Abstract
Objective
Amphetamine improves vigilance as assessed by continuous performance tests (CPT) in children and adults with attention deficit hyperactivity disorder (ADHD). Less is known however, regarding amphetamine effects on vigilance in healthy adults. Thus, it remains unclear whether amphetamine produces general enhancement of vigilance or if these effects are constrained to the remediation of deficits in patients with ADHD.
Method
We tested 69 healthy adults (35 female) on a standardized CPT (Conner’s CPT-2) after receiving 10 or 20 mg d-amphetamine or placebo. To evaluate potential effects on learning, impulsivity, and perseveration, participants were additionally tested on the Iowa Gambling Task (IGT) and Wisconsin Card Sorting Task (WCST).
Results
Participants receiving placebo exhibited the classic vigilance decrement, demonstrated by a significant reduction in attention (D′) across the task. This vigilance decrement was not observed however, after either dose of amphetamine. Consistent with enhanced vigilance, the 20 mg dose also reduced reaction time variability across the task and the ADHD confidence index. The effects of amphetamine appeared to be selective to vigilance since no effects were observed on the IGT, WCST, or response inhibition/perseveration measures from the CPT.
Conclusion
The present data support the premise that amphetamine improves vigilance irrespective of disease state. Given that amphetamine is a norepinephrine/dopamine transporter inhibitor and releaser, these effects are informative regarding the neurobiological substrates of attentional control.
Keywords: Attention, Vigilance, Stimulant, ADHD, Dopamine, Norepinephrine
Preparations of amphetamine have been used medicinally in the United States for nearly a century, originating with Benzedrine, intended for use as a decongestant. Initial marketing of amphetamine compounds focused on treatment of narcolepsy, postencephalitic parkinsonism, and depression, while a large unregulated market developed for diet pills containing amphetamine. The cognition-enhancing properties of amphetamine were recognized early in development, but it was not marketed for this purpose due to concerns of addiction and safety (For a review see Rasmussen, 2006). In the late 1950s however, amphetamine began to gain favor as a treatment for “minimal brain dysfunction”, a pediatric condition characterized by hyperactivity, inattention, and poor impulse control (For a review see: Lange, Reichl, Lange, Tucha, & Tucha, 2010). This diagnosis served as a precursor to the modern diagnosis of Attention Deficit Hyperactivity Disorder (ADHD), for which amphetamine and related stimulants are accepted treatments (Huang & Tsai, 2011).
Meta-analyses have confirmed the short-term effectiveness of amphetamines in the treatment of ADHD for children, adolescents (Punja et al., 2016), and adults (Castells, Ramos-Quiroga, Bosch, Nogueira, & Casas, 2011). The studies included in both of these reviews compared amphetamine preparations to placebo on validated ADHD symptom rating measures provided by the patient, parent, clinician, or experimenter. The effectiveness of amphetamine in the treatment of ADHD has also been studied using objective behavioral assessment for phenotypes of the disorder. A popular approach has been to evaluate effects on attention as measured by computerized tests of attention/vigilance such as the Continuous Performance Test (CPT). The CPT, introduced by Rosvold et al. (1956), presents stimuli (e.g., letters or numbers) consecutively and requires participants to respond only when an infrequent “target” stimulus (e.g., the letter X) appears. Variants of the CPT include requiring responses to the target (e.g., “X”) only when it is preceded by a separate specific stimulus (e.g., “A”). Although many variants exist, each task includes target stimuli (requiring a response) and non-target stimuli (e.g., Connors CPT).
Standardized CPTs have been developed as a result of concerns that cross-study comparisons were hindered by varying design parameters (Corkum & Siegel, 1993). The Conners’ CPT uses a “not-x” format in which targets (any letter except “x”) are more frequent than non-targets (“x”) allowing for a greater number of responses and hence more reliable estimates of response RTs. Such standardized tests enabled normative data on Conners’ CPT performance to be collected for children and adults (Conners, 2000; Conners, Epstein, Angold, & Klaric, 2003), enabling assessment relative to age- and sex-matched peers. Modern versions of the Conners’, such as the CPT-2, utilize normative data and a combination of response measures to provide an “ADHD confidence interval”. This measure uses a function of weighted measures from the CPT to suggest the likelihood of ADHD given the respondents’ performance and is increasingly being used to aid in diagnosis. The CPT-2 – as with other CPTs – also assess variability in reaction time (RT), which may relate most closely with ADHD symptoms (Epstein et al., 2006).
The CPT aids in characterizing attentional deficits in children with ADHD, who generally produce greater omission (failing to respond to a target) and commission (responding to a non-target) errors, as well as slowed RTs (Epstein et al., 2006; Fischer, Barkley, Edelbrock, & Smallish, 1990; Halperin et al., 1988; Klee & Garfinkel, 1983; Sykes, Douglas, & Morgenstern, 1973) and increased RT variability (Epstein et al., 2006; Tamm et al., 2014). Importantly, stimulant medications including dextroamphetamine (D-amp) remediate such CPT impairments in children (Losier, McGrath, & Klein, 1996; Riccio, Waldrop, Reynolds, & Lowe, 2001; Sostek, Buchsbaum, & Rapoport, 1980) and adults (Matochik et al., 1993) with ADHD. In contrast with methylphenidate, the most popular pharmacological treatment for ADHD, the effects of amphetamine on human attention has received less focus. As such, there remains a paucity of data on the attentional effects of amphetamine in healthy participants.
In an early trial of D-amp amongst 14 healthy prepubertal boys, Rapoport and colleagues (1978) reported that D-amp (0.50 mg/kg) improved attention on the Rosvold’s CPT by reducing errors of omission. In a mixed sex sample of 46 healthy young adults, participants reported perceived enhancement of cognition with mixed amphetamine salts, but enhancement was not observed on a cognitive battery which included several tasks related to attention (e.g., digit span forward, Go/No-go; Ilieva et al., 2013). In a large within-subject trial of 165 young adults D-amp decreased lapses of attention as reflected by reduced reaction times on the simple reaction time task, and reduced impulsive action on the stop task (Weafer & de Wit, 2013).
Results have also been mixed in the few studies in which amphetamine has been tested with healthy participants on modern CPT variants. Amongst 9 male volunteers, 5, 10, and 20 mg doses of D-amp failed to produce effects on a visual CPT (Slattum, Venitz, & Barr, 1996). In a mixed sex sample of 13 healthy participants (8 female) D-amp altered glucose metabolism in brain regions implicated in attentional processing but failed to improve CPT performance (Ernst et al., 1997). A 0.25 mg/kg dose of D-amp speeded RT but not accuracy on a CPT in 17 healthy individuals (Fleming, Bigelow, Weinberger, & Goldberg, 1995). The lack of evidence for amphetamine facilitation of CPT performance in healthy adult participants is particularly notable in that D-amp has been reported to improve CPT performance in prepubescent, but not older children, whom generally exhibit better performance (Sostek et al., 1980). It is conceivable that CPT performance in fully mature healthy adults is sufficiently accurate as to preclude the detection of facilitation effects. Alternatively, the attention-facilitating effect of amphetamine may be restricted to augmenting immature attentional processes and attention-related pathophysiology. Evaluating these possibilities remains important; if amphetamine is only efficacious in the context of psychopathology, it may serve as a useful tool for delineating the pathophysiology of the condition. Conversely, if amphetamine improves CPT performance in healthy adults, then such findings would be informative with regard to the neural substrates of attention; to the extent that attention can be dissociated from other task-relevant processes such as arousal, impulsivity, or perseveration, which can be assessed through alternative cognitive tasks.
