Abstract
In this study, we applied Environmental Scanning Electron Microscopy-Energy Dispersive Spectroscopy (ESEM-EDS) and Atomic Force Microscopy (AFM) analysis to three different cereal caryopses: barley, oat and einkorn wheat. The morphological structures, chemical elemental composition and surface characteristics of the three cereals were described. Regarding the morphology, barley showed the thickest pericarp, providing a strong barrier to digestion and absorption of nutrients. The aleurone layer of each cereal type contained protein body globoids within its cells. Large type-A and small type-B starchy granules were revealed in the endosperm of barley and einkorn wheat, whereas irregular starchy granules were found in oats. The starchy granule elemental composition, detected by ESEM-EDS, was rather homogenous in the three cereals, whereas the pericarp and protein body globoids showed heterogeneity. In the protein body globoids, oats showed higher P and K concentrations than barley and einkorn wheat. Regarding the topographic profiles, detected by AFM, einkorn wheat starchy granules showed a surface profile that differed significantly from that of oats and barley, which were quite similar to one another. The present work provides insights into the morphological and chemical makeup of the three grains shedding light on the higher bio-accessibility of einkorn wheat nutrients compared to barley and oats, providing important suggestions for human nutrition and technological standpoints.
Key words: Avena sativa L, elemental composition, Hordeum vulgare L, microstructure, phytic acid, Triticum monococcum
Introduction
Food microscopy analysis plays an important role in food science,1,2 not only for the morphology of microstructures, but also for providing nutrient localization3 and their bioaccessibility.4 Optical microscopy, the oldest and most widely used method, is suitable to detect the structural organization of nutrients in sections of raw and processed foods, such as: cereal caryopses,5-7 flour and bran,8 pasta or bread.9 For example, using optical microscopy with the aid of specific staining, it is possible to describe the histological localization of proteins, starch, polyphenols and β-glucan in the cross sections of several cereals.3
Accurate information on cereal microstructures can also be obtained by means of environmental scanning electron microscopy (ESEM) and atomic force microscopy (AFM).10
The ESEM technology represents an evolution of the conventional scanning electron microscope (SEM). In fact, the ESEM allows the observation of samples, even at high resolution, avoiding any conductive coating on the specimen, at different vacuum levels (high, low, atmospheric). This kind of opportunities permits the morphological analysis also of biological samples, without any preliminary treatment before the observation, by modulating vacuum, emission and electron’s detectors. Moreover, when equipped with an energy dispersive spectrometer (EDS), ESEM allows the semi-quantitative detection of the chemical elements constituting the ultrastructural components of the specimen (point or area analysis), with the same spatial high resolution of the morphological analysis and without the interference of the chemical stabilization, dehydration and conductive coating as it is necessary by utilizing a conventional SEM.11,12
Additional information on the architecture of caryopses can be obtained using AFM, a scanning probe microscopy involving the exploration of the sample surface with a sharp tipped probe, whose interaction with the sample can be recorded to generate various images of its surface structure.13-15 The advantages of AFM include minimal work in sample preparation,16 high resolution and quantification at a nanometric level17 through different surface descriptors.10,18
The caryopsis is a type of fruit, being surrounded by the pericarp fused to the seed coat. Next, the endosperm is observed, where the outermost layer is the aleurone layer. Finally there is the embryo (germ) within it.19 Each layer has its own structural organization and chemical composition, since the main function of the mature caryopsis is to store nutrients in a compartmentalized fashion.
The starchy endosperm, for example, is the tissue with the highest nutritional density, storing 80% of the starch weight of the caryopsis in the form of granules. The content and organization of the starchy granules depend on the cereal type and strongly influence the weight of the caryopsis and the quality of the endosperm.20 Starchy granules are constituted by amylopectin and amylose in an approximate ratio of 3:1.4 The starchy granules of wheat and barley are divided into type-A and type-B.20 The former have a lenticular shape and are large in size (15-40 μm); the latter are spherical and small (2-10 μm). The configuration of starchy granules has been investigated using AFM on natural and gelatinized starch from different vegetables, as recently reviewed by Zhu.21
The aleurone layer contains cells in one, two or three rows, depending on the cereal type, divided by walls which are distinct in the inner periclinal cell walls (which separate aleurone cells from endosperm cells) and the anticlinal cell walls (which separate two adjacent aleurone cells), composed mostly of arabinoxilan, β-glucan and phenolic acids.7 The aleurone cells are filled with protein bodies or aleurone grains, which represent important substructures involved in storage. The aleurone grains are embedded in a porous matrix, spherical in shape and surrounded by numerous organelles, including spherosomes.22 Depending on the cereal type and the tissue involved, aleurone grains may consist of several types of inclusions, among which the electron-dense globoids represent the main storage site of phosphorous and metal ions.23-27
In the present investigation, we performed an imaging study based on an ESEM-EDS and AFM integrated analysis, in order to characterize the main structural layers of barley, oat and einkorn wheat caryopses and to determine the chemical elemental composition of protein body globoids of the aleurone layer and starchy granules. The data on the three cereals yielded by the present investigation may prove useful from a nutritional and technological standpoint.
