Abstract
Background:
Currently, the standard protocol regarding the performance of procedures on patients receiving or having recently received isotretinoin (13-cis-retinoic acid) states that the procedures should not be performed. The recommendations in standard books and drug insert require discontinuation of isotretinoin for 6 months before performing cosmetic procedures, including waxing, dermabrasion, chemical peels, laser procedures, or incisional and excisional cold-steel surgery. These recommendations have been followed for over two decades despite little evidence for the stated increased risk of scarring.
Objective:
The Association of Cutaneous Surgeons (I) constituted a task force to review the evidence and to recommend consensus guidelines regarding the safety of skin procedures, including resurfacing, energy-device treatments, and dermatosurgical procedures in patients with concurrent or recent isotretinoin administration.
Materials and Methods:
Data were extracted from the literature through a PubMed search using the keywords “isotretinoin,” “safety,” “scarring,” “keloids,” “hypertrophic scarring,” and “pigmentation.” The evidence was then labeled and circulated to all members of task force for review.
Results:
The task force is of the opinion that there is insufficient evidence to support the current protocol of avoiding and delaying treatments in the patient group under consideration and recommends that the current practice should be discontinued. The task force concludes that performing procedures such as laser hair removal, fractional lasers for aging and acne scarring, lasers for pigmented skin lesions, fractional radio-frequency microneedling, superficial and medium-depth peels, microdermabrasion, dermaroller, biopsies, radio-frequency ablation, and superficial excisions is safe in patients with concurrent or recent isotretinoin administration.
Keywords: Dermatosurgical procedures, guidelines, isotretinoin, safety
BACKGROUND
Isotretinoin, a retinol derivative of vitamin A widely used in the treatment of acne vulgaris, has many pharmacological actions that affect epidermis, sebaceous gland, and collagen formation. The propensity of isotretinoin to affect these functions has led to questions about the possibility of poor wound healing, keloid development, and hypertrophic scarring, particularly in patients who undergo dermatosurgical procedures while on this drug. A detailed discussion of the mechanisms of action of the drug with respect to wound healing and scar formation has been reported in several standard publications, to which the reader is referred to.[1,2,3,4,5]
It has been the standard recommendation for over two decades that it is not safe to perform procedures in patients currently receiving or having recently completed systemic therapy with isotretinoin as the drug affects healing of wounds and hence may lead to hypertrophic scarring and keloid formation. Although this has generally been adhered to by practitioners, the recommendation has been questioned by a number of studies that have documented safety of cutaneous procedures in such patients. The Indian