Abstract
Background
Long-term publicly waitlisted bariatric surgery patients typically experience debilitating physical/psychosocial obesity-related comorbidities that profoundly affect their quality of life.
Objectives
We sought to measure quality-of-life impacts in a study population of severely obese patients who had multiyear waitlist times and then underwent bariatric surgery.
Methods
Participants were recruited opportunistically following a government-funded initiative to provide bariatric surgery to morbidly obese long-term waitlisted patients. Participants self-completed the EQ-5D-5L and AQoL-8D questionnaires pre- and postoperatively. Utility valuations (utilities) and individual/super dimension scores (AQoL-8D only) were generated.
Results
Participants’ (n = 23) waitlisted time was mean [standard deviation (SD)] 6.5 (2) years, body mass index reduced from 49.3 (9.35) kg/m2 preoperatively to 40.8 (7.01) 1 year postoperatively (p = 0.02). One year utilities revealed clinical improvements (both instruments). AQoL-8D improved significantly from baseline to 1 year, with the change twice that of the EQ-5D-5L [EQ-5D-5L: mean (SD) 0.70 (0.25) to 0.78 (0.25); AQoL-8D: 0.51 (0.24) to 0.67 (0.23), p = 0.04], despite the AQoL-8D’s narrower algorithmic range. EQ-5D-5L utility plateaued from 3 months to 1 year. AQoL-8D 1-year utility improvements were driven by Happiness/Coping/Self-worth (p < 0.05), and the Psychosocial super dimension score almost doubled at 1 year (p < 0.05). AQoL-8D revealed a wider dispersion of individual utilities.
Conclusions
Ongoing improvements in psychosocial parameters from 3 months to 1 year post-surgery accounted for improvements in overall utilities measured by the AQoL-8D that were not detected by EQ-5D-5L. Selection of a sensitive instrument is important to adequately assess changes in quality of life and to accurately reflect changes in quality-adjusted life-years for cost-utility analyses and resource allocation in a public healthcare resource-constrained environment.
Key Points for Decision Makers
| Psychosocial health status is an important health-related quality-of-life (HRQoL) outcome for long-waiting bariatric surgery patients. Whilst the EQ-5D is prevalent in the economic evaluation of bariatric surgery, compared with the EQ-5D-5L, the AQoL-8D preferentially captures and assesses psychosocial health for this study population. |
| If used in the clinical setting, utility valuations and individual and super dimension scores could provide both clinicians and healthcare decision-makers with important information regarding HRQoL impacts for people who have waited many years in the public health system for their bariatric surgery. |
| Long-waiting bariatric patients should not be ‘written-off’ by healthcare planners; they can still realise significant improvements in HRQoL outcomes when ultimately treated, and this should be factored into patient prioritisation decisions. |
Introduction
Obesity is a profoundly complex global public health, economic, and strategic policy problem [1–5]. Bariatric (obesity or metabolic) surgery is generally considered the most efficacious and cost-effective treatment intervention for people with intractable severe or morbid obesity, particularly for subgroups of patients such as people with type 2 diabetes [6–13]. Nonetheless, a recent comprehensive systematic review of 77 diverse health economics studies that reported on bariatric surgery from 1995 to 2015 found the EQ-5D is the prevalent multi-attribute utility instrument (MAUI) used in cost-utility analyses of bariatric surgery worldwide, and the impact of time delay for publicly waitlisted patients on the clinical, quality-of-life, and economic outcomes of bariatric surgery has been largely ignored [14].
As an important subgroup of bariatric surgery patients, long-term morbidly obese, publicly waitlisted, bariatric surgery patients generally experience increased physical and psychosocial comorbidity loads that ultimately translate to ‘sicker’ patients demanding proportionally more of the scarce healthcare dollar [14–17]. Recent qualitative evidence has indicated that waiting for bariatric surgery can lead to development of new or worsening obesity-related comorbidity or decline in mobility and be emotionally challenging (‘frustrating’, ‘depressing’, ‘stressful’) [18]. Additionally, the need to assess the psychosocial health status of bariatric patients in the short, medium, and longer terms has been increasingly identified [19] and recognised as crucial for bariatric surgery patients [20–24]. Moreover, the psychosocial health status of bariatric surgery patients is dynamic, some studies suggesting an improvement up to 4 years and declining thereafter [22, 24]. Other studies suggest that quality of life significantly improves up to 1 year and is maintained at 2 years [25]. Importantly, there is a paucity of quantitative evidence concerning the health-related quality-of-life (HRQoL) impacts for the group of long-waiting, public healthcare patients who then undergo bariatric surgery.
Within resource-constrained healthcare budgets, funders’ perceptions of ‘affordability’ are changing as bariatric surgery has increasingly become accepted as more than a cosmetic procedure for obesity and as the scale of the epidemic of severe obesity has become clearer [14]. Furthermore, the allocation of public-sector budgets is one part of a tremendously complex healthcare landscape that results in severely obese bariatric surgery candidates (with complex obesity-related comorbidities that translate to diminished HRQoL) experiencing multiyear wait times [14, 15]. A key reason for these multiyear wait times is the disproportionate rate of increase in severe obesity, and therefore the ever increasing demand for bariatric surgery surpassing the relatively static supply [17, 26].
Recent evidence has highlighted the differences and disagreements regarding the prioritisation of quality-of-life outcomes by health professionals and patients [27], revealing that patients prioritised seven quality of life items, none of which were prioritised by professionals. Surgeons prioritised only one quality-of-life outcome (versus four to 11 in the other health professionals’ subgroups, e.g. nurses and dieticians) [27]. These findings highlight the importance of individual, self-reported patient assessments of HRQoL in the bariatric surgery population.
Standardised outcomes reporting guidelines for metabolic and bariatric surgery were developed by the American Society of Metabolic and Bariatric Surgeons (ASMBS) to drive consistency of reporting clinical and HRQoL outcomes within the field [20, 21]. These guidelines acknowledge that whilst bariatric surgery produces marked weight loss and improvement of physical comorbidities, the impact on HRQoL is less well established [20, 21]. The guidelines did not provide specific recommendations regarding the reporting of health state utility values, also described as utility valuations or utilities [24].
Utility valuations are important health economic metrics that assess the strength of preference for an individual’s health state relative to perfect health and death, and importantly have inherent independent meaning [24, 28]. Utilities are assessed relative to a 0.00–1.00 scale, where 1.00 represents perfect health and 0.00 represents death, and therefore indicates the strength of preference for quality versus quantity of life [29]. Utilities are also a vital component of cost-utility analysis (a commonly used form of full economic evaluation that assesses the incremental costs of an intervention versus the incremental gains in quality-adjusted life-years) [12, 29, 30].
MAUIs are designed to rapidly and simply assess an individual or study population’s utility valuation(s) through the application of pre-established formulae/weights to the array of patient-reported responses to the instrument’s questions (generally self-reported through, for example, clinic visits, mail-outs, or the Internet) [24, 31, 32]. Based on patient-reported responses to MAUIs’ questionnaires, the algorithm of a given instrument generates utility valuations. Many instruments generate utilities that are less than zero described as a health state perceived to be worse than death (e.g. the most recent EQ-5D-5L UK value set range: −0.281 to 1.0 [33, 34]). Most instruments report minimal clinically important differences or minimal important differences for their utilities [35–37].
The EQ-5D-5L is an internationally prevalent MAUI used in the assessment of patient-reported quality-of-life outcomes and full economic evaluations of treatment interventions (including bariatric surgery) [14, 38]. Recent evidence has suggested that for the EQ-5D-3L (precursor to the 5L), a 1.0-unit decrease in body mass index (BMI) is associated with a 0.0051- to 0.0075-utility point increase. For a 1.0-unit decrease in BMI, the study reported a 0.0051 increase in utility when adjusted for baseline presence of comorbidity (stepwise approach); a 0.0052 increase in utility when adjusted for age, sex, and baseline BMI; a 0.0068 increase in utility when adjusted for age, sex, baseline BMI, and baseline comorbidity; and a 0.0075 increase in utility associated with the primary (baseline) analysis [39].
The AQoL-8D MAUI is informed by psychometric principles and testing and has been found to preferentially capture psychosocial health for people who had already undergone bariatric surgery many years previously in the private healthcare system {median [interquartile range (IQR)] 5 (3–8) years} [24, 40, 41]. This study also found that the AQoL-8D and EQ-5D-5L instruments were not interchangeable for the study population and that body weight is just one factor contributing to the complex HRQoL [24]. A recent study that investigated cross-sectional quality-of-life data using the Moorehead–Ardelt Quality of Life Questionnaire II also found that quality of life after bariatric surgery is not just dependent on weight loss [42]. Nevertheless, the Moorehead–Ardelt Quality of Life Questionnaire II is not an MAUI.