It is important to keep in mind however, that an ADHD diagnosis can be met with a presentation of inattentive symptoms, hyperactive/impulsive symptoms, or their combination. Accordingly, deficits in behavioral measures of impulsivity, and risk taking are also observed in individuals with ADHD. For example, ADHD has been associated with poor performance on the Iowa Gambling Task (IGT) in children/adolescents (Garon, Moore, & Waschbusch, 2006; Hobson, Scott, & Rubia, 2011; Masunami, Okazaki, & Maekawa, 2009; Miller, Sheridan, Cardoos, & Hinshaw, 2013; Toplak, Jain, & Tannock, 2005) and adults (Abouzari, Oberg, Gruber, & Tata, 2015; Malloy-Diniz, Fuentes, Leite, Correa, & Bechara, 2007). However, there are data to suggest that deficits in adults with ADHD may be more indicative of co-morbid problematic gambling (Abouzari et al., 2015; Abouzari, Oberg, & Tata, 2016). While experimental studies evaluating the effect of amphetamine or other stimulants on IGT performance amongst patients with ADHD are lacking, there is however some evidence suggesting that IGT deficits are absent in adults with ADHD who are medicated (Abouzari et al., 2015).
Children with ADHD also exhibit deficits on the Wisconsin Card Sorting Test (WCST; Biederman et al., 2009; Li et al., 2014; Martel, Nikolas, & Nigg, 2007; Uran & Kilic, 2015; Vaurio, Riley, & Mattson, 2008), a measure of set-shifting, perseveration, and feedback-based learning. Results in adults with ADHD are mixed (Antshel et al., 2010; Biederman et al., 2009; Silva et al., 2014; L. L. Weyandt & DuPaul, 2006; Lisa L. Weyandt, Linterman, & Rice, 1995), and thus, it remains unclear whether the WCST deficits observed with ADHD persist into adulthood. While there is some evidence that stimulant medications may improve WCST performance in children with ADHD (Ince Tasdelen, Karakaya, & Oztop, 2015; Kempton et al., 1999; Tannock & Schachar, 1992; Yildiz, Sismanlar, Memik, Karakaya, & Agaoglu, 2011; Zheng et al., 2015) limited study in adults does not support stimulant medications enhancing WCST performance (Advokat, 2010). Clarification on the effect of D-amp on these domains in healthy adults is required.
The present study therefore sought to evaluate the effects of D-amp on vigilance in healthy adult participants using the Conners’ CPT-2 (version 5). Healthy adult participants - after receiving placebo or D-amp (10 or 20 mg) - performed the CPT-2, and computerized versions of the IGT and WCST, the latter to evaluate effects on other ADHD-relevant domains (e.g., impulsivity, risk-taking, learning, and perseverative responding) which may potentially be augmented by D-amp confounding CPT results. We hypothesized that D-amp would enhance vigilance on the CPT-2 while producing little or no impact on IGT and WCST measures.
METHODS
Participants
Healthy adults between the age of 18 and 35 were recruited from the San Diego community and screened for psychiatric illness by a trained clinician using the Structured Clinical Interview for DSM-IV (SCID-CT; First, Williams, Spitzer, & Gibbon, 2007). Participants were excluded if they met criteria for an axis I or II disorder, a substance use disorder within the past month, reported a current neurological condition, or presented with impaired motor function. Participants provided a urine sample for toxicological analysis and were excluded for recent substance use. Participants were excluded if their sample tested positive for pregnancy or if they reported a possibility of becoming pregnant during the study. During study intake, participants were queried with regard to gender and responses are qualified as sex here and throughout this report. All study procedures were approved by the UC San Diego Human Research Protections Program and were conducted in accordance with the Helsinki Declaration.
Procedures
A single-session, double-blind, placebo-controlled design was used. Research staff reviewed study procedures and received consent from participants prior to data collection. To reduce the potential impact of expectancies regarding D-amp effects, participants were informed that during the study they may receive caffeine, amphetamine, modafinil, or placebo. Participants meeting inclusion/exclusion requirements were subsequently evaluated by a study physician who reviewed health history, and physiological measures (e.g., ECG, blood pressure, heart rate) to verify medical appropriateness. Participants then ingested an oral dose of amphetamine (10 or 20 mg) or placebo and were tested by research staff who were blind to the participants’ condition. Randomization of participants to dose conditions was completed by the UCSD investigational pharmacy to maintain experimenter blinding. During the latter phases of data collection, the pharmacy was instructed to oversample the placebo and 20 mg conditions as these groups were anticipated to produce the hypothesized contrasts. Participants began the Conners’ CPT-2 at ~80, IGT at ~ 95, and the WCST at ~ 120 min post treatment ingestion.
Behavioral Assessment
Conners’ Continuous Performance Test
The Conners’ CPT-2 (V5; Conners, 2000) is a computerized vigilance task in which participants are presented with a series of letters sequentially and instructed to respond on a keyboard to all letters except “X”, for which they are instructed to withhold responses. Each letter presentation is separated by an Inter-Stimulus Interval of 1, 2, or 4 seconds and participants complete 18 blocks of 20 trials for a total of 360 trials. During the task, the non-target stimulus (“X”) appears on 10% of trials. Target trials are categorized as a hit (response) or an omission (no response) and non-target trials are categorized as an error of commission (false alarm) when followed by a response, or a correct rejection (non-response). Response latencies are recorded trials in response trials.
The primary measures of inattention evaluated in the present study included errors of omission, errors of commission, hit reaction time (HRT), HRT error, D′, and RT variability. RT Variability is a measure of the consistency of HRT error across segments of the CPT relative to the participants own level of HRT error. D′ [z(false alarms) + z(misses)] is a measure of vigilance performance. The β measure [z(misses) − z(false alarms)/z(total errors) was analyzed to evaluate responsivity; bias towards response vs. non-response. Perseverations, responses occurring less than 100ms after stimulus onset, were evaluated as a measure of impulsivity, which can also impact attention measures, most notably false alarms and HRT. The change in HRT and HRT across blocks of the task were included to assess vigilance, the ability to maintain attention for infrequent stimuli across time. Performance across time (vigilance decrement) was evaluated by combining blocks 1 & 2, 3 & 4, and 5 & 6 of the original task. The original 6 blocks of the CPT are composed of 18 sub-blocks (3 per block) such that a group of trials from each ISI level (1, 2, and 4 second) appear once in each block, counterbalanced so that each of the six blocks has a unique order of ISI presentations. Thus, groupings of trials from each ISI level appear twice in each of the aggregated blocks however, the order of ISI level presentation varies between block. Lastly, the ADHD confidence interval was included as a global measure of attention/vigilance. The ADHD confidence interval is derived from a discriminant function of the following variables listed in order of weighting (from least to greatest): standard error (SE) by ISI, SE by block, D′, perseverations, HRT SE, RT by ISI, β, age, sex, percent omissions. The ADHD confidence function yields a value which reflects the percentage of respondents with this performance profile likely to meet criteria for ADHD diagnosis.