Materials and Methods
Plant material
Husked barley (Hordeum vulgare L., cv. Cometa) was provided by the Council for Agricultural Research and Economics – Genomics Research Centre (Fiorenzuola d’Arda, Italy); naked oats (Avena sativa L., cv. Leda) by Terra Bio Soc. Coop (Urbino, Italy); dehulled einkorn wheat (T. monococcum, cv. Monlis) by Prometeo srl (Urbino, Italy).
ESEM–EDS
The FEI Quanta 200 FEG Environmental Scanning Electron Microscope (FEI, Hillsboro, OR, USA), equipped with an energy dispersive X-ray spectrometer (EDAX Inc., Mahwah, NJ, USA), was used.
Briefly, immediately prior to the analyses, cereal grains were cut in perpendicular slices (transversal sections) with a sharp stainless steel razor. The slices of each cereal were deposited into the aluminium specimen stubs, previously covered with a conductive carbon adhesive disk (TAAB Ltd., Berks, UK).
The analyses were performed by using a focalised electron beam in a vacuum electron gun pressure of 5.0 e-6 mbar. The ESEM was utilized in low vacuum mode, with a specimen chamber pressure set from 0.80 to 0.91 mbar, an accelerating voltage of 25 kV, and a magnification of 800-22000x. The images were obtained by means of a secondary electron detector or a back-scattered electron detector.
The spectrometer unit was equipped with an ECON (Edax Carbon Oxigen Nitrogen) 6 utw x-ray detector and Genesis Analysis software. Each sample was measured with a time count of 100 sec. and an Amp Time of 51, while the probe current was 290 μA.
AFM
The XE-100 atomic force microscope (PARK Systems Inc., Suwon, Korea), equipped with a 50 μm scanner controlled by the XEP 1.8.1 software, was used.
Cross-sections of each cereal were prepared as previously reported.3 Briefly, the caryopses were first soaked in distilled water for three hours. They were then fixed in 10% formalin solution, dehydrated with alcohol and immersed in liquid paraffin. The paraffin blocks were cut into 5 μm thick cross-sections with a rotary microtome. The sections were collected on slides and subsequently deparaffinized with xylene and hydrated with alcohol and distilled water.3
For cross-section imaging, the microscope was set in the Non-Contact Mode (NCM), with the X-Y stage in the close loop and high voltage modes. The Z scanner was also set in the close loop and high voltage modes with a resolution of 1.8Å. The speed scan was set between 0.2 and 1 Hz. The cantilevers used in this study were the NCHR tips, with a nominal spring constant of 42 N/m and a typical resonant frequency between 200 and 300 kHz. The data collection was performed in air at controlled temperature.
In addition to topography, amplitude and phase detection microscopy signals were acquired. AFM images were analyzed by XEI software (PARK Systems Inc.). The reported roughness data were obtained from the same NCM topographic and phase images.
The topographic parameters, used as surface amplitude descriptors, were the following: the roughness profile values peakto- valley (Rpv), the standard deviation of the height valley (Rq), the average roughness (Ra), ten point average roughness (Rz). The topographic statistical parameters and functions, i.e., skewness (Rsk) and kurtosis (Rku), were used to measure asymmetry and flatness. All measures were from 1x1μm image size.
The phase signal roughness was used as a descriptor for the chemical surface variance of the investigated area.28 The detected zones with a phase signal contrast were named HPZ (high phase zone, brighter surface) and LPZ (low phase zone, darker area).
Results
Morphology of the caryopses using ESEM-EDS
Figure 1 shows the morphology of transversal sections of barley, oats and einkorn wheat, analyzed using the ESEMEDS technique.
Figure 1.
