Key Points
Question
In people with lower extremity peripheral artery disease (PAD), do granulocyte-macrophage colony-stimulating factor (GM-CSF) and supervised treadmill exercise improve 6-minute walk performance when the 2 interventions are combined and when each is used alone?
Findings
In a randomized clinical trial of 210 participants with PAD, supervised treadmill exercise significantly improved the 6-minute walk by 34 m at 12-week follow-up, compared with control. The combination of treadmill exercise with GM-CSF did not significantly improve the 6-minute walk distance more than exercise alone or more than GM-CSF alone, and GM-CSF alone did not improve the 6-minute walk distance more than placebo at 12-week follow-up.
Meaning
These results confirm the benefits of exercise but do not support using GM-CSF either alone or with exercise to treat walking impairment in PAD.
Abstract
Importance
Benefits of granulocyte-macrophage colony-stimulating factor (GM-CSF) for improving walking ability in people with lower extremity peripheral artery disease (PAD) are unclear. Walking exercise may augment the effects of GM-CSF in PAD, since exercise-induced ischemia enhances progenitor cell release and may promote progenitor cell homing to ischemic calf muscle.
Objectives
To determine whether GM-CSF combined with supervised treadmill exercise improves 6-minute walk distance, compared with exercise alone and compared with GM-CSF alone; to determine whether GM-CSF alone improves 6-minute walk more than placebo and whether exercise improves 6-minute walk more than an attention control intervention.
Design, Setting, and Participants
Randomized clinical trial with 2 × 2 factorial design. Participants were identified from the Chicago metropolitan area and randomized between January 6, 2012, and December 22, 2016, to 1 of 4 groups: supervised exercise + GM-CSF (exercise + GM-CSF) (n = 53), supervised exercise + placebo (exercise alone) (n = 53), attention control + GM-CSF (GM-CSF alone) (n = 53), attention control + placebo (n = 51). The final follow-up visit was on August 15, 2017.
Interventions
Supervised exercise consisted of treadmill exercise 3 times weekly for 6 months. The attention control consisted of weekly educational lectures by clinicians for 6 months. GM-CSF (250 μg/m2/d) or placebo were administered subcutaneously (double-blinded) 3 times/wk for the first 2 weeks of the intervention.
Main Outcomes and Measures
The primary outcome was change in 6-minute walk distance at 12-week follow-up (minimum clinically important difference, 20 m). P values were adjusted based on the Hochberg step-up method.
Results
Of 827 persons evaluated, 210 participants with PAD were randomized (mean age, 67.0 [SD, 8.6] years; 141 [67%] black, 82 [39%] women). One hundred ninety-five (93%) completed 12-week follow-up. At 12-week follow-up, exercise + GM-CSF did not significantly improve 6-minute walk distance more than exercise alone (mean difference, −6.3 m [95% CI, −30.2 to +17.6]; P = .61) or more than GM-CSF alone (mean difference, +28.7 m [95% CI, +5.1 to +52.3]; Hochberg-adjusted P = .052). GM-CSF alone did not improve 6-minute walk more than attention control + placebo (mean difference, −1.4 m [95% CI, −25.2 to +22.4]; P = .91). Exercise alone improved 6-minute walk compared with attention control + placebo (mean difference, +33.6 m [95% CI, +9.4 to +57.7]; Hochberg-adjusted P = .02).
Conclusions and Relevance
Among patients with PAD, supervised treadmill exercise significantly improved 6-minute walk distance compared with attention control + placebo, whereas GM-CSF did not significantly improve walking performance, either when used alone or when combined with supervised treadmill exercise. These results confirm the benefits of exercise but do not support using GM-CSF to treat walking impairment in patients with PAD.
Trial Registration
clinicaltrials.gov Identifier: NCT01408901
This 2 × 2 factorial trial compared the effects of granulocyte-macrophage colony-stimulating factor (GM-CSF) vs placebo and of supervised treadmill exercise vs education control on 6-minute walk distance at 12 weeks in patients with peripheral artery disease.
Introduction
Preliminary evidence supports the hypothesis that interventions that increase circulating endothelial progenitor cells may improve walking performance in patients with lower extremity peripheral artery disease (PAD). Colony-stimulating factors mobilize progenitor cells from bone marrow and spleen into the peripheral circulation and may improve walking performance in people with PAD by promoting angiogenesis and improving endothelial function and cardiovascular health. However, evidence is mixed regarding colony-stimulating factors and improved walking performance for people with PAD.
In people with PAD, walking exercise induces lower extremity ischemia and has been shown to increase circulating progenitor cells. Exercise-induced ischemia may promote homing of progenitor cells to ischemic muscle. Therefore, treadmill exercise combined with granulocyte-macrophage colony-stimulating factor (GM-CSF) may enhance the effect of GM-CSF on walking performance in people with PAD. The Progenitor Cell Release Plus Exercise to Improve Functional Performance in PAD (PROPEL) randomized trial used a 2 × 2 factorial design to determine whether combining GM-CSF with supervised treadmill exercise improved walking ability more than GM-CSF alone and more than supervised exercise alone, respectively. This trial also assessed whether GM-CSF was better than placebo and whether supervised treadmill exercise was better than an attention control intervention for improving walking performance in PAD.
