Abstract
Introduction and Objectives:
The restoration of the epithelium after injury takes place by migration of epithelial cells adjoining a wound or by centrifugal migration from hair follicles. To evaluate the feasibility and potential healing capacity of scalp follicular unit grafts transplanted into the wound bed of chronic leg ulcers.
Materials and Methods:
Patients with chronic nonhealing ulcers of more than 6 weeks duration were selected for the study. Those with infected ulcers and uncontrolled diabetes were excluded from the study. Fifteen patients were included in the study. Follicular unit grafts were harvested under local anesthesia using small-diameter (1 mm) circular punches. A density of 5 follicular grafts/cm2 was implanted into the ulcer bed. The ulcer was dressed with Vaseline gauze and elastic bandage for 24 h. The wound area and volume were calculated by length × width × 0.7854 and length × width × depth × 0.7854, respectively. The treatment outcome was defined as the percentage in change of area and volume of the ulcer, 18 weeks after intervention.
Results:
A total of 15 patients with 17 ulcers were treated with the above method. Of these 17 ulcers, 11 were venous ulcers, 2 were pyoderma gangrenosum associated with varicose veins, 2 were traumatic ulcers, and 2 were trophic ulcers. The baseline mean area of the ulcer was 6.72 cm2 (SD 5.65) and baseline volume was 2.87 cm3 (SD 2.9). The final area of the ulcer at the end of 18 weeks after the procedure was 3.84 cm2 (SD 5.43) and the final volume was 1.21 cm3 (SD 2.45), which was statistically significant. The mean percentage improvement in the area and volume of the ulcer was 48.8% and 71.98%, respectively. Two patients did not respond to the treatment. There were no adverse events after the procedure.
Conclusion:
We conclude that follicular unit grafting into wound beds is feasible and represents a promising therapeutic alternative for managing nonhealing chronic leg ulcers.
Keywords: Follicular unit graft; bulge stem cells; leg ulcers; wound healing Key message: Chronic non healing ulcers are very challenging to manage, with compression therapy being the mainstay along with ulcer management. The use of scalp as donor area in this study is based on the concept that, a lot of stem cells such as the bulge stem cells and mesenchymal stem cells are transferred to the ulcer bed thereby acting as hubs from where reinnervation and capillary sprouting of wound beds proceed. This has shown a promising healing response.
INTRODUCTION
Leg ulcers that do not heal even after 6 weeks are called chronic nonhealing leg ulcers. They are painful, debilitating, recurrent, and an economic burden for the patients, disturbing their quality of life. The condition affects 1% of the adult population and 3.6% of people older than 65 years.[1] They are of multifactorial etiology with comorbidities posing a great management challenge. It has been reported that ulcers related to venous insufficiency constitute 70%, arterial disease 10%, and ulcers of mixed etiology 15% of leg ulcer presentations. The remaining 5% of leg ulcers result from less common pathophysiological causes.[2]
Managing chronic leg ulcers is very challenging. When all the conservative and medical methods fail, we opt for surgical management after taking care of the etiological causes of ulcers. Various surgical modalities used are wound debridement and regular dressing, platelet-rich plasma or fibrin therapy, and skin grafting or flap surgeries. Skin grafting methods used so far are split-thickness skin grafting, pinch grafting, postage stamp grafting, and punch grafting.
The restoration of the epithelium after injury takes place by migration of epithelial cells from the old epithelium adjoining a wound or by centrifugal migration from any hair follicles (HFs) remaining within the wound.[3] It has been found that any scalp defect heals quickly because of the high density of terminal HFs and also that re-epithelialization of ulcers is quicker when scalp tissue is the donor.
Because there is a lot of published evidence that HFs act as wound promoters,[4] we, in our study, have harvested follicular unit grafts from the occiput and implanted into chronic nonhealing ulcers to evaluate the feasibility and wound-healing capacity of the implanted grafts.
