Key Points
Question
Are women with a history of gestational diabetes at an elevated risk of major cardiovascular disease (CVD) events later in life?
Findings
In this cohort study, with follow-up of nearly 90 000 US women older than 26 years, women with a history of gestational diabetes had 43% greater risk of CVD (myocardial infarction or stroke) compared with women without prior gestational diabetes , although absolute rates in this cohort were low. Adhering to healthy lifestyle factors over follow-up mitigated this modestly elevated risk.
Meaning
Although women with prior gestational diabetes have a higher risk of CVD, adhering to a healthy lifestyle may offset this risk.
Abstract
Importance
Previous studies identify gestational diabetes (GD) as a risk factor for intermediate markers of cardiovascular disease (CVD) risk; however, few are prospective, evaluate hard CVD end points, or account for shared risk factors including body weight and lifestyle.
Objective
To prospectively evaluate history of GD in relation to incident CVD risk.
Design, Setting, and Participants
The Nurses’ Health Study II (NHS II) is an observational cohort study of US female nurses established in 1989, with ongoing follow-up. Biennial questionnaires updated behavioral characteristics, health outcomes, and lifestyle factors. Multivariable Cox models estimated the hazard ratio (HR) and 95% CI for CVD risk. We included 89 479 women who reported at least 1 pregnancy and were free of CVD and cancer at baseline. Follow-up through May 31, 2015, was complete for more than 90% of eligible participants.
Exposures
History of GD was self-reported at baseline (1989) via questionnaire and updated every 2 years.
Main Outcomes and Measures
We observed 1161 incident self-reported nonfatal or fatal myocardial infarction or stroke, confirmed via medical records.
Results
Participants had a mean (SD) age of 34.9 (4.7) years. Adjusting for age, prepregnancy body mass index, and other covariates, GD vs no GD was associated with subsequent CVD (HR, 1.43; 95% CI, 1.12-1.81). Additional adjustment for weight gain since pregnancy and updated lifestyle factors attenuated the association (HR, 1.29; 95% CI, 1.01-1.65). Classifying GD by progression to T2D in relation to CVD risk indicated a positive association for GD with progression to T2D vs no GD or T2D (HR, 4.02; 95% CI, 1.94-8.31), and an attenuated relationship for GD only (HR, 1.30; 95% CI, 0.99-1.71).
Conclusions and Relevance
Gestational diabetes was positively associated with CVD later in life, although the absolute rate of CVD in this younger cohort of predominantly white women was low. This relationship is possibly mediated in part by subsequent weight gain and lack of healthy lifestyle.
This cohort study of US women participating in the Nurses’ Health Study II prospectively evaluates the association of a history of gestational diabetes with incident cardiovascular risk.
Introduction
Gestational diabetes (GD) is the onset or recognition of impaired glucose metabolism in pregnancy. Approximately 6% of pregnancies in the United States are complicated by GD, with trends indicating increases in prevalence in recent decades. The American Heart Association identifies GD as a risk factor for cardiovascular disease (CVD) in women, based on consistent evidence for the relationships between GD with subsequent hypertension, dyslipidemia, type 2 diabetes, vascular dysfunction and atherosclerosis, and other markers of cardiometabolic risk. In addition to accumulating evidence supporting GD as a risk factor for intermediate phenotypes, retrospective analyses indicate a positive relationship between GD with major CVD events (eg, myocardial infarction [MI], stroke). However, the relationship between GD with subsequent CVD has not been evaluated in prospectively with careful control for common risk factors or mediating lifestyle characteristics.
We sought to prospectively determine the association between a history of GD and risk of major CVD events, including MI and stroke, in the Nurses’ Health Study II (NHS II) cohort. The NHS II longitudinal cohort benefits from long-term prospective follow-up with information on reproductive history and repeated ascertainment of numerous lifestyle and health-related characteristics. We previously demonstrated among NHS II participants that a history of GD was associated with an elevated risk of hypertension over 16 years of follow-up. In addition, we observed that healthful dietary and lifestyle factors were associated with lower risk of progression from GD to weight gain and CVD-related chronic diseases, including hypertension and type 2 diabetes. Identifying women at high CVD risk several years prior to an event may provide individuals and health care professionals the opportunity to intervene with preventive therapy and lifestyle modifications. Thus, we also examined whether the relationship between GD and CVD events differed by participants’ behavioral and lifestyle factors during follow-up after pregnancy, to assess the potential for prevention of CVD after GD by interventions with healthy lifestyle modifications. Given that up to half of women with GD progress to type 2 diabetes within 10 years of pregnancy, we also sought to prospectively account for intermediate type 2 diabetes.
Methods
Study Population
This analysis was conducted in the NHS II, an ongoing longitudinal prospective cohort that began in 1989 with the enrollment of 116 430 female US nurses ages 24 to 44 years. Questionnaires are distributed every 2 years to update lifestyle characteristics and health-related information, with possible person-time exceeding 90% for each biennial cycle. This study was approved by the institutional review board of the Partners Health Care System (Boston, Massachusetts). Participants did not provide written informed consent; consent was indicated by the return of the questionnaires. Participants were not compensated.