Methods
The institutional review board at Northwestern University and all recruitment sites approved the protocol. Participants provided written informed consent. Participants were randomized between January 6, 2012, and December 22, 2016. The final follow-up visit was on August 15, 2017. Detailed methods are provided in the protocol in Supplement 1.
Participant Identification
Participants were identified from Chicago-area medical centers, through newspaper or radio advertisements, and from mailings to people 55 years and older in the Chicago area. People with PAD who previously participated in research with the principal investigator (M.M.M.) and expressed interest in future research were contacted. All data collection was carried out by Northwestern staff, and all study interventions were delivered at Northwestern.
Inclusion Criteria
Inclusion criteria included an ankle-brachial index (ABI) of 0.90 or less at the baseline visit. Potential participants with an ABI greater than 0.90 at the baseline visit were eligible, if a hospital-affiliated vascular laboratory report or lower extremity angiogram demonstrated PAD. Participants with an ABI of 0.90 to 1.00 at baseline and those with a normal ABI and prior lower extremity revascularization were eligible if their ABI decreased by 20% after a heel-rise test. Heel-rise testing consisted of 50 heel rises at a rate of 1 per second, followed by repeat ABI.
Exclusion Criteria
Potential participants were excluded if they had a below- or above-knee amputation; wheelchair confinement; use of a walking aid other than a cane; inability or unwillingness to attend exercise sessions; walking impairment for a reason other than PAD; foot ulcer or critical limb ischemia; or significant visual or hearing impairment. Potential participants were also excluded if they did not complete the run-in. The run-in consisted of attending 1 weekly health education session and 1 treadmill exercise session over a 3-week period. Potential participants with major surgery or revascularization during the previous 3 months or planned during the next 6 months; current participation or participation in the past 3 months in a clinical trial or cardiac rehabilitation; Parkinson disease; and those requiring oxygen with activity were excluded. Potential participants for whom exercise may be unsafe, including those with greater than class II New York Heart Association heart failure or angina, increase in angina pectoris during the prior 6 months, or abnormal baseline stress test findings, were excluded. Participants were excluded if they were already exercising at a level similar to the intervention. Participants treated for cancer in the past 2 years were excluded unless the cancer was early stage and their prognosis was excellent. Those with a Mini-Mental Status Examination score less than 23 were excluded.
Randomization
Participants were randomized using R (R Project for Statistical Computing) to 1 of 4 groups: supervised exercise + GM-CSF (exercise + GM-CSF), attention control + GM-CSF (GM-CSF alone), supervised exercise + placebo (exercise alone), or attention control + placebo. Randomization was stratified by diabetes mellitus, since patients with diabetes have fewer progenitor cells than those without diabetes. Block randomization was used, with block sizes randomly selected from 8 and 12.
Interventions
GM-CSF and Placebo
GM-CSF (250 μg/m2/d) or placebo was administered subcutaneously 3 times weekly for 2 weeks, in a double-blinded fashion.
Supervised Treadmill Exercise and Attention Control
Treadmill exercise was provided 3 times weekly with an exercise physiologist. Walking exercise duration was increased gradually until 50 minutes of exercise per session was achieved. Participants were asked to exercise to maximal ischemic leg symptoms. Participants randomized to attention control attended weekly 1-hour educational sessions by Northwestern faculty on health topics including cancer screening, immunizations, and hypertension.
Outcomes
Outcomes were measured before randomization and at 6 weeks, 12 weeks, and 6 months after randomization by an individual blinded to group assignment.
The primary outcome was change in 6-minute walk distance between baseline and 12-week follow-up. Twelve-week follow-up was selected for the primary outcome time point because potential beneficial changes such as angiogenesis may require time after GM-CSF therapy. Furthermore, prior studies of participants with PAD suggested that benefits of GM-CSF were greater at 12-week follow-up than at 6-month follow-up.
Secondary outcomes were 12-week change in maximal treadmill walking time and brachial artery flow–mediated dilation (FMD). Exploratory outcomes were change in 6-minute walk distance, maximal treadmill walking time, and brachial artery FMD at 6-week and 6-month follow-up and were included to determine the temporal pattern of response to study interventions.
Six-Minute Walk Test
Following a standardized protocol, participants walked up and down a 100-ft (30-m) hallway for 6 minutes after receiving instructions to cover as much distance as possible. The distance completed after 6 minutes was recorded. A small clinically meaningful change has been defined as 20 m and a large meaningful change as 50 m.
Treadmill Walking Performance
Maximal treadmill walking distance was measured using the Gardner-Skinner protocol.
Brachial Artery FMD
FMD of the proximal brachial artery was assessed using B-mode and Doppler ultrasound with a linear array vascular ultrasound transducer. Doppler blood flow in the brachial artery was recorded at rest and immediately after hyperemia induction. Brachial artery diameters were recorded at rest and then 60 and 90 seconds after cuff deflation. FMD was defined as the ratio of the maximum brachial artery diameter after reactive hyperemia to the resting diameter, expressed as percent. Images were interpreted by a single reader unaware of group assignment.
Progenitor Cell Measures
Progenitor cells were measured in peripheral blood using standard methods. Detailed methods are reported as eMethods in Supplement 2.
