Key Points
Question
Does high-cutoff hemodialysis (allowing efficient serum immunoglobulin light chain removal through very large membrane pores) improve kidney function in patients with myeloma cast nephropathy and severe acute kidney injury?
Findings
In this randomized clinical trial that included 98 patients, the hemodialysis independence rate at 3 months was 41% in the high-cutoff hemodialysis group and 33% in the conventional hemodialysis group, a difference that was not statistically significant but had a wide confidence interval.
Meaning
Although this study found no significant difference in hemodialysis independence at 3 months, further research may be warranted.
Abstract
Importance
Cast nephropathy is the main cause of acute kidney injury in multiple myeloma and persistent reduction in kidney function strongly affects prognosis. Strategies to rapidly remove nephrotoxic serum-free light chains combined with novel antimyeloma agents have not been evaluated prospectively.
Objective
To compare the hemodialysis independence rate among patients newly diagnosed with myeloma cast nephropathy treated with hemodialysis using a high-cutoff dialyzer (with very large membrane pores and high permeability to immunoglobulin light chains) or a conventional high-flux dialyzer (with small pores and lower permeability).
Design, Setting, and Participants
Randomized clinical trial involving 98 patients with biopsy-proven myeloma cast nephropathy requiring hemodialysis treated at 48 French centers between July 2011 and June 2016; the final date of follow-up was June 29, 2016.
Interventions
Intensive hemodialysis (eight 5-hour sessions over 10 days) with either a high-cutoff dialyzer (46 patients) or a conventional high-flux dialyzer (48 patients). All patients received the same chemotherapy regimen of bortezomib and dexamethasone.
Main Outcomes and Measures
Primary end point was hemodialysis independence at 3 months; secondary end points: hemodialysis independence rates at 6 and 12 months, hemodialysis- and chemotherapy-related adverse events, and death.
Results
Among 98 randomized patients, 94 (96%) (median age, 68.8 years [interquartile range, 61.2-75.3 years]; 45% women) were included in the modified intent-to-treat analysis. The hemodialysis independence rate at 3 months was 41.3% (n = 19) in the high-cutoff hemodialysis group vs 33.3% (n = 16) in the conventional hemodialysis group (between-group difference, 8.0% [95% CI, −12.0% to 27.9%], P = .42); at 6 months, the rate was 56.5% (n = 26) vs 35.4% (n = 17), respectively (between-group difference, 21.1% [95% CI, 0.9% to 41.3%], P = .04); and at 12 months, the rate was 60.9% (n = 28) vs 37.5% (n = 18) (between-group difference, 23.4% [95% CI, 3.2% to 43.5%], P = .02). The incidence of hemodialysis-related adverse events was 43% in the high-cutoff hemodialysis group vs 39% in the conventional hemodialysis group; chemotherapy-related serious adverse events, 39% vs 37%, respectively; and at 12 months, 9 patients vs 10 patients died.
Conclusions and Relevance
Among patients with myeloma cast nephropathy treated with a bortezomib-based chemotherapy regimen, the use of high-cutoff hemodialysis compared with conventional hemodialysis did not result in a statistically significant difference in hemodialysis independence at 3 months. However, the study may have been underpowered to identify an early clinically important difference.
Trial Registration
clinicaltrials.gov Identifier: NCT01208818
This randomized clinical trial compares the effects of hemodialysis using a high-cutoff dialyzer (with very large membrane pores and high permeability to immunoglobulin light chains) vs a conventional high-flux dialyzer (with small pores and lower permeability) on hemodialysis independence among patients with biopsy-proven myeloma cast nephropathy requiring hemodialysis in France.
Introduction
Symptomatic multiple myeloma presents at diagnosis in about 30% of patients with acute kidney injury (AKI), a minority of whom require hemodialysis. Acute kidney injury is mostly related to myeloma cast nephropathy characterized by precipitation of monoclonal light chains with uromodulin in distal tubules of the kidneys. Cast nephropathy occurs in multiple myeloma with massive light chain secretion, and is favored by factors enhancing light chain precipitability, reducing tubular flow, or both. Persistent impairment of kidney function (particularly end-stage kidney disease) induces higher morbidity and mortality, strongly affects quality of life, and is associated with increased costs.
Improvement of kidney function is achievable if a diagnosis is made early. Treatment of cast nephropathy addresses urgent symptomatic measures such as adequate fluid replacement, correction of precipitating factors, and administration of high-dose steroids. In addition, suppression of light chain production with chemotherapy is mandatory. The proteasome inhibitor bortezomib, which does not require dose adaptation to kidney function, is the current standard of care. However, because patients with severe AKI have been excluded from most randomized trials, these recommendations are based on retrospective studies.
The benefit of rapidly removing circulating monoclonal light chains by mechanical approaches has not been established. A randomized clinical trial, which was limited by a lack of histological confirmation of cast nephropathy, failed to demonstrate an advantage of plasma exchange. High-cutoff hemodialysis with new-generation membranes (allowing efficient light chain removal through their large pores) has been evaluated in retrospective studies in which hemodialysis independence was achieved in about 60% of patients. This compares favorably with the hemodialysis independence rate of around 30% reported in most cohorts of patients treated with conventional intermittent hemodialysis during the era of novel antimyeloma agents.
With the objective of assessing the effect of high-cutoff hemodialysis on the hemodialysis independence (discontinuation) rate, this randomized clinical trial was designed to assess patients newly diagnosed with myeloma and severe AKI secondary to biopsy-proven cast nephropathy.
Methods
Signed informed consent was obtained from all patients at trial entry. Research was performed in accordance with the Declaration of Helsinki and good clinical practice guidelines and with approval from an institutional review board (Comité de Protection des Personnes, Ile-de-France VI, France).
The MYRE study was conducted between July 2011 and June 2016 within hematology and nephrology departments at 48 French centers. The study enrolled patients with cast nephropathy, who were classified by the severity of kidney impairment. Patients who did not require hemodialysis were enrolled in a part of the MYRE trial that compared 2 chemotherapy regimens and their data are not reported herein.