Another recent study suggested that clear preoperative predictive markers of well-defined postoperative success following bariatric surgery would be invaluable and facilitate a more refined and evidence-based mechanism by which to select patients for bariatric surgery [43]. The study found that it is important to explore the relationships between preoperative clinical parameters and HRQoL in those morbidly obese patients who are eligible for bariatric surgery, and that identifying those clinical and psychosocial predictors of success assumes great significance when selecting (or prioritising) patients for bariatric surgery [43]. A recent systematic review that investigated quality-of-life outcomes for bariatric surgery patients found that the SF-36 was the most commonly used HRQoL instrument in the review’s 13 included studies (control group was one of the inclusion criteria) [25]. Utility valuations were not generated in these studies [25]. Importantly, utility valuations have been shown to be independent predictors of patient outcomes in patients with type 2 diabetes, including all-cause mortality and development of complications [44]. Clinicians have also found that measuring utilities is of benefit to patient–clinical assessment, relationships, communication, and management [32].
Our study arose from a targeted State Government of Tasmania, Australia, policy decision to reduce Tasmanian public hospital surgical waiting lists. This initiative provided us with a novel and exploratory opportunity to recruit a cohort of morbidly obese, long-term waitlisted, bariatric surgery patients who then underwent bariatric surgery as a result of this policy initiative. This provided us with the opportunity to investigate an important and increasingly prevalent study population of bariatric surgery patients who inherently carry complex physical and psychosocial HRQoL needs. We aimed to investigate the physical and psychosocial HRQoL changes in these patients by using the EQ-5D-5L and AQoL-8D MAUIs to generate utility valuations (both instruments), and the AQoL-8D’s individual dimensional scores (namely, Independent Living, Senses, Pain, Happiness, Coping, Relationships, Self-worth, and Mental Health) and super dimensional scores (namely, the composite Physical super dimension of Independent Living, Senses, and Pain; and the composite Psychosocial super dimension of Happiness, Coping, Relationships, Self-worth, and Mental Health) preoperatively and at two postoperative time points (namely, 3 months and 1 year). We also aimed to explore the HRQoL benefits of bariatric surgery for long-term waitlisted patients and concomitance with BMI changes. We further aimed to investigate whether the MAUIs would reveal significant psychosocial HRQoL impacts at 1 year postoperatively. We also aimed to explore whether utility valuations and individual and super dimension scores could provide healthcare decision-makers with important information regarding HRQoL impacts for people who had waited many years in the public health system for their bariatric surgery.
Methods
Study Design
Recruitment of Participants
A Tasmanian government policy decision was made in 2014 to allocate additional and targeted public funds to provide morbidly obese, long-term waitlisted patients with bariatric surgery in 2015. The policy decision provided us with an opportunity to recruit bariatric surgery patients who had waited for their surgery in a public healthcare system for many years. Appropriate ethics approvals were obtained from our University’s Health and Medical Human Research Ethics Committee before commencement of our study’s recruitment of participants.
We subsequently invited patients who were identified for bariatric surgery to participate in our study. Participants were provided an information package and consent materials before their bariatric surgery pre-admission clinic. The process for participants’ questionnaire completion after consenting to participate in the study is outlined in Sect. 2.1.2.
Participants who consented to participate in our quality-of-life study underwent publicly funded laparoscopic adjustable gastric banding (LAGB) surgery by the same surgeon in the Hobart Private Hospital. Laparoscopic banding was carried out using Apollo APS or APL bands, with adjustment ports attached to the left anterior rectus sheath [45]. Postoperative fluid diets were maintained for 3 weeks, with subsequent transition to normal foods, accompanied by instruction on eating technique and exercise.
The Multi-attribute Utility Instruments and Questionnaire Completion
Our earlier study comparing the EQ-5D-5L and AQoL-8D MAUIs for people who had undergone LAGB surgery many years previously provided a detailed summary of the divergent characteristics of the two purposively selected MAUIs [24]. Table 1 provides an overview of these characteristics. In summary, the EQ-5D-5L is an internationally prevalent instrument (e.g. from 2005 to 2010, the EQ-5D was used in 63% of economic evaluations) [38]; the EQ-5D instrument is prevalent in the full economic evaluation of bariatric surgery [14]; it describes 3125 health states (compared with 243 health states of the EQ-5D-3L precursor to the 5L); four of the five instrument’s health domains/classifications focus on physical HRQoL; and it takes less than 1 min to complete the EQ-5D-5L’s questionnaire. The EQ-5D-5L also contains a visual analogue scale (EQ-VAS). In contrast, the AQoL-8D’s classification system is supported by psychometric principles and testing, and 25 of the instrument’s 35 items capture and assess five (from eight) psychosocial domains of health (Happiness, Coping, Self-worth, Relationships, and Mental Health). The AQoL-8D describes billions of health states and takes 5 min to complete [40, 41].
Table 1.
Comparison of the dimensions of the EQ-5D-5L and AQoL-8D multi-attribute utility instruments
| Characteristics | EQ-5D-5L | AQoL-8D |
|---|---|---|
| Number of health states described | 3125 | 2.4 × 1023 |
| Total number of dimensions | Five dimensions, 1 item in each. Each item has 5 levels of severity scored as 1 (best) to 5 (worst) | Eight dimensions of between 3 and 8 items; 35 items in total. 25 of the 35 items capture and assess psychosocial domains of health. |
| Number of dimensions of physical health | Four dimensions: mobility, self-care, usual activities, and pain/discomfort | Three dimensions: (1) Independent Living, 4 items (household tasks, getting around, mobility, self-care); (2) Senses, 3 items (vision, hearing, communication); and (3) Pain, 3 items (frequency of pain, degree of pain, pain interference) |
| Number of dimensions of psychosocial health | One dimension: anxiety/depression with five levels of severity: (1) I am not anxious or depressed (2) I am slightly anxious or depressed (3) I am moderately anxious or depressed (4) I am severely anxious or depressed (5) I am extremely anxious or depressed |
Five dimensions: (4) Happiness, 4 items (contentment, enthusiasm, degree of feeling happiness, pleasure); (5) Coping, 3 items (energy, being in control, coping with problems); (6) Relationships, 7 items (relationship with family and friends, social isolation, social exclusion, intimate relationship, family role and community role); (7) Self-worth, 3 items (feeling like a burden, worthlessness, confidence); (8) Mental Health, 8 items (feelings of depression, trouble sleeping, feelings of anger, self-harm, feeling despair, worry, sadness, tranquillity/agitation) |
| Super dimensions of physical and psychosocial health | No super dimensions | Two super dimensions: Physical super dimension (PSD) and Psychosocial super dimension (MSD). PSD includes independent living, senses, and pain; MSD includes happiness, coping, relationships, self-worth, and mental health |
Participants were asked to self-complete both instruments’ questionnaires before their bariatric surgery at the pre-admission preoperative clinics and at two postoperative reportable time points, namely 3 months and 1 year after their bariatric surgery. Postoperative questionnaires were mailed out for self-completion with an explanatory cover letter and reply paid envelope enclosed. We evaluated the EQ-5D-5L and AQoL-8D questionnaire completion by assessing the overall proportion of participants who completed the questionnaire(s) at the study’s three time points for whom an individual utility value could be generated (outlined in Sect. 2.2).
Data Analysis
Participants with patient-reported HRQoL assessments for one or both instruments, for at least one time point where the MAUI algorithm (either instrument) could generate the instrument’s utility valuations or scores were included in the analyses (Table 2).
Table 2.