Ankle-Brachial Index
A handheld Doppler probe (Vascular Pocket Dop II, Nicolet Vascular Inc) was used to measure systolic blood pressures in the right brachial, dorsalis pedis, and posterior tibial arteries and left dorsalis pedis, posterior tibial, and brachial arteries. Measurements were repeated. The ABI was calculated by dividing mean pressures in each leg by the mean of the 4 brachial pressures.
Other Measures
Medical history, race, and demographics were obtained using patient report. Collecting information on race was required by the funding agency and also allowed for assessing the generalizability of the results. An open-ended question was used to obtain race. Height and weight were measured, and body mass index was calculated as weight in kilograms divided by height in meters squared.
Leg Symptoms
Leg symptoms were characterized using the San Diego claudication questionnaire. Intermittent claudication was defined as exertional calf pain that did not begin at rest, caused the participant to stop walking, and resolved within 10 minutes of rest. Participants without intermittent claudication were either asymptomatic (ie, reported no exertional leg symptoms) or had exertional leg symptoms that did not meet criteria for intermittent claudication.
Power Calculations
The trial was designed to test 4 primary hypotheses: (1) combining GM-CSF with supervised treadmill exercise would improve 6-minute walk distance more than GM-CSF alone; (2) combining GM-CSF with supervised treadmill exercise would improve 6-minute walk distance more than supervised treadmill exercise alone; (3) GM-CSF would improve 6-minute walk distance more than placebo; (4) supervised treadmill exercise would improve 6-minute walk distance more than an attention control intervention.
A previous randomized trial of supervised treadmill exercise in PAD showed that changes in 6-minute walk distance at 6-month follow-up were +21.28 m (SD, 46.82) in the exercise group and −15.02 (SD, 55.54) in the control group, representing an observed effect size of 0.69 SD. Assuming that the differences in each comparison were all at least 0.61 SD (SD, 51 m), then 50 participants per group provided more than 57.6% power to reject all 4 hypotheses, 75.9% power to reject 3 or more hypotheses, 96.4% power to reject 2 or more hypotheses, and 98.9% power to reject at least 1 hypothesis. The 0.61 SD corresponded to a difference of 31 m for the 6-minute walk test, which is between the previously established values for small (20 m) and large (50 m) minimum clinically important differences.
Statistical Analysis
Statistical analyses were performed according to intention to treat. Changes in 6-minute walk distance and maximal treadmill walking time between baseline and 12-week follow-up were compared between groups using a 2-sample t test. Changes in brachial artery FMD were compared using the Wilcoxon rank sum test, since data were skewed. Statistical testing was 2-sided.
The planned sample size was 240 participants. Investigators were recruited for as long as funding allowed, resulting in a sample size of 210 participants. Because of the reduced sample size, prior to any data analyses, investigators proposed a new statistical analysis plan using the Hochberg step-up method to adjust for multiple comparisons. This method provided more statistical power than the originally proposed Bonferroni method. Investigators and the data and safety monitoring board were blinded to all efficacy results until after this decision was made.
In the Hochberg method, P values are sorted from smallest to largest (P1 ≤ P2 ≤ P3 ≤ P4). In this analysis plan, the “hypothesis” refers to the null hypothesis. Rejecting all 4 hypotheses requires that P4 < .05. If P4 ≥ .05, the remaining 3 hypotheses are rejected if P3 < .05/2. If P3 ≥ .05/2, the remaining 2 hypotheses are rejected if P2 < .05/3. If P2 ≥ .05/3, the single remaining hypothesis is rejected if P1 < .05/4. Hochberg-adjusted P values were also calculated as follows: adjusted P4 = P4; adjusted P3 = minimum of (adjusted P4, 2 × P3); adjusted P2 = minimum of (adjusted P3, 3 × P2); and adjusted P1 = minimum of (adjusted P2, 4 × P1). An adjusted Hochberg P value is statistically significant if Hochberg-adjusted P < .05. Similar methods were used for secondary and exploratory outcomes.
Analyses were performed using multiple imputation for missing data using SAS Proc MI to obtain 20 imputed data sets. Results were combined to account for both between- and within-imputation variability. Variables used for imputation were age, ABI, body mass index, sex, race, smoking status, baseline outcome values, leg symptoms, and comorbidities. People who missed follow-up testing because of death were excluded from analyses at the time points that occurred after their death.
Interaction testing was performed for the effects of GM-CSF and exercise on study outcomes, using regression analyses. In the absence of a significant interaction, the additive effects of GM-CSF and exercise were analyzed. Mixed-effects regression analyses were used to compare study outcomes longitudinally at 6-week, 12-week, and 6-month follow-up.
SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
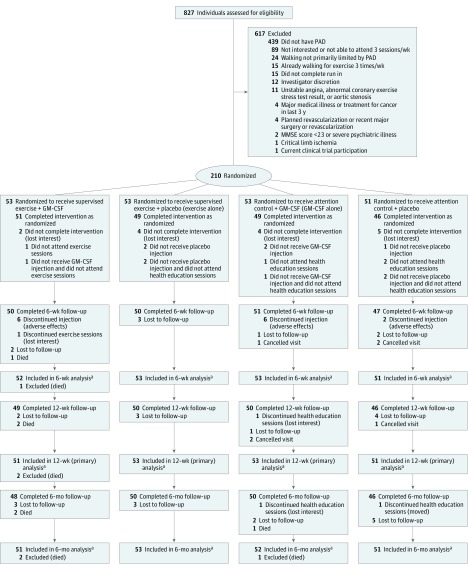
Of 827 people evaluated, 617 were excluded and 210 were randomized (Figure 1). One-hundred ninety-five (93%) completed 12-week follow-up. Sixty-seven percent were black; 39% were women. Table 1 reports characteristics of participants.