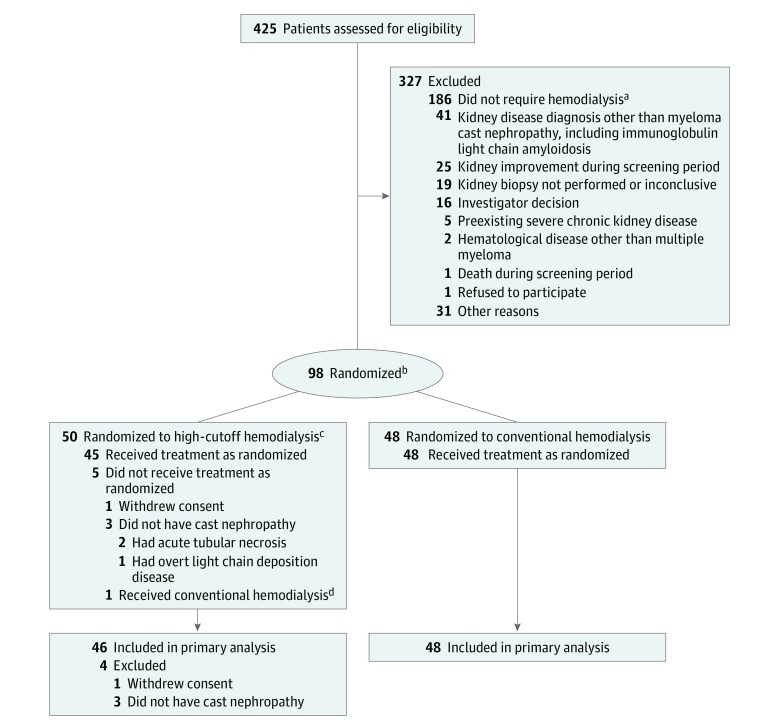
Patients with an indication for hemodialysis entered the part of the MYRE trial that evaluated high-cutoff hemodialysis compared with conventional hemodialysis. The study used a 2-group parallel randomized clinical trial design (Figure 1).
Figure 1. Study Flowchart of High-Cutoff vs Conventional Hemodialysis.
aEntered another randomized study comparing 2 bortezomib-based chemotherapeutic regimens (bortezomib and dexamethasone vs bortezomib, cyclophosphamide, and dexamethasone) for kidney outcome.
bRequired treatment with hemodialysis because of the presence of at least 1 of the following: hyperkalemia, metabolic acidosis, fluid overload, or symptoms of uremia.
cAllows efficient serum immunoglobulin light chain removal through very large membrane pores.
dIn accordance with the intention-to-treat principle, this patient was included in the analysis of the high-cutoff hemodialysis group.
Patients were selected by investigators based on presence of previously untreated monoclonal gammopathy and AKI (defined according to the Acute Kidney Injury Network criteria). The patients entered a 4- to 15-day screening period that involved correction of dehydration and other precipitating factors. Treatment of hypercalcemia with intravenous bisphosphonates was allowed. All patients received a 4-day oral course of 40 mg/d of dexamethasone or 400 mg/d of intravenous methylprednisolone. Multiple myeloma and cast nephropathy were diagnostically confirmed. In addition, histological confirmation of cast nephropathy was required.
After the screening period, patients with (1) a diagnosis of secreting multiple myeloma (according to criteria from the International Myeloma Working Group), (2) biopsy-proven cast nephropathy, (3) clinical indication for hemodialysis, and (4) neutrophil counts of 1.0 × 109/L or greater and platelet counts of 70 × 109/L or greater were randomized by investigators.
Patients were excluded if they (1) had preexisting chronic kidney disease with an estimated glomerular filtration rate (eGFR) of less than 30 mL/min/1.73 m2 for longer than 3 months (calculated using the simplified Modification of Diet in Renal Disease Study equation), (2) received more than 1 previous course of chemotherapy for myeloma, (3) had an uncontrolled malignant disorder, infection, or peripheral neuropathy, or (4) had associated immunoglobulin light chain amyloidosis in the kidney or overt light chain deposition disease with nodular glomerulosclerosis.
All patients were started on 21-day chemotherapy cycles with bortezomib (1.3 mg/m2 on days 1, 4, 8, and 11) and dexamethasone (20 mg on days 1-2, 4-5, 8-9, and 11-12). After the first chemotherapy cycle, patients who were older than 70 years were continued on 28-day cycles of bortezomib (1.3 mg/m2 weekly) and dexamethasone. Bortezomib was administered after the hemodialysis session on dialysis days, and was initially administered intravenously. Six months after study initiation, the protocol was amended for using the subcutaneous route (the original trial protocol appears in Supplement 1 and the amended trial protocol appears in Supplement 2).
Bortezomib dose was adapted according to standard recommendations for patients who experienced hematologic and neurological toxic effects. Corticosteroid dose could be reduced at the individual investigator’s discretion. Supportive and prophylactic measures included antibiotic therapy with penicillin and combination trimethoprim and sulfamethoxazole. Chemotherapy was started immediately after randomization. After the third chemotherapy cycle, reinforcement with cyclophosphamide (750 mg/m2 administered intravenously on day 1) was recommended for patients without a hematologic response. After the sixth chemotherapy cycle, treatment was determined by the individual investigator. High-dose melphalan with autologous stem cell transplantation was recommended for patients who were younger than 65 years and had an eGFR of 40 mL/min/1.73 m2 or greater.
Initiation of hemodialysis was recommended if patients had hyperkalemia, metabolic acidosis, fluid overload, or symptoms of uremia. In both groups, 8 hemodialysis sessions (5-hour duration, blood flow ≥250 mL/min, dialysate flow ≥500 mL/min) were planned over the first 10 days. If needed, patients received 3 additional weekly hemodialysis sessions using the same dialyzer until completion of 3 cycles of chemotherapy. In the high-cutoff hemodialysis group, the 2.1-m2 Theralite dialyzer (Gambro) was used. In the conventional hemodialysis group, a polyacrylonitrile, polysulfone, or polymethylmethacrylate high-flux dialyzer (eg, ultrafiltration coefficient >14 mL/min and surface ≥1.8 m2) could be used. Other hemodialysis procedures were performed according to local practice. If serum albumin level was less than 25 g/L prior to dialysis, a postdialysis perfusion of 20 g of albumin was performed. Local monitoring of serum-free light chain levels was recommended before and after the first 3 hemodialysis sessions, then twice weekly until the predialysis level of involved serum-free light chain was less than 500 mg/L.
An interactive web-response system was used to randomize patients in a 1:1 allocation ratio. The randomization was stratified by center and age (<65 years vs ≥65 years), with prespecified lists that were computer-generated by the study statistician using R software version 3.3.2 (R Project for Statistical Computing), based on permuted block sizes of 4 that remained blinded to investigators. Randomization was intended to occur before the first hemodialysis session. Among patients requiring urgent hemodialysis, a maximum of 7 previous sessions was allowed.
The primary end point was the rate of hemodialysis independence at 3 months after randomization (defined by an eGFR ≥15 mL/min/1.73 m2 15 days after the last hemodialysis session). Individual investigators determined hemodialysis withdrawal. Secondary end points included the hemodialysis independence rate after the first chemotherapy cycle at 6 and 12 months; complete recovery of kidney function (defined by return to baseline level of serum creatinine or eGFR if known or by eGFR ≥60 mL/min/1.73 m2 after the first and third chemotherapy cycles at 6 months and 1 year); eGFR level after the first and third chemotherapy cycles at 6 and 12 months; hematologic response after the first and third chemotherapy cycles at 6 and 12 months; event-free survival, relapse-free survival (myeloma progression-free survival), time to next myeloma therapy, and overall survival; and tolerance to treatment (including hemodialysis-related adverse events). The final date of follow-up was June 29, 2016.