Iowa Gambling Task
In the IGT (Bechara, Damasio, Damasio, & Anderson, 1994), participants are presented with 4 decks of cards (A-D) on a computer screen and instructed that they will be selecting cards sequentially from the deck(s) of their choosing. Each deck contains cards which indicate a theoretical amount of money won or lost (no actual money was provided). Decks A and B frequently result in a modest reward ($1.00) and occasionally in a large loss ($2.50 – 12.50), while decks C and D frequently provide a small reward ($0.50) and occasionally a modest to large loss ($0.25 – 2.50). Selecting from deck A or deck B exclusively results in a net loss, while selecting exclusively from decks C or D results in a net gain. Given that the order of cards in each deck is random, overall reward is generally maximized by selecting cards from decks C and D and avoiding decks A and B. Participants are informed that they will receive feedback on the amount won/loss after each choice, which will subsequently be added to or subtracted from a bar on the screen representing the overall amount won/lost during the task. While participants are told that some decks are worse than others, no information is provided about the amount or frequency of reward associated with each deck. The IGT provides a total score as well as well as the net gains/losses 5 times across the task and the number of selections from each of the four decks. Measures derived from the IGT provide an index of risk-taking and/or learning.
Wisconsin Card Sorting Test
Completion of the computerized WCST (WCST-64; Greve, 2001) requires participants to sort consecutively presented cards into one of four piles based upon a particular feature of the figure on the card. Each card presents 1–4 repetitions of a shape (a circle, star, square, or plus), in one of four colors (red, green, blue, or yellow). At the beginning of the task, participants are presented with four “target cards” presenting a single red circle, two green stars, three blue squares, or four yellow pluses. Instructions are to sort each new card into a pile below the target card that it matches. Thus, each new card can be matched to a target card based on either a consistent shape, color, or number of symbols. Participants are not told what attribute to match cards based upon but, are given feedback (correct/incorrect) after each match. Based upon this feedback, participants can determine which attribute is being matched and respond accordingly however, unbeknownst to the participant, the match attribute changes after every tenth consecutive correct match. Accurate responding depends upon repeatedly learning which attribute is being matched through the feedback provided after each match. Measures derived from the WCST included total correct matches, total errors, perseverative errors, non-perseverative errors, conceptual level responses, categories completed, trials to 1st category completed, and failure to maintain set. These measures reflect learning, set-shifting, and perseveration.
Drug treatment
Unmarked pills containing D-amp (10 or 20 mg) and an identical placebo preparation were provided by an Investigational Pharmacist who conducted study randomization. Participants were offered a small snack to minimize the risk of gastrointestinal discomfort following oral drug ingestion, which was monitored by the pharmacist. Given the pharmacokinetic profile of D-amp, plasma concentrations were expected to peak at approximately 1.5 – 2.0 hours post-ingestion (Wong et al., 1998). Thus, it was expected that testing would occur primarily during the ascending limb of drug distribution.
Statistical Analyses
Equivalence of demographic characteristics across groups were evaluated with ANOVA for continuous variables (age and years of education) and Chi Squared tests of independence for categorical variables (sex, race, and ethnicity). Outcome measures derived from each task were submitted to a two-way (dose by sex) between-subjects analysis of variance (ANOVA) with age included as a covariate. For each of the three tasks, the significance threshold was adjusted by dividing α (set at 0.05) by the number of measures evaluated from each task. Thus, the adjusted significance level was < .005 for CPT measures, and < .006 for the IGT and WCST. Significant effects of D-amp were followed with Tukey’s post hoc analyses. To evaluate the a priori hypothesis regarding the effects of D-amp on vigilance in the CPT, performance was aggregated into 3 time blocks (120 trials per block). After aggregation, data were submitted to three-way (dose by sex by block) mixed ANOVAs with age entered as a covariate. A traditional significance level (α < 0.05) was used to evaluate the a priori hypothesis of an interaction between dose and block (reflecting drug augmentation of the vigilance decrement). Significant interactions with dose were followed-up with one-way ANOVAs evaluating the effect of block or sex at each dose. All post-hoc tests related to significant effects of dose compared each active dose (10 or 20mg amphetamine) to placebo.
RESULTS
Sixty-nine participants (34 males) met study inclusion/exclusion criteria and completed all three experimental tasks. Participants ranged from 18 to 34 years of age with a median age of 22. Experimental groups did not significantly differ with regard to age, sex, years of education or ethnic/racial composition. Characteristics are presented in Table 1.
Table 1.
Demographic Characteristics
| Placebo | 10mg Amphetamine | 20mg Amphetamine | |
|---|---|---|---|
| Sex | |||
| Male | 15 | 7 | 12 |
| Female | 14 | 9 | 12 |
| Ethnicity (%) | |||
| Hispanic or Latino | 31.0 | 31.3 | 25.0 |
| Not Hispanic or Latino | 69.0 | 68.8 | 75.0 |
| Race (%) | |||
| Caucasian | 62.1 | 50.0 | 54.2 |
| African American | 13.8 | 8.3 | |
| Asian | 24.1 | 43.8 | 37.5 |
| Multiracial | 6.3 | ||
| Age (years) | 23.4 | 22.4 | 23.0 |
| Years of Education | 14.8 | 15.7 | 15.2 |
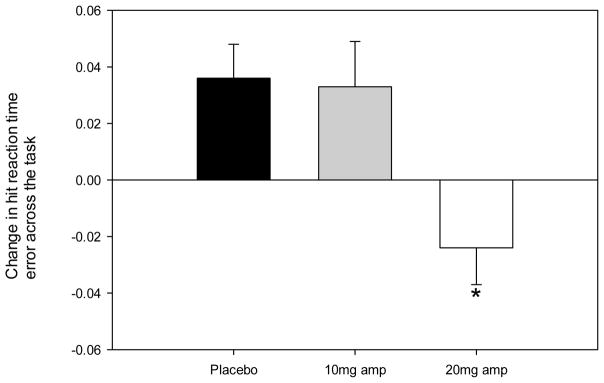
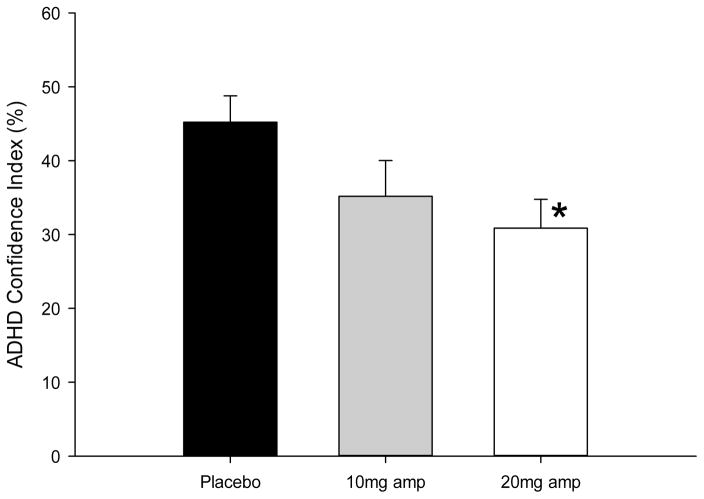
Significant effects of D-amp were detected on change in HRT error across the task (F(2,62) = 6.5, p = .003, ηp2 = .17), with post-hoc analyses revealing a significant difference between placebo, wherein HRT error increased across the task, compared with the 20 mg dose of D-amp, for which a reduction in HRT error was observed (p < .042; see Figure 1 and Table 2). As presented in Figure 2, D-amp also impacted the ADHD Confidence Index measure (F(2,62) = 3.9, p = .026, ηp2 = .11) with a lower confidence index observed at the 20mg dose relative to placebo (p = .009). This effect is considered trend level however, as the adjusted α threshold (< .005) was not met. A significant effect of sex was detected on the CPT with male participants producing a higher ADHD confidence Index (F(1,62) = 22.9, p < .001, ηp2 = .27). This effect was expected as sex is a weighted variable included in the discriminant function which produces the ADHD confidence Index. A trend was also observed in which males produced more perseverative responses on the CPT (F(1,62) = 4.7, p = .033, ηp2 = .07). Significant effects were not observed on any other measure of the CPT with regard to sex (p range: .319 – .877) or interactions between dose and sex (p range: .147 – .991). No effects were detected on the IGT or WCST with regard to Dose (p range: .161 – .957), sex (p range: .057 – .988), or their interaction (p range: .122 – .990).
