Abstract
Background
Selective CD28 inhibition is actively pursued as an alternative to B7 blockade using CTLA4-Ig based on the hypothesis that the checkpoint immune regulators CTLA-4 and PD-L1 will induce tolerogenic immune signals. We previously showed that blocking CD28 using a monovalent nonactivating reagent (single chain anti-CD28 Fv fragment linked to alpha-1 anti-trypsin: sc28AT) synergizes with calcineurin inhibitors in nonhuman primate (NHP) kidney and heart transplantation. Here, we explored the efficacy of combining a 3-week ‘induction” sc28AT treatment with prolonged CD154 blockade.
Methods
Cynomolgus monkey heterotopic cardiac allograft recipients received sc28AT (10 mg/kg, d0-20, n=3), hu5C8 (10–30 mg/kg, d0–84, n=4), or combination (n=6). Graft survival was monitored by telemetry. Protocol biopsies and graft explants were graded according to ISHLT AR and CAV scores. Alloantibody, T cell phenotype and Tregs were analyzed by flow cytometry. Immunochemistry and gene expression (Nanostring) characterized intra-graft cellular infiltration.
Results
Relative to modest prolongation of median graft survival time with sc28AT alone (34 days), hu5C8 (133 days) and sc28AT+hu5C8 (141 days) prolonged survival to a similar extent. CD28 blockade at induction, added to hu5C8, significantly attenuated the severity of acute rejection and cardiac allograft vasculopathy (CAV) during the first 3 months after transplantation relative to hu5C8 alone. These findings were associated with decreased proportions of circulating CD8+ and CD3+CD28− T cells, and modulation of inflammatory gene expression within allografts.
Conclusions
Induction with sc28AT promotes early cardiac allograft protection in hu5C8-treated NHPs. These results support further investigation of prolonged selective CD28 inhibition with CD40/CD154 blockade in NHP transplants.
Introduction
The CD28:B7 (CD80/CD86) costimulatory pathway is critically involved in primary T cell activation and differentiation, and as such represents an attractive therapeutic target for modulating pathogenic and protective T cell-mediated immune responses. Blockade of the B7 pathway with cytotoxic T lymphocyte antigen 4 Ig (CTLA4-Ig) reagents inhibits transplant rejection and autoimmune diseases in rodents and nonhuman primates (NHPs) (1–4), and belatacept (a high affinity variant of CTLA4-Ig) has translated clinically as an effective alternative to conventional small-molecule-based immunosuppressive regimens in renal transplantation (5). Targeting B7 is further effective in preclinical models when combined with antagonists of the CD40:CD154 pathway (anti-CD40 or anti-CD154 mAbs) (6–10).
However, experimental and emerging clinical data in transplant models support theoretical concern that B7-directed strategies interfere with CTLA-4:B7-mediated signals that are crucial to the development and function of donor-antigen-specific regulatory T cells (Tregs) (11). CTLA-4 engagement attenuates T cell responses, prevents development of autoimmunity (12), and plays a critical role in the induction of peripheral T cell tolerance to allografts (13, 14), whereas the absence of CTLA-4 (15) or its selective blockade (16) is linked to autoimmune disorders. Inhibition of anti-inflammatory and tolerogenic CTLA-4-driven pathways in T cells may explain why targeting the CD28:B7 pathway by B7 ligation, using either CTLA4-Ig (17) or anti-B7 antibodies (18), fails to lead to uniform transplant tolerance in selected rodent models, rigorous primate models, or human clinical trials (13, 19).
As predicted based on the opposing effects of engagement of CD28 and CTLA-4 by B7 family ligands on adaptive immunity, we and others have shown that selectively blocking CD28, without directly inhibiting potentially tolerogenic signals through CTLA-4, is relatively effective to modulate pathogenic T cell responses. A nonactivating anti-CD28 scFv antagonist (monovalent recombinant single-chain (sc) antibody fragment containing the F-variable (Fv) region) prevents allograft rejection in mice and exhibits additive or synergistic effects when combined with calcineurin inhibition or CD154 pathway blockade (14), and this has translated successfully in 2 primate transplant models (20). In contrast, a prior study combining CTLA4-Ig and anti-CD154, also designed based on promising evidence of synergistic effects in mice (7), did not exhibit synergism in a translational primate renal allograft model (8).
Using the noncross-linking anti-human CD28 scFv fragment linked to alpha-1 anti-trypsin (AT) (sc28AT) as previously described (20) to selectively inhibit CD28 signaling, here we evaluate the efficacy of combining sc28AT “induction” with CD154 blockade in cynomolgus monkey heart allograft recipients. Moreover, we describe the effect of selective anti-CD28 treatment on putative Treg number in both peripheral blood and the allograft and on gene expression within the allograft.
Materials and Methods
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine and were conducted in compliance with the NIH guidelines for the animals in research. Captive-bred and wild-caught Chinese cynomolgus monkeys (Macaca fascicularis) weighing between 2.7 and 7.7 kg were obtained from Alphagenesis (Yemassee, SC) and Primate Products (Miami, FL). The selection of donor-recipient pairs was based upon blood group compatibility and major histocompatibility complex (MHC) incompatibility by mixed lymphocyte reaction (MLR) (stimulatory index > 5; data not shown).
Heterotopic heart allograft transplantation and treatment protocol
All recipient animals underwent intra-abdominal heterotopic cardiac allotransplantation (21). Donor monkeys received heparin (3000 IU bolus IV) prior to graft removal and recipients received heparin (200 IU/kg IV) prior to graft implantation. Postoperatively, graft function (electrocardiogram; left ventricular systolic and diastolic pressure; heart rate) and core temperature were monitored daily by implanted telemetry device (D70-PCTP, Data Sciences International, St. Paul, MN). When clinical conditions allowed, open cardiac biopsies were performed by protocol at intervals until graft failure. Graft failure was defined by either a diminished pulse pressure (<30 mmHg for 2 consecutive days) or a drop in heart rate (<120 beats/min or a decline of >40 beats/min from a stable baseline) and confirmed by allograft biopsy or ultrasound and by direct visualization at graft explantation. Grafts were explanted if the animal met experimental termination or graft failure criteria, developed a life-threatening complication, or by protocol on postoperative day (POD) 90.
Humanized anti-human CD154 antibody 5C8H1, a mouse-human IgG1κ anti-CD154 antibody (hu5C8), was obtained from the NIH Nonhuman Primate Reagent Resource (Boston, MA). Single chain anti-human CD28 antibody fragment linked to alpha-1-anti-trypsine (sc28AT) was produced by Cell Essentials from a clone kindly provided by Bernard Vanhove (INSERM U643, Nantes, France). The specific activity of sc28AT was verified as previously described (16). Monkey recipients were untreated or received sc28AT (10 mg/kg/day IV on days 0–20), hu5C8 (30 mg/kg IV on days 0, 3, 7 and 14; 10 mg/kg on days 21, 28, 35 and 42; 20 mg/kg on days 56 and 84) (22), or the combination of sc28AT plus hu5C8.