NHS II participants were eligible for inclusion in this analysis if they reported a previous birth at age 18 years or older on the 1989 baseline questionnaire or an incident first birth during follow-up, through 2001. The 2001 questionnaire is the last cycle in which incident GD and other pregnancy outcomes were ascertained because most NHS II participants had passed reproductive age. We excluded participants reporting a history of type 1 diabetes (n = 64), CVD (n = 909), or cancer (n = 1548) prior to baseline or first pregnancy.
Exposure Assessment
History of GD was captured on the baseline questionnaire and updated every 2 years through 2001 from self-report of a physician’s diagnosis. Participants were considered exposed from the time of their first pregnancy in which GD was reported through the end of follow-up. Women were not considered exposed to GD if previous type 2 diabetes was reported. Self-reported GD was previously validated against medical records in a subgroup of NHS II participants reporting GD on the 1991 questionnaire, with 94% of cases confirmed.
Outcome Ascertainment
The primary outcome of interest was a composite of incident nonfatal and fatal MI and stroke occurring from baseline through return of the 2013 questionnaire. Participants reported a physician’s diagnosis of an MI or stroke on each biennial questionnaire. Medical records were obtained with participants’ consent. Nonfatal MI was confirmed through a physician’s review according to the World Health Organization criteria that include symptoms plus either diagnostic changes in an electrocardiograph or elevated levels of cardiac-specific enzymes. Nonfatal stroke was confirmed according to the National Survey of Stroke criteria that include a new focal neurologic deficit with sudden onset and persisting 24 hours or more, excluding pathologic abnormalities due to infection, traumatic injury, or malignant disease. Deaths were identified from next of kin, postal authorities, or a search of the National Death Index. Fatal CVD events were confirmed by autopsy records or if MI or stroke was listed as the cause of death on the death certificate with evidence of prior CVD available in the medical records. We considered secondary end points of MI and stroke separately, as well as fatal CVD.
Covariate Assessment
Self-reported race and ethnicity were captured at baseline. Family histories of diabetes, MI in a parent younger than 60 years, and stroke in a parent younger than 60 years were ascertained at baseline and updated approximately every 4 years. Lifestyle- and health-related characteristics, including smoking, medication use, and incident diagnoses of other health conditions, were updated every 2 years. Previously validated body weight was captured at baseline and every 2 years. Recalled body weight at various ages at baseline was also validated and used to derive prepregnancy body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) for pregnancies that occurred prior to the baseline questionnaire. Reproductive characteristics included pregnancies and pregnancy-related complications, oral contraceptive use, and menopausal status. Pregnancy hypertensive disorders included report of pregnancy-related high blood pressure or preeclampsia. A validated physical activity questionnaire was distributed at baseline and approximately every 4 years, asking participants’ to report their usual frequency of engaging in common recreational activities. These responses were converted into metabolic equivalent tasks (METs) and summed across activities to calculate total physical activity in METs per week. Usual dietary intake was assessed every 4 years via a validated semiquantitative food frequency questionnaire. We computed a healthy dietary pattern adherence score for each participant according to the 2010 Alternative Healthy Eating Index (aHEI-2010), with a higher score indicating greater overall diet quality.
Statistical Analysis
Participants’ person-time was calculated from time of first birth, and women were followed until first major CVD event (MI or stroke), death, or May 31, 2015, whichever occurred first. Exposure status (history of GD) was updated every 2 years through 2001, and participants were considered exposed from their first report of GD through the end of follow-up, regardless of GD occurrence in subsequent pregnancies. Thus, if a woman reported a non-GD pregnancy with a subsequent GD pregnancy, she would contribute both unexposed and exposed person-time over follow-up.
We computed age- and multivariable-adjusted Cox proportional hazards models to estimate the hazard ratios (HRs) and 95% CIs for the association between a history of GD vs no history with risk of CVD, adjusting for continuous age and years since index birth as the underlying time strata. Multivariable models were adjusted for prepregnancy BMI (continuous), parity (0, 1, 2, 3, ≥4 prior pregnancies ≥6 months), history of pregnancy hypertensive disorders up to or concurrently with a GD pregnancy (yes/no), family history of MI or stroke (yes/no), menopausal status (premenopausal, postmenopausal, unsure/biologically uncertain), postmenopausal hormone therapy use (never, past, current), and white race/ethnicity. In a secondary multivariable model, we also adjusted for potential mediating lifestyle factors, including weight change from prepregnancy (continuous), current alcohol consumption (0, 1-14, ≥15 g/d), daily low-dose aspirin (yes/no), smoking status (never, former, current), total physical activity (quartiles), and aHEI-2010 score (quartiles). Time-varying covariates were updated. Information from the previous questionnaire was carried forward for missing data and a missing indicator category was created for categorical covariates when necessary.
We secondarily categorized exposure by intermediate type 2 diabetes status to assess whether GD independently elevates CVD risk, or if its relationship with CVD is explained by its progression to type 2 diabetes, an established CVD risk factor. Participants without a history of GD or type 2 diabetes served as the reference group.