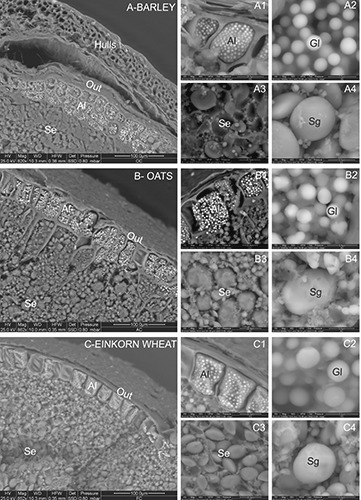
ESEM-EDS micrographs of barley (A), oats (B) and einkorn wheat (C) caryopses. Out, pericarp; Al, aleurone layer; Gl, protein body globoids of the aleurone layer; Se, starchy endosperm; Sg, starchy granules of the endosperm.
Barley (Figure 1A), the only husked cereal analyzed, showed a thick pericarp (Out) and seed coat.
Regarding the aleurone layer (Al), barley was characterized by two or three layers of cells (Figure 1A), whereas oat (Figure 1B) and einkorn wheat (Figure 1C) showed one regular layer of cells. In all the cereals, the cells of the aleurone layer showed several protein body globoids (Gl), which appeared as white beads, 1-2 μm in diameter (Figure 1 A2,B2,C2).
The starchy endosperm (Se) of the three cereals were characterized by granules of different shapes and sizes, appearing smaller and closer together in the upper part, near the aleurone layer, and larger towards the center of the caryopsis (Figures 1 A,B,C). At higher-magnification, the starchy endosperm of barley (Figure 1 A3) showed some empty pockets, likely due to the loss of the granules during the section cutting. Large type-A and small type-B starchy granules were evident in barley (Figures 1 A3,A4) and einkorn wheat (Figures 1 C3,C4), whereas in oat sections, irregular starch granules were identified (Figures 1 B3,B4), appearing as if they had formed through the aggregation of several smaller granules.
Elemental composition revealed by ESEM-EDS
We made use of our ESEM-EDS equipment to determine the elemental composition of the caryopsis layers.
Table 1 shows the average (%) composition of the pericarp of the three cereals. C and O represented about 98.7±0.6% of the outer layer elements. The higher C value compared to O, found in all cereals, may be due to the conductive carbon adhesive disk. In fact, no subtraction of the background was performed. All the cereals were found to contain P, S, K and Ca, with Ca which did not show any significant difference among the three cereals. Mg and Pb were only found in barley, Si in barley and oat, Mn in oat and einkorn wheat (Table 1).
Table 1.
Average elemental composition of the pericarp revealed by ESEM-EDS analysis.
| Elements | Barley | Oats | Einkorn wheat |
|---|---|---|---|
| C | 78.49±1.46 (b) | 78.91±1.93 (b) | 85.26±1.82 (a) |
| O | 19.40±1.18 (a) | 19.90±1.81 (a) | 14.01±1.60 (b) |
| Mg | 0.18±0.03 | nd | nd |
| Si | 0.08±0.01 (b) | 0.26±0.11 (a) | nd |
| P | 0.30±0.07 (a) | 0.15±0.04 (b) | 0.05±0.02 (c) |
| S | 0.16±0.02 (a) | 0.13±0.05 (ab) | 0.10±0.02 (b) |
| K | 0.97±0.13 (a) | 0.38±0.05 (b) | 0.25±0.06 (b) |
| Ca | 0.38±0.13 (a) | 0.23±0.12 (a) | 0.28±0.11 (a) |
| Mn | nd | 0.06±0.01 (a) | 0.11±0.03 (a) |
| Pb | 0.05±0.03 | nd | nd |
Values are reported as atomic percent (%) of the elements and are the mean ± SD of 10 independent analysis. (a,b) Different letters, for the same element, indicate statistically significant differences among the three cereals (P≤0.05, one-way ANOVA); nd, non detected element.
Table 2 shows the average elemental composition of the protein body globoids of the aleurone layer. C and O accounted for about 89.3±0.8% of the globoid composition, with no statistically significant difference among cereals. All the cereals were found to contain N, P, Mg, K, S. Notably, oat protein body globoids were the only in which Ca was found (Table 2).
Table 2.
Average elemental composition of protein body globoids of the aleurone layer revealed by ESEM-EDS analysis.
| Elements | Barley | Oats | Einkorn wheat |
|---|---|---|---|
| C | 60.23±2.23 (a) | 59.24±1.13 (a) | 57.96±3.30 (a) |
| N | 4.90±0.65 (a) | 4.00±0.16 (b) | 4.02±0.16 (b) |
| O | 29.10±2.06 (a) | 29.00±1.49 (a) | 32.15±2.81 (a) |
| Mg | 1.52±0.21 (a) | 1.75±0.04 (a) | 1.54±0.15 (a) |
| P | 2.79±0.25 (b) | 3.81±0.26 (a) | 2.76±0.25 (b) |
| S | 0.09±0.02 (a) | 0.10±0.03 (a) | 0.11±0.01 (a) |
| K | 1.38±0.06 (b) | 1.89±0.19 (a) | 1.45±0.09 (b) |
| Ca | nd | 0.22±0.05 | nd |
Values are reported as atomic percent (%) of the elements and are the mean ± SD of 10 independent analysis. (a,b) Different letters, for the same element, indicate statistically significant differences among the three cereals (P≤0.05, one-way ANOVA); nd, non detected element.