Histology and morphology in monkey allograft
Biopsy and explanted cardiac allograft tissue specimens were fixed in 10% formalin solution, embedded in paraffin, sectioned, and stained with H&E. Histopathological features of acute cardiac allograft rejection were quantified on a scale of 0 to 3 based on the revised 2005 ISHLT grading system (23). Cardiac allograft vasculopathy (CAV) was graded on a scale from 0 to 3 (0, normal artery; 1, 1%–10% occlusion; 2, 11–50% occlusion; 3, >50% occlusion) (24). ISHLT and CAV scores were performed by 3 evaluators (T. Zhang, L. Burdorf, and R.N. Pierson III) blinded to the treatment group and expressed as the median of individual scores.
Flow cytometry staining of peripheral blood
Recipient peripheral blood was collected at regular intervals for flow cytometric analysis of Tregs and T cell memory markers (See Supplementary Methods, Fig. S1).
Immunohistochemistry
Intra-graft ICOS expression and infiltrating Tregs were assessed by immunofluorescence as previously described with minor modifications (20, 25) (See Supplementary Methods).
NanoString Gene Expression Assay
Gene expression in cardiac allograft biopsies and explants was quantified for a custom codeset (60 inflammatory and immune marker genes as well as 5 Rhesus macaque housekeeping genes and 14 reference genes, see SI Table 1) using the NanoString platform. Total RNA was isolated from snap-frozen heart tissue using the Rneasy® Plus Universal Minikit (for explants) or the miRNeasy® Micro Kit (for biopsy specimens) according to the manufacturer’s instructions (Qiagen, Valencia, CA). After genomic DNA digestion (RNase-Free DNase I, Qiagen), purified RNA was assessed (Agilent Bioanalyzer; Nanodrop), and 200ng of RNA per sample processed through the Nanostring nCounter System (NanoString Technologies Inc., Seattle, WA) (26) by the Deep Sequencing Core at Johns Hopkins University (Baltimore, MD). Normalization and data analysis was carried out with the NanoString nSolver® Analysis Software v3.0 based on the geometric mean of the positive controls and the 4 reference genes that were most stable across all samples (HPRT1, RPL32, TBP, and YWHAZ). Values lower than the mean + 3 standard deviations of the 8 negative control counts were excluded from statistical analysis.
Detection of anti-donor alloantibodies
IgM and IgG donor-reactive alloantibodies were retrospectively measured by flow cytometry using archived frozen donor splenocytes and recipient serum samples as previously reported (24). Alloantibody reactivity was considered detectable when the proportion of IgM- or IgG-positive donor cells relative to donor serum before transplant was more than 8%.
Statistical analysis
Graft survival was evaluated with use of the Kaplan-Meier method using the log-rank test for significance analysis with censoring for animals that died or underwent euthanasia or elective explantation with functional allografts. Statistical significance of other data was analyzed using Mann-Whitney U test. Differences were considered significant when the P value was less than 0.05.
Results
Cardiac allograft survival
As previously reported, in cynomolgus macaques treated for 21 days with sc28AT monotherapy at 10 mg/kg, cardiac allografts survived for 22, 34, and 36 days (median survival time (MST) 34 days), significantly longer than in untreated monkeys (MST 6 days; n=5; p<0.05) (20). Blockade of CD154 with hu5C8 significantly increased graft survival (MST 133 days; n=4; p=0.01 vs. untreated or sc28AT alone) and prevented acute graft failure during hu5C8 therapy (Fig. 1, A and B). One functional heart graft was electively explanted by protocol on POD 90, and the other 3 eventually failed on POD113, 133, and 152 days, which was 29 to 68 days after the final hu5C8 treatment on POD 84 (22). With sc28AT+hu5C8, 1 recipient (MDJ2C) was euthanized with a beating graft on POD 76 for a surgical complication (intestinal injury) during protocol cardiac allograft biopsy. Two recipients were diagnosed with reactivation of occult prior malarial infection, treated with chloroquine: 1 (MDL1L) died on POD 54 with a beating graft due to severe malarial anemia despite clearance of the parasite from the blood; the other recipient (MDL33) recovered fully and underwent elective protocol explantation of the functioning graft on POD 90. The remaining 3 recipients in this group retained vigorously contracting grafts during sc28AT+hu5C8 treatment and exhibited further extended graft survival for up to 86 days beyond cessation of antibody administration (Fig. 1, A and B). There was no significant difference in survival time between the hu5C8 (MST 133 days) and sc28AT+hu5C8 (MST 141 days) groups, demonstrating that short-term “induction” (d0 – 21) sc28AT did not prolong allograft survival compaired with an optimized hu5C8 dosing regimen.
Figure 1. Individual graft survival time and histological analysis of monkey cardiac allografts.
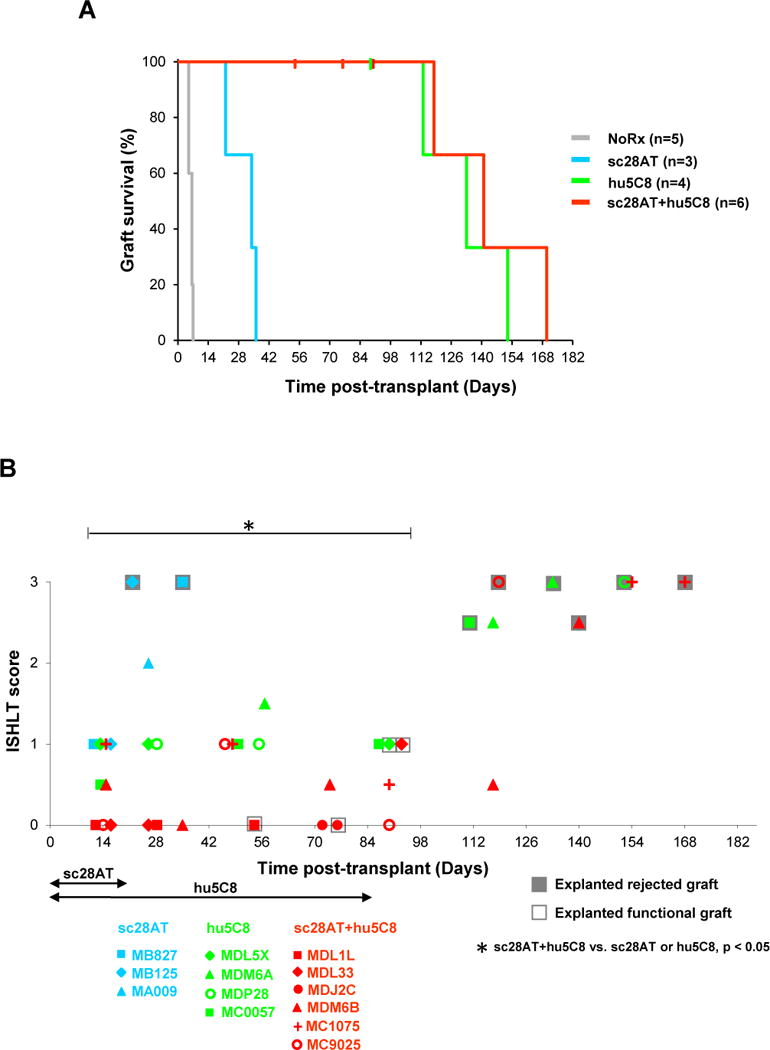
(A) sc28AT (blue) significantly prolonged allograft survival relative to no treatment (grey) (median survival time (MST), 34 vs. 6 days; P<0.05). There was no statistically significant difference in allograft survival comparing hu5C8-treated recipients (green, MST 133 days) with sc28AT+hu5C8-treated recipients (red, MST 141 days). Vertical hash marks represent animals that died or underwent euthanasia or elective explantation with functional allografts. (B) During the first 3 months after transplantation, ISHLT rejection scores of biopsy and functional (open boxes) or rejected (grey boxes) explanted cardiac allografts were significantly lower in sc28AT+hu5C8 group compared to sc28AT or hu5C8 monotherapy groups. Each dot represents the median of ISHLT scores for 1 tissue specimen. Multiple data points from individual animals over time are represented with a specific symbol, while symbol color identifies treatment group.
