Key Points
Question
Is supplementation with calcium, vitamin D, or combined calcium and vitamin D associated with a lower fracture incidence in community-dwelling older adults?
Findings
In this meta-analysis of 33 randomized clinical trials that included 51 145 participants, the use of supplements that included calcium, vitamin D, or both was not associated with a significant difference in the risk of hip fractures compared with placebo or no treatment (risk ratio, 1.53, 1.21, and 1.09, respectively).
Meaning
These findings do not support the routine use of these supplements in community-dwelling older adults.
Abstract
Importance
The increased social and economic burdens for osteoporosis-related fractures worldwide make the prevention of such injuries a major public health goal. Previous studies have reached mixed conclusions regarding the association between calcium, vitamin D, or combined calcium and vitamin D supplements and fracture incidence in older adults.
Objective
To investigate whether calcium, vitamin D, or combined calcium and vitamin D supplements are associated with a lower fracture incidence in community-dwelling older adults.
Data Sources
The PubMed, Cochrane library, and EMBASE databases were systematically searched from the inception dates to December 24, 2016, using the keywords calcium, vitamin D, and fracture to identify systematic reviews or meta-analyses. The primary randomized clinical trials included in systematic reviews or meta-analyses were identified, and an additional search for recently published randomized trials was performed from July 16, 2012, to July 16, 2017.
Study Selection
Randomized clinical trials comparing calcium, vitamin D, or combined calcium and vitamin D supplements with a placebo or no treatment for fracture incidence in community-dwelling adults older than 50 years.
Data Extraction and Synthesis
Two independent reviewers performed the data extraction and assessed study quality. A meta-analysis was performed to calculate risk ratios (RRs), absolute risk differences (ARDs), and 95% CIs using random-effects models.
Main Outcomes and Measures
Hip fracture was defined as the primary outcome. Secondary outcomes were nonvertebral fracture, vertebral fracture, and total fracture.
Results
A total of 33 randomized trials involving 51 145 participants fulfilled the inclusion criteria. There was no significant association of calcium or vitamin D with risk of hip fracture compared with placebo or no treatment (calcium: RR, 1.53 [95% CI, 0.97 to 2.42]; ARD, 0.01 [95% CI, 0.00 to 0.01]; vitamin D: RR, 1.21 [95% CI, 0.99 to 1.47]; ARD, 0.00 [95% CI, −0.00 to 0.01]. There was no significant association of combined calcium and vitamin D with hip fracture compared with placebo or no treatment (RR, 1.09 [95% CI, 0.85 to 1.39]; ARD, 0.00 [95% CI, −0.00 to 0.00]). No significant associations were found between calcium, vitamin D, or combined calcium and vitamin D supplements and the incidence of nonvertebral, vertebral, or total fractures. Subgroup analyses showed that these results were generally consistent regardless of the calcium or vitamin D dose, sex, fracture history, dietary calcium intake, and baseline serum 25-hydroxyvitamin D concentration.
Conclusions and Relevance
In this meta-analysis of randomized clinical trials, the use of supplements that included calcium, vitamin D, or both compared with placebo or no treatment was not associated with a lower risk of fractures among community-dwelling older adults. These findings do not support the routine use of these supplements in community-dwelling older people.
This meta-analysis summarizes the effects of calcium, vitamin D, or combined calcium and vitamin D supplements on fracture incidence among community-dwelling older adults.
Introduction
Approximately 40% of 50-year-old women will have major osteoporotic fractures during the remainder of their lifetimes, and these fractures are associated with major morbidity. Hip fractures are generally considered the most serious type of osteoporotic fracture. A cohort study conducted between 2000 to 2010 showed that more than one-fifth of patients died within 1 year after hip fracture. Survivors may require greater social and nursing care. The increased social and economic burdens associated with osteoporosis-related fractures worldwide make their prevention a major public health goal.
Practice guidelines recommend calcium and vitamin D supplements for older people to prevent fractures in people with osteoporosis. However, meta-analyses published to date have not reached consistent conclusions regarding the association between calcium, vitamin D, or combined calcium and vitamin D supplements and fracture risk.
Older people living in institutions such as nursing homes and residential care facilities have a higher risk of fracture compared with people living in the community. Therefore, the association of calcium and vitamin D supplementation with fracture risk may differ between community-dwelling men and women and people living in institutions. In addition, trials assessing calcium and vitamin D supplementation and risk of fracture have recently been published and add to the evidence base of the associations of calcium and vitamin D with fracture risk. Therefore, a systematic review and meta-analysis was performed to separately compare calcium, vitamin D, and combined calcium and vitamin D supplements with a placebo or no treatment for fracture incidence in community-dwelling older adults.
Methods
This meta-analysis was performed according to the Cochrane Handbook for Systematic Reviews of Interventions and presented based on Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. The protocol for this meta-analysis is available in PROSPERO (CRD42016053867).
Search Trials
We searched the PubMed, Cochrane library, and EMBASE databases from the inception dates to December 24, 2016, using the keywords calcium, vitamin D, and fracture to identify published systematic reviews or meta-analyses evaluating the association between calcium, vitamin D, or combined calcium and vitamin D supplements and the incidence of fracture. There were no language restrictions, but the search was restricted to systematic reviews or meta-analyses published in the last 10 years from December 24, 2006, toDecember 24, 2016, and excluded systematic reviews or meta-analyses that included only populations living in institutions (detailed search strategies are reported in eTable 1 in the Supplement). We identified original randomized clinical trials (RCTs) included in the systematic reviews or meta-analyses. An additional search was performed to identify recently published RCTs (from July 16, 2012, to July 16, 2017) meeting inclusion criteria, using the databases and keywords described above.
Inclusion Criteria
Trials were selected based on the following inclusion criteria: (1) RCTs comparing calcium, vitamin D, or combined calcium and vitamin D supplements with a placebo or no treatment group; (2) trials enrolling adults older than 50 years and living in their communities; and (3) trials providing fracture data. Exclusion criteria were (1) randomized trials without a placebo or no treatment group; (2) trials of participants with corticosteroid-induced secondary osteoporosis; (3) trials in which supplementation with calcium, vitamin D, or combined calcium and vitamin D was combined with other treatments (eg, an antiosteoporotic drug); (4) trials in which vitamin D analogues (eg, calcitriol) or hydroxylated vitamin D were used; and (5) trials in which dietary intake of calcium or vitamin D (eg, from milk) was evaluated.
Risk-of-Bias Assessments
The methodological quality for the included RCTs was assessed independently by 2 researchers (J.-G.Z., L.L.) based on Cochrane risk-of-bias criteria, and each quality item was graded as low risk, high risk, or unclear risk. The 7 items used to evaluate bias in each trial included the randomization sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. We defined other bias as trials sponsored by drug companies and trials in which baseline characteristics were not similar between different intervention groups. The included trials were graded as low quality, high quality, or moderate quality based on the following criteria: (1) trials were considered low quality if either randomization or allocation concealment was assessed as a high risk of bias, regardless of the risk of other items; (2) trials were considered high quality when both randomization and allocation concealment were assessed as a low risk of bias, and all other items were assessed as low or unclear risk of bias in a trial; (3) trials were considered moderate quality if they did not meet criteria for high or low risk.
Data Extraction
Two researchers (J.-G.Z., L.L.) independently extracted the following information from each study: lead author; publication year; country of origin; participant characteristics; doses of calcium, vitamin D, or their combination; dietary calcium intake; baseline serum 25-hydroxyvitamin D concentration; and trial duration. Disagreements were resolved by consensus. If the trials had more than 2 groups or factorial designs and permitted multiple comparisons, we extracted only the information and data of interest reported in the original articles. If a meta-analysis noted that unpublished data were provided by the primary authors, we extracted those fracture data from forest plots of the meta-analysis and reviewed original articles to confirm whether the trials met our inclusion criteria. When those data were our outcomes of interest, we pooled them with the data from primary trials.
The number of participants with hip fracture was the primary outcome because hip fracture can lead to more serious consequences than other fractures for older people. The secondary outcomes were the number of participants with nonvertebral fracture, vertebral fracture, and total fractures. Fractures were defined as total fractures when they occurred at all sites or when trials did not describe the sites of fractures in detail. If a trial only reported the number of participants with fractures at a single site, such as hip fracture, we did not consider it to be a total fracture.