Table 3 shows the average elemental composition of endosperm starchy granules. C and O accounted for about 99.6%, with no statistically significant difference among the three cereals, while P, S, and K constituted the remaining 0.4%. Traces of Cl were found in the starchy granules of barley and einkorn wheat.
Table 3.
Average elemental composition of endosperm starchy granules revealed by ESEM-EDS analysis.
| Elements | Barley | Oats | Einkorn wheat |
|---|---|---|---|
| C | 74.37±2.78 (a) | 78.14±3.64 (a) | 75.44±3.69 (a) |
| O | 25.26±2.84 (a) | 21.34±3.61 (a) | 24.21±3.68 (a) |
| P | 0.10±0.02 (b) | 0.21±0.02 (a) | 0.08±0.03 (b) |
| S | 0.05±0.03 (b) | 0.13±0.02 (a) | 0.09±0.04 (ab) |
| Cl | 0.05±0.02 (a) | nd | 0.06±0.03 (a) |
| K | 0.18±0.03 (ab) | 0.20±0.02 (a) | 0.13±0.05 (b) |
Values are reported as atomic percent (%) of the elements and are the mean ± SD of 10 independent analysis. (a,b) Different letters, for the same element, indicate statistically significant differences among the three cereals (P≤0.05, one-way ANOVA); nd, non detected element.
Figure S1 shows further micrographs of starchy granules of barley, oats and einkorn wheat after the ESEM-EDS analysis. Interestingly, after the passage of the electron flow, all the granules showed a deeper hole in their center (c) compared to that of the periphery (p), which remained smaller (Figure S1). The average elemental composition showed higher C and lower O in the periphery of the granules compared to the center, as well as the significantly increased P in their periphery (Table S1).
Morphology of the caryopses using AFM
We made use of AFM technique to show the 3D topographic images of the pericarp, aleurone layer and starchy endosperm of barley (Figure 2A), oats (Figure 2B) and einkorn wheat (Figure 2C).
Figure 2.
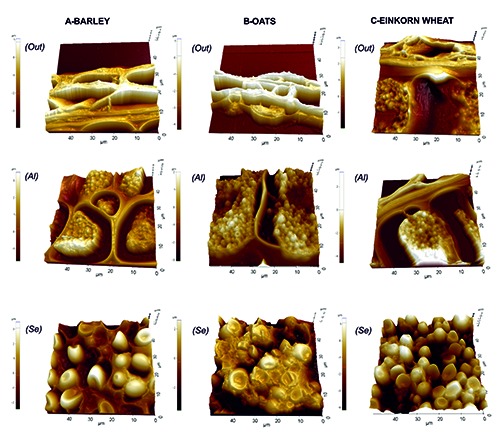
AFM images of barley (A), oats (B) and einkorn wheat (C) caryopses. Out, pericarp; Al, aleurone layer; Se, starchy endosperm.
The pericarp (Figure 2, Out) clearly appeared to be composed of several coatings, with barley showing the highest number of layers. Moreover, in barley, we observed the thickest walls dividing the aleurone cells (Figure 2, Al). The void space, which appears between the walls and the aleurone cells, is due to the dehydration and manipulation of the sample during the section preparation. Regarding the starchy endosperm (Figure 2, Se), barley starchy granules were regularly distributed; oats showed a strict aggregation of starchy granules to form larger polymorphic structures, whereas einkorn wheat showed small independent granules.
Surface characteristics by AFM
Using AFM, the topographic images of the caryopsis layers were acquired, together with the amplitude images, which exclusively represented a feedback drive, thus rendering the phase signal completely dependent on the surface roughness (Figures S2, S3, S4).
Table 4 shows the topographic parameters obtained by AFM image processing, thus providing quantitative data on the surface profile. Regarding the pericarp (Table 4), einkorn wheat showed the greatest topographic roughness with a platykurtic height distribution (Rku = 1.50), whereas barley and oats were mesokurtic (Rku = 3.47 and 3.04, respectively).
