Abstract
Objective
This study investigated whether daily and laboratory assessed pain differs as a function of the temporal stability and valence of affect in individuals with chronic knee osteoarthritis (KOA).
Methods
One hundred fifty-one men and women with KOA completed 14 days of electronic diaries assessing positive affect (PA), negative affect (NA), and clinical pain. A subset of participants (n =79) engaged in quantitative sensory testing (QST). State PA and NA were assessed prior to administration of stimuli that induced suprathreshold pain and temporal summation. Multilevel modeling and multiple regression evaluated associations of affect and pain as a function of valence (i.e., positive versus negative) and stability (i.e., stable versus state).
Results
In the diary, stable NA (B = −.63, standard error [SE] = .13, p < .001) was a stronger predictor of clinical KOA pain than stable PA (B = −.18, SE = .11, p = .091), and state PA (B = −.09, p < .001) was a stronger predictor of concurrent daily clinical pain than state NA (B = .04, SE = .02, p = .068). In the laboratory, state PA (B = −.05, SE = .02, p = .042), but not state NA (p = .46), predicted diminished temporal summation of mechanical pain.
Conclusions
Stable NA is more predictive of clinical pain than stable PA, whereas state PA is more predictive of both clinical and laboratory pain than state NA. The findings suggest that dynamic affect-pain processes in the field may reflect individual differences in central pain facilitation.
Keywords: positive affect, negative affect, chronic pain, daily diary, quantitative sensory testing, temporal summation
INTRODUCTION
The prevailing view of emotions is that “bad is stronger than good” (1,2). Indeed, widely accepted psychosocial interventions for chronic pain largely focus on the modification of negative cognitions and pain-related behaviors to improve emotional, physical, and social functioning (e.g., cognitive behavioral therapy for pain) (3). Bias toward the assessment and treatment of the negative affective element of pain is understandable; pain is an aversive experience, and negative affect (NA) is an expression of aversion. Despite this, there is emerging evidence that positive affect (PA) may be a source of resilience for some individuals with chronic pain (4–6). These findings support psychometric evidence of the independence of PA and NA (7) and suggest that both valences should be considered in studies of chronic pain. Relatively few studies, however, have used a dual-valence approach to the study of affect and pain.
In addition to valence (i.e., positive versus negative), the experience of pain on any given day may also differ as a function of the temporal stability of affect (i.e., stable versus state; Litt et al. (8)). Stable affects reflect an individual’s average mood over time and motivate general behavioral response repertoires. Although stable affects may be influenced by genetics (9,10), environmental circumstances also contribute to their development. Chronic exposure to aversive events or physical states, such as those experienced by patients with chronic pain, should therefore engender stable NA in a portion of the population (11,12). State affects, on the other hand, reflect temporary changes in affective tone and do not slavishly adhere to the dictates of a stable affective style (13). Everyday positive events, for example, produce reliable increases in state PA among patients with chronic pain (14). If both the valence and stability of affect reveal individual differences in chronic pain, assessment and therapeutic approaches may be more optimally tailored to person-level characteristics by focusing on the multidimensionality of the affective experience of pain. The purpose of the current study was to determine if daily and laboratory-assessed pain differs as a function of the temporal stability and valence of affect in individuals with chronic knee osteoarthritis (KOA).
Daily process methodology has been applied to chronic pain research to understand the time-varying and “real-time” relations of affect and pain (15). Owing to the nested structure of daily data, observations can be centered within person to create a state-like index of daily change (16). Another advantage of daily diaries is that repeatedly assessed affect reports can be averaged over time to create a stable, trait-like indicator for each individual (17). We refer to aggregate diary affect reports as “stable affects” rather than “trait affects” because they are not considered immutable personality characteristics, but rather, they reflect a reliable assessment of affects obtained over time.
Self-reports of affect, pain, and other subjective states are plagued by systematic biases engendered by the use of heuristics when recalling events or experiences. For example, self-reports of past experiences are influenced by the frequency of the experience (18), as well as its recency and relative intensity (19,20). Microlongitudinal assessment of affect is intended to minimize the potential influence of recall biases by rendering the proximity of the moment of recall closer to the actual moment of experience (21).
Some diary studies have investigated the association of state PA and NA with concurrent (e.g., same-day) pain reports in clinical samples of patients with temporal mandibular disorder and rheumatoid arthritis (5,8,22). Litt et al. (8) and Strand et al. (5) found that both state PA and state NA were significantly associated with concurrent pain levels, whereas a subsequent study of Litt et al. (22) found that only state PA was associated with concurrent pain. Interestingly, the study of Strand et al. (5) also averaged PA and NA measures across diary days to create stable indices and found that stable NA but not stable PA predicted rheumatoid arthritis pain across diary days. These preliminary data support a dimensional approach to studying the relations of affect and pain, but differing methods and results between them suggest that further examination is warranted.
The first aim of the current study was to extend our limited understanding of how dimensions of affect are differentially associated with pain by examining daily diary data from a sample of patients with KOA. We operationalized stable affect as the average of affect reports across diary days and state affect as each person’s daily deviation from his or her stable affect value. We hypothesized that stable NA but not stable PA would be associated with pain reports averaged across diary days. In contrast, we expected both positive and negative affective states to be associated with concurrent daily pain. On the basis of recent research that has uncovered the surprising availability and use of resilience resources in chronic illness populations—and their contribution to health outcomes independent of stress and NA levels (e.g., Moskowitz et al. (23) and Pressman and Cohen (24))—we reasoned that changes in state PA would be associated with changes in pain, irrespective of state NA levels.