Figure 1.
Amphetamine reduced the variability in hit reaction time across trial blocks of healthy human participants performing the Conners’ CPT-2 irrespective of sex. Asterisks represent a significant difference from placebo (p < 0.05).
Table 2.
Descriptive statistics and analysis of primary outcome measures
| Placebo n = 29 |
10mg Amphetamine n = 16 |
20mg Amphetamine n = 24 |
Amphetamine effects | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | F | DF | p | ηp2 | Adiusted α | |
| Conners’ CPT | |||||||||||
| Omissions (Misses) | 8.03 | 23.55 | 1.88 | 2.92 | 2.63 | 3.10 | 1.20 | 2, 62 | 0.308 | 0.04 | 0.005 |
| Comissions (False Alarm) | 11.66 | 7.78 | 11.94 | 7.25 | 11.33 | 6.21 | 0.02 | 2, 62 | 0.982 | 0.00 | 0.005 |
| Hit Reaction Time (HRT) | 392.58 | 64.23 | 357.22 | 48.32 | 365.09 | 47.94 | 2.42 | 2, 62 | 0.098 | 0.07 | 0.005 |
| HRT Error | 6.50 | 4.96 | 4.58 | 1.84 | 4.54 | 1.59 | 2.71 | 2, 62 | 0.075 | 0.08 | 0.005 |
| HRT Variability | 10.24 | 13.89 | 6.86 | 6.01 | 7.91 | 9.66 | 1.58 | 2, 62 | 0.215 | 0.05 | 0.005 |
| Detectability (D′) | 0.81 | 0.47 | 0.78 | 0.40 | 0.81 | 0.42 | 0.02 | 2, 62 | 0.982 | 0.00 | 0.005 |
| Response Style (β) | 0.97 | 1.05 | 0.49 | 0.40 | 0.69 | 0.69 | 1.70 | 2, 62 | 0.191 | 0.05 | 0.005 |
| Perseverations | 1.03 | 2.87 | 1.00 | 3.48 | 0.58 | 2.45 | 0.21 | 2, 62 | 0.815 | 0.01 | 0.005 |
| HRT Across Block | −0.005 | 0.023 | −0.003 | 0.019 | −0.009 | 0.021 | 0.45 | 2, 62 | 0.637 | 0.01 | 0.005 |
| HRT Error Across Block | 0.035 | 0.068 | 0.033 | 0.077 | −0.024 | 0.056 | 6.50 | 2, 62 | 0.003 | 0.17 | 0.005 |
| ADHD Confidence Index | 45.31 | 21.85 | 33.78 | 22.08 | 30.88 | 21.86 | 3.88 | 2, 62 | 0.026 | 0.11 | 0.005 |
| Iowa Gambling Task | |||||||||||
| Total Score | 9.31 | 27.73 | 12.88 | 21.20 | 14.25 | 33.99 | 0.23 | 2, 62 | 0.795 | 0.01 | 0.006 |
| Deck A | 14.34 | 4.78 | 16.25 | 6.89 | 14.58 | 5.30 | 0.54 | 2, 62 | 0.585 | 0.02 | 0.006 |
| Deck B | 31.00 | 13.72 | 27.31 | 9.89 | 28.29 | 13.79 | 0.56 | 2, 62 | 0.577 | 0.02 | 0.006 |
| Deck C | 22.34 | 11.30 | 23.56 | 6.25 | 23.92 | 15.53 | 0.12 | 2, 62 | 0.888 | 0.00 | 0.006 |
| Deck D | 32.31 | 13.62 | 32.88 | 11.40 | 33.21 | 14.48 | 0.04 | 2, 62 | 0.957 | 0.00 | 0.006 |
| Net 1 | −4.21 | 7.32 | −2.63 | 7.75 | −6.42 | 6.59 | 1.74 | 2, 62 | 0.185 | 0.05 | 0.006 |
| Net 2 | −0.48 | 6.56 | 0.50 | 4.16 | 3.25 | 9.47 | 1.78 | 2, 62 | 0.178 | 0.05 | 0.006 |
| Net 3 | 4.14 | 9.90 | 1.75 | 8.03 | 4.00 | 11.05 | 0.39 | 2, 62 | 0.678 | 0.01 | 0.006 |
| Net 4 | 1.86 | 8.77 | 5.25 | 8.29 | 6.42 | 10.06 | 1.88 | 2, 62 | 0.161 | 0.06 | 0.006 |
| Wisconsin Card Sorting Task | |||||||||||
| Total Correct | 48.21 | 8.17 | 51.63 | 5.40 | 48.67 | 7.84 | 0.00 | 2, 62 | 0.988 | 0.00 | 0.006 |
| Total Errors | 15.72 | 8.16 | 12.38 | 5.40 | 15.33 | 7.84 | 0.00 | 2, 62 | 0.972 | 0.00 | 0.006 |
| Persevarative Errors | 9.48 | 6.52 | 7.00 | 3.31 | 7.50 | 2.98 | 0.50 | 2, 62 | 0.484 | 0.01 | 0.006 |
| Nonperseverative Errors | 6.24 | 3.82 | 5.38 | 3.36 | 7.83 | 6.38 | 0.61 | 2, 62 | 0.439 | 0.01 | 0.006 |
| Conceptual Level Responses | 43.90 | 11.92 | 48.13 | 9.93 | 44.92 | 10.66 | 0.00 | 2, 62 | 0.954 | 0.00 | 0.006 |
| Categories Copleted | 3.52 | 1.35 | 4.06 | 1.24 | 3.63 | 1.38 | 0.03 | 2,62 | 0.867 | 0.00 | 0.006 |
| Trials to 1st Category Complete | 14.45 | 11.07 | 14.00 | 9.31 | 11.46 | 2.62 | 0.87 | 2, 62 | 0.422 | 0.03 | 0.006 |
| Failure to Maintain Set | 0.45 | 0.83 | 0.31 | 0.48 | 0.46 | 0.72 | 1.84 | 2, 62 | 0.180 | 0.03 | 0.006 |
Statistical tests meeting traditional significance have been highlighted in bold. Effect size and the Bonferroni corrected significance are also highlighted in bold when adjusted significance criteria were met.
Figure 2.
Amphetamine treatment reduced ADHD Confidence index produced by the Conners’ CPT-2 in healthy human participants irrespective of sex. Asterisks represent a significant difference from placebo (p < 0.05).