No clinical or pathologic signs of thrombosis and embolism were detected in animals treated with hu5C8 or sc28AT+hu5C8.
Effect of sc28AT on cellular infiltrate and CAV of cardiac allografts
Rejected grafts in all groups revealed intensive myocardial infiltration of mononuclear cells (ISHLT score 2–3) with and without haemorrhagic necrosis at explant, including both T and B cells (Fig. S2A). During the first 3 months after transplantation, the mean acute rejection score (mean ± standard error of the mean (SEM)) of biopsied or explanted functional grafts for sc28AT+hu5C8 group (0.32 ± 0.1) was significantly lower than for groups treated with sc28AT alone (2.0 ± 0.89) or hu5C8 alone (1.0 ± 0.08) (P<0.05 for both groups vs sc28AT+hu5C8; Fig. 1B).
During ongoing treatment, sc28AT+hu5C8 was generally associated with lower CAV scores (0.65 ± 0.08, mean ± SEM) than hu5C8 alone (1.13 ± 0.20; p<0.05) (Fig. 2, A and B). Rejected grafts in all groups revealed moderate to severe CAV lesions and C4d deposition (Fig. S2B) at explant. Taken together, these results demonstrate that induction using sc28AT effectively modulated pathogenic alloimmune responses in NHP while under treatment with hu5C8 (d0 – 90).
Figure 2. Cardiac allograft vasculopathy.
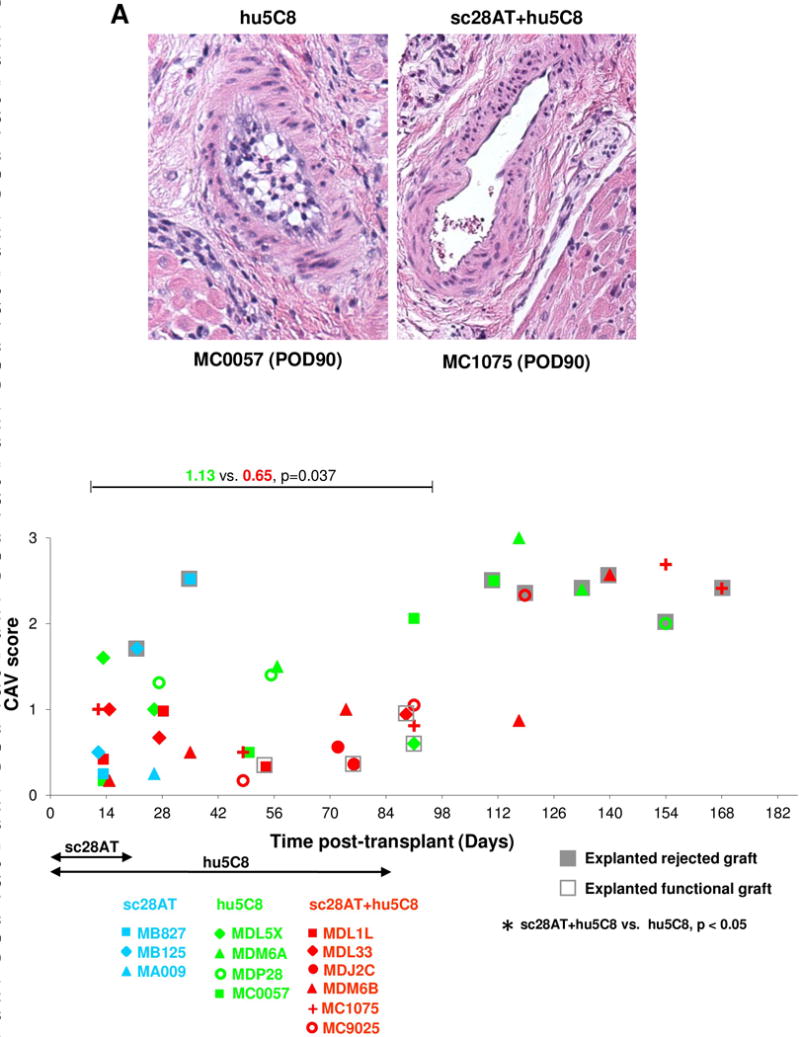
(A) Representative vessel from a hu5C8-treated cardiac allograft (day 90, left panel) shows grade 2 cardiac allograft vasculopathy (CAV) with distinct neointimal thickening (10–50% luminal narrowing). In contrast, a representative artery from a sc28AT+hu5C8-treated graft (day 90, right panel) shows absence of neointimal proliferation (H&E staining, original magnification ×200). (B) CAV severity score for biopsy and functional (open boxes) or rejected (grey boxes) explanted cardiac allograft tissue specimens. CAV scores with sc28AT+hu5C8 (red) were significantly lower than with hu5C8 alone (green) during the first 3 months. Each data point represents the median of CAV scores for 1 tissue specimen. Multiple data points from individual animals over time are represented with a specific symbol, while symbol color identifies treatment group.
Peripheral blood T cell phenotypes
To determine whether T cell phenotype was associated with the attenuated pathogenic response, T cell subsets in peripheral blood were characterized by flow cytometry. Following sc28AT induction treatment from POD 28 to 56, sc28AT+hu5C8-treated animals had a significantly higher proportion of CD4+ (41 ± 2.0% vs. 27.9 ± 3.5%; p= 0.001), a lower proportion of CD8+ (54 ± 2.1% vs. 66.6 ± 3.2%; p=0.002), and a lower proportion of CD28− T cells (33 ± 3.4% vs. 45.4 ± 3.7%; p=0.046) than hu5C8-treated animals (Fig. 3, left panels). A similar trend was observed for absolute numbers of CD8+ and CD3+CD28− circulating T cells although it did not reach statistical significance (Fig. 3 right panels). All together, these data suggest that sc28AT+hu5C8 combination treatment may limit the expansion/differentiation of CD8+ and CD3+CD28− effector/memory T cell subsets, both thought to mediate costimulation blockade-resistant rejection.
Figure 3. Peripheral blood T lymphocyte phenotype.
