Abstract
Calcium channel blockers (CCBs), particularly dihydropyridine-CCBs, (DHP-CCBs), have an established role in antihypertensive therapy, either as monotherapy or in combination with other antihypertensive drugs. Two hundred and fifty-one papers published in PubMed in English between January 1, 1990, and October 31, 2016, were identified using the keyword “lercanidipine.” Lercanidipine is a lipophilic third-generation DHP-CCB, characterized by high vascular selectivity and persistence in the smooth muscle cell membranes. Lercanidipine is devoid of sympathetic activation, and unlike the first and second generation of DHP-CCBs, it dilates both the afferent and the efferent glomerular arteries, while preserving the intraglomerular pressure. In addition, lercanidipine prevents renal damage induced by angiotensin II and demonstrates anti-inflammatory, antioxidant, and anti-atherogenic properties through an increasing bioavailability of endothelial nitric oxide. It is associated with a regression of microvascular structural modifications in hypertensive patients. The efficacy of lercanidipine has been demonstrated in patients with different degrees of hypertension, in the young and elderly and in patients with isolated systolic hypertension. In patients with diabetes and renal impairment, lercanidipine displays a renal protection with a significant decrease of microalbuminuria and improvement of creatinine clearance. Lercanidipine is well tolerated and is associated with a very low rate of adverse events, particularly ankle edema, compared with amlodipine and nifedipine. In conclusion, lercanidipine produces a sustained blood pressure-lowering activity with a high rate of responder/normalized patients, associated with a favorable tolerability profile.
Keywords: Dihydropyridine calcium channel blockers, hypertension, lercanidipine
INTRODUCTION
Hypertension is one of the most important risk factors for cardiovascular (CV) mortality and morbidity and is the most common chronic disorder seen in primary care.[1,2,3] Evidence from large randomized clinical trials and meta-analyses[4,5,6] has shown the benefits of high blood pressure (BP) reduction to prevent target organ damage, as well as mortality in elderly patients.[7,8] Lowering systolic BP (SBP) by 10 mm of mercury (mmHg) or diastolic BP (DBP) by 5 mmHg significantly decreases the risk of CV events with a larger reduction using a drug combination regimen.[4,7,9] Unfortunately, inadequate control of hypertension has been reported by several cross-sectional studies.[7,10,11,12] This paper aims to provide a critical review on BP-lowering, metabolic, and CV effects of lercanidipine in the treatment of hypertension, based on the results of published studies, with specific focus on the most recent data.
METHODOLOGY
A total of 251 articles available in PubMed, published in English between January 1, 1990, and October 31, 2016, using the keyword “lercanidipine,” were identified (including 45 randomized controlled trials, 2 observational studies, 40 reviews, 11 case reports, and 1 editorial). The articles focused on BP-lowering, metabolic, and CV effects of lercanidipine.
GENERALITIES
With regard to antihypertensive agents, it is recognized that calcium channel blockers (CCBs) play an important role in starting and maintaining antihypertensive therapy, either as monotherapy or in combination with renin-angiotensin (AT)-aldosterone system (RAAS) antagonists.[13,14,15] CCBs are a heterogeneous class, including dihydropyridine (DHP) and non-DHP (NDHP) subgroups. Both subgroups have a similar action mechanism: they inhibit the calcium influx into vascular smooth muscle cell through the L-type voltage-gated calcium channels. The lower intracellular calcium ion concentration induces vasodilation, reduction of peripheral vascular resistance, and consequently BP. However, unlike NDHP-CCBs, DHP-CCBs have higher vascular selectivity and are devoid of pharmacological effects at the myocardium level, such as negative inotropic and chronotropic activity.[16] The antihypertensive activity of a once-daily administration of DHP-CCBs – amlodipine, lercanidipine, lacidipine, manidipine, nifedipine gastrointestinal therapeutic system (GITS, an extended-release formulation of nifedipine) – is quite similar and stable throughout the 24-h dosing interval.[16]
Different meta-analyses have shown that DHP-CCBs have cardioprotective effects decreasing the risk of CV events,[4,17,18] such as strokes (-21%), coronary artery disease (-18%), and heart failure (-28%), in patients with or without CV disease and regardless of BP values before treatment. Moreover, compared to β-blockers and diuretics, CCBs lead to less new-onset type-2 diabetes,[19,20] decrease SBP variability, which accounts for stroke prevention,[21] and are not associated with the risk of new-onset atrial fibrillation.[22]
Lercanidipine hydrochloride (HCl) is a third-generation DHP-CCB characterized by high vascular selectivity and high lipophilicity, which enables easy penetration and considerable concentration and persistence in the phospholipids bilayer of the smooth muscle cell membranes, from which it is gradually released to reach the L-type calcium channels.[23,24] In vitro lercanidipine has shown a lower negative inotropic effect than other DHPs such as lacidipine, amlodipine, nitrendipine, nifedipine, and felodipine.[24]
PHARMACOKINETICS
Unlike other DHP-CCBs, the high lipophilicity of lercanidipine HCl provides a slow onset of action, a long-lasting smooth muscle relaxation, and a peripheral vasodilation.[23,24] After oral administration, lercanidipine is well absorbed by the gastrointestinal tract, with a peak plasma concentration reached after 1.5–3 h. The drug appears to have a biphasic elimination profile: first phase with elimination plasma half-life of 3–5 h,[23,25,26] followed by a second phase with terminal half-life of 10.5 h.[23,27] In hypertensive patients, the mean terminal elimination half-life after a single oral dose of 10–20 mg is 8–10.5 h.[27,28] However, the prolonged duration of the pharmacological activity is not dependent on the plasma drug half-life, but on the smooth muscle membrane kinetics;[25] therefore, despite the short plasma half-life, the pharmacodynamic action covers 24 h.
Lercanidipine is metabolized in the liver by cytochrome CYP3A4 and converted into inactive metabolites which are eliminated in urine and feces.[27,28] Lercanidipine should not be administered with inhibitors of CYP3A4 or cyclosporine.[28]
Pharmacokinetic properties are not modified by age or mild or moderate hepatic or renal impairment,[26] whereas in patients with severe renal insufficiency – estimated glomerular filtration rate (eGFR) <30 ml/min/m2 – the dosage has to be reduced to avoid high plasma concentrations.[25,26,27,28] The absorption of lercanidipine is increased by high-fat meals, and it should thus be administered before eating.[26,28] Concomitant administration of cimetidine or digoxin does not modify the pharmacokinetics of lercanidipine, whereas as with other DHP-CCBs, an interaction with simvastatin has been reported (increased plasma concentration of simvastatin). It is therefore recommended to administer simvastatin in the evening and lercanidipine in the morning.[28,29]
Taken together, these findings show that lercanidipine is a long-acting CCB allowing for once-daily administration. This effect is not dependent on plasma drug half-life but on smooth muscle membrane kinetics.