Hematologic response was assessed based on involved serum-free light chain level. Partial hematologic response was defined by a serum-free light chain level reduction of 50% or greater. A very good partial hematologic response was defined by a serum-free light chain level reduction of 90% or greater. Complete hematologic response was defined by a normal serum-free light chain level and a normal ratio of κ to λ with negative serum and urine immunofixation. Among patients who received hemodialysis, progression-free survival referred to the time until myeloma progression or death; progressive disease was defined by an increase of 25% or greater of involved serum-free light chain level from baseline (or entire serum monoclonal immunoglobulin level if present) or by any non–kidney myeloma-defining event. Otherwise, International Myeloma Working Group criteria were used. Adverse events (graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events) were systematically recorded. Data were periodically reviewed by an independent data and safety monitoring committee.
Based on experience and literature data, the hemodialysis independence rate was estimated at 30% at 3 months among patients with cast nephropathy and persistent severe AKI treated with bortezomib-containing chemotherapy regimens and hemodialysis with conventional high-flux dialyzers. Given the incidence of myeloma cast nephropathy, the potential risks, and the constraints of intensive hemodialysis, we determined that the minimal clinically important difference would be achievement of a 2-fold hemodialysis independence rate (ie, 60%) in the high-cutoff hemodialysis group. Such a difference was considered achievable because hemodialysis independence rates as high as 60% have been reported using high-cutoff hemodialysis. To detect a hemodialysis independence rate increase from 30% to 60% at 3 months after randomization, a sample size of 98 patients (49 in each group) was computed based on a 2-sided χ2 test (power of 80% and type I error rate of 5%). The statistical analysis plan appears in Supplement 3.
The analyses were performed on a modified intention-to-treat basis. Only patients without all eligible conditions or who withdrew consent (according to French regulations) were excluded. Summary statistics were reported as medians, interquartile ranges (IQRs), and percentages. Point estimates were computed with 95% CIs.
The χ2 test was used to compare hemodialysis independence at 3, 6, and 12 months and event-free survival (proportion of patients alive and still withdrawn from hemodialysis) at 12 months. Patients with missing data on kidney outcomes because of death were considered as treatment failures. Cumulative incidence of hemodialysis independence was estimated in a competing-risks framework because of deaths prior to hemodialysis independence, and then compared across randomized groups using the Gray test. A logistic regression model was used to provide effect size estimates based on odds ratios (ORs), and then adjust for the treatment comparisons based on potential prognostic factors selected by univariate analyses at the 10% level. In the regression model, missing values of serum-free light chain level reduction were imputed by the modal class.
Progression-free survival and overall survival from date of randomization were estimated using the Kaplan-Meier method and compared using the log-rank test, with treatment effect size measured by hazard ratios (HRs) estimated from Cox models. Proportional hazards assumptions were checked based on weighted residuals (P = .66). Safety was evaluated by the rates of reported severe adverse events.
A secondary analysis used a mixed-effects logistic model to account for potential center effects. For the post hoc analysis, a generalized estimating equation model for hemodialysis independence over the 12 months of follow-up was fitted to estimate the time × treatment interaction. Center effects were accounted for in the post hoc analysis using a random-effects estimator that compares the change in the odds for a treated patient vs a control patient from the same center.
All tests were 2-sided and 2-sided P values of <.05 were considered statistically significant. For the secondary end point analyses, there was no adjustment for multiple comparisons. Accordingly, these findings should be considered exploratory. Statistical analysis was performed using SAS version 9.3 (SAS Institute Inc) or R version 3.3.2. In addition, the R package geepack was used.
Results
Among 98 randomized patients, 94 (96%) (median age, 68.8 years [IQR, 61.2-75.3 years]; 45% women) were included in the modified intent-to-treat analysis. Fifty patients were randomized to the high-cutoff hemodialysis group and 48 patients were randomized to the conventional hemodialysis group (Figure 1). Four patients were excluded (3 because of incorrect diagnosis and 1 withdrew consent).
Baseline characteristics were similar in the 2 groups (Table 1). Light chain isotypes were equally distributed; 50% of patients in the high-cutoff hemodialysis group had light-chain multiple myeloma only compared with 46% of patients in the conventional hemodialysis group. All patients displayed high levels of serum-free light chain. After symptomatic measures were addressed such as fluid replacement, correction of precipitating factors, and administration of high-dose steroids, the median serum creatinine level was 6.4 mg/dL in the high-cutoff hemodialysis group and 7.3 mg/dL in the conventional hemodialysis group. At least 1 precipitating factor (most commonly use of nonsteroidal anti-inflammatory drugs) was recorded in 51% of the patients.
Table 1. Baseline Characteristics.