MATERIALS AND METHODS
This is a clinical interventional study carried out at a tertiary care center after taking institutional ethical board approval. We included 15 patients in the age group of 18–60 years, with chronic nonhealing leg ulcers of more than 6 weeks duration, who had received conventional therapies with no improvement. They were of various etiologies such as venous, diabetic, neuropathic, and traumatic causes. Only patients who gave written informed consent for the procedure were included in the study. Patients with bleeding disorders, uncontrolled diabetes, and infected ulcers were excluded from the study. Demographic details of all patients were recorded. Age, onset and progression of the ulcers, and treatment history were noted. Selected patients were thoroughly examined for the length, depth, and breadth of the ulcer by “clockface method” described by Sussman[5] using cotton-tip applicator and disposable paper ruler. A set of basic investigations were conducted on patients to find out underlying causes of ulcer, which included complete blood count with ESR (erythrocyte sedimentation rate), random blood sugar, pus culture and sensitivity for infected wounds, skin biopsy, venous and arterial Doppler if indicated, bleeding time, clotting time if needed, and other relevant investigations depending on the etiology.
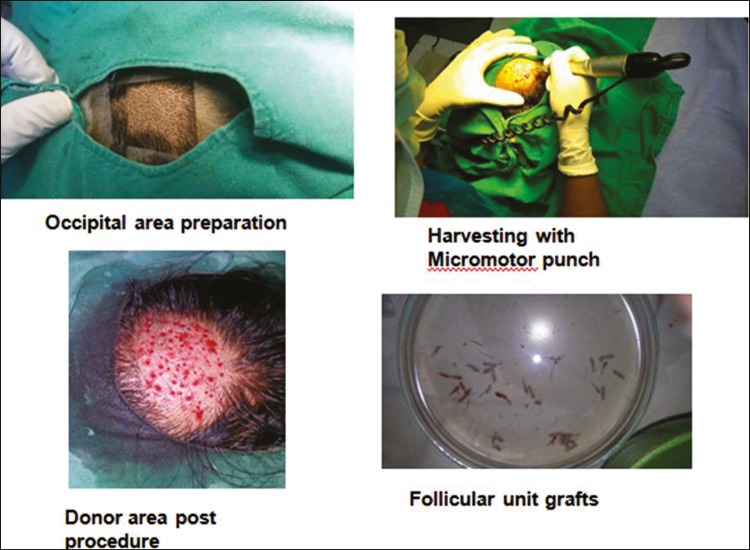
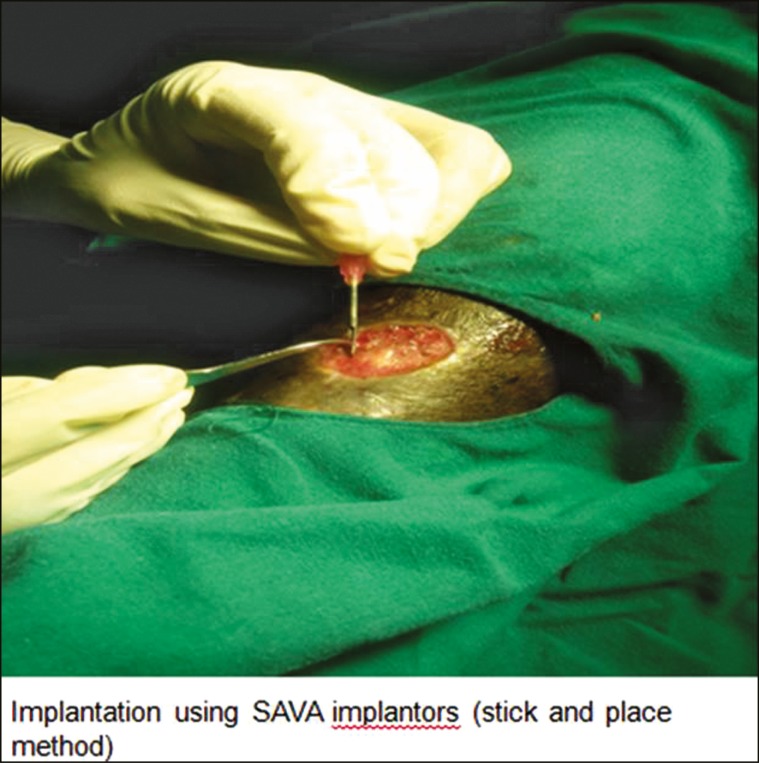
The procedure was performed under local anesthesia in an outpatient setting and involved the extraction of follicular unit grafts from the occipital area of scalp and their subsequent implantation into the wound bed. Hair grafts were harvested one at a time using a micromotor device with small-diameter (1 mm) circular punches [Figure 1]. A transplant density of 5 follicular grafts/cm2 was grafted as this was considered the minimum density required to guarantee tissue regeneration of the ulcer in a pilot study conducted by Jimenez et al.[6] in 2012. The nonhealing ulcers were first debrided to remove the infected and necrotic tissues. Only those hair grafts containing intact full-length follicular unit were used for transplantation using SAVA implanters by stick-and-place method [Figure 2]. Donor area was left to heal by secondary intention. A local dressing with antibiotic ointment, gauze, and tape was left on the donor wound for the first 24 h. Once the hair grafts were implanted in the ulcer bed, the ulcer was covered with Vaseline gauze and closed with elastic bandage for 24 h. Each patient was put on antibiotics and pain killers after the procedure for 10 days.