skin is brown and reacts differently to procedures with pigmentation. Hence, it was felt that there is a need for guidelines oriented to the Indian situation. The Association of Cutaneous Surgeons (I), as part of a presidential project by the then President Dr. Venkataram Mysore, conducted a multicentric study to examine the issue in the Indian setting, the findings of which were published recently. A task force was then constituted to formulate and recommend new guidelines appropriate to brown skin.
MATERIALS AND METHODS
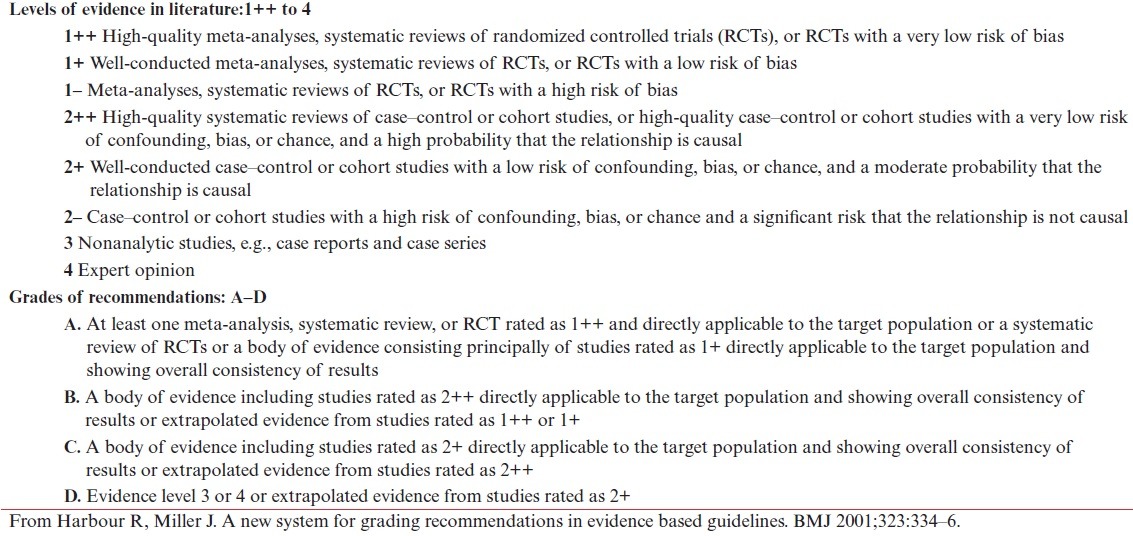
The task force performed a PubMed search using the keywords “isotretinoin,” “isotretinoin side effects,” “isotretinoin,” AND “laser”; “isotretinoin” AND “surgery”; “isotretinoin” AND “keloid”; “isotretinoin” AND “would healing”; “Isotretinoin” AND “hypertrophic scarring, isotretinoin, and pigmentation.” A total of 403, 62, and 27 articles were found with respect to “isotretinoin” AND “surgery”; “Isotretinoin” AND “wound healing”; and “Isotretinoin” AND “laser,” respectively. Of these, 27 articles were found to be of relevance as they specifically referred to the issue of scar formation and keloids. The task force also took into account recent consensus guidelines published by other associations such as the American Society for Dermatologic Surgery and a task force by JAMA (Journal of American Medical Association). The publications were studied in depth with respect to the evidence levels (as per Harbour and Miller’s revised grading system [Table 1]).
Table 1.
Harbour and Miller’s revised grading system for recommendations in evidence-based guideline
EXISTING GUIDELINES
All standard textbooks[1,2,3] state that performing dermatological procedures is not safe in patients currently or recently administered with isotretinoin and that a safe window period of 6 months after stopping the drug is advisable before performing procedures.
The patient information leaflet of the drug advises patients to “avoid chemical dermabrasion and laser treatment of the skin and wax depilation during and for at least 6 months after treatment as they could cause scarring or irritation of the skin.”[4]
The US Food and Drug Administration (FDA) also advises patients as follows: “Do not have cosmetic procedures to smooth your skin, including waxing, dermabrasion, or laser procedures, while you are using Accutane and for at least 6 months after you stop. Accutane can increase your chance of scarring from these procedures. Check with your doctor for advice about when you can have cosmetic procedures.”[5]
Historical basis for the existing guidelines
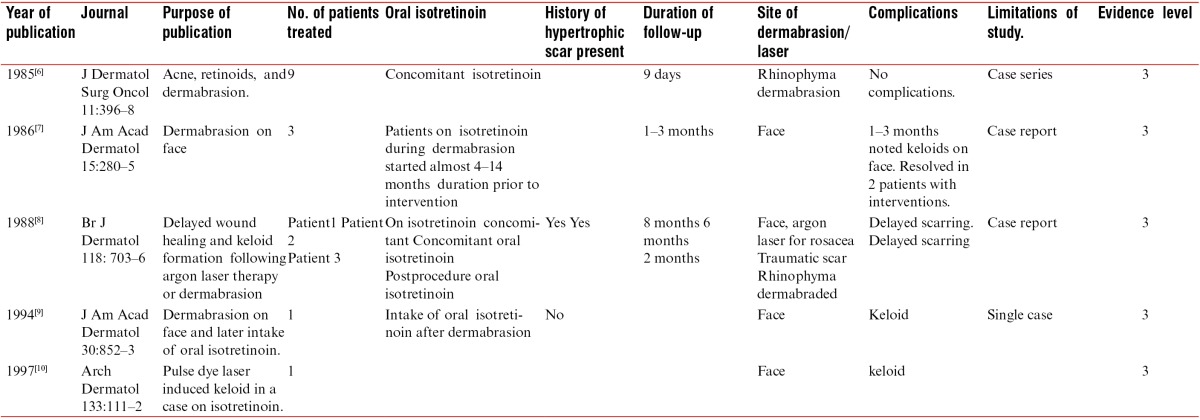
Some studies, mostly case reports from the 1980s to the 1990s, document delayed healing, hypertrophic scarring, and keloid formation after procedures in patients on oral isotretinoin.[6,7,8,9,10] The data of these studies, along with their evidence level, are shown in Table 2. During the same period, several other in vitro studies have shown that wound-healing processes can be affected by the administration of retinoids, topically and systemically.[11,12,13,14,15]
Table 2.
