Key Points
Question
Does the use of tumor-treating fields (TTFields), consisting of low-intensity, alternating electric fields delivered via transducer arrays applied to the scalp, when added to maintenance temozolomide chemotherapy, improve progression-free survival for patients with glioblastoma?
Findings
In this randomized clinical trial involving 695 patients with glioblastoma who had completed initial radiochemotherapy, median progression-free survival from randomization was 6.7 months in the TTFields plus temozolomide group and 4.0 months in the temozolomide-alone group (hazard ratio, 0.63), a significant difference.
Meaning
Among patients with glioblastoma, the addition of TTFields to maintenance temozolomide chemotherapy resulted in statistically significant improvement in survival. These results are consistent with those reported in a previous interim analysis.
Abstract
Importance
Tumor-treating fields (TTFields) is an antimitotic treatment modality that interferes with glioblastoma cell division and organelle assembly by delivering low-intensity alternating electric fields to the tumor.
Objective
To investigate whether TTFields improves progression-free and overall survival of patients with glioblastoma, a fatal disease that commonly recurs at the initial tumor site or in the central nervous system.
Design, Setting, and Participants
In this randomized, open-label trial, 695 patients with glioblastoma whose tumor was resected or biopsied and had completed concomitant radiochemotherapy (median time from diagnosis to randomization, 3.8 months) were enrolled at 83 centers (July 2009-2014) and followed up through December 2016. A preliminary report from this trial was published in 2015; this report describes the final analysis.
Interventions
Patients were randomized 2:1 to TTFields plus maintenance temozolomide chemotherapy (n = 466) or temozolomide alone (n = 229). The TTFields, consisting of low-intensity, 200 kHz frequency, alternating electric fields, was delivered (≥ 18 hours/d) via 4 transducer arrays on the shaved scalp and connected to a portable device. Temozolomide was administered to both groups (150-200 mg/m2) for 5 days per 28-day cycle (6-12 cycles).
Main Outcomes and Measures
Progression-free survival (tested at α = .046). The secondary end point was overall survival (tested hierarchically at α = .048). Analyses were performed for the intent-to-treat population. Adverse events were compared by group.
Results
Of the 695 randomized patients (median age, 56 years; IQR, 48-63; 473 men [68%]), 637 (92%) completed the trial. Median progression-free survival from randomization was 6.7 months in the TTFields-temozolomide group and 4.0 months in the temozolomide-alone group (HR, 0.63; 95% CI, 0.52-0.76; P < .001). Median overall survival was 20.9 months in the TTFields-temozolomide group vs 16.0 months in the temozolomide-alone group (HR, 0.63; 95% CI, 0.53-0.76; P < .001). Systemic adverse event frequency was 48% in the TTFields-temozolomide group and 44% in the temozolomide-alone group. Mild to moderate skin toxicity underneath the transducer arrays occurred in 52% of patients who received TTFields-temozolomide vs no patients who received temozolomide alone.
Conclusions and Relevance
In the final analysis of this randomized clinical trial of patients with glioblastoma who had received standard radiochemotherapy, the addition of TTFields to maintenance temozolomide chemotherapy vs maintenance temozolomide alone, resulted in statistically significant improvement in progression-free survival and overall survival. These results are consistent with the previous interim analysis.
Trial Registration
clinicaltrials.gov Identifier: NCT00916409
This randomized trial compares the effects of tumor-treating fields plus temozolomide vs temozolomide alone on progression-free and overall survival in patients with glioblastoma who had undergone resection or biopsy and radiochemotherapy.
Introduction
Glioblastoma is the most common and aggressive primary brain tumor with an annual incidence of 3.19 per 100 000.1,2,3,4,5 The disease course is typically rapid, with only approximately 1 in 4 patients alive 2 years after diagnosis, and only 5% to 10% of patients alive at 5 years.1,6,7
Since the current standard of care was established, consisting of surgical resection or biopsy, followed by radiotherapy with concomitant temozolomide chemotherapy, followed by maintenance temozolomide for 6 to 12 months,6 little progress has been made in the treatment of this disease.3,8,9 Most trials have shown median progression-free survival and median overall survival from diagnosis of 6.2 to 7.5 months and 14.6 to 16.7 months, respectively.4,5,6,8
Tumor-treating fields (TTFields) are an antimitotic treatment that selectively affects dividing glioblastoma cells by delivering low-intensity, intermediate-frequency (200 kHz) alternating electric fields via transducer arrays applied to the scalp.10,11 Tumor-treating fields cause mitotic arrest and apoptosis of rapidly dividing cells.10,11 Preclinical studies demonstrated increased sensitivity to chemotherapy with the addition of TTFields in human glioblastoma cell lines and in animal tumor models.12 In a randomized phase 3 trial involving 237 patients with recurrent glioblastoma whose several lines of prior therapy had failed, TTFields monotherapy was compared with the treating physicians’ best choice of salvage chemotherapy. Although no survival difference was observed, the higher objective response rate (12% vs 7%) suggested single-modality activity of TTFields.13
In 2009, this randomized phase 3 clinical trial was initiated, comparing maintenance temozolomide alone with maintenance temozolomide in combination with TTFields among patients with glioblastoma. A preplanned interim analysis involving the first 315 patients randomized was previously reported and demonstrated improved progression-free and overall survival.14 This article reports the final analysis involving all 695 randomized patients, with a median follow-up of 40 months and a minimum follow-up of 24 months.
Methods
The study was approved by the institutional review boards or ethics committees of all participating centers, and all patients provided written informed consent before entering the study. The trial protocol and statistical analysis plan are included in Supplement 1.
Study Population
Patients eligible for this study were aged 18 years or older, had a Karnofsky performance score of 70 or higher (a score of ≥70 ensures independence in activities of daily living), and had newly diagnosed and histologically confirmed supratentorial glioblastoma (World Health Organization [WHO] grade IV astrocytoma15). All participants had undergone maximal safe debulking surgery when feasible or biopsy and had completed standard radiotherapy with concomitant temozolomide at the time of enrollment. Prior use of implanted carmustine wafers was allowed. Patients with evidence of progressive disease following radiochemotherapy, infratentorial tumor location, and severe comorbidities were excluded. Adequate hematological, liver, and kidney function tests to allow for temozolomide chemotherapy were required.6,14,16
Study Design and Treatment
This multicenter, open-label, randomized clinical phase 3 trial, recruited 695 patients at 83 sites in North America, Europe, the Republic of Korea, and Israel. The trial was designed to test the efficacy and safety of TTFields in combination with best standard of care in the treatment of newly diagnosed glioblastoma. Patients were randomized after the end of radiochemotherapy at a ratio of 2:1 to receive standard maintenance temozolomide chemotherapy (150-200 mg/m2/d for 5 days every 28 days for 6 cycles) with or without the addition of TTFields. Tumor treating fields treatment was to be initiated at least 4 weeks but not more than 7 weeks from the last day of radiotherapy. Maintenance temozolomide was delivered in 28-day cycles according to the protocol established by the European Organisation for Research and Treatment of Cancer (EORTC) Brain Tumor and Radiotherapy Groups and the National Cancer Institute of Canada (NCIC) Clinical Trials Group.6 Extension of the duration of maintenance temozolomide beyond 6 cycles was allowed per local practice. Randomization was performed using a central web-based randomization system and was stratified by extent of resection (biopsy, partial resection, gross total resection) and by the methylation status of the O6-methylguanine-DNA methyltransferase (MGMT) gene promoter (methylated, unmethylated, unknown).
