Key Points
Question
Is warfarin use a protective factor for cancer?
Findings
In this population-based cohort study of 1 256 725 persons, there was a significantly lower age- and sex-adjusted incidence rate ratio of cancer among warfarin users vs nonusers.
Meaning
Warfarin, used by millions of adults worldwide, may be associated with lower cancer incidence across a broad range of malignant neoplasms.
Abstract
Importance
In cancer models, warfarin inhibits AXL receptor tyrosine kinase–dependent tumorigenesis and enhances antitumor immune responses at doses not reaching anticoagulation levels. This study investigates the association between warfarin use and cancer incidence in a large, unselected population-based cohort.
Objective
To examine the association between warfarin use and cancer incidence.
Design, Setting, and Participants
This population-based cohort study with subgroup analysis used
the Norwegian National Registry coupled with the Norwegian Prescription Database and the Cancer Registry of Norway. The cohort comprised
all persons (N = 1 256 725) born between January 1, 1924, and December 31, 1954, who were residing in Norway from January 1, 2006, through December 31, 2012. The cohort was divided into 2 groups—warfarin users and nonusers; persons taking warfarin for atrial fibrillation or atrial flutter were the subgroup. Data were collected from January 1, 2004, to December 31, 2012. Data analysis was conducted from October 15, 2016, to January 31, 2017.
Exposures
Warfarin use was defined as taking at least 6 months of a prescription and at least 2 years from first prescription to any cancer diagnosis. If warfarin treatment started after January 1, 2006, each person contributed person-time in the nonuser group until the warfarin user criteria were fulfilled.
Main Outcomes and Measures
Cancer diagnosis of any type during the 7-year observation period (January 1, 2006, through December 31, 2012).
Results
Of the 1 256 725 persons in the cohort, 607 350 (48.3%) were male, 649 375 (51.7%) were female, 132 687 (10.6%) had cancer, 92 942 (7.4%) were classified as warfarin users, and 1 163 783 (92.6%) were classified as nonusers. Warfarin users were older, with a mean (SD) age of 70.2 (8.2) years, and were predominantly men (57 370 [61.7%]) as compared with nonusers, who had a mean (SD) age of 63.9 (8.6) years and were mostly women (613 803 [52.7%]). Among warfarin users and compared with nonusers, there was a significantly lower age- and sex-adjusted incidence rate ratio (IRR) in all cancer sites (IRR, 0.84; 95% CI, 0.82-0.86) and in prevalent organ-specific sites (lung, 0.80 [95% CI, 0.75-0.86]; prostate, 0.69 [95% CI, 0.65-0.72]; and breast, 0.90 [95% CI, 0.82-1.00]). There was no observed significant effect in colon cancer (IRR, 0.99; 95% CI, 0.93-1.06). In a subgroup analysis of patients with atrial fibrillation or atrial flutter, the IRR was lower in all cancer sites (IRR, 0.62; 95% CI, 0.59-0.65) and in prevalent sites (lung, 0.39 [95% CI, 0.33-0.46]; prostate, 0.60 [95% CI, 0.55-0.66]; breast, 0.72 [95% CI, 0.59-0.87]; and colon, 0.71 [95% CI, 0.63-0.81]).
Conclusions and Relevance
Warfarin use may have broad anticancer potential in a large, population-based cohort of persons older than 50 years. This finding could have important implications for the selection of medications for patients needing anticoagulation.
This population-based cohort study uses the Norwegian national registries to determine the association between warfarin use and cancer incidence among persons aged 52 to 82 years.
Introduction
Warfarin sodium is the most used anticoagulant worldwide and is prescribed to 5% to 10% of the adult population in Western countries for a range of indications, including atrial fibrillation, prosthetic heart valves, and venous thromboembolism. The antitumor potential of warfarin is demonstrated in different experimental cancer model systems. Previous epidemiological studies differ regarding the association between warfarin and cancer, with one suggesting a specific association with urogenital cancers and another indicating no association with cancer incidence. A clearly defined molecular mechanism of how vitamin K antagonism affects cancer development and a population-based cohort study are needed to resolve this controversy.