Statistical Analysis
The association of calcium, vitamin D, and combined calcium and vitamin D supplements with fracture incidence was assessed, and each type of supplement was separately compared with a placebo or no treatment group. We performed meta-analysis to calculate risk ratios (RRs), absolute risk differences (ARDs), and 95% CIs using the Mantel-Haenszel statistical method. If zero events were reported for one group in a comparison, a value of 0.5 was added to both groups for each such study. Based on the practice recommendation of the Cochrane Handbook, trials with zero events in both the intervention and the control groups were not included in the meta-analysis when RRs were calculated.
A random-effects model was used to pool the data, and statistical heterogeneity between summary data was evaluated using the I2 statistic. Sensitivity analysis was performed by excluding low-quality studies, trials recruiting participants with particular conditions, or trials with characteristics different from the others.
When an inconsistency was detected between the RR and ARD for the same outcome, we explained the results based on the RR because the RR model is more consistent than ARD, particularly for an intervention aimed at preventing an undesirable event.
To evaluate whether the association between calcium, vitamin D, or combined calcium and vitamin D supplements and fractures was modified by clinical characteristics, we specified subgroups based on dose and frequency of calcium supplementation (≥1 or <1 g/d), vitamin D supplementation (≥800 IU/d; <800 IU/d; intermittent high-dose given as once every year; intermittent high-dose given as other frequencies, including once every 3 or 4 months and once every 1 week or month), or combined calcium and vitamin D supplementation; sex (women-only trials or trials that include both men and women); fracture history (participants with a history of fractures or other conditions, including participants with fracture history in trials in which not all participants had a history of fracture before the start of the trial, no previous fracture history, and missing fracture data); dietary calcium intake (≥900 or <900 mg/d); and baseline serum 25-hydroxyvitamin D concentration (≥20 or <20 ng/mL). Analysis was performed to assess whether the difference between the subgroups was statistically significant. We assessed publication bias by examining funnel plots when the number of trials reporting the primary outcomes was 10 or more.
All meta-analyses were performed using Revman version 5.3 (Cochrane Collaboration). All tests were 2-tailed, and P < .05 was considered statistically significant.
Results
Studies Retrieved and Characteristics
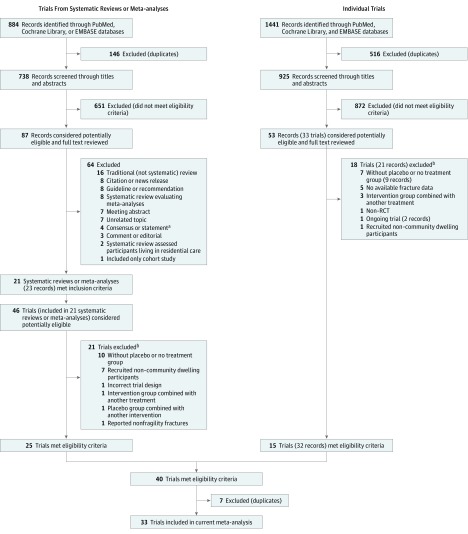
From the searches for systematic reviews or meta-analyses, 884 potentially eligible records were identified. Titles and abstracts of these records were screened for inclusion. Full texts of 87 records were read, and 21 met the inclusion criteria (Figure 1). Four systematic reviews or meta-analyses evaluated calcium supplementation with or without vitamin D for fracture prevention in older people. Seventeen systematic reviews or meta-analyses evaluated vitamin D with or without calcium for fracture incidence. Twenty-five RCTs met the inclusion criteria from 21 included systematic reviews or meta-analyses. eTables 2 and 3 in the Supplement summarize the RCTs included in the systematic reviews or meta-analyses.
Figure 1. Literature Search and Screening Process.
aIndicates a consensus or statement endorsed by a professional or other society.
bIncludes 1 trial excluded from both the search of systematic reviews and meta-analyses and the individual-trial search.
The searches for recently published RCTs yielded 1441 records, and 53 full texts of these records were reviewed. Of these, 15 trials met inclusion criteria. After excluding duplicate trials, 33 RCTs involving 51 145 participants were ultimately included in this meta-analysis (Figure 1). Hansson et al did not report the residential status of participants, although a previous meta-analysis classified this status as community. The trial by Hansson et al was included, but a sensitivity analysis was performed that excluded that trial. Table 1 reports the characteristics of the included RCTs. We excluded 38 trials for the reasons listed in eTable 4 in the Supplement.
Table 1. Characteristics of the Included Trials and Participants.
| Included Trials | Treatment | Women, No. (%) | Mean Age, y | Previous Fracture | Calcium Intake, mg/d | Baseline 25OHD, ng/mL | Treatment Duration |
|---|---|---|---|---|---|---|---|
| Calcium vs Placebo or No Treatment | |||||||
| Inkovaara et al, 1983 (Finland) |
1.2 g/d (n = 42) Placebo (n = 42) |
69 (82) | 80.1 | NA | NA | NA | 9 mo |
| Hansson and Roos, 1987 (Sweden) |
1 g/d (n = 25) Placebo (n = 25) |
50 (100) | 65.9 | Yes | NA | NA | 3 y |
| Reid et al, 1993 (New Zealand) |
1 g/d (n = 68) Placebo (n = 67) |
135 (100) | 58 | No vertebral fracture | 750 | 37.5 | 4 y |
| Recker et al, 1996 (United States) |
1.2 g/d (n = 95) Placebo (n = 102) |
197 (100) | 73.5 | Partialc | 434 | 25.5e | 4 y |
| Riggs et al, 1998 (United States) |
1.6 g/d (n = 119) Placebo (n = 117) |
236 (100) | 66.2 | No | 714 | 30.1 | 4 y |
| Baron et al, 1999 (United States) |
1.2 g/d (n = 464) Placebo (n = 466) |
258 (28) | 61.0 | NA | 877 | NA | 4 y |
| Ruml et al, 1999 (United States) |
0.8 g/d (n = 29) Placebo (n = 34) |
63 (100) | 52 | No | 613 | NA | 2 y |
| Peacock et al, 2000 (United States) |
0.75 g/d (n = 126) Placebo (n = 135) |
187 (72) | 73.8 | Partialc | 597 | 25.0 | 4 y |
| Avenell et al, 2004 (United Kingdom) |
1 g/d (n = 29) No treatment (n = 35) |
NAa (83) | 78b | Yes | NA | NA | 3.8 y |
| RECORD Grant et al, 2005 (United Kingdom) |
1 g/d (n = 1311) Placebo (n = 1332) |
2241 (85) | 77 | Yes | NA | 15.2e,f | 2-5 y |
| Prince et al, 2006 (Australia) |
0.48 g/d (n = 730) Placebo (n = 730) |
1460 (100) | 75.2 | Partialc | 915 | 31.0e | 5 y |
| Reid et al, 2006 (New Zealand) |
1 g/d (n = 732) Placebo (n = 739) |
1471 (100) | 74.3 | Partialc | 857 | 20.7 | 5 y |
| Mitri et al, 2011 (United States) |
0.8 g/d (n = 22) Placebo (n = 24) |
23 (50) | 58.0 | NA | 923 | 24.5 | 4 mo |
| Aloia et al, 2013 (United States) |
1.2 g/d (n = 35) Placebo (n = 31) |
66 (100) | 59.3 | NA | 898 | 26.6 | 6 mo |
| Vitamin D vs Placebo or No Treatment | |||||||
| Inkovaara et al, 1983 (Finland) |