Our second aim was to evaluate whether the affect-pain patterns observed in the field would be similarly evident on laboratory pain tests conducted in a controlled environment. Very little is known about the relevance of daily affective processes to pain sensitivity measured through quantitative sensory testing (QST). One QST phenomenon, the temporal summation of pain, may be particularly relevant to affect, given its links to functional alterations in cortical structures known to be involved in emotion regulation, including the insula and cingulate cortices (25–27). Furthermore, those functional alterations were especially prominent in patients with fibromyalgia (25), a chronic widespread pain disorder associated with poor PA regulation in daily life (28). No study, to our knowledge, has examined dimensions of affect in the context of QST measures designed to stimulate these central afferent pathways. A subset of our sample completed measures of state PA and state NA before undergoing laboratory assessments of pain in response to the administration of repeated phasic noxious stimuli, a QST modality designed to induce temporal summation (29–32). We expected that patterns observed in the field would be reflected in the laboratory. Specifically, we hypothesized that a) stable NA (measured in the diary) and b) both state PA and state NA measured at baseline of the QST session would predict differences in response to phasic noxious stimuli.
METHODS
Participants
The data from the current study were taken from the Sleep in Osteoarthritis Project (National Institutes of Health [NIH]/National Institute of Arthritis and Musculoskeletal and Skin Diseases R01 AR05487) designed to characterize and evaluate the role of sleep in pain modulation and study the effects of cognitive behavior therapy of insomnia in KOA. The study was approved by the Johns Hopkins Medicine institutional review board, and all participants provided informed consent. The data presented here were taken exclusively from the baseline assessment performed before randomization. Data collection for the participants included in the present study occurred between July 2008 and May 2012. Participants were 48 men and 103 women (n = 151) who met the American College of Rheumatology criteria for KOA (33). Participants were recruited via advertisements in community media outlets and physician offices. To be included in the study, participants had to meet the American College of Rheumatology criteria for KOA, diagnosed by a board-certified rheumatologist based on history and physical examination, bilateral standing, and semiflexion view radiographs; have at least one knee rated at least 1 on the Kellgren-Lawrence scale (34); and report knee pain greater than 2/10 on a near-daily basis (>4 d/wk) for at least 6 months before entering the study. Participants with serious medical illnesses such as congestive heart disease, history of cerebral vascular events, cancer, or other chronic pain or rheumatic disorders were excluded. Participants were also excluded if they were diagnosed as having severe or unstable psychopathology, cognitive impairment/dementia, current substance abuse disorder (or positive toxicology screening), or other major medical illness that would affect sleep or pain. Participants agreed to discontinue all pain-relieving and sedative medications 24 hours before pain testing. Most (76%) of the sample carried an insomnia diagnosis.
Procedure
All participants completed an informed consent, were administered a battery of questionnaires, and underwent clinical interviews and bilateral knee x-rays. Participants were trained on the use of a personal digital assistant (Palm Pilot) and were instructed to complete evening diaries before bed each evening for 2 weeks.1 A total of 2 participants were excluded from these analyses for having completed fewer than 7 days of diaries. Of the remaining 151 participants, adherence to the diary protocol was good; diaries were completed for 1753 (83%) of a possible 2114 days. At a second baseline visit, participants returned the devices and engaged in three QST measures of phasic pain. A subset of participants were enrolled in an anciallary study of inflammatory responsivity to pain (NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases R01 AR059410; n = 79). As part of this ancillary study, we added PA and NA measures immediately before the QST procedures. Participants included in this set of analyses did not differ from the remainder of the sample in age, race, sex, or clinical pain.
Daily Measures
Daily Pain
Each day, participants rated the severity of pain they experienced on average throughout the day on a 0 to 100 visual analog scale (35), where 0 represented no pain and 100 represented worst possible pain.
Daily PA and NA
Each day, participants were asked to rate the extent to which they felt three positive emotions (happy, calm, and agreeable) and three negative emotions (unhappy, anxious, and annoyed). The individual items appear on full scales of the Positive and Negative Affect Schedule-X (PANAS-X) (36) and the Profile of Mood States-Bipolar (POMS-Bipolar) (37). Participants rated each item on a 0 to 100 numeric rating scale, where 0 being not at all and 100 being extremely. The positive and negative items were averaged to create daily indices of PA and NA, respectively. “Unhappy” was dropped from the calculation of NA because of concern that its similarity to the “Happy” item in the PA scale would bias intermeasure correlation estimates and increase Type 2 errors in models for which NA was included as a covariate. Therefore, an average of “anxious” and “annoyed” was computed to create a daily index of NA. Across days, the PA items were highly correlated (r values range from 0.72 to 0.80), and internal consistency was excellent (Cronbach α = .91). Similarly, the daily NA items were highly correlated (r = 0.74), and internal consistency was excellent (Cronbach α = .85).
Clinical Pain
Clinical Knee Pain
The Western Ontario MacMaster Universities Arthritis Index (WOMAC) (38) was used to assess lower extremity baseline osteoarthritis pain severity for the 48 hours before the diary study. Only the pain subscale was used in the present study. The pain subscale assesses pain in five domains (i.e., during walking, using stairs, in bed, sitting or lying, and standing) on a 100-mm visual analog scale. Internal consistency for this subscale is excellent (Cronbach α = .71–.91) (38–40).
Laboratory Measures
Laboratory PA and NA
At the baseline of the laboratory session, participants rated the extent to which they felt three positive emotions (happy, calm, and pleasant) and six negative emotions (anxious, nervous, tense, frustrated, irritable, and annoyed) at that moment. The individual items appear on full scales from the PANAS-X and POMS-Bipolar. Participants rated each item on a 0 to 100 numeric rating scale, where 0 being not at all and 100 being extremely. The positive and negative items were averaged to create baseline indices of PA and NA, respectively. For PA, interitem correlation was moderate (r values range from 0.43 to 0.66), and internal consistency was adequate (Cronbach α = .75). For NA, interitem correlation was moderate to high (r values range from 0.42 to 0.97), and internal consistency was excellent (Cronbach α = .90).