Vitamin K in humans serves as a cofactor for gamma-glutamyl carboxylase that converts glutamate to γ-carboxyglutamate in substrate N-terminal domains of vitamin K–dependent proteins. This conversion allows vitamin K–dependent proteins to bind calcium and membrane phosphatidylserine moieties with high affinity. The reduced (hydroquinone) active cofactor form of vitamin K is maintained through the action of 2 vitamin K oxidoreductases (VKORC1 and VKORC1L1), which are both potently inhibited by warfarin. Treatment with warfarin depletes vitamin K pools, leading to reduced gamma-glutamyl carboxylase activity and noncarboxylated γ-carboxyglutamate domains of vitamin K–dependent proteins. These proteins are comparatively rare in the human genome (numbering ≤14), comprising mainly proteins involved in blood clotting and bone homeostasis. A notable exception is GAS6 (growth arrest–specific 6), the ligand of the AXL receptor tyrosine kinase family associated with immune regulation and cancer. Warfarin treatment inhibits AXL receptor signaling, blocking malignant traits of aggressive carcinoma cells and enhancing antitumor natural killer cell activity at doses that do not affect coagulation. Importantly, noncarboxylated GAS6 retains full AXL binding affinity but is unable to induce receptor activation, thus converting the ligand into a selective AXL antagonist.
New oral anticoagulants that target different factors in the coagulation cascade and prevent the need for repeated blood monitoring are gaining favor. Hence, it is important to more clearly determine the cancer-protective potential of warfarin to better inform the decisions on appropriate anticoagulation regimens.
We conducted a population-based study to examine the association between warfarin use and cancer incidence in a large cohort of the Norwegian population.
Methods
Data Sources and Study Design
We conducted a nationwide cohort study using the Norwegian National Registry, a national database that contains information on every person who is currently or has in the past been a resident of Norway. Norway has comprehensive, population-based national health registries with broad coverage that are supported by a universal health care system. A unique identification number assigned to each citizen and permanent resident enables the retrieval of coupled information for different health conditions with a high level of accuracy and completeness. The 11-digit personal identification number includes an individual’s date of birth and sex, and the use of this number yields linked information from different registries. Using such a system, we coupled a population cohort with information from the Cancer Registry of Norway (CRN) and the Norwegian Prescription Database (NorPD). Data from the Norwegian National Registry included year of birth, date of death, sex, and emigration status. This study was approved with a patient consent exemption by the Regional Ethics Committee; this exemption was granted because the data used were already registered and anonymized. Data were collected from January 1, 2004, to December 31, 2012. Data analysis was conducted from October 15, 2016, to January 31, 2017.
Established in 1951, the CRN is a nationwide registry of all cancer occurrences in Norway. Mandatory reporting from several independent sources ensures the completeness and high quality of CRN data. The CRN uses the International Classification of Diseases, Seventh Edition (ICD-7) and the International Classification of Diseases, Tenth Edition, Clinical Modification (ICD-10-CM) for diagnosis classification and the International Classification of Diseases for Oncology, Third Edition for morphological classification of the different lesions. Data from the CRN included cancer diagnosis, date of diagnosis, and localization.
Established in 2004, the NorPD collects information on all drugs dispensed by Norwegian pharmacies but not the drugs used by hospitalized or otherwise institutionalized patients. The NorPD uses the Anatomical Therapeutic Classification System for categorizing registered drugs. The information retrieved from the NorPD included the name of prescription; date of drug dispensing; number of doses dispensed; and ICD-7, ICD-10-CM, and International Classification of Primary Care (ICPC), Second Edition codes for indication of medication use. Both the NorPD and the CRN have a 99% nationwide coverage, which means 99% of all cancer diagnoses and 99% of all prescriptions in the country are registered in these registries.
The study cohort (N = 1 256 725) included all persons born between January 1, 1924, and December 31, 1954, who were living in Norway from January 1, 2006, through December 31, 2012. Using identification numbers, we coupled this cohort (1) with the CRN to retrieve information on all cancers in the cohort diagnosed between January 1, 2006, and December 31, 2012, and (2) with the NorPD to retrieve information on warfarin prescriptions in the cohort filled from January 1, 2004, through December 31, 2012. The NorPD provided the linkage, and the data were analyzed anonymously.
Warfarin Exposure Assessment
In Norway, warfarin is only available by prescription. To define regular prediagnostic users, we divided the study cohort into 2 groups—warfarin users and nonusers. According to the hypothesized molecular mechanism, a minimal warfarin exposure period along with a minimal interval between exposure and cancer diagnosis was assumed to be required for warfarin to affect cancer development. The warfarin users group comprised persons who received a warfarin prescription at least 2 years before any cancer diagnosis and with an interval between first and last prescription of at least 6 months. For warfarin users without a cancer diagnosis, the interval between first and last prescription had to be at least 6 months. All other person-years in the cohort were classified as nonusers. The duration of warfarin use was calculated according to the time interval between the first and last prescription of warfarin, with the assumption that the use was continuous between these two dates.