| Characteristic | No. (%) of Patientsa |
|
|---|---|---|
| High-Cutoff Hemodialysis (n = 46) |
Conventional Hemodialysis (n = 48) |
|
| Age, y | ||
| Median (IQR) | 68.4 (60.9-74.6) | 68.8 (62.2-75.7) |
| <65 | 17 (37) | 14 (29) |
| ≥65 | 29 (63) | 34 (71) |
| Male sex | 23 (50) | 29 (60) |
| Medical history | ||
| Hypertension | 23 (50) | 30 (63) |
| Diabetes | 7 (15) | 7 (15) |
| Known monoclonal gammopathy of undetermined significance or indolent multiple myeloma | 4 (9) | 14 (29) |
| Time from diagnosis of acute kidney injury to chemotherapy, median (IQR), d | 8.0 (5.0-12.0) | 9.0 (5.5-13.0) |
| 1 Cycle of chemotherapy before randomization | ||
| Bortezomib, cyclophosphamide, and dexamethasone | 1 (2) | 1 (2) |
| Cyclophosphamide, thalidomide, and prednisone | 0 | 1 (2) |
| Multiple Myeloma | ||
| Light chain isotype | ||
| κ | 24 (52) | 27 (56) |
| λ | 22 (48) | 21 (44) |
| Secreted monoclonal immunoglobulin | ||
| IgG | 11 (24) | 16 (33) |
| IgA | 12 (26) | 7 (15) |
| IgD | 3 (6) | |
| Light chain only | 23 (50) | 22 (46) |
| Serum-free light chain level, median (IQR), mg/L | 6590 (3421-12 528) | 5230 (3023-14 110) |
| Bone marrow plasma cells, median (IQR), % | 38 (22-56) | 31 (15-60) |
| Hemoglobin, median (IQR), g/dL | 8.9 (8.2-9.6) | 9.5 (8.7-10.2) |
| Platelet count, median (IQR), ×109/L | 186 (157-228) | 170 (151-209) |
| β2 microglobulin, median (IQR), mg/L | 20.8 (14.9-28.4) | 21.8 (14.6-36.0) |
| Albumin, median (IQR), g/L | 32 (28-35) | 34 (29-38) |
| Lactate dehydrogenase level greater than upper limit of normal | 6 (13) | 9 (19) |
| Lytic bone lesions | 32 (70) | 28 (58) |
| Cytogenetics, No./total (%) | ||
| Standard risk | 23/27 (85) | 23/31 (74) |
| High riskb | 4/27 (15) | 8/31 (26) |
| Kidney Presentation | ||
| De novo acute kidney injury | 43 (93) | 40 (83) |
| Known preexisting chronic kidney disease (estimated glomerular filtration rate ≥30 mL/min/1.73 m2)c | 3 (6) | 8 (17) |
| Admission to intensive care unit before randomization | 7 (15) | 8 (17) |
| Serum creatinine at randomization, median (IQR), mg/dL | 6.4 (5.3-8.1) | 7.3 (5.2-9.2) |
| Proteinuria, median (IQR), g/24 h | 2.2 (1.4-4.1) | 1.7 (1.1-2.7) |
| Ratio of urine protein to creatinine, median (IQR), mg/mmoL | 418 (296-907) | 387 (258-623) |
| Ratio of urine albumin to protein, median (IQR), % | 8.5 (4.0-19.8) | 8.5 (4.3-20.3) |
| No. of precipitating factors of cast nephropathy | ||
| 0 | 21 (46) | 25 (52) |
| 1 | 13 (28) | 14 (29) |
| ≥2 | 12 (26) | 9 (19) |
| Type of precipitating factor | ||
| Angiotensin-converting enzyme inhibitor or angiotensin receptor antagonist use | 2 (4) | 2 (4) |
| Diuretic use | 1 (2) | 6 (13) |
| Infection | 5 (11) | 2 (4) |
| Hypercalcemia | 9 (20) | 4 (8) |
| Dehydration | 6 (13) | 5 (10) |
| Nonsteroidal anti-inflammatory drug use | 18 (39) | 15 (31) |
| Kidney Pathology | ||
| Severe adverse event (bleeding) after biopsy | 1 (2) | 1 (2) |
| Typical myeloma casts | 46 (100) | 46 (96) |
| Probable cast nephropathyd | 0 | 2 (4) |
| Concomitant light chain–related kidney disease | 6 (13) | 6 (13) |
| Light chain deposition diseasee | 4 (9) | 6 (13) |
| Light chain proximal tubulopathy | 2 (4) | 0 |
Abbreviation: IQR, interquartile range.
SI conversion factor: To convert creatinine to μmol/L, multiply by 88.4.
Unless otherwise indicated.
Presence of t(4;14), Del17p, or both.
An estimated glomerular filtration rate of less than 30 mL/min/1.73 m2 at the time of randomization was an exclusion criterion.
In 2 cases with inadequate biopsy samples, the diagnosis was made using indirect lesions (eg, tubular lumen dilatation in the absence of any other cause of AKI).
By immunofluorescence only. Overt disease with typical glomerular lesions was an exclusion criterion.
Urine protein electrophoresis (when available) invariably showed prominent light-chain proteinuria and the median albuminuria level was less than 10%. Kidney biopsy was complicated by severe bleeding in 2 patients, including 1 in the high-cutoff hemodialysis group who died 5 months later after multiple complications. Typical myeloma casts were observed in 92 patients and indirect lesions of cast nephropathy in 2 patients. Eighty-two patients had pure cast nephropathy, 2 had associated light chain crystals in proximal tubules, and 10 had concomitant light chain deposition disease without significant glomerular lesions by light microscopy.
Primary Outcome
The hemodialysis independence rate at 3 months was 41.3% (n = 19) in the high-cutoff hemodialysis group vs 33.3% (n = 16) in the conventional hemodialysis group (between-group difference, 8.0% [95% CI, −12.0% to 27.9%], P = .42). The estimate in the post hoc secondary analysis accounting for center effects was similar (OR, 1.27 [95% CI, 0.50 to 3.27]; P = .62).
Secondary Outcomes
Kidney Outcomes at 6 and 12 Months
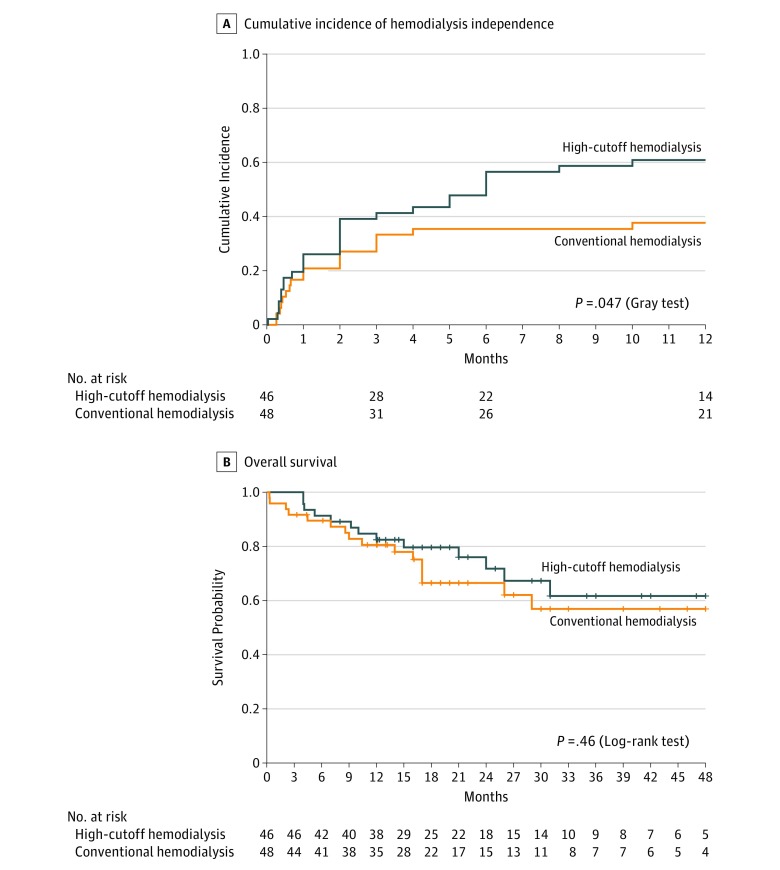
At 6 months, the hemodialysis independence rate in the high-cutoff hemodialysis group was 56.5% (n = 26) compared with 35.4% (n = 17) in the conventional hemodialysis group (between-group difference, 21.1% [95% CI, 0.9%-41.3%]; OR, 2.37 [95% CI, 1.03-5.44]; P = .04); at 12 months, it was 60.9% (n = 28) vs 37.5% (n = 18), respectively (between-group difference, 23.4% [95% CI, 3.2%-43.5%]; OR, 2.59 [95% CI, 1.13-5.96]; P = .02). Kidney recovery (hemodialysis independence) occurred after a median of 2 months in the high-cutoff hemodialysis group and after a median of 1 month in the conventional hemodialysis group (Table 2). The cumulative incidence of hemodialysis independence within 12 months after randomization appears in Figure 2A; the 12-month estimate was 60.9% (95% CI, 47.0%-74.8%) in the high-cutoff hemodialysis group and 38.4% (95% CI, 24.5%-52.2%) in the conventional hemodialysis group (P = .04).