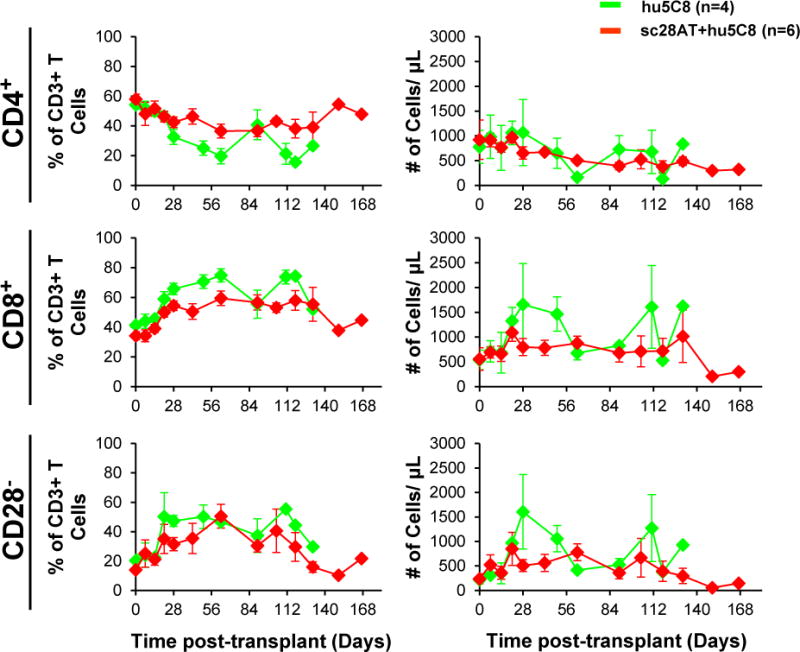
The mean (±SEM) of proportions (%) of CD4+ (top), CD8+ (middle), and CD28− (bottom) among CD3+ lymphocytes are shown on the left panels, and absolute numbers in the right panels. Animals treated with sc28AT+hu5C8 (red) maintained lower proportion of CD8+ T cells (p=0.002) and CD28− CD3+ T cells (p=0.046) compared to hu5C8-treated animals (green) during the second month after transplant. Absolute numbers of these 3 T cell populations were similar between the 2 groups.
CD4+CD25+CD127loFoxp3+ T cells (putative Tregs, Fig. 4) tended to increase during the first weeks following transplantation in all treatment groups. Relative to each individual animal’s day 0 pretransplant value (Fig. S3), both the number (left panel) and proportion (right panel) of Tregs were increased in association with sc28AT (1.2–2-fold increase at days 14 and 21), sc28AT+hu5C8 (1.1–2.2-fold increase from POD 7 through 28), and, to a lesser extent, hu5C8 (1.2–1.5-fold increase at days 14 and 28). From POD 7 to 35, sc28AT-treated animals had significantly higher absolute numbers of peripheral blood Tregs than hu5C8 or sc28AT+hu5C8-treated animals. However, there was not a significant difference in Treg numbers between hu5C8 group and sc28AT+hu5C8 group.
Figure 4. Quantification of circulating CD4+CD25+CD127lo Foxp3+ Tregs by flow cytometry.
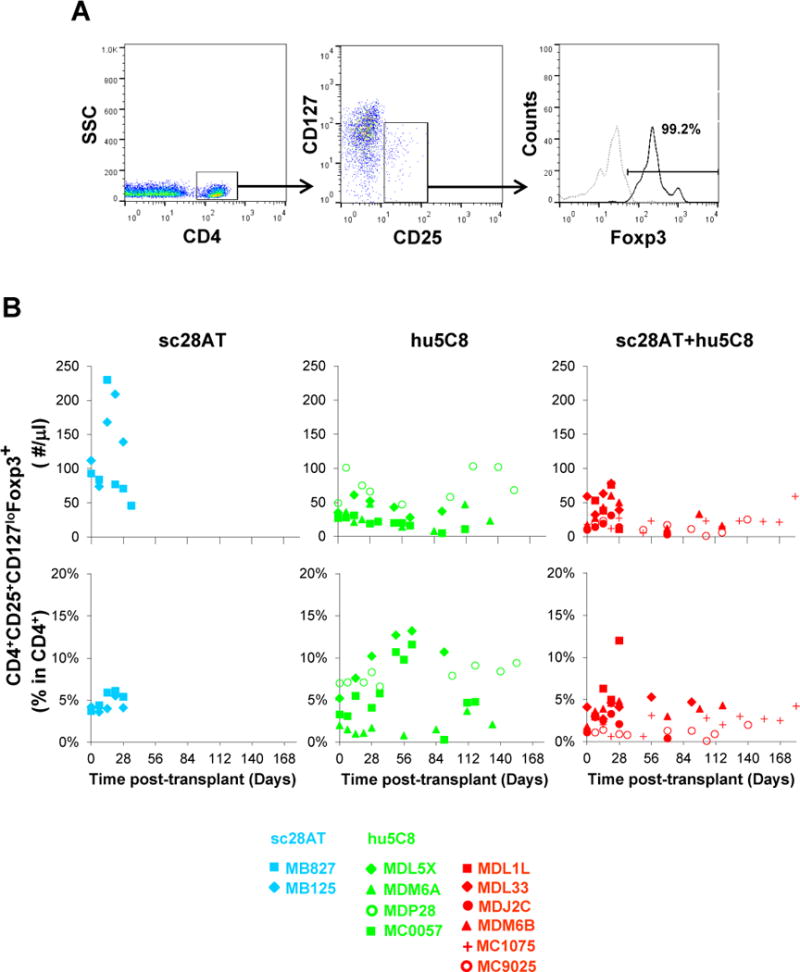
(A) Representative flow cytometry analysis of blood putative Tregs in a recipient treated with sc28AT+hu5C8 (MDM6B) at 4 weeks after transplantation when CD4+CD25+CD127loFoxP3+ subset representes 4.8% of total CD4+ cells. Dotted gray line represents the isotype control. (B) Treg absolute numbers (upper panel) and Treg proportion (CD25+CD127loFoxp3+ Tregs as a % among CD4+ cells) (lower panel) generally rose in most animals during the first weeks of treatment, but were similar (not significantly different) between hu5C8 and sc28AT+hu5C8 groups. Color coding (blue, sc28AT; green, hu5C8; red, sc28AT+hu5C8) and recipient-specific symbols are as in Figure 1.
The ratio of TEM (effector memory T cells) to Tregs among CD3+ T cells in peripheral blood is shown in Fig. S4. TEM:Treg ratio in the hu5C8 and sc28AT+hu5C8 group generally increased from POD 14 – 28, suggesting the early increase in peripheral Treg may have been counteracted by a TEM response.
Immunohistochemistry
On POD 7–28 after transplantation, graft biopsies from animals treated with sc28AT and sc28AT+hu5C8 generally trended to increase the number of CD4+ cells (Fig. S5) and percentages (Fig. 5B) of Foxp3-expressing CD4+ putative Tregs compared to grafts treated with hu5C8 monotherapy, but this did not reach statistical significance.
Figure 5. Immunofluorescent staining of graft infiltrating CD4+Foxp3+ T cells.