Indication for warfarin use was drawn from the NorPD based on the refund code registered on the prescriptions. For prescriptions dated before March 3, 2008, the coding system did not specify the indication for the warfarin use but used larger groups of diagnosis. After March 3, 2008, patients on warfarin for atrial fibrillation or atrial flutter (AF) were identified by 2 different systems: the ICD-10-CM (code I48) for prescriptions made by hospital physicians and the ICPC (code K78) for prescriptions made by general practitioners. First warfarin prescriptions after March 3, 2008, were used for the AF subgroup analysis; warfarin user criteria were otherwise identical. Warfarin users contributed person-time in the nonusers group until the warfarin user criteria were fulfilled.
Statistical Analysis
Descriptive analyses are given as frequencies and proportions and means with SDs. The study cohort was followed from inclusion to the date of cancer diagnosis, date of death, or end of follow-up (December 31, 2012), whichever occurred first. The primary cancer diagnosis for each person was used for the calculations. We calculated the person-years of observation for each group to account for differences in follow-up time between the groups. To examine the cancer risk for warfarin users compared with nonusers, we calculated the incidence rate ratios (IRRs), adjusting for sex and age (2-year age groups), using the Mantel-Haenszel test. A 95% CI was applied to determine statistical significance. If warfarin treatment started after January 1, 2006, each person contributed person-time to the nonusers group until the person fulfilled the criteria of a warfarin user to avoid immortal-time bias. Site-specific results are presented if the number of cancer incidences in the user group was at least 10 cases. Competing risk regression (method of Fine and Gray) was conducted with death as a competing event.
To address the increased risk of cancer after venous thromboembolism and pulmonary embolism, we conducted a sensitivity analysis among the AF subgroup, who were patients prescribed warfarin to prevent thromboembolic events after AF. The IRRs were analyzed for all cancer sites and site-specific cancers of the AF subgroup and compared with those of the nonusers group.
A separate sensitivity analysis addressed the influence of different time intervals between warfarin prescription and cancer diagnosis in the user definition. All the statistical calculations were performed using Stata/IC, version 13.1 and Stata/IC, version 14 (StataCorp LLC).
Results
Characteristics of the Cohort
The study cohort comprised all persons aged 52 to 82 years living in Norway from January 1, 2006, through December 31, 2012. Of the 1 256 725 persons included in this cohort, 92 942 (7.4%) were classified as warfarin users. During the study follow-up period (January 1, 2006, to December 31, 2012), 132 687 persons (10.6%) were diagnosed with cancer over 8 008 218 person-years. The mean (SD) follow-up time was 6.15 (1.8) years, and the median (interquartile range) duration of warfarin use was 4.7 (2.2-7.8) years. Most warfarin users (71 688 [77.1%]) were treated for more than 2 years. Warfarin users were older, with a mean (SD) age of 70.2 (8.2) years, and were predominantly men (57 370 [61.7%]) compared with nonusers, who had a mean (SD) age of 63.9 (8.6) years and were mostly women (613 803 [52.7%]). The baseline characteristics of the cohort are listed in Table 1.
Table 1. Characteristics of the Study Population.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Warfarin Users | Nonusers | Total | |
| Sex | |||
| Male | 57 370 (61.7) | 549 980 (47.3) | 607 350 (48.3) |
| Female | 35 572 (38.3) | 613 803 (52.7) | 649 375 (51.7) |
| Total | 92 942 (7.4) | 1 163 783 (92.6) | 1 256 725 |
| Person-years | 430 266 (5.4) | 7 577 952 (94.6) | 8 008 218 |
| Follow-up time, mean (SD), y | 4.6 (2.2) | 6.3 (1.7) | 6.2 (1.8) |
| Year of birth | |||
| 1924-1933 | 42 830 (46.1) | 226 079 (19.4) | 268 909 (21.4) |
| 1934-1943 | 30 166 (32.4) | 323 003 (27.8) | 353 169 (28.1) |
| 1944-1954 | 19 942 (21.5) | 614 701 (52.8) | 634 647 (50.5) |
| Age, mean (SD), y | 70.2 (8.2) | 63.9 (8.6) | |
| Deaths in study period | 20 502 (22.1) | 124 480 (10.7) | 144 982 (11.5) |
| Cancer diagnosis | 8754 (9.4) | 123 933 (10.6) | 132 687 (10.6) |
| Duration of warfarin use, mo | |||
| 6 to 11 | 9520 (10.2) | NA | |
| 12-24 | 11 734 (12.7) | NA | |
| >24 | 71 688 (77.1) | NA | |
| Duration of use, mean (SD), y | 4.8 (2.8) | NA | |
| Duration of use, median (IQR), y | 4.7 (2.2-7.8) | NA | |
| Reason for warfarin usea | |||
| Atrial fibrillation or atrial flutter (ICD-10-CM I48; ICPC K78) | 33 313 (35.8) | NA | |
| Pulmonary embolism (ICD-10-CM I26; ICPC K93) | 3141 (3.4) | NA | |
| Phlebitis (ICD-10-CM I80, I82; ICPC K94) | 2173 (2.3) | NA | |
| Ischemic heart disease (ICD-10-CM -22, I20-I22, I25; ICPC -22, -26, -27, K74-K76) | 9055 (9.7) | NA | |
| Cerebral ischemia (ICD-10-CM G45, I63; ICPC K89, K90) | 2121 (2.3) | NA | |
| Valvular defect (ICD-10-CM I34, I35, Z94, Z95; ICPC -51, A89, K83) | 1425 (1.5) | NA | |
| Other | 41 714 (44.8) | NA | |
Abbreviations: ICD-10-CM, International Classification of Diseases, Tenth Edition, Clinical Modification; ICPC, International Classification of Primary Care, Second Edition; IQR, interquartile range; NA, not applicable.
