Abstract
It is widely accepted that cAMP signaling is compartmentalized within cells. However, our knowledge of how receptors, cAMP signaling enzymes, effectors, and other key proteins form specific signaling complexes to regulate specific cell responses is limited. The multicomponent nature of these systems and the spatiotemporal dynamics involved as proteins interact and move within a cell make cAMP responses highly complex. Adenylyl cyclases, the enzymatic source of cAMP production, are key starting points for understanding cAMP compartments and defining the functional signaling complexes. Three basic elements are required to form a signaling compartment. First, a localized signal is generated by a G protein-coupled receptor paired to one or more of the nine different transmembrane adenylyl cyclase isoforms that generate the cAMP signal in the cytosol. The diffusion of cAMP is subsequently limited by several factors, including expression of any number of phosphodiesterases (of which there are 24 genes plus spice variants). Finally, signal response elements are differentially localized to respond to cAMP produced within each locale. A-kinase-anchoring proteins, of which there are 43 different isoforms, facilitate this by targeting protein kinase A to specific substrates. Thousands of potential combinations of these three elements are possible in any given cell type, making the characterization of cAMP signaling compartments daunting. This review will focus on what is known about how cells organize cAMP signaling components as well as identify the unknowns. We make an argument for adenylyl cyclases being central to the formation and maintenance of these signaling complexes.
Introduction
The classic model of G protein-coupled receptor (GPCR) signaling, where receptors, G proteins, effector enzymes, and downstream signaling proteins float in a sea of phospholipids and interact with one another stochastically and generate a global signal via a second messenger, does not explain pharmacological reality. If one bathes cells in a catecholamine, such as epinephrine, β-adrenergic receptors are activated and a cAMP signal is generated in the cytosol. However, cAMP is a soluble messenger that can diffuse everywhere, theoretically making the location of its generation irrelevant. Moreover, cAMP can elicit hundreds of different cellular responses based on protein kinase A (PKA) phosphorylation events and activation of exchange protein directly activated by cAMP (Schmidt et al., 2013). Recent efforts using RNA sequencing to define the full range of GPCR expressed in a given cell type reveal that most cells express approximately 100 different heptahelical receptors (Insel et al., 2015). Thus, the pharmacological reality is that at least 20–30 of these receptors couple Gαs, stimulate adenylyl cyclase (AC) activity, and promote cAMP production. The classic model of cAMP signaling does not differentiate Gαs-coupled responses from different GPCR agonists. Ligands, such as prostaglandin E2 (PGE2), that activate Gαs-coupled receptors generate cAMP in the cytosol and should lead to the exact same set of cellular responses as epinephrine, but they do not. Most will readily agree that a model where epinephrine and PGE2 yield the same cellular response is not teleologically supported, and in fact experimental observations debunking this idea date back nearly 40 years (Brunton et al., 1979; Hayes et al., 1979). Indeed, the idea that cAMP signaling is compartmentalized has been readily accepted for decades but has never been incorporated into the general model of cAMP signaling. In fact, the first description of cAMP signaling compartments was made in 1983 by Ian Buxton and Larry Brunton (Buxton and Brunton, 1983).
The arrangement of GPCR signaling proteins to create and maintain strict cAMP compartments is poorly understood, despite the concept of cAMP signaling compartmentation being both palatable and not new. Examples of specific physiologic responses mediated by distinct cAMP signaling compartments are also equally lacking. The three basic elements required to define cAMP signaling compartments are localized signal generation, restricted diffusion of the signal, and localized signal response elements. All cells express a number of different proteins that perform each of these functions in cAMP signaling. This review will discuss these three elements and illustrate the evidence that various signaling proteins contribute to cAMP signaling compartments in different cell models.
Localized Signal Generation
GPCRs are the first step in the cascade leading to second messenger generation and are critical elements in determining the nature of the subsequent localized cAMP signal. The initial organization of cell responses to activation of any given GPCR is based on AC partnering with the receptor within discrete signaling microdomains. While GPCRs can be quite mobile with respect to different plasma membrane domains and even intracellular organelles (depending on the nature of the receptor and its level of activity), AC isoforms appear quite steady in their localization. A primary distinction between isoforms of AC is their residence in caveolar/lipid raft microdomains or in nonraft plasma membrane domains (Ostrom and Insel, 2004; Dessauer et al., 2017). For example AC1, AC3, AC5, AC6, and AC8 associate with lipid rafts, which in many cells also contain one or more forms of caveolin. On the other hand, AC2, AC4, AC7, and AC9 are found within nonraft plasma membrane microdomains. ACs within these microdomains appear constant, with no differences among species, cell types, activation states, or even caveolin expression (Ostrom and Insel, 2004; Cooper and Tabbasum, 2014). The availability of Gαs appears to be in excess of both GPCR and AC and relatively uniform in its distribution across membrane microdomains (Post et al., 1995; Ostrom et al., 2001).
AC localization to lipid raft or nonraft complexes correlates with GPCR coupling with specific ACs, implying that the juxtaposition of receptor and effector with a single microdomain is necessary for efficient coupling. Specific GPCR/AC coupling has been elucidated using knockdown or overexpression of different AC isoforms. For example, AC6 overexpression selectively enhances beta-2 adrenergic receptor (β2AR) signaling in airway smooth muscle, lung fibroblasts, and neonatal cardiac myocytes, but not signaling from other Gαs-coupled receptors (Ostrom et al., 2000, 2002; Liu et al., 2008; Bogard et al., 2011). In contrast, prostanoid EP receptors in human airway smooth muscle (HASM) specifically colocalize with and couple to AC2 in nonraft domains based on AC2 overexpression studies (Bogard et al., 2012). Table 1 shows what is known about the localization and GPCR coupling of each of the nine transmembrane forms of AC. Also noted are the cells that have been used for these studies and notes on other key pieces of information. What is clear from Table 1 is that most ACs are significantly understudied in terms of their receptor coupling.
TABLE 1.