Table 4.
Topographic roughness parameters revealed by AFM analysis.
| Region/species | Rpv | Rq | Ra | Rz | Rsk | Rku |
|---|---|---|---|---|---|---|
| Pericarp | ||||||
| Barley | 0.318 | 0.067 | 0.053 | 0.316 | 1.185 | 3.478 |
| Oat | 0.473 | 0.098 | 0.082 | 0.469 | -0.798 | 3.041 |
| Einkorn wheat | 1.411 | 0.478 | 0.437 | 1.406 | -0.3 | 1.504 |
| Protein body globoids | ||||||
| Barley | 0.666 | 0.131 | 0.105 | 0.654 | -0.551 | 2.905 |
| Oat | 0.291 | 0.044 | 0.035 | 0.282 | 1.269 | 4.639 |
| Einkorn wheat | 0.462 | 0.118 | 0.102 | 0.461 | 0.003 | 1.827 |
| Starchy granules | ||||||
| Barley | 0.726 | 0.17 | 0.145 | 0.724 | 0.118 | 2.004 |
| Oat | 0.626 | 0.155 | 0.133 | 0.623 | 0.2 | 1.979 |
| Einkorn wheat | 0.363 | 0.057 | 0.042 | 0.357 | 2.084 | 7.299 |
Rpv, peak-to-valley roughness (μm); Rq, root mean square roughness (μm); Ra, average roughness (μm); Rz, Ten point average roughness (μm); Rsk, skewness; Rku, kurtosis. Topographic roughness parameters were calculated from AFM topographic images (1x1 μm).
The topographic signal of protein body globoids revealed oats as an outlier sample (Table 4). In fact, in terms of roughness, oats were the flattest grain, with a positive skewness moment (Rsk = 1.26) and leptokurtic distribution (Rku = 4.63) compared to barley and einkorn wheat.
Regarding the topographic profile of starchy granules, einkorn wheat showed a surface profile that differed significantly from that of oats and barley, which were quite similar to one another (Table 4). In particular, the starchy granule surface of einkorn wheat was fairly smooth, with an extremely positive asymmetrical scattering (more peaks than valleys), due to the positive moment of the skewness value (Rsk = 2.08) and the leptokurtic distribution (Rku = 7.29). On the contrary, oats and barley showed a moderately symmetrical and platykurtic distribution of shapes, as extrapolated by their skewness and kurtosis values, as well as their roughness parameters, which were similar to those previously described by Barrera et al.10
Observing the AFM phase images, the pericarp (Figure S2) showed marker phase signals with respect to protein body globoids (Figure S3) and starchy granules (Figure S4). In fact, the most important characteristic was the presence of continuous, dark lanes (indicated as LPZ), which wrapped around the pericarp of each cereal (Figure S2). Interestingly, two marked wrapping lanes were present in barley and einkorn wheat, while only one tiny lane was observable in oats (Figure S2, Phase 50 μm).
Table S2 shows the main phase signal roughness parameters obtained for the entire imaged zone (1x1 μm), as well as for the two selected sub-zones, labeled as HPZ (bright surface) and LPZ (dark surface).
Considering the whole phase signal of the pericarp (Table S2, Out), only barley showed a positive middle value (31.91 degree), while oats and einkorn wheat showed negative middle values (-14.58 and -24.20 degrees, respectively). However, in all cereals, the sub-micrometric analysis of the pericarp revealed a divergent phase signal between the HPZ and LPZ zone within the whole area (Table S2).
The phase signals of the globoid surfaces (Table S2, Al) showed divergences compared to the topographic data (Table 4). In fact, the whole phase signal showed einkorn wheat to have a negative middle value (-24.16 degree), whereas the middle phase signal was positive in oats (16.66 degree) and barley (53.81 degree). Moreover, einkorn wheat showed a divergent mean phase signal difference between HPZ and LPZ (7.62 and -59.4 degrees, respectively), whereas no significant differences were observed between LPZ and HPZ mean phase signals of oat and barley (Table S2).
The phase signals of the starchy granule surfaces (Table S2, Se) were similar to the topographic data (Table 4). In fact, the middle values of the whole phase signals were positive in oats (22.64 degree) and barley (65.83 degree), and negative in einkorn wheat (-40.89 degree). The investigated phase signals of the HPZ and LPZ starchy granule sub-zones showed an interesting variation among the three species (Table S2). While in oats and barley the mean phase signal was slightly different between HPZ and LPZ, in einkorn wheat, the two phases differed notably, thus indicating a very large surface alteration of the two zones.