Figure 1.
Harvesting follicular units from the occipital area of scalp
Figure 2.
Implantation of the follicular unit grafts in the ulcer bed using SAVA implanters
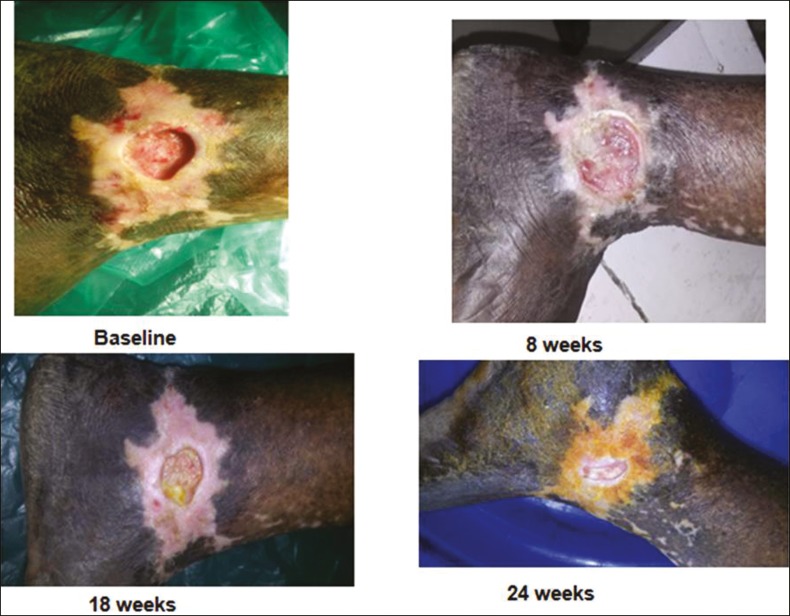
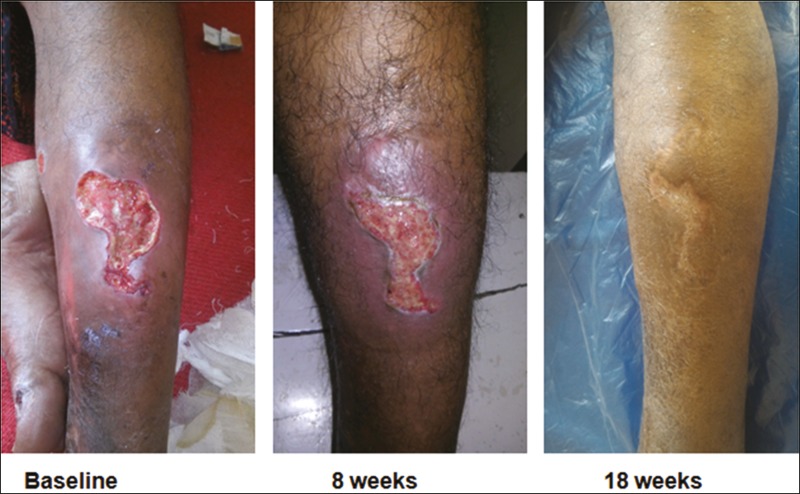
Outcome measurement: The wound area and volume were calculated by length × width × 0.7854 and length × width × depth × 0.7854, respectively. The treatment outcome was defined as the percentage in change of area and volume of the ulcer and was calculated as initial measurement minus assessment-day measurement divided by initial measurement, 18 weeks after intervention, which is the end point of the study. Digital photographs were taken at 0, 8, and 18 weeks [Figures 3–6]. Secondary outcome included an analysis of the safety of the procedure.
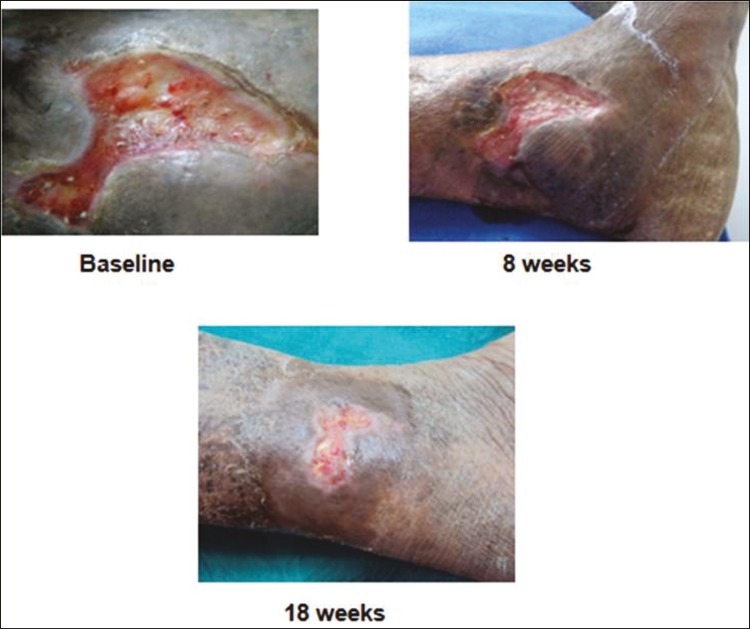
Figure 3.
Before and after procedure photos of a venous ulcer
Figure 6.
Before and after procedure photos of a pyoderma gangrenosum ulcer
Figure 4.
Before and after procedure photos of another venous ulcer
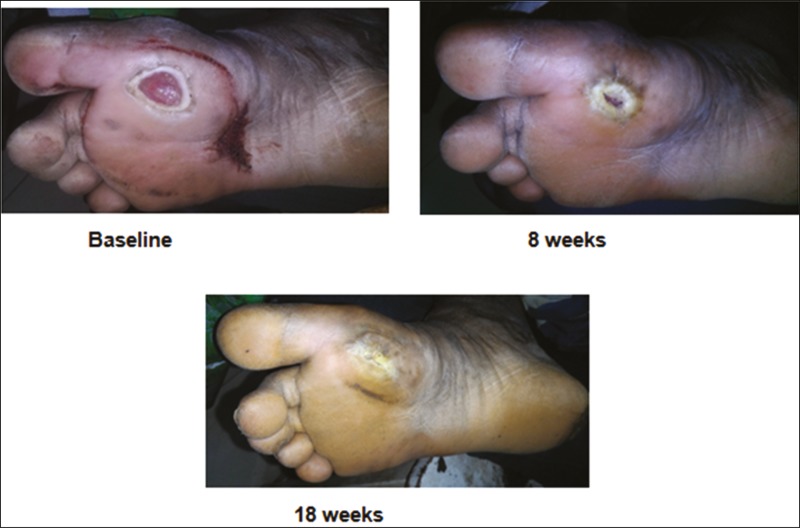
Figure 5.