Figure 1. Eligible Participants and Follow-up Among People Evaluated for the PROPEL Trial.
GM-CSF indicates granulocyte-macrophage colony-stimulating factor; MMSE, Mini–Mental State Examination; PAD, peripheral artery disease; PROPEL, Progenitor Cell Release Plus Exercise to Improve Functional Performance in PAD.
aData were imputed for participants who were lost to follow-up or who canceled the visit.
Table 1. Baseline Characteristics of PROPEL Study Participants by Group.
| Baseline Characteristics | No. (%) | ||||
|---|---|---|---|---|---|
| Overall (N = 210) |
Exercise + GM-CSF (n = 53) |
Exercise + Placebo (Exercise Alone) (n = 53) |
Attention Control + GM-CSF (GM-CSF Alone) (n = 53) |
Attention Control+ Placebo (n = 51) |
|
| Age, mean (SD), y | 67.0 (8.6) | 66.6 (9.5) | 67.5 (8.7) | 67.9 (7.5) | 66.0 (8.6) |
| Ankle-brachial index, mean (SD) | 0.70 (0.19) | 0.70 (0.20) | 0.69 (0.19) | 0.71 (0.17) | 0.69 (0.19) |
| Body mass index, mean (SD) | 30.5 (6.6) | 29.7 (6.5) | 31.7 (6.0) | 30.7 (6.9) | 29.9 (6.8) |
| Women | 82 (39) | 20 (38) | 23 (43) | 19 (36) | 20 (39.) |
| Race | |||||
| White | 64 (30) | 18 (34) | 19 (36) | 16 (30) | 11 (22) |
| Black | 141 (67) | 35 (66) | 33 (62) | 35 (66) | 38 (75) |
| Current smoker | 71 (34) | 21 (40) | 20 (38) | 12 (23) | 18 (35) |
| Diabetes | 80 (38) | 18 (34) | 21 (40) | 21 (40) | 20 (39) |
| Myocardial infarction | 46 (22) | 16 (30) | 5 (9) | 14 (26) | 11 (22) |
| Hypertension | 175 (83) | 44 (83) | 44 (83) | 42 (79) | 45 (88) |
| Cancer | 38 (18) | 11 (21) | 7 (13) | 8 (15) | 12 (24) |
| Stroke | 32 (15) | 9 (17) | 5 (9) | 8 (15) | 10 (20) |
| Pulmonary disease | 43 (20) | 12 (23) | 10 (19) | 11 (21) | 10 (20) |
| Intermittent claudication | 64 (30) | 19 (36) | 18 (34) | 11 (21) | 16 (31) |
| Ischemic leg symptoms other than intermittent claudication | 136 (65) | 30 (57) | 33 (62) | 39 (74) | 34 (67) |
| No exertional leg symptoms | 9 (4) | 3 (6) | 2 (4) | 3 (6) | 1 (2) |
| Total treadmill time, mean (SD), min | 7.5 (4.6) | 7.8 (5.4) | 6.5 (4.5) | 8.8 (4.7) | 6.9 (3.4) |
| Maximum relative brachial artery FMD, median (IQR), %a | 5.2 (3.1-7.4) | 4.9 (2.4-7.6) | 5.0 (3.0-7.8) | 5.5 (3.8-7.1) | 5.3 (2.7-7.3) |
| Progenitor cells, mean (SD), %b | |||||
| CD34+CD45lo | 0.03 (0.02) | 0.03 (0.02) | 0.03 (0.01) | 0.03 (0.01) | 0.03 (0.02) |
| CD34+CD45loCD133+ | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) |
| CD34+CD45loCD31+ | 0.02 (0.01) | 0.03 (0.02) | 0.02 (0.01) | 0.02 (0.01) | 0.03 (0.02) |
| CD34+CD45loCD31+CD133+ | 0.02 (0.01) | 0.02 (0.02) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.01) |
Abbreviations: FMD, flow-mediated dilation; IQR, interquartile range; PROPEL, Progenitor Cell Release Plus Exercise to Improve Functional Performance in PAD.
Defined as ratio of the maximum brachial artery diameter after reactive hyperemia to the resting diameter. Brachial artery diameters recorded at rest and then 60 and 90 seconds after cuff deflation.
Values are percent of total live white blood cells. Notations indicate the specific progenitor cell types. A plus sign indicates the presence of the marker immediately preceding.
Study drug injections were completed for 278 of 318 possible injections (87.4%) in the exercise + GM-CSF group, 294 of 318 (92.5%) in the exercise-alone group, 272 of 318 (85.5%) in the GM-CSF–alone group, and 285 of 306 (93.1%) in the attention control + placebo group. Exercise session attendance was 2441 of 3504 sessions (69.7%) in the exercise + GM-CSF group and 2623 of 3619 (72.5%) in the exercise-alone group. Attendance at attention control sessions was 863 of 1159 (74.5%) in the GM-CSF-alone group and 810 of 1079 (75.1%) in the attention control + placebo group.