We stratified by CVD lifestyle risk factors to assess whether risk differed according to current healthy behavior status. For this we considered BMI (above or below a BMI of 25.0), diet quality (above or below aHEI-2010 median), physical activity (above or below 500 MET-minutes/week), and smoking status (never vs ever). We also stratified by whether women met 0 to 2 or 3 to 4 of any combination of these healthy lifestyle factors. Lifestyle was updated over time to reflect current behavior. Finally, we examined heterogeneity by additional risk factors, including age at first birth (<30 vs ≥30 years), prepregnancy BMI (normal, <25.0, vs overweight/obese, ≥25.0), and family history of CVD. P values for interaction were derived from likelihood ratio tests comparing models with and without the multiplicative interaction term added to the main effects multivariable model. We used SAS statistical software (version 9.1; SAS Institute Inc).
Results
There were 89 479 parous NHS II participants eligible for inclusion in our analysis, with a mean (SD) age at first birth of 26.6 (4.6) years and a mean age of 34.9 (4.7) years at study enrollment in 1989. A total of 5292 women (5.9%) reported a history of GD in at least 1 pregnancy either at baseline (58%) or follow-up (42%). Age-adjusted characteristics by history of GD are presented in Table 1. Women with a history of GD were more likely to be at least 30 years old at first birth and overweight or obese prepregnancy. A history of pregnancy hypertensive disorders was also more common among women with GD, as was a family history of type 2 diabetes and CVD. Women with prior GD reported slightly less physical activity and alcohol intake but did not differ by smoking status, daily aspirin use, history of oral contraceptive use, or diet quality score at baseline.
Table 1. Age-Adjusted Baseline (1989) Characteristics by History of Gestational Diabetes (GD) of 89 479 Parous Women in the US-Based Nurses’ Health Study II Cohorta.
| Characteristic | No History of GD | History of GD |
|---|---|---|
| No. | 84 187 | 5292 |
| Age, mean (SD), yb | 34.9 (4.7) | 33.8 (4.4) |
| Age at first birth, mean (SD), yb | 26.5 (4.5) | 27.5 (4.8) |
| <30, % | 77 | 69 |
| ≥30, % | 23 | 31 |
| Prepregnancy BMI, mean (SD) | 21.0 (3.0) | 21.5 (3.6) |
| Normal weight (<25.0), % | 92 | 88 |
| Overweight/obese (≥25.0), % | 8 | 12 |
| Baseline BMI, mean (SD) | 23.9 (4.7) | 25.8 (5.9) |
| Normal weight (<25.0), % | 71 | 55 |
| Overweight (25.0-29.9), % | 19 | 25 |
| Obese (≥30.0), % | 10 | 20 |
| Parity (pregnancies ≥6 mo), mean (SD) | 1.8 (1.1) | 1.9 (1.2) |
| History of pregnancy hypertension disorders, % | 10 | 21 |
| Total physical activity, mean (SD), METs/wk | 23.8 (35.3) | 21.6 (32.7) |
| aHEI-2010 dietary pattern score, mean (SD) | 46.9 (10.7) | 46.4 (10.4) |
| Alcohol intake, mean (SD), g/d | 2.9 (5.7) | 2.3 (4.9) |
| Smoking status, % | ||
| Never | 66 | 66 |
| Past | 22 | 21 |
| Current | 12 | 13 |
| Current regular aspirin use, %c | 10 | 10 |
| Ever oral contraceptive use, % | 85 | 84 |
| Family history of diabetes, % | 16 | 30 |
| Family history of MI or stroke, % | 14 | 18 |
| Race/ethnicity, % | ||
| White | 92 | 89 |
| African American | 1 | 2 |
| Asian American | 1 | 3 |
| Other | 1 | 1 |
| Multirace | 2 | 2 |
| Hispanic | 2 | 3 |
Abbreviations: aHEI-2010, Alternative Healthy Eating Index (higher score indicates higher dietary quality); BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MET, metabolic equivalent tasks; MI, myocardial infarction.
Values are given as means (SDs) or percentages and are standardized to the age distribution of the study population. Values of polytomous variables may not sum to 100% owing to rounding.
Value is not age-adjusted.
Aspirin or aspirin-containing products used regularly at least once per week in the past 2 years.
Incident primary CVD events occurred in 1161 parous women during a median of 25.7 years of follow-up (1.1 events per 1000 person-years), including 612 MIs and 553 strokes (eFigure in the Supplement). Compared with parous women without GD, those with a history of GD experienced a 60% greater risk of CVD during follow-up (HR, 1.60; 95% CI, 1.26-2.04) (Table 2). The positive relationship remained after adjustment for risk factors (HR, 1.43; 95% CI, 1.12-1.81). Additional adjustment for potentially mediating lifestyle and behavioral risk factors, including weight gain since prepregnancy, current smoking status, diet quality score, alcohol intake, aspirin use, and physical activity, moderately attenuated the association (HR, 1.29; 95% CI, 1.01-1.65). When we considered MI and stroke risks separately, GD was positively associated with MI risk in the fully adjusted model (HR, 1.45; 95% CI, 1.05-1.99), but was not associated with stroke risk (HR, 1.10; 95% CI, 0.75-1.61). GD was not associated with fatal CVD events (n = 72), although this analysis may have been underpowered (HR, 1.21; 95% CI, 0.45-3.30).