RELEVANT PHARMACOLOGICAL AND CLINICAL PHARMACOLOGICAL ASPECTS OF LERCANIDIPINE
Sympathetic activation
Unlike nifedipine GITS and felodipine, lercanidipine decreases sympathetic overdrive associated with hypertension. During chronic treatment in hypertensive patients, at similar BP reduction, norepinephrine plasma concentration was not modified by lercanidipine (10–20 mg/daily), whereas it was increased by nifedipine GITS and felodipine.[30,31] Moreover, muscle sympathetic nerve traffic, assessed via microneurography, was decreased by lercanidipine and increased by felodipine, suggesting that lercanidipine as monotherapy,[31] or combined with enalapril[32] during chronic administration, does not induce sympathetic activation, secondary to peripheral vasodilation. This aspect has an important clinical relevance considering that sympathetic overdrive can be associated with the development and progression of target organ damage and CV events in hypertensive patients.[30,33]
Antioxidant and anti-inflammatory activity
Lercanidipine increases nitric oxide (NO) bioavailability and endothelium-dependent vasodilation in hypertensive patients.[34] It also reduces the markers of oxidative stress, such as plasma lipoperoxides, isoprostanes, myeloperoxidase, a leukocyte-derived vascular NO oxidase,[35] malondialdehyde,[34,36] asymmetric dimethylarginine (ADMA), an endogenous NO synthase inhibitor,[34,36,37] and metalloproteinase-9.[38] In addition, the drug inhibits vascular neointimal and smooth muscle cell proliferation as well as cholesterol accumulation through the reduction of cellular reactive oxygen species.[37,39,40,41]
In hypertensive patients, lercanidipine decreases the plasma white blood cells, C-reactive protein, E-selectin, P-selectin,[42] lipoprotein-a, and intracellular adhesion molecules involved in thrombotic process and vascular/tissue injury.[43]
Lercanidipine may exert anti-atherogenic effects as well as antihypertensive activity. A significant reduction of atherosclerotic lesions and cholesterol accumulation has been demonstrated in animals,[39,40] and a 35% decrease of low-density lipoprotein-cholesterol (LDL-C) oxidation has been observed in hypertensive patients with diabetes mellitus.[44]
Renal effects
At renal level, lercanidipine acts differently from other first- and second-generation DHP-CCBs. It dilates both the afferent and the efferent glomerular arteries, with intraglomerular capillary pressure remaining unchanged.[45,46,47] This ability is thought to be a consequence of inhibition of both L-type (preglomerular) and T-type (postglomerular) calcium channels at renal level. Postglomerular arteries are rich in T-type calcium channels, and the third-generation CCBs have been proved to inhibit T-type channels in postglomerular vessels.[48,49] In addition, lercanidipine decreases tubule-interstitial fibrosis and microalbuminuria in spontaneously hypertensive rats,[46,47] demonstrating a renal protection independent of BP reduction. The renal protection of lercanidipine has been confirmed in a double-transgenic rat (dTGR) model, with overexpression of human renin and angiotensinogen genes.[37] Lercanidipine treatment prevented renal damage and mortality induced by AT-II. In treated animals, proteinuria decreased and plasma creatinine levels were maintained in the normal range compared with untreated dTGR rats. Moreover, a decrease of monocyte infiltration, extracellular matrix formation, and fibrosis was observed in renal vessels. These effects may result from inhibition of tissue inflammation and from improved NO bioavailability. At cellular level, the action of lercanidipine in this experimental model seems related to intracellular protein kinase C isoforms inhibition and activation of the dimethylarginine dimethylaminohydrolase enzyme involved in ADMA metabolism, as demonstrated by reduced ADMA plasma concentration in dTGR animals. Consequently, intracellular NO bioavailability increases since ADMA is an inhibitor of NO synthase. These intracellular effects of lercanidipine are caused by reduced intracellular calcium concentration.
Effects on microvascular structure in hypertensive patients
In hypertensive patients, lercanidipine treatment is associated with a regression of microvascular structural changes,[50] the effect being confirmed through the evaluation of the retinal arteriolar morphology.[51] The wall-to-lumen ratio of retinal arterioles was assessed using scanning laser Doppler flowmetry to evaluate the retinal perfusion. The results show that lercanidipine significantly decreases the wall-to-lumen ratio [Figure 1], as well as the wall thickness and the wall cross-sectional area of the retinal arteries.[51] This effect may be related to the antioxidant and anti-inflammatory properties of lercanidipine. These effects, together with BP reduction, have a high clinical relevance, considering the role of endothelial dysfunction, oxidative stress, low-grade inflammation, and arterial stiffness in the pathogenesis of atherosclerosis and the importance of organ damage in long-term CV disease.
Figure 1.
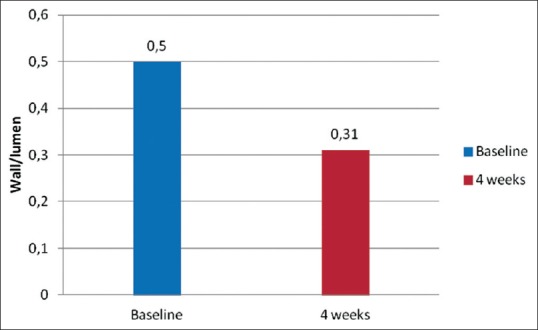
Reduction in the wall-to-lumen ratio after lercanidipine treatment (4 weeks) (P < 0.001 vs. baseline)[51]
Preclinical data demonstrate that lercanidipine is highly selective for vascular tissue and produces smooth muscle relaxation through binding to L-type calcium channels. Moreover, it has a lower negative inotropic effect compared with other DHP CCBs, does not cause significant reflex tachycardia, shows a renal protection, and exerts anti-atherogenic, anti-inflammatory, and antioxidant effects.
ANTIHYPERTENSIVE ACTIVITY
The therapeutic efficacy of lercanidipine (10–20 mg/daily) has been evaluated in double-blind, randomized, comparative trials and in large observational studies, in patients with mild-to-moderate hypertension, severe hypertension, isolated systolic hypertension, hypertensive patients with diabetes mellitus, kidney disease, or concomitant different CV risk factors, as well in elderly subjects.
In patients with mild-to-moderate hypertension or with isolated systolic hypertension,[52] lercanidipine significantly reduces the augmentation index, as well the central SBP and pulse pressure [Figure 2].
Figure 2.
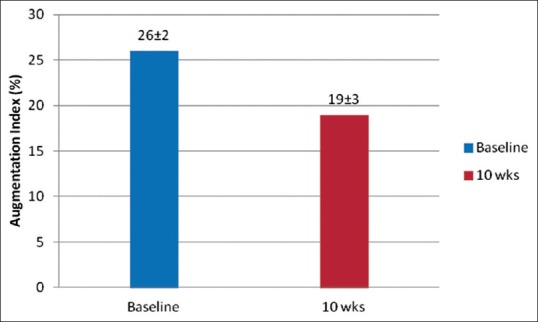
Effects of 10 weeks' lercanidipine treatment on augmentation index (P < 0.005 vs. baseline)
The most important clinical studies are reported in Tables 1 and 2. A significant reduction of SBP and DBP, associated with a high rate of responder patients and BP normalizations, was obtained after 4–6 weeks of therapy.[53] The antihypertensive effect is maintained for 24 h, with a favorable smoothness index and a significant decrease of morning BP rise as well as BP variability [Figure 3].[51,54,55,56,57]
Table 1.
Comparative randomized studies versus other calcium channel blockers
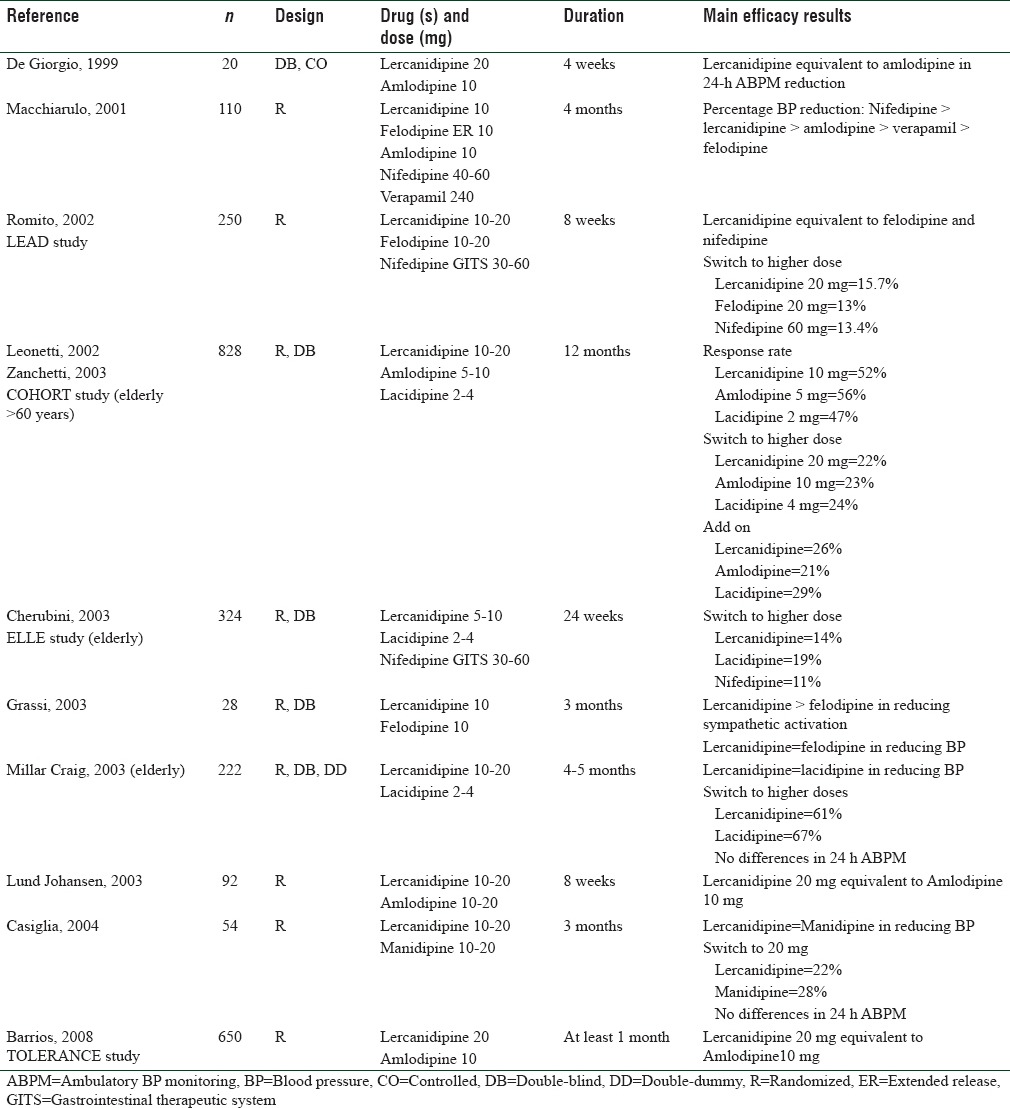
Table 2.