1000 IU/d (n = 45) Placebo (n = 42) |
71 (82) | 79.6 | NA | NA | NA | 9 mo |
| Lips et al, 1996 (The Netherlands) |
400 IU/d (n = 1291) Placebo (n = 1287) |
1916 (74) | 80.0 | No hip fracture | 868 | 10.6e | 3-4 y |
| Trivedi et al, 2003 (United Kingdom) |
100 000 IU every 4 mo (n = 1345) Placebo (n = 1341) |
649 (24) | 74.8 | NA | 742 | NA | 5 y |
| Avenell et al, 2004 (United Kingdom) |
800 IU/d (n = 35) No treatment (n = 35) |
NAa (83) | 78b | Yes | NA | NA | 3.8 y |
| NONOF Harwood et al, 2004 (United Kingdom) |
300 000 IU once (n = 38) No treatment (n = 37) |
75 (100) | 80.5 | Yes | NA | 11.6 | 1 y |
| RECORD Grant et al, 2005 (United Kingdom) |
800 IU/d (n = 1343) Placebo (n = 1332) |
2264 (85) | 77 | Yes | NA | 15.2e,f | 2-5 y |
| Smith et al, 2007 (United Kingdom) |
300 000 IU every year (n = 4727) Placebo (n = 4713) |
5086 (54) | 79.1 | Partialc | 625d | 22.6e | 3 y |
| Vital D Sanders et al, 2010 (Australia) |
500 000 IU every year (n = 1131) Placebo (n = 1127) |
2258 (100) | 76.1 | Partialc | 976 | 19.8e | 3-5 y |
| Mitri et al, 2011 (United States) |
2000 IU/d (n = 23) Placebo (n = 24) |
25 (53) | 58.0 | NA | 926 | 25.3 | 4 mo |
| Glendenning et al, 2012 (Australia) |
150 000 IU every 3 mo (n = 353) Placebo (n = 333) |
686 (100) | 76.7 | NA | 864 | 26.3e | 9 mo |
| TIDE Punthakee et al, 2012 (Canada) |
1000 IU/d (n = 607) Placebo (n = 614) |
499 (41) | 66.6 | Partialc | NA | NA | 4 mo |
| Aloia et al, 2013 (United States) |
4000 IU/d (n = 47) Placebo (n = 31) |
78 (100) | 59.3 | NA | 881 | 26.1 | 6 mo |
| VitDISH Witham et al, 2013 (United Kingdom) |
100 000 IU every 3 mo (n = 80) Placebo (n = 79) |
77 (49) | 76.8 | NA | 1125 | 18.0 | 1 y |
| VitaDial Massart et al, 2014 (Belgium) |
25 000 IU every week (n = 26) Placebo (n = 29) |
21 (38) | 64.1 | NA | 881 | 17.8 | 3 mo |
| DEX Uusi-Rasi et al, 2015 (Finland) |
800 IU/d (n = 102) Placebo (n = 102) |
204 (100) | 73.9 | NA | 1082 | 26.7 | 2 y |
| BEST-D Hin et al, 2017 (United Kingdom) |
4000 IU/d (n = 102) 2000 IU/d (n = 102) Placebo (n = 101) |
150 (49) | 71.7 | Partialc | 710 | 20.1 | 1 y |
| ViDA Khaw et al, 2017 (New Zealand) |
200 000 IU followed by 100 000 IU monthly (n = 2558) Placebo (n = 2550) |
2139 (42) | 65.9 | Partialc | 810d | 25.2 | 3.4 y |
| Calcium Plus Vitamin D vs Placebo or No Treatment | |||||||
| Inkovaara et al, 1983 (Finland) |
Calcium (1.2 g/d) + D3 (1000 IU/d) (n = 46) Placebo (n = 42) |
69 (78) | 79.0 | NA | NA | NA | 9 mo |
| Dawson-Hughes et al, 1997 (United States) |
Calcium (0.5 g/d) + D3 (700 IU/d) (n = 187) Placebo (n = 202) |
213 (54) | 71.1 | NA | 729 | 29.6e | 3 y |
| Avenell et al, 2004 (United Kingdom) |
Calcium (1 g/d) + D3 (800 IU/d) (n = 35) No treatment (n = 35) |
NAa (83) | 78b | Yes | NA | NA | 3.8 y |
| NONOF Harwood et al, 2004 (United Kingdom) |
Calcium (1g/d) + D2 (300 000 IU once) (n = 36) Calcium (1 g/d) + D3 (800 IU/d) (n = 39) No treatment (n = 37) |
112 (100) | 81.7 | Yes | NA | 11.9 | 1 y |
| Porthouse et al, 2005 (United Kingdom) |
Calcium (1 g/d) + D3 (800 IU/d) (n = 1321) No treatment (n = 1993) |
3314 (100) | 76.8 | Partialc | 1080 | NA | 1.5-3.5 y |
| RECORD Grant et al, 2005 (United Kingdom) |
Calcium (1 g/d) + D3 (800 IU/d) (n = 1306) Placebo (n = 1332) |
2232 (85) | 77.5 | Yes | NA | 15.2e,f | 2-5 y |
| WHI Jackson et al, 2006 (United States) |
Calcium (1 g/d) + D3 (400 IU/d) (n = 4015) Placebo (n = 3957) |
7972 (100) | 62.4 | Partialc | 1151 | 18.9e | 7 y |
| Bolton-Smith et al, 2007 (United Kingdom) |
Calcium (1 g/d) + D3 (400 IU/d) (n = 62) Placebo (n = 61) |
123 (100) | 68.6 | NA | 1073 | 23.9 | 2 y |
| OSTPRE-FPS Salovaara et al, 2010 (Finland) |
Calcium (1g/d) + D3 (800 IU/d) (n = 1718) No treatment (n = 1714) |
3432 (100) | 67.3 | Partialc | 957 | 19.8e | 3 y |
| Mitri et al, 2011 (United States) |
Calcium (0.8 g/d) + D3 (2000 IU/d) (n = 23) Placebo (n = 24) |
25 (53) | 58.0 | NA | 979 | 23.3 | 4 mo |
| Aloia et al, 2013 (United States) |
Calcium (1.2 g/d) + D3 (4000 IU/d) (n = 46) Placebo (n = 31) |
77 (100) | 58.0 | NA | 900 | 27.3 | 6 mo |
| Liu et al, 2015 (China) |
Calcium (1.5 g/d) + D3 (600 IU/d) (n = 50) Placebo (n = 48) |
98 (100) | 62.1 | No | 1500 | NA | 1 y |
| Xue et al, 2017 (China) |
Calcium (0.6 g/d) + D3 (800 IU/d) (n = 139) Placebo (n = 173) |
312 (100) | 63.6 | Partialc | NA | 30.8 | 1 y |
Abbreviation: 25OHD, 25-hydroxyvitamin D.
Women accounted for 83% of total participants in this trial, but detailed data not available for each group.
Mean age is 78 y for total participants in this trial, but detailed data not available for each group.
This trial reported partial participants with fracture history.
Partial participants were assessed for dietary calcium intake.
Partial participants received measurement of baseline 25OHD concentrations.
The RECORD trial reported that the mean baseline 25OHD concentrations for a sample of 60 participants was 15.2 ng/mL, but detailed data were not available for each group.
eFigures 1 and 2 in the Supplement show the assessment of the risk of bias. All studies were randomized; 25 were double-blind, placebo-controlled trials; 19 trials described an adequate random sequence generation process; and 13 trials described the methods used for allocation concealment. One trial was low quality, 6 were high quality, and the others were moderate quality. Inkovaara et al did not report whether the data represent the number of fractures or participants with fracture (3 fractures in the placebo group, 1 fracture in the calcium group, 1 fracture in the vitamin D group, and no fracture in the combined calcium and vitamin D group). The data were included, but a sensitivity analysis was performed that excluded that trial. We obtained unpublished data for 6 trials from a Cochrane review. Two included trials enrolled 222 participants, accounting for 0.4% of total participants, and reported that no fracture events occurred in their intervention and control groups during the follow-up period. Publication bias was not reported because the number of trials reporting hip fracture (primary outcome) was less than 10 for each comparison.
Calcium was administered as calcium carbonate in 14 trials; calcium citrate in 3 trials; calcium citrate malate in 2 trials; a combination of bicarbonate, lactate, and gluconate in 1 trial; in combination with lactate, gluconate, and carbonate in 1 trial; and in unclear form in 1 trial. Vitamin D was administered as vitamin D3 in 23 trials and vitamin D2 in 2 trials (Table 1).