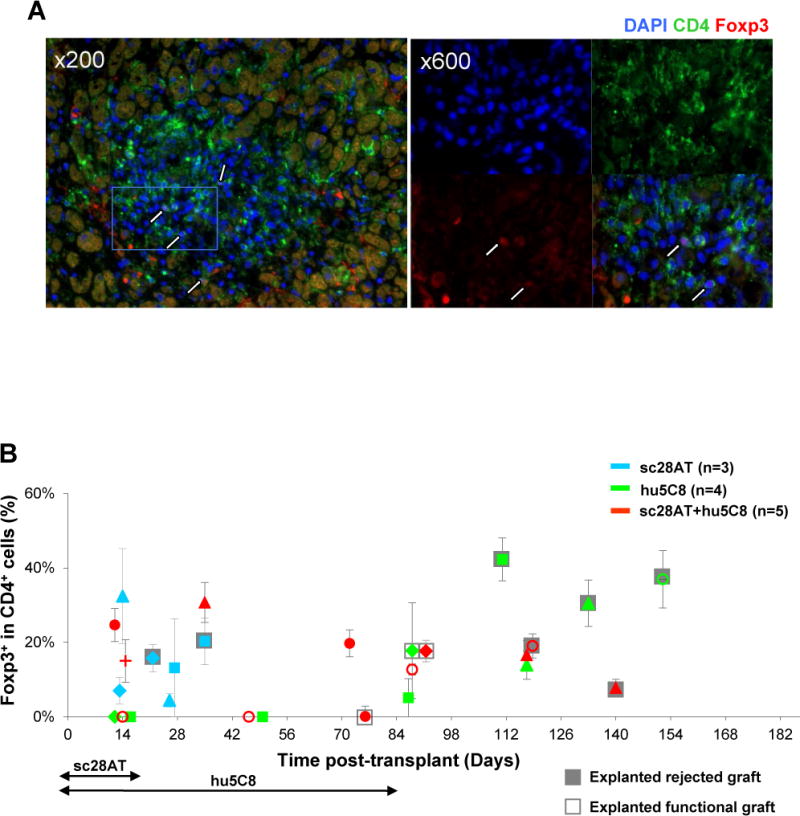
(A) Representative pictures of a cardiac allograft from sc28AT+hu5C8 group (MC9025, d118). Blue, DAPI nuclear staining; green, CD4; red, Foxp3. Several putative Tregs are identified by nuclear staining of Foxp3 surrounded by CD4 staining (white arrows). Original magnification, ×200 (left), × 600 (right). The right panel represents a magnification of the white rectangle indicated in the left panel. (B) Percentages of Foxp3+ cells among CD4+ lymphocytes in graft biopsies and functional (open boxes) or rejected (grey boxes) explanted cardiac allografts. Each dot represents the mean ± SEM of 2~7 fields from the same tissue specimen.
To explore intragraft T cell activation, we further evaluated biopsies and explants for ICOS. During the first month after transplantation, graft biopsies from animals treated with hu5C8 showed lower numbers of CD3+ cells (Fig. 6, Fig. S6) and proportions of ICOS-expressing CD3+ cells compared to sc28AT or sc28AT+hu5C8 (Fig. 6B). Rejected allografts exhibited higher ICOS+ and CD3+ infiltrating cells than nonrejecting biopsies in the same animal (Fig. 6B, Fig. S6).
Figure 6. Immunofluorescent staining of intra-graft CD3+ICOS+ T cells.
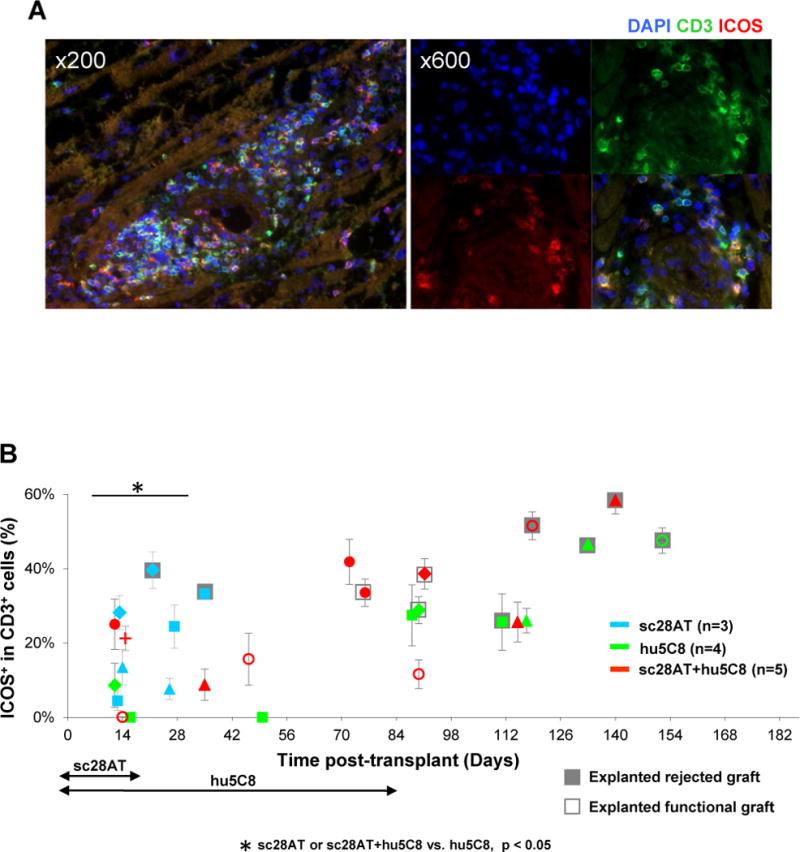
(A) Representative pictures of a cardiac allograft from sc28AT+hu5C8 group (MC9025, d118). Blue, DAPI nuclear staining; green, CD3; red, ICOS. A majority of CD3+ T cells express ICOS in this rejected allograft. (B) Percentages of ICOS+ cells among CD3+ cells in biopsies and explanted cardiac allografts. Each dot represents the mean±SEM of 2~7 fields from the same tissue specimen. *P<0.05 for the comparisons indicated.
Graft Gene Expression Analysis
Allografts treated with sc28AT monotherapy had significantly lower levels of IFNG (p<0.05), IDO1 (p<0.05), PDL-2 (P<0.05) and GZMB (p=0.05), and higher levels of PD-1 (p=0.03) compared to acutely rejected untreated allografts (Fig. 7). Compared to sc28AT monotherapy, sc28AT+hu5C8-treated allografts expressed significantly lower levels of many genes including inflammatory (GZMB, IFNG, IDO1, and IL-6) and T cell costimulation (CTLA-4, PDL-2, and PD-1; Fig. 7, Table S1). IDO1 levels were significantly less (p=0.02) and ICAM-1, CD3E and IL-6 tended to be lower (p=0.07) in the sc28AT+hu5C8 treatment group compared to hu5C8 monotherapy. IL-21 transcript levels were low in normal heart (2.5±0.3 normalized counts) and significantly elevated in acutely rejected allografts (31±23, p=0.0003). While sc28AT monotherapy did not decrease IL-21 (41±18) compared to no treatment, both hu5C8 (11±21, p=0.13) and sc28AT+hu5C8 (12±9, p=0.06) regimens were associated with low IL-21 levels. FOXP3 was increased in rejecting untreated heart allografts and in all treatment groups compared to normal myocardium, but it did not differ significantly between the experimental groups. Finally, a cluster of additional pro-inflammatory genes (IL1B, IL-2, IL-12A) and anti-inflammatory (TIM3, PD-L2, CD163) tended to be expressed at lower levels in protocol biopsies collected under combined drug treatment (POD 14–28) than in either monotherapy groups over this interval following transplant (p<0.2), which resembled expression patterns of nontransplanted monkey heart tissue (Figure 7, rectangle box with red contours in the center).