Comparison of study participants’ (total participants, n = 23) BMI, summary health state utility valuations for the EQ-5D-5L and the AQoL-8D, and EQ-VAS scores before and 3 months and 1 year after bariatric surgery, and sensitivity analyses for full completers (n = 9)
| Before surgery | 3 months after surgery | 1 year after surgery | Change in mean from 3 months to 1 year after surgery and ToS** (p ≤ 0.05) | Change in mean from before surgery to 1 year after surgery and ToS** (p ≤ 0.05) | |
|---|---|---|---|---|---|
| Years on public waiting list, mean (SD) | 6.5 (2.0)† | ||||
| BMI (kg/m2) Mean (SD) |
(n = 21) 49.3 (9.3)* |
(n = 21) 43.5 (7.2) |
(n = 22) 40.8 (7.0) |
−2.7 BMI points, p = 0.40 | −8.5 BMI points, p = 0.02** |
| %TWL, mean (SD) | NA | NA | 16% (7.1) | NA | 16% |
| %EWL, mean (SD) | NA | NA | 34% (14.9) | NA | 34% |
| MAUIs’ HSUVs and EQ-VAS scores (n = x) | |||||
| EQ-5D-5L | (n = 16) | (n = 19) | (n = 18) | ||
| Mean (SD) | 0.70 (0.25) | 0.80 (0.25) | 0.78 (0.25) | −0.02 utility points, p = 0.92 | +0.08 utility points, p = 0.25 |
| Median (IQR) | 0.73 (0.54–0.91) | 0.84 (0.59–0.86) | 0.86 (0.67–0.93) | ||
| AQoL-8D | (n = 15) | (n = 18) | (n = 17) | ||
| Mean (SD) | 0.51 (0.24) | 0.61 (0.24) | 0.67 (0.23) | +0.06 utility points, p = 0.66 | +0.16 utility points, p = 0.04** |
| Median (IQR) | 0.51 (0.29–0.78) | 0.58 (0.43–0.78) | 0.67 (0.48–0.86) | ||
| EQ-VAS | (n = 16) | (n = 19) | (n = 18) | ||
| Mean (SD) | 57 (25) | 67 (24) | 73 (19) | +6 points, p = 0.31 | +16 VAS score, p = 0.08 |
| Median (IQR) | 65 (34–73) | 65 (48–90) | 80 (56–90) | ||
| Subgroup analysis* (n = 9) | |||||
| BMI (kg/m2), mean (SD) | 47.6 (7.4) | 43.6 (6.1) | 39.6 (6.4) | 4.0 BMI points | −8.0 BMI points |
| %TWL, mean (SD) | NA | NA | 16.6% (7.3) | NA | 16.6% |
| %EWL, mean (SD) | NA | NA | 36.3% (15.8) | NA | 36.3% |
| EQ-5D-5L utility, mean (SD) | 0.69 (0.21) | 0.80 (0.15) | 0.73 (0.20) | −0.07 utility points, p = 0.52 | +0.04 utility points, p = 0.26 |
| AQoL-8D utility, mean (SD) | 0.45 (0.19) | 0.57 (0.21) | 0.60 (0.22) | +0.03 utility points, p = 0.07 | +0.15 utility points, p = 0.01** |
| EQ-VAS, mean (SD) | 59 (22) | 66 (22) | 67 (21) | +1 VAS score | + 8 VAS score, p = 0.18 |
BMI body mass index, EWL excess weight lost, HSUV health state utility valuation, IQR interquartile range, MAUI multi-attribute utility instrument, NA not applicable, SD standard deviation, ToS test of significance, TWL total weight lost, VAS visual analogue scale
† One long-term waitlisted patient's time on the waiting list not available
* Full-completers subgroup analysis before and 3 months and 1 year after bariatric surgery
** ToS Wilcoxon rank test (p ≤ 0.05)
Baseline socio-demographic and clinical data were analysed descriptively as mean [standard deviation (SD)] for continuous variables and frequency (%) for categorical variables. BMI was calculated as weight (kg)/[height (m)]2. Percentage total weight loss was calculated as weight loss (kg)/initial weight (kg) × 100, and percentage excess weight loss was calculated as total weight loss/{initial weight − [25 × height (m)2]} × 100. Height and weight data were collected from medical records at the study’s three time points.
Utility valuations were generated for the EQ-5D-5L using the most recent UK value based on directly elicited preferences [33, 34] (range −0.281 to 1.00 utility points) and for the AQoL-8D using the most recent Australian scoring algorithm available on the AQoL group’s website (http://www.aqol.com.au) (range +0.09 to 1.0 utility points). Summary statistics of both MAUIs’ utility valuations and EQ-VAS were assessed as mean (SD) and median (IQR), and for individual and super dimension scores (AQoL-8D), they were assessed as mean (SD).
A minimal clinically important difference or minimal important difference is the smallest difference in score in the outcome of interest which patients perceive as beneficial and which would mandate a change in the patient’s management [46, 47]. A recently reported composite minimal important difference for the EQ-5D-5L of selected chronic health conditions including hypertension, heart disease, arthritis, asthma or chronic obstructive pulmonary disease, cancer, diabetes, chronic back pain, and anxiety or depression has been calculated as 0.04 utility points [35]. We adopted this recent EQ-5D-5L composite measure for our study because of the array of complex physical and psychosocial health conditions included in the measure of minimal important difference. There is no reported minimal important difference for the AQoL-8D; however, there is a reported minimal important difference for the AQoL-4D. This is a composite measure that also includes chronic health conditions [37]. We therefore conservatively reported a minimal important difference for the AQoL-8D as the upper bound of the confidence interval (CI) of the AQoL-4D’s minimal important difference (95% CI 0.03–0.08), namely, 0.08 points [37].
AQoL-8D Australian population norms for the total population and the 45- to 54-year-old age group were sourced from recently derived and published norms for the instrument [48].
Given that the MAUI-generated data are not normally distributed and also the relatively small sample size, this study employed the Wilcoxon signed rank test to test for statistical significance at the 5% level (p ≤ 0.05). The Wilcoxon signed rank test for significance is the non-parametric counterpart of the paired t test, and corresponds to a test of whether the median of the differences between paired observations is zero in the population from which the sample is drawn [49].
We undertook sensitivity analyses on the subgroup of individuals who fully completed both MAUIs’ questionnaires for all three reported time points (called ‘full-completers’) to test the robustness of all results including significance testing.
Statistical analyses were undertaken using IBM SPPS (version 22) or R (version 3.0.2).
Results
Participants’ Clinical and Socio-demographic Characteristics and Questionnaire Completion
Twenty-three participants were recruited to the study and completed at least one of the MAUIs’ questionnaires at one of the reportable time points to enable the generation of utility valuations (both instruments) and individual and super dimension scores (AQoL-8D only) (Table 2).
For these participants (n = 23), mean (SD) age was 50 (10) years, 43% were males, and mean (SD) time on the public waiting list for bariatric surgery was 6.5 (2.0) years. Table 2 (supported by Appendix) also provides results regarding changes in BMI, percentage total weight lost, and percentage excess weight lost. At 1 year postoperatively, the percentage of total weight lost was mean (SD) 16% (7.1%). BMI decreased from mean (SD) 49.3 (9.3) kg/m2 before surgery to 43.5 (7.2) (3 months) to 40.8 (7.0) (1 year) after surgery, giving rise to a significant reduction of 8.5 BMI units preoperatively to 1 year postoperatively (p = 0.02).
Appendix provides the socio-demographic characteristics of all participants (n = 23), the subgroup of full-completers of both questionnaires at all three reportable time points (n = 9), and the subgroup of participants who did not fully complete all questionnaires at the three reportable time points (n = 14). There was no substantial difference in age or sex [all participants (n = 23) males 43%; full-completers (n = 9) males 44%; partial-completers (n = 14) males 42%]. The order of magnitude for the number of years on the public waiting list was also similar. The postoperative obesity classifications and mean (SD) BMI measures were similar between the subgroups, and the magnitudes of change between obesity classifications were also similar between the subgroups.
Questionnaire completion for the entire cohort across the three reported time points is outlined in detail in Table 2. Overall, utility valuations could be assessed for 75% of participants for both MAUIs, and for individual and super dimension scores for the AQoL-8D only. Subgroup analyses were conducted for full-completers of both instruments’ questionnaires across all three time points (n = 9) (outlined below and Tables 2 and 3).
Table 3.
