Abstract
Background/Objectives:
The possible relationship between smoking and risk of colonic diverticulosis has been suggested by recent epidemiological studies, although the results were inconsistent. This meta-analysis was conducted to summarize all available data.
Methods:
A comprehensive literature review was conducted using the MEDLINE and EMBASE databases through May 2017 to identify all studies that compared the risk of colonic diverticulosis among current and former smokers versus nonsmokers. Effect estimates from each study were extracted and combined together using the random-effect, generic inverse variance method of DerSimonian and Laird.
Results:
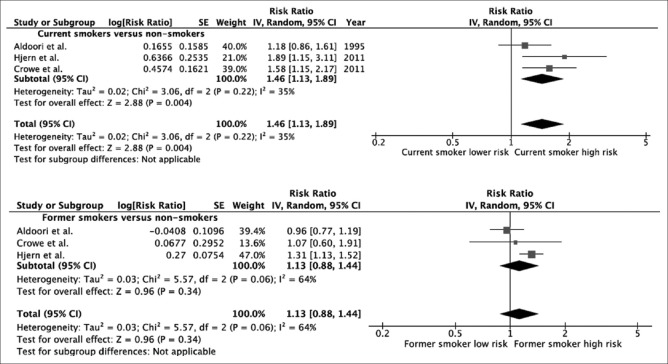
Of 465 potentially eligible articles, three prospective cohort studies with 130,520 participants met the eligibility criteria and were included in the meta-analysis. The risk of colonic diverticulosis in current smokers was significantly higher than nonsmokers with the pooled risks ratio of 1.46 (95% confidence interval [CI], 1.13–1.89). However, the risk of colonic diverticulosis in former smokers was not significantly higher than nonsmokers with the pooled risk ratio of 1.13 (95% CI, 0.88–1.44).
Conclusions:
A significantly increased risk of colonic diverticulosis among current smokers is demonstrated in this study.
KEY WORDS: Diverticular disease, diverticulosis, meta-analysis, obesity, overweight
Introduction
Colonic diverticula are sac-like structures protruding through the defect of circular muscle in the colonic wall. They are usually detected by colonoscopy.[1] The prevalence of colonic diverticulosis increases with age, affecting approximately 50% of population at the age of 60 years and 70% population at the age of 80 years.[2,3] Lifestyle and environmental factors play an important role in the development of colonic diverticulosis. Known risk factors include obesity, low-fiber diet, and lack of exercise.[4,5,6]
Smoking is one of the leading preventable causes of mortality in the United States and worldwide. Studies have showed that smoking increases the risk of several gastrointestinal diseases such as Crohn's disease, peptic ulcer, and gastroesophageal reflux disease.[7,8,9,10] Interestingly, some epidemiologic studies have also suggested a possible relationship between smoking and colonic diverticulosis, although the results were inconsistent.[5,11,12] This systematic review and meta-analysis were conducted to summarize all available studies to better characterize the relationship.
Methods
Information sources and search strategy
A systematic literature search was conducted using EMBASE and MEDLINE database from inception to May 2017 to identify all original studies that investigated the association between smoking and the risk of colonic diverticulosis. The systematic literature review was independently conducted by three investigators (K.W., B.B., and P.U.) using the search strategy that included the terms for “smoking” and “colonic diverticulosis” as described in [Online Supplementary Data 1 (171KB, pdf) available online]. A manual search for additional potentially relevant studies using references of the included articles was also performed. No language limitation was applied. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement which is provided as [Online Supplementary Data 2 (202.4KB, pdf) available online].
Search Strategy
PRISMA 2009 Checklist
Selection criteria
Eligible studies must be case–control, cross-sectional, or cohort studies that investigated the association between smoking and colonic diverticulosis. They must provide the effect estimates (odds ratios [ORs], relative risks [RRs], hazard ratios, or standardized incidence ratio) with 95% confidence intervals (CI). Inclusion was not restricted by the study size. When more than one article using the same database/cohort was available, the study with the most comprehensive data/analyses was included.
Retrieved articles were independently reviewed for their eligibility by the same three investigators. The discrepancy was resolved by conference with all investigators. Newcastle–Ottawa quality assessment scale was used to appraise the quality of study in three areas including the recruitment of cases and controls, the comparability between the two groups, and the ascertainment of the outcome of interest for cohort study and the exposure for case–control study.[13] Score of more than or equal to 7 was considered as of adequate quality.[14] The modified Newcastle–Ottawa scale as described by Herzog et al. was used for cross-sectional study.[15]
Data abstraction
A structured data collection form was used to extract the following data from each study: title of the study, publication year, name of the first author, year when the study was conducted, country where the study was conducted, number of participants, demographic data of participants, definition of obesity, methods used to identify colonic diverticulosis, adjusted effect estimates with 95% CI, and covariates that were adjusted in the multivariable analysis.