Figure 7. Nanostring gene expression analysis.
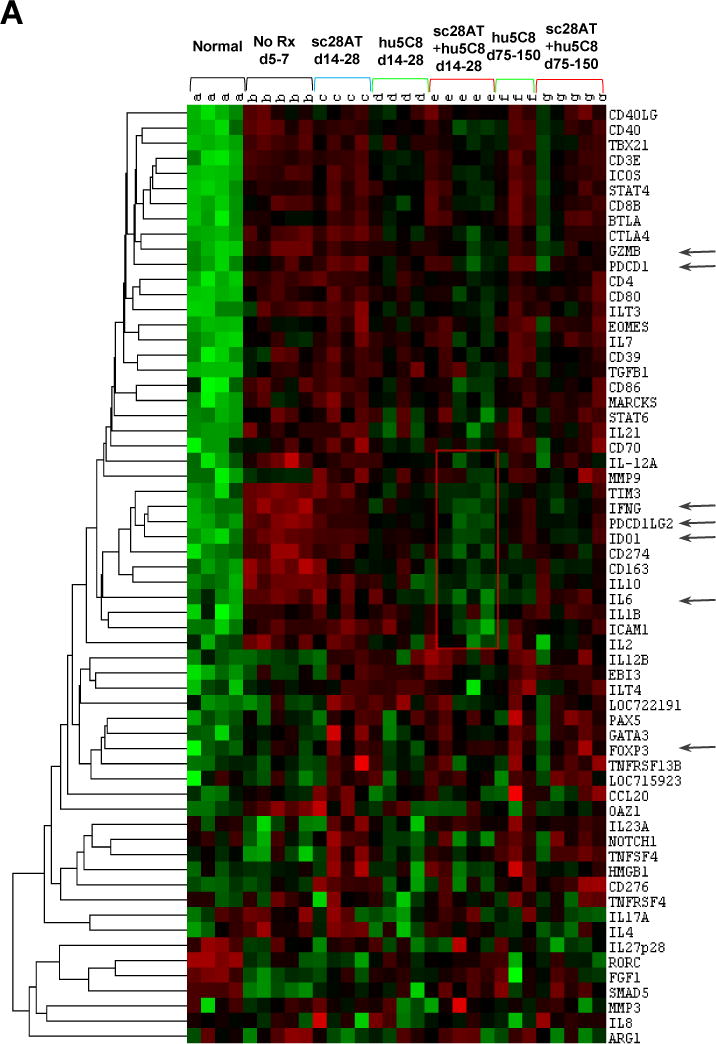
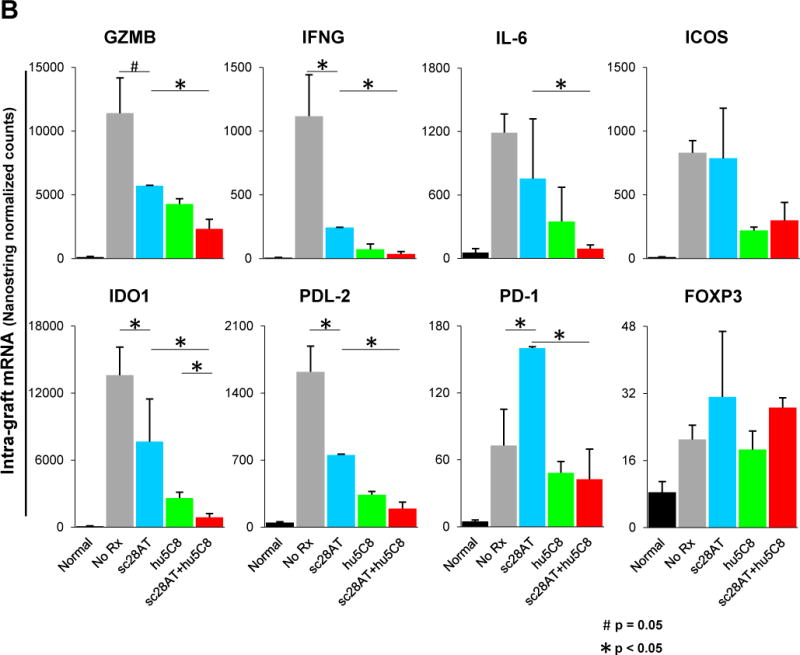
Intra-graft gene expression profiling in naive monkey heart (Normal, n=4), untreated allografts (No Rx, n=5), or allografts from recipients treated with sc28AT (blue, n=4), hu5C8 (green, n=4) or sc28AT+hu5C8 (red, n=5). (A) Heat-map analysis for the entire custom panel of 60 genes. Each column represents 1 allograft tissue specimen. Hu5C8±sc28AT-treated groups were analyzed both during therapy (d14–28 posttransplant) and around the time of hu5C8-therapy withdrawal (d75–150). The rectangular box with red contours in the centre delineates a cluster of genes that tend to be expressed at lower levels in protocol biopsies collected under combined drug treatment (d14–28) than in either monotherapy groups at the same time, and more similar to nontransplanted monkey heart tissue (Normal). Arrows denote 6 most differentially expressed or pathway relevant genes shown in more details in panel B. Results represent the mean ± SEM of normalized counts for each group. (B) During the first month after transplantation, sc28AT is associated with significantly increased expression of GZMB, IFNG, IL-6, IDO1, PDL-2, and PD-1, and MARCKS and CTLA-4 (not illustrated) compared to sc28AT+hu5C8. Expression of ICOS and Foxp3 was increased in rejecting (untreated and sc28AT-treated) allografts and in grafts protected by either hu5C8 alone or sc28AT+hu5C8. Asterisks denote p values < 0.05 between groups for the indicated comparisons, determined using the NanoString nSolver® Analysis Software.
Effect of sc28AT on anti-donor Ab
All 3 recipients treated with sc28AT alone elaborated strong levels (40–100%) of IgM and IgG alloantibody (Ab) around the time of graft failure (Fig. 8, left panel). Treatment with either hu5C8 or sc28AT+hu5C8 was associated with absent or low levels (<25%) of donor-specific antibody. With sc28AT+hu5C8, weak IgM was detected in 2 recipients infected with malaria (MDL1L and MDL33), and weak IgG was detected in recipient MDJ2C on the day of euthanasia due to intestinal complication after allograft biopsy. It is of note that at the time of graft demise, all 3 hu5C8 monotherapy animals elaborated donor reactive IgG antibody, whereas in 2 of 3 sc28AT+hu5C8 recipients serum IgG were undetectable. These results suggest that sc28AT+hu5C8 may be more effective than hu5C8 in attenuating humoral responses.
Figure 8. Donor-specific alloantibodies from the day of transplant to graft explant.