Table 2. Hematologic and Kidney Responses.
| No. (%) of Patientsa | Between-Group Difference (95% CI), % |
P Value | ||
|---|---|---|---|---|
| High-Cutoff Hemodialysis (n = 46) |
Conventional Hemodialysis (n = 48) |
|||
| Primary Outcome | ||||
| Cumulative hemodialysis independence within 3 mo | 19 (41.3) | 16 (33.3) | 8.0 (−12.0 to 27.9) | .42 |
| Secondary Outcomes | ||||
| Cumulative hemodialysis independence within 6 mo | 26 (56.5) | 17 (35.4) | 21.1 (0.9 to 41.3) | .04 |
| Cumulative hemodialysis independence within 12 mo | 28 (60.9) | 18 (37.5) | 23.4 (3.2 to 43.5) | .02 |
| Time to hemodialysis independence, median (IQR), mo | 2.0 (0.4 to 5.7) | 1.0 (0.4 to 3.0) | .26 | |
| Alive without hemodialysis at 12 mo, No./total (%) | 24/37 (64.9) | 17/38 (44.7) | 20.2 (−2.6 to 42.9) | .15 |
| Hematologic response after first chemotherapy cycle | ||||
| Reduction in serum-free light chain, median (IQR), % | 89 (61 to 99) | 71 (22 to 91) | .02 | |
| Serum-free light chain level <500 mg/L | 20 (43.5) | 15 (31.2) | 12.3 (−7.7 to 32.1) | .29 |
| Hematologic response at 3 mo | ||||
| Overallb | 41 (89.1) | 30 (62.5) | 26.6 (9.8 to 43.4) | .003 |
| Very good partial or complete | 28 (60.9) | 21 (43.7) | 17.2 (−3.3 to 37.5) | .22 |
| Hematologic response at 6 mo | ||||
| Overallb | 36 (78.3) | 29 (60.4) | 17.9 (−0.8 to 36.5) | .06 |
| Very good partial or complete | 32 (69.6) | 23 (47.9) | 21.7 (1.8 to 41.5) | .03 |
| Serum-Free Light Chain Reduction With Hemodialysis | ||||
| Reduction after first hemodialysis session, median (IQR), % | ||||
| Overall | 68 (60 to 79) | 31 (9 to 49) | <.001 | |
| κ Light chain level | 77 (67 to 86) | 35 (19 to 48) | <.001 | |
| λ Light chain level | 63 (52 to 72) | 18 (6 to 56) | <.001 | |
| Reduction after third hemodialysis session, median (IQR), % | ||||
| Overall | 72 (64 to 80) | 34 (16 to 57) | <.001 | |
Abbreviation: IQR, interquartile range.
Unless otherwise indicated.
Indicates partial response, very good partial response, and complete response.
Figure 2. Kidney and Patient Outcomes.
A, The median follow-up (until hemodialysis independence or, if not, within the 12-month observation period) was 5.1 months (interquartile range [IQR], 1.3-12.1 months) in the high-cutoff hemodialysis group and 8.0 months (IQR, 1.8-12.1 months) in the conventional hemodialysis group. After 3 months, 9 patients in the high-cutoff hemodialysis group reached hemodialysis independence compared with 2 patients in the conventional hemodialysis group.
B, The short vertical bars on the curves indicate censored observations. The median duration of follow-up was 19.5 months (IQR, 12.0-30.8 months) in the high-cutoff hemodialysis group and 17.0 months (IQR, 10.9-27.5 months) in the conventional hemodialysis group.
Event-Free and Overall Survival
At 12 months, among 75 patients, 41 were still alive and no longer receiving hemodialysis (24/37 [64.9%] in the high-cutoff hemodialysis group and 17/38 [44.7%] in the conventional hemodialysis group; between-group difference, 20.2% [95% CI, −2.6% to 42.9%]; OR, 2.28 [95% CI, 0.90 to 5.77]; P = .15) (Table 2) and had median eGFR values of 36 mL/min/1.73 m2 (IQR, 25 to 63 mL/min/1.73 m2) and 39 mL/min/1.73 m2 (IQR, 27 to 53 mL/min/1.73 m2), respectively. One patient in each group was restarted on hemodialysis (at 22 months in the high-cutoff hemodialysis group and at 13 months in the conventional hemodialysis group).
At 12 months, 19 patients had died (9 [20%] in the high-cutoff hemodialysis group and 10 [21%] in the conventional hemodialysis group). Of these 19 patients, 9 had been withdrawn from hemodialysis (5 in the high-cutoff hemodialysis group and 4 in the conventional hemodialysis group). Within a median follow-up of 17.5 months (IQR, 12.0-30.0 months), 29 patients had died (13 in the high-cutoff hemodialysis group and 16 in the conventional hemodialysis group; HR, 0.76 [95% CI, 0.36-1.58]; log-rank test P = .46; Figure 2B). Causes of death were myeloma progression (n = 15; 9 in the high-cutoff hemodialysis group and 6 in the conventional hemodialysis group), infections (n = 7; 1 and 6, respectively), cardiovascular (n = 3; all in the conventional hemodialysis group), hemorrhage (n = 1 in the high-cutoff hemodialysis group), and other or unknown (n = 3; 2 in the high-cutoff hemodialysis group and 1 in the conventional hemodialysis group).
Chemotherapy and Hematologic Response
The median time from diagnosis of AKI to initiation of bortezomib was 8.0 days (IQR, 5.0-12.0 days) in the high-cutoff hemodialysis group and 9.0 days (IQR, 5.5-13.0 days) in the conventional hemodialysis group. Forty patients in each group received at least 3 courses of bortezomib and dexamethasone. Cyclophosphamide was added for 13 patients (28%) in the high-cutoff hemodialysis group after a mean of 4.4 months (SD, 1.0 month) and for 11 patients (23%) in the conventional hemodialysis group after a mean of 4.2 months (SD, 0.6 months).