The following designations represent reimbursement codes used by the Norwegian Medicines Agency in addition to ICD-10 and ICPC: -22 indicates secondary prophylaxis after heart attack; -26, atherosclerotic disease (secondary prophylaxis); -27, increased risk of developing atherosclerotic disease (primary prophylaxis); and -51, organ transplant (transplanted heart valve).
Warfarin Use and Cancer Incidence
In the warfarin users group, 8754 persons (9.4%) received a diagnosis of cancer in 430 266 person-years. In the nonusers group, 123 933 persons (10.6%) received a diagnosis of cancer in 7 577 952 person-years. As expected, the most prevalent cancer sites were prostate, lung, colon, and breast, representing a total of 65 747 persons (49.6%). We observed a significantly lower IRR for all cancer sites in the warfarin users group compared with the nonusers group (adjusted IRR, 0.84; 95% CI, 0.82-0.86) (Table 2; eTable 1 in the Supplement). A significantly lower IRR in 3 of the 4 most prevalent cancer sites was found in the warfarin users group compared with that in the nonusers group: prostate (IRR, 0.69; 95% CI, 0.65-0.72), lung (IRR, 0.80; 95% CI, 0.75-0.86), and female breast cancer (IRR, 0.90; 95% CI, 0.82-1.00). For lung cancer, subtype analysis revealed a lower IRR for non–small-cell lung carcinoma (IRR, 0.80; 95% CI, 0.74-0.87) and small-cell lung carcinoma (IRR, 0.71; 95% CI, 0.59-0.86) in the warfarin users group compared with the nonusers group (Table 2).
Table 2. Incidence Rate Ratio of Cancer in Warfarin Users vs Nonusers.
| Cancer Site | IRR (95% CI) |
|---|---|
| All cancers | 0.84 (0.82-0.86) |
| Prostate | 0.69 (0.65-0.72) |
| Lung | 0.80 (0.75-0.86) |
| NSCLC | 0.80 (0.74-0.87) |
| SCLC | 0.71 (0.59-0.86) |
| Colon | 0.99 (0.93-1.06) |
| Breast | 0.90 (0.82-1.00) |
| Ductal | 0.90 (0.81-1.02) |
| Lobular | 1.00 (0.75-1.32) |
| Skina | 1.09 (1.01-1.18) |
| Gynecological | 0.85 (0.74-0.97) |
| Ovary | 0.81 (0.65-1.02) |
| Uterus | 0.91 (0.77-1.08) |
| Cervix | 1.03 (0.69-1.54) |
| Vulva and vagina | 0.64 (0.39-1.04) |
| Bone marrow | 1.00 (0.91-1.10) |
| Leukemia | 0.99 (0.89-1.11) |
| CLL | 0.89 (0.71-1.11) |
| AML | 0.70 (0.51-0.98) |
| Bladder | 0.82 (0.75-0.90) |
| Malignant melanoma | 0.96 (0.87-1.07) |
| Rectum and anus | 0.77 (0.69-0.86) |
| Rectum | 0.76 (0.68-0.85) |
| Anus | 0.90 (0.51-1.58) |
| Lymphoma | 0.92 (0.82-1.04) |
| Hodgkin | 0.66 (0.34-1.26) |
| Non-Hodgkin | 0.92 (0.81-1.04) |
| Brain | 0.74 (0.64-0.87) |
| Astrocytoma | 0.75 (0.57-0.98) |
| Meningioma | 0.66 (0.51-0.86) |
| Glioblastoma | 0.75 (0.56-1.00) |
| Kidney and ureters | 0.93 (0.82-1.06) |
| Kidney | 0.96 (0.84-1.10) |
| Pancreas | 0.92 (0.80-1.04) |
| Stomach | 0.77 (0.65-0.90) |
| Head and neck | 0.64 (0.51-0.81) |
| Tongue | 0.30 (0.16-0.56) |
| Throat | 0.59 (0.39-0.90) |
| Larynx | 0.75 (0.55-1.04) |
| Salivary gland | 1.34 (0.74-2.41) |
| Mouth | 0.80 (0.56-1.14) |
| Esophagus | 1.03 (0.83-1.27) |
| Gallbladder | 0.94 (0.71-1.24) |
| Endocrine glandsb | 0.53 (0.36-0.80) |
| Thyroid | 0.81 (0.56-1.18) |
Abbreviations: AML, acute myeloid leukemia; CLL, chronic lymphatic leukemia; IRR, incidence rate ratio (adjusted for sex and age); NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer.