To ensure the accuracy, this data extraction process was independently performed by two investigators (K.W. and B. B.) and was reviewed by the senior investigator (P.U.).
Statistical analysis
We used the Review Manager 5.3 software from the Cochrane Collaboration (London, UK) for the data analysis. Adjusted point estimates from each study were combined by the generic inverse variance method of DerSimonian and Laird, which assigned the weight of each study in the pooled analysis inversely to its variance.[16] As the outcome interest was relatively uncommon, we planned to use OR of case–control study and cross-sectional study as an estimate for RR to calculate the pooled effect estimate with cohort study. Considering the high likelihood of between-study variance because of different study designs and underlying populations, a random-effect model was used. Cochran's Q-test and I2 statistic were used to determine the between-study heterogeneity. A value of I2 of 0%–25% represents insignificant heterogeneity, 26-50% represents low heterogeneity, 51%–75% represents moderate heterogeneity, and more than 75% represents high heterogeneity.[17]
We planned to use funnel plot to assess the presence of publication bias if we have a sufficient number of included studies.
Results
A total of 465 potentially eligible articles were identified using our search strategy (140 articles from Medline and 325 articles from EMBASE). After the exclusion of 123 duplicated articles, 342 articles underwent title and abstract review. Three hundred and twenty-eight articles were excluded at this stage since they were case reports, correspondences, review articles, in vitro studies, animal studies or interventional studies, and leaving 14 articles for full-text review. Nine of them were excluded after the full-length review as they did not report the outcome of interest while two articles were excluded since they were descriptive studies without comparative analysis. Three prospective cohort studies with 130,520 participants met the eligibility criteria[5,11,12] and were included in the meta-analysis. The literature review and selection process are shown in Figure 1. The characteristics and quality assessment of the studies are presented in Table 1. The inter-rater agreement for the quality assessment was high with the kappa statistics of 0.80.
Figure 1.
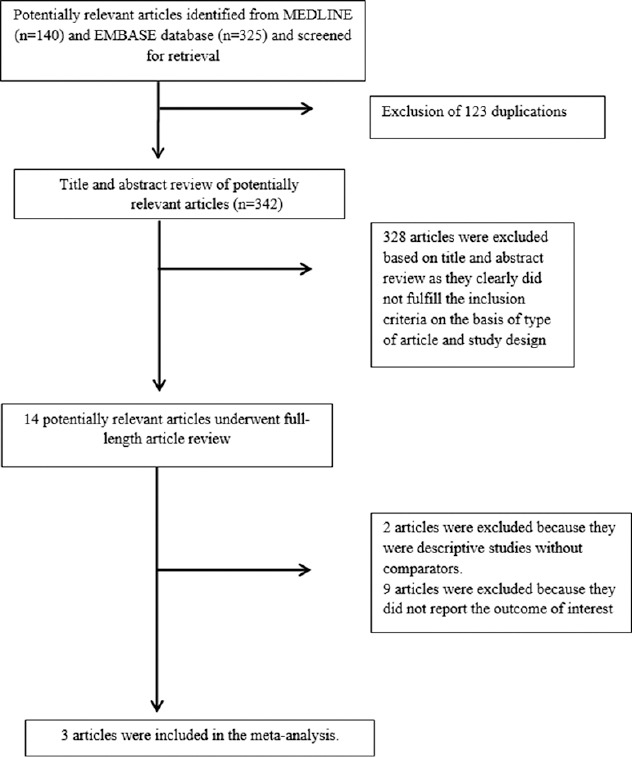
Literature review process
Table 1.