Large, effectiveness studies in real-life setting
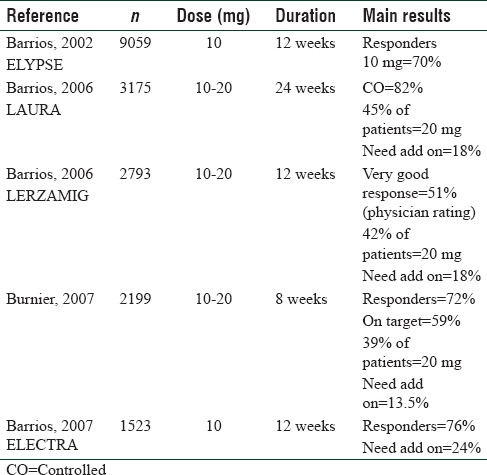
Figure 3.
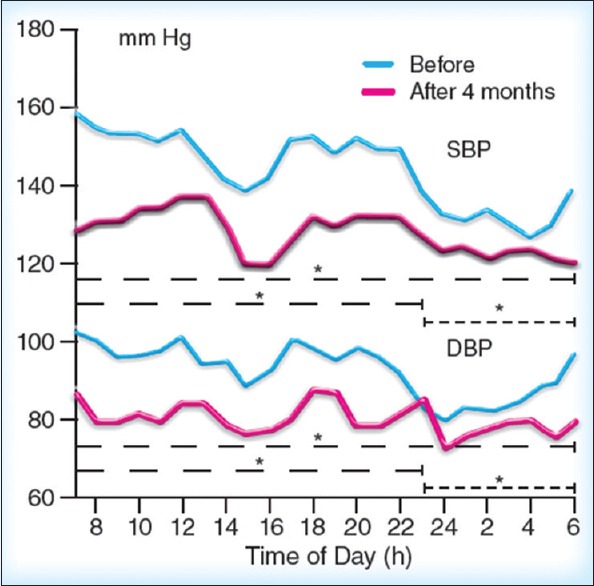
Twenty-four hours ambulatory blood pressure monitoring-Fourier analysis for systolic (upper panel) and diastolic (lower panel) blood pressure before and after 4 months lercanidipine treatment. Asterisks refer (* P < 0.05) to the between curves statistical significance both for 24-h and for the day and night periods[57]
Recently, an international, multicenter, randomized, placebo-controlled, parallel group, factorial design study[58] was performed in 1000 patients with Stage 2 hypertension with lercanidipine (10–20 mg/daily) as monotherapy or combined with enalapril. The results have shown that in patients treated with 10 mg of lercanidipine office and home, SBP/DBP decreased by 11.0/10.4 mmHg and 8.8/4.6 mmHg with a responder rate (defined as office SBP and DBP reductions of >20 and >10 mmHg) of 47% and 53%, respectively. The values obtained with the 20 mg dose of lercanidipine were -13.0/-13.0 mmHg and -7.7/-5.5 mmHg with a responder rate of 46% and 62%, respectively.
Large-scale, open observational studies performed in clinical practice in patients treated with lercanidipine10 mg as monotherapy uptitrated to 20 mg in 36%–47% of patients[59,60] reported a high percentage of patients (46.4%–63.0%) with normalized BP.
Comparison with other dihydropyridine calcium channel blockers
Globally, the antihypertensive efficacy of lercanidipine, assessed either as office BP and 24-h BP monitoring, does not differ statistically from other DHP-CCBs such as amlodipine, felodipine, nifedipine GITS, lacidipine, and manidipine, as reported in two meta-analyses of comparative studies.[61,62] Lercanidipine has also been compared with other drug classes such as atenolol,[63] hydrochlorothiazide,[64] captopril,[65] losartan,[66] and candesartan,[67] showing a similar antihypertensive efficacy.
Direct comparison trials with amlodipine show equivalence of the antihypertensive efficacy between the two drugs,[68,69,70] a finding recently confirmed in a controlled randomized trial performed in acute stroke patients.[54] In these patients, lercanidipine and amlodipine significantly reduced clinical BP, as well mean 24-h, day-time, and night-time BP and decreased early morning BP surge. No statistically significant difference was observed in BP reduction, trough-peak ratio, smoothness index, and response and normalization rates between lercanidipine and amlodipine.
The clinical efficacy of lercanidipine is also similar to that of felodipine,[71,72] nifedipine GITS,[72,73,74] and lacidipine.[56,70,74]
ANTIHYPERTENSIVE ACTIVITY IN SPECIFIC PATIENTS
Elderly patients
The antihypertensive efficacy of lercanidipine in elderly patients with mild-to-moderate hypertension has been evaluated in three multicenter, double-blind randomized trials, as well as in other studies and surveys.[70,74,75,76,77]
The COHORT study[70] compared lercanidipine with amlodipine and lacidipine in hypertensive patients with a mean age of 69–70 years. After 6 months, BP significantly decreased with lercanidipine (−29.6/−14.5 mmHg) as well as with amlodipine and lacidipine, with no significant difference between drugs. Similar results were observed with regard to the responder rate also (around 50% at lower dose–up to 80% after increasing the dose).
In the ELderly and LErcanidipine trial[74] performed on patients with a mean age of 73 years, lercanidipine and nifedipine GITS decreased DBP more than lacidipine, whereas the efficacy on SBP was not different. The AGATE study investigated the antihypertensive activity of lercanidipine in patients aged < 65 and ≥65 years,[77] showing a similar antihypertensive effect in the two groups (SBP/DBP -17/9 and -21/10 mmHg, respectively).
This finding has been confirmed in a large survey in general practice,[59] with a similar reduction of SBP/DBP in patients < 65 or ≥65 years old (-24/14 mmHg vs. -29/13 mmHg) and similar rate of BP normalization (65% and 60%). Moreover, this survey demonstrated comparable changes in BP (SBP/DBP -26/-14 mmHg and -24/-14 mmHg) and in BP normalization rate (66% and 61%) in females and males, respectively. Overall, the responder rate was 72% with a 10 mg dose, whereas 29% of subjects needed the 20 mg dose to obtain BP control.
Therefore, the therapeutic activity of lercanidipine is not age or gender dependent. This finding has an important therapeutic relevance if we consider that: (a) the majority of hypertensive patients are older than 65 years, (b) it is more difficult to achieve BP control in elderly women than in elderly men,[7] and (c) international guidelines suggest that CCBs are suitable drugs for treating hypertension in the elderly.[15]
Patients of different populations
Efficacy and tolerability of antihypertensive drugs may vary between populations. In clinical studies with antihypertensive drugs, most of the patients are Caucasians. African-Americans are more likely to have chronic kidney diseases and end-stage renal disease than Caucasians.[78] In general, the former have a better response in terms of BP reduction treatment with CCB monotherapy and diuretics than β-blockers and inhibitors of the renin-AT axis.[79] BP response to CCB monotherapy is qualitatively similar in Blacks and Whites.[80]
There are limited data specifically evaluating BP-lowering and long-term outcomes in Asian populations, but the response to antihypertensive drugs is likely to be similar to Caucasians.[81] A dedicated study with lercanidipine has been performed among Asians of different ethnic groups (Chinese, Malays, and Indians) and the results confirm that lercanidipine is effective in lowering BP in the Asian population, similarly to other studies involving Caucasians.[82]
It therefore can be stated that the therapeutic effect of lercanidipine is not race or ethnicity dependent.