Excluding malignant melanoma and basal cell carcinoma.
Excluding thyroid.
Sensitivity analyses on all cancer sites’ risk stratified by age, sex, and duration of warfarin use are shown in eTable 2 in the Supplement. We detected in the warfarin users group a lower IRR with the shortest and longest warfarin exposures as well as a lower IRR for men than women. No difference in cancer risk was detected when the minimal time interval between warfarin treatment and cancer diagnosis was less than 2 years. Competing risk analysis with death as a competing event confirmed the results despite more deaths occurring in the warfarin users group.
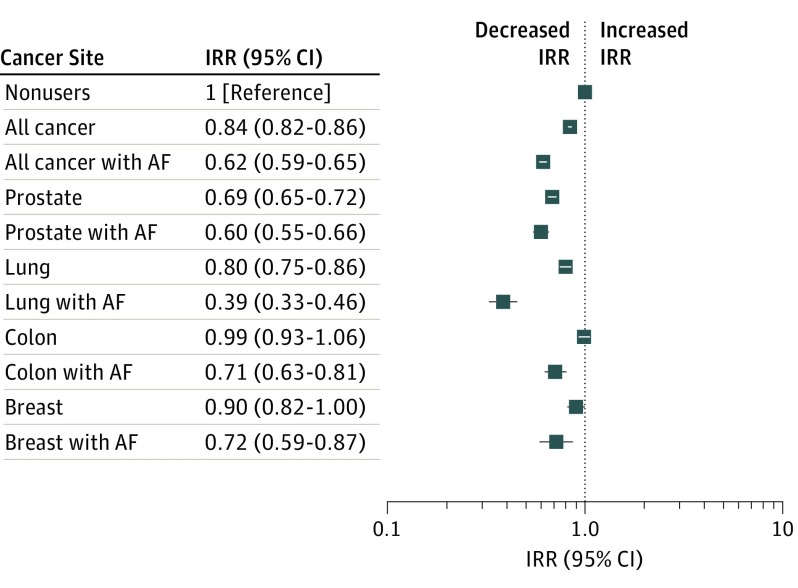
Subgroup analysis of the AF subgroup, which comprised 33 313 persons (35.8%) in 135 617 person-years of warfarin use, showed a significantly lower IRR for all cancer sites (IRR, 0.62; 95% CI, 0.59-0.65) and most prevalent sites (compared with nonusers): IRR for prostate was 0.60 (95% CI, 0.55-0.66), lung was 0.39 (95% CI, 0.33-0.46), and female breast was 0.72 (95% CI, 0.59-0.87) (eTable 3 in the Supplement). Differences in adjusted IRRs between all warfarin users and warfarin users with AF for all cancer sites combined and the 4 most prevalent cancer sites are shown in the Figure.
Figure. Adjusted Incidence Rate Ratios (IRRs) for Nonusers, Warfarin Users, and Warfarin Users With Atrial Fibrillation or Atrial Flutter (AF).
There were 1 163 783 nonusers (7 577 952 person-years), 92 942 warfarin users (430 266 person-years), and 33 313 warfarin users with AF (135 617 person-years).
Discussion
Our large population-based study with a subgroup analysis of patients with arrhythmia reveals a remarkable association between warfarin use and lower cancer incidence across a broad range of malignant neoplasms. The well-known challenges of warfarin dosing that necessitate regular monitoring have fueled a transition to new oral anticoagulants. An unintended consequence of this switch to new oral anticoagulants may be an increased incidence of cancer, which is an important consideration for public health.
Thromboembolic disease is associated with an elevated cancer risk, especially the first year after the diagnosis, and this is an important potential confounder. Patients with AF routinely receive warfarin treatment to prevent embolic strokes. Hence, this subgroup lacks the occult preexisting malignant neoplasm characteristic associated with patients with thromboembolic events. A large number of patients with AF were identified in the cohort, and the association between warfarin use and lower cancer incidence in this subgroup was stronger compared with that in groups in the main analysis. This finding supports the hypothesis that warfarin may exert considerable cancer protection against major carcinoma types.