Old studies on oral isotretinoin intake and scarring postinterventions: summary of data with evidence levels
Detailed examination of these studies shows that these are all limited studies in a small number of patients, or isolated case reports of an inadequate number of procedures that were mostly aggressive procedures or conducted with early generation laser devices. The evidence would at best qualify for level 3. Furthermore, these studies were conducted with respect to only certain procedures such as argon laser (which is not no longer used), dermabrasion (which is rarely performed nowadays), and early generation of pulse dye laser (which was far inferior to currently available machines). There were no reports in context to other currently common, noninvasive, or minimally invasive procedures, such as microdermabrasion, microneedling, laser hair removal, fractional laser, and superficial peels. There was no documentation of ethnicity of patients or any other conflicting factors. It is therefore somewhat perplexing that such limited and basic data in a few procedures could lead to profound and sweeping recommendations applicable to all procedures including noninvasive and minor procedures, and that such recommendations could last for over two decades in clinical practice. It was also made at a time when isotretinoin itself was being subjected to strict evaluation and scrutiny, with respect to various issues such as dosage schedules, side-effect profiles such as psychological effects, teratogenicity, and effects on cholesterol. This was perhaps a knee-jerk and premature reaction and not evidence-based medicine.
Basis for revision of current guidelines
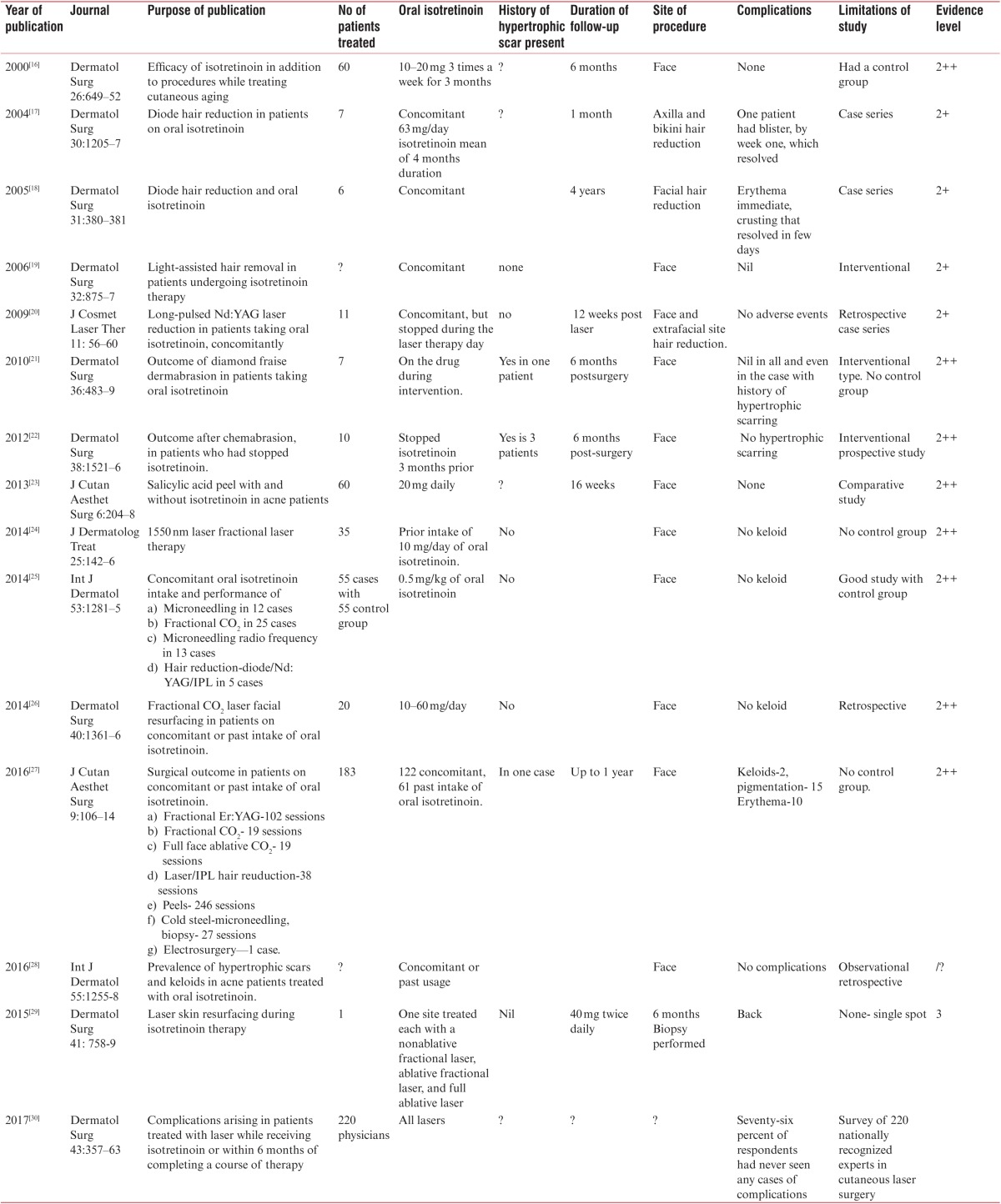
It is interesting and important to note that the existing guidelines did not go unquestioned and reports were published from the very beginning documenting the safety of procedures in this patient group. However, such studies tended to be small, possibly because physicians were deterred from performing large studies due to medicolegal implications. Hence, although these studies were far more in number than the studies that supported the need for a window period in procedures, they did not create sufficient academic impact to alter the recommended guideline. An examination of such studies in each procedure with the available evidence is shown in Table 3.