Quantitative Sensory Tests
Suprathreshold Thermal Phasic Pain
Pain ratings (0–100) were gathered in response to each of ten 0.5-second heat pulses of equal temperature (51°C)2 applied to the left dorsal forearm by a 9-cm2 probe attached to the Medoc Contact Heat-Evoked Potential Stimulator (41). The interstimulus interval between each pulse was set at 2.5 seconds in accordance with prior studies of temporal summation (42). If a participant discontinued before the 10th pulse, the last pain rating was carried forward. After precedent (43,44), we characterized suprathreshold pain as the peak pain ratings obtained from the middle of the series of 10 phasic stimuli. On average, peak pain ratings occurred between the fourth and seventh pulses. Therefore, an average of the pain ratings after the fourth, fifth, sixth, and seventh pulses was calculated and used for analysis.
Temporal Summation of Mechanical Phasic Pain
Pain ratings were gathered in response, first, to a single stimulus and then after a sequence of 10 stimuli of identically weighted punctate noxious probes applied on a flat contact area of 0.2-mm diameter, separately, to the dorsal surface of the middle finger (nondominant) and the patella (index knee). The index knee was the knee with greater self-reported pain. If both knees had equal levels of pain, the “index knee” was chosen at random. The 10-stimulus train was delivered with an interstimulus interval of 1 second, guided by a metronome. Participants rated the stimuli on a numerical rating scale from 0 to 100. In contrast to the thermal phasic pain procedure, pain ratings for mechanical phasic pain were only obtained once after the initial probe and then once more after the 10-probe train (i.e., maximal pain experienced during the 10-stimulus train). An index was created by dividing the rating obtained after the 10th probe by the rating obtained after the initial probe. Separate indices were calculated for mechanical phasic pain at the finger and the patella. Similar procedures have been used to index temporal summation of mechanical pain in healthy participants (45) and patients with neuropathic pain (46,47).
Two separate weights were trialed: 256 and 512 mN. Both weights produced temporal summation across participants (i.e., higher ratings after the 10th stimulus compared with the first). To minimize the number of outcomes tested, we evaluated the variance in both indices and included the stimulus for which there was greater variance. We found that the 256-mN finger probe (σ2 finger = 23.85) produced greater variance in pain response across participants than the 512-mN finger probe (σ2 finger = 9.05), whereas the 512-mN patella probe (σ2 patella = 97.67) produced greater variance in pain response across participants than the 256-mN patella probe (σ2 patella = 72.58). Therefore, the analyses for this study included the index created from the pain ratings obtained in response to the 256-mN stimulus at the finger and the 512-mN stimulus at the patella.
Data Analytic Strategy
Our overarching goal was to evaluate the association of state versus stable measures of PA and NA with pain in patients with chronic KOA. The research design afforded two opportunities to measure the associations of pain and affect: a) in the course of daily life via diary reporting and b) in the laboratory in response to QST.
Stable Affect Analyses
In the current study, stable PA and NA were assessed by averaging daily reports over time within participants. The first set of models tested the association of stable PA and NA with daily pain. A mixed-models approach was used to account for dependencies in the data associated with the repeated assessment of the dependent variable, daily pain. First, separate models tested the Level 2 (i.e., between-person) effect of stable PA and stable NA, respectively, on daily pain. Next, both stable PA and stable NA were entered together in the same model, along with their interaction term. Age, race, and sex were covaried in all models. Following the methods described previously (48), we allowed the intercept to vary randomly across people and controlled for autoregression between observations by modeling an autoregressive 1 function in the variance/covariance matrix.
To evaluate the roles of stable PA and NA on response to QST, the diary-based stable PA and NA variables were entered as independent variables in separate multiple regression models predicting temporal summation of mechanical phasic pain at the finger and the patella, and suprathreshold thermal phasic pain at the forearm. Age, sex, and race were entered as covariates in all models. In addition, we controlled for the oppositely valenced state and trait affect in each model.
State Affect Analyses
Multilevel modeling was again used to evaluate the associations of state affects with daily clinical pain. Diary state PA and NA variables were computed by centering the daily scores around each individual’s mean across days, resulting in daily within-person deviations. This process, known as group-mean centering, is an essential component of measuring state-based variation in time-variant, nested data structures (16). Following the methods described in Singer and Willet (48) and Nezlek (49), we then tested a Level 1 (i.e., within-person) model in which daily pain was the dependent variable, state PA (or NA) was the independent variable, the intercept was allowed to randomly vary, and autoregression was controlled. To test the independence of the relation between one state affective valence and pain from that of the other affective valence, we centered the oppositely valenced affect around the grand mean and included it as a covariate.
Laboratory resting states PA and NA were operationalized as the baseline PA and NA reports, given by participants immediately before engaging in the series of QST tests. These variables were entered as predictors in separate multiple regressions, with the mechanical and thermal phasic pain variables entered as dependent variables and age, sex, and race entered as covariates. The oppositely valenced affect was also tested as a covariate in each model and was removed if not significant.
Insomnia diagnosis was tested as an interaction term with each affect predictor to determine if any effects were qualified by the presence of insomnia. All analyses were conducted in SPSS version 19 (IBM Corporation).
