Mullenix PS, Steele SR, Elliott DC. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg. 2003;185(5):485–91. doi: 10.1016/s0002-9610(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 12.Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peltz ED, D’Alessandro A, Moore EE, Chin T, Silliman CC, Sauaia A, Hansen KC, Banerjee A. Pathologic metabolism: An exploratory study of the plasma metabolome of critical injury. J Trauma Acute Care Surg. 2015;78(4):742–51. doi: 10.1097/TA.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blais EM, Rawls KD, Dougherty BV, Li ZI, Kolling GL, Ye P, Wallqvist A, Papin JA. Reconciled rat and human metabolic networks for comparative toxicogenomics and biomarker predictions. Nat Commun. 2017;8:14250. doi: 10.1038/ncomms14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vernon PJ, Paredes RM, Sooter AJ, Schaub LJ, Grossman HM, Pusateri AE, Glaser JJ, Sheppard FR. Severe Hemorrhagic Shock Induces Acute Activation and Expansion of IL-8+/IL-10+ Neutrophils with Enhanced Oxidative Reactivity in Non-Human Primates. Shock. 2016;46(3 Suppl 1):129–36. doi: 10.1097/SHK.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 16.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom. 2017;31(8):663–73. doi: 10.1002/rcm.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogren LK, Murphy CJ, Johnston EL, Sinha N, Serkova NJ, Drew KL. 1H–NMR Metabolomic Biomarkers of Poor Outcome after Hemorrhagic Shock are Absent in Hibernators. PLOS ONE. 2014;9(9):e107493. doi: 10.1371/journal.pone.0107493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rixen D, Raum M, Holzgraefe B, Sauerland S, Nagelschmidt M, Neugebauer EA. A pig hemorrhagic shock model: oxygen debt and metabolic acidemia as indicators of severity. Shock. 2001;16(3):239–44. doi: 10.1097/00024382-200116030-00012. [DOI] [PubMed] [Google Scholar]
- 19.Macko AR, Moore HB, Cap AP, Meledeo MA, Moore EE, Sheppard FR. Tissue injury suppresses fibrinolysis after hemorrhagic shock in nonhuman primates (rhesus macaque) J Trauma Acute Care Surg. 2017;82(4):750–7. doi: 10.1097/TA.0000000000001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilgour E, Vernon RG. Catecholamine activation of pyruvate dehydrogenase in white adipose tissue of the rat in vivo. Biochem J. 1987;241(2):415–9. doi: 10.1042/bj2410415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.