Table 3.
Recent studies of oral isotretinoin intake and study of scarring postinterventions: summary with evidence level (Harbour and Miller’s revised grading system)
ANALYSIS OF STUDIES AND EVIDENCE FOR EACH PROCEDURE AND TASK FORCE RECOMMENDATIONS
Laser hair reduction
Selective photothermolysis is the principle that laser hair removal is based on, where the target chromophore is the melanin in the hair follicle.[3] By this principle, the collagen is untouched and the epidermis is not affected. Hence, theoretically the procedure should be safe even among patients on isotretinoin.[3] A number of studies have shown the safety of the procedure[17,18,19,20,25,27] In contrast to this, there has never been a single study that establishes that the procedure was unsafe.
Level of evidence in literature: 2++
Recommendation: In view of this, the task force recommends that hair removal by both laser (of different types) and intense pulsed light is safe. The procedure can safely be conducted among patients on isotretinoin.
Recommendation level: Grade B
Lasers for resurfacing
Fractional lasers (ablative lasers [CO2 and erbium-doped yttrium aluminum garnet (Er:YAG) laser] and nonablative lasers erbium glass) are used for postacne scarring. Lasers for acne scarring are a more aggressive treatment than laser hair removal as the target tissue is collagen and thus it affects collagen synthesis. However, several studies have been conducted that do not confirm any risk for development of keloids or hypertrophic scars.[24,25,26,27,28]
Level of evidence in literature: 2++
Recommendation: The task force recommends that fractional ablative and nonablative lasers can be used safely among patients on isotretinoin.
Recommendation level: Grade B
Laser procedures for other indications
A single case where a patient who had acne vulgaris with nevus of hori underwent four sessions with q-switched Nd:YAG laser and showed no evidence of hypertrophic scar or keloid formation.[27]
Level of evidence in literature: 3
Recommendation: Because q-switched lasers cause limited damage to the epidermis as well as dermal collagen and because there are no documented studies of any adverse events with this laser, the task force recommends that the procedure can be safely performed in patients.
Recommendation level: Grade D
Dermabrasion and full-face ablative CO2 laser resurfacing
Dermabrasion and ablative CO2 laser resurfacing are perhaps the most invasive cutaneous aesthetic procedures, though seldom used after the advent of fractional lasers, particularly in the Indian setting. Early reports indicated the development of hypertrophic scarring after dermabrasion, and in fact was responsible for the recommendation currently being followed.[7,8,9] However, one recent study suggested that the procedure is safe without any tendency for hypertrophic scarring.[29]
Level of evidence in literature: 2+
Recommendation: The task force feels that dermabrasion and full-face CO2 laser resurfacing as a treatment modality is not widely performed in current practice as safer options are available and any recommendation is not relevant in this procedure. If any physician still wishes to resort to this procedure, it would be safer to wait for the window period of 6 months after stopping the drug, and the procedure performed after receiving the appropriate informed consent and following protocols.