Table 2. History of GD and Long-term Risk of Cardiovascular Disease Among 89 479 Parous US Women in the Nurses’ Health Study II Cohort, 1989-2015.
| Characteristic | HR (95% CI) | P Value | |
|---|---|---|---|
| No History of GD | History of GD | ||
| Total CVD | |||
| Events, No. | 1079 | 82 | |
| Person-years, No. | 976 307 | 60 050 | |
| Incidence rate, per 1000 person-years | 1.10 | 1.37 | |
| Age-adjusted | 1 [Reference] | 1.60 (1.26-2.04) | <.001 |
| Multivariable modela | 1 [Reference] | 1.43 (1.12-1.81) | .004 |
| Multivariable model + current lifestyle factorsb | 1 [Reference] | 1.29 (1.01-1.65) | .04 |
| MI | |||
| Events, No. | 563 | 49 | |
| Person-years | 976 456 | 60 054 | |
| Incidence rate, per 1000 person-years | 0.58 | 0.82 | |
| Age-adjusted | 1 [Reference] | 1.83 (1.35-2.50) | <.001 |
| Multivariable modela | 1 [Reference] | 1.59 (1.16-2.17) | .004 |
| Multivariable model + current lifestyle factorsb | 1 [Reference] | 1.45 (1.05-1.99) | .02 |
| Stroke | |||
| Events, No. | 520 | 33 | |
| Person-years, No. | 981 145 | 61 632 | |
| Incidence rate, per 1000 person-years | 0.53 | 0.54 | |
| Age-adjusted | 1 [Reference] | 1.33 (0.92-1.94) | .10 |
| Multivariable modela | 1 [Reference] | 1.22 (0.83-1.78) | .30 |
| Multivariable model + current lifestyle factorsb | 1 [Reference] | 1.10 (0.75-1.61) | .60 |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease (myocardial infarction [MI] or stroke); GD, gestational diabetes; HR, hazard ratio.
Multivariable model age, years since first birth age, menopausal status, current hormone therapy use, white race/ethnicity, family history of MI or stroke, history of pregnancy hypertensive disorders, prepregnancy BMI, and parity.
Multivariable model also adjusts for current weight change from prepregnancy, aspirin use, alcohol intake, smoking status, physical activity, and Alternative Healthy Eating Index 2010 diet quality score. Time-varying covariates are updated every 2 to 4 years.
Among women with a history of GD, 1008 (19.0%) subsequently developed type 2 diabetes vs 4078 (4.8%) among women without prior GD. Compared with the reference group of parous women without GD or type 2 diabetes, we observed an elevated risk of CVD for women with GD only, type 2 diabetes only, or both GD and type 2 diabetes in the age-adjusted model (Table 3). The fully adjusted model indicated a greater than 3-fold elevated risk of CVD for women with both GD and type 2 diabetes or type 2 diabetes only vs women without any diabetes (HR, 3.71; 95% CI, 1.79-7.67 and HR, 3.74; 95% CI, 1.85-7.53, respectively). Women with a history of GD but without progression to type 2 diabetes did not experience an elevated CVD risk after adjusting for weight change and other lifestyle factors (HR, 1.20; 95% CI, 0.91-1.58). Similar trends were observed for MI and stroke outcomes individually.
Table 3. History of GD by Progression to Type 2 Diabetes and Long-term Cardiovascular Disease Among 89 479 Parous US Women in the Nurses’ Health Study II Cohort, 1989-2015.
| Characteristic | HR (95% CI) | |||
|---|---|---|---|---|
| No GD or Type 2 Diabetes | GD Only | Type 2 Diabetes Only | GD and Type 2 Diabetes | |
| Total CVD | ||||
| Events, No. | 994 | 61 | 85 | 21 |
| Person-years, No. | 958 775 | 54 787 | 23 964 | 7654 |
| Incidence rate, per 1000 person-years | 1.04 | 1.11 | 3.55 | 2.74 |
| Age-adjusted model | 1 [Reference] | 1.41 (1.07-1.85) | 6.44 (3.26-12.71) | 5.55 (2.72-11.33) |
| Multivariable modela | 1 [Reference] | 1.30 (0.99-1.71) | 4.48 (2.25-8.91) | 4.04 (1.96-8.36) |
| Multivariable model + current lifestyle factorsb | 1 [Reference] | 1.20 (0.91-1.58) | 3.74 (1.85-7.53) | 3.71 (1.79-7.67) |
| MI | ||||
| Events, No. | 513 | 36 | 50 | 13 |
| Person-years, No. | 958 890 | 54 789 | 23 986 | 7655 |
| Incidence rate, per 1000 person-years | 0.53 | 0.66 | 2.08 | 1.70 |
| Age-adjusted model | 1 [Reference] | 1.56 (1.09-2.23) | 7.46 (3.03-18.37) | 7.15 (2.70-18.90) |
| Multivariable modela | 1 [Reference] | 1.42 (0.99-2.03) | 5.09 (2.02-12.82) | 5.04 (1.86-13.65) |
| Multivariable model + current lifestyle factorsb | 1 [Reference] | 1.32 (0.92-1.89) | 3.81 (1.45-10.05) | 4.27 (1.54-11.87) |
| Stroke | ||||
| Events, No. | 485 | 25 | 35 | 8 |
| Person-years, No. | 958 892 | 54 801 | 23 986 | 7661 |
| Incidence rate, per 1000 person-years | 0.51 | 0.46 | 1.46 | 1.04 |
| Age-adjusted model | 1 [Reference] | 1.22 (0.80-1.86) | 5.51 (1.94-15.68) | 4.13 (1.44-11.90) |
| Multivariable modela | 1 [Reference] | 1.15 (0.75-1.75) | 3.95 (1.39-11.23) | 3.24 (1.11-9.49) |
| Multivariable model + current lifestyle factorsb | 1 [Reference] | 1.05 (0.68-1.60) | 3.40 (1.19-9.75) | 2.87 (0.98-8.41) |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease (myocardial infarction or stroke); GD, gestational diabetes; HR, hazard ratio; MI, myocardial infarction.