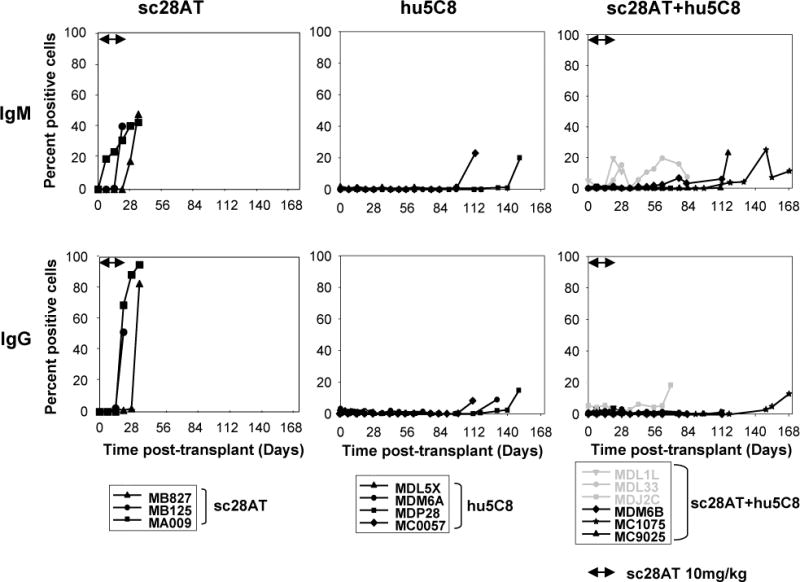
The presence of IgM (upper panel) and IgG (lower panel) anti-donor serum alloantibodies was measured by flow cytometry. In sc28AT+hu5C8 animals (right panels), weak IgM Abs were detected in recipients that suffered from malaria infection (MDL1L and MDL33, grey lines) and weak IgG Ab was detected in recipient (MDJ2C) on the day of euthanasia due to post biopsy intestinal complication (grey line). Significant (>20%) elaboration of donor-specific antibody was consistently prevented during treatment with either hu5C8 or sc28AT+hu5C8.
Discussion
In this study, we evaluate sc28AT, a nonactivating, noncross-linking monovalent anti-CD28 antibody fragment, in a stringent preclinical cynomolgus monkey heart allograft model. Using induction sc28AT in combination with hu5C8, to inhibit the CD40/CD154 costimulation pathway, we demonstrate that the addition of sc28AT attenuates the severity of acute rejection (ISHLT scores) and CAV for up to 2 months after clearance of the sc28AT from the circulation. Transient CD28 inhibition did not offer a significant graft survival benefit when combined with prolonged hu5C8, suggesting that extending anti-CD28 treatment duration, particularly with the use of improved reagents with enhanced bioavailability is a logical approach to further explore possible synergy with CD40/CD154 blockade. We chose to optimize the hu5C8 dosing regimen because our long-term goal is to induce tolerance in this NHP model. Nevertheless, the significant attenuation of CAV associated with combined treatment is particularly remarkable given the short duration of CD28 blockade, the short half-life of the reagent, and the long interval between cessation of CD28 “induction” treatment and eventual CAV progression once “maintenance” anti-CD154 treatment was discontinued.
Because naturally occurring Tregs require CD28 signals for their survival, concern has been expressed that selective CD28 inhibition might abrogate the strategy’s tolerogenic potential. While the primate model does not lend itself to directly test this hypothesis, our data are consistent with the alternative hypothesis that selective CD28 blockade permits or promotes expansion of Tregs via one or more CD28-independent mechanisms. The interactions of CTLA-4 with B7-1 and B7-2 play a pivotal role in the suppression of pathogenic T cell activation and enhanced CTLA-4 engagement can result in the induction of adaptive Tregs with donor alloantigen specificity (27). Our previous observation that blockade of CTLA-4 during selective CD28 inhibition triggers murine cardiac allograft rejection accompanied with profound decrease in intra-graft Foxp3 gene expression is also consistent with this working model (14). Aside from CTLA-4, the newly discovered PD-L1 interacts with B7-1 to inhibit T cell proliferation and cytokine production (28) and promote the development and function of induced Tregs (29). Prior studies demonstrated that sc28AT-based immunotherapy increases the production of Tregs in a kidney baboon transplant model (20). In the current study, putative Tregs were present in peripheral blood and in biopsies from stable, well-functioning grafts during sc28AT treatment, supporting our working hypothesis that selective CD28 blockade promotes Treg-mediated protective immunomodulation.
In an attempt to understand how sc28AT synergizes with anti-CD154 to mediate a protective effect on CAV, we analyzed a panel of immune and inflammatory genes in the graft. Allografts treated with sc28AT+hu5C8 had significantly lower levels of proinflammatory markers (IFNG, IL-6, t-bet), as well as CD4, CD8- (GZMB) and antigen-presenting cells- (CD80, CD86) associated gene transcripts compared to sc28AT-treated allografts. Moreover, IL-6 tended to be decreased with sc28AT+hu5C8 compared to hu5C8 alone (p=0.07). Lower IL-6 levels may reflect decreased activation of antigen presenting cells via CD28- and CD40/CD154-driven costimulation. Low levels of IL-6 may in turn favor the differentiation of activated T cells into Tregs instead of Th17, increase the susceptibility of effector T cells to suppression by Tregs, promote activation-induced cell death (30), improve endothelial cell integrity (31) and constrain differentiation of activated B cells into plasma cells (32).
ICOS is a CD28-dependent costimulatory molecule that is involved in T cell activation, differentiation, and chronic rejection (33). In the current study, increases in allograft ICOS expression during rejection development, especially at time of graft demise, confirms our prior observations (25). Meanwhile, based on the hypothesis that the majority of ICOS+ cells are pathogenic, we are currently investigating whether selective inhibition, especially “delayed” blockade of the ICOS:ICOS-L pathway, can prevent/abort pathogenic alloimmunity in the context of CD28:B7 and/or CD40:CD154 pathway blockade in NHPs.
Low levels of anti-donor IgM and IgG antibodies were detected transiently in 3 CD28/CD154 animals during acute infectious challenge or associated with bowel injury. We believe these findings are likely assay artifacts associated with acute phase reaction and do not reflect incomplete inhibition of the 2 costimulation pathways targeted in this group. Nevertheless, one exciting result in this study was the observation that animals treated with sc28AT+hu5C8 had better controlled IgG humoral response than hu5C8 at the time of graft loss, although we were unable to verify this statistically due to limited animal numbers. Whether an apparent trend toward delayed anti-donor antibody elaboration is associated with addition of sc28AT to efficient CD154 pathway inhibition will require further study.
In summary, our results suggest that “induction” with a selective CD28 blockade promotes protective alloimmune responses in the early posttransplant period in anti-CD154-treated NHPs. Whether prolonged selective CD28 inhibition, alone or in combination with CD40/CD154 blockade, is capable of conferring significant survival benefit to transplanted allografts in primates is under study. Together with our prior work showing synergy between sc28AT and calcineurin inhibitors (20), our preclinical observations support efforts to explore selective CD28 blockade to prevent allograft rejection in human transplantation.
Supplementary Material
Acknowledgments
We are grateful to Bernard Vanhove for validating the activity of the generic form of sc28AT. This work was supported by the NIH (UO1 AI 066719), an ASTS Mid-Career Award, a contract from the DOD ORD (N00014-04-1-0821), and an AHA Grant-in-Aid, all to RNP; by the Other Tobacco Related Diseases research grant from the Maryland Restitution Fund Program, to AA and RNP; and by the NIH Nonhuman Primate Reagent Resource (OD010976 and AI126683).