Treatment with TTFields was delivered through 4 transducer arrays with 9 insulated electrodes each placed on the shaved scalp and connected to a portable device set to generate 200-kHz electric fields within the brain (Optune, Novocure Inc). Transducer array layouts were determined using a TTFields mapping software system to optimize field intensity within the treated tumor (NovoTAL, Novocure Inc). Patients were trained by the nursing staff and device technician to operate the device independently, replace transducer arrays, and troubleshoot any alarm conditions (eg, disconnected cables). All treatment was delivered on an outpatient basis and at home. The transducer arrays were supplied in individual sterile packages, and replaced by the patient, a caregiver, or a device technician twice a week. Although uninterrupted treatment was recommended, the patient could take short treatment breaks to tend to personal needs. The patient was advised to continue treatment for no fewer than 18 hours a day.
If tumor progression occurred, second-line therapy was offered per local practice. However, in the experimental group, TTFields could be continued until second radiologic progression occurred or for a maximum of 24 months.
Patient Surveillance and Follow-up
Patients diagnosed with glioblastoma who had undergone surgical resection or biopsy and had received standard radiochemotherapy were randomized to receive either TTFields plus temozolomide or temozolomide alone between July 2009 and December 2014 (Figure 1). The database was locked for final analysis on December 28, 2016. Baseline contrast–enhanced magnetic resonance imaging (MRI) of the brain was required within 2 weeks before starting treatment with maintenance temozolomide with or without TTFields. A complete physical examination and laboratory parameters were performed within 1 week of treatment start. Evaluation also included the EORTC QLQ-C30 quality-of-life questionnaire with its brain-specific module (BN-20)17,18 and a Mini-Mental State Examination (a test result of 27-30 points is considered normal function). Patients were seen monthly for medical follow-up and routine laboratory examinations. Quality of life was assessed every 3 months.
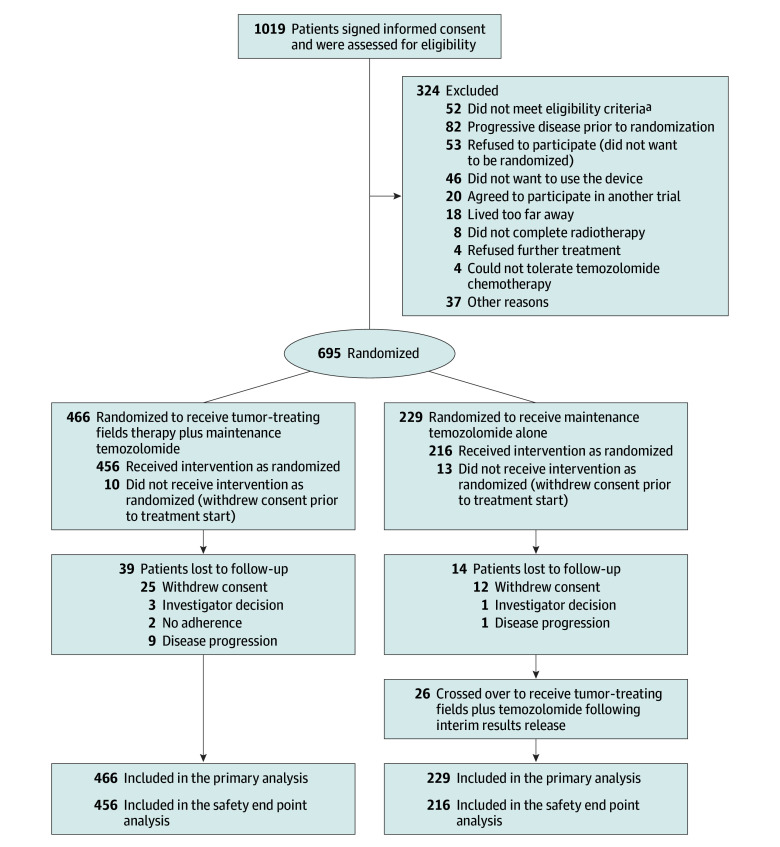
Figure 1. Recruitment and Inclusion of Patients in the Study.
aTen patients were out of randomization window; 8 had low platelet counts; 17, infratentorial disease; 4, elevated liver enzymes; 3, programmable shunts; 10, pacemakers or defibrillators.
Adverse events were recorded for 2 months after treatment discontinuation according to National Cancer Institute Common Toxicity Criteria (NCI-CTC) v3.0. Adverse events were presented descriptively as number and percentage of patients with each adverse event term for all patients available at the time of the analysis.
Independent Radiological Review
Magnetic resonance imaging was performed at 2-month intervals until second progression. In the event of clinical progression, MRI was to be performed within 1 week after the investigator had become aware of it. All MRIs were reviewed by 2 blinded central independent radiologists (BioClinica Inc) and were evaluated for tumor response and progression (Macdonald criteria19). For cases in which the 2 reviewers were not in agreement, a third blinded radiologist adjudicated between them.
Central MGMT Testing, Pathology Review, and Molecular Analyses
In patients with paraffin-embedded tumor tissue available, evaluation of the MGMT methylation status was performed using quantitative methylation-specific polymerase chain reaction3,20 by a central laboratory licensed by MDxHealth. If the MGMT methylation status could not be determined centrally prior to randomization, local MGMT methylation status was used for stratification. All data analyses were based on the central blinded assessment.