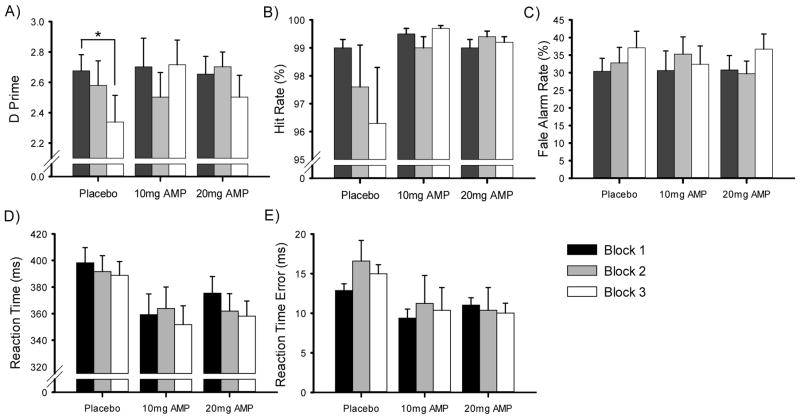
Given that D-amp is reputed to exert effects on the maintenance of attention over time, with vigilance decrements commonly observed in CPTs, select measures (D′, Hit Rate, False Alarm Rate, HRT, and HRT Error) from the task were additionally evaluated across 3 trial blocks. Significant main effects were not detected on any CPT measure with regard to dose (p range: .294 – .985), sex (p range: .778 – .918), or trial block (p range: .285 – .710). In contrast, and consistent with our a priori hypothesis, a significant dose by block interaction on D′ was observed (F(4,124) = 2.7, p = .036, ηp2 = .08). One-way ANOVAs revealed a significant effect of trial block on D′ for participants receiving placebo (F(2,56) = 5.0, p = .010, ηp2 = .15), i.e., a vigilance decrement, but not for those receiving either the 10 mg (F(2,30)=2.1, p = .14, ηp2 = .12) or 20 mg (F(2,46)=2.3, p = 0.11, ηp2 = 0.09) doses of D-amp, as illustrated in Figure 3A.
Figure 3.
Vigilance decrement was observed in placebo- but not amphetamine-treated healthy human participants performing the Conners’ CPT-2 as measured by D′ (A), driven in part by changes in target responding (Hit Rate; B) and responses to non-targets (False Alarm Rate; C), across trial blocks. Reaction time and reaction time variability are presented in panels D and E, respectively. Asterisks represent a significant difference from block 1 performance (p < 0.05).
DISCUSSION
The present findings demonstrate that in addition to improving attention in disease states, D-amp at 10 and 20 mg was effective for the enhancement of attention in healthy adults. D-amp enhanced vigilance by blocking the progressive deterioration of attentional performance over time; the classic vigilance decrement (for a review; See, Howe, Warm, & Dember, 1995). Consistent with improved vigilance, participants receiving the 20 mg dose of D-amp also exhibited statistically significant reductions in magnitude of HRT error across the task. A dose-dependent reduction in the ADHD Confidence Index was also observed, though this trend did not meet the adjusted significance threshold.
Prior studies have demonstrated D-amp-induced improvement of CPT performance amongst children (Sostek et al., 1980), but not adults (Ernst et al., 1997; Slattum et al., 1996; Sostek et al., 1980), while global reductions in RT have been observed with both D-amp (Fleming et al., 1995), and another ADHD-approved stimulant medication, methylphenidate (Cooper et al., 2005; Klorman et al., 1984; Strauss et al., 1984). The present study benefitted from a larger sample size than is typical of studies stimulant effects on CPT performance in healthy adults. Given the strong performance of healthy participants under placebo conditions, it is not surprising that facilitative effects were somewhat subtle, potentially explaining the failure of prior studies to detect performance-enhancing effects. The present findings however, do support the feasibility of detecting pro-cognitive drug effects with a standardized CPT in healthy adults. The most prominent effect of amphetamine was a reduction of the change in HRT error across the task which is consistent with recent reports of amphetamine improving simple reaction time task performance by reducing the occurrence of long reaction times, considered to reflect lapses of attention (Weafer & de Wit, 2013). Notably, increased reaction time variability is a prominent feature of ADHD (Gu et al., 2013; Lin et al., 2015) that significantly impairs reading ability (Tamm et al., 2014). While reaction time variability may serve as a useful endophenotype of ADHD, amphetamine effects appear nonspecific to individuals with deficits in this domain.
Though not marketed for this purpose, reports of amphetamine and other stimulant use for cognitive enhancement amongst students, academics, and other professions requiring vigilance has long been reported (Smith & Farah, 2011). The relative paucity of positive findings regarding cognitive enhancement in modern studies of healthy individuals however, has led investigators to question whether such use is even effective (Ilieva & Farah, 2013). The present study lends support for acute enhancement of vigilance in healthy individuals, but further work is required to establish who benefits most from stimulant treatment, whether such gains are maintained with extended use, and to evaluate the balance of these gains with adverse side effects (see Farah, 2015).
Notably, a prominent trend towards dose-dependent reduction of the ADHD confidence index was observed. The confidence index is a composite of demographic and performance measures (sex, age, percent omissions, standard error by ISI, HRT, response style, D′, and RT by block) which has been demonstrated to prospectively predict ADHD diagnosis from childhood performance (Breaux, Griffith, & Harvey, 2016). This trend suggests that, in healthy adults, D-amp may enhance those aspects of CPT performance that are related to impairment in individuals with ADHD. Hence, the neuro-enhancing properties of amphetamine are likely not constrained to rectifying attentional deficits associated with ADHD.
Of the Conner’s CPT measures, RT variability has been reported to have the strongest relationship to ADHD symptoms, with evidence that stimulant treatment has an enduring effect on RT deficits (Epstein et al., 2006). It is important to consider however, accuracy and RT in conjunction since faster RTs may result in more errors, reflecting a speed-accuracy trade-off. In the present study, improvements were observed with regard to vigilance (D′) at both doses, without a significant RT cost and in the context of reduced RT error variability at 20 mg. The beneficial effect of D-amp could relate to its greater selectivity of norepinephrine vs. dopamine transporters (NET and DAT respectively), but mechanistic studies using more selective treatments are required.
It is also critical to consider whether the effect of D-amp is selective to attention or whether the observed enhancement of attention derives from an effect on other task-relevant processes such as impulsivity or arousal. Importantly, while D-amp improved measures of attention and vigilance (D′ and change in RT variability), it did not affect response style (β; tendency towards responding or non-responding). D-amp also did not affect response inhibition (i.e., false alarms), or perseverative responses on the CPT, which are indicative of impulsivity. Prior studies in healthy adults have found D-amp to reduce impulsivity as measured by false alarms on a Go/No-Go task (de Wit et al., 2002), relative stop reaction time on the stop task (de Wit et al., 2002; Hamidovic et al., 2009; Weafer & de Wit, 2013) and delay discounting (de Wit et al., 2002). These findings are further supported by preclinical data demonstrating D-amp induced reduction of impulsive responses on a fixed consecutive number schedule in mice (Rivalan et al., 2007) and delay discounting in mice (Helms et al., 2006) and rats (Bizot et al., 2011). In the present study, the lack of an amphetamine effect on impulsivity-related measures may have resulted from a floor effect as healthy subjects produced few false alarms and perseverations. It should also be noted that in both humans and rodents, general performance and the effects of amphetamine across disparate measures of impulsivity are inconsistent, suggesting the influence of distinct neural correlates on aspects of impulsivity.