Characteristics of studies included
| Study | Aldoori et al.[11] | Crowe et al.[12] | Hjern et al.[5] |
|---|---|---|---|
| Country | United States | United Kingdom | Sweden |
| Study design | Prospective cohort | Prospective cohort | Prospective cohort |
| Year of publication | 1995 | 2011 | 2011 |
| Participants | This study utilized the resource of the health professionals follow-up study which recruited United States male health professionals aged 40-75 years in 1986. History of smoking was obtained at the entrance of cohort | This study utilized the resource of the EPIC-Oxford cohort, the recruited adult participants across the United Kingdom between 1993 and 1999. History of smoking was obtained at the entrance of cohort | This study utilized the Swedish mammography cohort which recruited women from central Sweden who were aged 40-75 years between 1987 and 1990 |
| Follow-up | The follow-up was done through questionnaires every 2 years until 1992 | The follow-up was done through medical record review until September 30, 2009 | The follow-up was done through medical record review until December 31, 2009 |
| Assessment of smoking status | Self-report through questionnaire | Self-report through questionnaire | Self-report through questionnaire |
| Diagnosis of diverticulosis | Self-report through follow-up questionnaires (medical records of sample participants were reviewed, diagnosis confirmed in 95%) | Review of the National Health Service database for the presence of diagnostic codes for diverticulosis | Review of the Swedish patient register database for the presence of diagnostic codes for diverticulosis |
| Number of participants | 47,678 | 47,033 | 35,809 |
| Mean age of participants in years | Not available | Not available | Nonsmokers: 62.0 Current smokers: 59.2 Former smokers: 59.2 |
| Percentage of female | 0 | 76 | 100 |
| Confounder adjustment | Age, physical activity, energy-adjusted dietary fiber, and energy-adjusted total fat | Sex, method of recruitment, and region of residence | Age, intake of dietary fiber, diabetes, hypertension, medications, alcohol, body mass index, physical activity, and educational level |
| Quality assessment (Newcastle=Ottawa scale) | Selection: 3 Comparability: 2 Outcome: 2 | Selection: 3 Comparability: 2 Outcome: 3 | Selection: 3 Comparability: 2 Outcome: 3 |
We found a significantly increased risk of colonic diverticulosis among current smokers compared with nonsmokers with the pooled RR of 1.46 (95% CI, 1.13–1.89). The heterogeneity between studies of the overall analysis was low with an I2 of 35%. The risk of colonic diverticulosis in former smokers was not significantly higher than nonsmokers with the pooled risk ratio of 1.13 (95% CI, 0.88–1.44, I2 = 64%). Forest plots of both analyses are shown as Figure 2.
Figure 2.
Forest plot of this meta-analysis
Evaluation for publication bias
We did not perform the evaluation for publication bias as the number of studies included in the meta-analysis was too small.[18]
Discussion
This is the first systematic review and meta-analysis that summarizes all available studies on the relationship between smoking and the risk of colonic diverticulosis. We found an approximately 1.4-fold increased risk of colonic diverticulosis among current smokers compared to nonsmokers. The risk was attenuated and did not reach statistical significance for former smokers.
The reason behind this association is not clear. Cigarette smoking is often associated with other poor health habits including lack of exercise and low-fiber diet,[19,20] which are predisposing factors for colonic diverticulosis. Thus, it is possible that the apparent association is not causal and is a result of confounding effect. On the other hand, nicotine in cigarette is known to cause relaxation of smooth muscle in gastrointestinal tract through the release of nitric oxide.[21] In fact, studies have demonstrated the effect of nicotine to reduce tone and activity of smooth muscle in sigmoid colon, resulting in reduction of the colonic contraction.[21,22] This effect of nicotine may predispose smokers to development of diverticulosis.
Although the literature review process of this meta-analysis was robust and the quality of the included prospective cohort studies was high, we acknowledge that the study has some limitations. Thus, the results should be interpreted with caution.
First, the number of included studies was quite small which may undermine the validity our results. We also could not evaluate our study for publication bias due to the small number of included studies and publication bias in favor of positive studies might have been present. Second, the primary studies included in this meta-analysis were conducted in 2 regions (The United States and Europe). Therefore, the results might not be generalizable to other populations. Third, all included studies used self-reported questionnaire to determine smoking status which may have a lower accuracy compared to other methods such as structured interview. Finally, subgroup analysis based on sex could be helpful in this meta-analysis as sex distribution varied considerably across the primary studies which may affect the homogeneity of the effect estimates. Unfortunately, such analysis could not be performed as the primary studies did not provide sex-specific data. Nonetheless, the statistical heterogeneity was not high in both analyses (low in current smokers versus nonsmokers analysis and moderate in former smokers versus nonsmokers analysis).