Patients were included based on initial local histological diagnosis. A retrospective pathology review and evaluation of molecular testing was performed by a neuropathologist (B.L.) and molecular biologist (M.E.H.). Deletion of chromosomal arms 1p and 19q and amplification of the epidermal growth factor receptor (EGFR) were evaluated by fluorescent in situ hybridization (FISH), immunohistochemistry (IHC), or both; and the mutation status of the isocitrate dehydrogenase 1 (IDH1) gene was determined by immunohistochemistry for the most common mutant IDH1-R132H as described previously.21 For cases in which insufficient tissue was available for EGFR FISH, the result of EGFR IHC was used as a surrogate (Hirsch score, ≥200 amplified; <200, not amplified).22
Outcomes
Primary and Secondary End Points
The primary end point was progression-free survival, and the secondary end point was overall survival, with analyses conducted in the intent-to-treat population.
The protocol defined that overall survival would be analyzed in a per-protocol population including only patients who received their original allocated treatments. However, 26 patients (11%) in the temozolomide-alone control group crossed over and received TTFields after December 2014, following release of the results of the interim analysis of the trial. These 26 patients had more favorable baseline characteristics than the rest of the control patients (MGMT methylated, 48%; Karnofsky performance score, 80-100; time from end of radiotherapy to randomization, 31 days) and received more cycles of temozolomide (median, 10.5 cycles). To avoid possible bias, these patients were analyzed as randomized in the control group according to the intent-to-treat principle.
Exploratory End Points
Other predefined exploratory end points were percentage of patients alive and progression free at 6 months, annualized survival rates, quality of life, Mini-Mental State Examination, and Karnofsky performance score. The quality-of-life data are not reported in this article.
Statistical Analysis
Primary and Secondary End Points
For the primary end point of progression-free survival, the calculated sample size was 700 patients aimed to detect a hazard ratio (HR) of 0.78 or less, with 80% power allowing for 10% loss to follow-up and a 2-sided α = .05. Overall survival was a powered secondary end point in the study (80% power; HR, 0.76; 2-sided α = .05). To avoid multiplicity, overall survival was to be tested statistically only if the primary end point of the study was met.
To allow for 2 analyses in the trial, the final type I error of 0.05 was split between the interim and final analyses based on a standard α spending function (Lan and DeMets23,24). The primary end point at the final analysis would be achieved if progression-free survival was significantly longer in the TTFields plus temozolomide group using a stratified log-rank test (stratified by the randomization strata) with an α of .046 (an α of 0.014 was spent on the interim analysis).
The secondary end point would be achieved at the final analysis if overall survival was significantly longer in the TTFields plus temozolomide group using a stratified log-rank test with an α of .048 (an α of .006 was spent on the interim analysis).
Missing Data
For the analysis of progression-free survival patients were censored for progression when treatment was changed before evidence of progression (at the date of treatment change), at the date of their last MRI if lost to follow up, or upon reaching the cutoff date without progression. For the analysis of overall survival, patients without a known date of death were censored at the last known date they were documented to be alive.
Exploratory End Points
The exploratory end points of annual survival rates and the rate of progression-free survival at 6 months were compared between groups using a 1-sided Z distribution of the Kaplan-Meier estimates of the survival rates at the defined time point. In addition, the Cox proportional hazards model was used to analyze both progression-free survival and overall survival controlling for treatment group, age, sex, MGMT methylation status (as determined by the central laboratory), tumor location in the brain, and country of residence (United States vs all other countries). The threshold for significant interactions in the model was specified at an α of .05.
Post Hoc Analysis
Post hoc analyses of prespecified subgroups (MGMT promoter methylation status, extent of resection (complete, partial resection, or biopsy), age (continuous), performance status (90-100 vs ≤80), sex, and geographic region (United States vs the rest of the world) was performed using a multivariate analysis testing the difference between treatment groups while controlling for the other prognostic factors.
Analysis of Adverse Events and Tolerability
Differences in the incidence of adverse events between groups was tested using a χ2 test at an α of .05. The incidence of adverse events was also compared between groups after normalizing the incidence to the average treatment duration per group. Differences in the time to decline in Karnofsky performance score and Mini-Mental State Examination were tested using a log-rank test at an α of .05. All analyses were performed using SAS version 9.4.
Results
Study Participants
Four hundred and sixty-six patients were randomized to receive TTFields plus temozolomide and 229 to receive temozolomide alone (Figure 1). Patient baseline characteristics were balanced between the 2 groups (Table 1). The median age was 56 years (interquartile range [IQR], 48-63 years), 68% were men, and median Karnofsky performance score was 90%. Eighty-nine percent of patients were white, and 49% of the patients were treated in the United States.
Table 1. Patient and Treatment Characteristics.
| Characteristics | No. (%) of Patients | |
|---|---|---|
| TTFields + Temozolomide (n = 466) |
Temozolomide Alone (n = 229) |
|
| Age, y | ||
| Median (range) | 56.0 (19-83) |
57.0 (19-80) |
| ≥65 | 89 (19) | 45 (20) |
| <65 | 377 (81) | 184 (80) |
| Karnofsky performance scorea | ||
| Median (range) | 90.0 (60-100) |
90.0 (70-100) |
| 90-100 | 308 (66) | 149 (65) |
| ≤80 | 154 (33) | 74 (32) |
| Missing | 4 (1) | 6 (3) |
| Sex | ||
| Men | 316 (68) | 157 (69) |
| Women | 150 (32) | 72 (31) |
| Region | ||
| United States | 221 (47) | 118 (52) |