Patients with isolated systolic hypertension
In patients with isolated systolic hypertension,[56] the responder rate after 8 weeks of treatment was significantly higher with lercanidipine than with lacidipine (65% vs. 50% P = 0.04), whereas no significant difference was observed at the end of the study (67% vs. 58%). Another placebo-controlled study[83] reported a high percentage of patients with normalized BP (62%) after treatment with lercanidipine.
Patients with concomitant cardiovascular risk factors: Obesity, metabolic syndrome, dyslipidemia, diabetes, target organ damage
The LERZAMIG study,[84] performed in obese or overweight patients, showed that the antihypertensive efficacy of lercanidipine is independent of body mass index or excessive body fat. At the end of treatment, a “very good response” was rated by 51% of evaluating physicians. In this high-risk population, 42% of patients required the 20 mg dose.
This finding has been recently confirmed by the results of a study performed on hypertensive patients with severe obesity, in which the 24-h BP-lowering effects of lercanidipine, combined with enalapril, were shown to be similar in magnitude to those detected with the felodipine-enalapril combination.[32] In the lercanidipine/enalapril combination-treated group, the antihypertensive effects were associated with a much lesser tachycardia and sympathetic activation than in the felodipine/enalapril-treated group.
The LAURA study,[85] a multicenter, observational, open-label investigation performed on 3175 patients in a real-life setting, evaluated lercanidipine effectiveness in patients with hypertension and concomitant CV risk factors, such as dyslipidemia, smoking, family history of CV disease, and target organ damage. After 6 months of lercanidipine treatment, BP significantly decreases by 18.5/13.8 mmHg in patients at low risk and by 23/15.2, 24.4/16.1, and 27.4/17.4 mmHg in patients with medium, high, and very high risk, respectively. A BP control rate was achieved in 55% of patients treated with 10 mg/day of lercanidipine and in 82% of those uptitrated to 20 mg/day. Therefore, a significant antihypertensive effect was obtained across all CV risk levels, more evident in patients at highest risk.
Data obtained in hypertensive patients with type-2 diabetes have shown that the antihypertensive efficacy of lercanidipine as monotherapy was not associated with impairment of glucose homeostasis. In these patients, fasting blood glucose significantly decreased from 153 to 133 mg/dl, as well as the glycosylated hemoglobin level (5.8%–5.5%), fructosamine (from 280 to 230 mg/dl), and the area under the curve obtained during the oral glucose tolerance test, without significant differences between 10 and 20 mg/day of lercanidipine. At baseline, patients were randomized to receive 10 mg or 20 mg of lercanidipine. The dose could be increased after 4 weeks to 20 mg or 30 mg according to the clinical response. At the study end, 55% of patients responded to 10 mg and 50% of patients responded to 20 mg. The response reached 95% of the patients after uptitration to 20 mg.[86]
Patients with chronic renal disease/albuminuria
CCBs could be particularly indicated for renal protection during long-term treatment of hypertension.[87,88] However, DHP-CCBs have a heterogeneous impact on renal hemodynamics. Unlike other DHP-CCBs, which dilate only the afferent artery, lercanidipine dilates both the afferent and the efferent glomerular arteries[46] avoiding the increase of intraglomerular capillary pressure involved in renal damage and progression. While amlodipine displays a renal protection only when combined with angiotensin-converting enzyme (ACE) inhibitors or with angiotensin receptor blockers, otherwise lercanidipine, thanks to its renal hemodynamic effects, protects renal function as single-drug regimen.
The “Diabete Ipertensione Albuminuria Lercanidipina” study[89] which evaluated the effectiveness of lercanidipine monotherapy in comparison with ramipril in mild-to-moderate hypertensive patients with type-2 diabetes and persistent microalbuminuria, showed a >50% reduction of microalbuminuria) in 34.2% and 22.2% of patients treated with lercanidipine and ramipril, respectively. This finding can explain the improvement of creatinine clearance in hypertensives, with or without type-2 diabetes and with chronic mild renal failure, uncontrolled with ACE inhibitors or AT receptor blockers, observed in the ZAndip en Function Renal Alterada study.[90] Lercanidipine and RAAS inhibitors have a synergic effect in reducing microalbuminuria.[91] Indeed, 10–20 mg/day of lercanidipine as add-on to renin-AT axis blocking drugs significantly reduces proteinuria by 20%–35% in patients with proteinuric renal disease.[92,93] Most recently, the REnal Disease: LErcanidipine Valuable Effect on urinary albumin Loses trial compared the effects of lercanidipine associated with enalapril versus the amlodipine plus enalapril combination; investigators found a reduction of albuminuria in the lercanidipine-treated patients but not in the group treated with amlodipine.[94]
The ability of lercanidipine 20 mg to reduce albuminuria in patients with hypertension can be a protective effect against renal damage since the kidney is one of the targets for end-organ damage in hypertensive and diabetic patients.[15] The improvement of renal function with lercanidipine has been evaluated in patients after renal artery intervention for atherosclerotic lesions.[95] Six months after the intervention, the eGFR significantly increased from 71 ± 21 ml/min/1.73 m2 to 78 ± 23 ml/min/1.73 m2 and 24-h urine protein excretion decreased significantly from 0.03 g to 0.02 g. This evidence, while confirming the renal protection of lercanidipine, has important clinical relevance because it has been demonstrated that microalbuminuria/proteinuria in hypertensives is an important predictor of CV disease and chronic renal impairment.[96]
Therefore, lercanidipine significantly improves BP in young and elderly hypertensive patients, as well as in patients with diabetes, or CV risk factors. The antihypertensive efficacy does not differ statistically from other DHP-CCBs.
In patients with kidney disease, lercanidipine improves creatinine clearance and reduces microalbuminuria, particularly when associated with renin AT system inhibitors.
TOLERABILITY
Adverse events
The most frequent adverse events (AEs) induced by DHP-CCBs are related to systemic vasodilation and include ankle edema, dizziness, headache, flushing, palpitations, and vertigo. Numerous studies have shown that treatment with lercanidipine is associated with a very low rate of AEs[53] [Table 3] and withdrawal from the therapy. Even if the rate of AEs differs across studies, overall 11.5%–11.8% of patients reported AEs[53,85] and a very low percentage (1%–2%) discontinued lercanidipine treatment due to AEs.[59] In the ELYPSE study,[97] which included 9000 patients, AEs were recorded in 6.5% of patients.
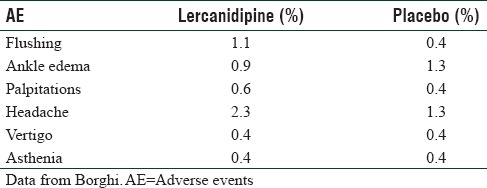
Table 3.
Adverse effects of lercanidipine compared with placebo
Compared with other DHP-CCBs (amlodipine, felodipine, lacidipine, nifedipine, and nitrendipine), the tolerability of lercanidipine was globally higher,[53,70,74,98] providing again a strong evidence of a good tolerability during prolonged therapy.
The “lercanidipine challenge trial,”[99] performed in patients with AEs during treatment with different DHP-CCBs and who switched to lercanidipine, showed lercanidipine treatment to be associated with a significant reduction of flushing, headache, dizziness, and particularly ankle edema.