Effects of D-amp were not detected on the WCST, a task sensitive to learning, set-shifting, and perseveration effects, consistent with prior studies in healthy adults (Fleming et al., 1995; Mattay et al., 1996; Mattay et al., 2003). Additionally, no effect of D-amp was observed on IGT performance, a task sensitive to effects on perseveration, learning, and risk-taking. With regard to arousal, a prior report on the effects of the D-amp on activity in healthy adults with the doses used presently revealed that the 20 mg dose protected individuals from significant declines in activity during behavioral pattern monitoring (Minassian et al., 2016). This effect was not observed however, at the 10 mg dose and neither dose produced effects on exploration or the pattern of spatial of spatial movement. Thus, while D-amp-induced enhancement of CPT performance is not easily accounted for by effects on response strategy, perseveration, impulsivity, or risk-taking, it may not be dissociable from increased arousal in healthy adults.
As a whole, the present data support the conclusion that D-amp improves the vigilance of healthy adults in a manner similar to effects observed in patients with ADHD. Thus, in addition to clarifying that D-amp improves attention alone and may not simply be remediating pathological processes involved in ADHD, it also reveals neurobiological substrates underlying attentional control. Amphetamine increases synaptic availability of both dopamine and norepinephrine in large part through its inhibitory action on catecholamine transporters (i.e., DAT and NET). With regard to vigilance, the relative contributions of dopaminergic and noradrenergic signaling remain ambiguous and critical circuits have yet to be delineated. A full understanding of these processes is likely to require animal models in which relevant physiological assessments and manipulations can be performed. This effort can be significantly aided by the use of cross-species tasks that enable analogous testing in humans and animals, such as the 5-Choice CPT (5C-CPT). The 5C-CPT is available for testing in mice (Young, Light, Marston, Sharp, & Geyer, 2009), is human fMRI (McKenna, Young, Dawes, Asgaard, & Eyler, 2013) and EEG (Young, Bismark, Sun, Zhang, McIlwain, Grootendorst, & Light, 2017) compatible, and is sensitive to clinical pathology (Young et al., 2013; 2017). Thus, the use of the 5C-CPT is likely to aid in delineating the contribution of dopaminergic and noradrenergic signaling to vigilance performance. For example, mice with reduced D4 receptor expression exhibit response disinhibition and poor vigilance on the 5C-CPT (Young, Powell, Scott, Zhou, & Geyer, 2011), suggesting a potential therapeutic target. Indeed when Hayward and colleagues (2016) treated rats with high response disinhibition with a dopamine D4 receptor agonist remediated their deficits. Conducting human within-subject dose response studies with potential catecholaminergic treatments may also reveal individuals sensitive to such treatments. Evidence that amphetamine can improve human and rodent 5C-CPT performance consistent with the present studies is first required.
Several limitations are worth noting. For example, at the doses used, D-amp produced physiological and psychological effects that may be detectable to participants and spoil the blinding tactic. Participants were informed however, that they may receive caffeine, amphetamine, modafinil, or placebo, limiting the potential expectancy confound. Although we previously reported D-amp inducing modest hyperactivity in healthy human participants (Minassian et al, 2016), no effect on response bias was detected in the current study and it is unlikely that the pro-cognitive effects observed resulted from changes in learning, perseveration, or non-specific arousal. In sum, we report that D-amp enhanced vigilance amongst healthy adult participants, consistent with reports on its effects in patients with ADHD. Detection of these subtle effects required a sample size larger than typical of earlier studies.
Acknowledgments
We thank all of the participants who volunteered for these studies, which were made possible by funding from NIMH grants R01MH104344-03, and R01 MH071916, as well as by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center. The authors do not have a financial relationship with the funding organizations, and had full control of all primary data. The authors thank Dr. Harriet De Wit for her assistance in designing this study, and Dustin Kreitner, Mahalah Buell, Elise Winbrock, and Karen Kloezeman for their contributions to data collection and analysis.
References
- Abouzari M, Oberg S, Gruber A, Tata M. Interactions among attention-deficit hyperactivity disorder (ADHD) and problem gambling in a probabilistic reward-learning task. Behav Brain Res. 2015;291:237–243. doi: 10.1016/j.bbr.2015.05.041. [DOI] [PubMed] [Google Scholar]
- Abouzari M, Oberg S, Tata M. Theta-band oscillatory activity differs between gamblers and nongamblers comorbid with attention-deficit hyperactivity disorder in a probabilistic reward-learning task. Behav Brain Res. 2016;312:195–200. doi: 10.1016/j.bbr.2016.06.031. [DOI] [PubMed] [Google Scholar]
- Advokat C. What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/hyperactivity disorder (ADHD) Neuroscience & Biobehavioral Reviews. 2010;34(8):1256–1266. doi: 10.1016/j.neubiorev.2010.03.006. doi: http://dx.doi.org/10.1016/j.neubiorev.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Faraone SV, Maglione K, Doyle AE, Fried R, Seidman LJ, Biederman J. Executive functioning in high-IQ adults with ADHD. Psychol Med. 2010;40(11):1909–1918. doi: 10.1017/S0033291709992273. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Ball SW, Fried R, Doyle AE, Cohen D, … Faraone SV. Are cognitive deficits in attention deficit/hyperactivity disorder related to the course of the disorder? A prospective controlled follow-up study of grown up boys with persistent and remitting course. Psychiatry Res. 2009;170(2–3):177–182. doi: 10.1016/j.psychres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizot JC, David S, Trovero F. Effects of atomoxetine, desipramine, d-amphetamine and methylphenidate on impulsivity in juvenile rats, measured in a T-maze procedure. Neurosci Lett. 2011;489(1):20–24. doi: 10.1016/j.neulet.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Breaux RP, Griffith SF, Harvey EA. Preschool Neuropsychological Measures as Predictors of Later Attention Deficit Hyperactivity Disorder. J Abnorm Child Psychol. 2016 doi: 10.1007/s10802-016-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells X, Ramos-Quiroga JA, Bosch R, Nogueira M, Casas M. Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813. doi: 10.1002/14651858.CD007813.pub2. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ Continuous Performance Test (CPT-2) computer program for windows, technical guide, and software manual. Toronto, ON: Multi Health Systems, Inc; 2000. [Google Scholar]
- Conners CK, Epstein JN, Angold A, Klaric J. Continuous performance test performance in a normative epidemiological sample. J Abnorm Child Psychol. 2003;31(5):555–562. doi: 10.1023/a:1025457300409. [DOI] [PubMed] [Google Scholar]
- Cooper NJ, Keage H, Hermens D, Williams LM, Debrota D, Clark CR, Gordon E. The dose-dependent effect of methylphenidate on performance, cognition and psychophysiology. J Integr Neurosci. 2005;4(1):123–144. doi: 10.1142/s0219635205000744. [DOI] [PubMed] [Google Scholar]