| Outside the United States | 245 (53) | 111 (48) |
| Race/ethnicity | ||
| White | 416 (89) | 201 (88) |
| African American | 3 (1) | 1 (<1) |
| Asian | 27 (6) | 19 (8) |
| Hispanic | 18 (4) | 7 (3) |
| American Indian | 1 (<1) | 1 (<1) |
| Antiepileptic drug use at baseline | 205 (44) | 95 (41) |
| Corticosteroid use at baseline | 135 (29) | 64 (28) |
| Mini-Mental State Examination scoreb | ||
| 27-30 | 356 (76) | 160 (70) |
| ≤26 | 88 (19) | 48 (21) |
| Missing | 22 (5) | 21 (9) |
| Extent of resection | ||
| Biopsy | 60 (13) | 29 (13) |
| Partial resection | 157 (34) | 77 (33) |
| Gross total resection | 249 (53) | 123 (54) |
| MGMT promotor region methylation status | ||
| Tissue available and tested | 386 (83) | 185 (81) |
| Methylated | 137 (36) | 77 (42) |
| Unmethylated | 209 (54) | 95 (51) |
| Invalid | 40 (10) | 13 (7) |
| Slides available for central pathology review | 296 (64) | 138 (60) |
| Confirmed glioblastoma | 285 (96) | 134 (97) |
| WHO grade II or III glioma | 4 (1) | 2 (1) |
| Insufficient quality for diagnosis | 7 (2) | 2 (1) |
| IDH1-R132H status | ||
| Tissue available and tested | 260 (56) | 119 (52) |
| Mutated | 19 (7) | 6 (5) |
| Negative test results | 240 (92) | 113 (95) |
| Invalid | 1 (<1) | |
| EGFR status | ||
| Tissue available and tested | 252 (54) | 112 (49) |
| Amplified | 102 (41) | 43 (38) |
| Not amplified | 147 (58) | 68 (61) |
| Invalid | 3 (1) | 1 (1) |
| Tumor tissue chromosomes 1p and 19q | ||
| Tissue available and tested | 259 (56) | 112 (49) |
| Codeletion | 2 (1) | |
| Loss 1p only | 4 (2) | 1 (1) |
| Loss 19q only | 3 (1) | 3 (3) |
| Retained | 239 (92) | 102 (91) |
| Invalid | 11 (4) | 6 (5) |
| Tumor positionc | ||
| Corpus callosum | 25 (5) | 12 (5) |
| Frontal lobe | 190 (41) | 84 (37) |
| Occipital lobe | 58 (12) | 27 (12) |
| Parietal lobe | 146 (31) | 89 (39) |
| Temporal lobe | 191 (41) | 90 (40) |
| Missing | 3 (1) | 3 (1) |
| Tumor locationc | ||
| Left hemisphere | 214 (46) | 99 (43) |
| Right hemisphere | 249 (53) | 127 (55) |
| Both hemispheres | 4 (1) | 2 (1) |
| Corpus callosum | 15 (3) | 9 (4) |
| Missing | 1 (<1) | 1 (<1) |
| Treatment delivery | ||
| Completed standard radiation therapy | ||
| 57-63 Gy | 422 (91) | 212 (93) |
| <57 Gy | 21 (5) | 11 (5) |
| >63 Gy | 18 (4) | 3 (1) |
| Dose not reported | 5 (1) | 3 (1) |
| Concomitant radiation therapy and temozolomide | ||
| Yes | 433 (93) | 212 (93) |
| No record available | 33 (7) | 17 (7) |
| Time from last day of radiation treatment to randomization, median (range), d | 37 (15-128) |
36 (15-70) |
| Time from initial diagnosis to randomization, median (range), mo | 3.8 (1.7-6.2) |
3.7 (1.4-6.3) |
| Temozolomide cycles, median (range) | 6 (0-51) |
5 (0-33) |
| Tumor-treatming fields therapy | ||
| Duration, median (range), mo | 8.2 (0-82) | |
| ≥18 h/d (first 3 mo of treatment), mean | 347 (75) | |
Abbreviations: EGFR, epidermal growth factor receptor gene; IDH1-R132H, socitrate dehydrogenase 1 (IDH1) R132H mutation site; MGMT, O6-methylguanine-DNA-methyltransferase gene; TTFields, tumor-treating fields; WHO, World Health Organization.
Karnofsky performance score ranges from 0 to 100 in 10-point increments, with a higher score representing better performance status.
Scores range from 1 to 30, with a higher score representing better cognitive function.
Multiple positions for each patient allowed (for multifocal tumors).
Fifty-four percent had undergone a gross total resection (>95% of the tumor removed; as assessed and reported by the surgeon), 13% of patients had a diagnostic biopsy only. Histological slides for central pathology review were available for 434 of 695 patients (62%). The local diagnosis of glioblastoma was confirmed in 419 of 434 patients (97%). For 6 cases WHO grade II or III diagnoses were made, and for the remaining 9 patients, the available tissue for review did not allow for a definitive diagnosis or showed no tumor, yet all these patients were included in the intent-to-treat analysis. Tumor tissue for MGMT testing was available for 82% of the patients; of the cases with a valid test (518 of 571) 41% were MGMT methylated (40% TTFields plus temozolomide group and 45% for the temozolomide-only group). In 7% of tumors, expression of the IDH1-R132H mutant was demonstrated by a positive immunohistochemistry, EGFR was amplified in 40%.
Tumor location (lobe, hemisphere) in the brain was also comparable between the groups. The median time from histological diagnosis to randomization was 3.8 months (range, 1.7-6.2 months) for patients in the TTFields plus temozolomide group, and 3.7 months (range, 1.4-6.3 months) for those in the temozolomide-only group. Median time from the end of radiotherapy to randomization was 37 days in the TTFields plus temozolomide group and 36 days in the temozolomide-only group and occurred in most patients after starting of the first cycle of maintenance temozolomide. Median time from randomization to TTFields was 5 days (IQR, 3-7 days).
Treatment Delivery
All patients had completed radiotherapy and concomitant temozolomide as per local practice. The median number of temozolomide cycles until first tumor progression was 6 (range, 0-51) for the TTFields plus temozolomide group and 5 (range, 0-33) for the temozolomide-only group; the median duration of TTFields treatment was 8.2 months (range, 0-82 months), 51% (n = 237) of patients continued TTFields after the first progression.
Efficacy End points
After a median follow-up of 40 months (IQR, 34-66 months), and a minimum follow-up of 24 months, the primary end point of median progression-free survival was 6.7 months (95% CI, 6.1-8.1 months) for patients treated with TTFields plus temozolomide vs 4.0 months (95% CI, 3.8-4.4 months) for patients treated with temozolomide alone, for a proportional hazard ratio (HR) of 0.63 (95% CI, 0.52-0.76; P < .001; stratified log-rank test; Figure 2A). For the secondary end point of overall survival, the median survival duration from randomization was 20.9 months (95% CI, 19.3-22.7 months) in the TTFields plus temozolomide group vs 16.0 months (95% CI, 14.0-18.4 months) in the temozolomide-only group, proportional HR of 0.63 (95% CI, 0.53-0.76; P < .001; stratified log-rank test; Figure 2B).
Figure 2. Kaplan-Meier Survival Curves for Patients Included in the Final Analysis in the Intent-to-Treat Population.