Funding: This work was supported by the NIH (UO1 AI 066719), an ASTS Mid-Career Award, a contract from the DOD ORD (N00014-04-1-0821), and an AHA Grant-in-Aid, all to RNP; by the Other Tobacco Related Diseases research grant from the Maryland Restitution Fund Program, to AA and RNP; and by the NIH Nonhuman Primate Reagent Resource (OD010976 and AI126683).
Abbreviations
- Ab
alloantibody
- CAV
cardiac allograft vasculopathy
- CTLA-4
cytotoxic T lymphocyte antigen 4
- Foxp3
forkhead box P3
- ICOS
inducible costimulator
- MHC
major histocompatibility complex
- MLR
mixed lymphocyte reaction
- MST
median survival time
- NHP
nonhuman primate
- PBMC
peripheral blood mononuclear cells
- PD-L1
programmed death ligand 1
- POD
postoperative day
- scFv
single-chain fragment variable
- sc28AT
CD28 scFv fragment linked to alpha-1 anti-trypsin
- SEM
standard error of the mean
- TEM
effector memory T cell
- Tregs
regulatory T cells
Footnotes
Authorship Contributions: T.Z., A.M.A., and R.N.P. III designed the experiments, analysed the data, and wrote the article. W.S., N.A.O., E.B., and G.B. performed experiments and analysed data. E.S., L.B., X.C., T.M., S.D., D.H., E.R., E.W., A.K., and C.A. conducted all experiments.
Disclosure : The authors declare no conflict of interest.
References
- 1.Ford ML, Adams AB, Pearson TC. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat Rev Nephrol. 2014;10(1):14. doi: 10.1038/nrneph.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams AB, Ford ML, Larsen CP. Costimulation Blockade in Autoimmunity and Transplantation: The CD28 Pathway. J Immunol. 2016;197(6):2045. doi: 10.4049/jimmunol.1601135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malvezzi P, Jouve T, Rostaing L. Costimulation Blockade in Kidney Transplantation: An Update. Transplantation. 2016;100(11):2315. doi: 10.1097/TP.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oshima S, Karrer EE, Kawato Y, et al. The Effect of ASP2409, a Novel CD86-Selective Variant of CTLA4-Ig, on Renal Allograft Rejection in Nonhuman Primates. Transplantation. 2016;100(12):2611. doi: 10.1097/TP.0000000000001397. [DOI] [PubMed] [Google Scholar]
- 5.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. 2016;374(4):333. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 6.Rigby MR, Trexler AM, Pearson TC, Larsen CP. CD28/CD154 blockade prevents autoimmune diabetes by inducing nondeletional tolerance after effector t-cell inhibition and regulatory T-cell expansion. Diabetes. 2008;57(10):2672. doi: 10.2337/db07-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381(6581):434. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 8.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94(16):8789. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang T, Pierson RN, 3rd, Azimzadeh AM. Update on CD40 and CD154 blockade in transplant models. Immunotherapy. 2015;7(8):899. doi: 10.2217/IMT.15.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Hu M, Qian YW, et al. In Vivo Costimulation Blockade-Induced Regulatory T Cells Demonstrate Dominant and Specific Tolerance to Porcine Islet Xenografts. Transplantation. 2017;101(7):1587. doi: 10.1097/TP.0000000000001482. [DOI] [PubMed] [Google Scholar]
- 11.Vasu C, Prabhakar BS, Holterman MJ. Targeted CTLA-4 engagement induces CD4+CD25+CTLA-4high T regulatory cells with target (allo)antigen specificity. J Immunol. 2004;173(4):2866. doi: 10.4049/jimmunol.173.4.2866. [DOI] [PubMed] [Google Scholar]
- 12.Fife BT, Griffin MD, Abbas AK, Locksley RM, Bluestone JA. Inhibition of T cell activation and autoimmune diabetes using a B cell surface-linked CTLA-4 agonist. J Clin Invest. 2006;116(8):2252. doi: 10.1172/JCI27856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng XX, Markees TG, Hancock WW, et al. CTLA4 signals are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J Immunol. 1999;162(8):4983. [PubMed] [Google Scholar]
- 14.Zhang T, Fresnay S, Welty E, et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant. 2011;11(8):1599. doi: 10.1111/j.1600-6143.2011.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 16.Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187(3):427. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5(3):443. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 18.Haanstra KG, Ringers J, Sick EA, et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003;75(5):637. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- 19.Lin H, Rathmell JC, Gray GS, Thompson CB, Leiden JM, Alegre ML. Cytotoxic T lymphocyte antigen 4 (CTLA4) blockade accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA4 can function independently of CD28. J Exp Med. 1998;188(1):199. doi: 10.1084/jem.188.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poirier N, Azimzadeh AM, Zhang T, et al. Inducing CTLA-4 dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Science Translational Medicine. 2010;2(17):17ra10. doi: 10.1126/scitranslmed.3000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierson RN, 3rd, Chang AC, Blum MG, et al. Prolongation of primate cardiac allograft survival by treatment with ANTI-CD40 ligand (CD154) antibody. Transplantation. 1999;68(11):1800. doi: 10.1097/00007890-199912150-00026. [DOI] [PubMed] [Google Scholar]
- 22.O’Neill NA, Zhang T, Braileanu G, et al. Comparative Evaluation of alphaCD40 (2C10R4) and alphaCD154 (5C8H1 and IDEC-131) in a Nonhuman Primate Cardiac Allotransplant Model. Transplantation. 2017;101(9):2038. doi: 10.1097/TP.0000000000001836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Kelishadi SS, Azimzadeh AM, Zhang T, et al. Preemptive CD20+ B cell depletion attenuates cardiac allograft vasculopathy in cyclosporine-treated monkeys. J Clin Invest. 2010;120(4):1275. doi: 10.1172/JCI41861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azimzadeh AM, Pfeiffer S, Wu G, et al. Alloimmunity in primate heart recipients with CD154 blockade: evidence for alternative costimulation mechanisms. Transplantation. 2006;81(2):255. doi: 10.1097/01.tp.0000190099.62847.e6. [DOI] [PubMed] [Google Scholar]
- 26.Geiss GK, Bumgarner RE, Birditt B, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26(3):317. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 27.Li R, Perez N, Karumuthil-Melethil S, Prabhakar BS, Holterman MJ, Vasu C. Enhanced engagement of CTLA-4 induces antigen-specific CD4+CD25+Foxp3+ and CD4+CD25− TGF-beta 1+ adaptive regulatory T cells. J Immunol. 2007;179(8):5191. doi: 10.4049/jimmunol.179.8.5191. [DOI] [PubMed] [Google Scholar]
- 28.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 31.von Rossum A, Rey K, Enns W, et al. Graft-Derived IL-6 Amplifies Proliferation and Survival of Effector T Cells That Drive Alloimmune-Mediated Vascular Rejection. Transplantation. 2016;100(11):2332. doi: 10.1097/TP.0000000000001227. [DOI] [PubMed] [Google Scholar]
- 32.Roll P, Muhammad K, Schumann M, et al. In vivo effects of the anti-interleukin-6 receptor inhibitor tocilizumab on the B cell compartment. Arthritis Rheum. 2011;63(5):1255. doi: 10.1002/art.30242. [DOI] [PubMed] [Google Scholar]
- 33.Ozkaynak E, Gao W, Shemmeri N, et al. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2(7):591. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