Ankle edema is the most common AE during treatment with DHP-CCBs and may reduce patients' compliance to the therapy and favor drug withdrawal. Globally, the incidence of ankle edema during lercanidipine treatment is between 0.6% and 9%,[53,70] much lower than the 23%–29% reported with other DHP-CCBs.[100,101] Three studies[69,102,103] have assessed the ankle edema intensity and the incidence induced by lercanidipine using an objective measurement: leg volume (water-displacement volume) and pretibial subcutaneous tissue pressure. The increase in leg volume after 8 weeks of treatment was significantly lower in the lercanidipine group compared with amlodipine,[69,103] and a significantly lower percentage of patients had clinical signs of leg edema with lercanidipine than with amlodipine (9.8% vs. 33.3%). Similar findings have been obtained comparing lercanidipine with nifedipine GITS during 12 weeks of therapy. Ankle-foot volume and pretibial subcutaneous tissue pressure were significantly lower with lercanidipine than with nifedipine.[102] This objective evidence confirms the observations reported in clinical trials and particularly in two meta-analyses of randomized trials.[61,104] Compared with the pooled data reported for amlodipine, nifedipine, and felodipine,[61] lercanidipine was associated with a significantly lower percentage of patients with peripheral edema (7.0% vs. 14.0%, P < 0.001), with 56% relative risk reduction, while compared with the pooled data of lacidipine and manidipine, there were no significant differences (8.5% vs. 6.6%). Therefore, lipophilic DHP-CCBs induce a significant 57% risk reduction for ankle edema compared with hydrophilic DHP-CCBs.[104] The low incidence of ankle edema with lercanidipine is independent of age,[70,74,99] gender,[59,69] ethnic group,[82] presence of concomitant CV disease,[59,85] or BP reduction.[69,102]
Withdrawal rate and persistence
In all probability, the good tolerability profile of lercanidipine has given a very low withdrawal rate for AEs of 2.1%–<1%.[59,70,97] Compared with amlodipine, nifedipine, and felodipine,[61,105] lercanidipine treatment was associated with a 76% decrease in the relative risk of withdrawal. A very high adherence and persistence to therapy (90%–99%) during lercanidipine treatment[59,60,97,106] have been reported, compared with the 39%–72% reported with other DHP-CCBs.[106,107]
Metabolic adverse events
Overall, no clinically meaningful changes in any of the laboratory parameters during treatment with lercanidipine have been reported during the studies. Chronic therapy with lercanidipine has a neutral effect on glucose or lipid metabolism. On the contrary, it has been shown to have a favorable effect on fasting glucose, glucose tolerance test, insulin sensitivity, glycosylated hemoglobin – both in diabetics and not diabetics – total cholesterol, LDL-C, high-density lipoprotein-cholesterol, and triglycerides.[27,60,84,93,86] The absence of negative effects of lercanidipine on lipid and glucose metabolism is an added advantage in the treatment of hypertension, frequently associated with impaired metabolic parameters.
Globally, lercanidipine is well tolerated with a significantly lower incidence of AEs, particularly peripheral edema, compared with amlodipine, nifedipine, and felodipine. No clinical changes in laboratory parameters have been reported during the studies.
CONCLUSIONS
Pharmacological characteristics
Lercanidipine is a third-generation lipophilic DHP-CCB. Due to its high lipophilicity, lercanidipine has an easy penetration and a considerable concentration and persistence in the phospholipids bilayer of the smooth muscle cell membranes, from which it is gradually released to reach the calcium channels; therefore, despite its relatively short half-life, the pharmacological activity of lercanidipine is prolonged.
Antihypertensive efficacy
Globally, the antihypertensive efficacy of lercanidipine is not inferior, and in some studies even superior, to that of other DHP-CCBs or other antihypertensive agents. The dosage of lercanidipine in the different studies was 10–20 mg/day. The antihypertensive efficacy of lercanidipine has been demonstrated in patients with mild-moderate hypertension, as well in patients with type-2 diabetes, renal disease, and isolated systolic hypertension or with several concomitant CV risk factors.
Renal effects
Studies in hypertensive patients with diabetes or renal impairment have shown that lercanidipine has protective effects on the kidneys because it dilates the afferent and efferent glomerular arteries, preserving the intraglomerular pressure. Unlike other CCBs, lercanidipine has also been shown to reduce albuminuria, a recognized risk factor for CV events in hypertensive patients.
Tolerability
Lercanidipine has a favorable tolerability profile with lower incidence of adverse effects, particularly peripheral edema, and withdrawals because of peripheral edema, compared with amlodipine, nifedipine GITS, and felodipine. Moreover, the adherence of patients to lercanidipine therapy is higher than that reported with first- and second-generation DHP-CCBs.
These evidences are of great value for everyday clinical practice and can help physicians to better tailor the treatment according to patients' needs and therapeutic response.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. WRITING GROUP MEMBERS. Heart disease and stroke statistics-2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Lawes CM, Vander Hoorn S, Rodgers A. International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–8. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 3.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–83. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong HT. Cardiovascular outcomes in the comparative hypertension drug trials: More consensus than controversy. Singapore Med J. 2008;49:599–605. [PubMed] [Google Scholar]
- 6.Blood Pressure Lowering Treatment Trialists' Collaboration. Ninomiya T, Perkovic V, Turnbull F, Neal B, Barzi F, et al. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: Meta-analysis of randomised controlled trials. BMJ. 2013;347:f5680. doi: 10.1136/bmj.f5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aronow WS. Treatment of systemic hypertension. Am J Cardiovasc Dis. 2012;2:160–70. [PMC free article] [PubMed] [Google Scholar]
- 8.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 9.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens. 2014;32:2285–95. doi: 10.1097/HJH.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 10.Giannattasio C, Cairo M, Cesana F, Alloni M, Sormani P, Colombo G, et al. Blood pressure control in Italian essential hypertensives treated by general practitioners. Am J Hypertens. 2012;25:1182–7. doi: 10.1038/ajh.2012.108. [DOI] [PubMed] [Google Scholar]
- 11.Grassi G, Cifkova R, Laurent S, Narkiewicz K, Redon J, Farsang C, et al. Blood pressure control and cardiovascular risk profile in hypertensive patients from central and eastern European countries: Results of the BP-CARE study. Eur Heart J. 2011;32:218–25. doi: 10.1093/eurheartj/ehq394. [DOI] [PubMed] [Google Scholar]
- 12.Kotseva K, Wood D, De Backer G, De Bacquer D, Pyörälä K, Reiner Z, et al. EUROASPIRE III. Management of cardiovascular risk factors in asymptomatic high-risk patients in general practice: Cross-sectional survey in 12 European countries. Eur J Cardiovasc Prev Rehabil. 2010;17:530–40. doi: 10.1097/HJR.0b013e3283383f30. [DOI] [PubMed] [Google Scholar]
- 13.Krause T, Lovibond K, Caulfield M, McCormack T, Williams B. Guideline Development Group. Management of hypertension: Summary of NICE guidance. BMJ. 2011;343:d4891. doi: 10.1136/bmj.d4891. [DOI] [PubMed] [Google Scholar]
- 14.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 15.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Blood Press. 2013;22:193–278. doi: 10.3109/08037051.2013.812549. [DOI] [PubMed] [Google Scholar]