Microdomain localization and receptor coupling of mammalian AC isoforms
| AC Isoform | Microdomain Localization | GPCR Coupling | Systems Studied | Notes |
|---|---|---|---|---|
| AC1 | Lipid raft | β2AR | Vascular smooth muscle cells | AC1 overexpression used to demonstrate GPCR coupling |
| AC2 | Nonraft | EP2/4R | Airway smooth muscle cells | AC2 overexpression used to demonstrate GPCR coupling |
| AC3 | Lipid raft | β2AR, D1R glucagon | HEK-293 | Glucagon receptors were exogenously expressed |
| AC4 | Nonraft | Unknown | Unknown | |
| AC5 | Lipid raft | β1AR, β2AR, A2AR | Cardiac myocytes, hepatocytes | D5R couple to AC5 in nonrafts of renal tubule cells |
| AC6 | Lipid raft | β1AR, β2AR, IPR, D1, A2AR, PAC1 | HEK-293, cardiac myocytes, airway and GI smooth muscle cells, vascular smooth muscle cells, cardiac fibroblasts, lung fibroblasts, hepatocytes, platelets | AC6 overexpression, knockdown, and antibody inactivation have been used to determine GPCR-AC6 coupling |
| AC7 | Nonraft | D1R-EP1R | HEK-293 | Regulation of AC7 by D1R is via Gβγ and requires EP1R |
| AC8 | Lipid raft | None | HEK-293, pancreatic cells, hippocampal neurons | Stimulated by store-operated and L-type calcium channels |
| AC9 | Nonraft | Unknown | Unknown |
Cardiac myocytes also appear to exhibit some correlation between AC localization and GPCRs. There is evidence that AC5 is preferentially expressed in the T-tubules, while AC6 is found in the peripheral sarcolemma (Timofeyev et al., 2013). Furthermore, beta-1 adrenergic receptors (β1ARs) have been reported to couple selectively to AC5 in neonatal cardiac myocytes (Tsunematsu et al., 2015). On the other hand, in adult cardiac myocytes, both β1ARs and β2ARs are found in T-tubules, where they couple to AC5, while β1ARs alone are found in the peripheral sarcolemma, where they couple specifically to AC6 (Nikolaev et al., 2010; Timofeyev et al., 2013). However, Cros and Brette (2013) found that β2AR couples in the peripheral sarcolemma, but not in the T-tubules. Thus, GPCRs display AC-specific coupling that reflects receptor colocalization to AC isoforms in specific plasma membrane environments that can give rise to different cellular responses.
Numerous other GPCRs show differential coupling to AC activity based on localization to specific membrane domains. D1 dopamine receptors are coupled to lipid-raft-dependent AC3, AC5, and AC6 activity, whereas D5 dopamine receptors interact with AC5 in a nonraft localized manner (Yu et al., 2014). Lipid rafts are a specific location for prostacyclin receptor inhibition of platelet activation since IP receptors and AC5/6 are coexpressed in these types of microdomains (Liu et al., 2008; Raslan and Naseem, 2015). Downstream signaling from family B GPCRs depend on colocalization to domains with specific ACs since adenylate cyclase–activating polypeptide type I receptor signaling can be mitigated with AC6, but not AC7, knockdown (Emery et al., 2015). GPCR receptor types can switch their coupling to ACs in a regulated manner. Uterine smooth muscle AC activity from α2-adrenergic receptor stimulation is inhibitory in early pregnancy and becomes stimulatory after midterm pregnancy (Zhou et al., 2000). This change in AC activity is probably due to an upregulation of uterine AC2 expression (Zhou et al., 2007). Coexpressed GPCRs can even influence AC activity of another GPCR and its cognate AC. For example, PGE2 may regulate neuronal dopamine signaling through EP1 receptors that facilitate dopamine D1 activation of cAMP production in an AC7/Gβγ signaling–dependent manner (Ehrlich et al., 2013).
A structural component must exist in all ACs that confers affinity for one domain or the other since AC isoforms are either lipid raft or nonraft localized. Likely mechanisms that stabilize these interactions include sequence-specific protein-protein or protein-lipid interactions, and post-translational modifications. Evidence for the critical role of the intracellular C1 and C2 domains in targeting ACs to lipid rafts come from studies with chimeras of raft and nonraft isoforms (Crossthwaite et al., 2005) and approaches examining fragments of ACs (Thangavel et al., 2009). These studies show AC isoforms have key protein-protein interactions that confer their lipid raft localization. Protein-lipid interactions may also be critical for raft targeting of ACs. Mutation of N-linked glycosylation sites on an extracellular loop of AC8 causes this lipid-raft localized isoform to appear in nonraft membranes (Pagano et al., 2009). Mutation of similar glycosylation sites in AC6 also causes nonraft targeting (unpublished data). Other known components of lipid raft microdomains, such as caveolin, appear to not have a direct functional role in the lipid raft localization of ACs. Cell lines without caveolin-1, whether by knockout approaches or naturally absent, do not show differences in AC expression or localization (Thangavel et al., 2009).
Nine isoforms of AC exist in the human genome and each isoform has long been known to display specific regulatory properties (Dessauer et al., 2017). AC isoform-specific regulation can be mediated by a diverse array of intracellular components including Gβγ proteins, calcium-calmodulin, protein kinases, and nitric oxide (Tang and Gilman, 1992; Sunahara et al., 1996; Hanoune and Defer, 2001; Patel et al., 2001; Cooper, 2003). Any one particular intracellular regulator can differentially influence the direction of AC activity depending on the isoform. For example, Gβγ subunits stimulate AC2 and AC4; inhibit AC1, AC5, and AC6 (Gao and Gilman, 1991; Tang and Gilman, 1991; Taussig et al., 1993; Bayewitch et al., 1998); and produce conditional stimulation of AC5 or AC6 based on differences in the structural homology of each isoform (Thomas and Hoffman, 1996; Gao et al., 2007). Most AC isoforms are stimulated by protein kinase C (PKC) except for AC6, which is inhibited (Jacobowitz et al., 1993; Kawabe et al., 1994; Lai et al., 1997). Likewise, calcium-calmodulin stimulates AC1, AC3, and AC8 isoforms and AC5 and AC6 are inhibited by the presence of divalent calcium (Tang et al., 1991; Choi et al., 1992; Katsushika et al., 1992; Yoshimura and Cooper, 1992; Cali et al., 1994). The differential effects of other regulatory proteins of AC isoforms continue to be identified including annexin A4, a calcium-dependent phospholipid binding protein that inhibits AC activity in HEK293 and cardiomyocyte cells (Heinick et al., 2015).