Multivariable model adjust for age, years since index pregnancy, menopausal status, current hormone therapy use, white race/ethnicity, family history of MI or stroke, history of pregnancy hypertensive disorders, prepregnancy BMI, and parity.
Multivariable model also adjusts for current weight change from prepregnancy, aspirin use, alcohol intake, smoking status, physical activity, and Alternative Healthy Eating Index 2010 diet quality score. Time-varying covariates, and lifestyle factors were updated every 2 to 4 years throughout follow-up.
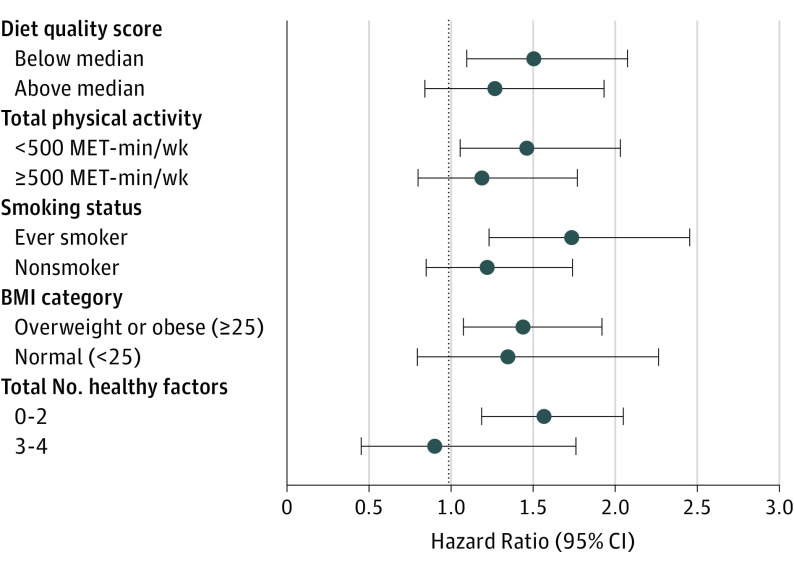
Stratification by updated healthy behavior risk factors for CVD suggested maintaining a healthier lifestyle in midlife may attenuate the association between a history of GD and CVD risk (Figure). GD was not associated with CVD risk during follow-up among women in the top half of diet quality score (HR, 1.27; 95% CI, 0.84-1.93), with at least 500 MET-minutes per week of physical activity (HR, 1.19; 95% CI, 0.80-1.77), never smokers (HR, 1.22; 95% CI, 0.85-1.74), or BMI of less than 25.0 (HR, 1.35; 95% CI, 0.80-2.27). Furthermore, among women with at least 3 of these 4 healthy lifestyle factors, a history of GD was not associated with CVD risk (HR, 0.90; 95% CI, 0.46-1.76). Conversely, a positive association between GD and CVD risk remained among women with 2 or fewer healthy factors (HR, 1.57; 95% CI, 1.19-2.05) (P value for interaction = .20). Additional a priori stratified analyses indicated a similar relationship between a history of GD with CVD risk by age at first birth (P value for interaction = .60), family history of CVD (P value for interaction = .10), and years since index pregnancy, <10 vs ≥10 (P value for interaction = .20). We did observe effect modification by prepregnancy BMI (P value for interaction = .01), with women who were overweight or obese (BMI ≥ 25.0) experiencing greater than 2-fold CVD risk with a history of GD vs no GD (HR, 2.04; 95% CI, 1.23-3.38), and no relationship among women who were not overweight or obese prepregnancy (BMI <25.0) (HR, 1.19; 95% CI, 0.89-1.59). Interaction by prepregnancy BMI remained after adjusting for weight change since prepregnancy and other updated lifestyle factors (data not shown).
Figure. Multivariable-Adjusted Models for the Relationship Between History of GD With Incident CVD Risk, Stratified by Updated Lifestyle Factors.
BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); CVD, cardiovascular disease; GD, gestational diabetes; MET, metabolic equivalent task.