Before and after procedure photos of a trophic ulcer
Statistical analysis
We compared the area and volume at baseline, after 8 weeks, and after 18 weeks by repeated-measures analysis of variance and post hoc Tukey test. A P-value of <0.05 was considered significant. We also compared the number of ulcers showing varied percentage improvement in area and volume of ulcers.
RESULTS
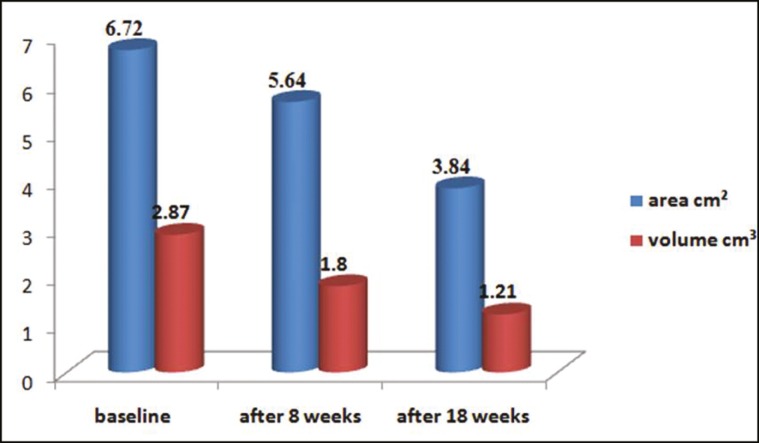
A total of 15 patients with 17 ulcers were treated with the earlier-mentioned method. Of these ulcers, 11 were venous ulcers, 2 were pyoderma gangrenosum associated with varicose veins, 2 were traumatic ulcers, and 2 were trophic ulcers [Table 1]. Among the included patients, 14 (93.3%) were males and 1 (6.67%) was female, with a mean age of 37.59 ± 15.38 years. Of 15 patients, 2 (13.3%) were above 60 years of age, 3 (20%) were in the age group of 41–60 years, and 10 (66.67%) were in the age group of 21–40 years. The duration of the nonhealing ulcers presented by the patients ranged from 2 to 48 months with a mean duration of 9 months. The baseline mean area of the ulcer was 6.72 cm2 (SD 5.65) and baseline volume was 2.87 cm3 (SD 2.9). The final area of the ulcer at the end of 18 weeks after the procedure was 3.84 cm2 (SD 5.43) and the final volume was 1.21 cm3 (SD 2.45), which was statistically significant. The mean percentage improvement in the area and volume of the ulcer was 48.8% and 71.98%, respectively [Table 2, Figure 7]. We found the improvement in the volume was significantly more compared to the improvement in area (P = 0.02). Three (13.33%) patients showed more than 75% reduction in the area of ulcer and five showed 50%–75% reduction in the area of the ulcer. Two patients did not respond to the treatment, one was having varicose vein that needed surgical management and the other was having pyoderma gangrenosum in the background of venous stasis. There were no adverse events following the procedure. Donor site healed well in all cases [Figure 8]. Outer hair shafts were visible in the recipient area in the early postprocedural stages, which however disappeared later.
Table 1.
Demographic and clinical data of patients in the study

Table 2.