The localization of GPCRs and specific ACs to lipid rafts is also responsible for coupling capacitive calcium entry with calcium-dependent AC isoform activity (Cooper et al., 1995; Fagan et al., 1998, 2000; Smith et al., 2002). The importance of calcium-sensitive AC isoforms, such as AC6 or AC8, and their proximity to capacitive calcium entry channels is evident since AC coupling is lost if lipid rafts are disrupted. Thus, regulation of AC isoforms and their downstream cellular functions are also influenced by positioning within different cAMP signaling compartments (Ostrom et al., 2012; Cooper and Tabbasum, 2014). Therefore, both AC isoform-specific regulatory properties and cAMP signaling compartments must be considered as additional dimensions that regulate cAMP effects in a spatiotemporal manner specific to cell type and external stimuli conditions.
Restriction of Signal Diffusion
Generating an intracellular signal in a specific locale has little consequence if the signal freely diffuses through the cell. Rich et al. (2000) described some of the first data showing membrane-delimited cAMP signals that were not detected in the cytosol. Ample evidence has emerged since then that cAMP does not freely diffuse inside cells (Agarwal et al., 2016; Richards et al., 2016); however, the manner in which this second messenger’s movement is restricted remains poorly defined. One can readily imagine that both phosphodiesterase (PDE) activity and physical barriers to diffusion could play a role. Experimental data and modeling of cell signaling in cardiac myocytes reveals that PDE activity cannot account for all of the restricted diffusion of cAMP (Saucerman et al., 2014). Thus, long-distance diffusion of cAMP through a cell must reckon with a variety of factors including the buffering effects of PKA, structural components of the cell that are impervious to small molecules in the cytosol, and enzymes that catalyze its breakdown (Yang et al., 2016).
Davare et al. (2001) were among the first to describe that β2AR signal in a preassembled complex to regulate L-type Ca2+ channels. While no PDE (or A-kinase-anchoring protein [AKAP]) was specifically identified in these early studies, it was clear that a membrane-delimited cAMP signaling event occurred due to formation of specific signaling complexes that contained a GPCR and a downstream effector protein. PDEs possess the essential qualities to play roles in maintaining compartmentalized cAMP signaling (Kokkonen and Kass, 2017). In the cAMP degradation realm, most work has focused on PDE4 (Houslay and Adams, 2003). PDE4D binds to β-arrestin, meaning it can be brought into close association with recently activated GPCRs (Baillie et al., 2003). β-arrestin-PDE4 recruitment regulates β-adrenergic receptors switching from Gs to Gi and coupling to the mitogen-activated protein kinase pathway (Baillie et al., 2003; Lynch et al., 2005). PDEs also bind to certain AKAP isoforms to target their enzymatic activity to specific signaling complexes (discussed in more detail susbequently).
Many different GPCRs form distinct cAMP signaling complexes at the plasma membrane (as discussed previuosly), and PDE activity has been found to play a key role in compartmentation of these responses (Jurevicius and Fischmeister, 1996; Zaccolo and Pozzan, 2002). Considering that the human genome expresses 24 PDE genes (along with a large number of possible splice variants) it is not surprising that different PDE isoforms play distinct roles in cAMP signaling compartments and regulate specific cellular responses (Rochais et al., 2006). Targeted cAMP Forster resonance energy transfer sensors also reveal evidence for differences in basal cAMP levels in lipid raft versus nonraft domains of some cell types (Iancu et al., 2008; Agarwal et al., 2014), but not others (Agarwal et al., 2017). While differences in basal AC activity could partly explain these observations (due to different AC isoforms being expressed in these two domains), nonuniform distribution of PDE activity might also contribute to such effects. Heterogeneity of PDE4 isoforms appears to also contribute to the regulation of different cAMP signals. For example, an examination of signaling from mouse embryonic fibroblasts isolated from PDE4B and PDE4D knockouts reveals that PDE4B has a role in regulating membrane-delimited cAMP signals while PDE4D has a more global role in regulating cytosolic cAMP levels (Blackman et al., 2011). Furthermore, splice variants of PDE4 can also have roles in different cAMP compartments. In cardiac myocytes, PDE4D3 is targeted to various locations where it is involved in spatial control over cAMP/PKA-dependent regulation of ryanodine receptor signaling complexes involving AKAP6 (Lehnart et al., 2005) and IKs potassium channel complexes involving AKAP9 (Terrenoire et al., 2009). As mentioned previously, PDE4 is recruited to the β2AR upon agonist stimulation. This is specifically the PDE4D5 splice variant. On the other hand, the β1AR forms a complex with PDE4D8 that dissociates upon agonist binding (Richter et al., 2008). The β2AR has also been shown to interact with PDE4D9 and PDE4D8 in an agonist-dependent manner as well (Liu et al., 2009).
While the cAMP signaling community has mostly focused on PDE4 isoforms, many other PDEs capable of regulating cAMP levels are encoded in the human genome. We have recently described the expression of PDE8A in HASM cells (Johnstone et al., 2017). This is an interesting PDE in that it is less widely expressed and is not inhibited by 3-isobutyl-1-methylxanthine, a broad spectrum PDE inhibitor historically used in biochemical assays of cAMP. PDE8A appears to selectively localize in the lipid raft fractions from these cells where β2AR and AC6 are enriched. Based on shRNA knockdown as well as pharmacological inhibition, PDE8A activity regulates cAMP generated from stimulation of β2AR but has no effect on cAMP signaling stimulated by PGE2. β2AR-mediated inhibition of HASM cell proliferation is also selectively regulated by PDE8A activity. Therefore, there is at least one PDE isoform that participates in a specific cAMP signaling compartment. The role of all other PDE isoforms in specific cAMP compartments is essentially unknown. We hypothesize that some isoforms are global regulators of cAMP signals (that is, they are not localized to specific compartments), others are specifically localized (as with PDE8A), and still others might regulate a specific complex contextually by moving into a location based on certain stimuli (such as PDE4, bound to β-arrestin, moving to the site of a recently activated GPCR).