- 16.Godfraind T. Calcium channel blockers in cardiovascular pharmacotherapy. J Cardiovasc Pharmacol Ther. 2014;19:501–15. doi: 10.1177/1074248414530508. [DOI] [PubMed] [Google Scholar]
- 17.Costanzo P, Perrone-Filardi P, Petretta M, Marciano C, Vassallo E, Gargiulo P, et al. Calcium channel blockers and cardiovascular outcomes: A meta-analysis of 175,634 patients. J Hypertens. 2009;27:1136–51. doi: 10.1097/HJH.0b013e3283281254. [DOI] [PubMed] [Google Scholar]
- 18.Wu L, Deng SB, She Q. Calcium channel blocker compared with angiotensin receptor blocker for patients with hypertension: A meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) 2014;16:838–45. doi: 10.1111/jch.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancia G, Grassi G, Zanchetti A. New-onset diabetes and antihypertensive drugs. J Hypertens. 2006;24:3–10. doi: 10.1097/01.hjh.0000194119.42722.21. [DOI] [PubMed] [Google Scholar]
- 20.Eleftheriadou I, Tsioufis C, Tsiachris D, Tentolouris N, Stefanadis C. Choice of antihypertensive treatment in subjects with pre-diabetes. Is there a dream after the Navigator. Curr Vasc Pharmacol. 2011;9:715–22. doi: 10.2174/157016111797484099. [DOI] [PubMed] [Google Scholar]
- 21.Rothwell PM, Howard SC, Dolan E, Dobson JE, Dahlöf B, Poulter NR, et al. Effects of β blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–80. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 22.Jong GP, Chen HY, Li SY, Liou YS. Long-term effect of antihypertensive drugs on the risk of new-onset atrial fibrillation: A longitudinal cohort study. Hypertens Res. 2014;37:950–3. doi: 10.1038/hr.2014.104. [DOI] [PubMed] [Google Scholar]
- 23.Meredith PA. Lercanidipine: A novel lipophilic dihydropyridine calcium antagonist with long duration of action and high vascular selectivity. Expert Opin Investig Drugs. 1999;8:1043–62. doi: 10.1517/13543784.8.7.1043. [DOI] [PubMed] [Google Scholar]
- 24.Angelico P, Guarneri L, Leonardi A, Testa R. Vascular-selective effect of lercanidipine and other 1,4-dihydropyridines in isolated rabbit tissues. J Pharm Pharmacol. 1999;51:709–14. doi: 10.1211/0022357991772844. [DOI] [PubMed] [Google Scholar]
- 25.Herbette LG, Vecchiarelli M, Sartani A, Leonardi A. Lercanidipine: Short plasma half-life, long duration of action and high cholesterol tolerance. Updated molecular model to rationalize its pharmacokinetic properties. Blood Press Suppl. 1998;2:10–7. [PubMed] [Google Scholar]
- 26.Barchielli M, Dolfini E, Farina P, Leoni B, Targa G, Vinaccia V, et al. Clinical pharmacokinetics of lercanidipine. J Cardiovasc Pharmacol. 1997;29(Suppl 2):S1–15. [Google Scholar]
- 27.McClellan KJ, Jarvis B. Lercanidipine: A review of its use in hypertension. Drugs. 2000;60:1123–40. doi: 10.2165/00003495-200060050-00009. [DOI] [PubMed] [Google Scholar]
- 28.Bang LM, Chapman TM, Goa KL. Lercanidipine: A review of its efficacy in the management of hypertension. Drugs. 2003;63:2449–72. doi: 10.2165/00003495-200363220-00013. [DOI] [PubMed] [Google Scholar]
- 29.Zhou YT, Yu LS, Zeng S, Huang YW, Xu HM, Zhou Q, et al. Pharmacokinetic drug-drug interactions between 1,4-dihydropyridine calcium channel blockers and statins: Factors determining interaction strength and relevant clinical risk management. Ther Clin Risk Manag. 2014;10:17–26. doi: 10.2147/TCRM.S55512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fogari R, Mugellini A, Zoppi A, Corradi L, Rinaldi A, Derosa G, et al. Differential effects of lercanidipine and nifedipine GITS on plasma norepinephrine in chronic treatment of hypertension. Am J Hypertens. 2003;16:596–9. doi: 10.1016/s0895-7061(03)00901-4. [DOI] [PubMed] [Google Scholar]
- 31.Grassi G, Seravalle G, Turri C, Bolla G, Mancia G. Short-versus long-term effects of different dihydropyridines on sympathetic and baroreflex function in hypertension. Hypertension. 2003;41:558–62. doi: 10.1161/01.HYP.0000058003.27729.5A. [DOI] [PubMed] [Google Scholar]
- 32.Seravalle G, Brambilla G, Pizzalla DP, Casati A, Riva M, Cuspidi C, et al. Differential effects of enalapril-felodipine versus enalapril-lercanidipine combination drug treatment on sympathetic nerve traffic and metabolic profile in obesity-related hypertension. J Am Soc Hypertens. 2016;10:244–51. doi: 10.1016/j.jash.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–90. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taddei S, Virdis A, Ghiadoni L, Versari D, Salvetti G, Magagna A, et al. Calcium antagonist treatment by lercanidipine prevents hyperpolarization in essential hypertension. Hypertension. 2003;41:950–5. doi: 10.1161/01.HYP.0000063361.70525.3C. [DOI] [PubMed] [Google Scholar]
- 35.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–4. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 36.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–28. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Menne J, Park JK, Agrawal R, Lindschau C, Kielstein JT, Kirsch T, et al. Cellular and molecular mechanisms of tissue protection by lipophilic calcium channel blockers. FASEB J. 2006;20:994–6. doi: 10.1096/fj.05-4087fje. [DOI] [PubMed] [Google Scholar]
- 38.Martinez ML, Lopes LF, Coelho EB, Nobre F, Rocha JB, Gerlach RF, et al. Lercanidipine reduces matrix metalloproteinase-9 activity in patients with hypertension. J Cardiovasc Pharmacol. 2006;47:117–22. doi: 10.1097/01.fjc.0000196241.96759.71. [DOI] [PubMed] [Google Scholar]
- 39.Soma MR, Natali M, Donetti E, Baetta R, Farina P, Leonardi A, et al. Effect of lercanidipine and its (R)-enantiomer on atherosclerotic lesions induced in hypercholesterolemic rabbits. Br J Pharmacol. 1998;125:1471–6. doi: 10.1038/sj.bjp.0702221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canavesi M, Baldini N, Leonardi A, Sironi G, Bellosta S, Bernini F, et al. In vitro inhibitory effect of lercanidipine on cholesterol accumulation and matrix metalloproteinases secretion by macrophages. J Cardiovasc Pharmacol. 2004;44:416–22. doi: 10.1097/01.fjc.0000139448.56713.3d. [DOI] [PubMed] [Google Scholar]
- 41.Wu JR, Liou SF, Lin SW, Chai CY, Dai ZK, Liang JC, et al. Lercanidipine inhibits vascular smooth muscle cell proliferation and neointimal formation via reducing intracellular reactive oxygen species and inactivating ras-ERK1/2 signaling. Pharmacol Res. 2009;59:48–56. doi: 10.1016/j.phrs.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Farah R, Khamisy-Farah R, Shurtz-Swirski R. Calcium channel blocker effect on insulin resistance and inflammatory markers in essential hypertension patients. Int Angiol. 2013;32:85–93. [PubMed] [Google Scholar]
- 43.Cominacini L, Pasini AF, Pastorino AM, Garbin U, Davoli A, Rigoni A, et al. Comparative effects of different dihydropyridines on the expression of adhesion molecules induced by TNF-alpha on endothelial cells. J Hypertens. 1999;17:1837–41. doi: 10.1097/00004872-199917121-00009. [DOI] [PubMed] [Google Scholar]
- 44.Rachmani R, Levi Z, Zadok BS, Ravid M. Losartan and lercanidipine attenuate low-density lipoprotein oxidation in patients with hypertension and type 2 diabetes mellitus: A randomized, prospective crossover study. Clin Pharmacol Ther. 2002;72:302–7. doi: 10.1067/mcp.2002.127110. [DOI] [PubMed] [Google Scholar]
- 45.Sabbatini M, Leonardi A, Testa R, Tomassoni D, Vitaioli L, Amenta F, et al. Effects of dihydropyridine-type ca2+ antagonists on the renal arterial tree in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2002;39:39–48. doi: 10.1097/00005344-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Sabbatini M, Leonardi A, Testa R, Vitaioli L, Amenta F. Effect of calcium antagonists on glomerular arterioles in spontaneously hypertensive rats. Hypertension. 2000;35:775–9. doi: 10.1161/01.hyp.35.3.775. [DOI] [PubMed] [Google Scholar]
- 47.Sabbatini M, Vitaioli L, Baldoni E, Amenta F. Nephroprotective effect of treatment with calcium channel blockers in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2000;294:948–54. [PubMed] [Google Scholar]