Improvement in area and volume of ulcers after treatment with follicular unit grafting
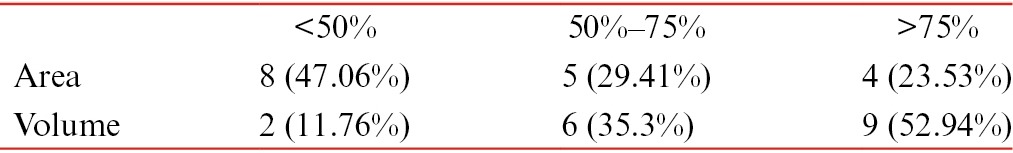
Figure 7.
Graph comparing area and volume at baseline, after 8 weeks, and after 18 weeks (P < 0.001)
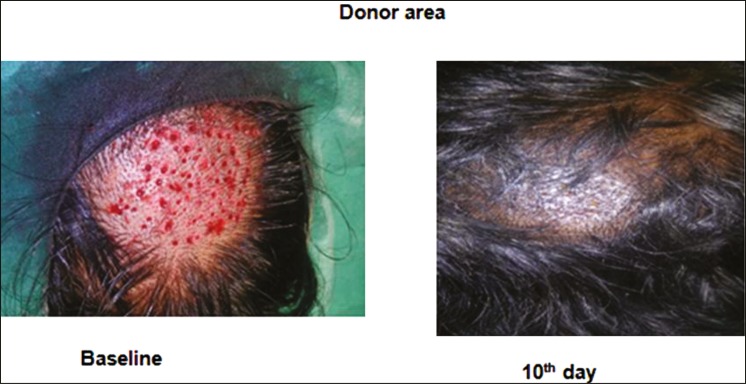
Figure 8.
Donor area heals completely by 10 days
DISCUSSION
Management of chronic nonhealing leg ulcers poses a great challenge because of the chronicity, cost involved, need for regular follow-up, and the quality of living of the patients. Compression bandage or stocking is the mainstay of managing venous ulcers conservatively. Various surgical grafting methods such as split-thickness skin grafting,[7] punch grafting,[8] and pinch grafting[9] have been used to promote re-epithelialization and healing of ulcers. None of the aforementioned methods bear HFs in the grafts. Punch grafting in chronic leg ulcers has been used as surgical management, but the donor area is usually thigh, which is devoid of HFs. Here, we have used the scalp as donor area, thereby a lot of stem cells such as the bulge stem cells and mesenchymal stem cells are transferred to the ulcer bed.
HFs and other skin appendages have a wound-healing promoting role by acting simply as hubs from where reinnervation[10] and capillary sprouting[11] of wound beds proceed. But a major capability of the HF seems to be the outsourcing of precursor cells for immediate local skin repair.[12] A major contribution by Bishop[13] in 1945 considered the HF to be the principal cutaneous structure whose presence or absence conditions the outcome of the healing response. He reported that re-epithelialization starts around the HFs and from the marginal epithelium, granulation tissue develop at the sites of follicles, and scar formation occurs when the HFs are destroyed. It has been clinically demonstrated that, when scalp is the donor for split-thickness graft, the donor wound heals faster than when the donor area is thigh or some other region, suggesting a role of HF density in the speed of re-epithelialization.[14] At the cellular level, upon wounding, bulge epithelial follicular stem cells migrate to the epidermis to aid with the rapid re-epithelialization of wounded skin.[15] Perifollicular mesenchymal cells from the dermal sheath and/or dermal papillae also appear to participate in the wound-healing response, moving out of the follicles into the wounds, where they contribute to dermal fibroblasts and myofibroblasts.[16] Therefore, HF neogenesis suggests a complex mesenchymal–epithelial interaction. A recent study describes the use of a hydrogel formulation composed of leucine-rich repeat-containing G-protein-coupled receptor 6 (LGR6+) follicular stem cells that appears to enhance the wound healing and angiogenic response when transplanted into full-thickness skin wounds in mice.[11]
Although there is large body of evidence about the role of HF stem cells in wound healing, abundant clinical reports demonstrating the same are lacking. First original hair-related and commercially available (EpiDex; EuroDerm Biotec and Aesthetics, Stuttgart, Germany) therapy for chronic leg ulcers was plucking hairs from a patient’s scalp to obtain epidermal autografts that could be transplanted to the wound bed.