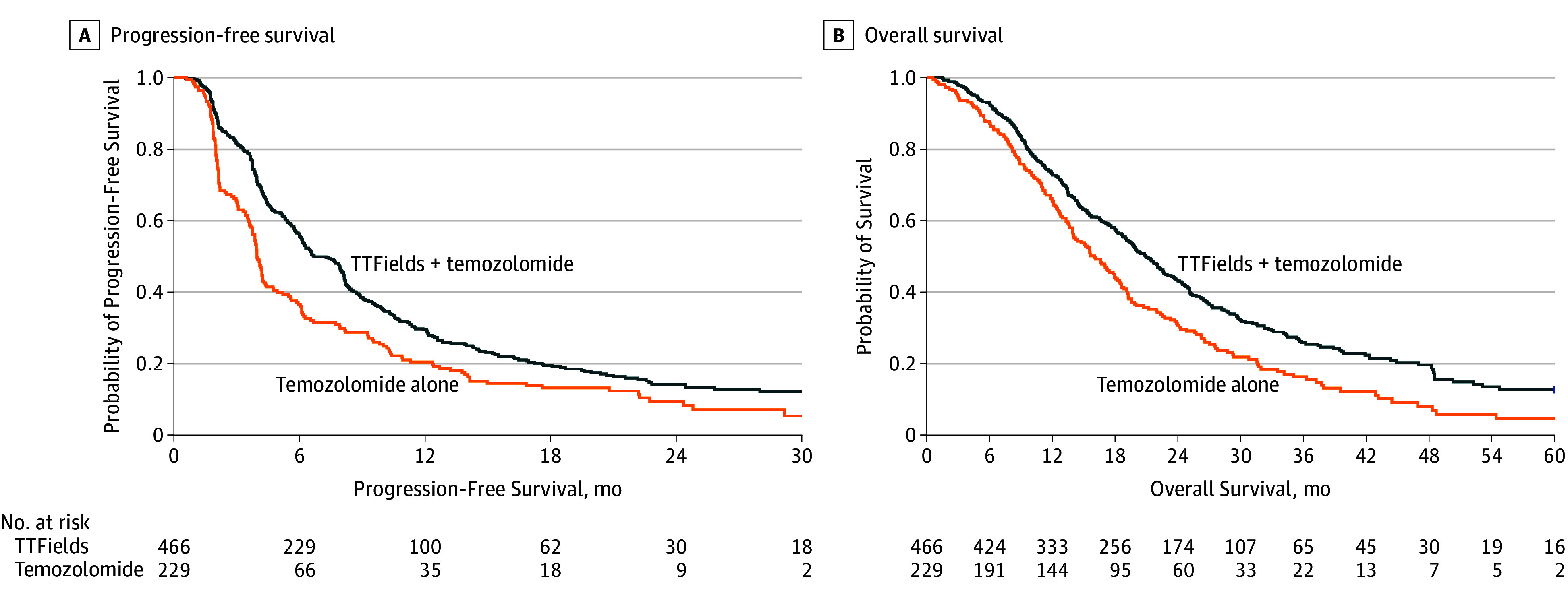
A, Median progression-free survival from randomization for the tumor-treating fields (TTFields) plus temozolomide group was 6.7 months and was 4.0 months for the temozolomide-alone group (hazard ratio [HR], 0.63; 95% CI, 0.52-0.76; P < .001). B, Median survival from randomization was 20.9 for the TTFields plus temozolomide group vs 16.0 months for the temozolomide-alone group (HR, 0.63; 95% CI, 0.53-0.76; P < .001). Median follow up was 44 months (range, 25-91 months) in both groups.
In exploratory analyses, the percentage of patients alive at 2 years from randomization was 43% (95% CI, 39%-48%); at 3 years, 26% (95% CI, 22%-31%), and at 5 years, 13% (95% CI, 9%-18%) in the TTFields plus temozolomide group and for the temozolomide-only group at 2 years was 31% (95% CI, 25%-38%; P < .001); at 3 years, 16% (95% CI, 12%-23%; P = .009); and at 5 years, 5% (95% CI, 2%-11%; P = .004). Progression-free survival at 6 months was 56% (95% CI, 51%- 61%) for patients treated with TTFields plus temozolomide and 37% (95% CI, 30%-44%) with temozolomide only (P < .001) (Table 2).
Table 2. Summary of Study End Pointsa.
| TTFields + Temozolomide (n = 466) |
Temozolomide Alone (n = 229) |
Between-Group Differences | |
|---|---|---|---|
| Progression-free survival | |||
| Primary end point, median (95% CI), mo | 6.7 (6.1-8.1) | 4.0 (3.8-4.4) | 2.7 (2.1-4.2) |
| Overall survival | |||
| Secondary end point, median (95% CI), mo | 20.9 (19.3-22.7) | 16.0 (14.0-18.4) | 4.9 (2.3-7.9) |
| Exploratory end points, % (95% CI | |||
| Progression-free 6-mo survival rate | 56 (51-61) | 37 (30-44) | 19 (15-23) |
| Annual survival rates, y | |||
| 1 | 73 (69-77) | 65 (59-72) | 8 (0-16) |
| 2 | 43 (39-48) | 31 (25-38) | 12 (4-18) |
| 3 | 26 (22-31) | 16 (12-23) | 10 (3-17) |
| 4 | 20 (16-25) | 8 (4-14) | 12 (5-19) |
| 5 | 13 (9-18) | 5 (2-11) | 8 (2-14) |
Abbreviation: TTFields, tumor-treating fields.
Survival rates are actuarial estimates according to the Kaplan-Meier method.
An exploratory Cox proportional hazards model adjusting for Karnofsky performance score, MGMT promotor methylation status, geographic region, age, tumor location, and extent of resection were consistent with the findings of the progression-free and overall survival analyses. The following factors were associated with longer overall survival: TTFields plus temozolomide treatment (HR, 0.63; 95% CI, 0.53-0.76; P < .001), female sex (HR, 0.76, 95% CI, 0.63-0.92; P = .005), methylated MGMT promoter (HR, 0.50; 95% CI, 0.41-0.62; P < .001), younger age (as a continuous variable; HR, 0.978 per year; 95% CI, 0.969-0.985; P < .001) and higher Karnofsky performance score (as a categorical variable in 10 point increments; P < .001). Patients with frontal tumors had non-significantly longer survival (HR = 0.82, CI 0.67-1.01, P = .061). Country of treatment and extent of resection were not associated with a significant difference in survival (P = .101 and P = .183, respectively).
Post Hoc Subgroup Analysis
In post hoc analyses, TTFields plus temozolomide was associated with an increase in progression-free survival and overall survival (Figure 3; Cox proportional hazards, P < .05 for the treatment effect within each subgroup) in all subgroups of patients regardless of age, sex, Karnofsky performance score, MGMT promoter methylation status, geographic region, or extent of resection. Patients 65 years or older had shorter survival than patients younger than 65 years. In both age groups, TTFields plus temozolomide was associated with significantly increased survival compared with temozolomide alone for older (HR, 0.51; 95% CI, 0.33-0.77) and younger patients (HR, 0.67; 95% CI, 0.55-0.82; Figure 4A and Figure 4B). Patients with tumors that lacked MGMT promoter methylation had a significantly shorter survival than patients with tumors with MGMT promoter methylation, although use of TTFields with temozolomide was associated with longer survival (HR, 0.66; 95% CI, 0.49-0.85 both in patients with tumors that were MGMT methylated and tumors that were unmethylated, respectively; Figure 4C and Figure 4D). In the TTFields plus temozolomide group, 265 patients who were treated with TTFields for 18 hours a day or more (monthly average in the first 6 months of treatment) had longer survival than 185 patients treated less than 18 hours a day (22.6 months, 95% CI, 19.7-25.1 months vs 19.1 months, 95% CI, 16.5-21.9; HR, 0.65; 95% CI, 0.49-0.85; P = .009).