Recommendation level: Grade C
Chemical peeling
Chemical peels are performed for a number of indications including patients of acne and scarring. In today’s practice, superficial peels and occasionally, medium-depth peels are used. Studies have recognized the safety of salicylic acid, glycolic acid, combination peels, and trichloroacetic acid among patients on isotretinoin.[22,23,24,25] However, a case report of persistent hyperpigmentation after glycolic acid peel in one patient has been published[30] There have been very few reports of deep peels in patients on isotretinoin, perhaps because of lack of use of deep peels in current practice.
Level of evidence in literature: 2+
Recommendation: The task force considers the superficial and medium-depth peels to be safe in patients on isotretinoin
Recommendation level: Grade C
Microdermabrasion and dermaroller
Microdermabrasion and dermaroller are increasingly used in dermatology practice for a number of reasons. These are generally minimally invasive and safe procedures. There are two studies[25,27] that document the safety of procedures in patients administered with isotretinoin.
Level of evidence in literature: 2+
Recommendation: The task force recommends that microneedling and microdermabrasion treatment can safely be performed in patients administered with isotretinoin.
Recommendation level: Grade C
Radio-frequency procedures
Radio-frequency devices are used in dermatology practice either to cut/coagulate—exophytic lesions or for collagen stimulation in scars and rejuvenation. The limited data show both ablative radio-frequency and fractional microneedling radio-frequency to be safe.[25,27] One single compound nevus excision on face lead to keloid.[27]
Level of evidence in literature: 2+
Recommendation: The task force feels that superficial lesions can safely be removed by radio-frequency devices in patients administered with isotretinoin. However, for deep lesions, caution should be exercised during the window period of 6 months and such decisions should be based on the medical needs of the patient with the given condition.
Recommendation level: Grade B
Skin biopsies
Skin biopsies that involve collagen damage did not produce keloid in eight patients.[27]
Level of evidence in literature: 2+
Recommendation: A biopsy is crucial for diagnosis and needs to be performed for medical reasons. Therefore, there cannot be a recommendation restricting this essential diagnostic procedure.
Recommendation level: Grade B
DISCUSSION
The aforementioned review shows rather overwhelmingly that the risks of hypertrophic scarring, keloid formation, delayed wound healing, and pigmentation are not significant in most dermatological procedures. As such, the recommendation to avoid procedures in these patients was, in our opinion, based on flawed reasoning. As Goodman[31] stated in a commentary “Many different ‘standards’ of practice dictated how long after Isotretinoin was completed it was considered safe to consider chemical peels, dermabrasion, or laser resurfacing. No science followed but many firmly held opinions ranging from 6 to 24 months were considered to be the prudent time after Isotretinoin before procedures were safe.”
The task force is of the firm opinion that such a recommendation based on poor quality evidence would never have been accepted in the current day with emphasis on evidence-based practice if it had been made. Therefore, the recommendation has only served to deny patients safe treatments and put unnecessary fear in the minds of doctors. This was confirmed by a recent survey of nationally recognized experts of laser surgery regarding the treatment of patients administered isotretinoin therapy currently or within 6 months.[32] In this report, most of the respondents (70%) affirmed that medicolegal concerns guided their decision-making regarding this patient population, in contrast to concerns about atypical or poor wound healing (69%), scarring (66%), and hypertrophic or keloidal scarring (49%). This was despite the fact that 76% respondents had never seen any complications in their own clinical practice. It was rightly summarized by Goldman[33] that “What becomes clear is that the overwhelming majority of physicians are curtailing laser treatments because of the fear of litigation.”
The task force, therefore, opines that the current recommendation needs to be withdrawn and summarizes its recommendations as follows:
There is no need to delay procedures such as laser hair removal, fractional lasers, radio frequency, microneedling, microdermabrasion for which there have never been any reports of adverse effects. These procedures can be performed safely: “Level B.”
Aggressive procedures such as dermabrasion, full-face ablative laser resurfacing, and deep peels, where there are small reports of adverse effects, are rarely performed in current practice, particularly in brown skin. However, if a physician still wishes to perform these procedures, caution needs to be exercised and such procedures may be performed after a window period of 6 months of stopping the drug. Appropriate informed consent needs to be taken in such cases and the physician should follow all the protocols applicable to each procedure: “Level C.”
During its survey, the task force found that most of the studies quoted above used a dosage ranging between 10 and 80 mg. Although there is no evidence to judge whether higher doses carry greater risk, it may be prudent at the current time to restrict the dosage to less than 0.5 mg/kg body weight in patients: “Level C.”