Discussion
Findings from this large prospective cohort suggest that women with a history of GD experience a modest elevated long-term risk of CVD compared with parous women without a history of GD, although the absolute risk difference was quite low (0.5 additional events/1000 person-years), likely owing to the younger age of the cohort. Adjusting for updated lifestyle risk factors for CVD, including current diet, physical activity, smoking status, and weight gain, explained some, but not all, of this relationship. Furthermore, GD was not associated with CVD risk among women with healthier behavior profiles (healthy diet, physically active, never smoker, or not overweight or obese). The association between GD and CVD risk persisted among women with both GD and subsequent type 2 diabetes compared with women without GD or type 2 diabetes. Together, our findings suggest GD is possibly related to CVD risk through a sustained unhealthy lifestyle after pregnancy.
Most, but not all, previous studies of GD and CVD reported findings consistent with ours, with most evidence based on retrospective cohorts, case-control studies, and registry databases. GD was not associated with incident composite CVD end point (HR, 1.07; 95% CI, 0.81-1.42), heart disease (HR, 1.21; 95% CI, 0.84-1.73), or stroke (HR, 1.04; 95% CI, 0.60-1.82) in the European Prospective Investigation into Cancer and Nutrition (EPIC) Dutch cohort. However, GD was recalled at mean age of 51 years, many years after pregnancy, which may have led to exposure misclassification and underestimation of the association. Five retrospective analyses of cohorts, registries, and administrative databases reported positive relationships between GD with CVD risk. Estimates in 4 of the studies were attenuated after adjusting for subsequent type 2 diabetes status, while the fifth did not account for this. An administrative database study of more than 1 million pregnancies in Ontario, Canada, observed findings similar to our age-adjusted models, with elevated CVD risk for all GD pregnancies, with or without intermediate type 2 diabetes. Their analysis, however, was unadjusted for BMI, menopausal status, race/ethnicity, and other potential major confounders, which largely attenuated the elevated CVD risk among women with GD but without type 2 diabetes in our study. While these analyses provide preliminary evidence to support a positive relationship between GD with CVD risk, they are limited by their retrospective assessment of GD and lack of adjustment for relevant confounders (eg, prepregnancy BMI), which may be prone to recall bias and/or misclassification when asked several years after pregnancy.
Our longitudinal analysis of a prospective cohort indicates a positive relationship between a history of GD and CVD risk later in life, and several pathways may underlie this observation. First, experiencing a GD pregnancy may have a direct impact on cardiometabolic function. Previous prospective and cross-sectional analyses indicate that women with a history of GD experience higher levels of atherosclerosis, impaired vascular function, and are more likely to have dyslipidemia and elevated blood pressure or hypertension compared with women without GD. Differences in these intermediate phenotypes are seen prior to the development of type 2 diabetes, and in some studies are evident after relatively short-term follow-up from the index pregnancy. Thus, it is plausible that GD induces lasting cardiovascular effects in the mother, independent of progression to type 2 diabetes, a known CVD risk factor. Few of these studies, however, controlled for prepregnancy characteristics, and were thus unable to account for whether underlying cardiometabolic risk existed prior to pregnancy, predisposing women to both GD and CVD later in life. Accumulating evidence indicates that differences in GD vs non-GD pregnancies are apparent even prior to pregnancy for many CVD risk factors, including dyslipidemia, adipokines, and poor lifestyle. Therefore, prospective studies with carefully phenotyped CVD markers prepregnancy and postpregnancy are warranted to tease out whether GD itself induces adverse cardiovascular changes, is simply a marker for underlying high risk, or is some combination of these (ie, experiencing a GD pregnancy exacerbates underlying subclinical dysfunction). We also observed heterogeneity by prepregnancy overweight/obesity status, which may indicate differences in GD etiologies among women with lower BMI vs conventional higher-risk profiles.
Strengths and Limitations
Strengths of the present study include prospective longitudinal follow-up of a large cohort of women for incident confirmed CVD events. Our analysis was carefully adjusted for a number of potential confounders, including reproductive and lifestyle characteristics. Furthermore, our ability to conduct a well-powered prospective analysis to evaluate clinical end points builds on previous evidence of intermediate biomarkers and phenotypes. Limitations include the racially/ethnically homogeneous study population, precluding our ability to address the relationship between GD and CVD in potentially higher-risk minority populations. Generalizability to older populations also requires additional investigation, given the relatively young age of the NHS II population and correspondingly low rates of incident CVD. Furthermore, there is evidence that even mild glucose intolerance below the threshold of a GD diagnosis predicts subsequent cardiometabolic risk; however, our binary diagnosis of GD (yes/no) prevented us from examining the spectrum of exposure. In addition, our reliance on self-report of GD may introduce some level of exposure misclassification, although a validation study in a subgroup demonstrated the validity of this approach. Misclassification may be greater among women recalling pregnancy history at baseline, and among women with pregnancies prior to universal GD screening practices, potentially leading us to underestimate the true association between GD and CVD. Although we controlled for potential confounders, including reproductive characteristics, prepregnancy BMI, and several lifestyle risk factors, residual confounding from measurement error in the covariates is possible. Furthermore, we cannot rule out that preclinical CVD may be preexisting among women with GD.