Figure 3. Overall Survival for Each Prognostic Patient Subgroup of Patients Treated With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone.
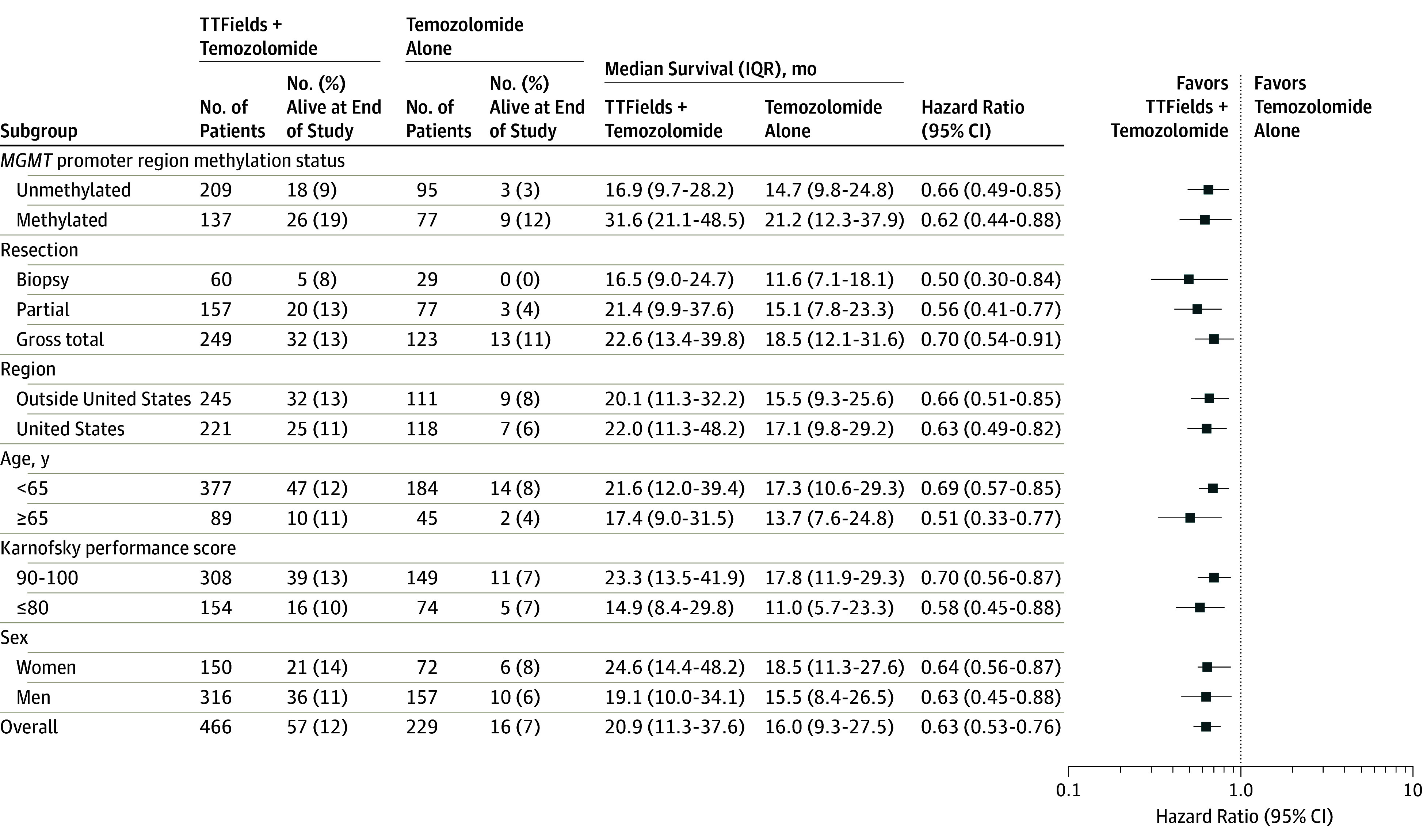
Data points represent Cox hazard ratios of overall survival in each subgroup of patients treated with tumor-treating fields (TTFields) plus temozolomide compared with temozolomide alone and were adjusted for the other subgroups. Error bars represent 95% CIs of the hazard ratios. The Karnofsky performance score is measured from 0 to 100 in 10-point increments, with higher scores indicating better the patient performance status.
IQR, indicates interquartile range; MGMT, O6-methylguanine-DNA methyltransferase promotor region methylation status.
Figure 4. Overall Survival by Patient Age and by MGMT Promotor Region Methylation Status.