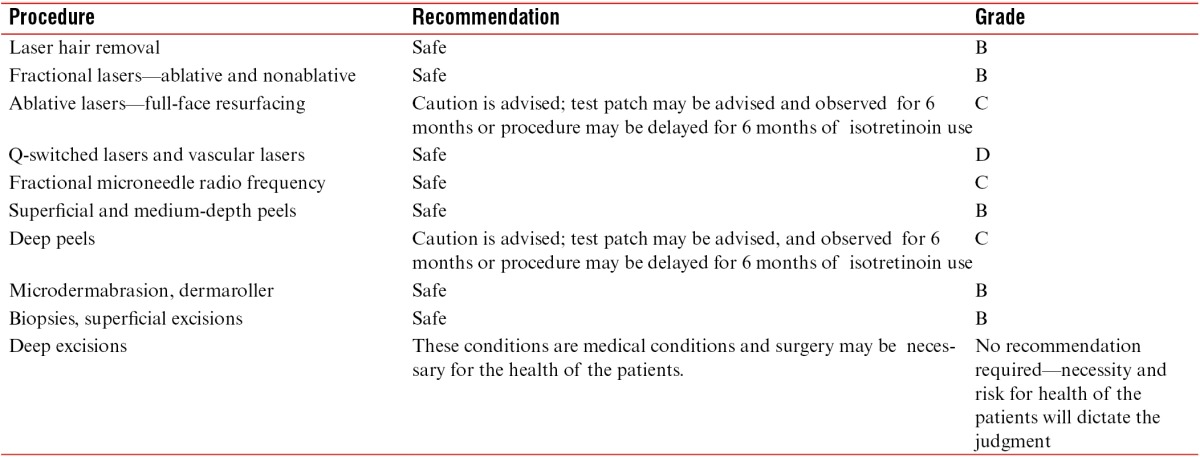
The summary of recommendations for each procedure is shown in Table 4.
Table 4.
Summary of recommendations and grade recommendation in patients on isotretinoin
The task force is aware that the recommendations made here are based on level B or C evidence. It is the task force’s hope that the formulation of these recommendations will remove the apprehension from the minds of physicians and lead to more procedures being performed in patients administered with isotretinoin. This will lead to the further accumulation of evidence, and the formulation of guidelines will be based on stronger evidence in the future. It is also heartening to note that two recent guidelines[34,35] have been published on this subject and the task force concurs with those recommendations. A recent textbook edited by the first author of this article also dealt with the subject and suggested that the procedures can be safely performed in these patients.[36] In addition to this, some recent textbooks discuss at length the adverse events of isotretinoin such as inflammatory bowel disease and depression but have stopped mentioning keloid and hypertrophic scarring.[37,38]
Finally, these recommendations have been made with particular reference to Indian or brown skin. It is noteworthy that two of the largest studies[25,27] that support the recommendation have been on Indian skin.
Financial support and sponsorship
Nil.
Conflict of interest
The authors have indicated no significant interest with commercial supporters.
REFERENCES
- 1.Christopher RL. Keloids and hypertrophic scars. In: Griffiths CEM, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook’s textbook of dermatology. Oxford, UK: John Wiley; 2016. pp. 96.45–96.46. [Google Scholar]
- 2.Thomas PH. Acne, rosacea and related disorders. In: Thomas PH, editor. Clinical dermatology. Philadelphia, USA: Elsevier; 2016. pp. 247–8. [Google Scholar]
- 3.Zaenglein AL, Thiboutot DM. Acne vulgaris. In: Bolognia JL, Jurizzo JL, Schaffer JV, editors. Dermatology. Philadelphia, USA: Elsevier, Saunders; 2012. pp. 557–8. [Google Scholar]
- 4.Isotretinoin 5 mg and 20 mg capsules [Patient information leaflet] Chippenham, UK: Alliance Pharmaceuticals Limited; 2017. [Accessed September 5, 2016]. Available from: https://www.medicines.org.uk/emc/PIL.15643.latest.pdf . [Google Scholar]
- 5.FDA drug information on Isotretinoin. [Accessed January 13, 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/18662s051lbl.pdf .