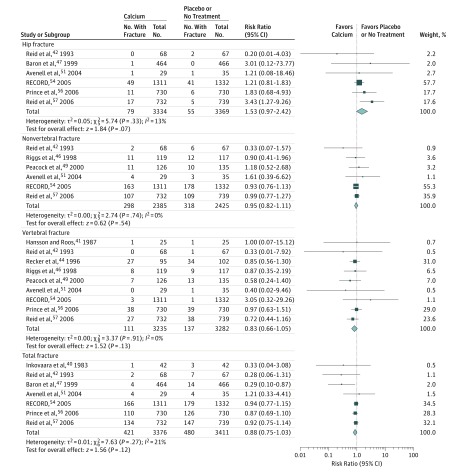
Calcium Supplementation and Fracture Risk
Fourteen trials compared calcium supplements with a placebo or no treatment. As shown in Figure 2, there was no significant association of calcium supplementation with hip fracture (RR, 1.53 [95% CI, 0.97 to 2.42]; ARD, 0.01 [95% CI, 0.00 to 0.01]). There was no statistically significant association of calcium supplementation with nonvertebral fractures (RR, 0.95 [95% CI, 0.82 to 1.11]; ARD, −0.01 [95% CI, −0.02 to 0.01]), vertebral fractures (RR, 0.83 [95% CI, 0.66 to 1.05]; ARD, −0.01 [95% CI, −0.03 to 0.01]), or total fractures (RR, 0.88 [95% CI, 0.75 to 1.03]; ARD, −0.02 [95% CI, −0.03 to −0.01]) compared with placebo or no treatment. Baron et al recruited only participants with a recent history of colorectal adenomas. A sensitivity analysis in which the trias by Baron et al, Inkovaara et al, and Hansson and Roos were excluded showed that the results did not change (eTable 5 in the Supplement). There was no significant association of calcium with fracture risk in subgroups for hip, nonvertebral, vertebral, and total fractures based on calcium dose, sex, fracture history, dietary calcium intake, or baseline serum 25-hydroxyvitamin D concentration (Table 2).
Figure 2. Meta-analysis Results of Calcium Supplementation for the Incidence of Hip, Nonvertebral, Vertebral, and Total Fractures.
Size of data markers is proportional to the weight of each trial. Risk ratios and 95% CIs were calculated using the Mantel-Haenszel method, with a random-effects model used to pool data. Error bars indicate 95% CIs. Risk ratio data are rounded to 2 decimal places; error bars reflect unrounded values. Trials with zero events in both the intervention and control groups are not included in the meta-analysis.
Table 2. Subgroup Analysis of Association Between Calcium Supplementation and Fracture Incidence for Each Variable.
| Variable | No. of Trials | No. of Participants | Fracture, RR (95% CI) | P Valuea | |
|---|---|---|---|---|---|
| With Fracture | Total | ||||
| Hip Fracture (Primary Outcome) | |||||
| Calcium dose, g/d | |||||
| ≥1 | 5 | 117 | 5243 | 1.55 (0.79-3.05) | .79 |
| <1 | 1 | 17 | 1460 | 1.83 (0.68-4.93) | |
| Sex | |||||
| Women-only trials | 3 | 41 | 3066 | 1.97 (0.74-5.28) | .39 |
| Trials with men and women | 3 | 93 | 3637 | 1.23 (0.83-1.84) | |
| Previous fractures | |||||
| Yes | 2 | 92 | 2707 | 1.21 (0.81-1.82) | .18 |
| Otherb | 4 | 42 | 3996 | 2.17 (1.02-4.62) | |
| Calcium intake, mg/d | |||||
| ≥900 | 1 | 17 | 1460 | 1.83 (0.68-4.93) | .99 |
| <900 | 3 | 25 | 2536 | 1.86 (0.38-9.14) | |
| Baseline 25OHD, ng/mL | |||||
| ≥20 | 3 | 41 | 3066 | 1.97 (0.74-5.28) | .37 |
| <20 | 1 | 90 | 2643 | 1.21 (0.81-1.83) | |
| Nonvertebral Fracture | |||||
| Calcium dose, g/d | |||||
| ≥1 | 5 | 595 | 4549 | 0.95 (0.82-1.10) | .61 |
| <1 | 1 | 21 | 261 | 1.18 (0.52-2.68) | |
| Sex | |||||
| Women-only trials | 3 | 247 | 1842 | 0.96 (0.76-1.21) | .96 |
| Trials with men and women | 3 | 369 | 2968 | 0.95 (0.79-1.15) | |
| Previous fractures | |||||
| Yes | 2 | 348 | 2707 | 0.94 (0.77-1.14) | .82 |
| Otherb | 4 | 268 | 2103 | 0.97 (0.78-1.22) | |
| Calcium intake, mg/d | |||||
| ≥900 | 0 | 0 | 0 | Not estimable | NA |
| <900 | 4 | 268 | 2103 | 0.97 (0.78-1.22) | |
| Baseline 25OHD, ng/mL | |||||
| ≥20 | 4 | 268 | 2103 | 0.97 (0.78-1.22) | .76 |
| <20 | 1 | 341 | 2643 | 0.93 (0.76-1.13) | |
| Vertebral Fracture | |||||
| Calcium dose, g/d | |||||
| ≥1 | 7 | 151 | 4796 | 0.81 (0.60-1.08) | .78 |
| <1 | 2 | 97 | 1721 | 0.87 (0.57-1.33) | |
| Sex | |||||
| Women-only trials | 6 | 223 | 3549 | 0.85 (0.66-1.08) | .64 |
| Trials with men and women | 3 | 25 | 2968 | 0.69 (0.31-1.54) | |
| Previous fractures | |||||
| Yes | 3 | 7 | 2757 | 1.34 (0.29-6.15) | .54 |
| Otherb | 6 | 241 | 3760 | 0.82 (0.65-1.05) | |
| Calcium intake, mg/d | |||||
| ≥900 | 1 | 77 | 1460 | 0.97 (0.63-1.51) | .37 |
| <900 | 5 | 164 | 2300 | 0.77 (0.58-1.02) | |
| Baseline 25OHD, ng/mL | |||||
| ≥20 | 6 | 241 | 3760 | 0.82 (0.65-1.05) | .26 |
| <20 | 1 | 4 | 2643 | 3.05 (0.32-29.26) | |
| Total Fractures | |||||
| Calcium dose, g/d | |||||
| ≥1 | 6 | 665 | 5327 | 0.85 (0.67-1.09) | .90 |
| <1 | 1 | 236 | 1460 | 0.87 (0.69-1.10) | |
| Sex | |||||
| Women-only trials | 3 | 526 | 3066 | 0.88 (0.74-1.06) | .51 |
| Trials with men and women | 4 | 375 | 3721 | 0.71 (0.37-1.35) | |
| Previous fractures | |||||
| Yes | 2 | 353 | 2707 | 0.95 (0.78-1.15) | .31 |
| Otherb | 5 | 548 | 4080 | 0.79 (0.60-1.06) | |
| Calcium intake, mg/d | |||||
| ≥900 | 1 | 236 | 1460 | 0.87 (0.69-1.10) | .27 |
| <900 | 3 | 308 | 2536 | 0.50 (0.20-1.30) | |
| Baseline 25OHD, ng/mL | |||||
| ≥20 | 3 | 526 | 3066 | 0.88 (0.74-1.06) | .64 |
| <20 | 1 | 345 | 2643 | 0.94 (0.77-1.15) | |
Abbreviations: RR, relative risk; 25OHD, 25-hydroxyvitamin D.
P value for heterogeneity between subgroups.
Includes previous no fracture, partial fracture, and missing fracture data.
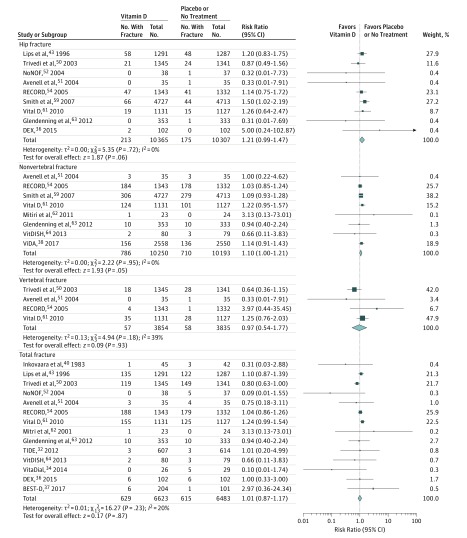
Vitamin D Supplementation and Fracture Risk
Seventeen trials compared vitamin D supplementation with a placebo or no treatment. Figure 3 shows the result of the traditional meta-analysis comparing vitamin D with a placebo or no treatment. There was no significant association of vitamin D with hip fracture (RR, 1.21 [95% CI, 0.99 to 1.47]; ARD, 0.00 [95% CI, −0.00 to 0.01]) or nonvertebral fractures (RR, 1.10 [95% CI, 1.00 to 1.21]; ARD, 0.01 [95% CI, −0.00 to 0.01]), compared with placebo or no treatment. There was no significant association of vitamin D with risk for vertebral fractures (RR, 0.97 [95% CI, 0.54 to 1.77]; ARD, 0.00 [95% CI, −0.00 to 0.01]), or total fractures (RR, 1.01 [95% CI, 0.87 to 1.17]; ARD, 0.00 [95% CI, −0.01 to 0.01]).