RESULTS
Sample Characteristics and Measure Descriptives
Table 1 displays the demographic characteristics of the sample. Table 2 presents the correlations between the primary study measures, as well as means and standard deviations. Notably, the correlation between stable PA from the diary and laboratory-assessed state PA (r = 0.40) was modest, as was the correlation between stable NA and laboratory-assessed state NA (r = 0.40). Also of note, daily pain was not significantly correlated with the phasic pain measures. Of the 79 participants for whom laboratory affect measures were administered before QST, 37 discontinued the 51° phasic pain procedure before the 10th pulse. These individuals were not significantly different from those who completed the thermal phasic pain task in stable PA (p = .20), state PA in the laboratory (p = .16), or mechanical phasic pain of the finger (p = .35) and patella (p = .45). However, they did report significantly greater stable NA (p < .001), state NA in the laboratory (p < .001), and clinical pain from the diary (p = .016)
TABLE 1.
Sample Demographics
| KOA (n = 36) | KOA-I (n = 115) | Total (n = 151) | |
|---|---|---|---|
| Age, y, M (SD) | 67.25 (9.99) | 59.34 (8.47) | 61.23 (9.45) |
| Sex | |||
| % Female | 52.8 | 73.0 | 68.2 |
| Race | |||
| % African American | 30.6 | 36.5 | 35.1 |
| % White | 66.7 | 60.0 | 6.16 |
| Education | |||
| % College graduate | 66.7 | 48.1 | 53.3 |
| Employment | |||
| % Employed | 38.9 | 41.2 | 40.7 |
| % Retired | 55.6 | 35.0 | 40.0 |
| % Unemployed | 0.0 | 17.4 | 13.3 |
| Household income | |||
| % >$50,000 | 51.5 | 46.0 | 47.4 |
KOA = knee osteoarthritis; KOA-I = knee osteoarthritis with comorbid insomnia; M = mean; SD = standard deviation.
TABLE 2.
Means and Correlations of Primary Measures
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | M (SD) | |
|---|---|---|---|---|---|---|---|---|---|
| Average daily PA (0–100) | 1 | −.63** | .49** | −.15 | −.23** | .13 | .08 | .04 | 65.41 (14.49) |
| Average daily NA (0–100) | 1 | −.40** | .40** | .40** | −.13 | −.04 | .05 | 25.80 (16.91) | |
| Laboratory PA (0–100) | 1 | −.40** | .04 | −.28* | .08 | −.08 | 74.54 (18.56) | ||
| Laboratory NA (0–100) | 1 | .20 | .02 | .004 | .11 | 12.49 (17.68) | |||
| Daily pain (0–100) | 1 | −.11 | −.13 | .14 | 43.83 (21.32) | ||||
| Mechanical QST, finger (index) | 1 | .09 | −.03 | 3.33 (4.88) | |||||
| Mechanical QST, patella (index) | 1 | .11 | 4.69 (9.88) | ||||||
| Thermal QST (0–100) | 1 | 64.43 (23.74) |
M = mean; SD = standard deviation; PA = positive affect; NA = negative affect; QST = quantitative sensory testing.
Associations of Stable Affect and Clinical Pain
The first set of models tested whether stable levels of PA and NA were associated with between-person differences in daily pain. Analyses revealed that stable PA (B = −.18, standard error [SE] = .11; t(145) = −1.70, p = .091) was not significantly associated with lower pain across diary days. In contrast, stable NA (B =.63, SE = .13; t(145) = 5.81, p < .001) was significantly associated with higher pain across diary days. These effects remained significant when controlling for stable PA. The results showed that a 1-unit increase in stable NA resulted in an approximately threefold greater change in daily pain than a 1-unit increase in stable PA. The interaction term of stable NA × stable PA was not significant (p = .96), indicating that effects at different levels of each affect were not qualified by levels of the oppositely valenced affect. Insomnia diagnosis did not moderate the effect of stable NA or PA on pain.
The pattern of findings for stable affects on daily clinical pain was also evident when WOMAC pain was the criterion in place of averaged daily pain. Stable PA was not significantly associated with WOMAC pain (B = −.11, SE = .07; t(143) = −.15, p = .13). In contrast, NA was significantly associated with higher WOMAC pain (B =.43, SE = .07; t(143) = 6.34, p < .001). The interaction of stable PA and NA on WOMAC pain was not significant (p = .15).
Associations of Stable Affect and Laboratory Pain
We next sought to determine if stable measures of PA and NA assessed in the diary predicted individual differences on mechanical and thermal phasic pain in the laboratory. Stable PA did not significantly predict temporal summation of mechanical pain at the finger (p = .21) or patella (p = .57), or suprathreshold thermal pain (p = .57). Stable NA did not significantly predict temporal summation of mechanical pain at the finger (p = .24) or patella (p = .94), or suprathreshold thermal pain (p = .16).
Associations of State Affect and Daily Clinical Pain
The next set of analyses evaluated the extent to which changes in daily state PA and state NA were associated with changes in daily pain. The first model yielded a significant main effect for state PA (B = −.11, SE = .02; t(1585) = −4.75, p < .001), indicating that when state PA was elevated relative to a participant’s mean, pain was reduced. When NA was added to the model as a covariate, the state PA effect remained significant (B = −.09, SE = .026; t(1581) = −3.58, p < .001), suggesting that the daily coupling between state PA and pain is independent of the association between NA and pain. Insomnia did not significantly moderate the state PA–pain effect (p = .45).
Next, we tested the extent to which changes in state NA were associated with daily pain across diary days. The basic model yielded a significant main effect (B =.07, SE = .03; t(1573) = 3.60, p < .001), indicating that on days when state NA was higher relative to a person’s mean, pain was also elevated. However, the addition of PA to the model as a covariate attenuated the state NA effect to nonsignificance (B =.04, SE = .02; t(1564) = 1.83, p = .068). Thus, daily within-person variation in NA was not significantly associated with variation in daily pain when the PA-pain relation was taken into account. As with the stable affects, the interaction term of state NA × state PA was not significant (p = .94), and insomnia diagnosis was not a significant moderator in this model (p = .28).