Conclusion
This study may suggest an increased risk of colonic diverticulosis among current smokers compared to nonsmokers. Whether this association is causal or is a result of confounding effect requires further investigations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Online Supplementary Data's
Online Supplementary Data 1 (171KB, pdf) : Search strategy
Online Supplementary Data 2 (202.4KB, pdf) : PRISMA checklist
References
- 1.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: Lower gastrointestinal diseases. Gastroenterology. 2009;136:741–54. doi: 10.1053/j.gastro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Painter NS, Burkitt DP. Diverticular disease of the colon, a 20th century problem. Clin Gastroenterol. 1975;4:3–21. [PubMed] [Google Scholar]
- 3.Peery AF, Barrett PR, Park D, Rogers AJ, Galanko JA, Martin CF, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142:266–720. doi: 10.1053/j.gastro.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dore MP, Pes GM, Marras G, Soro S, Rocchi C, Loria MF, et al. Risk factors associated with colonic diverticulosis among patients from a defined geographic area. Tech Coloproctol. 2016;20:177–83. doi: 10.1007/s10151-015-1401-7. [DOI] [PubMed] [Google Scholar]
- 5.Hjern F, Wolk A, Håkansson N. Smoking and the risk of diverticular disease in women. Br J Surg. 2011;98:997–1002. doi: 10.1002/bjs.7477. [DOI] [PubMed] [Google Scholar]
- 6.Peery AF, Keku TO, Martin CF, Eluri S, Runge T, Galanko JA, et al. Distribution and characteristics of colonic diverticula in a United States screening population. Clin Gastroenterol Hepatol. 2016;14:980–50. doi: 10.1016/j.cgh.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breuer-Katschinski BD, Holländer N, Goebell H. Effect of cigarette smoking on the course of Crohn's disease. Eur J Gastroenterol Hepatol. 1996;8:225–8. doi: 10.1097/00042737-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Kohata Y, Fujiwara Y, Watanabe T, Kobayashi M, Takemoto Y, Kamata N, et al. Long-term benefits of smoking cessation on gastroesophageal reflux disease and health-related quality of life. PLoS One. 2016;11:e0147860. doi: 10.1371/journal.pone.0147860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuzaki J, Suzuki H, Kobayakawa M, Inadomi JM, Takayama M, Makino K, et al. Association of visceral fat area, smoking, and alcohol consumption with reflux esophagitis and barrett's esophagus in Japan. PLoS One. 2015;10:e0133865. doi: 10.1371/journal.pone.0133865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: The adverse effects of tobacco smoking on the natural history of Crohn's disease. Aliment Pharmacol Ther. 2016;43:549–61. doi: 10.1111/apt.13511. [DOI] [PubMed] [Google Scholar]
- 11.Aldoori WH, Giovannucci EL, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, et al. Prospective study of physical activity and the risk of symptomatic diverticular disease in men. Gut. 1995;36:276–82. doi: 10.1136/gut.36.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe FL, Appleby PN, Allen NE, Key TJ. Diet and risk of diverticular disease in oxford cohort of European prospective investigation into cancer and nutrition (EPIC): Prospective study of British vegetarians and non-vegetarians. BMJ. 2011;343:d4131. doi: 10.1136/bmj.d4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.McPheeters ML, Peterson NB, Idowu RT, Jerome RN, Potter SA, Andrews JC. Newcastle-Ottawa Scale (N-O-S) for Observational Studies (e.g., cohort studies and case control studies) Evid Rep Technol Assess (Full Rep): Vanderbilt University Evidence-Based Practice Center; August. 2012 [Google Scholar]
- 15.Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á, et al. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes. A systematic review? BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannah R, Rothstein AJ, Borenstein M. New Jersey: John Wiley & Sons; 2005. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. [Google Scholar]
- 19.Al-Tannir MA, Kobrosly SY, Elbakri NK, Abu-Shaheen AK. Prevalence and predictors of physical exercise among nurses. A cross-sectional study. Saudi Med J. 2017;38:209–12. doi: 10.15537/smj.2017.2.15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Brook JS, Leukefeld CG, De La Rosa M, Brook DW. Lack of preventive health behaviors in the early forties: The role of earlier trajectories of cigarette smoking from adolescence to adulthood. Subst Use Misuse. 2017;52:1527–37. doi: 10.1080/10826084.2017.1281310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green JT, McKirdy HC, Rhodes J, Thomas GA, Evans BK. Intra-luminal nicotine reduces smooth muscle tone and contractile activity in the distal large bowel. Eur J Gastroenterol Hepatol. 1999;11:1299–304. doi: 10.1097/00042737-199911000-00020. [DOI] [PubMed] [Google Scholar]
- 22.McKirdy HC, Richardson CE, Green JT, Rhodes J, Williams GT, Marshall RW, et al. Differential effect of nitric oxide synthase inhibition on sigmoid colon longitudinal and circular muscle responses to nicotine and nerve stimulation in vitro . Br J Surg. 2004;91:229–34. doi: 10.1002/bjs.4395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search Strategy
PRISMA 2009 Checklist