Progenitor cells increased significantly in the exercise + GM-CSF group and in the GM-CSF–alone groups at 2-week follow-up, but subsequently there were no statistically significant increases in progenitor cells in any group compared with baseline (eFigures 1 and 2 in Supplement 2).
Primary Outcomes
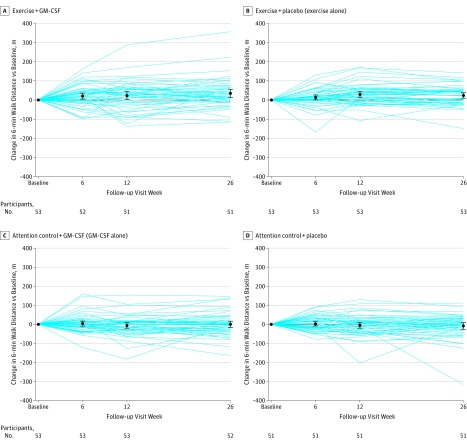
P values for the 4 primary comparisons, ranked from smallest to largest, were P1 = .006, P2 = .017, P3 = .61, P4 = .91. Using the Hochberg method to adjust for multiple comparisons, exercise + GM-CSF did not significantly improve 6-minute walk distance more than exercise alone (from 334.1 to 356.4 m in the exercise + GM-CSF group vs 338.7 to 367.3 m in the exercise-alone group; mean difference, −6.3 m [95% CI, −30.2 to +17.6]; P = .61; Hochberg-adjusted P = .91) or more than GM-CSF alone (from 339.8 to 333.4 m in the GM-CSF–alone group; mean difference, +28.7 [95% CI, +5.1 to +52.3]; P = .02 [unrounded value P = .0173, which was greater than the value required for statistical significance, P < .0167]; Hochberg-adjusted P = .052). GM-CSF alone did not improve 6-minute walk distance more than attention control + placebo (339.1 to 334.1 m in the attention control + placebo group; mean difference, −1.4 m [95% CI, −25.2 to +22.]; P = .91; Hochberg-adjusted P = .91) at 12-week follow-up. Exercise alone improved 6-minute walk distance more than attention control + placebo (mean difference, +33.6 m [95% CI, +9.4 to +57.7]; P = .006; Hochberg-adjusted P = .02) at 12-week follow-up (Table 2). Figure 2 shows changes in 6-minute walk distances for the 4 study interventions.
Table 2. Effect of GM-CSF and Exercise on Primary and Secondary Outcomes at 12-Week Follow-up.
| Exercise + GM-CSF (n = 51) |
Exercise + Placebo (Exercise Alone) (n = 53) |
Attention Control + GM-CSF (GM-CSF Alone) (n = 53) |
Attention Control + Placebo (n = 51) |
|
|---|---|---|---|---|
| Primary Outcome: 6-Minute Walk Distance, m | ||||
| Baseline, mean (SD) | 334.1 (107.3) | 338.7 (95.6) | 339.8 (101.0) | 339.1 (92.0) |
| 12-wk follow-up, mean (SD) | 356.4 (97.0) | 367.3 (91.2) | 333.4 (104.9) | 334.1 (91.2) |
| Within-group 12-wk change, mean (95% CI) | 22.2 (5.4 to 39.0) | 28.5 (11.7 to 45.4) | −6.4 (−23.0 to +10.1) | −5.0 (−22.3 to +12.2) |
| 12-wk change (95% CI)a | ||||
| Relative to attention control + GM-CSF (GM-CSF alone) | 28.7 (5.1 to 52.3) P = .02b Hochberg-adjusted P = .052 |
1 [Reference] | ||
| Relative to exercise + placebo (exercise alone) | −6.3 (−30.2 to +17.6) P = .61 Hochberg-adjusted P = .91 |
1 [Reference] | ||
| Relative to attention control + placebo | 33.6 (9.4 to 57.7) P = .006 Hochberg-adjusted P = .02 |
−1.4 (−25.2 to +22.4) P = .91 Hochberg-adjusted P = .91 |
1 [Reference] | |
| Maximal Treadmill Walking Time, min | ||||
| Baseline, mean (SD) | 7.8 (5.3) | 6.5 (4.5) | 8.8 (4.7) | 6.9 (3.4) |
| 12-wk follow-up, mean (SD) | 11.3 (5.6) | 10.7 (5.0) | 8.7 (4.2) | 7.4 (4.0) |
| Within-group 12-wk change, mean (95% CI) | 3.5 (2.5 to 4.5) | 4.2 (3.2 to 5.2) | −0.1 (−1.1 to +0.9) | +0.5 (−0.6 to +1.6) |
| 12-wk change (95% CI)a | ||||
| Relative to attention control + GM-CSF (GM-CSF alone) | 3.6 (2.1 to 5.0) P < .001 Hochberg-adjusted P < .001 |
1 [Reference] | ||
| Relative to exercise + placebo (exercise alone) | −0.7 (−2.1 to +0.8) P = .35 Hochberg-adjusted P = .44 |
1 [Reference] | ||
| Relative to attention control + placebo | — | 3.7 (2.2 to 5.2) P < .001 Hochberg-adjusted P < .001 |
−0.6 (−2.1 to +0.9) P = .44 Hochberg-adjusted P = .44 |
1 [Reference] |
| Brachial Artery Flow–Mediated Dilation, % | ||||
| Baseline, median (IQR) | 4.95 (2.71 to 7.62) | 5.06 (2.98 to 7.81) | 5.49 (3.83 to 7.05) | 5.40 (2.67 to 7.29) |
| 12-wk follow-up, median (IQR) | 4.25 (2.44 to 6.62) | 5.12 (2.96 to 7.67) | 5.90 (3.55 to 8.36) | 3.96 (2.38 to 8.22) |
| Within-group 12-wk change, median (IQR) | −0.37 (−2.50 to +1.28) | +0.14 (−1.53 to +1.50) | +0.10 (−1.49 to +1.18) | −0.72 (−2.02 to +1.64) |
| 12-wk change (95% CI)c | ||||
| Relative to attention control + GM-CSF (GM-CSF alone) | −0.61 (−1.67 to +0.44) P = .26 Hochberg-adjusted P = .49 |
1 [Reference] | ||
| Relative to exercise + placebo (exercise alone) | −0.67 (−1.84 to +0.51) P = .27 Hochberg-adjusted P = .49 |
1 [Reference] | ||
| Relative to attention control + placebo | +0.48 (−0.64 to +1.59) P = .40 Hochberg-adjusted P = .49 |
+0.36 (−0.66 to +1.38) P = .49 Hochberg-adjusted P = .49 |
1 [Reference] | |
Abbreviation: IQR, interquartile range.