Chemotherapy was prematurely discontinued because of toxic effects in 5 patients (3 in the high-cutoff hemodialysis group and 2 in the conventional hemodialysis group). The incidence of grade 3 or greater cytopenia and infectious complications was not significantly different. Grade 3 or greater peripheral neuropathy was observed in 1 patient in the high-cutoff hemodialysis group and in 4 patients in the conventional hemodialysis group. At least 1 serious adverse event was recorded in 39% of the patients in the high-cutoff hemodialysis group and in 37% of the patients in the conventional hemodialysis group.
At 3 months, the rate of hematologic response (partial response, very good partial response, and complete response) was 89.1% (n = 41) in the high-cutoff hemodialysis group and 62.5% (n = 30) in the conventional hemodialysis group (between-group difference, 26.6% [95% CI, 9.8% to 43.4%]; OR, 4.9 [95% CI, 1.6 to 14.7]; P = .003; Table 2). At 3 months, the rate of very good partial or complete hematologic response was 60.9% (n = 28) in the high-cutoff hemodialysis group and 43.7% (n = 21) in the conventional hemodialysis group (between-group difference, 17.2% [95% CI, −3.3% to 37.5%]; OR, 2.00 [95% CI, 0.88 to 4.55]; P = .22).
After the first cycle of bortezomib and dexamethasone, the median reduction rate in involved serum-free light chain level was 89% (95% CI, 61% to 99%) in the high-cutoff hemodialysis group and 71% (95% CI, 22% to 91%) in the conventional hemodialysis group (P = .02). Twenty patients (43.5%) in the high-cutoff hemodialysis group achieved a serum-free light chain level of less than 500 mg/L compared with 15 patients (31.2%) in the conventional hemodialysis group (between-group difference, 12.3% [95% CI, −7.7% to 32.1%]; OR, 1.69 [95% CI, 0.59 to 3.94]; P = .29) (Table 2).
Autologous stem cell transplantation was performed in 13 patients in the high-cutoff hemodialysis group and in 6 patients in the conventional hemodialysis group. Of these patients, 4 in the high-cutoff hemodialysis group and 1 in the conventional hemodialysis group still required hemodialysis and none achieved subsequent kidney response.
The median progression-free survival was 35 months (95% CI, 26 months-not available) in the high-cutoff hemodialysis group and 20 months (95% CI, 17-31 months) in the conventional hemodialysis group (HR, 0.57 [95% CI, 0.33-0.99]; P = .046).
Hemodialysis Procedures
Compared with standard membranes used in hemodialysis, the membrane used for patients in the high-cutoff hemodialysis group produced a significantly higher reduction of both κ and λ light chain levels after the first and third hemodialysis session (P < .001; Table 2). Incidence of hemodialysis-related adverse events of any grade was 43% in the high-cutoff hemodialysis group and 39% in the conventional hemodialysis group (P = .83). One patient in the high-cutoff hemodialysis group discontinued the procedure because of intolerance. A postdialysis perfusion of 20 g of albumin was required for 41% of the high-cutoff hemodialysis sessions and 4% of the conventional hemodialysis sessions because the predialysis albumin level was less than 25 g/L.
Variables Associated With Kidney Outcome
The bivariable analysis of the variables associated with hemodialysis independence within 12 months appears in Table 3. In the multivariable analysis, the main variables were whole immunoglobulin–secreting myeloma, involved serum-free light chain level of 500 mg/L or less after 1 cycle of chemotherapy, and randomization to the high-cutoff hemodialysis group. After adjusting on myeloma subtype (whole immunoglobulin or myeloma-secreting light chain only) and serum-free light chain level reduction of 500 mg/L or less, randomization to the high-cutoff hemodialysis group was still associated with an increased occurrence of hemodialysis independence within 12 months (OR, 2.78 [95% CI, 1.13-6.80]; P = .03). Post hoc analysis based on the generalized estimating equation model showed that there was a significant time × treatment interaction for the evolution of response during the 12 months of follow-up (P = .03).
Table 3. Variables Associated With Kidney Outcome Within 12 Months.
| No. of Patients |
No. of Events |
Bivariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |||
| Age group, y | ||||||
| 65 | 63 | 30 | 1 [Reference] | .72 | ||
| ≥65 | 31 | 16 | 0.85 (0.36-2.02) | |||
| Monoclonal gammopathy of undetermined significance | ||||||
| No preexisting | 76 | 38 | 1 [Reference] | .67 | ||
| Preexisting | 18 | 8 | 0.80 (0.28-2.25) | |||
| Chronic kidney disease | ||||||
| No preexisting | 83 | 40 | 1 [Reference] | .69 | ||
| Preexistinga | 11 | 6 | 1.29 (0.36-4.56) | |||
| Type of myelomab | ||||||
| Light chain | 45 | 16 | 1 [Reference] | .02 | 1 [Reference] | .03 |
| Whole immunoglobulin | 49 | 30 | 2.62 (1.14-6.05) | 2.75 (1.11-6.80) | ||
| Light chain isotype | ||||||
| κ | 51 | 24 | 1 [Reference] | .69 | ||
| λ | 43 | 22 | 1.18 (0.52-2.66) | |||
| Cytogenetics | ||||||
| Standard risk | 46 | 23 | 1 [Reference] | >.99 | ||
| High riskc | 12 | 6 | 1.00 (0.28-3.56) | |||
| Baseline serum-free light chain level, mg/Ld | ||||||
| <3000 | 20 | 13 | 1 [Reference] | |||
| 3000-5999 | 26 | 11 | 0.39 (0.12-1.32) | .13 | ||
| 6000-11 999 | 17 | 10 | 0.77 (0.20-2.92) | .70 | ||
| ≥12 000 | 18 | 12 | 0.40 (0.12-1.32) | .13 | ||
| After first chemotherapy cycle, level of involved serum-free light chain, mg/Le | ||||||
| >500 | 59 | 23 | 1 [Reference] | .01 | 1 [Reference] | .049 |
| ≤500 | 35 | 23 | 3.00 (1.25-7.18) | 2.51 (1.00-6.33) | ||
| Randomization groupf | ||||||
| Conventional hemodialysis | 48 | 18 | 1 [Reference] | .02 | 1 [Reference] | .03 |
| High-cutoff hemodialysis | 46 | 28 | 2.59 (1.13-5.97) | 2.78 (1.13-6.80) | ||
Defined by an estimated glomerular filtration rate of 30 mL/min/1.73 m2 or greater.
At baseline, the median serum-free light chain level was 7627 mg/L (interquartile range, 3564-14 110 mg/L) in patients with light chain myeloma and was 5040 mg/L (interquartile range, 2505-12 425 mg/L) in patients with whole immunoglobulin myeloma (P = .16).