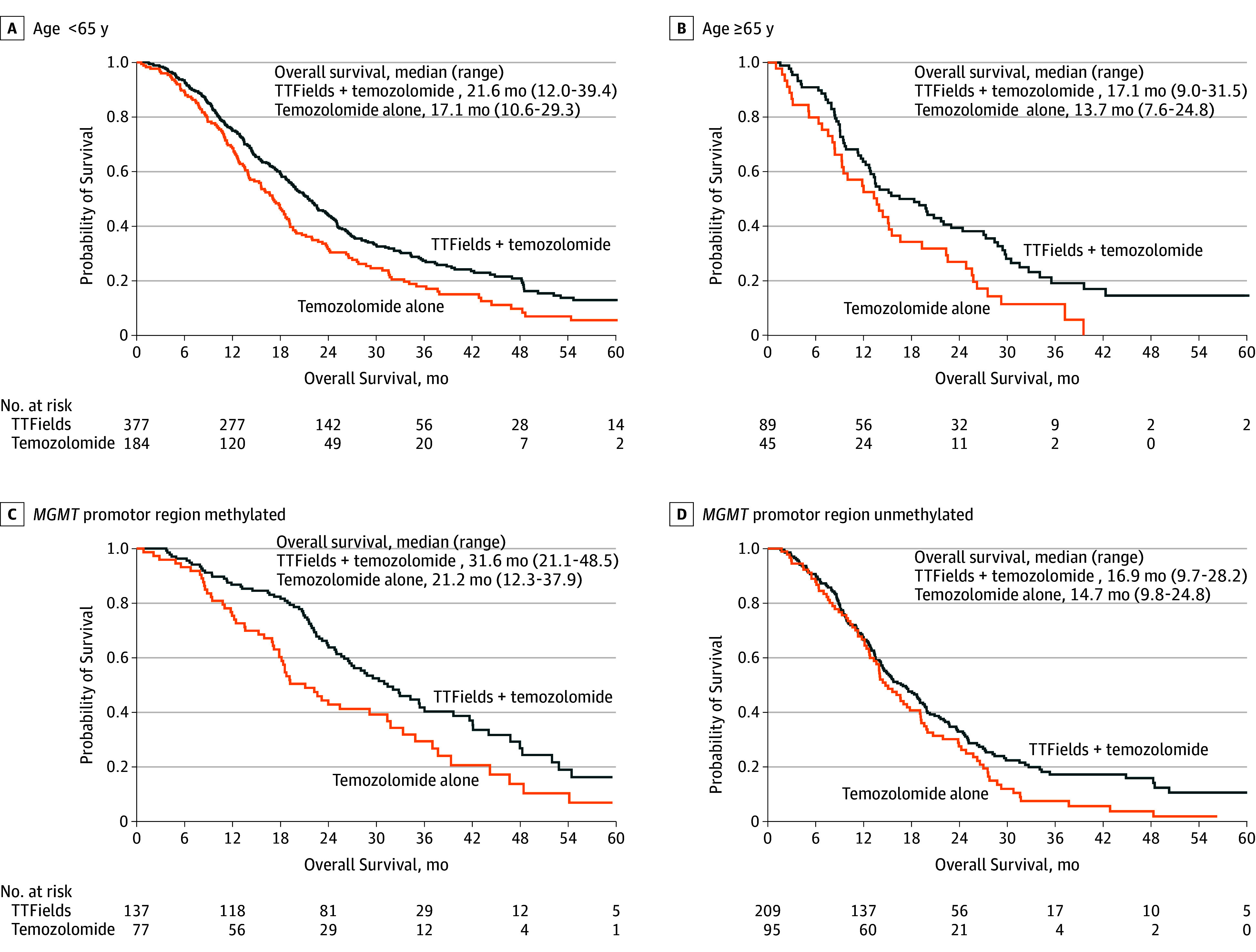
A, In comparing tumor-treating fields (TTFields) plus temozolomide vs temozolomide alone among patients younger than 65 years the hazard ratio (HR) was 0.67 (95% CI, 0.55-0.82). B, In comparing the 2 treatments among patients 65 years or older, the HR was 0.51 (95% CI, 0.22-0.77). C, In comparing the treatments among patients with O6-methylguanine-DNA methyltransferase MGMT promotor region methylation, the HR was 0.62 (95% CI, 0.43-0.88). D, In comparing the treatments among patients without the MGMT promotor region methylation, the HR was 0.66 (95% CI, 0.49-0.85). The median follow-up of patients was 44 months (range, 25-91 months) in all groups.
Adverse Events and Tolerability
The addition of TTFields to temozolomide therapy was not associated with any significant increase in rates of systemic adverse events compared with temozolomide therapy alone (48% vs 44%, respectively; P = .58; Table 3), and the overall incidence, distribution, and severity of adverse events were not statistically different in patients in the 2 treatment groups. The numerically higher incidence of some adverse events in the TTFields plus temozolomide group was a reflection of the longer duration of temozolomide treatment in this group due to delayed occurrence of progression. When adverse event incidence normalized to duration of treatment was analyzed, these differences disappeared. The only exception was a higher incidence of localized skin toxic effects (medical device site reaction beneath the transducer arrays) in patients treated with TTFields plus temozolomide; mild to moderate skin irritation was observed in 52% of patients, and severe (grade 3) skin involvement occurred in 2%. Anxiety, confusion, insomnia, and headaches which were reported more frequently (statistically nonsignificant) in patients treated with TTFields at the interim analysis were not seen in the final adverse event analysis of the trial. The incidence of seizures was identical in the 2 groups.
Table 3. Adverse Events by Body System and Severity (≥5% Incidence in Any Group).
| Grade 3-4 Events, No. (%) of Patients | ||
|---|---|---|
| TTFields + Temozolomide (n = 456) |
Temozolomide Alone (n = 229) |
|
| ≥1 Adverse event | 218 (48) | 94 (44) |
| Blood and lymphatic system disordersa | 59 (13) | 23 (11) |
| Thrombocytopenia | 39 (9) | 11 (5) |
| Gastrointestinal disorders | 23 (5) | 8 (4) |
| Asthenia, fatigue, and gait disturbance | 42 (9) | 13 (6) |
| Infections | 32 (7) | 10 (5) |
| Injury, poisoning, and procedural complications (falls and medical device site reaction) | 24 (5) | 7 (3) |
| Metabolism and nutrition disorders (anorexia, dehydration, and hyperglycemia) | 16 (4) | 10 (5) |
| Musculoskeletal and connective tissue disorders | 21 (5) | 9 (4) |
| Nervous system disorders | 109 (24) | 43 (20) |
| Seizures | 26 (6) | 13 (6) |
| Respiratory, thoracic and mediastinal disorders (pulmonary embolism, dyspnea, and aspiration pneumonia) | 24 (5) | 11 (5) |
Abbreviation: TTFields, tumor-treating fields.
The numerically slightly higher incidence of hematological toxicity, fatigue, and some other adverse effects are due to the longer treatment duration and observation time in the experimental group. The differences disappear when data are normalized to treatment duration.
To estimate tolerability, prespecified exploratory analyses of the association of TTFields device use with patients’ activities of daily life and cognition were performed using the Karnofsky performance score and the Mini-Mental State Examination. Time to a sustained 6-point decline in the Mini-Mental State Examination score was significantly longer in the TTFields plus temozolomide group than the temozolomide-alone group (16.7 months, 95% CI, 14.7-19.0 months vs 14.2 months, 95% CI, 12.7-17.0 months, respectively; HR, 0.79; 95% CI, 0.66- 0.95; P = .01). Time to a sustained 10-point decrease in Karnofsky performance score was also significantly longer in the TTFields plus temozolomide group than in the temozolomide-alone group (5.5 months;, 95% CI, 5.0-6.3 months vs 3.9 months; 95% CI, 3.1-5.2 months, respectively; HR, 0.80; 95% CI, 0.67-0.95; P = .009).