Associations of State Affect and Laboratory Pain
The final set of models tested the association of laboratory-measured state PA and NA with phasic pain in a subset of participants for whom laboratory affect measures were available. State PA significantly predicted lower temporal summation of mechanical phasic pain on the finger (B = −.05, SE = .02; t(67) = −20, p = .042; R2 = 0.08). This effect is graphically displayed in Figure 1.3 Insomnia diagnosis was not a significant moderator in this model (p = .54).
Figure 1.
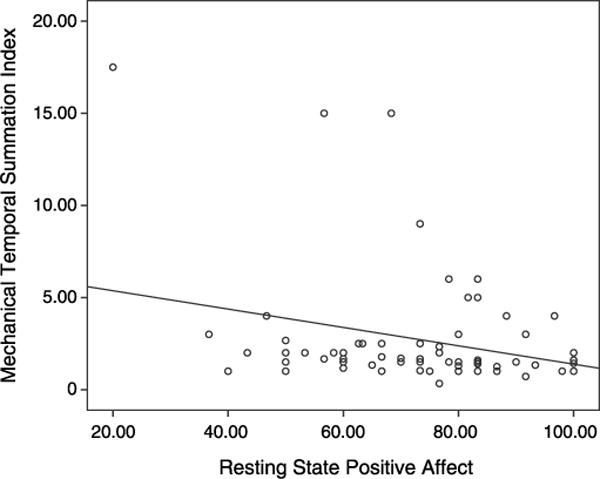
Laboratory state positive affect is associated with diminished temporal summation of mechanical phasic pain.
State PA did not predict significant differences in mechanical phasic pain on the patella (p = .85) and evidenced a trend toward predicting thermal phasic pain (B = −.25, SE = .14, t(69) = −1.8, p = .075) that was attenuated (p = .37) when NA was covaried. State NA did not predict significant differences in mechanical phasic pain on the finger (p = .46) or patella (p = .93). State NA alone predicted higher suprathreshold phasic pain (B =.34, SE = .15, t(69) = 2.22, p = .030), but this effect was nonsignificant when state PA was covaried (p = .13). The interaction of state PA × NA was not predictive of mechanical phasic pain on the finger (p = .13) or the patella (p = .20), as well as suprathreshold thermal phasic pain (p = .91).
DISCUSSION
The purpose of this study was to evaluate whether experiences of clinical and experimentally induced pain among patients with chronic KOA differed as a function of the temporal stability and valence of affect. In support of our hypotheses, results revealed that stable, trait-like NA was strongly associated with clinical pain, resulting in a threefold greater difference in pain reports than stable PA. In contrast, state-based measures of PA, but not NA, were associated with variance in both clinical and experimental phasic pain. Broadly, the results suggest that the temporal stability and valence of affect are important considerations in the assessment of individual differences in chronic pain.
The finding that stable NA was a more robust predictor of clinical osteoarthritis pain than stable PA supports previous findings from a daily diary study that included a sample of patients with rheumatoid arthritis (5). When stable indicators of affect and clinical pain are derived from concurrent assessments from a daily diary averaged across days, it could be argued that such an association is merely an artifact of measurement, whereby the reporting of an aversive painful experience is certain to couple with the affective evaluation of that state of aversion over time. However, we extended the diary clinical pain finding by demonstrating the same pattern of results for the associations of stable affects and WOMAC pain, a reliable, disease-specific measure of clinical pain assessed in this study at a single time point, separate from the diary pain assessment. Together, the findings suggest that a stable negative affective style is more strongly associated with clinical pain than a stable positive affective style. These findings may be relevant to the assessment of patient characteristics in a clinical setting and may help clinicians better understand which patients could benefit from closer follow-up and, perhaps, a broader array of prevention and/or treatment options as KOA disease progresses.
Departing from the general premise that negative is always stronger than positive (1,2), we found that daily variation in state PA was more strongly associated with daily clinical pain than state NA. Owing to the temporal dynamics of state-based fluctuations in affect and pain in the flow of daily life, it is understandable that effects observed for stable affects do not always translate to the state level. The examination of within-person microlongitudinal changes in affect and pain introduces daily processes not observable in an aggregate analysis, such as affective gains and losses relative to an individual’s typical level. PA is generally higher and more variable than NA (50,51) and has been shown to buffer against the situational impact of stress and NA on pain (6,52). A loss of PA, then, should leave the individual vulnerable to an increase in pain irrespective of NA level, whereas a gain in PA should protect against an increase in pain when NA is high. Thus, a daily change in PA may be altogether more destabilizing to the individual than a change in NA, a process that would be reflected in the greater amount of variance in daily pain accounted for by state PA relative to state NA.
These findings are not without precedent. Watson (53), for example, found that trait NA assessed with the Multidimensional Personality Questionnaire was a much stronger predictor of self-reported health complaints among college students than trait PA. State PA and state NA, however, were similarly predictive of health complaints (53). Early arguments for the consistent association of NA with negative health outcomes rested on the belief that people with higher NA were more likely to complain about poor health, rather than actually be in poorer health, than those lower in NA (54). Such an argument is fundamentally a trait-based approach to understanding the association of NA and health. Although we did not explicitly distinguish in the present study between somaticization and physiological measures of health, we did observe that stable, trait-like NA styles were strongly associated with subjective clinical pain complaints, but not responses to controlled nociceptive stimuli. In contrast, our state affect analyses revealed a different pattern of affect-pain associations that shed light on the importance of assessing within-person changes over time. Although NA and pain are expected to be highly correlated when assessed near a single time point, our analyses revealed that the daily within-person change in NA had a relatively weak influence on the fluctuation of pain states.