- Corkum PV, Siegel LS. Is the Continuous Performance Task a valuable research tool for use with children with Attention-Deficit-Hyperactivity Disorder? J Child Psychol Psychiatry. 1993;34(7):1217–1239. doi: 10.1111/j.1469-7610.1993.tb01784.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27(5):813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Conners CK, Hervey AS, Tonev ST, Arnold LE, Abikoff HB … Group MTACS. Assessing medication effects in the MTA study using neuropsychological outcomes. J Child Psychol Psychiatry. 2006;47(5):446–456. doi: 10.1111/j.1469-7610.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik J, Schmidt M, Jons PH, Liebenauer LL, … Cohen RM. Intravenous dextroamphetamine and brain glucose metabolism. Neuropsychopharmacology. 1997;17(6):391–401. doi: 10.1016/S0893-133X(97)00088-2. [DOI] [PubMed] [Google Scholar]
- Farah MJ. NEUROSCIENCE. The unknowns of cognitive enhancement. Science. 2015;350(6259):379–380. doi: 10.1126/science.aad5893. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT) New York: Biometrics Research, New York State Psychiatric Institute; 2007. [Google Scholar]
- Fischer M, Barkley RA, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: II. Academic, attentional, and neuropsychological status. J Consult Clin Psychol. 1990;58(5):580–588. doi: 10.1037//0022-006x.58.5.580. [DOI] [PubMed] [Google Scholar]
- Fleming K, Bigelow LB, Weinberger DR, Goldberg TE. Neuropsychological effects of amphetamine may correlate with personality characteristics. Psychopharmacol Bull. 1995;31(2):357–362. [PubMed] [Google Scholar]
- Garon N, Moore C, Waschbusch DA. Decision making in children with ADHD only, ADHD-anxious/depressed, and control children using a child version of the Iowa Gambling Task. J Atten Disord. 2006;9(4):607–619. doi: 10.1177/1087054705284501. [DOI] [PubMed] [Google Scholar]
- Greve KW. The WCST-64: a standardized short-form of the Wisconsin Card Sorting Test. Clin Neuropsychol. 2001;15(2):228–234. doi: 10.1076/clin.15.2.228.1901. [DOI] [PubMed] [Google Scholar]
- Gu SL, Gau SS, Tzang SW, Hsu WY. The ex-Gaussian distribution of reaction times in adolescents with attention-deficit/hyperactivity disorder. Res Dev Disabil. 2013;34(11):3709–3719. doi: 10.1016/j.ridd.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Wolf LE, Pascualvaca DM, Newcorn JH, Healey JM, O’Brien JD, … Young JG. Differential assessment of attention and impulsivity in children. J Am Acad Child Adolesc Psychiatry. 1988;27(3):326–329. doi: 10.1097/00004583-198805000-00010. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Dlugos A, Skol A, Palmer AA, de Wit H. Evaluation of genetic variability in the dopamine receptor D2 in relation to behavioral inhibition and impulsivity/sensation seeking: an exploratory study with d-amphetamine in healthy participants. Exp Clin Psychopharmacol. 2009;17(6):374–383. doi: 10.1037/a0017840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward A, Tomlinson A, Neill JC. Low attentive and high impulsive rats: A translational animal model of ADHD and disorders of attention and impulse control. Pharmacol Ther. 2016;158:41–51. doi: 10.1016/j.pharmthera.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, Mitchell SH. Impact of strain and D-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology (Berl) 2006;188(2):144–151. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Hobson CW, Scott S, Rubia K. Investigation of cool and hot executive function in ODD/CD independently of ADHD. J Child Psychol Psychiatry. 2011;52(10):1035–1043. doi: 10.1111/j.1469-7610.2011.02454.x. [DOI] [PubMed] [Google Scholar]
- Huang YS, Tsai MH. Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge. CNS Drugs. 2011;25(7):539–554. doi: 10.2165/11589380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Ilieva I, Boland J, Farah MJ. Objective and subjective cognitive enhancing effects of mixed amphetamine salts in healthy people. Neuropharmacology. 2013;64:496–505. doi: 10.1016/j.neuropharm.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Ilieva I, Farah MJ. Cognitive Enhancement with Amphetamine: History Repeats Itself. AJOB Neuroscience. 2013;4(1):24–25. doi: 10.1080/21507740.2012.762069. [DOI] [Google Scholar]
- Ince Tasdelen B, Karakaya E, Oztop DB. Effects of Atomoxetine and Osmotic Release Oral System-Methylphenidate on Executive Functions in Patients with Combined Type Attention-Deficit/Hyperactivity Disorder. J Child Adolesc Psychopharmacol. 2015;25(6):494–500. doi: 10.1089/cap.2014.0155. [DOI] [PubMed] [Google Scholar]
- Kempton S, Vance A, Maruff P, Luk E, Costin J, Pantelis C. Executive function and attention deficit hyperactivity disorder: stimulant medication and better executive function performance in children. Psychological Medicine. 1999;29(3):527–538. doi: 10.1017/s0033291799008338. [DOI] [PubMed] [Google Scholar]
- Klee SH, Garfinkel BD. The computerized continuous performance task: a new measure of inattention. J Abnorm Child Psychol. 1983;11(4):487–495. doi: 10.1007/BF00917077. [DOI] [PubMed] [Google Scholar]
- Klorman R, Bauer LO, Coons HW, Lewis JL, Peloquin J, Perlmutter RA, … Strauss J. Enhancing effects of methylphenidate on normal young adults’ cognitive processes. Psychopharmacol Bull. 1984;20(1):3–9. [PubMed] [Google Scholar]
- Lange KW, Reichl S, Lange KM, Tucha L, Tucha O. The history of attention deficit hyperactivity disorder. Atten Defic Hyperact Disord. 2010;2(4):241–255. doi: 10.1007/s12402-010-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, He N, Li Y, Chen L, Huang X, Lui S, … Gong Q. Intrinsic brain abnormalities in attention deficit hyperactivity disorder: a resting-state functional MR imaging study. Radiology. 2014;272(2):514–523. doi: 10.1148/radiol.14131622. [DOI] [PubMed] [Google Scholar]
- Lin HY, Hwang-Gu SL, Gau SS. Intra-individual reaction time variability based on ex-Gaussian distribution as a potential endophenotype for attention-deficit/hyperactivity disorder. Acta Psychiatr Scand. 2015;132(1):39–50. doi: 10.1111/acps.12393. [DOI] [PubMed] [Google Scholar]
- Losier BJ, McGrath PJ, Klein RM. Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without ADHD: a meta-analytic review. J Child Psychol Psychiatry. 1996;37(8):971–987. doi: 10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A. Impulsive behavior in adults with attention deficit/hyperactivity disorder: characterization of attentional, motor and cognitive impulsiveness. J Int Neuropsychol Soc. 2007;13(4):693–698. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- Martel M, Nikolas M, Nigg JT. Executive function in adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1437–1444. doi: 10.1097/chi.0b013e31814cf953. [DOI] [PubMed] [Google Scholar]
- Masunami T, Okazaki S, Maekawa H. Decision-making patterns and sensitivity to reward and punishment in children with attention-deficit hyperactivity disorder. Int J Psychophysiol. 2009;72(3):283–288. doi: 10.1016/j.ijpsycho.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Nordahl TE, Gross M, Semple WE, King AC, Cohen RM, Zametkin AJ. Effects of acute stimulant medication on cerebral metabolism in adults with hyperactivity. Neuropsychopharmacology. 1993;8(4):377–386. doi: 10.1038/npp.1993.38. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Berman KF, Ostrem JL, Esposito G, Van Horn JD, Bigelow LB, Weinberger DR. Dextroamphetamine enhances “neural network-specific” physiological signals: a positron-emission tomography rCBF study. J Neurosci. 1996;16(15):4816–4822. doi: 10.1523/JNEUROSCI.16-15-04816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, … Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100(10):6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna BS, Young JW, Dawes SE, Asgaard GL, Eyler LT. Bridging the bench to bedside gap: validation of a reverse-translated rodent continuous performance test using functional magnetic resonance imaging. Psychiatry Res. 2013;212(3):183–191. doi: 10.1016/j.pscychresns.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Miller M, Sheridan M, Cardoos SL, Hinshaw SP. Impaired decision-making as a young adult outcome of girls diagnosed with attention-deficit/hyperactivity disorder in childhood. J Int Neuropsychol Soc. 2013;19(1):110–114. doi: 10.1017/S1355617712000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Young JW, Cope ZA, Henry BL, Geyer MA, Perry W. Amphetamine increases activity but not exploration in humans and mice. Psychopharmacology (Berl) 2016;233(2):225–233. doi: 10.1007/s00213-015-4098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punja S, Shamseer L, Hartling L, Urichuk L, Vandermeer B, Nikles J, Vohra S. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2:CD009996. doi: 10.1002/14651858.CD009996.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Buchsbaum MS, Zahn TP, Weingartner H, Ludlow C, Mikkelsen EJ. Dextroamphetamine: cognitive and behavioral effects in normal prepubertal boys. Science. 1978;199(4328):560–563. doi: 10.1126/science.341313. [DOI] [PubMed] [Google Scholar]