Discussion
In the final analysis of this randomized phase 3 trial, the addition of the TTFields treatment to standard temozolomide maintenance therapy, compared with standard temozolomide maintenance therapy alone, resulted in increased progression-free survival and overall survival in patients with newly diagnosed glioblastoma. After a median follow-up of 40 months, the addition of TTFields to temozolomide, compared with temozolomide alone, resulted in longer median progression-free survival from the time of randomization, 6.7 months vs 4.0 months and longer median overall survival from randomization, 20.9 months vs 16.0 months, respectively. These findings are consistent with the preliminary results reported based on a planned interim analysis of the first 315 patients enrolled, after a median follow-up of 38 months, in which median progression-free survival in the intent-to-treat population was 7.1 months (95% CI, 5.9-8.2 months) in the TTFields plus temozolomide group (210 patients analyzed) and 4.0 months (95% CI, 3.3-5.2 months) in the temozolomide-alone group (105 patients analyzed).
In the current study, exploratory end points were consistent with the primary and secondary end points in this trial. In a post hoc analysis the effect of TTFields was observed in all clinical and molecular subgroups, including patients older than age 65 years and patients with MGMT unmethylated tumors.
To assess whether the improved outcome may have been related to other factors than the TTFields therapy the data were scrutinized for possible imbalances, unexpected poor performance of the control group, or differences in supportive care administered to patients between the 2 groups. Both clinical factors and molecular tumor characteristics were well balanced and comparable between the 2 groups. MGMT promoter methylation, the strongest predictive factor for outcome in temozolomide-treated patients,25 was more prevalent in the control group (45% vs 40% of samples with a valid result). Patients with early tumor progression occurring during the first 3 months after diagnosis were not included in this trial, and so the randomized patient population had a better prognosis, for both groups, compared with other trials that had randomized patients before radiation therapy. The reported survival times were measured from randomization, not from diagnosis, so for an estimation of the overall outcome 3.8 months should be added in both groups. The RTOG 0525/Intergroup study, which evaluated dose-dense temozolomide, also randomized patients only after completion of radiochemotherapy.8 Outcome of the control group in the current study and of the RTOG study were very similar, and in both studies, the median survival from randomization was 16 months.
In this trial, the rates of systemic adverse effects were not significantly different in the 2 treatment groups. The occurrence of mild to moderate skin irritation related to reaction beneath the transducer arrays of the device occurred in more than half of patients in the TTFields plus temozolomide group.
These findings are in contrast to the more than 23 randomized trials conducted over the last decade that have evaluated novel agents or intensified treatment strategies (eg, dose-dense temozolomide, cilengitide, nimotuzumab, bevacizumab, and rindopepimut3,5,8,26) for treatment of patients with newly diagnosed glioblastoma and have failed to demonstrate improved survival. Innovative treatments for glioblastoma are needed.
Limitations
This study has several limitations. First, the current trial was open-label because it was considered practically unfeasible (heat and easy measure of current associated with TTFields) and ethically unacceptable to expose patients to a sham device. Although a placebo effect may affect subjective end points like quality of life or even progression-free survival by influencing the frequency of imaging and its interpretation, in the current trial a consistent benefit was observed in progression-free survival as assessed by blinded central radiology review, as well as in the gold standard of objective outcome, overall survival. Second, delivery of TTFields therapy requires the patient to continuously carry a device on a shaved scalp and may create burdens for patients. Nevertheless, the majority of patients were able to handle the device independently or with some help from a caregiver. The fact that 75% of patients achieved treatment adherence of 75% or more (ie, using the device for ≥18 hours per day) indicated good tolerability. The effects of the TTFields treatment and the need for continuous use of the device on quality of life will be reported separately.
Conclusions
In the final analysis of this randomized clinical trial of patients with glioblastoma who had received standard radiochemotherapy, the addition of TTFields to maintenance temozolomide chemotherapy vs maintenance temozolomide alone, resulted in statistically significant improvement in progression-free survival and overall survival. These results are consistent with the previous interim analysis.
Trial protocol and statistical analysis plan
eAppendix. Listing of all participating centers and local principal investigators
References
- 1.Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18(suppl 5):v1-v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25(26):4127-4136. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Gorlia T, et al. ; European Organisation for Research and Treatment of Cancer (EORTC); Canadian Brain Tumor Consortium; CENTRIC study team . Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100-1108. [DOI] [PubMed] [Google Scholar]
- 4.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709-722. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westphal M, Heese O, Steinbach JP, et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer. 2015;51(4):522-532. [DOI] [PubMed] [Google Scholar]
- 10.Kirson ED, Dbalý V, Tovarys F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104(24):10152-10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giladi M, Schneiderman RS, Voloshin T, et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep. 2015;5:18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirson ED, Schneiderman RS, Dbalý V, et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med Phys. 2009;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192-2202. [DOI] [PubMed] [Google Scholar]
- 14.Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535-2543. [DOI] [PubMed] [Google Scholar]
- 15.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganiere V, Christen G, Bally F, et al. Listeria brain abscess, Pneumocystis pneumonia and Kaposi's sarcoma after temozolomide. Nat Clin Pract Oncol. 2006;3(6):339-343. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. [DOI] [PubMed] [Google Scholar]
- 18.Taphoorn MJ, Claassens L, Aaronson NK, et al. ; EORTC Quality of Life Group, and Brain Cancer, NCIC and Radiotherapy Groups . An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46(6):1033-1040. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277-1280. [DOI] [PubMed] [Google Scholar]
- 20.Vlassenbroeck I, Califice S, Diserens AC, et al. Validation of real-time methylation-specific PCR to determine O6-methylguanine-DNA methyltransferase gene promoter methylation in glioma. J Mol Diagn. 2008;10(4):332-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegi ME, Janzer RC, Lambiv WL, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group . Presence of an oligodendroglioma-like component in newly diagnosed glioblastoma identifies a pathogenetically heterogeneous subgroup and lacks prognostic value: central pathology review of the EORTC_26981/NCIC_CE.3 trial. Acta Neuropathol. 2012;123(6):841-852. [DOI] [PubMed] [Google Scholar]
- 22.Coulibaly B, Nanni I, Quilichini B, et al. Epidermal growth factor receptor in glioblastomas: correlation between gene copy number and protein expression. Hum Pathol. 2010;41(6):815-823. [DOI] [PubMed] [Google Scholar]
- 23.DeMets DL, Lan G. The alpha spending function approach to interim data analyses. Cancer Treat Res. 1995;75:1-27. [DOI] [PubMed] [Google Scholar]
- 24.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994;13(13-14):1341-1352. [DOI] [PubMed] [Google Scholar]
- 25.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003. [DOI] [PubMed] [Google Scholar]
- 26.Weller M, Butowski N, Tran DD, et al. ; ACT IV trial investigators . Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373-1385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eAppendix. Listing of all participating centers and local principal investigators