Our dimensional approach with diary data was also partly supported in analyses involving QST. Importantly, state PA, but not state NA, predicted diminished temporal summation of mechanical phasic pain on the finger. The phasic stimuli chosen for analysis induced temporal summation across participants, a phenomenon thought to be associated with central sensitization (31,32). The consistency of the findings across measurement modalities (i.e., diary and laboratory), to some degree, supports the ecological validity of our diary and laboratory findings. However, these results should be considered preliminary because they were conducted with a reduced sample and were not consistent across QST modalities. Furthermore, they cannot be seen as a “laboratory replication” per se because the state affect measures were from a single time point, rather than a deviation from an individual’s mean. These caveats notwithstanding, this is the first study, to our knowledge, to report parallel findings from the laboratory and the field on specific dimensions of affect and pain. That the laboratory findings were observed on measures shown previously to be sensitive to the summation of pain suggests that elevated state PA may protect against the neuronal cascade evoked by repeated nociceptive stimulation.
These findings have potentially important implications for our understanding of how some individuals with chronic pain are psychologically resilient to the pathophysiological progression of disease. They suggest that short-term increases in PA may be effective in damping the persistence of pain by reducing the sensitization gating of central pain processing pathways. From a biopsychosocial perspective (55), an accumulation of such PA-pain effects on a daily basis may attenuate one’s vulnerability to the development of depression secondary to chronic pain. Clinically, it may be useful to teach patients how to recognize daily PA-pain contingencies such as instances when increases in PA precede decreases in pain. Such self-regulatory knowledge may be a potentially powerful tool in the effort to promote a greater sense of pain control and improve chronic pain outcomes. The development of positive affective resources promotes prosocial behavior by fostering an interest in helping others (56,57) and reduces vulnerability to anhedonia and depression (58,59), perhaps by improving coping efforts (60). By targeting PA-pain contingencies and encouraging the development of positive affective resources, clinicians may help patients understand their inherent sources of resilience to chronic pain so that they may flourish in the face of adversity (61–63).
Future studies should include a broader assessment of positive affective functioning during QST. For example, it would be interesting to know if hedonic capacity (e.g., Gard et al. (64)), motivation, and reward responsiveness (e.g., Pizzagalli et al. (65)) are related to central pain facilitation because these components of the broader endogenous reward system cannot be extracted from the brief emotion–based measures used in this study.
Limitations
This study was limited in several respects. First, given prior evidence that PA buffers the association of NA and pain (6,66), it was surprising that the interaction between PA and NA did not yield significant results in any diary model. It is possible that the relatively short diary assessment period (i.e., 2 weeks) limited our ability to find more complex contingent relations revealed through moderation analyses.
A second limitation is that the laboratory state affect measures were only administered to a portion of the sample and were secondary to the parent study’s primary aims. Unfortunately, a more comprehensive affect battery is not available. As a result, we may have been underpowered to detect true associations between state affect and laboratory pain sensitivity. Thus, the laboratory state affect findings should be considered preliminary until they are replicated in a study designed specifically to evaluate the dynamics of affect and pain across multiple methodological modalities.
The high rate of comorbid insomnia in the sample (76%) is also a potential limit to the generality of the findings. This limitation, however, is tempered by the fact that as many as 58% of patients with osteoarthritis experience insomnia symptoms at least 3 days per week (67), and greater than 80% of patients across chronic pain disorders report at least one insomnia symptom (68).
Finally, the diary measures of PA and NA lack formal psychometric data. As is common in diary studies, the number of items administered must be kept at a level that will promote daily adherence. For that reason, we could only select a subset of items from the PANAS and POMS-Bipolar measures with face validity for PA and NA, rather than administer a full validated scale. Although our diary scales have not been psychometrically validated, they did evidence excellent internal consistency within valence and were moderately correlated between valences, suggesting they were related but not identical constructs.
SUMMARY AND CONCLUSIONS
This study offers three principle findings. First, stable NA was a stronger predictor of clinical KOA pain than stable PA. Second, time-variant state PA was a stronger predictor of concurrent daily clinical pain than state NA. Third, resting state PA but not state NA predicted diminished temporal summation of mechanical phasic pain. These findings advance our understanding of the relations between affect and pain in KOA and provide novel preliminary evidence that dynamic affect-pain processes observed in the field may reflect individual differences in central pain facilitation.
Acknowledgments
Source of Funding and Conflicts of Interest: Funding for work on the current study was provided by NIH R01 AR05487 (M.T.S.), NIH R01AR059410 (M.T.S.), and NIH T32 NS070201 (P.H.F.).
Abbreviations
- NA
negative affect
- PA
positive affect
- KOA
knee osteoarthritis
- QST
quantitative sensory testing
- WOMAC
Western Ontario MacMaster Universities Arthritis Index.
Footnotes
Morning diaries of sleep-related variables were also completed. These variables were outside the scope of the current analyses and therefore are not described here.
Three separate temperatures were administered: 49°C, 51°C, and a tailored temperature set to the lowest temperature between 40°C and 51°C at which the participant rated pain greater than or equal to 35 on the 0 to 100 scale. Preliminary analyses revealed that, across participants, only the 51°C temperature resulted in an increase in pain ratings across the 10 heat pulse train (i.e., temporal summation), whereas the other temperatures resulted in a decrease in ratings across pulses. Therefore, we chose to only include the 51°C trial in the present analyses.