Localization of Downstream Effectors
The organization of the GPCR-AC signaling is not limited to just the regional localization of receptor and effector elements based on raft or nonraft compartments. The ramification of cAMP responses also critically depends on the spatial organization of the elements that link PKA activity to a cellular response. The systematic way in which the cAMP signal is sculpted in various cell types to produce differential response has been primarily attributed to the apposition of AC and attendant PDE isoforms (Houslay and Milligan, 1997). However, this view is quickly being expanded with an understanding of the role of AKAPs to direct cAMP actions. AKAPs organize PKA signaling with a wide variety of other molecules via their defining PKA binding motif (Colledge and Scott, 1999). AKAPs distribute regional signals throughout the cell by scaffolding PKA to its intracellular substrates involved in various signaling pathways. These critical functions of AKAPs can be anchored to the site of cAMP generation (Kapiloff et al., 2014). Several diverse members of the AKAP family interact with ACs in an isoform-specific manner and regulate cAMP signaling (Bauman et al., 2006). To date, the specific AKAP-AC interactions have been defined are AKAP9/Yotiao with AC1, AC2, AC3, and AC9 (Piggott et al., 2008); mAKAP with AC2 and AC5 (Kapiloff et al., 2009); and AKAP79/150 with AC2, AC3, AC5, AC6, AC8, and AC9 (Efendiev et al., 2010; Delint-Ramirez et al., 2011; Shen and Cooper, 2013).
The taxonomy of AC-AKAP complexes and their roles in AC regulation continues to be a focus of ongoing research (Dessauer, 2009). The AKAP9/Yotiao complex illustrates the differential role AKAP complexes can have on how associated ACs operate. For example, AKAP9/Yotiao promotes normal functioning of AC1 and AC9, whereas it inhibits activity of AC2 and AC3 upon association (Piggott et al., 2008). Another AKAP, AKAP5 (AKAP79/150), decreases the sensitivity of AC8 to Ca++, while it can also interact with AC5 and AC6 via β2ARs (Bauman et al., 2006; Willoughby et al., 2010). In this configuration PKA associates and phosphorylates β2AR to initiate desensitization, receptor translocation, and even G protein switching (Daaka et al., 1997). PKA can then phosphorylate AC5 and AC6 to inhibit activity or alter AC8 sensitivity to store-operated, AKAP5-mediated calcium entry (Beazely and Watts, 2006; Delint-Ramirez et al., 2011). Therefore, one of the main roles of an AKAP is to provide a substrate for feed forward and backward regulation of the cAMP signaling cascade.
AKAPs also regulate AC activity via PKC phosphorylation. In general, PKC activity will increase activity of certain ACs (AC1, AC2, AC3, AC5, and AC7) but inhibit the activity of others (AC6) (Sunahara et al., 1996; Lai et al., 1997). For example, muscarinic receptor activation of cAMP production occurs via recruitment of PKC to a complex containing the PKC-stimulable AC2, which is organized specifically by AKAP5, with PDE4 and PKA as contributing elements in the complex (Shen and Cooper, 2013). α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid–type glutamate receptors in mouse forebrain slices depend upon AKAP5 anchoring both PKA and AC (Zhang et al., 2013). Mutations of the KCNQ1 subunit of the slow outward potassium channel interrupts its binding to Yotiao, causing loss of PKA regulation of the channel and alteration in the action potential that causes arrhythmias (Marx et al., 2002). Other AKAP-AC complexes have other clear pathophysiological implications (Efendiev and Dessauer, 2011).
AKAPs also bind PDEs. As discussed previously, AKAP9 brings PDE4D3, but not PDE4D5, to a complex with cardiac IKs channels to create tightly integrated signaling (Terrenoire et al., 2009). Previous studies have shown that mAKAP binds PDE4 to facilitate a negative feedback loop by which local cAMP would activate mAKAP-tethered PKA, which in turn would phosphorylate colocalized PDE4 and upregulate its cAMP hydrolyzing activity (Dodge et al., 2001). This operational model was further expanded by findings that AKAP12 (Gravin) also binds PDE4D to allow it to selectively regulate near-membrane cAMP signaling events (Willoughby et al., 2006). Guinzberg et al. (2017) recently described that adenosine A2A receptors couple to an AC6/AKAP79/PDE3A complex in hepatocytes, while in these same cells A2B receptors couple to an AC5/D-AKAP2 complex. The interplay between ACs, AKAPs, PKA, and PDEs highlights the manifold intracellular signaling circuitry that can be organized to elicit specific, tuned responses throughout different cell types. However, much work remains to fully characterize these complexes and understand how they shape cAMP signaling in differentiated cells.
From a Signal to a Response
cAMP signaling compartmentation is of little consequence if the separate signaling pools do not yield distinct cellular responses. We, and others, have described that AC6 has the unique ability to regulate arborization, a form of cytoskeletal reorganization, in HASM cells (Gros et al., 2006; Bogard et al., 2012). AC2 overexpressed in the same cells is unable to mediate the arborization response unless PDE4 activity is inhibited. We have also recently described that overexpression of different AC isoforms yields drastically different gene expression responses. Overexpression of AC2 or AC6 in HASM cells and subsequent stimulation by forskolin leads to gene expression changes that are heterogeneous (Bogard et al., 2013). For example, interleukin 6 production in airway smooth muscle cells is directed by AC2-generated cAMP, whereas somatostatin production was shown to be activated by AC6-derived cAMP (Bogard et al., 2013). In vascular smooth muscle cells and HEK-293 cells, AC1 selectively slowed cell proliferation while AC2, AC5, and AC6 had little effect (Gros et al., 2006). These data suggest the cellular responses depend on the loci of cAMP production within the cell and provide rationale for continuing efforts to define the signaling complexes that lead to cAMP signaling compartmentation in differentiated cells. However, cAMP can elicit hundreds of potential responses in a given cell and we know very little about how many different responses any given localized pool of cAMP can affect.