Comparison of AQoL-8D individual and super dimension scores before and 3 months and 1 year after surgery (total participants, n = 23), Australian population norms, and subgroup (sensitivity) analysis
| Before surgery (n = 15), mean (SD) | 3 months after surgery (n = 18), mean (SD) | 1 year after surgery (n = 17), mean (SD) | Improvement in mean score preoperatively to 3 months and 1 year postoperatively (change) | Australian population norms | Subgroup (sensitivity) analysis (n = 9) |
||||
|---|---|---|---|---|---|---|---|---|---|
| 45- to 54-year-old age group, mean (SD) | Total, mean (SD) | Before surgery, mean (SD) | 1 year after surgery, mean (SD) | Test of significance* (p ≤ 0.05) |
|||||
| AQoL-8D individual and super dimensions | |||||||||
| Individual dimensions of physical health | |||||||||
| Independent Living | 0.69 (0.22) | 0.75 (0.19) | 0.79 (0.20) | +0.06; 0.10 | 0.93 (0.12) | 0.94 (0.11) | 0.65 (0.20) | 0.73 (0.21) | p = 0.14 |
| Senses | 0.81 (0.13) | 0.83 (0.13) | 0.84 (0.11) | +0.02; 0.03 | 0.88 (0.10) | 0.91 (0.10) | 0.81 (0.14) | 0.86 (0.12) | p = 0.29 |
| Pain | 0.56 (0.34) | 0.62 (0.32) | 0.67 (0.31) | +0.06; 0.11 | 0.84 (0.21) | 0.86 (0.19) | 0.51 (0.31) | 0.61 (0.30) | p = 0.22 |
| Individual dimensions of psychosocial health | |||||||||
| Happiness | 0.65 (0.16) | 0.75 (0.15) | 0.77 (0.13) | +0.10; 0.12 | 0.77 (0.16) | 0.80 (0.15) | 0.61 (0.16) | 0.76 (0.11) | p = 0.01** |
| Coping | 0.67 (0.15) | 0.76 (0.15) | 0.79 (0.12) | +0.09; 0.12 | 0.80 (0.16) | 0.83 (0.15) | 0.62 (0.10) | 0.78 (0.09) | p = 0.01** |
| Relationships | 0.62 (0.16) | 0.67 (0.18) | 0.71 (0.18) | +0.05; 0.09 | 0.78 (0.16) | 0.79 (0.16) | 0.59 (0.17) | 0.66 (0.16) | p = 0.08 |
| Self-worth | 0.65 (0.21) | 0.76 (0.18) | 0.75 (0.19) | +0.11; 0.10 | 0.84 (0.16) | 0.85 (0.15) | 0.73 (0.19) | 0.73 (0.18) | p = 0.03** |
| Mental Health | 0.54 (0.12) | 0.60 (0.15) | 0.62 (0.19) | +0.06; 0.08 | 0.67 (0.17) | 0.69 (0.17) | 0.53 (0.09) | 0.59 (0.18) | p = 0.25 |
| Super dimensions | |||||||||
| Physical super dimension | 0.51 (0.29) | 0.56 (0.27) | 0.62 (0.26) | +0.05; 0.11 | 0.79 (0.20) | 0.83 (0.18) | 0.46 (0.27) | 0.55 (0.24) | p = 0.13 |
| Psychosocial super dimension | 0.25 (0.15) | 0.37 (0.25) | 0.41 (0.25) | +0.12; 0.16 | 0.47 (0.24) | 0.50 (0.24) | 0.20 (0.11) | 0.34 (0.23) | p = 0.008** |
| HSUV | 0.51 (0.24) | 0.61 (0.24) | 0.67 (0.23) | +0.10; 0.16 | 0.77 (0.20) | 0.80 (0.19) | 0.45 (0.19) | 0.60 (0.22) | p = 0.01** |
HSUV health state utility valuation, SD standard deviation
* Wilcoxon signed rank test significant at p ≤ 0.05
** Significant result at (p < 0.05)
Changes in Both Instruments’ Utility Valuations Compared to BMI
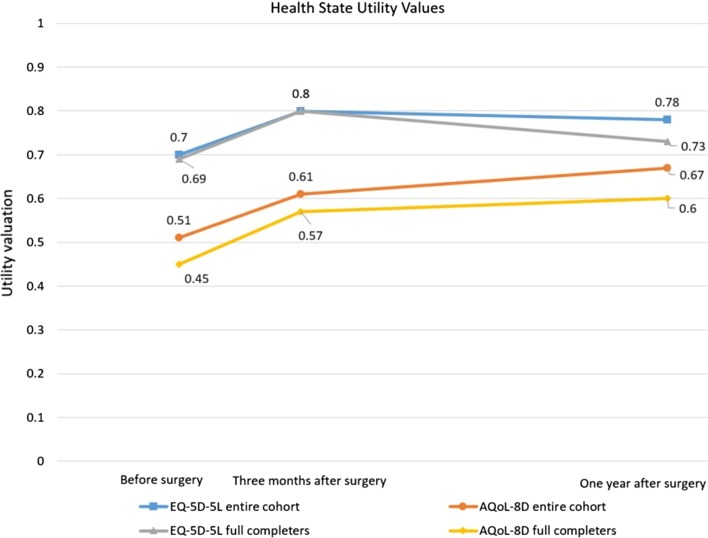
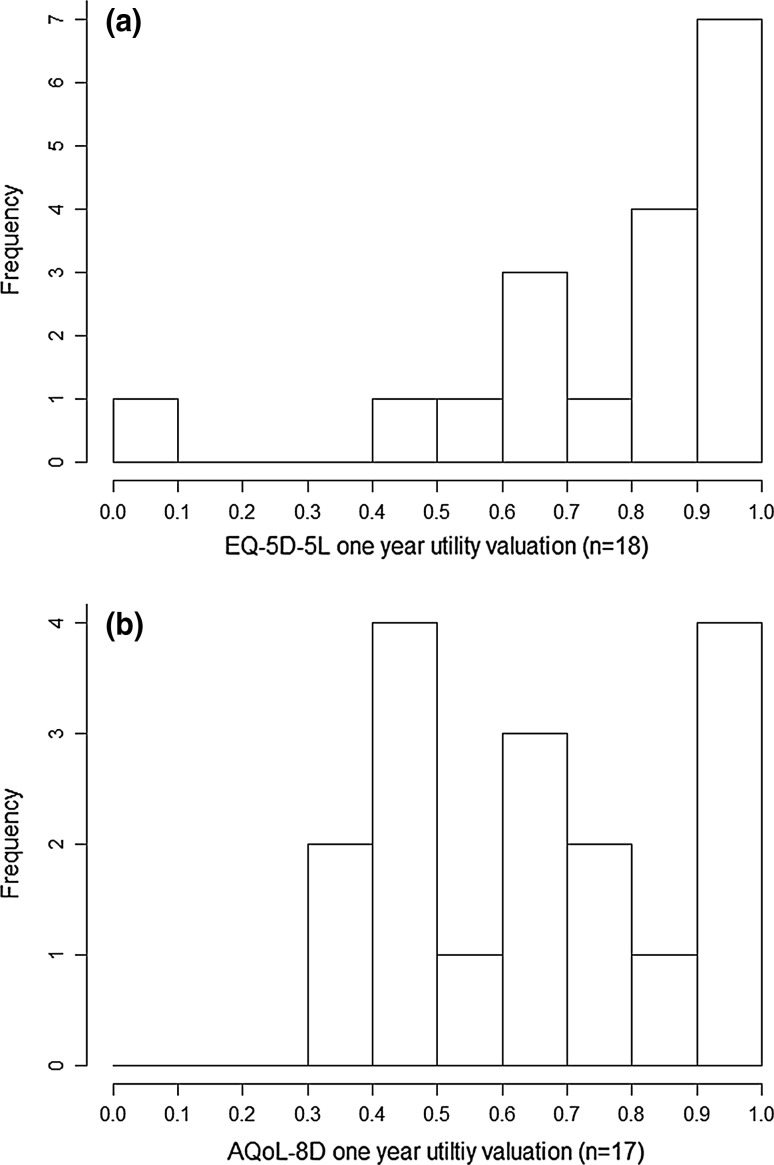
Table 2 provides summary results for changes in BMI, utility valuations (both instruments), and EQ-VAS scores at the three reported time points. Figure 1 also provides a schematic representation of utility changes for the entire cohort (n = 23) and full-completers (n = 9). Figure 2 provides the distribution of utility valuations at the individual level for both instruments (Fig. 2a EQ-5D-5L and Fig. 2b AQoL-8D) 1 year after surgery.
Fig. 1.
Comparison of the EQ-5D-5L and AQoL-8D health state utility valuations before surgery and 3 months and 1 year after surgery
Fig. 2.