Inspection of the scatterplot raised concerns that outliers were driving the observed effect. The mean of the mechanical temporal summation index across participants was 2.74, and 80% of the observations were below 3. Therefore, to investigate how heavily they influenced the results, we reran the analysis, excluding cases in which temporal summation index was greater than 3. In this smaller sample, the effect was still evident (R2 = 0.04) but was slightly attenuated to a statistical trend (p = .11). We then reran the analysis excluding cases in which the temporal summation index was less than 3. In this sub-sample at the higher end of the temporal summation range (n = 11), the influence of positive affect on temporal summation was evident, was statistically significant (p = .010), and had a much larger effect size (R2 = 0.48). Together, these supplemental analyses support the overall interpretation that state PA is associated with diminished mechanical temporal summation, but raise the question of whether the magnitude of the effect differs at extreme ends of the temporal summation spectrum.
The authors declare no conflicts of interest.
Contributor Information
Patrick H. Finan, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Phillip J. Quartana, Department of Behavioral Biology, Walter Reed Army Institute of Research, Silver Spring, Maryland.
Michael T. Smith, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland.
References
- 1.Baumeister RF, Bratlavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev Gen Psychol. 2001;5:323–70. [Google Scholar]
- 2.Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: the negativity bias in evaluative categorizations. J Pers Soc Psychol. 1998;75:887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- 3.Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2009:CD007407. doi: 10.1002/14651858.CD007407.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Smith BW, Zautra AJ. Vulnerability and resilience in women with arthritis: test of a two-factor model. J Consult Clin Psychol. 2008;76:799–810. doi: 10.1037/0022-006X.76.5.799. [DOI] [PubMed] [Google Scholar]
- 5.Strand EB, Kerns RD, Christie A, Haavik-Nilsen K, Klokkerud M, Finset A. Higher levels of pain readiness to change and more positive affect reduce pain reports—a weekly assessment study on arthritis patients. Pain. 2007;127:204–13. doi: 10.1016/j.pain.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol. 2005;73:212–20. doi: 10.1037/0022-006X.73.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 8.Litt MD, Shafer D, Napolitano C. Momentary mood and coping processes in TMD pain. Health Psychol. 2004;23:354–62. doi: 10.1037/0278-6133.23.4.354. [DOI] [PubMed] [Google Scholar]
- 9.Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- 10.Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:85–9. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- 11.Okun MA, Melichar JF, Hill MD. Negative daily events, positive and negative social ties, and psychological distress among older adults. Gerontologist. 1990;30:193–9. doi: 10.1093/geront/30.2.193. [DOI] [PubMed] [Google Scholar]
- 12.Smith BW, Zautra AJ. The role of personality in exposure and reactivity to interpersonal stress in relation to arthritis disease activity and negative affect in women. Health Psychol. 2002;21:81–8. [PubMed] [Google Scholar]
- 13.Kenny DA, Zautra A. The trait-state-error model for multiwave data. J Consult Clin Psychol. 1995;63:52–9. doi: 10.1037//0022-006x.63.1.52. [DOI] [PubMed] [Google Scholar]
- 14.Isen AM. Positive affect. In: Dalgleish T, Power M, editors. Handbook of Cognition and Emotion. New York: Wiley; 1999. pp. 521–39. [Google Scholar]
- 15.Affleck G, Zautra A, Tennen H, Armeli S. Multilevel daily process designs for consulting and clinical psychology: a preface for the perplexed. J Consult Clin Psychol. 1999;67:746–54. doi: 10.1037//0022-006x.67.5.746. [DOI] [PubMed] [Google Scholar]
- 16.Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12:121–38. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- 17.Zautra A, Smith B, Affleck G, Tennen H. Examinations of chronic pain and affect relationships: applications of a dynamic model of affect. J Consult Clin Psychol. 2001;69:786–95. doi: 10.1037//0022-006x.69.5.786. [DOI] [PubMed] [Google Scholar]
- 18.Tversky A, Kahneman D. Availability: A heuristic for judging frequency and probability. Cognit Psychol. 1973;5:207–32. [Google Scholar]
- 19.Redelmeier DA, Katz J, Kahneman D. Memories of colonoscopy: a randomized trial. Pain. 2003;104:187–94. doi: 10.1016/s0304-3959(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 20.Stone AA, Broderick JE, Kaell AT, DelesPaul PA, Porter LE. Does the peak-end phenomenon observed in laboratory pain studies apply to real-world pain in rheumatoid arthritics? J Pain. 2000;1:212–7. doi: 10.1054/jpai.2000.7568. [DOI] [PubMed] [Google Scholar]
- 21.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 22.Litt MD, Shafer DM, Ibanez CR, Kreutzer DL, Tawfik-Yonkers Z. Momentary pain and coping in temporomandibular disorder pain: exploring mechanisms of cognitive behavioral treatment for chronic pain. Pain. 2009;145:160–8. doi: 10.1016/j.pain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moskowitz JT, Epel ES, Acree M. Positive affect uniquely predicts lower risk of mortality in people with diabetes. Health Psychol. 2008;27(Suppl 1):S73–82. doi: 10.1037/0278-6133.27.1.S73. [DOI] [PubMed] [Google Scholar]
- 24.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131:925–71. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 25.Craggs JG, Staud R, Robinson ME, Perlstein WM, Price DD. Effective connectivity among brain regions associated with slow temporal summation of C-fiber–evoked pain in fibromyalgia patients and healthy controls. J Pain. 2012;13:390–400. doi: 10.1016/j.jpain.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129:130–42. doi: 10.1016/j.pain.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12:1078–89. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finan PH, Zautra AJ, Davis MC. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosom Med. 2009;71:474–82. doi: 10.1097/PSY.0b013e31819e0a8b. [DOI] [PubMed] [Google Scholar]