By inhibiting GAS6-AXL signaling, warfarin blocks tumorigenesis (independent of anticoagulation) in murine models. Importantly, AXL signaling suppresses antitumor immunity. Hence, the observed association between warfarin use and lower cancer incidence is likely due in part to an enhanced antitumor immune surveillance of early cancer. Congruently, warfarin is associated with immune activation, and autoimmune disorders are reported as an adverse effect.
Results from this study show that warfarin therapy is associated with a lower incidence for many but not all cancer sites. Clinical practice in Norway ensures that suspected cancer is biopsied and biopsied also for persons receiving warfarin treatment. Nevertheless, the incidence of superficial cancer (eg, skin) is just as common in warfarin users as in nonusers, whereas several screening-detected cancers (eg, prostate, breast) have a lower incidence. On the other hand, we observed no difference in colorectal cancer, which is suspected to be more apparent when a patient receives anticoagulation therapy (such as in colorectal neoplasia with hematochezia). Interestingly, these observations correspond with reported AXL expression, which is prevalent in lung and breast cancers, for example, but lower in nonmelanoma skin cancers and lymphomas.
To our knowledge, this observational cohort study is the first to use large, nationwide cancer and prescription registries to examine the association between warfarin therapy and cancer incidence. The coupled Norwegian health registries provided a reliable basis for our investigation and enabled the formation of a population-level cohort with numerous diverse cancer cases and long-term follow-up. Selection bias is unlikely as the cohort was extracted from a national registry that includes all residents within the selected age group. Cancer cases were identified using the CRN with 99% coverage. The risk of recall bias for warfarin use is eliminated by the NorPD, which has had nearly complete coverage since 2004.
Prescription data may overestimate drug exposure as not all prescriptions were filled. Our warfarin user definition minimizes this bias. Short-term or unfilled prescriptions were excluded to avoid compliance issues. Furthermore, patients’ international normalized ratio levels were closely monitored by health professionals to adjust warfarin dosage and optimize anticoagulation. This monitoring contributes to the detection of symptoms of undiagnosed cancer. Focusing on cancer incidence 2 years after treatment highlights the influence of warfarin on early neoplasia. Most patients (71 688 [77.1%]) continued warfarin therapy for more than 2 years, with a mean (SD) duration of 4.8 (2.8) years. Patients with transient risk factors generally did not receive warfarin treatment beyond 3 to 6 months, although this decision remains at the discretion of the treating physician. This discontinuation may in part account for the lower IRR in the small fraction of patients who received warfarin therapy for 6 to 24 months. Differences in cancer risk between patients who received short-term and those who received long-term warfarin treatment could influence the observed IRR differences in treatment duration. Overall, most patients treated with warfarin have poorer health and higher cancer risk compared with a healthy population. Many of the risk factors for cancer are the same risk factors for AF and thromboembolic disease, such as alcohol use, obesity, and smoking.
Another source of potential bias is that warfarin treatment criteria may exclude patients with complicating comorbidities or other tolerance issues. No alternative anticoagulation treatments were available for comparison during the study period. Patients with severe comorbidities may be less likely to participate in established cancer screening programs (eg, mammography, Papanicolaou test).
Limitations
This study has several limitations. First, an important but quantitatively modest limitation is that the NorPD lacks information on medications dispensed in hospitals and nursing homes, and this missing data could have led to the systematic misclassification of some individuals as nonusers. In addition, we did not have access to prescription data before 2004. Thus, individuals may have used warfarin longer than the time registered, or warfarin therapy may have been discontinued prior to 2004 and users were misclassified as nonusers. These misclassifications are expected to decrease the observed warfarin-associated cancer protection. Interruptions in treatment between first and last prescription among warfarin users may not be detected, leading to the misclassification of person-years without treatment in the warfarin users group and potentially increasing the observed association between warfarin use and lower cancer incidence.
Second, information was missing on comedications used by patients that may affect cancer development, such as statins, β-blockers, and angiotensin-converting enzyme inhibitors. However, a case-control study identified a protective association of warfarin with prostate cancer (IRR, 0.67-0.80) depending on the length of treatment and after controlling for potential confounders by other drugs.
Third, there was a potential bias due to the lack of information on cancer diagnoses prior to the study start. Thus, some registered cancer occurrences might have been recurrences.
Fourth, information on lifestyle choices (eg, diet, weight management, or smoking) was unavailable in the registries and could not be addressed. Alcoholism and liver disease are contraindications for warfarin treatment and risk factors for cancer development. We observed a significantly lowered IRR for alcohol-related cancers, such as head and neck cancer and liver cancer. Some of this observed association could be explained by a lack of these risk factors in the warfarin users group.