Conclusions
Overall, we found that women with a history of GD have a modest elevated risk of CVD, particularly for MI events, compared with parous women without a history of GD. The association was attenuated among women without subsequent type 2 diabetes and those adhering to low-risk lifestyle behaviors, including maintaining a healthful diet, physical activity, nonoverweight/nonobese body weight, and nonsmoking. Collectively, these findings support the role of lifestyle for the prevention of CVD among high-risk women with a history of GD, although the small absolute rate increase of approximately 0.3 CVD events per 1000 person-years observed for those with a history of GD among these younger, predominantly white women may preclude widespread costly long-term interventions. Future data with continuous follow-up of these women are warranted to evaluate longer-term health implications of GD history.
eFigure. Cumulative incidence of cardiovascular disease according to history of gestational diabetes mellitus
References
- 1.Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124(5):804-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosca L, Benjamin EJ, Berra K, et al. ; American Heart Association . Effectiveness-based guidelines for the prevention of cardiovascular disease in women: 2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57(12):1404-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobias DK, Hu FB, Forman JP, Chavarro J, Zhang C. Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study. Diabetes Care. 2011;34(7):1582-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunderson EP, Chiang V, Pletcher MJ, et al. . History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: the Coronary Artery Risk Development in Young Adults study. J Am Heart Assoc. 2014;3(2):e000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajala O, Jensen LA, Ryan E, Chik C. Women with a history of gestational diabetes on long-term follow up have normal vascular function despite more dysglycemia, dyslipidemia and adiposity. Diabetes Res Clin Pract. 2015;110(3):309-314. [DOI] [PubMed] [Google Scholar]
- 6.O’Higgins AC, O’Dwyer V, O’Connor C, Daly SF, Kinsley BT, Turner MJ. Postpartum dyslipidaemia in women diagnosed with gestational diabetes mellitus. Ir J Med Sci. 2017;186(2):403-407. [DOI] [PubMed] [Google Scholar]
- 7.Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia. 2016;59(7):1403-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lekva T, Bollerslev J, Norwitz ER, Aukrust P, Henriksen T, Ueland T. Aortic stiffness and cardiovascular risk in women with previous gestational diabetes mellitus. PLoS One. 2015;10(8):e0136892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu J, Norman M, Wallensteen M, Gennser G. Increased large arterial stiffness and impaired acetylcholine induced skin vasodilatation in women with previous gestational diabetes mellitus. Br J Obstet Gynaecol. 1998;105(12):1279-1287. [DOI] [PubMed] [Google Scholar]
- 10.Heitritter SM, Solomon CG, Mitchell GF, Skali-Ounis N, Seely EW. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. 2005;90(7):3983-3988. [DOI] [PubMed] [Google Scholar]
- 11.Li JW, He SY, Liu P, Luo L, Zhao L, Xiao YB. Association of gestational diabetes mellitus (GDM) with subclinical atherosclerosis: a systemic review and meta-analysis. BMC Cardiovasc Disord. 2014;14:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentley-Lewis R. Late cardiovascular consequences of gestational diabetes mellitus. Semin Reprod Med. 2009;27(4):322-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appiah D, Schreiner PJ, Gunderson EP, et al. . Association of gestational diabetes mellitus with left ventricular structure and function: the CARDIA Study. Diabetes Care. 2016;39(3):400-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr DB, Utzschneider KM, Hull RL, et al. . Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29(9):2078-2083. [DOI] [PubMed] [Google Scholar]
- 15.Fadl H, Magnuson A, Östlund I, Montgomery S, Hanson U, Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG. 2014;121(12):1530-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freibert SM, Mannino DM, Bush H, Crofford LJ. The association of adverse pregnancy events and cardiovascular disease in women 50 years of age and older. J Womens Health (Larchmt). 2011;20(2):287-293. [DOI] [PubMed] [Google Scholar]
- 17.Goueslard K, Cottenet J, Mariet AS, et al. . Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc Diabetol. 2016;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tobias DK, Zhang C, Chavarro J, et al. . Healthful dietary patterns and long-term weight change among women with a history of gestational diabetes mellitus. Int J Obes (Lond). 2016;40(11):1748-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Zhu Y, Chavarro JE, et al. . Healthful dietary patterns and the risk of hypertension among women with a history of gestational diabetes mellitus: a prospective cohort study. Hypertension. 2016;67(6):1157-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao W, Tobias DK, Bowers K, et al. . Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: a prospective cohort study. JAMA Intern Med. 2014;174(7):1047-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobias DK, Hu FB, Chavarro J, Rosner B, Mozaffarian D, Zhang C. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch Intern Med. 2012;172(20):1566-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773-1779. [DOI] [PubMed] [Google Scholar]