Expressed as difference in changes. Unadjusted P values from the 2-sample t test.
Unrounded value P = .0173 (threshold for statistical significance, P < .0167).
Expressed as Hodges-Lehmann estimation of location shift (95% confidence limits). Unadjusted P values from Wilcoxon rank sum test. Statistical significance is determined using the Hochberg step-up method to adjust for multiple comparisons. In the Hochberg method, P values are sorted from from smallest to largest (P1≤P2≤P3≤P4). In this analysis plan, the “hypothesis” refers to the null hypothesis. Rejecting all 4 hypotheses requires that P4 < .05. If P4 ≥ .05, the remaining 3 hypotheses are rejected if P3 < .05/2. If P3 ≥ .05/2, the remaining 2 hypotheses are rejected if P2 < .05/3. If P2 ≥ .05/3, the single remaining hypthesis is rejected if P1 < .05/4. Hochberg-adjusted P values can also be calculated as follows: adjusted P4 = P4; adjusted P3 = minimum of (adjusted P4, 2 × P3); adjusted P2 = minimum of (adjusted P3, 3 × P2); and adjusted P1 = minimum of (adjusted P2, 4 × P1). An adjusted Hochberg P value is statistically significant if Hochberg-adjusted P < .05. Similar methods were used for secondary and exploratory outcomes.
Figure 2. Changes in 6-Minute Walk Distance by Study Intervention (N = 208).
Results are based on multiple imputation and exclude 2 participants who died before 12-week follow-up testing and 1 participant who died before 24-week follow-up. Data markers indicate means; error bars, 95% confidence intervals.
Secondary Outcomes
Using the Hochberg method, at 12-week follow-up, exercise + GM-CSF significantly improved maximal treadmill walking time more than GM-CSF alone (+3.6 minutes [95% CI, +2.1 to +5.0]; P < .001; Hochberg-adjusted P < .001) (Table 2). Exercise + GM-CSF did not significantly improve maximal treadmill walking time more than exercise alone (Table 2). GM-CSF alone did not significantly improve maximal treadmill walking time more than attention control + placebo (Table 2). The exercise-alone intervention significantly improved maximal treadmill walking time more than attention control + placebo (+3.7 minutes [95% CI, +2.2 to +5.2]; P < .001; Hochberg-adjusted P < .001) (Table 2; eFigure 3 in Supplement 2). There were no significant differences in brachial artery FMD for any prespecified comparison (Table 2; eFigure 4 in Supplement 2). There were no changes over time in resting brachial artery diameter or hyperemic stimulus (see Supplement 2).
Exploratory Outcomes
Adjusting for multiple comparisons, at 6-week follow-up the exercise + GM-CSF intervention significantly improved maximal treadmill walking distance compared with GM-CSF alone. Exercise + placebo significantly improved maximal treadmill walking distance more than attention control + placebo (eTable 1 in Supplement 2). At 6-week follow-up, there were no significant differences in 6-minute walk distance or brachial artery FMD for any prespecified comparison (eTable 1 in Supplement 2).
Adjusting for multiple comparisons, at 6-month follow-up, exercise + GM-CSF significantly improved 6-minute walk distance and maximal treadmill walking time more than GM-CSF alone (eTable 2 in Supplement 2). Exercise alone improved 6-minute walk and maximal treadmill walking distance, respectively, more than attention control + placebo (eTable 2 in Supplement 2). At 6-month follow-up, there were no other significant differences in the prespecified comparisons.
Two participants randomized to exercise + GM-CSF died. One died between the third and fourth study drug injections; the death certificate listed “crack cocaine overdose” as cause of death. The second participant collapsed while entering the exercise facility for an exercise session during week 11 and died within 24 hours. One participant randomized to GM-CSF alone died of heart failure at week 14 after randomization. The 3 deaths were determined unlikely related to study participation.