- 48.Hayashi K, Ozawa Y, Fujiwara K, Wakino S, Kumagai H, Saruta T, et al. Role of actions of calcium antagonists on efferent arterioles – With special references to glomerular hypertension. Am J Nephrol. 2003;23:229–44. doi: 10.1159/000072054. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi K, Homma K, Wakino S, Tokuyama H, Sugano N, Saruta T, et al. T-type ca channel blockade as a determinant of kidney protection. Keio J Med. 2010;59:84–95. doi: 10.2302/kjm.59.84. [DOI] [PubMed] [Google Scholar]
- 50.Grassi G, Quarti-Trevano F, Scopelliti F, Seravalle G, Cuspidi C, Mancia G, et al. Effects of long-term lercanidipine or hydrochlorothiazide administration on hypertension-related vascular structural changes. Blood Press. 2006;15:268–74. doi: 10.1080/08037050600963669. [DOI] [PubMed] [Google Scholar]
- 51.De Ciuceis C, Salvetti M, Rossini C, Muiesan ML, Paini A, Duse S, et al. Effect of antihypertensive treatment on microvascular structure, central blood pressure and oxidative stress in patients with mild essential hypertension. J Hypertens. 2014;32:565–74. doi: 10.1097/HJH.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 52.Mackenzie IS, McEniery CM, Dhakam Z, Brown MJ, Cockcroft JR, Wilkinson IB, et al. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension. 2009;54:409–13. doi: 10.1161/HYPERTENSIONAHA.109.133801. [DOI] [PubMed] [Google Scholar]
- 53.Borghi C. Lercanidipine in hypertension. Vasc Health Risk Manag. 2005;1:173–82. [PMC free article] [PubMed] [Google Scholar]
- 54.Cao TS, Huynh VM, Tran VH. Effects of lercanidipine versus amlodipine in hypertensive patients with cerebral ischemic stroke. Curr Med Res Opin. 2015;31:163–70. doi: 10.1185/03007995.2014.964855. [DOI] [PubMed] [Google Scholar]
- 55.Omboni S, Zanchetti A. Antihypertensive efficacy of lercanidipine at 2.5, 5 and 10 mg in mild to moderate essential hypertensives assessed by clinic and ambulatory blood pressure measurements. Multicenter study investigators. J Hypertens. 1998;16:1831–8. doi: 10.1097/00004872-199816120-00017. [DOI] [PubMed] [Google Scholar]
- 56.Millar-Craig M, Shaffu B, Greenough A, Mitchell L, McDonald C. Lercanidipine vs. lacidipine in isolated systolic hypertension. J Hum Hypertens. 2003;17:799–806. doi: 10.1038/sj.jhh.1001614. [DOI] [PubMed] [Google Scholar]
- 57.Macchiarulo C, Pieri R, Mitolo DC, Pirrelli A. Antihypertensive effects of six calcium antagonists: Evidence from Fourier analysis of 24-hour ambulatory blood pressure recordings. Curr Ther Res Clin Exp. 2001;62:236–53. [Google Scholar]
- 58.Mancia G, Coca A, Chazova I, Girerd X, Haller H, Pauletto P, et al. Effects on office and home blood pressure of the lercanidipine-enalapril combination in patients with stage 2 hypertension: A European randomized, controlled clinical trial. J Hypertens. 2014;32:1700–7. doi: 10.1097/HJH.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burnier M, Gasser UE. Efficacy and tolerability of lercanidipine in patients with hypertension: Results of a phase IV study in general practice. Expert Opin Pharmacother. 2007;8:2215–23. doi: 10.1517/14656566.8.14.2215. [DOI] [PubMed] [Google Scholar]
- 60.Barrios V, Escobar C, de la Figuera M, Honorato J, Llisterri JL, Segura J, et al. High doses of lercanidipine are better tolerated than other dihydropyridines in hypertensive patients with metabolic syndrome: Results from the TOLERANCE study. Int J Clin Pract. 2008;62:723–8. doi: 10.1111/j.1742-1241.2008.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Makarounas-Kirchmann K, Glover-Koudounas S, Ferrari P. Results of a meta-analysis comparing the tolerability of lercanidipine with the 1st and 2nd generation dihydropyridine calcium channel blockers. Clin Ther. 2009;31:1652–63. doi: 10.1016/j.clinthera.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Ghamami N, Chiang SH, Dormuth C, Wright JM. Time course for blood pressure lowering of dihydropyridine calcium channel blockers. Cochrane Database Syst Rev. 2014;31:CD010052. doi: 10.1002/14651858.CD010052.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morisco C, Trimarco B. Efficacy and tolerability of comparison to and in combination with atenol in patients with mild to moderate hypertension in a double-bind controlled study. J Cardiovasc Pharmacol. 1997;29(Suppl 2):S26–30. [Google Scholar]
- 64.Notarbartolo A, Rengo F, Scafidi V, Acanfora D. Long-term effects of lercanidipine on the lipoprotein and apolipoprotein profile of patients with mild-to-moderate essential hypertension. Curr Ther Res Clin Exp. 1999;60:228–36. [Google Scholar]
- 65.Sangiorgi GB, Putignano E, Calcara L, Barbagallo M. Efficacy and tolerability of lercanidipine vs. captopril in patients with mild to moderate hypertension in a double controlled study. J Cardiovasc Pharmacol. 1997;29(Suppl 2):S36–9. [Google Scholar]
- 66.James IG, Jones A, Davies P. A randomised, double-blind, double-dummy comparison of the efficacy and tolerability of lercanidipine tablets and losartan tablets in patients with mild to moderate essential hypertension. J Hum Hypertens. 2002;16:605–10. doi: 10.1038/sj.jhh.1001430. [DOI] [PubMed] [Google Scholar]
- 67.Aranda P, Aranda FJ, Bianchi JL, Cerezo S, Gonzales L, Michan A, et al. Therapeutic efficacy and tolerability of lercanidipine versus candesartan, alone or in combination, in mild-moderate essential hypertensives. J Hypertens. 2000;18(Suppl 2):S152. [Google Scholar]
- 68.De Giorgio LA, Orlandini F, Malasoma P, Zappa A. Double-blind, cross-over study of lercanidipine versus amlodipine in the treatment of mild-to-moderate essential hypertension. Curr Ther Res. 1999;60:511–20. [Google Scholar]
- 69.Lund-Johansen P, Stranden E, Helberg S, Wessel-Aas T, Risberg K, Rønnevik PK, et al. Quantification of leg oedema in postmenopausal hypertensive patients treated with lercanidipine or amlodipine. J Hypertens. 2003;21:1003–10. doi: 10.1097/00004872-200305000-00026. [DOI] [PubMed] [Google Scholar]
- 70.Leonetti G, Magnani B, Pessina AC, Rappelli A, Trimarco B, Zanchetti A, et al. Tolerability of long-term treatment with lercanidipine versus amlodipine and lacidipine in elderly hypertensives. Am J Hypertens. 2002;15:932–40. doi: 10.1016/s0895-7061(02)03000-5. [DOI] [PubMed] [Google Scholar]
- 71.Wu Y, Xu M, Wang H, Xu X, Zhao S, Zhang M, et al. Lercanidipine hydrochloride versus felodipine sustained-release for mild-to-moderate hypertension: A multi-center, randomized clinical trial. Curr Med Res Opin. 2015;31:171–6. doi: 10.1185/03007995.2014.960073. [DOI] [PubMed] [Google Scholar]
- 72.Romito R, Pansini MI, Perticone F, Antonelli G, Pitzalis M, Rizzon P, et al. Comparative effect of lercanidipine, felodipine, and nifedipine GITS on blood pressure and heart rate in patients with mild to moderate arterial hypertension: The lercanidipine in adults (LEAD) study. J Clin Hypertens (Greenwich) 2003;5:249–53. doi: 10.1111/j.1524-6175.2003.01960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Policicchio D, Magliocca R, Malliani A. Efficacy and tolerability of lercanidipine in patients with mild to moderate essential hypertension: A comparative study with slow release nifedipine. J Cardiovasc Pharmacol. 1997;29(Suppl 2):S31–5. [Google Scholar]
- 74.Cherubini A, Fabris F, Ferrari E, Cucinotta D, Antonelli Incalzi R, Senin U, et al. Comparative effects of lercanidipine, lacidipine, and nifedipine gastrointestinal therapeutic system on blood pressure and heart rate in elderly hypertensive patients: The ELderly and LErcanidipine (ELLE) study. Arch Gerontol Geriatr. 2003;37:203–12. doi: 10.1016/s0167-4943(03)00047-5. [DOI] [PubMed] [Google Scholar]
- 75.Ninci MA, Magliocca R, Malliani A. Efficacy and tolerability of lercanidipine in elderly patients with mild to moderate hypertension in a placebo-controlled, double-blind study. J Cardiovasc Pharmacol. 1997;29:S40–4. [Google Scholar]
- 76.Marteil N, Lopez-Eady MD, Castro P, Mortoneda E, Luque M. Modifications of the pulse pressure in elderly hypertensives treated with lercanidipine. J Hypertens. 2004;22(Suppl 2):S121. [Google Scholar]