Figure 3. Meta-analysis Results of Vitamin D Supplementation for the Incidence of Hip, Nonvertebral, Vertebral, and Total Fractures.
Size of data markers is proportional to the weight of each trial. Risk ratios and 95% CIs were calculated using the Mantel-Haenszel method, with a random-effects model used to pool data. Error bars indicate 95% CIs. Risk ratio data are rounded to 2 decimal places; error bars reflect unrounded values. Trials with zero events in both the intervention and control groups are not included in the meta-analysis.
Mitri et al included only participants who had a high risk of diabetes; the TIDE trial included only people with type 2 diabetes and glycated hemoglobin concentration of 6.5% to 9.5% who were at risk of cardiovascular disease; the VitDISH trial enrolled only participants with isolated systolic hypertension; and the VitaDial trial included only people receiving hemodialysis (chronic kidney disease stage 5) with serum 25-hydroxyvitamin D levels less than 30 ng/mL. A sensitivity analysis was performed that separately excluded the TIDE trial, VitDISH trial, and VitaDial trial, as well as the trials by Mitri et al and Inkovaara et al, and the results remained unchanged (eTable 5 in the Supplement).
Table 3 summarizes results of subgroup analyses for vitamin D and the incidence of fracture. Vitamin D supplementation was associated with a significantly higher incidence of hip fracture in participants with baseline serum 25-hydroxyvitamin D concentrations of 20 ng/mL or greater (RR, 1.49 [95% CI, 1.03 to 2.17]; ARD, 0.00 [95% CI, −0.00 to 0.01]), but interaction term compared with people with serum 25-hydroxyvitamin D concentrations less than 20 ng/mL was not statistically significant. Intermittent high-dose vitamin D given once yearly was associated with a higher incidence of hip fracture (RR, 1.41 [95% CI, 1.02 to 1.96]; ARD, 0.00 [95% CI, 0.00 to 0.01]) (Table 3).
Table 3. Subgroup Analysis of Association Between Vitamin D Supplementation and Fracture Incidence for Each Variable.
| Variable | No. of Trials | No. of Participants | Fracture, RR (95% CI) | P Valuea | |
|---|---|---|---|---|---|
| With Fracture | Total | ||||
| Hip Fracture (Primary Outcome) | |||||
| Vitamin D dose and frequency | |||||
| ≥800 IU/d | 3 | 91 | 2949 | 1.14 (0.76-1.72) | .48 |
| <800 IU/d | 1 | 106 | 2578 | 1.20 (0.83-1.75) | |
| Intermittent high-dose (given as once every year) | 3 | 145 | 11773 | 1.41 (1.02-1.96) | |
| Intermittent high-dose (given as other frequenciesb) | 2 | 46 | 3372 | 0.84 (0.48-1.50) | |
| Sex | |||||
| Women-only trials | 4 | 38 | 3223 | 1.20 (0.64-2.26) | .99 |
| Trials with men and women | 5 | 350 | 17449 | 1.21 (0.98-1.49) | |
| Previous fractures | |||||
| Yes | 3 | 90 | 2820 | 1.09 (0.73-1.64) | .60 |
| Otherc | 5 | 296 | 17648 | 1.24 (0.99-1.55) | |
| Calcium intake, mg/d | |||||
| ≥900 | 2 | 36 | 2462 | 1.35 (0.70-2.59) | .80 |
| <900 | 4 | 262 | 15390 | 1.23 (0.96-1.58) | |
| Baseline 25OHD, ng/mL | |||||
| ≥20 | 3 | 113 | 10330 | 1.49 (1.03-2.17) | .30 |
| <20 | 4 | 229 | 7586 | 1.18 (0.91-1.52) | |
| Nonvertebral Fracture | |||||
| Vitamin D dose and frequency | |||||
| ≥800 IU/d | 3 | 369 | 2792 | 1.03 (0.85-1.24) | .72 |
| <800 IU/d | 0 | 0 | 0 | Not estimable | |
| Intermittent high-dose (given as once every year) | 2 | 810 | 11698 | 1.13 (0.99-1.29) | |
| Intermittent high-dose (given as other frequenciesb) | 3 | 317 | 5953 | 1.12 (0.90-1.39) | |
| Sex | |||||
| Women-only trials | 2 | 245 | 2944 | 1.20 (0.94-1.52) | .44 |
| Trials with men and women | 6 | 1251 | 17499 | 1.08 (0.97-1.20) | |
| Previous fractures | |||||
| Yes | 2 | 368 | 2745 | 1.02 (0.85-1.24) | .39 |
| Otherc | 6 | 1128 | 17698 | 1.13 (1.01-1.26) | |
| Calcium intake, mg/d | |||||
| ≥900 | 3 | 231 | 2464 | 1.22 (0.95-1.55) | .50 |
| <900 | 3 | 897 | 15234 | 1.11 (0.97-1.26) | |
| Baseline 25OHD, ng/mL | |||||
| ≥20 | 4 | 898 | 15281 | 1.11 (0.98-1.26) | .88 |
| <20 | 3 | 592 | 5092 | 1.09 (0.94-1.27) | |
| Vertebral Fracture | |||||
| Vitamin D dose and frequency | |||||
| ≥800 IU/d | 2 | 6 | 2745 | 1.51 (0.14-16.14) | .22 |
| <800 IU/d | 0 | 0 | 0 | Not estimable | |
| Intermittent high-dose (given as once every year) | 1 | 63 | 2258 | 1.25 (0.76-2.03) | |
| Intermittent high-dose (given as other frequenciesb) | 1 | 46 | 2686 | 0.64 (0.36-1.15) | |
| Sex | |||||
| Women-only trials | 1 | 63 | 2258 | 1.25 (0.76-2.03) | .52 |
| Trials with men and women | 3 | 52 | 5431 | 0.85 (0.29-2.47) | |
| Previous fractures | |||||
| Yes | 2 | 6 | 2745 | 1.51 (0.14-16.14) | .69 |
| Otherc | 2 | 109 | 4944 | 0.91 (0.48-1.75) | |
| Calcium intake, mg/d | |||||
| ≥900 | 1 | 63 | 2258 | 1.25 (0.76-2.03) | .09 |
| <900 | 1 | 46 | 2686 | 0.64 (0.36-1.15) | |
| Baseline 25OHD, ng/mL | |||||
| ≥20 | 0 | 0 | 0 | Not estimable | NA |
| <20 | 2 | 68 | 4933 | 1.34 (0.78-2.30) | |
| Total Fractures | |||||
| Vitamin D dose and frequency | |||||
| ≥800 IU/d | 7 | 404 | 4609 | 1.04 (0.86-1.25) | .15 |
| <800 IU/d | 1 | 257 | 2578 | 1.10 (0.87-1.39) | |
| Intermittent high-dose (given as once every year) | 2 | 285 | 2333 | 0.49 (0.04-5.88) | |
| Intermittent high-dose (given as other frequenciesb) | 4 | 298 | 3586 | 0.79 (0.64-0.99) | |
| Sex | |||||
| Women-only trials | 4 | 317 | 3223 | 1.10 (0.75-1.62) | .55 |
| Trials with men and women | 10 | 927 | 9883 | 0.97 (0.84-1.12) | |
| Previous fractures | |||||
| Yes | 3 | 379 | 2820 | 0.81 (0.36-1.81) | .58 |
| Otherc | 9 | 848 | 10027 | 1.02 (0.85-1.23) | |
| Calcium intake, mg/d | |||||
| ≥900 | 4 | 298 | 2668 | 1.22 (0.98-1.51) | .16 |
| <900 | 5 | 557 | 6310 | 0.94 (0.69-1.27) | |
| Baseline 25OHD, ng/mL | |||||
| ≥20 | 4 | 40 | 1242 | 1.12 (0.59-2.11) | .94 |
| <20 | 6 | 919 | 7800 | 1.09 (0.91-1.31) | |
Abbreviations: RR, relative risk; 25OHD, 25-hydroxyvitamin D.
P value for heterogeneity between subgroups.
Other frequencies include once every 3 or 4 months-and once every 1 week or month.