- 24.Bao Y, Bertoia ML, Lenart EB, et al. . Origin, methods, and evolution of the three Nurses’ Health Studies. Am J Public Health. 2016;106(9):1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon CG, Willett WC, Rich-Edwards J, et al. . Variability in diagnostic evaluation and criteria for gestational diabetes. Diabetes Care. 1996;19(1):12-16. [DOI] [PubMed] [Google Scholar]
- 26.Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular Survey Methods. Geneva, Switzerland: World Health Organization; 1982. [Google Scholar]
- 27.Walker AE, Robins M, Weinfeld FD. The national survey of stroke: clinical findings. Stroke. 1981;12(2, pt 2)(suppl 1):I13-I44. [PubMed] [Google Scholar]
- 28.Stampfer MJ, Willett WC, Speizer FE, et al. . Test of the National Death Index. Am J Epidemiol. 1984;119(5):837-839. [DOI] [PubMed] [Google Scholar]
- 29.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466-473. [DOI] [PubMed] [Google Scholar]
- 30.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19(8):570-572. [PubMed] [Google Scholar]
- 31.Wolf AM, Hunter DJ, Colditz GA, et al. . Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991-999. [DOI] [PubMed] [Google Scholar]
- 32.Willett WC. Nutritional Epidemiology. 3rd ed Oxford, England: Oxford University Press; 2012. [Google Scholar]
- 33.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343(1):16-22. [DOI] [PubMed] [Google Scholar]
- 34.Heida KY, Franx A, van Rijn BB, et al. . Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus. Hypertension. 2015;66(6):1116-1122. [DOI] [PubMed] [Google Scholar]
- 35.Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ. 2009;181(6-7):371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Retnakaran R, Shah BR. Role of type 2 diabetes in determining retinal, renal, and cardiovascular outcomes in women with previous gestational diabetes mellitus. Diabetes Care. 2017;40(1):101-108. [DOI] [PubMed] [Google Scholar]
- 37.Karoli R, Siddiqi Z, Fatima J, Shukla V, Mishra PP, Khan FA. Assessment of noninvasive risk markers of subclinical atherosclerosis in premenopausal women with previous history of gestational diabetes mellitus. Heart Views. 2015;16(1):13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caliskan M, Caklili OT, Caliskan Z, et al. . Does gestational diabetes history increase epicardial fat and carotid intima media thickness? Echocardiography. 2014;31(10):1182-1187. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira AP, Calderon IM, Costa RA, Roscani MG, Magalhães CG, Borges VT. Assessment of structural cardiac abnormalities and diastolic function in women with gestational diabetes mellitus. Diab Vasc Dis Res. 2015;12(3):175-180. [DOI] [PubMed] [Google Scholar]
- 40.Anastasiou E, Lekakis JP, Alevizaki M, et al. . Impaired endothelium-dependent vasodilatation in women with previous gestational diabetes. Diabetes Care. 1998;21(12):2111-2115. [DOI] [PubMed] [Google Scholar]
- 41.Hannemann MM, Liddell WG, Shore AC, Clark PM, Tooke JE. Vascular function in women with previous gestational diabetes mellitus. J Vasc Res. 2002;39(4):311-319. [DOI] [PubMed] [Google Scholar]
- 42.Meyers-Seifer CH, Vohr BR. Lipid levels in former gestational diabetic mothers. Diabetes Care. 1996;19(12):1351-1356. [DOI] [PubMed] [Google Scholar]
- 43.Davis CL, Gutt M, Llabre MM, et al. . History of gestational diabetes, insulin resistance and coronary risk. J Diabetes Complications. 1999;13(4):216-223. [DOI] [PubMed] [Google Scholar]
- 44.Hakkarainen H, Huopio H, Cederberg H, Pääkkönen M, Voutilainen R, Heinonen S. The risk of metabolic syndrome in women with previous GDM in a long-term follow-up. Gynecol Endocrinol. 2016;32(11):920-925. [DOI] [PubMed] [Google Scholar]
- 45.Winhofer Y, Tura A, Thomas A, et al. . Hidden metabolic disturbances in women with normal glucose tolerance five years after gestational diabetes. Int J Endocrinol. 2015;2015:342938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumfeld Y, Novack L, Wiznitzer A, et al. . Pre-conception dyslipidemia is associated with development of preeclampsia and gestational diabetes mellitus. PLoS One. 2015;10(10):e0139164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harville EW, Viikari JS, Raitakari OT. Preconception cardiovascular risk factors and pregnancy outcome. Epidemiology. 2011;22(5):724-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han ES, Krauss RM, Xu F, et al. . Prepregnancy adverse lipid profile and subsequent risk of gestational diabetes. J Clin Endocrinol Metab. 2016;101(7):2721-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iliodromiti S, Sassarini J, Kelsey TW, Lindsay RS, Sattar N, Nelson SM. Accuracy of circulating adiponectin for predicting gestational diabetes: a systematic review and meta-analysis. Diabetologia. 2016;59(4):692-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hedderson MM, Darbinian J, Havel PJ, et al. . Low prepregnancy adiponectin concentrations are associated with a marked increase in risk for development of gestational diabetes mellitus. Diabetes Care. 2013;36(12):3930-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tobias DK, Zhang C, van Dam RM, Bowers K, Hu FB. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta-analysis. Diabetes Care. 2011;34(1):223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tobias DK, Zhang C, Chavarro J, et al. . Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am J Clin Nutr. 2012;96(2):289-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C, Tobias DK, Chavarro JE, et al. . Adherence to healthy lifestyle and risk of gestational diabetes mellitus: prospective cohort study. BMJ. 2014;349:g5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Cumulative incidence of cardiovascular disease according to history of gestational diabetes mellitus