Conclusions
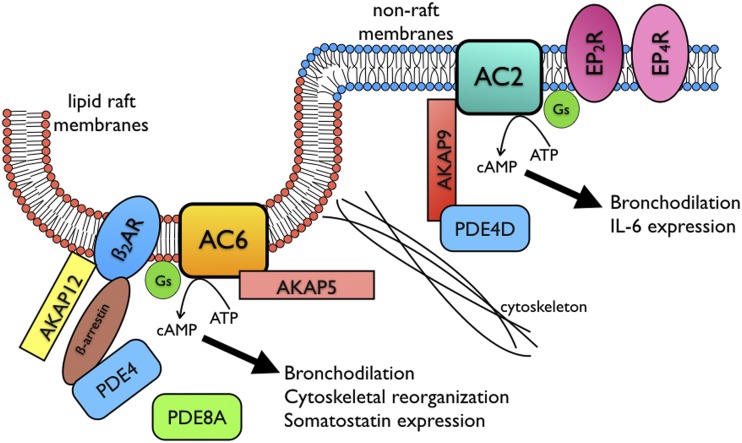
Compartmentation of cAMP signaling allows different GPCRs to use the same second messenger to regulate a unique array of cellular responses. AC isoforms appear to anchor these cAMP signaling compartments since their localization does not change across cell types or activation states. AC isoforms couple to specific GPCRs, synthesize cAMP in distinct subcellular locations, and bind to many other proteins that together can form large signaling complexes. AKAPs are of particular importance because they form specific interactions with GPCRs, ACs, and downstream signaling elements (targets of PKA phosphorylation); cAMP regulatory enzymes (PDEs); and probably other key signaling proteins. However, there are likely other proteins that play critical roles in scaffolding these signaling molecules together. Proteomic and other unbiased approaches are needed to identify the whole range of proteins participating in these signaling complexes. The result of the formation of these multiprotein complexes is that each compartment can have a tailored magnitude, duration, and scope of the cAMP signal generated. These compartmentalized signals are likely dynamic and contextual, as well as cell specific. The proximal elements of two main cAMP signaling compartments in HASM cells have been defined; therefore, we present a diagram that illustrates some of what is known (see Fig. 1). Understanding how these signaling complexes form, what responses they mediate, and how they may be altered in disease should lead to new therapeutic strategies that have far greater efficacy for the desired response with fewer unwanted effects.
Fig. 1.
Schematic diagram of proposed cAMP signaling compartments in HASM cells. Two main cAMP signaling compartments have been defined, one centered around β2AR-AC6 in lipid raft domains (consisting of sphingolipid, shown in red, and cholesterol) and another around EP2/4R-AC2 in nonraft domains (consisting of phospholipid, shown in blue). Illustrated are the main AKAP and PDE isoforms known to assemble in these signaling complexes or regulate cAMP emanating from each location. Cytoskeletal elements likely also form barriers to cAMP diffusion. Cell responses that have been linked to specific compartments are listed. A number of AKAP and PDE isoforms are expressed in HASM but the locations of some are unknown, and thus are not shown. These include PDE3, AKAP2, AKAP3, ezrin, and Map2B. It is also unknown if PDE4D splice variants have different localizations. PDE8A is located in lipid rafts where it regulates β2AR-AC6 signaling, but it is unknown how it is tethered specifically in this location. AKAP12 (gravin), AKAP5 (AKAP79), AKAP9 (yotiao).
Abbreviations
- AC
adenylyl cyclase
- AKAP
A-kinase-anchoring protein
- β1AR
beta-1 adrenergic receptor
- β2AR
beta-2 adrenergic receptor
- GPCR
G protein-coupled receptor
- HASM
human airway smooth muscle
- PDE
phosphodiesterase
- PGE2
prostaglandin E2
- PKA
protein kinase A
- PKC
protein kinase C
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Johnstone, Agarwal, Harvey, Ostrom.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM107094].
References
- Agarwal SR, Clancy CE, Harvey RD. (2016) Mechanisms restricting diffusion of intracellular cAMP. Sci Rep 6:19577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal SR, Miyashiro K, Latt H, Ostrom RS, Harvey RD. (2017) Compartmentalized cAMP responses to prostaglandin EP2 receptor activation in human airway smooth muscle cells. Br J Pharmacol 174:2784–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal SR, Yang PC, Rice M, Singer CA, Nikolaev VO, Lohse MJ, Clancy CE, Harvey RD. (2014) Role of membrane microdomains in compartmentation of cAMP signaling. PLoS One 9:e95835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. (2003) β-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates β-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci USA 100:940–945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DM, Dessauer CW, et al. (2006) Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell 23:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayewitch ML, Avidor-Reiss T, Levy R, Pfeuffer T, Nevo I, Simonds WF, Vogel Z. (1998) Inhibition of adenylyl cyclase isoforms V and VI by various Gβγ subunits. FASEB J 12:1019–1025. [DOI] [PubMed] [Google Scholar]
- Beazely MA, Watts VJ. (2006) Regulatory properties of adenylate cyclases type 5 and 6: a progress report. Eur J Pharmacol 535:1–12. [DOI] [PubMed] [Google Scholar]
- Blackman BE, Horner K, Heidmann J, Wang D, Richter W, Rich TC, Conti M. (2011) PDE4D and PDE4B function in distinct subcellular compartments in mouse embryonic fibroblasts. J Biol Chem 286:12590–12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogard AS, Adris P, Ostrom RS. (2012) Adenylyl cyclase 2 selectively couples to E prostanoid type 2 receptors, whereas adenylyl cyclase 3 is not receptor-regulated in airway smooth muscle. J Pharmacol Exp Ther 342:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogard AS, Birg AV, Ostrom RS. (2013) Non-raft adenylyl cyclase 2 defines a cAMP signaling compartment that selectively regulates IL-6 expression in airway smooth muscle cells: differential regulation of gene expression by AC isoforms. Naunyn Schmiedebergs Arch Pharmacol 387:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogard AS, Xu C, Ostrom RS. (2011) Human bronchial smooth muscle cells express adenylyl cyclase isoforms 2, 4, and 6 in distinct membrane microdomains. J Pharmacol Exp Ther 337:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton LL, Hayes JS, Mayer SE. (1979) Hormonally specific phosphorylation of cardiac troponin I and activation of glycogen phosphorylase. Nature 280:78–80. [DOI] [PubMed] [Google Scholar]
- Buxton IL, Brunton LL. (1983) Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem 258:10233–10239. [PubMed] [Google Scholar]
- Cali JJ, Zwaagstra JC, Mons N, Cooper DM, Krupinski J. (1994) Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem 269:12190–12195. [PubMed] [Google Scholar]