There was no statistically significant interaction of supervised exercise on the association of GM-CSF with change in 6-minute walk distance (P = .78). Therefore, data from the 2 GM-CSF groups, 2 exercise groups, and 2 placebo groups were combined. There was no statistically significant effect of GM-CSF on change in 6-minute walk distance at 12-week follow-up compared with placebo (−3.8 m [95% CI, −20.6 to +12.9]; P = .65). Exercise improved 6-minute walk distance by 31.1 m (95% CI, +14.3 to +47.9]; P < .001). In exploratory longitudinal analyses, exercise alone significantly improved 6-minute walk distance during follow-up compared with attention control + placebo (mean difference, +25.5 m [95% CI, +7.3 to +43.8]; P = .006; Hochberg-adjusted P = .02) (see Supplement 2 for results of remaining longitudinal analyses).
Discussion
In this trial, combining GM-CSF with supervised treadmill exercise did not significantly improve 6-minute walk distance more than supervised exercise alone or more than GM-CSF alone. GM-CSF alone did not significantly improve 6-minute walk distance more than placebo. Supervised treadmill exercise alone significantly improved 6-minute walk distance more than attention control + placebo.
By promoting lower extremity ischemia, a stimulus for endothelial progenitor cell release, treadmill exercise could potentially stimulate circulating endothelial cell increases and migration to ischemic lower extremity muscles in patients with PAD. Combining ischemia-inducing treadmill exercise with GM-CSF had potential to enhance therapeutic benefits of GM-CSF. However, combining exercise with GM-CSF was not better than exercise alone, and GM-CSF alone did not significantly improve any outcome more than placebo.
In contrast to results reported here, a pilot randomized trial of 45 participants with PAD demonstrated that GM-CSF improved treadmill walking performance and brachial artery FMD at 12-week follow-up compared with placebo, and these benefits were observed as early as 2 weeks after GM-CSF injections. However, a subsequent randomized trial of 159 participants with PAD reported no significant improvement in maximal treadmill walking time at 12-week follow-up among participants randomized to GM-CSF compared with placebo (mean difference, 53 seconds; P = .08). Two of 9 secondary outcomes, the Walking Impairment Questionnaire distance score and the 36-Item Short Form Health Survey physical functioning score, significantly improved in the GM-CSF group compared with placebo. There were no significant differences in any outcomes between GM-CSF and placebo at 6-month follow-up.
This study had unique features. First, it combined supervised exercise with GM-CSF, to determine whether exercise-induced leg ischemia enhanced the benefits of GM-CSF. Second, the study assessed changes in walking performance at 3 time points, ranging from 6 weeks to 6 months after injection. This characteristic is important because colony-stimulating factors increase circulating progenitor cells immediately, but angiogenesis and other favorable systemic changes may require more time. Third, the study included participants with and without classic symptoms of intermittent claudication. Since most people with PAD do not have classic claudication symptoms, this trial is more generalizable to patients with PAD encountered by practicing clinicians. Fourth, the study assessed changes in 6-minute walk performance, which is more relevant to walking in daily life than treadmill walking.
Additional characteristics of the results should be noted. First, supervised treadmill exercise improved treadmill walking performance as early as 6-week follow-up but had a more gradual effect on 6-minute walk distance. This may be because the treadmill exercise intervention specifically trained the participant to the treadmill outcome measure. Second, a previous study reported that supervised treadmill exercise improved brachial artery FMD in PAD, but results reported here showed no effect of exercise on FMD. The reason for this discrepancy is unclear. Third, results reported here are not consistent with those from a prior report that treadmill exercise significantly increased circulating endothelial progenitor cells in patients with PAD.
Limitations
This study has several limitations. First, the trial randomized 88% of the targeted sample size of 240 participants. Second, a longer duration of GM-CSF therapy may have achieved greater benefit. The 2-week therapy duration was based on a smaller randomized trial in which 6 injections of GM-CSF over 2 weeks achieved significant improvement in treadmill walking performance at 12-week follow-up. Third, it is possible that granulocyte colony-stimulating factor (rather than GM-CSF) would have been more effective.
Conclusions
Among patients with PAD, supervised treadmill exercise significantly improved 6-minute walk distance compared with attention control + placebo, whereas GM-CSF did not significantly improve walking performance, either when used alone or when combined with supervised treadmill exercise. These results confirm the benefits of exercise but do not support using GM-CSF to treat walking impairment in patients with PAD.