- 6.Roenigk HH Jr, Pinski JB, Robinson JK, Hanke CW. Acne, retinoids, and dermabrasion. J Dermatol Surg Oncol. 1985;11:396–8. doi: 10.1111/j.1524-4725.1985.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 7.Rubenstein R, Roenigk HH Jr, Stegman SJ, Hanke CW. Atypical keloids after dermabrasion of patients taking Isotretinoin. J Am Acad Dermatol. 1986;15:280–5. doi: 10.1016/s0190-9622(86)70167-9. [DOI] [PubMed] [Google Scholar]
- 8.Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during Isotretinoin treatment. Br J Dermatol. 1988;118:703–6. doi: 10.1111/j.1365-2133.1988.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 9.Katz BE, Mac Farlane DF. Atypical facial scarring after Isotretinoin therapy in a patient with previous dermabrasion. J Am Acad Dermatol. 1994;30:852–3. doi: 10.1016/s0190-9622(94)70096-6. [DOI] [PubMed] [Google Scholar]
- 10.Bernestein LJ, Geronemus RG. Keloid formation with the 585-nm pulsed dye laser during Isotretinoin treatment. Arch Dermatol. 1997;133:111–2. doi: 10.1001/archderm.1997.03890370123029. [DOI] [PubMed] [Google Scholar]
- 11.Brinckerhoff CE, McMillan RM, Dayer JM, Harris ED Jr. Inhibition by retinoic acid of collagenase production in rheumatoid synovial cells. N Engl J Med. 1980;303:432–6. doi: 10.1056/NEJM198008213030805. [DOI] [PubMed] [Google Scholar]
- 12.Bauer EA, Seltzer JL, Eisen AZ. Retinoic acid inhibition of collagenase and gelatinase expression in human skin fibroblast cultures: evidence for a dual mechanism. J Invest Dermatol. 1983;81:162–9. doi: 10.1111/1523-1747.ep12543590. [DOI] [PubMed] [Google Scholar]
- 13.Abergel RP, Meeker CA, Oikarinen H, Oikarinen AI, Uitto J. Retinoid modulation of connective tissue metabolism in keloid fibroblast cultures. Arch Dermatol. 1985;121:632–5. [PubMed] [Google Scholar]
- 14.Frosch PJ, Czarnetzki BM. Effect of retinoids on wound healing in diabetic rats. Arch Dermatol Res. 1989;281:424–6. doi: 10.1007/BF00455329. [DOI] [PubMed] [Google Scholar]
- 15.Moy RL, Moy LS, Bennett RG, Zitelli JA, Uitto J. Systemic Isotretinoin: Effects on dermal wound healing in a rabbit ear model in vivo. J Dermatol Surg Oncol. 1990;16:1142–6. doi: 10.1111/j.1524-4725.1990.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Perez E, Khawaja HA, Alvarez TY. Oral Isotretinoin as part of the treatment of cutaneous aging. Dermatol Surg. 2000;26:649–52. doi: 10.1046/j.1524-4725.2000.99210.x. [DOI] [PubMed] [Google Scholar]
- 17.Khatri KA. Diode laser hair removal in patients undergoing Isotretinoin therapy. Dermatol Surg. 2004;30:1205–7. doi: 10.1111/j.1524-4725.2004.30373.x. discussion 1207. [DOI] [PubMed] [Google Scholar]
- 18.Cassano N, Arpaia N, Vena GA. Diode laser hair removal and Isotretinoin therapy. Dermatol Surg. 2005;31:380–1. doi: 10.1111/j.1524-4725.2005.31097_1. [DOI] [PubMed] [Google Scholar]
- 19.Khatri KA, Garcia V. Light-assisted hair removal in patients undergoing Isotretinoin therapy. Dermatol Surg. 2006;32:875–7. doi: 10.1111/j.1524-4725.2006.32182.x. [DOI] [PubMed] [Google Scholar]
- 20.Khatri KA. The safety of long-pulsed Nd:YAG laser hair removal in skin types III-V patients during concomitant Isotretinoin therapy. J Cosmet Laser Ther. 2009;11:56–60. doi: 10.1080/14764170802612984. [DOI] [PubMed] [Google Scholar]
- 21.Bagatin E, dos Santos Guadanhim LR, Yarak S, Kamamoto CS, de Almeida FA. Dermabrasion for acne scars during treatment with oral Isotretinoin. Dermatol Surg. 2010;36:483–9. doi: 10.1111/j.1524-4725.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- 22.Picosse FR, Yarak S, Cabral NC, Bagatin E. Early chemabrasion for acne scars after treatment with oral Isotretinoin. Dermatol Surg. 2012;38:1521–6. doi: 10.1111/j.1524-4725.2012.02460.x. [DOI] [PubMed] [Google Scholar]