Discussion
The results of the present work showed the morphological and topographic aspects of the substructures of barley, oats and einkorn wheat, obtained by ESEM-EDS and AFM techniques. The analyzed substructures were: the pericarp; the aleurone layer, with its protein body globoids; the starchy endosperm, with its granules.
The pericarp and the aleurone layer resulted the most variegated structures in terms of thickness, surface profiles and elemental compositions. Barley showed the thickest pericarp with the aleurone layer constituted by three rows of cells, whereas oats and einkorn wheat were characterized by more delicate structures. A harder and more compact structure is functional for barley to pearl and malt.29 Pearling is the process utilized to remove the hulls and the bran layers of grains, in order to provide a form for human consumption, which cooks faster and allows short chewing.8,29 Malting is the controlled germination of cereals, set up by means of steeping, germination and kilning, to ensure physical and biochemical changes within the grain. Therefore, the cell wall properties are the most important characteristics, which better facilitate moisture and enzyme activity expression in the endosperm, with a minimal breakdown inside the caryopsis.30
A second consideration, linking morphology with technology and nutrition, derives from the elemental composition, which allowed us to obtain the mineral relative concentrations of the substructures. Our results show that the pericarp and the aleurone layer, beyond to the fiber, are rich of Ca, Mg and Mn, which are important elements for enzymatic activity and human tissue function.31 Since the pericarp of grains is lost with the bran during milling,8 only by consuming whole grain flour, we can benefit from their full mineral contents.3 Indeed, a mixture of two, out of the three cereal flours, better integrated the minerals for recommended daily intake.31
A third nutritional comment is related to the globoid structures of the aleurone layer. The protein body globoids have shown a remarkable concentration of P and Mg in all cereals, with oats showing the highest P concentration,32 which clearly points to the presence of phytic acid. Depending on the refining level, part of the aleurone layer is discarded as bran during milling,3 along with phytic acid. What this means from a nutritional standpoint is the object of an intense debate, since phytic acid has long been considered as an anti-nutritional factor, due to its ability to form insoluble and indigestible complexes with metal ions. Nevertheless, phytic acid is now viewed positively by nutrition experts, because it also shows interesting health protective functions.11,33,34 With the AFM analysis, we evidenced several differences among cereals, which suggest different chemical composition, organization of macromolecules and orientation.
We have to remind that the samples analyzed by AFM were prepared with the same procedure, as we did by optical microscopy in our previous report,3 i.e., the grain cuts went through a double process of hydration and dehydration, providing us the possibility to analyze the grain architecture on the caryopsis in situ. Therefore, the comparative analysis of optical microscopy and AFM analysis, allowed us to underlie the aggregation of starchy granules of oats (Figure 2B Se, present work), as it was possible to foresee from the picture obtained with the optical microscopy in our previous report.3 Similarly, we can observe a strong correspondence between the structures of the aleurone layers (Figure 2 Al, present work) and those, which appeared in our previous report, well separated from each other by a grey membrane and enclosed in a polysaccharide matrix stained in orangeyellow. 3
In terms of surface profiles, the AFM analysis indicates that the protein body globoids of oats show a topographic roughness, which differ from that of barley and einkorn wheat, but we do not know if this aspect could have implications in terms of nutrition.
As far as the starchy granules is concerned, our AFM results showed that their surfaces are dissimilar to one another, with the starchy granule of einkorn wheat showing the smoothest surface. The shift of phase signals, at the AFM analysis, suggested both a different chemical composition but also a different orientation of the functional groups, present on the surface of the granules.10
By the ESEM-EDS analysis, we provided evidence that starchy granules are harder in their periphery than they are in their center, 4 as shown by their elemental composition. Einkorn wheat starchy granules appear smaller than those of the other cereals, whereas oats showed large-sized starchy granules, which appear to be the aggregation of smaller granules. This characteristic of starchy granules, together with the thick walls, accounts for the more challenging nutrient bio-accessibility of oats and barley versus einkorn wheat. The possible link between the sub-structures of the caryopsis and their bio-accessibility, hypothesized herein, at a structural level, must be confirmed by further studies, in which the hydrolysis of starchy granules is monitored, as they undergo progressive structural changes under the attack of hydrolytic enzymes,4 using time course imaging, performed by ESEM-EDS analysis.
In conclusion, the present research underlines the main differences among the architectures of cereals. The first difference stems from the number and type of nucleated cells in the aleurone layer. The high number of aleurone cells contributes, for example, to the release of several enzymes involved in the hydrolysis of malt polysaccharides to produce fermentable carbohydrates during mashing.