- Rasmussen N. Making the first anti-depressant: amphetamine in American medicine, 1929–1950. J Hist Med Allied Sci. 2006;61(3):288–323. doi: 10.1093/jhmas/jrj039. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Waldrop JJ, Reynolds CR, Lowe P. Effects of stimulants on the continuous performance test (CPT): implications for CPT use and interpretation. J Neuropsychiatry Clin Neurosci. 2001;13(3):326–335. doi: 10.1176/jnp.13.3.326. [DOI] [PubMed] [Google Scholar]
- Rivalan M, Gregoire S, Dellu-Hagedorn F. Reduction of impulsivity with amphetamine in an appetitive fixed consecutive number schedule with cue for optimal performance in rats. Psychopharmacology (Berl) 2007;192(2):171–182. doi: 10.1007/s00213-007-0702-6. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Jr, Beck LH. A continuous performance test of brain damage. J Consult Psychol. 1956;20(5):343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- See JE, Howe SR, Warm JS, Dember WN. Meta-analysis of the sensitivity decrement in vigilance. Psychological Bulletin. 1995;117(2):230–249. doi: 10.1037/0033-2909.117.2.230. [DOI] [Google Scholar]
- Silva KL, Rovaris DL, Guimaraes-da-Silva PO, Victor MM, Salgado CA, Vitola ES, … Bau CH. Could comorbid bipolar disorder account for a significant share of executive function deficits in adults with attention-deficit hyperactivity disorder? Bipolar Disord. 2014;16(3):270–276. doi: 10.1111/bdi.12158. [DOI] [PubMed] [Google Scholar]
- Slattum PW, Venitz J, Barr WH. Comparison of methods for the assessment of central nervous system stimulant response after dextroamphetamine administration to healthy male volunteers. J Clin Pharmacol. 1996;36(11):1039–1050. doi: 10.1177/009127009603601108. [DOI] [PubMed] [Google Scholar]
- Smith ME, Farah MJ. Are prescription stimulants “smart pills”? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychol Bull. 2011;137(5):717–741. doi: 10.1037/a0023825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sostek AJ, Buchsbaum MS, Rapoport JL. Effects of amphetamine on vigilance performance in normal and hyperactive children. J Abnorm Child Psychol. 1980;8(4):491–500. doi: 10.1007/BF00916502. [DOI] [PubMed] [Google Scholar]
- Strauss J, Lewis JL, Klorman R, Peloquin LJ, Perlmutter RA, Salzman LF. Effects of methylphenidate on young adults’ performance and event-related potentials in a vigilance and a paired-associates learning test. Psychophysiology. 1984;21(6):609–621. doi: 10.1111/j.1469-8986.1984.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Sykes DH, Douglas VI, Morgenstern G. Sustained attention in hyperactive children. J Child Psychol Psychiatry. 1973;14(3):213–220. doi: 10.1111/j.1469-7610.1973.tb01189.x. [DOI] [PubMed] [Google Scholar]
- Tamm L, Epstein JN, Denton CA, Vaughn AJ, Peugh J, Willcutt EG. Reaction time variability associated with reading skills in poor readers with ADHD. J Int Neuropsychol Soc. 2014;20(3):292–301. doi: 10.1017/S1355617713001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock R, Schachar R. Methylphenidate and cognitive perseveration in hyperactive children. J Child Psychol Psychiatry. 1992;33(7):1217–1228. doi: 10.1111/j.1469-7610.1992.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Jain U, Tannock R. Executive and motivational processes in adolescents with Attention-Deficit-Hyperactivity Disorder (ADHD) Behav Brain Funct. 2005;1(1):8. doi: 10.1186/1744-9081-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uran P, Kilic BG. Comparison of neuropsychological performances and behavioral patterns of children with attention deficit hyperactivity disorder and severe mood dysregulation. Eur Child Adolesc Psychiatry. 2015;24(1):21–30. doi: 10.1007/s00787-014-0529-8. [DOI] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN. Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc. 2008;14(1):119–129. doi: 10.1017/S1355617708080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, de Wit H. Inattention, impulsive action, and subjective response to D-amphetamine. Drug Alcohol Depend. 2013;133(1):127–133. doi: 10.1016/j.drugalcdep.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyandt LL, DuPaul G. ADHD in college students. J Atten Disord. 2006;10(1):9–19. doi: 10.1177/1087054705286061. [DOI] [PubMed] [Google Scholar]
- Weyandt LL, Linterman I, Rice JA. Reported prevalence of attentional difficulties in a general sample of college students. Journal of Psychopathology and Behavioral Assessment. 1995;17(3):293–304. doi: 10.1007/bf02229304. [DOI] [Google Scholar]
- Wong YN, Wang L, Hartman L, Simcoe D, Chen Y, Laughton W, … Grebow P. Comparison of the single-dose pharmacokinetics and tolerability of modafinil and dextroamphetamine administered alone or in combination in healthy male volunteers. J Clin Pharmacol. 1998;38(10):971–978. doi: 10.1002/j.1552-4604.1998.tb04395.x. [DOI] [PubMed] [Google Scholar]
- Yildiz O, Sismanlar SG, Memik NC, Karakaya I, Agaoglu B. Atomoxetine and Methylphenidate Treatment in Children with ADHD: The Efficacy, Tolerability and Effects on Executive Functions. Child Psychiatry & Human Development. 2011;42(3):257–269. doi: 10.1007/s10578-010-0212-3. [DOI] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One. 2009;4(1):e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Scott CN, Zhou X, Geyer MA. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: Separating response inhibition from premature responding. Behav Brain Res. 2011;222(1):183–192. doi: 10.1016/j.bbr.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA, Rissling AJ, Sharp RF, Eyler LT, Asgaard G, Light GA. Reverse translation of the rodent 5C-CPT reveals that the impaired attention of people with schizophrenia is similar to scopolamine-induced deficits in mice. Translational Psychiatry. 2013;3:e324. doi: 10.1038/tp.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Bismark AW, Sun Y, Zhang W, McIlwain M, Grootendorst I, Light GA. Neurophysiological characterization of attentional performance dysfunction in schizophrenia patients in a reverse-translated task. Neuropsychopharmacology. 2017;42(6):1338–1348. doi: 10.1038/npp.2016.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Liang JM, Gao HY, Yang ZW, Jia FJ, Liang YZ, … Zhuo JM. An Open-label, Self-control, Prospective Study on Cognitive Function, Academic Performance, and Tolerability of Osmotic-release Oral System Methylphenidate in Children with Attention-deficit Hyperactivity Disorder. Chin Med J (Engl) 2015;128(22):2988–2997. doi: 10.4103/0366-6999.168948. [DOI] [PMC free article] [PubMed] [Google Scholar]