Conclusions
Conflicting conclusions on the association between warfarin anticoagulation therapy and cancer risk have been reported (eg, Tagalakis et al). A prospective randomized clinical trial comparing 6 months with 6 weeks of warfarin therapy following venous thromboembolism reported a significant reduction in incidence among those in the 6-month group after 8 years of follow-up. However, a separate study found no significant reduction in cancer after extending warfarin treatment to 1 year in patients with initial occurrence of venous thromboembolism. A case-control study found no significant difference in bladder cancer risk; similarly, a recent study did not observe a decreased risk of prostate cancer among warfarin users. Notably, these studies included any warfarin exposure, including short-term treatment and treatment prior to cancer diagnosis, leading to a higher number of warfarin users (13% to 16%) than observed in our study cohort and than expected in a general population (eg, 3% to 9%). In contrast, we applied a stricter definition of warfarin use, with a 2-year minimum time interval between start of warfarin treatment and cancer diagnosis, to test the hypothesis that warfarin may affect earlier-stage neoplasia. Cancer development is a protracted process spanning many years. Hence, enforcing a strict definition of warfarin use, with a minimum time interval between start of warfarin treatment and cancer diagnosis, is necessary for warfarin treatment to plausibly influence cancer incidence.
Our results document a lower incidence of cancer associated with warfarin in a population-level study. We observed a lower relative risk in a large cohort comprising many different cancer types, reinforced by a subgroup analysis of patients with AF who were receiving warfarin treatment to prevent thromboembolic events. Our data indicate that warfarin provides a possible cancer protection, a finding that may have important implications for choosing medications for patients who need anticoagulation. Further studies are warranted to fully elucidate the mechanisms underpinning these observations.
eTable 1. Number of Cases and Incidence Rate Ratio of Cancer in Warfarin Users Compared to Non-Users
eTable 2. Sensitivity Analyses
eTable 3. Number of Cases and Incidence Rate Ratio of Cancer in Warfarin Users After Atrial Fibrillation/Flutter Compared to Non-Users
References
- 1.Dossett LA, Riesel JN, Griffin MR, Cotton BA. Prevalence and implications of preinjury warfarin use: an analysis of the National Trauma Databank. Arch Surg. 2011;146(5):565-570. [DOI] [PubMed] [Google Scholar]
- 2.Keeling D, Baglin T, Tait C, et al. ; British Committee for Standards in Haematology . Guidelines on oral anticoagulation with warfarin—fourth edition. Br J Haematol. 2011;154(3):311-324. [DOI] [PubMed] [Google Scholar]
- 3.Tadros R, Shakib S. Warfarin—indications, risks and drug interactions. Aust Fam Physician. 2010;39(7):476-479. [PubMed] [Google Scholar]
- 4.Kirane A, Ludwig KF, Sorrelle N, et al. . Warfarin blocks Gas6-mediated Axl activation required for pancreatic cancer epithelial plasticity and metastasis. Cancer Res. 2015;75(18):3699-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan JJ, Ketcham AS, Wexler H. Reduced incidence of spontaneous metastases with long-term Coumadin therapy. Ann Surg. 1968;168(1):163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paolino M, Choidas A, Wallner S, et al. . The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507(7493):508-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson RC, Lyndon PJ, Tudway AJ. Effects of anticoagulation and ileal resection on the development and spread of experimental intestinal carcinomas. Br J Cancer. 1980;42(1):85-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tagalakis V, Tamim H, Blostein M, Collet JP, Hanley JA, Kahn SR. Use of warfarin and risk of urogenital cancer: a population-based, nested case-control study. Lancet Oncol. 2007;8(5):395-402. [DOI] [PubMed] [Google Scholar]
- 9.Kinnunen PT, Murtola TJ, Talala K, Taari K, Tammela TL, Auvinen A. Warfarin use and prostate cancer risk in the Finnish Randomized Study of Screening for Prostate Cancer. Scand J Urol. 2016;50(6):413-419. [DOI] [PubMed] [Google Scholar]
- 10.Rajotte I, Hasanbasic I, Blostein M. Gas6-mediated signaling is dependent on the engagement of its gamma-carboxyglutamic acid domain with phosphatidylserine. Biochem Biophys Res Commun. 2008;376(1):70-73. [DOI] [PubMed] [Google Scholar]
- 11.Tie JK, Jin DY, Stafford DW. Conserved loop cysteines of vitamin K epoxide reductase complex subunit 1-like 1 (VKORC1L1) are involved in its active site regeneration. J Biol Chem. 2014;289(13):9396-9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsh J, Dalen J, Anderson DR, et al. . Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1)(suppl):8S-21S. [DOI] [PubMed] [Google Scholar]
- 13.Cranenburg EC, Schurgers LJ, Vermeer C. Vitamin K: the coagulation vitamin that became omnipotent. Thromb Haemost. 2007;98(1):120-125. [PubMed] [Google Scholar]
- 14.Davra V, Kimani SG, Calianese D, Birge RB. Ligand activation of TAM family receptors—implications for tumor biology and therapeutic response. Cancers (Basel). 2016;8(12):E107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skiri H. Role and status of civil registration (population registration) and vital statistics systems in Norway. Statistics Norway. https://unstats.un.org/unsd/vitalstatkb/KnowledgebaseArticle50247.aspx. Published October 1995. Accessed September 11, 2017.