The second difference regards the thickness of the cell walls, which guarantees integrity of the caryopsis during processing. In this regard, the integrity of the caryopsis during pearling and malting is not assured by the thinnest structures found in oats and particularly in einkorn wheat, which require careful and mild manipulations to avoid seed breakages.
Concerning the milling process, which is needed for flours productions to prepare human foods, we can infer that a complete disruption of the bran is necessary in the production of whole grain flour, which guarantees the complete nutritional intake of macro- and micronutrients.8,35 Among the different flours, einkorn wheat results the most favored cereal in terms of bio-accessibility and digestibility, because of its small and smooth starchy granules and hence easily attached by the hydrolytic enzymes.
Acknowledgements
The authors wish to thank the ARPA Marche (Pesaro, PU, Italy), for the permission to utilize the ESEM-EDS, Prof. Federica Bortolotti and Dr. Anna Panato, Dept. of Diagnostic and Public Health, University of Verona (VR, Italy), for their help in the preparation of the cereals sections. We acknowledge Dr. Valeria Terzi of the Council for Agricultural Research and Economics (CREA) - Genomics Research Centre, Fiorenzuola d’Arda (PC, Italy), for providing barley samples, Terra Bio Soc. Coop. and Prometeo s.r.l. (Urbino, PU, Italy), for oats and einkorn wheat samples, Timothy Bloom for assistance in the English Language.
References
- Karoui R, Blecker C. Fluorescence spectroscopy measurement for quality assessment of food systems - a review. Food Bioproc Tech 2011;4:364-86. [Google Scholar]
- Kalab M. Practical aspects of electron microscopy in cheese research. Adv Exp Med Biol 1995;367:247-76. [DOI] [PubMed] [Google Scholar]
- Panato A, Antonini E, Bortolotti F, Ninfali P. The histology of grain caryopses for nutrient location: a comparative study of six cereals. Int J Food Sci Technol 2017;52:1238-45. [Google Scholar]
- Tang H, Mitsunaga T, Kawamura Y. Molecular arrangement in blocklets and starch granule architecture. Carbohydr Polym 2006;63:555-60. [Google Scholar]
- Miller SS, Fulcher RG, Sen A, Arnason JT. Oat endosperm cell walls. I. Isolation, composition, and comparison with other tissues. Cereal Chem 1995;72:421-7. [Google Scholar]
- Fulcher RG. Fluorescence Microscopy of Cereals. Food Struct 1982;1:167-75. [Google Scholar]
- Jääskeläinen AS, Holopainen-Mantila U, Tamminen T, Vourinen T. Endosperm and aleurone cell structure in barley and wheat as studied by optical and Raman microscopy. J Cereal Sci 2013;57:543-50. [Google Scholar]
- Antonini E, Diamantini G, Ninfali P. The effect of mechanical processing on avenanthramide and phenol levels in two organically grown Italian oat cultivars. J Food Sci Technol 2017;54:2279-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autio K, Salmenkallio-Marttila M. Light Microscopic investigations of cereal grains, doughs and breads. LWT - Food Sci Technol 2001;34:18-22. [Google Scholar]
- Barrera GN, Calderon-Dominguez G, Chanona-Perez J, Gutierrez-Lopez GF, Leon AE, Ribotta PD. Evaluation of the mechanical damage on wheat starch granules by SEM, ESEM, AFM and texture image analysis. Carbohydr Polym 2013;98:1449-57. [DOI] [PubMed] [Google Scholar]
- Ockenden I, Dorsch JA, Reid MM, Lin L, Grant LK, Raboy V, et al. Characterization of the storage of phosphorus, inositol phosphate and cations in grain tissues of four barley (Hordeum vulgare L.) low phytic acid genotypes. Plant Sci 2004;167:1131-42. [Google Scholar]
- Joyce C, Deneau A, Peterson K, Ockenden I, Raboy V, Lott JNA. The concentrations and distributions of phytic acid phosphorus and other mineral nutrients in wild-type and low phytic acid Js-12- LPA wheat (Triticum aestivum) grain parts. Can J Bot 2005;83:1599-607. [Google Scholar]
- Morris VJ, Woodward NC, Gunning AP. Atomic force microscopy as a nanoscience tool in rational food design. J Sci Food Agric 2011;91:2117-25. [DOI] [PubMed] [Google Scholar]
- Burgert I, Keplinger T. Plant micro- and nanomechanics: experimental techniques for plant cell-wall analysis. J Exp Bot 2013;64:4635-49. [DOI] [PubMed] [Google Scholar]