Frequency distributions of utility valuations at the individual level for the EQ-5D-5L (n = 18) (a) and AQoL-8D (n = 17) (b) for the entire cohort 1 year after bariatric surgery
Our study’s key finding was that change in both instruments’ summary utility valuations and also the EQ-VAS scores reported clinical improvements that exceeded the minimal important difference for all participants (n = 23) (EQ-5D-5L 0.08 utility points; AQoL-8D 0.16 utility points; EQ-VAS 16 points) from before surgery to 1 year after surgery. Importantly, the change in utility valuations derived for the AQoL-8D was twice that for the EQ-5D-5L (0.16 vs. 0.08 utility points) for the 1-year time horizon. Further, the AQoL-8D utility change from baseline to 1 year was statistically significant (p = 0.04), whereas only a trend was observed for the EQ-5D-5L (p = 0.25) (Table 2; Fig. 1). When we compared preoperative versus 1-year postoperative utility increases to BMI reductions over the same time horizon, we found that for every 1.0-unit reduction in BMI, the AQoL-8D utility valuation increased 0.02 units, compared with a 0.01 increase in utility for the EQ-5D-5L.
Another important finding was that from 3 months to 1 year postoperatively, the mean EQ-5D-5L utility valuation showed a slight decrease, by 0.02 utility points, whereas the mean AQoL-8D utility valuation gave rise to the third of the identified increases in utility for this instrument across the three time points (+0.06 utility points). An increase was also observed in the EQ-VAS scores from 3 months to 1 year. Notwithstanding these general trends, all changes from the reported 3 months to 1 year time point were not statistically significant (Table 2).
After surgery utility valuations at the individual level for both instruments were not normally distributed and the AQoL-8D revealed a wider dispersion (Fig. 2).
Subgroup analyses revealed that the orders of magnitudes, general trends, and significance testing of all our findings were robust when only the full-completers (n = 9) were analysed (Tables 2, 3). For example, from before surgery to 1 year after surgery, we found that the AQoL-8D’s improvement in utility score 1 year after surgery was 0.15 points and the BMI reduction was 8.0 BMI units as compared with 0.16 utility points and a BMI reduction of 8.5 BMI units for the entire cohort (Tables 2, 3).
Assessment of Individual Domains of HRQoL: AQoL-8D Individual and Super Dimension Scores
Table 3 provides a comparison of the AQoL-8D’s individual dimensions (Independent Living, Senses, Pain, Happiness, Coping, Self-worth, Relationships, and Mental Health) and Physical and Psychosocial super dimensions for the three reported time points, and subgroup analyses and significance testing for the full-completers subgroup from before surgery to 1 year after surgery.
A key finding for our particular study population of long-term waitlisted patients a year after bariatric surgery was that all individual and super dimension scores within the AQoL-8D improved. The individual psychosocial dimensions of Happiness, Coping, and Self-worth improved the most over this time horizon (0.12, 0.12, and 0.10 point improvements, respectively). The individual physical dimensions of Independent Living and Pain also improved (0.10 and 0.11 points, respectively). Additionally, Happiness and Coping approached general population norms [48]. These results were robust to subgroup analysis of full-completers of all three questionnaires (Table 3). Importantly, significance testing of the full-completers’ results revealed that Happiness (p = 0.01), Coping (p = 0.01), Self-worth (p = 0.03), and the Psychosocial super dimension (p = 0.008), and the summary AQoL-8D utility valuation (p = 0.01) were statistically significant (Table 3).
Discussion
Our exploratory study is the first study to investigate the HRQoL impacts using both the EQ-5D-5L and AQoL-8D for a study population of severely obese, long-term publicly waitlisted patients who were then able to access bariatric surgery in 2015 because of a 2014 State Government public policy decision to reduce waiting times and to surgically treat long-waiting patients.
We found that the participants’ responses to the EQ-5D-5L and AQoL-8D generated clinical improvements in utility valuations and EQ-VAS scores from before surgery to 1 year after surgery, where the minimal important differences were exceeded. Another important finding was that the AQoL-8D’s increase in utility valuation (0.16 utility points) was twice that of the EQ-5D-5L increase (0.08 utility points) at 1 year, with the AQoL-8D result statistically significant (and robust to subgroup analyses of the full-completers).
Public Resource Allocation to Bariatric Surgery: Waiting Lists and Patient Prioritisation
Our key findings highlighted two important and inextricably linked points regarding the assessment and utilisation of utility valuations for long-term waitlisted patients who subsequently undergo bariatric surgery. First, choice of an appropriate MAUI to preferentially capture and assess HRQoL for this study population is crucial. Second, suboptimal public resource allocation decisions regarding the ‘optimal’ amount of bariatric surgery will likely occur if utility valuations, as an input measure of health impact for health economic evaluation (specifically cost-utility analyses), are generated by an instrument that is not sensitive to this study population’s complex HRQoL.
Health economic evaluation is an important resource allocation methodology because it provides decision-makers with comparable analyses to underpin decisions about committing scarce healthcare resources to one use instead of another [14]. Cost-utility analyses of bariatric surgery to date have been dominated by use of the EQ-5D MAUIs [14]. Economic evaluation of interventions which affect HRQoL commonly employ cost-utility analyses which prioritise interventions according to the cost per quality-adjusted life-year. The estimation of quality-adjusted life-years is increasingly based upon the utility valuations predicted from an MAUI [50]. One of our study’s key findings was that the AQoL-8D’s utility changes/impacts from before surgery to 1 year after surgery were twice the magnitude of the EQ-5D-5L. Additionally, the EQ-5D-5L reported a plateauing utility valuation from 3 months to 1 year, in contrast to the AQoL-8D, which revealed a clinical improvement. If the nominated instrument lacks sensitivity within a particular health context (or health domain), interventions (such as bariatric surgery) affecting health states where the chosen instrument's sensitivity is low, will likely be disadvantaged [24, 50].
A recent study that investigated EQ-5D-5L utility valuations for patients who had undergone surgery at a Canadian Bariatric Centre for Excellence (n = 304 before surgery, n = 138 after surgery, 45% completion rate after surgery) found that mean utility valuation before and 1 year after surgery was 0.65 (before)/0.90 (after) utility points (for ‘other’ bariatric surgery) and 0.70 (before)/0.90 (after) utility points (for Roux-en-Y bariatric surgery) [51]. These results are similar to the order of magnitude of our exploratory study’s EQ-5D-5L preoperative results. We note that the higher postoperative valuation for the Canadian study could be explained by the low completion rate, arguably of patients who would rate themselves closer to perfect health (45% of patients only responding 1 year postoperatively), and the EQ-5D-5L’s inability to detect health impacts closer to perfect health (ceiling effects).
In contrast, our study’s AQoL-8D preoperative summary utility valuations of mean (SD) 0.51 (0.24) indicated a significantly diminished HRQoL for our study population before surgery that was also reflected in the AQoL-8D’s individual and super dimension scores. In turn, the AQoL-8D’s ability to preferentially capture HRQoL (compared with the EQ-5D-5L) for our study population of long-term waitlisted patients who then subsequently underwent bariatric surgery is reflected in the reduced utility valuations. One of the key findings of our earlier research that conducted a head-to-head comparison of the two instruments was that the EQ-5D-5L and AQoL-8D are not interchangeable for people who had undergone bariatric surgery many years previously [24]. This study of long-term waitlisted patients also suggests that the AQoL-8D preferentially captures HRQoL and that the two instruments are not interchangeable for long-term waitlisted patients who subsequently undergo bariatric surgery.
Recent evidence has found that utility valuations measured by the major MAUIs differ [namely, the EQ-5D-5L, SF-6D, Health Utilities Index (HUI) 3, 15D and AQoL-8D] [50]. Most of these differences can be explained by the descriptive/classification systems of the MAUIs. These ‘dominating’ differences are estimated to explain an average of 66% of the difference between utilities obtained by the MAUIs (i.e. EQ-5D-5L, SF-6D, HUI 3, 15D, and AQoL-8D) and 81% of the difference between the utilities of the EQ-5D-5L and AQoL-8D [50]. In turn, our study’s findings reflect the relative sensitivities of the EQ-5D-5L’s and AQoL-8D’s classification systems to our study population’s physical and psychosocial domains of health. The AQoL-8D’s changes in utility valuation were predominantly driven by the AQoL-8D’s individual psychosocial dimensions and Psychosocial super dimension scores.
The AQoL-8D’s utility valuations differed significantly from before surgery to 1 year after surgery, predominantly driven by the AQoL-8D’s individual psychosocial and Psychosocial super dimension scores. Cost-utility analyses of the health impacts for long-term waitlisted patients who subsequently undergo bariatric surgery should appropriately reflect these health impacts. Our findings are particularly important because cost-utility analyses of bariatric surgery are dominated by the EQ-5D MAUIs [14].