[17] This method involved isolating keratinocytes from the external root sheath of the plucked hairs and culturing them in vitro to obtain epidermal sheets that were transplanted to the wound bed of the ulcer. The practical advantages of this therapy are its noninvasive nature and easy handling in an outpatient setting with no need for anesthesia or donor-site surgical intervention. Disadvantages included the time required for processing the epidermal sheets. As chronic leg ulcers are devoid of HFs, the epidermis can regrow only via cells migrating from the edges of intact skin. Hair cycle phase influences wound-healing capacity of the HFs. So a more pragmatic approach of hair-related therapy for chronic leg ulcers would be to directly insert in the wound bed terminal anagen HFs (harvested from the patient) containing epithelial and mesenchymal stem cells that, when implanted within damaged tissue, would eventually proliferate, migrate away from the implant, and induce healing of the ulcer.[18]
Jiménez et al.[6] conducted a pilot study in which autologous grafts of follicular units were transplanted into experimental area of ulcer bed and found that there was a significant enhancement of wound healing compared to the control area. At the 18-week endpoint, a 27% ulcer area reduction in the experimental square was observed against 6.5% in the control square. Improvement of clinical symptoms (appearance of granulation tissue, wound border reactivation, and a lesser amount of exudation) was noted in 7 of 10 cases. The same method was replicated in this study, but grafting was done to the entire ulcer bed of chronic leg ulcers of 15 patients. We found a mean percentage improvement in the area and volume of the ulcer by 48.8% and 71.98%, respectively, at the end of 18 weeks. We found the improvement in the volume was significantly more compared to the improvement in area (P = 0.02). In their study, Jiménez et al.[18] found that the age of the patient had no bearing on the wound-healing process with respect to the follicular unit grafts because the density of HF bulge stem cells does not alter with aging. But in our study we had an elderly patient with gray hair in the donor area and found that his ulcer did not show significant improvement. This could be attributed to other comorbidities in the elderly age group.[19] There were no other adverse events noted in the study. The donor area healed well in all cases.
LIMITATIONS AND CONCLUSIONS OF THE STUDY
A trichoscope analysis was not done before the study to facilitate harvesting follicular grafts in the anagen phase. It was taken care during the harvesting process that only visibly thicker follicular units were selected. We did not compare the healing process in the grafted area with a control area because we performed the procedure to the entire ulcer bed. We did not compare this method with any other grafting method, as we will be taking it up in the future studies. The size of the ulcer, which we can treat by this method, is also a major limitation, as large-sized ulcers may require larger number of follicular unit grafts. The number of follicular units per unit area also needs to be standardized.
We found that the volume replenishment of the ulcer bed was quicker than the re-epithelialization of the ulcer. This has given us an impetus to study further with the help of mice models the epidermal and dermal characteristics of the replenished tissue in the ulcers after the follicular unit grafting procedure, results of which will be published in our next article.
Follicular unit grafting into chronic nonhealing leg ulcer shows a promising therapeutic intervention. We need more studies with larger sample size and randomized controlled designs.
Financial support and sponsorship
IADVL Grant.