Study Protocol and Statistical Analysis Plan
eTable 1. Effect of GM-CSF and Exercise on Study Outcomes at 6-Week Follow-up
eTable 2. Effect of GM-CSF and Exercise on Study Outcomes at 6-Month Follow-up
eTable 3. Effect of GM-CSF and Exercise on Changes in Reactive Hyperemia Flow, Reactive Hyperemic Average Peak Velocity, and Brachial Artery Diameter at 12-Week Follow-up
eTable 4. Results of Generalized Linear Mixed Models for Effect of GM-CSF and Exercise on Study Outcomes (Longitudinal Analyses)
eMethods. Detailed Methods for Measurement of Progenitor Cells
eFigure 1. Temporal Changes in Endothelial Progenitor Cells According to Study Group Assignment With Error Bars
eFigure 2. Temporal Changes in Endothelial Progenitor Cells According to Study Group Assignment Without Error Bars
eFigure 3. Changes in Treadmill Walking Time by Study Intervention (N = 208)
eFigure 4. Changes in Brachial Artery Flow-Mediated Dilation by Study Intervention (N = 208)
References
- 1.Subramaniyam V, Waller EK, Murrow JR, et al. Bone marrow mobilization with granulocyte macrophage colony-stimulating factor improves endothelial dysfunction and exercise capacity in patients with peripheral arterial disease. Am Heart J. 2009;158(1):53-60. [DOI] [PubMed] [Google Scholar]
- 2.Sandri M, Adams V, Gielen S, et al. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation. 2005;111(25):3391-3399. [DOI] [PubMed] [Google Scholar]
- 3.Laufs U, Werner N, Link A, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109(2):220-226. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964-967. [DOI] [PubMed] [Google Scholar]
- 5.Thijssen DHJ, Torella D, Hopman MTE, Ellison GM. The role of endothelial progenitor and cardiac stem cells in the cardiovascular adaptations to age and exercise. Front Biosci (Landmark Ed). 2009;14:4685-4702. [DOI] [PubMed] [Google Scholar]
- 6.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593-600. [DOI] [PubMed] [Google Scholar]
- 7.van Royen N, Schirmer SH, Atasever B, et al. START trial: a pilot study on STimulation of ARTeriogenesis using subcutaneous application of granulocyte-macrophage colony-stimulating factor as a new treatment for peripheral vascular disease. Circulation. 2005;112(7):1040-1046. [DOI] [PubMed] [Google Scholar]
- 8.Arai M, Misao Y, Nagai H, et al. Granulocyte colony-stimulating factor: a noninvasive regeneration therapy for treating atherosclerotic peripheral artery disease. Circ J. 2006;70(9):1093-1098. [DOI] [PubMed] [Google Scholar]
- 9.Poole J, Mavromatis K, Binongo JN, et al. Effect of progenitor cell mobilization with granulocyte-macrophage colony-stimulating factor in patients with peripheral artery disease: a randomized clinical trial. JAMA. 2013;310(24):2631-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saber R, Liu K, Ferrucci L, et al. Ischemia-related changes in circulating stem and progenitor cells and associated clinical characteristics in peripheral artery disease. Vasc Med. 2015;20(6):534-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domanchuk K, Ferrucci L, Guralnik JM, et al. PROgenitor cell release Plus Exercise to improve functionaL performance in peripheral artery disease: the PROPEL study. Contemp Clin Trials. 2013;36(2):502-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aboyans V, Criqui MH, Abraham P, et al. ; American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia . Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890-2909. [DOI] [PubMed] [Google Scholar]
- 13.Amirhamzeh MM, Chant HJ, Rees JL, Hands LJ, Powell RJ, Campbell WB. A comparative study of treadmill tests and heel raising exercise for peripheral arterial disease. Eur J Vasc Endovasc Surg. 1997;13(3):301-305. [DOI] [PubMed] [Google Scholar]
- 14.Heun R, Papassotiropoulos A, Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int J Geriatr Psychiatry. 1998;13(6):368-380. [DOI] [PubMed] [Google Scholar]
- 15.Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45(9):1449-1457. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301(2):165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130(1):61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013;310(1):57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743-749. [DOI] [PubMed] [Google Scholar]
- 20.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23(4):402-408. [PubMed] [Google Scholar]
- 21.McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32(6):1164-1171. [DOI] [PubMed] [Google Scholar]
- 22.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136(12):873-883. [DOI] [PubMed] [Google Scholar]
- 23.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1(1):65-71. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800-802. [Google Scholar]
- 25.Raghunathan TE. What do we do with missing data? some options for analysis of incomplete data. Annu Rev Public Health. 2004;25:99-117. [DOI] [PubMed] [Google Scholar]
- 26.Harel O, Zhou XH. Multiple imputation: review of theory, implementation and software. Stat Med. 2007;26(16):3057-3077. [DOI] [PubMed] [Google Scholar]
- 27.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286(13):1599-1606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol and Statistical Analysis Plan
eTable 1. Effect of GM-CSF and Exercise on Study Outcomes at 6-Week Follow-up
eTable 2. Effect of GM-CSF and Exercise on Study Outcomes at 6-Month Follow-up
eTable 3. Effect of GM-CSF and Exercise on Changes in Reactive Hyperemia Flow, Reactive Hyperemic Average Peak Velocity, and Brachial Artery Diameter at 12-Week Follow-up
eTable 4. Results of Generalized Linear Mixed Models for Effect of GM-CSF and Exercise on Study Outcomes (Longitudinal Analyses)
eMethods. Detailed Methods for Measurement of Progenitor Cells
eFigure 1. Temporal Changes in Endothelial Progenitor Cells According to Study Group Assignment With Error Bars
eFigure 2. Temporal Changes in Endothelial Progenitor Cells According to Study Group Assignment Without Error Bars
eFigure 3. Changes in Treadmill Walking Time by Study Intervention (N = 208)
eFigure 4. Changes in Brachial Artery Flow-Mediated Dilation by Study Intervention (N = 208)