Includes previous no fracture, partial fracture, and missing fracture data.
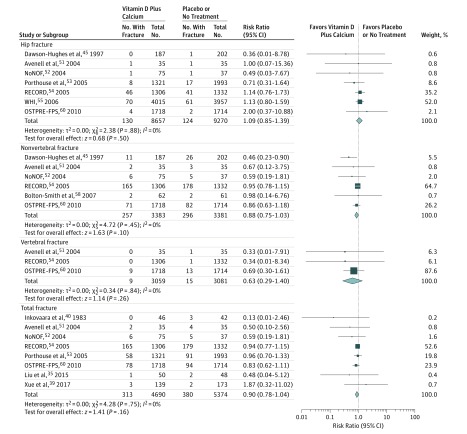
Combination Calcium and Vitamin D Supplementation and Fracture Risk
Thirteen trials compared results for participants receiving combination calcium and vitamin D supplementation vs placebo or no treatment. In the Women’s Health Initiative (WHI) trial, 36 282 women were randomized into 1 of 4 groups: (1) calcium combined with vitamin D; (2) combined supplementation with calcium, vitamin D, and hormone therapy; (3) hormone therapy alone; or (4) placebo alone. In this meta-analysis, only the data of the participants who did not receive hormone therapy were pooled. Figure 4 shows results of the meta-analysis. There was no significant association of combined calcium and vitamin D with hip fracture (RR, 1.09 [95% CI, 0.85 to 1.39]; ARD, 0.00 [95% CI, −0.00 to 0.00]), nonvertebral fracture (RR, 0.88 [95% CI, 0.75 to 1.03]; ARD, −0.01 [95% CI, −0.02 to 0.00]), vertebral fracture (RR, 0.63 [95% CI, 0.29 to 1.40]; ARD, −0.00 [95% CI, −0.00 to 0.00]), or total fractures (RR, 0.90 [95% CI, 0.78 to 1.04]; ARD, −0.01 [95% CI, −0.01 to 0.00]). The results of sensitivity analysis were not altered after excluding the trial by Inkovaara et al (eTable 5 in the Supplement).
Figure 4. Meta-analysis Results of Combined Calcium and Vitamin D Supplementation for the Incidence of Hip, Nonvertebral, Vertebral, and Total Fractures.
Size of data markers is proportional to the weight of each trial. Risk ratios and 95% CIs were calculated using the Mantel-Haenszel method, with a random-effects model used to pool data. Error bars indicate 95% CIs. Risk ratio data are rounded to 2 decimal places; error bars reflect unrounded values. Trials with zero events in both the intervention and control groups are not included in the meta-analysis.
The subgroup analysis did not show any significant differences within subgroups based on the dose of calcium or vitamin D, sex, fracture history, dietary calcium intake, and baseline serum 25-hydroxyvitamin D concentration (Table 4).
Table 4. Subgroup Analysis of Association Between Combined Calcium and Vitamin D Supplementation and Fracture Incidence for Each Variable.
| Variable | No. of Trials | No. of Participants | Fracture, RR (95% CI) | P Valuea | |
|---|---|---|---|---|---|
| With Fracture | Total | ||||
| Hip Fracture (Primary Outcome) | |||||
| Calcium and vitamin D dose and frequency | |||||
| ≥1 g/d and ≥800 IU/db | 5 | 122 | 9566 | 1.06 (0.74-1.51) | .83 |
| Otherc | 2 | 132 | 8361 | 1.12 (0.80-1.57) | |
| Sex | |||||
| Women-only trials | 4 | 164 | 14830 | 1.07 (0.79-1.46) | .86 |
| Trials with men and women | 3 | 90 | 3097 | 1.12 (0.75-1.68) | |
| Previous fractures | |||||
| Yes | 3 | 91 | 2820 | 1.12 (0.75-1.68) | .86 |
| Otherd | 4 | 163 | 15107 | 1.07 (0.79-1.46) | |
| Calcium intake, mg/d | |||||
| ≥900 | 3 | 162 | 14718 | 1.08 (0.79-1.47) | .50 |
| <900 | 1 | 1 | 389 | 0.36 (0.01-8.78) | |
| Baseline 25OHD, ng/mL | |||||
| ≥20 | 1 | 1 | 389 | 0.36 (0.01-8.78) | .48 |
| <20 | 4 | 226 | 14154 | 1.14 (0.88-1.48) | |
| Nonvertebral Fracture | |||||
| Calcium and vitamin D dose and frequency | |||||
| ≥1 g/d and ≥800 IU/db | 4 | 512 | 6252 | 0.91 (0.77-1.07) | .07 |
| Otherc | 2 | 41 | 512 | 0.50 (0.26-0.94) | |
| Sex | |||||
| Women-only trials | 3 | 168 | 3667 | 0.84 (0.63-1.13) | .63 |
| Trials with men and women | 3 | 385 | 3097 | 0.72 (0.42-1.26) | |
| Previous fractures | |||||
| Yes | 3 | 359 | 2820 | 0.93 (0.77-1.13) | .30 |
| Otherd | 3 | 194 | 3944 | 0.72 (0.46-1.13) | |
| Calcium intake, mg/d | |||||
| ≥900 | 2 | 157 | 3555 | 0.87 (0.64-1.18) | .09 |
| <900 | 1 | 37 | 389 | 0.46 (0.23-0.90) | |
| Baseline 25OHD, ng/mL | |||||
| ≥20 | 2 | 41 | 512 | 0.50 (0.26-0.94) | .07 |
| <20 | 3 | 507 | 6182 | 0.91 (0.77-1.08) | |
| Vertebral Fracture | |||||
| Calcium and vitamin D dose and frequency | |||||
| ≥1 g/d and ≥800 IU/db | 3 | 24 | 6140 | 0.63 (0.29-1.40) | NA |
| Otherc | 0 | 0 | 0 | Not estimable | |
| Sex | |||||
| Women-only trials | 1 | 22 | 3432 | 0.69 (0.30-1.61) | .56 |
| Trials with men and women | 2 | 2 | 2708 | 0.34 (0.04-3.20) | |
| Previous fractures | |||||
| Yes | 2 | 2 | 2708 | 0.34 (0.04-3.20) | .56 |
| Otherd | 1 | 22 | 3432 | 0.69 (0.30-1.61) | |
| Calcium intake, mg/d | |||||
| ≥900 | 1 | 22 | 3432 | 0.69 (0.30-1.61) | NA |
| <900 | 0 | 0 | 0 | Not estimable | |
| Baseline 25OHD, ng/mL | |||||
| ≥20 | 0 | 0 | 0 | Not estimable | NA |
| <20 | 2 | 23 | 6070 | 0.66 (0.29-1.50) | |
| Total Fractures | |||||
| Calcium and vitamin D dose and frequency | |||||
| ≥1 g/d and ≥800 IU/db | 6 | 685 | 9654 | 0.90 (0.78-1.04) | .74 |
| Otherc | 2 | 8 | 410 | 1.15 (0.28-4.74) | |
| Sex | |||||
| Women-only trials | 5 | 340 | 7268 | 0.88 (0.71-1.08) | .84 |
| Trials with men and women | 3 | 353 | 2796 | 0.83 (0.48-1.42) | |
| Previous fractures | |||||
| Yes | 3 | 361 | 2820 | 0.92 (0.76-1.11) | .78 |
| Otherd | 5 | 332 | 7244 | 0.88 (0.71-1.09) | |
| Calcium intake, mg/d | |||||
| ≥900 | 3 | 324 | 6844 | 0.88 (0.71-1.09) | NA |
| <900 | 0 | 0 | 0 | Not estimable | |
| Baseline 25OHD, ng/mL | |||||
| ≥20 | 1 | 5 | 312 | 1.87 (0.32-11.02) | .42 |
| <20 | 3 | 527 | 6182 | 0.90 (0.76-1.05) | |
Abbreviations: RR, relative risk; 25OHD, 25-hydroxyvitamin D.
P value for heterogeneity between subgroups.
Calcium dose 1 g/d or greater and vitamin D dose 800 IU/d or greater.
Includes calcium dose less than 1 g/d and/or vitamin D dose less than 800 IU/d.
Includes previous no fracture, partial fracture, and missing fracture data.