In summary, long-term publicly waitlisted patients are an important and emerging subgroup of bariatric surgery patients, yet there is a paucity of evidence regarding longitudinal HRQoL impacts for this population if they are successful in getting publicly funded bariatric surgery. Our findings show that previously long-waiting patients with substantially diminished HRQoL did show significant improvements in HRQoL after surgery. This is important in that it shows clearly that long-waiting patients should not be ‘written off’—they can still realise significant improvements in HRQoL outcome when ultimately treated. A recent cost-utility study from Sweden, the first study to quantify the potential impact of extensive waiting times on the costs and clinical outcomes of bariatric surgery, highlighted the necessity of reducing waiting lists and removing unnecessary barriers to allow greater utilisation of surgery for patients unresponsive to conservative medical management [10]. Nevertheless, addressing this issue, given the large gap between the demand for and supply of publicly funded bariatric surgery, which has resulted in protracted wait times for the procedure in countries such as Australia, Canada, and the UK [17, 52] and the longest of any surgical procedure in Canada (average 5 years) [17], would require significant commitment and investment.
Weight Status is Only One Factor Contributing to Complex HRQoL for Long-Term Waitlisted Patients Who Undergo Bariatric Surgery
Another important finding of our study is that the AQoL-8D’s individual and super dimension scores identify psychosocial health as an important driver of holistic postoperative health 1 year after bariatric surgery. The AQoL-8D’s Psychosocial super dimension almost doubled in magnitude from before surgery to 1 year after surgery, and this change was statistically significant. This result is validated by a recent systematic review of the literature regarding the quality-of-life outcomes of bariatric surgery, where the SF-36 was the most commonly used HRQoL instrument and the quality-of-life subscale for mental health showed improvements in three of the six included SF-36 studies [25]. Notably, none of these studies generated utility valuations or scores. Our study’s AQoL-8D Psychosocial super dimension result is also validated by recent literature which suggests that psychosocial health status increases up to 4 years after bariatric surgery, but declines after this timeframe [22, 23].
Utility valuations have also been found to be independent predictors of health impacts [44]. Our study’s results also support our earlier findings that if the choice of MAUI appropriately captures the individual and study population’s physical and psychosocial health status through the sensitivity of the MAUI’s dimensions/classification system, then the MAUI’s predictive qualities could be a useful clinical measurement tool to rapidly and conveniently assess the intervention’s likely health impacts in individuals and for the study population [24].
Our study also found that relative to BMI unit reductions, the AQoL-8D recorded 0.02-utility point increases for 1.0-BMI unit reductions, and for the EQ-5D-5L, 0.01-utility point increases for 1.0-BMI unit reductions. A recent study found that for the EQ-5D-3L, for a 1.0-unit BMI reduction there was a 0.0051–0.0075 increase in utility. Notwithstanding the differing classification systems and utility valuations of the two MAUIs, the AQoL-8D recorded a greater utility increase per unit of BMI reduction. We contend that this difference was driven by the impact of psychosocial health—the AQoL-8D’s broader (depth and breadth) psychosocial classification system captured and assessed domains of health that are not ‘weight change’ or ‘BMI change’ related. Our findings are also supported by a recent cross-sectional study that compared quality of life measured by the Moorehead–Ardelt Quality of Life Questionnaire in obese patients 12–18 months after bariatric surgery that found there is a limited relationship between BMI and HRQoL [42].
In summary, we contend that the importance of psychosocial factors in driving the measured improvements in HRQoL should not be lost on policy-makers in allocating resources. Much recent debate on bariatric surgery has focused on the physical health impacts of weight loss, especially on its potential to avoid or mitigate the worst effects of diabetes. However, if much of the real health gain observed derives from psychosocial impacts, this may have important consequences for patient selection and prioritisation decisions.
Increased Mobility
We also found that the AQoL-8D’s individual physical dimensions of Independent Living and Pain improved from before surgery to 1 year after surgery. A recent study that conducted proportional analysis for the EQ-5D-5L has found that mobility significantly increases 1 year after bariatric surgery [51]. The increases in the AQoL-8D individual physical dimensions of health and the Physical super dimension further support these findings.
Only 10 of the 35 items of the AQoL-8D capture and assess the physical domains of health that inform the individual physical dimensions of Independent Living, Senses, and Pain. A recent study suggests that the AQoL-8D’s descriptive system is preferential to psychosocial health rather than physical health [53].
Supporting Qualitative Evidence
Some of our study’s participants participated in long interviews for an associated qualitative study regarding the support needs of patients waiting for publicly funded bariatric surgery [18]. The findings of this study indicated that waiting for bariatric surgery was commonly associated with a range of deleterious consequences including weight gain and deteriorating physical and psychosocial health [18]. These qualitative findings both support and provide further contextualisation and nuance to our study’s baseline AQoL-8D utility valuations and individual and Psychosocial and Physical super dimension scores that revealed substantially reduced summary utility valuations and scores that were well below the relative Australian population norms (Table 3). Our study has shown that our cohort’s HRQoL was substantially diminished before surgery, and this qualitative evidence also suggests it is likely that utility valuations and individual and super dimension scores could have been measurably lower for our unique cohort if long-waiting patients were left untreated.
Limitations
There are a number of limitations to our study. The first limitation is sample size. Nevertheless, our study was exploratory and we were provided with a novel opportunity to recruit participants from the long-term waitlisted patients subsequently fast-tracked for bariatric surgery through a government policy decision to reduce waiting lists. The second limitation is that all participants were operated on by the same surgeon in the same hospital. This could affect the generalisability of our results if scaled up to all bariatric surgery patients. The third limitation is that there is no control arm in the study. The observational nature of our study did not enable the recruitment of a control arm to elicit utility valuations. The fourth limitation is the use of the UK value set for the EQ-5D-5L because there is no Australian value set available for the instrument. The final limitation is that the sample is at risk of participant selection bias, which could also affect the generalisability of our results. Recent evidence has found that public sector waiting times are years in duration in some countries and that there are physical (worsening of comorbidities and further weight gain) and psychosocial impacts for patients waiting for bariatric surgery.
A strength of our study is the high response rate of 75% to the questionnaires across all three reportable time points. Additionally, our study is an exploratory study of long-term waitlisted patients and could inform larger confirmatory studies of HRQoL (particularly assessed through utilities derived from generic MAUIs) for long-term waitlisted patients who subsequently undergo bariatric surgery.
Conclusions
Our exploratory study of long-term waitlisted patients recruited opportunistically following a government policy decision to reduce waiting lists suggests that long-waiting bariatric surgery patients should not be ‘written off’ by healthcare planners; they can still realise significant improvements in HRQoL outcomes when ultimately treated, and this should be factored into patient prioritisation decisions. Addressing this issue given the large gap between the demand for and supply of publicly funded bariatric surgery in many countries would require significant commitment and investment.
Ongoing improvements in psychosocial parameters from 3 months to a year post-surgery explained improvements in overall utility valuation measured by the AQoL-8D that were not detected by EQ-5D-5L. Selection of a sensitive instrument is crucial to adequately measure changes in utility valuation and to accurately reflect changes in quality-adjusted life-years generated for cost-utility analyses. Cost-utility analyses for long-term waitlisted patients for bariatric surgery should employ utility valuations from MAUIs that are sensitive to physical and psychosocial health changes. Only through comprehensive assessments of HRQoL impacts before and after surgery can we robustly inform public resource allocation decisions. We found that the AQoL-8D preferentially captures these health impacts compared with the EQ-5D-5L.
Coupled with BMI assessment, pre-surgery utility valuations should be investigated as independent predictors of post-surgery HRQoL (particularly psychosocial health status) for morbidly obese, long-term waitlisted, bariatric surgery patients.