- Choi EJ, Xia Z, Storm DR. (1992) Stimulation of the type III olfactory adenylyl cyclase by calcium and calmodulin. Biochemistry 31:6492–6498. [DOI] [PubMed] [Google Scholar]
- Colledge M, Scott JD. (1999) AKAPs: from structure to function. Trends Cell Biol 9:216–221. [DOI] [PubMed] [Google Scholar]
- Cooper DM. (2003) Regulation and organization of adenylyl cyclases and cAMP. Biochem J 375:517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DM, Mons N, Karpen JW. (1995) Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature 374:421–424. [DOI] [PubMed] [Google Scholar]
- Cooper DM, Tabbasum VG. (2014) Adenylate cyclase-centred microdomains. Biochem J 462:199–213. [DOI] [PubMed] [Google Scholar]
- Cros C, Brette F. (2013) Functional subcellular distribution of β1- and β2-adrenergic receptors in rat ventricular cardiac myocytes. Physiol Rep 1:e00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossthwaite AJ, Seebacher T, Masada N, Ciruela A, Dufraux K, Schultz JE, Cooper DM. (2005) The cytosolic domains of Ca2+-sensitive adenylyl cyclases dictate their targeting to plasma membrane lipid rafts. J Biol Chem 280:6380–6391. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. (1997) Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 390:88–91. [DOI] [PubMed] [Google Scholar]
- Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. (2001) A β2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science 293:98–101. [DOI] [PubMed] [Google Scholar]
- Delint-Ramirez I, Willoughby D, Hammond GR, Ayling LJ, Cooper DM. (2011) Palmitoylation targets AKAP79 protein to lipid rafts and promotes its regulation of calcium-sensitive adenylyl cyclase type 8 [published correction appears in J Biol Chem (2015)]. J Biol Chem 286:32962–32975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessauer CW. (2009) Adenylyl cyclase—A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol 76:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessauer CW, Watts VJ, Ostrom RS, Conti M, Dove S, Seifert R. (2017) International union of basic and clinical pharmacology. CI. Structures and small molecule modulators of mammalian adenylyl cyclases. Pharmacol Rev 69:93–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. (2001) mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J 20:1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efendiev R, Dessauer CW. (2011) A kinase-anchoring proteins and adenylyl cyclase in cardiovascular physiology and pathology. J Cardiovasc Pharmacol 58:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efendiev R, Samelson BK, Nguyen BT, Phatarpekar PV, Baameur F, Scott JD, Dessauer CW. (2010) AKAP79 interacts with multiple adenylyl cyclase (AC) isoforms and scaffolds AC5 and -6 to α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors. J Biol Chem 285:14450–14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich AT, Furuyashiki T, Kitaoka S, Kakizuka A, Narumiya S. (2013) Prostaglandin E receptor EP1 forms a complex with dopamine D1 receptor and directs D1-induced cAMP production to adenylyl cyclase 7 through mobilizing G(betagamma) subunits in human embryonic kidney 293T cells. Mol Pharmacol 84:476–486. [DOI] [PubMed] [Google Scholar]
- Emery AC, Liu XH, Xu W, Eiden MV, Eiden LE. (2015) Cyclic Adenosine 3′,5′-Monophosphate Elevation and Biological Signaling through a Secretin Family Gs-Coupled G Protein-Coupled Receptor Are Restricted to a Single Adenylate Cyclase Isoform. Mol Pharmacol 87:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan KA, Mons N, Cooper DMF. (1998) Dependence of the Ca2+-inhibitable adenylyl cyclase of C6-2B glioma cells on capacitative Ca2+ entry. J Biol Chem 273:9297–9305. [DOI] [PubMed] [Google Scholar]
- Fagan KA, Smith KE, Cooper DM. (2000) Regulation of the Ca2+-inhibitable adenylyl cyclase type VI by capacitative Ca2+ entry requires localization in cholesterol-rich domains. J Biol Chem 275:26530–26537. [DOI] [PubMed] [Google Scholar]
- Gao BN, Gilman AG. (1991) Cloning and expression of a widely distributed (type IV) adenylyl cyclase. Proc Natl Acad Sci USA 88:10178–10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Sadana R, Dessauer CW, Patel TB. (2007) Conditional stimulation of type V and VI adenylyl cyclases by G protein betagamma subunits. J Biol Chem 282:294–302. [DOI] [PubMed] [Google Scholar]
- Gros R, Ding Q, Chorazyczewski J, Pickering JG, Limbird LE, Feldman RD. (2006) Adenylyl cyclase isoform-selective regulation of vascular smooth muscle proliferation and cytoskeletal reorganization. Circ Res 99:845–852. [DOI] [PubMed] [Google Scholar]
- Guinzberg R, Díaz-Cruz A, Acosta-Trujillo C, Vilchis-Landeros MM, Vázquez-Meza H, Lozano-Flores C, Chiquete-Felix N, Varela-Echavarría A, Uribe-Carvajal S, Riveros-Rosas H, et al. (2017) Newly synthesized cAMP is integrated at a membrane protein complex signalosome to ensure receptor response specificity. FEBS J 284:258–276. [DOI] [PubMed] [Google Scholar]
- Hanoune J, Defer N. (2001) Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 41:145–174. [DOI] [PubMed] [Google Scholar]
- Hayes JS, Brunton LL, Brown JH, Reese JB, Mayer SE. (1979) Hormonally specific expression of cardiac protein kinase activity. Proc Natl Acad Sci USA 76:1570–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinick A, Husser X, Himmler K, Kirchhefer U, Nunes F, Schulte JS, Seidl MD, Rolfes C, Dedman JR, Kaetzel MA, et al. (2015) Annexin A4 is a novel direct regulator of adenylyl cyclase type 5. FASEB J 29:3773–3787. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. (2003) PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J 370:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Milligan G. (1997) Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem Sci 22:217–224. [DOI] [PubMed] [Google Scholar]