- 29.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–81. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Obermann M, Pleger B, de GA, Stude P, Kaube H, Diener HC, Katsarava Z. Temporal summation of trigeminal pain in human anterior cingulate cortex. Neuroimage. 2009;46:193–200. doi: 10.1016/j.neuroimage.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 31.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–75. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 32.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 33.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 34.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–26. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 36.Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded Form. Ames: The University of Iowa; 1994. [Google Scholar]
- 37.Lorr M, McNair DM. Profile of Mood States: Bipolar Form. San Diego: Educational and Industrial Testing Service; 1988. [Google Scholar]
- 38.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 39.Dunbar MJ, Robertsson O, Ryd L, Lidgren L. Appropriate questionnaires for knee arthroplasty. Results of a survey of 3600 patients from The Swedish Knee Arthroplasty Registry. J Bone Joint Surg Br. 2001;83:339–44. doi: 10.1302/0301-620x.83b3.11134. [DOI] [PubMed] [Google Scholar]
- 40.McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum. 2001;45:453–61. doi: 10.1002/1529-0131(200110)45:5<453::aid-art365>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 41.Vierck CJ, Jr, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 42.Edwards RR, Smith MT, Stonerock GL, Haythornthwaite J. Pain-related catastrophizing in healthy women is associated with greater temproal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22:730–7. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 43.Valencia C, Fillingim RB, George SZ. Suprathreshold heat pain response is associated with clinical pain intensity for patients with shoulder pain. J Pain. 2011;12:133–40. doi: 10.1016/j.jpain.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valencia C, Kindler LL, Fillingim RB, George SZ. Investigation of central pain processing in shoulder pain: converging results from 2 musculoskeletal pain models. J Pain. 2012;13:81–9. doi: 10.1016/j.jpain.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magerl W, Wilk SH, Treede RD. Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. Pain. 1998;74:257–68. doi: 10.1016/s0304-3959(97)00177-2. [DOI] [PubMed] [Google Scholar]
- 46.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–43. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 47.Vase L, Nikolajsen L, Christensen B, Egsgaard LL, Arendt-Nielsen L, Svensson P, Staehelin JT. Cognitive-emotional sensitization contributes to wind-up-like pain in phantom limb pain patients. Pain. 2011;152:157–62. doi: 10.1016/j.pain.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 49.Nezlek JB. Multilevel random coefficient analyses of event- and interval-contingent data in social and personality psychology research. Pers Soc Psychol Bull. 2001;27:771–85. [Google Scholar]
- 50.David JP, Green PJ, Martin R, Suls J. Differential roles of neuroticism, extraversion, and event desirability for mood in daily life: an integrative model of top-down and bottom-up influences. J Pers Soc Psychol. 1997;73:149–59. doi: 10.1037//0022-3514.73.1.149. [DOI] [PubMed] [Google Scholar]
- 51.Zautra AJ, Affleck GG, Tennen H, Reich JW, Davis MC. Dynamic approaches to emotions and stress in everyday life: Bolger and Zuckerman reloaded with positive as well as negative affects. J Pers. 2005;73:1511–38. doi: 10.1111/j.0022-3506.2005.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis MC, Zautra AJ, Smith BW. Chronic pain, stress, and the dynamics of affective differentiation. J Pers. 2004;72:1133–59. doi: 10.1111/j.1467-6494.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson D. Intraindividual and interindividual analyses of positive and negative affect: their relation to health complaints, perceived stress, and daily activities. J Pers Soc Psychol. 1988;54:1020–30. doi: 10.1037//0022-3514.54.6.1020. [DOI] [PubMed] [Google Scholar]
- 54.Watson D, Pennebaker JW. Health complaints, stress, and distress: exploring the central role of negative affectivity. Psychol Rev. 1989;96:234–54. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- 55.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 56.Diener E, Seligman ME. Very happy people. Psychol Sci. 2002;13:81–4. doi: 10.1111/1467-9280.00415. [DOI] [PubMed] [Google Scholar]
- 57.Lyubomirsky S, King L, Diener E. The benefits of frequent positive affect: does happiness lead to success? Psychol Bull. 2005;131:803–55. doi: 10.1037/0033-2909.131.6.803. [DOI] [PubMed] [Google Scholar]
- 58.Folkman S, Moskowitz JT. Positive affect and the other side of coping. Am Psychol. 2000;55:647–54. doi: 10.1037//0003-066x.55.6.647. [DOI] [PubMed] [Google Scholar]
- 59.Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001 J Pers Soc Psychol. 2003;84:365–76. doi: 10.1037//0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ben-Zur H. Coping syles and affect. Int J Stress Manag. 2009;16:87–101. [Google Scholar]
- 61.Aspinwall LG. Rethinking the role of positive affect in self-regulation. Motiv Emotion. 1998;22:1–32. [Google Scholar]
- 62.Fredrickson BL, Losada MF. Positive affect and the complex dynamics of human flourishing. Am Psychol. 2005;60:678–86. doi: 10.1037/0003-066X.60.7.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilbert DT, Pinel EC, Wilson TD, Blumberg SJ, Wheatley TP. Immune neglect: a source of durability bias in affective forecasting. J Pers Soc Psychol. 1998;75:617–38. doi: 10.1037//0022-3514.75.3.617. [DOI] [PubMed] [Google Scholar]
- 64.Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Pers. 2006;40:1086–102. [Google Scholar]
- 65.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strand EB, Zautra AJ, Thoresen M, Odegard S, Uhlig T, Finset A. Positive affect as a factor of resilience in the pain–negative affect relationship in patients with rheumatoid arthritis. J Psychosom Res. 2006;60:477–84. doi: 10.1016/j.jpsychores.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 67.Wilcox S, Brenes GA, Levine D, Sevick MA, Shumaker SA, Craven T. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Am Geriatr Soc. 2000;48:1241–51. doi: 10.1111/j.1532-5415.2000.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 68.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature Sleep Med Rev. 2004;8:119–32. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]