Appendix
Participants’ clinical and socio-demographic characteristics before and 1 year after surgery for the total fast-track cohort, the subgroup of participants who fully completed both MAUIs at all three time points, and the subgroup of participants who were not full-completers (n = 14)
| Characteristics | Fast-track cohort (n = 23) | Full-completers (n = 9) | Partial completers (n = 14) |
|---|---|---|---|
| Age years, mean (SD) | 50 (10) | 48 (11) | 52 (9) |
| Sex (n = x, %) | Male (10, 43%) Female (13, 57%) |
Male (4, 44%) Female (5, 56%) |
Male (6, 42%) Female (8, 58%) |
| Number of years on public waiting list, mean (SD) | 6.5 (2.0)** | 7.3 (2.5) | 6.1 (1.6) |
| Number of participants in obesity category (n = x, %) | |||
| Before surgery | |||
| BMI ≥ 30–34.9 kg/m2 (Class I) | (1, 4%) | 0 | (1, 7%) |
| BMI ≥ 35–39.9 kg/m2 (Class II) | 0 | 0 | 0 |
| BMI ≥ 40–49.9 kg/m2 (Class III) | (13, 57%) | (7, 78%) | (6, 43%) |
| BMI ≥ 50 kg/m2 * | (9, 39%) | (2, 11%) | (7, 50%) |
| 12 months after surgery | |||
| BMI ≥30–34.9 kg/m2 (Class I) | (2, 10%) | (2, 14%) | (3, 21%)† |
| BMI ≥35–39.9 kg/m2 (Class II) | (7, 33%) | (3, 21%) | (2, 14%) |
| BMI ≥40–49.9 kg/m2 (Class III) | (9, 43%) | (3, 21%) | (6, 43%) |
| BMI ≥50 kg/m2 | (3, 14%) | (1, 11%) | (2, 14%) |
| BMI (kg/m2) | |||
| Before surgery, mean (SD) | 49.3 (9.35) | 47.6 (7.4) | 49.9 (10.6) |
| After surgery, mean (SD) | 40.8 (7.0) | 39.6 (6.4) | 41.6 (7.5)† |
| % Total weight lost, mean (SD) | 16 (7.1) | NA | NA |
MAUI multi-attribute utility instrument, SD standard deviation, NA not applicable
* Super-obese [54]
** One long-term waitlisted patient's time on the waiting list not available
†One missing value
Authorship
Julie Campbell contributed to study design, data verification and analysis, and manuscript preparation and final approval. Martin Hensher contributed to study design and manuscript review and final approval. Amanda Neil contributed to study design and manuscript review and final approval. Alison Venn contributed to study design and manuscript review and final approval. Stephen Wilkinson contributed to study design and manuscript review and final approval. Andrew Palmer contributed to study design, data verification and analysis, and manuscript preparation and final approval.
Compliance with Ethical Standards
Ethical standards
The University of Tasmania’s Health and Medical Human Research Ethics Committee (HMHREC) approved this study, and informed consent was obtained from all participants in accordance with the HMHREC’s guidelines. This study was performed in accordance with the ethical standards of the Declaration of Helsinki.
Funding
This work was supported by a National Health and Medical Research Council (NHMRC) Partnership Project Grant (APP1076899). AV is supported by an NHMRC fellowship grant. AN is supported by a Select Foundation Research Fellowship.
Conflicts of interest
The authors Julie Campbell, Martin Hensher, Amanda Neil, Alison Venn, Stephen Wilkinson, and Andrew Palmer declare they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
The dataset used for this study contains the following: the participant-reported responses to the EQ-5D-5L and the AQoL-8D MAUIs’ questionnaires; the individual utility valuations (both instruments) and utility scores (AQoL-8D only) that were generated with the instruments’ specific algorithm; the participant-reported EQ-VAS scores; and participants’ socio-demographic and clinical data. The corresponding author will provide a de-identified dataset upon reasonable request for all or part of the data.
References
- 1.Cawley J. An economy of scales: a selective review of obesity’s economic causes, consequences, and solutions. J Health Econ. 2015;43:244–268. doi: 10.1016/j.jhealeco.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31(1):219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Gortmaker SL, Swinburn BA, Levy D, Carter R, Mabry PL, Finegood DT, et al. Changing the future of obesity: science, policy, and action. Lancet. 2011;378(9793):838–847. doi: 10.1016/S0140-6736(11)60815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke B, Swinburn B, Sacks G. The application of theories of the policy process to obesity prevention: a systematic review and meta-synthesis. BMC Public Health. 2016;16(1):1084. doi: 10.1186/s12889-016-3639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberto CA, Swinburn B, Hawkes C, Huang TT, Costa SA, Ashe M, et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet. 2015;385(9985):2400–2409. doi: 10.1016/S0140-6736(14)61744-X. [DOI] [PubMed] [Google Scholar]
- 6.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg. 2015;25(10):1822–1832. doi: 10.1007/s11695-015-1657-z. [DOI] [PubMed] [Google Scholar]
- 7.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23(4):427–436. doi: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 8.Keating CL, Bulfone L, Dixon JB, Maglianno DJ, Moodie ML, O’Brien PE, et al. Cost-effectiveness of surgically induced weight loss for the management of type 2 diabetes: modeled lifetime analysis. Diabetes Care. 2009;32(4):567–574. doi: 10.2337/dc08-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoerger TJ, Zhang P, Segel JE, Kahn HS, Barker LE, Couper S. Cost-effectiveness of bariatric surgery for severely obese adults with diabetes. Diabetes Care. 2010;33(9):1933–1939. doi: 10.2337/dc10-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borisenko O, Adam D, Funch-Jensen P, Ahmed A, Zhang R, Colpan Z, et al. Bariatric Surgery can lead to net cost savings to health care systems: results from a comprehensive european decision analytic model. Obes Surg. 2015;25(9):1559–1568. doi: 10.1007/s11695-014-1567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cawley J, Meyerhoefer C, Biener A, Hammer M, Wintfeld N. Savings in medical expenditures associated with reductions in body mass index among US adults with obesity, by diabetes status. PharmacoEconomics. 2015;33(7):707–722. doi: 10.1007/s40273-014-0230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song HJ, Kwon JW, Kim YJ, Oh SH, Heo Y, Han SM. Bariatric surgery for the treatment of severely obese patients in South Korea—is it cost effective? Obes Surg. 2013;23(12):2058–2067. doi: 10.1007/s11695-013-0971-6. [DOI] [PubMed] [Google Scholar]
- 13.Lee YY, Veerman JL, Barendregt JJ. The cost-effectiveness of laparoscopic adjustable gastric banding in the morbidly obese adult population of Australia. PLoS One. 2013;8(5):e64965. doi: 10.1371/journal.pone.0064965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell J, Venn A, Neil A, Hensher M, Sharman M, Palmer A. Diverse approaches to the health economic evaluation of bariatric surgery: a comprehensive systematic review. Obes Rev. 2016;17(9):850–894. doi: 10.1111/obr.12424. [DOI] [PubMed] [Google Scholar]
- 15.Padwal RS, Sharma AM. Treating severe obesity: morbid weights and morbid waits. CMAJ. 2009;181(11):777–778. doi: 10.1503/cmaj.081508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padwal RS, Majumdar SR, Klarenbach S, Birch DW, Karmali S, McCargar L, et al. Health status, quality of life, and satisfaction of patients awaiting multidisciplinary bariatric care. BMC Health Serv Res. 2012;12(1):139. doi: 10.1186/1472-6963-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill RS, Majumdar SR, Wang X, Tuepah R, Klarenbach SW, Birch DW, et al. Prioritization and willingness to pay for bariatric surgery: the patient perspective. Can J Surg. 2014;57(1):33–39. doi: 10.1503/cjs.021212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharman M, Venn AJ, Jose KA, Williams D, Hensher M, Palmer AJ, et al. The support needs of patients waiting for publicly-funded bariatric surgery—implications for health service planners. Clin Obes. 2016;7(1):46–53. doi: 10.1111/cob.12169. [DOI] [PubMed] [Google Scholar]
- 19.Lindekilde N, Gladstone BP, Lübeck M, Nielsen J, Clausen L, Vach W, et al. The impact of bariatric surgery on quality of life: a systematic review and meta-analysis. Obes Rev. 2015;16(8):639–651. doi: 10.1111/obr.12294. [DOI] [PubMed] [Google Scholar]
- 20.Brethauer SA, Kim J, El Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11(3):489–506. doi: 10.1016/j.soard.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Brethauer S, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg. 2015;25(4):587–606. doi: 10.1007/s11695-015-1645-3. [DOI] [PubMed] [Google Scholar]
- 22.Herpertz S, Muller A, Burgmer R, Crosby RD, de Zwaan M, Legenbauer T. Health-related quality of life and psychological functioning 9 years after restrictive surgical treatment for obesity. Surg Obes Relat Dis. 2015;11(6):1361–1370. doi: 10.1016/j.soard.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Burgmer R, Legenbauer T, Müller A, de Zwaan M, Fischer C, Herpertz S. Psychological outcome 4 years after restrictive bariatric surgery. Obes Surg. 2014;24(10):1670–1678. doi: 10.1007/s11695-014-1226-x. [DOI] [PubMed] [Google Scholar]
- 24.Campbell JA, Palmer AJ, Venn A, Sharman M, Otahal P, Neil A. A head-to-head comparison of the EQ-5D-5L and AQoL-8D multi-attribute utility instruments in patients who have previously undergone bariatric surgery. Patient. 2016;9(4):311–322. doi: 10.1007/s40271-015-0157-5. [DOI] [PubMed] [Google Scholar]
- 25.Hachem A, Brennan L. Quality of life outcomes of bariatric surgery: a systematic review. Obes Surg. 2016;26(2):395–409. doi: 10.1007/s11695-015-1940-z. [DOI] [PubMed] [Google Scholar]
- 26.Cawley J. The economics of obesity. Oxford: Oxford University Press; 2011. [Google Scholar]
- 27.Coulman KD, Howes N, Hopkins J, Whale K, Chalmers K, Brookes S, et al. A comparison of health professionals’ and patients’ views of the importance of outcomes of bariatric surgery. Obes Surg. Obes Surg. 2016;26(11):2738–2746. doi: 10.1007/s11695-016-2186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan MA, Richardson J, O’Brien P. The effect of obesity upon health related quality of life (HRQoL): a comparison of the AQoL-8D and SF-36 instruments. Farmeconomia Health Econ Therapeut Pathw. 2012;13(2):69–82. doi: 10.7175/fe.v13i2.208. [DOI] [Google Scholar]
- 29.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes, 3rd edn. Oxford, UK: Oxford University Press; 2015.