- 23.Kar BR, Tripathy S, Panda M. Comparative study of oral isotretinoin versus oral isotretinoin + 20% salicylic acid peel in the treatment of active acne. J Cutan Aesthet Surg. 2013;6:204–8. doi: 10.4103/0974-2077.123403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon JH, Park EJ, Kwon IH, Kim CW, Lee GS, Hann SK, et al. Concomitant use of an infrared fractional laser with low-dose Isotretinoin for the treatment of acne and acne scars. J Dermatolog Treat. 2014;25:142–6. doi: 10.3109/09546634.2013.768758. [DOI] [PubMed] [Google Scholar]
- 25.Chandrashekar BS, Varsha DV, Vasanth V, Jagadish P, Madura C, Rajashekar ML. Safety of performing invasive acne scar treatment and laser hair removal in patients on oral Isotretinoin: A retrospective study of 110 patients. Int J Dermatol. 2014;53:1281–5. doi: 10.1111/ijd.12544. [DOI] [PubMed] [Google Scholar]
- 26.Kim HW, Chang SE, Kim JE, Ko JY, Ro YS. The safe delivery of fractional ablative carbon dioxide laser treatment for acne scars in Asian patients receiving oral Isotretinoin. Dermatol Surg. 2014;40:1361–6. doi: 10.1097/DSS.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 27.Mahadevappa OH, Mysore V, Viswanath V, Thurakkal S, Majid I, Talwar S, et al. Surgical outcome in patients taking concomitant or recent intake of oral Isotretinoin: A multicentric study-ISO-AIMS study. J Cutan Aesthet Surg. 2016;9:106–14. doi: 10.4103/0974-2077.184054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guadanhim LR, Gonçalves RG, Bagatin E. Observational retrospective study evaluating the effects of oral Isotretinoin in keloids and hypertrophic scars. Int J Dermatol. 2016;55:1255–8. doi: 10.1111/ijd.13317. [DOI] [PubMed] [Google Scholar]
- 29.Khatri KA, Iqbal N, Bhawan J. Laser skin resurfacing during Isotretinoin therapy. Dermatol Surg. 2015;41:758–9. doi: 10.1097/DSS.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 30.Gerber PA, Kukova G, Bölke E, Homey B, Diedrichson E. Severe hyperpigmentation and scarring following glycolic acid peel treatment in combination with low-dose Isotretinoin. Eur J Med Res. 2014;19:60. doi: 10.1186/s40001-014-0060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman G. Commentary Dermatol Surg. 2004;30:1207. [Google Scholar]
- 32.Prather HB, Alam M, Poon E, Arndt KA, Dover JS. Laser safety in Isotretinoin use: A survey of expert opinion and practice. Dermatol Surg. 2017;43:357–63. doi: 10.1097/DSS.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 33.Goldman D. Commentary. JAMA Dermatol. 2017;153:810. [Google Scholar]
- 34.Spring LK, Krakowski AC, Alam M, Bhatia A, Brauer J, Cohen J, et al. Isotretinoin and timing of procedural interventions: A systematic review with consensus recommendations. JAMA Dermatol. 2017;153:802–9. doi: 10.1001/jamadermatol.2017.2077. [DOI] [PubMed] [Google Scholar]
- 35.Waldman A, Bolotin D, Arndt KA, Dover JS, Geronemus RG, Chapas A, et al. ASDS guidelines task force: Consensus recommendations regarding the safety of lasers, dermabrasion, chemical peels, energy devices, and skin surgery during and after Isotretinoin use. Dermatol Surg. 2017;43:1249–62. doi: 10.1097/DSS.0000000000001166. [DOI] [PubMed] [Google Scholar]
- 36.Venkataram M, Omprakash HM, Revantha S. Isotretinoin and procedures in Dermatology- Reviewing clinical significance. In: Venkataram M, editor. ACS(I) text book of cutaneous and aesthetic surgery. 1st ed. vol 2. New Delhi, India: Jaypee Brothers; 2017. pp. 1338–41. [Google Scholar]
- 37.James WD, Elston DM, Berger TG. Acne vulgaris. In: James WD, Elston DM, Berger TG, editors. Andrew’s diseases of the skin: Clinical dermatology. Philadelphia, USA: Elsevier; 2016. pp. 230–1. [Google Scholar]
- 38.Pattan TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, editor. Comprehensive dermatology drug therapy. Philadelphia, USA: Elsevier Saunders; 2013. pp. 252–68. [Google Scholar]