- Chichti E, George M, Delenne JY, Lullien- Pellerin V. Changes in the starch-protein interface depending on common wheat grain hardness revealed using atomic force microscopy. Plant Sci 2015;239:1-8. [DOI] [PubMed] [Google Scholar]
- Huh KW, Kim SH, Ku HH, Lee BM, Kim JY, Seo YW, et al. Surface imaging of barley aleurone cell by atomic force microscopy. K J Crop Sci 2004;49:36-40. [Google Scholar]
- Liu P, Chen L, Corrigan PA, Yu L, Liu Z. Application of atomic force microscopy on studying micro- and nano-structures of starch. Int J Food Eng 2008. doi:https://doi.org/10.2202/1556-3758.1510. [Google Scholar]
- Park H, Xu S, Seetharaman K. A novel in situ atomic force microscopy imaging technique to probe surface morphological features of starch granules. Carbohydr Res 2011;346:847-53. [DOI] [PubMed] [Google Scholar]
- Evers T, Millar S. Cereal grain structure and development: some implications for quality. J Cereal Sci 2002;36:261-84. [Google Scholar]
- Jing YP, Liu DT, Yu XR, Xiong F, Li DL, Zheng YK, et al. Development of endosperm cells and starch granules in common wheat. Cereal Res Commun 2014;42:514-24. [Google Scholar]
- Zhu F. Atomic force microscopy of starch systems. Crit Rev Food Sci Nutr 2017;57:3127-44. [DOI] [PubMed] [Google Scholar]
- Pomeranz Y. Structure and mineral composition of cereal aleurone cells as shown by scanning electron microscopy. AACC 1973;50:504-11. [Google Scholar]
- Regvar M, Eichert D, Kaulich B, Gianoncelli A, Pongrac P, Vogel-Mikus K, et al. New insights into globoids of protein storage vacuoles in wheat aleurone using synchrotron soft X-ray microscopy. J Exp Bot 2011;62:3929-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombi E, Smith E, Hansen TH, Paterson D, de Jonge MD, Howard DL, et al. Megapixel imaging of (micro)nutrients in mature barley grains. J Exp Bot 2011;62:273-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Tian S, Liao H, Zhang J, Yang X, Labavitch JM, et al. Analysis of metal element distributions in rice (Oryza sativa L.) seeds and relocation during germination based on X-ray fluorescence imaging of Zn, Fe, K, Ca, and Mn. PLoS One 2013;8:e57360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamme F, Robert P, Bouchet B, Saulnier L, Dumas P, Guillon F. Aleurone cell walls of wheat grain: high spatial resolution investigation using synchrotron infrared microspectroscopy. Appl Spectrosc 2008;62:895-900. [DOI] [PubMed] [Google Scholar]
- Lott JNA, Spitzer E. X-ray Analysis Studies of elements stored in protein body globoid crystals of Triticum grains. Plant Physiol 1980;66:494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao E, Dvorak JA. Phase imaging by atomic force microscopy: analysis of living homoiothermic vertebrate cells. Biophys J 1999;76:3289-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M, bu-Ghannam N, Gallaghar E. Barley for brewing: Characteristic changes during malting, brewing and applications of its by-products. Compr Rev Food Sci Food Saf 2010;9:318-28. [DOI] [PubMed] [Google Scholar]
- Ogushi K, Lim P, Barr AR, Takahashi S, Asakura T, Ito K. Japanese barley meets Australia: Quality performance of malting barley grown in different countries. J Inst Brew 2002;108:303-9. [Google Scholar]
- Frølich W. Bioavailability of minerals from cereals. Spiller GA, editor. CRC Handbook of Dietary fiber in human nutrition: CRC Press; 2001. p. 499-530. [Google Scholar]
- Gupta RK, Gangoliya SS, Singh NK. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol 2015;52:676-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abebe Y, Bogale A, Hambidge KM, Stoecker BJ, Bailey K, Gibson RS. Phytate, zinc, iron and calcium content of selected raw and prepared foods consumed in rural Sidama, Southern Ethiopia, and implications for bioavailability. J Food Compost Anal 2007;20: 161-8. [Google Scholar]
- Silva EO, Bracarense AP. Phytic acid: From antinutritional to multiple protection factor of organic systems. J Food Sci 2016;81:R1357-R1362. [DOI] [PubMed] [Google Scholar]
- van der Kamp JW, Poutanen K, Seal CJ, Richardson DP. The HEALTHGRAIN definition of ‘whole grain’. Food Nutr Res 2014;58:22100-107. [DOI] [PMC free article] [PubMed] [Google Scholar]