- 30.Castilla I, Mar J, Valcarcel-Nazco C, Arrospide A, Ramos-Goni JM. Cost-utility analysis of gastric bypass for severely obese patients in Spain. Obes Surg. 2014;24(12):2061–2068. doi: 10.1007/s11695-014-1304-0. [DOI] [PubMed] [Google Scholar]
- 31.Holland R, Smith RD, Harvey I, Swift L, Lenaghan E. Assessing quality of life in the elderly: a direct comparison of the EQ-5D and AQoL. Health Econ. 2004;13(8):793–805. doi: 10.1002/hec.858. [DOI] [PubMed] [Google Scholar]
- 32.Skinner EH, Denehy L, Warrillow S, Hawthorne G. Comparison of the measurement properties of the AQoL and SF-6D in critical illness. Crit Care Resusc. 2013;15(3):205. [PubMed] [Google Scholar]
- 33.Devlin N, Shah K, Feng Y, Mulhern B, van Hout B. Valuing Health-related quality of life: an EQ-5D-5L value set for England. OHE Research Paper 16/01. [DOI] [PMC free article] [PubMed]
- 34.Feng Y, Devlin N, Shah K, Mulhern B, Van Hout B. New methods for modelling EQ-5D-5L value sets: an application to English data. OHE Research Paper 16/02. [DOI] [PMC free article] [PubMed]
- 35.Tsiplova K, Pullenayegum E, Cooke T, Xie F. EQ-5D-derived health utilities and minimally important differences for chronic health conditions: 2011 Commonwealth Fund Survey of Sicker Adults in Canada. Qual Life Res. 2016;25(12):3009–3016. doi: 10.1007/s11136-016-1336-0. [DOI] [PubMed] [Google Scholar]
- 36.Warkentin LM, Majumdar SR, Johnson JA, Agborsangaya CB, Rueda-Clausen CF, Sharma AM, et al. Weight loss required by the severely obese to achieve clinically important differences in health-related quality of life: two-year prospective cohort study. BMC Med. 2014;12:175. doi: 10.1186/s12916-014-0175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawthorne G, Osborne R. Population norms and meaningful differences for the assessment of quality of life (AQoL) measure. Aust N Z J Public Health. 2005;29(2):136–142. doi: 10.1111/j.1467-842X.2005.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen G, Khan MA, Iezzi A, Ratcliffe J, Richardson J. Mapping between 6 multiattribute utility instruments. Med Decis Making. 2016;36(2):147–159. doi: 10.1177/0272989X15578127. [DOI] [PubMed] [Google Scholar]
- 39.Lester ELW, Padwal R, Majumdar SR, Ye F, Birch DW, Klarenbach SW. Association of preference-based health-related quality of life with weight loss in obese adults. Value Health. 2017. doi:10.1016/j.jval.2016.04.016(in press). [DOI] [PubMed]
- 40.Richardson J, Iezzi A, Khan MA, Maxwell A. Validity and reliability of the assessment of quality of life (AQoL)-8D multi-attribute utility instrument. Patient. 2014;7(1):85–96. doi: 10.1007/s40271-013-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson J, Sinha K, Iezzi A, Khan M. Modelling utility weights for the assessment of quality of life (AQoL)-8D. Qual Life Res. 2014;23(8):2395–2404. doi: 10.1007/s11136-014-0686-8. [DOI] [PubMed] [Google Scholar]
- 42.Janik MR, Rogula T, Bielecka I, Kwiatkowski A, Paśnik K. Quality of life and bariatric surgery: cross-sectional study and analysis of factors influencing outcome. Obes Surg. 2016;26(12):2849–2855. doi: 10.1007/s11695-016-2220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aftab SS, Halder L, Piya M, Reddy N, Fraser I, Menon V, et al. Predictors of weight loss at 1 year after laparoscopic adjustable gastric banding and the role of presurgical quality of life. Obes Surg. 2014;24(6):885–890. doi: 10.1007/s11695-014-1184-3. [DOI] [PubMed] [Google Scholar]
- 44.Clarke PM, Hayes AJ, Glasziou PG, Scott R, Simes J, Keech AC. Using the EQ-5D index score as a predictor of outcomes in patients with type 2 diabetes. Med Care. 2009;47(1):61–68. doi: 10.1097/MLR.0b013e3181844855. [DOI] [PubMed] [Google Scholar]
- 45.Thornton CM, Rozen WM, So D, Kaplan ED, Wilkinson S. Reducing band slippage in laparoscopic adjustable gastric banding: the mesh plication pars flaccida technique. Obes Surg. 2008;19(12):1702. doi: 10.1007/s11695-008-9672-y. [DOI] [PubMed] [Google Scholar]
- 46.Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 47.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395–407. doi: 10.1016/S0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 48.Maxwell A, Ozmen M, Iezzi A, Richardson J. Deriving population norms for the AQoL-6D and AQoL-8D multi-attribute utility instruments from web-based data. Qual Life Res. 2016;25(12):3209–3219. doi: 10.1007/s11136-016-1337-z. [DOI] [PubMed] [Google Scholar]
- 49.Kirkwood B, Sterne J. Medical statistics. 2. Oxford: Blackwell Science; 2003. [Google Scholar]
- 50.Richardson J, Iezzi A, Khan MA. Why do multi-attribute utility instruments produce different utilities: the relative importance of the descriptive systems, scale and ‘micro-utility’ effects. Qual Life Res. 2015;24(8):2045–2053. doi: 10.1007/s11136-015-0926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarride J-E, Breau R, Sharma AM, Hong D, Gmora S, Guertin JR, et al. The effect of bariatric surgery on mobility, health-related quality of life, healthcare resource utilization, and employment status. Obes Surg. 2017;27(4):1128. doi: 10.1007/s11695-017-2598-5. [DOI] [PubMed] [Google Scholar]
- 52.Owen-Smith A, Kipping R, Donovan J, Hine C, Maslen C, Coast J. A NICE example? Variation in provision of bariatric surgery in England. BMJ. 2013;1(346):f2453. doi: 10.1136/bmj.f2453. [DOI] [PubMed] [Google Scholar]
- 53.Richardson J, Khan MA, Iezzi A, Maxwell A. Comparing and explaining differences in the magnitude, content, and sensitivity of utilities predicted by the EQ-5D, SF-6D, HUI 3, 15D, QWB, and AQoL-8D multiattribute utility instruments. Med Decis Making. 2015;35(3):276–291. doi: 10.1177/0272989X14543107. [DOI] [PubMed] [Google Scholar]
- 54.Kakarla VR, Nandipati K, Lalla M, Castro A, Merola S. Are laparoscopic bariatric procedures safe in superobese (BMI ≥ 50 kg/m2) patients? An NSQIP data analysis. Surg Obes Relat Dis. 2011;7(4):452–458. doi: 10.1016/j.soard.2010.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used for this study contains the following: the participant-reported responses to the EQ-5D-5L and the AQoL-8D MAUIs’ questionnaires; the individual utility valuations (both instruments) and utility scores (AQoL-8D only) that were generated with the instruments’ specific algorithm; the participant-reported EQ-VAS scores; and participants’ socio-demographic and clinical data. The corresponding author will provide a de-identified dataset upon reasonable request for all or part of the data.