Discussion
Results of this meta-analysis showed that calcium, calcium plus vitamin D, and vitamin D supplementation alone were not significantly associated with a lower incidence of hip, nonvertebral, vertebral, or total fractures in community-dwelling older adults. Sensitivity analyses that excluded low-quality trials and studies that exclusively enrolled patients with particular medical conditions did not alter these results. Furthermore, these results were generally consistent regardless of the dose of calcium or vitamin D, sex, fracture history, calcium intake, and baseline serum 25-hydroxyvitamin D concentration.
A meta-analysis by Tang et al reported that calcium supplementation was significantly associated with prevention of osteoporosis-related fractures. However, the report by Tang et al included 2 large-sample cluster trials and did not adjust for the number of participants, which might have increased the probability of smaller P values and narrower CIs between the intervention and control groups. Bolland et al reported that calcium supplementation was significantly associated with a lower incidence of total fracture in community-dwelling participants. Results reported here showed that calcium supplementation was not associated with a lower rate of hip fracture. In the current analyses, the point estimate regarding the association of calcium supplementation with hip fracture was increased, but it did not reach statistical significance. These results suggest the possibility of a significant association of calcium supplementation with increased fracture incidence, but the current analyses may have lacked statistical power to show this association. However, the reason for this association is unclear. Overall, results reported here suggest that calcium should not be routinely recommended for fracture prevention.
Prior analyses also reported favorable associations of high-dose (≥800 IU daily) vitamin D supplementation and fracture incidence. Bischoff-Ferrari et al found that supplementation with 800 IU or more of vitamin D per day was associated with lower rates of hip fracture and nonvertebral fractures in adults 65 years or older. However, their findings may have been influenced by inclusion of the trial by Chapuy et al, which only enrolled participants living in an institution. Another possible reason for differences in conclusions of earlier meta-analyses and the current meta-analysis is that more recently published trials reported neutral or harmful associations of vitamin D supplementation and fracture incidence. Results reported here showed that vitamin D was associated with a higher risk for hip fracture, but this finding did not reach statistical significance. This finding may be attributable to lack of statistical power in this meta-analysis.
Avenell et al performed a Cochrane review that concluded that combined supplementation might be associated with reduced incidence of hip fracture or total fractures. Bolland et al found that supplementation with combined calcium and vitamin D did not decrease the incidence of hip fracture or total fractures in community-dwelling individuals. In the current meta-analysis, data from the WHI trial were updated compared with prior reports. The WHI trials demonstrated a significant interaction of hormone therapy for the association of supplementation with combined calcium and vitamin D and risk of hip fracture (P = .01). Supplementation with combined calcium and vitamin D was associated with lower fracture risk in participants who were also taking hormone therapy. In contrast, supplementation with combined calcium and vitamin D had no benefit in women not assigned to hormone therapy. However, previously published meta-analyses did not exclude patients receiving hormone therapy. In results reported here, data were pooled for 7972 participants (4015 participants randomly received combined calcium and vitamin D, and 3957 participants randomly received placebo) who did not receive hormone therapy.
In the meta-analysis reported here, few included trials specifically enrolled participants with established osteoporosis, but some trials enrolled participants with risk factors for osteoporosis, such as lower serum 25-hydroxyvitamin D concentration, low dietary calcium intake, previous fracture, and postmenopausal status. A subgroup analysis based on adherence was not performed in this meta-analysis because the definition of adherence substantially differed between included trials. A previous trial reported that calcium and vitamin D supplements lowered fracture risk for individuals living in residential institutions. These populations are more likely to have osteoporosis because of their poorer mobility, infrequent sun exposure, and poorer diet. For these reasons it is possible that older people living in residential care communities may benefit from calcium or vitamin D supplements. In summary, benefits of calcium and vitamin D supplementation may differ between people living in the community and people living in residential institutions.
This study has several limitations. First, RCTs from 21 published systematic reviews and meta-analyses were identified, which might lead to the omission of trials meeting inclusion criteria. Second, some included trials did not test baseline serum 25-hydroxyvitamin D concentrations for all participants. The subgroup results might have been different if all individuals were tested. Third, some RCTs were of poor quality and, for example, used unclear allocation concealment. Fourth, the methods used to classify studies as high quality may have been relatively lenient, and other researchers may have selected different definitions for study quality.
Conclusions
In this meta-analysis of randomized clinical trials, the use of supplements that included calcium, vitamin D, or both compared with placebo or no treatment was not associated with a lower risk of fractures among community-dwelling older adults. These findings do not support the routine use of these supplements in community-dwelling older people.
eTable 1. Search Strategy for Each Database
eTable 2. Randomized Trials Included in Systematic Reviews or Meta-Analyses Evaluating Calcium Supplements With or Without Vitamin D for Fracture Incidence
eTable 3. Randomized Trials Included In Systematic Reviews or Meta-Analyses Evaluating Vitamin D Supplements With or Without Calcium for Fracture Incidence
eTable 4. Excluded Trials and Reasons for Exclusion
eTable 5. Results of Sensitivity Analyses With Exclusion of the Listed Trials
eFigure 1. Number/Proportions of Trials That Met Each Criterion for Risk of Bias Across the 33 Included Trials
eFigure 2. Results of the Risk of Bias for 33 Included Trials
References
- 1.Si L, Winzenberg TM, Chen M, Jiang Q, Palmer AJ. Residual lifetime and 10 year absolute risks of osteoporotic fractures in Chinese men and women. Curr Med Res Opin. 2015;31(6):1149-1156. [DOI] [PubMed] [Google Scholar]
- 2.Dyer SM, Crotty M, Fairhall N, et al. ; Fragility Fracture Network (FFN) Rehabilitation Research Special Interest Group . A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016;16:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo JC, Srinivasan S, Chandra M, et al. Trends in mortality following hip fracture in older women. Am J Manag Care. 2015;21(3):e206-e214. [PubMed] [Google Scholar]
- 4.Bolland MJ, Grey A. A case study of discordant overlapping meta-analyses: vitamin D supplements and fracture. PLoS One. 2014;9(12):e115934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duque G, Lord SR, Mak J, et al. Treatment of osteoporosis in Australian residential aged care facilities: update on consensus recommendations for fracture prevention. J Am Med Dir Assoc. 2016;17(9):852-859.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27349626&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins JPT. Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011). Cochrane Collaboration website. http://training.cochrane.org/handbook. 2011. Accessed November 22, 2017.