Presence of t(4;14), Del17p, or both.
Missing data for 3 patients.
Missing data (n = 6) were imputed by the modal class (no reduction).
Adjusted for myeloma subtype (whole immunoglobulin or myeloma-secreting light chain only) and serum-free light chain level reduction of 500 mg/L or less.
Discussion
In this randomized clinical trial that included patients newly diagnosed with cast nephropathy treated with a bortezomib-based regimen, the primary outcome of an increase in hemodialysis independence rate from 30% to 60% at 3 months with high-cutoff hemodialysis was not reached. However, the study may have lacked power to detect such a difference at this early end point.
Randomization occurred after appropriate symptomatic measures were addressed such as adequate fluid replacement, correction of precipitating factors, and administration of high-dose steroids among patients with established AKI who still required hemodialysis. All patients received the same bortezomib-based regimen and hemodialysis intensity to specifically assess the effect of the 2.1-m2 high-cutoff dialyzer on kidney outcome. In both groups, the intensive hemodialysis schedule resulted in a good tolerance profile and acceptable feasibility in standard nephrology facilities. Higher albumin loss in the high-cutoff hemodialysis group did not result in more frequent complications with the postdialysis perfusion. High-cutoff hemodialysis allowed higher clearance of both κ and λ light chains.
In all participants, the diagnosis of myeloma cast nephropathy was histologically confirmed. The study highlights the characteristic features of cast nephropathy, including high serum-free light chain levels, predominant light chain proteinuria, and the frequency of precipitating factors, particularly nonsteroidal anti-inflammatory drugs. In routine practice, the risk-benefit ratio of kidney biopsy remains questionable because of the potential for hemorrhagic complications; therefore, biopsy should be primarily considered in patients with atypical features such as significant albuminuria.
Results of this trial are in keeping with most retrospective studies that evaluated patients treated with high-cutoff dialyzers and conventional dialyzers. Preliminary results of another unpublished randomized trial that addressed intensive high-cutoff hemodialysis in myeloma cast nephropathy showed a kidney function recovery rate of 60% in the high-cutoff group. A similar high hemodialysis independence rate was observed in that study’s control group. In contrast with the current study, randomization occurred without a screening period, allowing inclusion of patients who might have lost indication for hemodialysis after symptomatic measures were addressed. In addition, frontline use of a bortezomib-based triplet and differences in hemodialysis schedule (daily 8-hour hemodialysis sessions using two 1.1-m2 high-cutoff dialyzers vs 3 weekly 4-hour sessions in the conventional hemodialysis group) resulted in a high incidence of infections and decreased overall survival in the high-cutoff hemodialysis group.
This trial reinforces literature supporting the importance of rapid and deep serum-free light chain response for subsequent kidney outcome. An independent predictive factor of hemodialysis independence at 12 months was the achievement of a predialysis serum-free light chain level below 500 mg/L after the first cycle of chemotherapy, a value considered a threshold for cast formation. Baseline serum-free light chain level was not predictive, presumably because of limitations of the nephelometric assay for high serum-free light chain values. However, light chain only multiple myeloma, which is the most frequent cause of cast nephropathy through the high production rate of nephrotoxic light chains, was associated with a lower probability of kidney recovery. To remove nephrotoxic serum-free light chains in patients with severe AKI, the use of plasma exchange also has been advocated. High-cutoff hemodialysis may be preferred because it carries a lower risk of adverse effects and allows higher serum-free light chain removal with less rebound effect.
Additional studies are required to optimize the modalities of light chain removal in cast nephropathy. Because the cost of high-cutoff dialyzers is a concern, evaluation of global financial weight, taking into account the duration of hemodialysis support, is desirable. Future studies may include patients with AKI at the time of myeloma relapse, which is an increasingly observed situation for which the probability of a profound and swift serum-free light chain response with chemotherapy is less predictable than among patients with newly diagnosed myeloma.
Limitations
This study has several limitations. First, the expected increase in hemodialysis independence rate from 30% to 60% at 3 months used in the sample size computation was arbitrarily chosen from retrospective studies. A smaller difference in the kidney recovery rate may have been of clinical significance; therefore, this study could be considered underpowered. Second, although the trial showed significantly higher kidney recovery rates at 6 and 12 months in the high-cutoff hemodialysis group, these were secondary end points and need to be interpreted as exploratory because of the potential for type I error. It was not anticipated that high-cutoff hemodialysis would allow delayed kidney function response (Figure 2A) because this type of response was rarely reported. Delayed improvement of kidney function may reflect selection of patients with severe AKI because of the screening period before randomization, which presumably eliminated patients for whom supportive care and steroids alone led to kidney recovery.
Third, another potential limitation was the use of a bortezomib-dexamethasone doublet instead of a triplet because frontline bortezomib-based triplets are increasingly used in the treatment of myeloma to produce rapid tumor mass reduction. However, available data do not suggest a superiority of triplet combinations in AKI, except for a retrospective study of 31 patients, in which hemodialysis independence was higher among the 13 patients who received different triplets. The benefit-risk ratio of intensified chemotherapy (particularly regarding infectious complications) warrants careful investigation among frail patients with severe AKI who sometimes require referral to intensive care units at diagnosis (required by 16% of patients in the current study). Moreover, the potential nephrotoxicity of certain novel agents and the increased risk of tumor lysis syndrome with more effective antimyeloma combinations should be considered. In this trial, chemotherapy was reinforced with cyclophosphamide in cases of insufficient hematologic response. Chemotherapy was similarly delivered in both groups, and both groups had high hematologic response rates and good tolerance profiles. The achievement of more frequent partial response at 3 months in the high-cutoff hemodialysis group (Table 2) was likely caused by better serum-free light chain removal through hemodialysis.
Fourth, although the primary outcome was not achieved, the trial showed significantly higher kidney recovery rates at 6 and 12 months in the high-cutoff hemodialysis group. Nevertheless, those results should be considered exploratory given the potential inflation of the type I error rate because of the numerous secondary and post hoc analyses.
Conclusions
Among patients with myeloma cast nephropathy treated with a bortezomib-based chemotherapy regimen, the use of high-cutoff hemodialysis compared with conventional hemodialysis did not result in a statistically significant difference in hemodialysis independence at 3 months. However, the study may have been underpowered to identify an early clinically important difference.