- 77.Poncelet P, Ribstein J, Goullard L, Bassous M, Grès CS, Clerson P, et al. Efficacy and acceptability of lercanidipine are not age dependent in patients with essential hypertension: The AGATE study. Ann Cardiol Angeiol (Paris) 2004;53:123–30. doi: 10.1016/j.ancard.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O'Hare AM, et al. White/black racial differences in risk of end-stage renal disease and death. Am J Med. 2009;122:672–8. doi: 10.1016/j.amjmed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sica D. Optimizing hypertension and vascular health: Focus on ethnicity. Clin Cornerstone. 2004;6:28–38. doi: 10.1016/s1098-3597(04)80076-0. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen TT, Kaufman JS, Whitsel EA, Cooper RS. Racial differences in blood pressure response to calcium channel blocker monotherapy: A meta-analysis. Am J Hypertens. 2009;22:911–7. doi: 10.1038/ajh.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khan JM, Beevers DG. Management of hypertension in ethnic minorities. Heart. 2005;91:1105–9. doi: 10.1136/hrt.2004.044560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chia YC, Yeoh ES, Ng CJ, Khoo EM, Chua CT. Efficacy and tolerability of lercanidipine in mild to moderate hypertension among asians of different ethnic groups. Singapore Med J. 2009;50:500–5. [PubMed] [Google Scholar]
- 83.Barbagallo M, Barbagallo Sangiorgi G. Efficacy and tolerability of lercanidipine in monotherapy in elderly patients with isolated systolic hypertension. Aging (Milano) 2000;12:375–9. doi: 10.1007/BF03339863. [DOI] [PubMed] [Google Scholar]
- 84.Barrios V, Calderon A, Navarro A, Noya C, Herranz I, Prieto L. Lercanidipine effectiveness and tolerability profile is not influenced by overweight or body fat increase. The LERZAMIG study. J Hypertens. 2004;22(Suppl 2):S258–9. [Google Scholar]
- 85.Barrios V, Escobar C, Navarro A, Barrios L, Navarro-Cid J, Calderón A, et al. Lercanidipine is an effective and well tolerated antihypertensive drug regardless the cardiovascular risk profile: The LAURA study. Int J Clin Pract. 2006;60:1364–70. doi: 10.1111/j.1742-1241.2006.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Viviani GL. Lercanidipine in type II diabetic patients with mild to moderate arterial hypertension. J Cardiovasc Pharmacol. 2002;40:133–9. doi: 10.1097/00005344-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 87.Voyaki SM, Staessen JA, Thijs L, Wang JG, Efstratopoulos AD, Birkenhäger WH, et al. Follow-up of renal function in treated and untreated older patients with isolated systolic hypertension. Systolic hypertension in Europe (Syst-eur) trial investigators. J Hypertens. 2001;19:511–9. doi: 10.1097/00004872-200103000-00020. [DOI] [PubMed] [Google Scholar]
- 88.Robles NR. Calcium antagonists and renal failure progression. Ren Fail. 2008;30:247–55. doi: 10.1080/08860220701856946. [DOI] [PubMed] [Google Scholar]
- 89.Dalla Vestra M, Pozza G, Mosca A, Grazioli V, Lapolla A, Fioretto P, et al. Effect of lercanidipine compared with ramipril on albumin excretion rate in hypertensive type 2 diabetic patients with microalbuminuria: DIAL study (diabete, ipertensione, albuminuria, lercanidipina) Diabetes Nutr Metab. 2004;17:259–66. [PubMed] [Google Scholar]
- 90.Robles NR, Ocon J, Gomez CF, Manjon M, Pastor L, Herrera J, et al. Lercanidipine in patients with chronic renal failure: The ZAFRA study. Ren Fail. 2005;27:73–80. [PubMed] [Google Scholar]
- 91.Scholze J, Bramlage P, Trenkwalder P, Kreutz R. Efficacy and safety of a fixed-dose combination of lercanidipine and enalapril in daily practice. A comparison of office, self-measured and ambulatory blood pressure. Expert Opin Pharmacother. 2011;12:2771–9. doi: 10.1517/14656566.2011.626770. [DOI] [PubMed] [Google Scholar]
- 92.Robles NR, Pastor L, Manjón M, Ocón J, Gómez Campderá F, Herrera J, et al. Lercanidipine in diabetic patients with renal failure. Nefrologia. 2004;24:338–43. [PubMed] [Google Scholar]
- 93.Robles NR, Romero B, de Vinuesa EG, Sánchez-Casado E, Cubero JJ. Treatment of proteinuria with lercanidipine associated with renin-angiotensin axis-blocking drugs. Ren Fail. 2010;32:192–7. doi: 10.3109/08860220903541135. [DOI] [PubMed] [Google Scholar]
- 94.Robles NR, Calvo C, Sobrino J, Espinel E, Esteban R, Mateos L, et al. RED LEVEL trial (REnal Disease: LErcanidipine Valuable Effect on urinary albumin Loses) Curr Med Res Opin. 2016;32(Suppl 2):29–34. doi: 10.1080/03007995.2016.1218838. [DOI] [PubMed] [Google Scholar]
- 95.Peng M, Jiang XJ, Dong H, Zou YB, Zhang HM, Wu HY, et al. Can lercanidipine improve renal function in patients with atherosclerotic renal artery stenosis undergoing renal artery intervention? Curr Med Res Opin. 2015;31:177–82. doi: 10.1185/03007995.2014.960071. [DOI] [PubMed] [Google Scholar]
- 96.Schrader J, Lüders S, Kulschewski A, Hammersen F, Züchner C, Venneklaas U, et al. Microalbuminuria and tubular proteinuria as risk predictors of cardiovascular morbidity and mortality in essential hypertension: Final results of a prospective long-term study (MARPLE study)*. J Hypertens. 2006;24:541–8. doi: 10.1097/01.hjh.0000209991.48928.c4. [DOI] [PubMed] [Google Scholar]
- 97.Barrios V, Navarro A, Esteras A, Luque M, Romero J, Tamargo J, et al. Antihypertensive efficacy and tolerability of lercanidipine in daily clinical practice. The ELYPSE study. Eficacia de lercanidipino y su perfil de seguridad. Blood Press. 2002;11:95–100. doi: 10.1080/08037050211265. [DOI] [PubMed] [Google Scholar]
- 98.Beckey C, Lundy A, Lutfi N. Lercanidipine in the treatment of hypertension. Ann Pharmacother. 2007;41:465–73. doi: 10.1345/aph.1H299. [DOI] [PubMed] [Google Scholar]
- 99.Study Group of the Regional Unit of the Italian Society of Hypertension. Borghi C, Prandin MG, Dormi A, Ambrosioni E. Improved tolerability of the dihydropyridine calcium-channel antagonist lercanidipine: The lercanidipine challenge trial. Blood Press Suppl. 2003;1:14–21. doi: 10.1080/08038020310000087. [DOI] [PubMed] [Google Scholar]
- 100.Hollenberg NK. Observations on the safety of lercanidipine: Adverse event data from placebo-controlled trials. Am J Hypertens. 2002;15:58A–9A. [Google Scholar]
- 101.Weir MR. Incidence of pedal edema formation with dihydropyridine calcium channel blockers: Issues and practical significance. J Clin Hypertens (Greenwich) 2003;5:330–5. doi: 10.1111/j.1524-6175.2003.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fogari R, Malamani GD, Zoppi A, Preti P, Vanasia A, Fogari E, et al. Comparative effect of lercanidipine and nifedipine gastrointestinal therapeutic system on ankle volume and subcutaneous interstitial pressure in hypertensive patients: A double-blind, randomized, parallel-group study. Curr Ther Res. 2000;61:850–62. [Google Scholar]
- 103.Pedrinelli R, Dell'Omo G, Nuti M, Menegato A, Balbarini A, Mariani M, et al. Heterogeneous effect of calcium antagonists on leg oedema: A comparison of amlodipine versus lercanidipine in hypertensive patients. J Hypertens. 2003;21:1969–73. doi: 10.1097/00004872-200310000-00026. [DOI] [PubMed] [Google Scholar]
- 104.Makani H, Bangalore S, Romero J, Htyte N, Berrios RS, Makwana H, et al. Peripheral edema associated with calcium channel blockers: Incidence and withdrawal rate – A meta-analysis of randomized trials. J Hypertens. 2011;29:1270–80. doi: 10.1097/HJH.0b013e3283472643. [DOI] [PubMed] [Google Scholar]
- 105.Elliott HL, Meredith PA. Thrapeutic equivalence in the treatment of hypertension: Can lercanidipine and nifedipine GITS be considered to be interchangeable? World J Cardiol. 2014;6:507–13. doi: 10.4330/wjc.v6.i6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pruijm MT, Maillard MP, Burnier M. Patient adherence and the choice of antihypertensive drugs: Focus on lercanidipine. Vasc Health Risk Manag. 2008;4:1159–66. doi: 10.2147/vhrm.s3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Veronesi M, Cicero AF, Prandin MG, Dormi A, Cosentino E, Strocchi E, et al. A prospective evaluation of persistence on antihypertensive treatment with different antihypertensive drugs in clinical practice. Vasc Health Risk Manag. 2007;3:999–1005. [PMC free article] [PubMed] [Google Scholar]