- 16.Larsen IK, Småstuen M, Johannesen TB, et al. . Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218-1231. [DOI] [PubMed] [Google Scholar]
- 17.Furu K. Establishment of the nationwide Norwegian Prescription Database (NorPD)—new opportunities for research in pharmacoepidemiology in Norway. Nor Epidemiol. 2008;18(2):129-136. doi: 10.5324/nje.v18i2.23 [DOI] [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 19.Kumana CR, Cheung BM, Siu DC, Tse HF, Lauder IJ. Non-vitamin K oral anticoagulants versus warfarin for patients with atrial fibrillation: absolute benefit and harm assessments yield novel insights. Cardiovasc Ther. 2016;34(2):100-106. [DOI] [PubMed] [Google Scholar]
- 20.Sørensen HT, Mellemkjaer L, Steffensen FH, Olsen JH, Nielsen GL. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med. 1998;338(17):1169-1173. [DOI] [PubMed] [Google Scholar]
- 21.Baron JA, Gridley G, Weiderpass E, Nyrén O, Linet M. Venous thromboembolism and cancer. Lancet. 1998;351(9109):1077-1080. [DOI] [PubMed] [Google Scholar]
- 22.Björck F, Ek A, Johansson L, Själander A. Warfarin persistence among atrial fibrillation patients—why is treatment ended? Cardiovasc Ther. 2016;34(6):468-474. [DOI] [PubMed] [Google Scholar]
- 23.Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33:355-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gjerdrum C, Tiron C, Høiby T, et al. . Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci U S A. 2010;107(3):1124-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Li L, Xu J, Zhang C. Pharmaceutical care for a patient with warfarin-induced autoimmune hepatitis [in Chinese]. Beijing Da Xue Xue Bao. 2016;48(1):183-186. [PubMed] [Google Scholar]
- 26.Bobek V, Kovarík J. Antitumor and antimetastatic effect of warfarin and heparins. Biomed Pharmacother. 2004;58(4):213-219. [DOI] [PubMed] [Google Scholar]
- 27.Paccez JD, Vogelsang M, Parker MI, Zerbini LF. The receptor tyrosine kinase Axl in cancer: biological functions and therapeutic implications. Int J Cancer. 2014;134(5):1024-1033. [DOI] [PubMed] [Google Scholar]
- 28.Conen D, Wong JA, Sandhu RK, et al. . Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. 2016;1(4):389-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31(12):1514-1521. [DOI] [PubMed] [Google Scholar]
- 30.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29(19):2635-2644. [DOI] [PubMed] [Google Scholar]
- 31.Tagalakis V, Blostein M, Robinson-Cohen C, Kahn SR. The effect of anticoagulants on cancer risk and survival: systematic review. Cancer Treat Rev. 2007;33(4):358-368. [DOI] [PubMed] [Google Scholar]
- 32.Schulman S, Lindmarker P. Incidence of cancer after prophylaxis with warfarin against recurrent venous thromboembolism: duration of anticoagulation trial. N Engl J Med. 2000;342(26):1953-1958. [DOI] [PubMed] [Google Scholar]
- 33.Taliani MR, Agnelli G, Prandoni P, et al. ; Warfarin Optimal Duration Italian Trial (WODIT) Investigators . Incidence of cancer after a first episode of idiopathic venous thromboembolism treated with 3 months or 1 year of oral anticoagulation. J Thromb Haemost. 2003;1(8):1730-1733. [DOI] [PubMed] [Google Scholar]
- 34.Blumentals WA, Foulis PR, Schwartz SW, Mason TJ. Does warfarin therapy influence the risk of bladder cancer? Thromb Haemost. 2004;91(4):801-805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Number of Cases and Incidence Rate Ratio of Cancer in Warfarin Users Compared to Non-Users
eTable 2. Sensitivity Analyses
eTable 3. Number of Cases and Incidence Rate Ratio of Cancer in Warfarin Users After Atrial Fibrillation/Flutter Compared to Non-Users