- Iancu RV, Ramamurthy G, Warrier S, Nikolaev VO, Lohse MJ, Jones SW, Harvey RD. (2008) Cytoplasmic cAMP concentrations in intact cardiac myocytes. Am J Physiol Cell Physiol 295:C414–C422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel PA, Wilderman A, Zambon AC, Snead AN, Murray F, Aroonsakool N, McDonald DS, Zhou S, McCann T, Zhang L, et al. (2015) G protein-coupled receptor (GPCR) expression in native cells: “novel” endoGPCRs as physiologic regulators and therapeutic targets. Mol Pharmacol 88:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobowitz O, Chen J, Premont RT, Iyengar R. (1993) Stimulation of specific types of Gs-stimulated adenylyl cyclases by phorbol ester treatment. J Biol Chem 268:3829–3832. [PubMed] [Google Scholar]
- Johnstone TB, Smith KH, Koziol-White CJ, Li F, Kazarian AG, Corpuz ML, Shumyatcher M, Ehlert FJ, Himes BA, Panettieri RA, et al. (2017) PDE8 is expressed in human airway smooth muscle and selectively regulates cAMP signaling by β2AR-AC6. Am J Respir Cell Mol Biol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurevicius J, Fischmeister R. (1996) cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by β-adrenergic agonists. Proc Natl Acad Sci USA 93:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapiloff MS, Piggott LA, Sadana R, Li J, Heredia LA, Henson E, Efendiev R, Dessauer CW. (2009) An adenylyl cyclase-mAKAPβ signaling complex regulates cAMP levels in cardiac myocytes. J Biol Chem 284:23540–23546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapiloff MS, Rigatti M, Dodge-Kafka KL. (2014) Architectural and functional roles of A kinase-anchoring proteins in cAMP microdomains. J Gen Physiol 143:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsushika S, Chen L, Kawabe J, Nilakantan R, Halnon NJ, Homcy CJ, Ishikawa Y. (1992) Cloning and characterization of a sixth adenylyl cyclase isoform: types V and VI constitute a subgroup within the mammalian adenylyl cyclase family. Proc Natl Acad Sci USA 89:8774–8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe J, Iwami G, Ebina T, Ohno S, Katada T, Ueda Y, Homcy CJ, Ishikawa Y. (1994) Differential activation of adenylyl cyclase by protein kinase C isoenzymes. J Biol Chem 269:16554–16558. [PubMed] [Google Scholar]
- Kokkonen K, Kass DA. (2017) Nanodomain regulation of cardiac cyclic nucleotide signaling by phosphodiesterases. Annu Rev Pharmacol Toxicol 57:455–479. [DOI] [PubMed] [Google Scholar]
- Lai HL, Yang TH, Messing RO, Ching YH, Lin SC, Chern Y. (1997) Protein kinase C inhibits adenylyl cyclase type VI activity during desensitization of the A2a-adenosine receptor-mediated cAMP response. J Biol Chem 272:4970–4977. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, Richter W, Jin SL, Conti M, Marks AR. (2005) Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell 123:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Ramani B, Soto D, De Arcangelis V, Xiang Y. (2009) Agonist dose-dependent phosphorylation by protein kinase A and G protein-coupled receptor kinase regulates β2 adrenoceptor coupling to Gi proteins in cardiomyocytes. J Biol Chem 284:32279–32287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Thangavel M, Sun SQ, Kaminsky J, Mahautmr P, Stitham J, Hwa J, Ostrom RS. (2008) Adenylyl cyclase type 6 overexpression selectively enhances β-adrenergic and prostacyclin receptor-mediated inhibition of cardiac fibroblast function because of colocalization in lipid rafts. Naunyn Schmiedebergs Arch Pharmacol 377:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MJ, Baillie GS, Mohamed A, Li X, Maisonneuve C, Klussmann E, van Heeke G, Houslay MD. (2005) RNA silencing identifies PDE4D5 as the functionally relevant cAMP phosphodiesterase interacting with βarrestin to control the protein kinase A/AKAP79-mediated switching of the β2-adrenergic receptor to activation of ERK in HEK293B2 cells. J Biol Chem 280:33178–33189. [DOI] [PubMed] [Google Scholar]
- Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, Marks AR, Kass RS. (2002) Requirement of a macromolecular signaling complex for β adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 295:496–499. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. (2010) β2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327:1653–1657. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Bogard AS, Gros R, Feldman RD. (2012) Choreographing the adenylyl cyclase signalosome: sorting out the partners and the steps. Naunyn Schmiedebergs Arch Pharmacol 385:5–12. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. (2001) Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J Biol Chem 276:42063–42069. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Insel PA. (2004) The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol 143:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom RS, Liu X, Head BP, Gregorian C, Seasholtz TM, Insel PA. (2002) Localization of adenylyl cyclase isoforms and G protein-coupled receptors in vascular smooth muscle cells: expression in caveolin-rich and noncaveolin domains. Mol Pharmacol 62:983–992. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Violin JD, Coleman S, Insel PA. (2000) Selective enhancement of β-adrenergic receptor signaling by overexpression of adenylyl cyclase type 6: colocalization of receptor and adenylyl cyclase in caveolae of cardiac myocytes. Mol Pharmacol 57:1075–1079. [PubMed] [Google Scholar]
- Pagano M, Clynes MA, Masada N, Ciruela A, Ayling LJ, Wachten S, Cooper DM. (2009) Insights into the residence in lipid rafts of adenylyl cyclase AC8 and its regulation by capacitative calcium entry. Am J Physiol Cell Physiol 296:C607–C619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TB, Du Z, Pierre S, Cartin L, Scholich K. (2001) Molecular biological approaches to unravel adenylyl cyclase signaling and function. Gene 269:13–25. [DOI] [PubMed] [Google Scholar]
- Piggott LA, Bauman AL, Scott JD, Dessauer CW. (2008) The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc Natl Acad Sci USA 105:13835–13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post SR, Hilal-Dandan R, Urasawa K, Brunton LL, Insel PA. (1995) Quantification of signalling components and amplification in the beta-adrenergic-receptor-adenylate cyclase pathway in isolated adult rat ventricular myocytes. Biochem J 311:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raslan Z, Naseem KM. (2015) Compartmentalisation of cAMP-dependent signalling in blood platelets: The role of lipid rafts and actin polymerisation. Platelets 26:349–357. [DOI] [PubMed] [Google Scholar]
- Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. (2000) Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol 116:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, Lomas O, Jalink K, Ford KL, Vaughan-Jones RD, Lefkimmiatis K, Swietach P. (2016) Intracellular tortuosity underlies slow cAMP diffusion in adult ventricular myocytes. Cardiovasc Res 110:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W, Day P, Agrawal R, Bruss MD, Granier S, Wang YL, Rasmussen SG, Horner K, Wang P, Lei T, et al. (2008) Signaling from β1- and β2-adrenergic receptors is defined by differential interactions with PDE4. EMBO J 27:384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochais F, Abi-Gerges A, Horner K, Lefebvre F, Cooper DM, Conti M, Fischmeister R, Vandecasteele G. (2006) A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ Res 98:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucerman JJ, Greenwald EC, Polanowska-Grabowska R. (2014) Mechanisms of cyclic AMP compartmentation revealed by computational models. J Gen Physiol 143:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Dekker FJ, Maarsingh H. (2013) Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol Rev 65:670–709. [DOI] [PubMed] [Google Scholar]
- Shen JX, Cooper DM. (2013) AKAP79, PKC, PKA and PDE4 participate in a Gq-linked muscarinic receptor and adenylate cyclase 2 cAMP signalling complex. Biochem J 455:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Gu C, Fagan KA, Hu B, Cooper DM. (2002) Residence of adenylyl cyclase type 8 in caveolae is necessary but not sufficient for regulation by capacitative Ca2+ entry. J Biol Chem 277:6025–6031. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG. (1996) Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol 36:461–480. [DOI] [PubMed] [Google Scholar]
- Tang WJ, Gilman AG. (1991) Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science 254:1500–1503. [DOI] [PubMed] [Google Scholar]
- Tang WJ, Gilman AG. (1992) Adenylyl cyclases. Cell 70:869–872. [DOI] [PubMed] [Google Scholar]
- Tang WJ, Krupinski J, Gilman AG. (1991) Expression and characterization of calmodulin-activated (type I) adenylylcyclase. J Biol Chem 266:8595–8603. [PubMed] [Google Scholar]
- Taussig R, Quarmby LM, Gilman AG. (1993) Regulation of purified type I and type II adenylylcyclases by G protein βγ subunits. J Biol Chem 268:9–12. [PubMed] [Google Scholar]
- Terrenoire C, Houslay MD, Baillie GS, Kass RS. (2009) The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J Biol Chem 284:9140–9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel M, Liu X, Sun SQ, Kaminsky J, Ostrom RS. (2009) The C1 and C2 domains target human type 6 adenylyl cyclase to lipid rafts and caveolae. Cell Signal 21:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JM, Hoffman BB. (1996) Isoform-specific sensitization of adenylyl cyclase activity by prior activation of inhibitory receptors: role of beta gamma subunits in transducing enhanced activity of the type VI isoform. Mol Pharmacol 49:907–914. [PubMed] [Google Scholar]
- Timofeyev V, Myers RE, Kim HJ, Woltz RL, Sirish P, Heiserman JP, Li N, Singapuri A, Tang T, Yarov-Yarovoy V, et al. (2013) Adenylyl cyclase subtype-specific compartmentalization: differential regulation of L-type Ca2+ current in ventricular myocytes. Circ Res 112:1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunematsu T, Okumura S, Mototani Y, Ohnuki Y, Jin H, Cai W, Suita K, Sato I, Umemura M, Yokoyama U, et al. (2015) Coupling of β1-adrenergic receptor to type 5 adenylyl cyclase and its physiological relevance in cardiac myocytes. Biochem Biophys Res Commun 458:531–535. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Masada N, Wachten S, Pagano M, Halls ML, Everett KL, Ciruela A, Cooper DM. (2010) AKAP79/150 interacts with AC8 and regulates Ca2+-dependent cAMP synthesis in pancreatic and neuronal systems. J Biol Chem 285:20328–20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D, Wong W, Schaack J, Scott JD, Cooper DM. (2006) An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. EMBO J 25:2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PC, Boras BW, Jeng MT, Docken SS, Lewis TJ, McCulloch AD, Harvey RD, Clancy CE. (2016) A computational modeling and simulation approach to investigate mechanisms of subcellular cAMP compartmentation. PLOS Comput Biol 12:e1005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Cooper DM. (1992) Cloning and expression of a Ca2+-inhibitable adenylyl cyclase from NCB-20 cells. Proc Natl Acad Sci USA 89:6716–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Sun M, Villar VA, Zhang Y, Weinman EJ, Felder RA, Jose PA. (2014) Differential dopamine receptor subtype regulation of adenylyl cyclases in lipid rafts in human embryonic kidney and renal proximal tubule cells. Cell Signal 26:2521–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. (2002) Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295:1711–1715. [DOI] [PubMed] [Google Scholar]
- Zhang M, Patriarchi T, Stein IS, Qian H, Matt L, Nguyen M, Xiang YK, Hell JW. (2013) Adenylyl cyclase anchoring by a kinase anchor protein AKAP5 (AKAP79/150) is important for postsynaptic β-adrenergic signaling. J Biol Chem 288:17918–17931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XB, Lutz S, Steffens F, Korth M, Wieland T. (2007) Oxytocin receptors differentially signal via Gq and Gi proteins in pregnant and nonpregnant rat uterine myocytes: implications for myometrial contractility. Mol Endocrinol 21:740–752. [DOI] [PubMed] [Google Scholar]
- Zhou XB, Wang GX, Huneke B, Wieland T, Korth M. (2000) Pregnancy switches adrenergic signal transduction in rat and human uterine myocytes as probed by BKCa channel activity. JJ Physiol 24:339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]