Trial protocol
Amended trial protocol
Statistical analysis plan
References
- 1.Dimopoulos MA, Sonneveld P, Leung N, et al. International Myeloma Working Group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol. 2016;34(13):1544-1557. [DOI] [PubMed] [Google Scholar]
- 2.Hutchison CA, Batuman V, Behrens J, et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol. 2011;8(1):43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yadav P, Hutchison CA, Basnayake K, et al. Patients with multiple myeloma have excellent long-term outcomes after recovery from dialysis-dependent acute kidney injury. Eur J Haematol. 2016;96(6):610-617. [DOI] [PubMed] [Google Scholar]
- 4.Clark WF, Stewart AK, Rock GA, et al. Plasma exchange when myeloma presents as acute renal failure. Ann Intern Med. 2005;143(11):777-784. [DOI] [PubMed] [Google Scholar]
- 5.Hutchison CA, Bradwell AR, Cook M, et al. Treatment of acute renal failure secondary to multiple myeloma with chemotherapy and extended high cut-off hemodialysis. Clin J Am Soc Nephrol. 2009;4(4):745-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchison CA, Cockwell P, Stringer S, et al. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol. 2011;22(6):1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heyne N, Denecke B, Guthoff M, et al. Extracorporeal light chain elimination: high cut-off (HCO) haemodialysis parallel to chemotherapy allows for a high proportion of renal recovery in multiple myeloma patients with dialysis-dependent acute kidney injury. Ann Hematol. 2012;91(5):729-735. [DOI] [PubMed] [Google Scholar]
- 8.Hutchison CA, Heyne N, Airia P, et al. Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol Dial Transplant. 2012;27(10):3823-3828. [DOI] [PubMed] [Google Scholar]
- 9.Zannetti BA, Zamagni E, Santostefano M, et al. Bortezomib-based therapy combined with high cut-off hemodialysis is highly effective in newly diagnosed multiple myeloma patients with severe renal impairment. Am J Hematol. 2015;90(7):647-652. [DOI] [PubMed] [Google Scholar]
- 10.Gerth HU, Pohlen M, Görlich D, et al. Impact of high-cut-off dialysis on renal recovery in dialysis-dependent multiple myeloma patients. PLoS One. 2016;11(5):e0154993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curti A, Schwarz A, Trachsler J, et al. Therapeutic efficacy and cost effectiveness of high cut-off dialyzers compared to conventional dialysis in patients with cast nephropathy. PLoS One. 2016;11(7):e0159942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chanan-Khan AA, Kaufman JL, Mehta J, et al. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure. Blood. 2007;109(6):2604-2606. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Reversibility of renal impairment in patients with multiple myeloma treated with bortezomib-based regimens. Clin Lymphoma Myeloma. 2009;9(4):302-306. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig H, Adam Z, Hajek R, et al. Light chain-induced acute renal failure can be reversed by bortezomib-doxorubicin-dexamethasone in multiple myeloma. J Clin Oncol. 2010;28(30):4635-4641. [DOI] [PubMed] [Google Scholar]
- 15.Morabito F, Gentile M, Ciolli S, et al. Safety and efficacy of bortezomib-based regimens for multiple myeloma patients with renal impairment. Eur J Haematol. 2010;84(3):223-228. [DOI] [PubMed] [Google Scholar]
- 16.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network. Crit Care. 2007;11(2):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ecotière L, Thierry A, Debiais-Delpech C, et al. Prognostic value of kidney biopsy in myeloma cast nephropathy. Nephrol Dial Transplant. 2016;31(1):64-72. [DOI] [PubMed] [Google Scholar]
- 18.Nasr SH, Valeri AM, Sethi S, et al. Clinicopathologic correlations in multiple myeloma. Am J Kidney Dis. 2012;59(6):786-794. [DOI] [PubMed] [Google Scholar]
- 19.International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders. Br J Haematol. 2003;121(5):749-757. [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. [DOI] [PubMed] [Google Scholar]
- 21.Bridoux F, Leung N, Hutchison CA, et al. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015;87(4):698-711. [DOI] [PubMed] [Google Scholar]
- 22.Zand L, Nasr SH, Gertz MA, et al. Clinical and prognostic differences among patients with light chain deposition disease, myeloma cast nephropathy and both. Leuk Lymphoma. 2015;56(12):3357-3364. [DOI] [PubMed] [Google Scholar]
- 23.Lameire N, Kellum JA, et al. Contrast-induced acute kidney injury and renal support for acute kidney injury. Crit Care. 2013;17(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467-1473. [DOI] [PubMed] [Google Scholar]
- 25.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. [Google Scholar]
- 26.Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2006;15(2):1-11. [Google Scholar]
- 27.Hutchison CA, Cockwell P, Reid S, et al. Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma. J Am Soc Nephrol. 2007;18(3):886-895. [DOI] [PubMed] [Google Scholar]
- 28.Hutchison CA, Cockwell P, Heyne N, et al. European trial of free light chain removal by extended haemodialysis in cast nephropathy (EuLITE). J Am Soc Nephrol. 2016;27:8A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung N, Gertz MA, Zeldenrust SR, et al. Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int. 2008;73(11):1282-1288. [DOI] [PubMed] [Google Scholar]
- 30.Burnette BL, Leung N, Rajkumar SV. Renal improvement in myeloma with bortezomib plus plasma exchange. N Engl J Med. 2011;364(24):2365-2366. [DOI] [PubMed] [Google Scholar]
- 31.Campbell JP, Cobbold M, Wang Y, et al. Development of a highly-sensitive multi-plex assay using monoclonal antibodies for the simultaneous measurement of kappa and lambda immunoglobulin free light chains in serum and urine. J Immunol Methods. 2013;391(1-2):1-13. [DOI] [PubMed] [Google Scholar]
- 32.Knudsen LM, Hjorth M, Hippe E, et al. Renal failure in multiple myeloma. Eur J Haematol. 2000;65(3):175-181. [DOI] [PubMed] [Google Scholar]
- 33.Bladé J, Fernández-Llama P, Bosch F, et al. Renal failure in multiple myeloma. Arch Intern Med. 1998;158(17):1889-1893. [DOI] [PubMed] [Google Scholar]
- 34.Magarotto V, Bringhen S, Offidani M, et al. Triplet vs doublet lenalidomide-containing regimens for the treatment of elderly patients with newly diagnosed multiple myeloma. Blood. 2016;127(9):1102-1108. [DOI] [PubMed] [Google Scholar]
- 35.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Bortezomib-based triplets are associated with a high probability of dialysis independence and rapid renal recovery in newly diagnosed myeloma patients with severe renal failure or those requiring dialysis. Am J Hematol. 2016;91(5):499-502. [DOI] [PubMed] [Google Scholar]
- 36.Lodhi A, Kumar A, Saqlain MU, Suneja M. Thrombotic microangiopathy associated with proteasome inhibitors. Clin Kidney J. 2015;8(5):632-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oiwa K, Morita M, Kishi S, et al. High risk of tumor lysis syndrome in symptomatic patients with multiple myeloma with renal dysfunction treated with bortezomib. Anticancer Res. 2016;36(12):6655-6662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Amended trial protocol
Statistical analysis plan