- 7.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med. 2002;21(11):1575-1600. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, et al. Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr. 2007;86(6):1780-1790. [DOI] [PubMed] [Google Scholar]
- 10.Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657-666. [DOI] [PubMed] [Google Scholar]
- 11.Rabenda V, Bruyère O, Reginster JY. Relationship between bone mineral density changes and risk of fractures among patients receiving calcium with or without vitamin D supplementation: a meta-regression. Osteoporos Int. 2011;22(3):893-901. [DOI] [PubMed] [Google Scholar]
- 12.Bolland MJ, Leung W, Tai V, et al. Calcium intake and risk of fracture: systematic review. BMJ. 2015;351:h4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92(4):1415-1423. [DOI] [PubMed] [Google Scholar]
- 14.Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;(158):1-235. [PMC free article] [PubMed] [Google Scholar]
- 15.Cranney A, Weiler HA, O’Donnell S, Puil L. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr. 2008;88(2):513S-519S. [DOI] [PubMed] [Google Scholar]
- 16.Izaks GJ. Fracture prevention with vitamin D supplementation: considering the inconsistent results. BMC Musculoskelet Disord. 2007;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson C, Gaugris S, Sen SS, Hosking D. The effect of cholecalciferol (vitamin D3) on the risk of fall and fracture: a meta-analysis. QJM. 2007;100(4):185-192. [DOI] [PubMed] [Google Scholar]
- 18.Avenell A, Gillespie WJ, Gillespie LD, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2009;(2):CD000227. [DOI] [PubMed] [Google Scholar]
- 19.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(6):551-561. [DOI] [PubMed] [Google Scholar]
- 20.Bergman GJ, Fan T, McFetridge JT, Sen SS. Efficacy of vitamin D3 supplementation in preventing fractures in elderly women: a meta-analysis. Curr Med Res Opin. 2010;26(5):1193-1201. [DOI] [PubMed] [Google Scholar]
- 21.DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai JK, Lucas RM, Clements MS, Roddam AW, Banks E. Hip fracture risk in relation to vitamin D supplementation and serum 25-hydroxyvitamin D levels: a systematic review and meta-analysis of randomised controlled trials and observational studies. BMC Public Health. 2010;10:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(12):827-838. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention [published correction appears in N Engl J Med. 2012;367(5):481]. N Engl J Med. 2012;367(1):40-49. [DOI] [PubMed] [Google Scholar]
- 25.Geddes JA, Inderjeeth CA. Evidence for the treatment of osteoporosis with vitamin D in residential care and in the community dwelling elderly. Biomed Res Int. 2013;2013:463589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(4):307-320. [DOI] [PubMed] [Google Scholar]
- 28.LeBlanc E, Chou R, Zakher B, Daeges M, Pappas M. Screening for Vitamin D Deficiency: Systematic Review for the U.S. Preventive Services Task Force Recommendation. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 29.LeBlanc ES, Zakher B, Daeges M, Pappas M, Chou R. Screening for vitamin D deficiency: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(2):109-122. [DOI] [PubMed] [Google Scholar]
- 30.Zheng YT, Cui QQ, Hong YM, Yao WG. A meta-analysis of high dose, intermittent vitamin D supplementation among older adults. PLoS One. 2015;10(1):e0115850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27(1):367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Punthakee Z, Bosch J, Dagenais G, et al. ; TIDE Trial Investigators . Design, history and results of the Thiazolidinedione Intervention with vitamin D Evaluation (TIDE) randomised controlled trial. Diabetologia. 2012;55(1):36-45. [DOI] [PubMed] [Google Scholar]
- 33.Aloia JF, Dhaliwal R, Shieh A, Mikhail M, Islam S, Yeh JK. Calcium and vitamin D supplementation in postmenopausal women. J Clin Endocrinol Metab. 2013;98(11):E1702-E1709. [DOI] [PubMed] [Google Scholar]
- 34.Massart A, Debelle FD, Racapé J, et al. Biochemical parameters after cholecalciferol repletion in hemodialysis: results from the VitaDial randomized trial. Am J Kidney Dis. 2014;64(5):696-705. [DOI] [PubMed] [Google Scholar]
- 35.Liu BX, Chen SP, Li YD, et al. The effect of the modified eighth section of eight-section brocade on osteoporosis in postmenopausal women: a prospective randomized trial. Medicine (Baltimore). 2015;94(25):e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med. 2015;175(5):703-711. [DOI] [PubMed] [Google Scholar]
- 37.Hin H, Tomson J, Newman C, et al. Optimum dose of vitamin D for disease prevention in older people: BEST-D trial of vitamin D in primary care. Osteoporos Int. 2017;28(3):841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khaw KT, Stewart AW, Waayer D, et al. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol. 2017;5(6):438-447. [DOI] [PubMed] [Google Scholar]
- 39.Xue Y, Hu Y, Wang O, et al. Effects of enhanced exercise and combined vitamin D and calcium supplementation on muscle strength and fracture risk in postmenopausal Chinese women. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017;39(3):345-351. [DOI] [PubMed] [Google Scholar]
- 40.Inkovaara J, Gothoni G, Halttula R, Heikinheimo R, Tokola O. Calcium, vitamin D and anabolic steroid in treatment of aged bones: double-blind placebo-controlled long-term clinical trial. Age Ageing. 1983;12(2):124-130. [DOI] [PubMed] [Google Scholar]
- 41.Hansson T, Roos B. The effect of fluoride and calcium on spinal bone mineral content: a controlled, prospective (3 years) study. Calcif Tissue Int. 1987;40(6):315-317. [DOI] [PubMed] [Google Scholar]
- 42.Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ. Effect of calcium supplementation on bone loss in postmenopausal women. N Engl J Med. 1993;328(7):460-464. [DOI] [PubMed] [Google Scholar]
- 43.Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons: a randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124(4):400-406. [DOI] [PubMed] [Google Scholar]
- 44.Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res. 1996;11(12):1961-1966. [DOI] [PubMed] [Google Scholar]
- 45.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670-676. [DOI] [PubMed] [Google Scholar]
- 46.Riggs BL, O’Fallon WM, Muhs J, O’Connor MK, Kumar R, Melton LJ III. Long-term effects of calcium supplementation on serum parathyroid hormone level, bone turnover, and bone loss in elderly women. J Bone Miner Res. 1998;13(2):168-174. [DOI] [PubMed] [Google Scholar]
- 47.Baron JA, Beach M, Mandel JS, et al. ; Calcium Polyp Prevention Study Group . Calcium supplements for the prevention of colorectal adenomas. N Engl J Med. 1999;340(2):101-107. [DOI] [PubMed] [Google Scholar]
- 48.Ruml LA, Sakhaee K, Peterson R, Adams-Huet B, Pak CY. The effect of calcium citrate on bone density in the early and mid-postmenopausal period: a randomized placebo-controlled study. Am J Ther. 1999;6(6):303-311. [DOI] [PubMed] [Google Scholar]
- 49.Peacock M, Liu G, Carey M, et al. Effect of calcium or 25OH vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. J Clin Endocrinol Metab. 2000;85(9):3011-3019. [DOI] [PubMed] [Google Scholar]
- 50.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Avenell A, Grant AM, McGee M, McPherson G, Campbell MK, McGee MA; RECORD Trial Management Group . The effects of an open design on trial participant recruitment, compliance and retention—a randomized controlled trial comparison with a blinded, placebo-controlled design. Clin Trials. 2004;1(6):490-498. [DOI] [PubMed] [Google Scholar]
- 52.Harwood RH, Sahota O, Gaynor K, Masud T, Hosking DJ; Nottingham Neck of Femur (NONOF) Study . A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: the Nottingham Neck of Femur (NONOF) study. Age Ageing. 2004;33(1):45-51. [DOI] [PubMed] [Google Scholar]
- 53.Porthouse J, Cockayne S, King C, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330(7498):1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grant AM, Avenell A, Campbell MK, et al. ; RECORD Trial Group . Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621-1628. [DOI] [PubMed] [Google Scholar]
- 55.Jackson RD, LaCroix AZ, Gass M, et al. ; Women’s Health Initiative Investigators . Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683. [DOI] [PubMed] [Google Scholar]
- 56.Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med. 2006;166(8):869-875. [DOI] [PubMed] [Google Scholar]
- 57.Reid IR, Mason B, Horne A, et al. Randomized controlled trial of calcium in healthy older women. Am J Med. 2006;119(9):777-785. [DOI] [PubMed] [Google Scholar]
- 58.Bolton-Smith C, McMurdo ME, Paterson CR, et al. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res. 2007;22(4):509-519. [DOI] [PubMed] [Google Scholar]
- 59.Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women—a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford). 2007;46(12):1852-1857. [DOI] [PubMed] [Google Scholar]
- 60.Salovaara K, Tuppurainen M, Kärkkäinen M, et al. Effect of vitamin D(3) and calcium on fracture risk in 65- to 71-year-old women: a population-based 3-year randomized, controlled trial—the OSTPRE-FPS. J Bone Miner Res. 2010;25(7):1487-1495. [DOI] [PubMed] [Google Scholar]
- 61.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815-1822. [DOI] [PubMed] [Google Scholar]
- 62.Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr. 2011;94(2):486-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J Bone Miner Res. 2012;27(1):170-176. [DOI] [PubMed] [Google Scholar]
- 64.Witham MD, Price RJ, Struthers AD, et al. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Intern Med. 2013;173(18):1672-1679. [DOI] [PubMed] [Google Scholar]
- 65.Robbins JA, Aragaki A, Crandall CJ, et al. Women’s Health Initiative clinical trials: interaction of calcium and vitamin D with hormone therapy. Menopause. 2014;21(2):116-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. J Bone Miner Res. 2004;19(3):370-378. [DOI] [PubMed] [Google Scholar]
- 67.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327(23):1637-1642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Strategy for Each Database
eTable 2. Randomized Trials Included in Systematic Reviews or Meta-Analyses Evaluating Calcium Supplements With or Without Vitamin D for Fracture Incidence
eTable 3. Randomized Trials Included In Systematic Reviews or Meta-Analyses Evaluating Vitamin D Supplements With or Without Calcium for Fracture Incidence
eTable 4. Excluded Trials and Reasons for Exclusion
eTable 5. Results of Sensitivity Analyses With Exclusion of the Listed Trials
eFigure 1. Number/Proportions of Trials That Met Each Criterion for Risk of Bias Across the 33 Included Trials
eFigure 2. Results of the Risk of Bias for 33 Included Trials