REFERENCES
- 1.Ryan TJ. The epidemiology of leg ulcers. In: Westerhof W, editor. Leg ulcers: diagnosis and treatment. Amsterdam: Elsevier Science Publishers BV; 1993. pp. 19–27. [Google Scholar]
- 2.Casey G. Causes and management of leg and foot ulcers. Nurs Stand. 2004;18:57–8. doi: 10.7748/ns2004.07.18.45.57.c3653. [DOI] [PubMed] [Google Scholar]
- 3.Brown JB, McDowell F. Epithelial healing and the transplantation of skin. Ann Surg. 1942;115:1166–81. doi: 10.1097/00000658-194206000-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navsaria HA, Ojeh NO, Moiemen N, Griffiths MA, Frame JD. Reepithelialization of a full-thickness burn from stem cells of hair follicles micrografted into a tissue-engineered dermal template (Integra) Plast Reconstr Surg. 2004;113:978–81. doi: 10.1097/01.prs.0000105632.86651.ef. [DOI] [PubMed] [Google Scholar]
- 5.Sussman C. Wound measurements. In: Sussman C, Bates-Jensen B, editors. Wound care: a collaborative practice manual for physical therapists and nurses. Gaithersburg, MD: Aspen; 2001. pp. 120–41. [Google Scholar]
- 6.Jiménez F, Garde C, Poblet E, Jimeno B, Ortiz J, Martínez ML, et al. A pilot clinical study of hair grafting in chronic leg ulcers. Wound Repair Regen. 2012;20:806–14. doi: 10.1111/j.1524-475X.2012.00846.x. [DOI] [PubMed] [Google Scholar]
- 7.Ratner D. Skin grafting. From here to there. Dermatol Clin. 1998;16:75–90. doi: 10.1016/s0733-8635(05)70488-5. [DOI] [PubMed] [Google Scholar]
- 8.Nordström A, Hansson C. Punch-grafting to enhance healing and to reduce pain in complicated leg and foot ulcers. Acta Derm Venereol. 2008;88:389–91. doi: 10.2340/00015555-0443. [DOI] [PubMed] [Google Scholar]
- 9.Oien RF, Hansen BU, Håkansson A. Pinch grafting of leg ulcers in primary care. Acta Derm Venereol. 1998;78:438–9. doi: 10.1080/000155598442737. [DOI] [PubMed] [Google Scholar]
- 10.Cheng C, Guo GF, Martinez JA, Singh V, Zochodne DW. Dynamic plasticity of axons within a cutaneous milieu. J Neurosci. 2010;30:14735–44. doi: 10.1523/JNEUROSCI.2919-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lough DM, Yang M, Blum A, Reichensperger JD, Cosenza NM, Wetter N, et al. Transplantation of the LGR6+ epithelial stem cell into full-thickness cutaneous wounds results in enhanced healing, nascent hair follicle development, and augmentation of angiogenic analytes. Plast Reconstr Surg. 2014;133:579–90. doi: 10.1097/PRS.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 12.Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–61. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Bishop GH. Regeneration after experimental removal of skin in man. Am J Anat. 1945;76:153–81. [Google Scholar]
- 14.Barret JP, Dziewulski P, Wolf SE, Desai MH, Herndon DN. Outcome of scalp donor sites in 450 consecutive pediatric burn patients. Plast Reconstr Surg. 1999;103:1139–42. doi: 10.1097/00006534-199904040-00006. [DOI] [PubMed] [Google Scholar]
- 15.Ito M, Cotsarelis G. Is the hair follicle necessary for normal wound healing? J Invest Dermatol. 2008;128:1059–61. doi: 10.1038/jid.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gharzi A, Reynolds AJ, Jahoda CA. Plasticity of hair follicle dermal cells in wound healing and induction. Exp Dermatol. 2003;12:126–36. doi: 10.1034/j.1600-0625.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 17.Renner R, Harth W, Simon JC. Transplantation of chronic wounds with epidermal sheets derived from autologous hair follicles—the Leipzig experience. Int Wound J. 2009;6:226–32. doi: 10.1111/j.1742-481X.2009.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiménez F, Poblet E, Izeta A. Reflections on how wound healing-promoting effects of the hair follicle can be translated into clinical practice. Exp Dermatol. 2015;24:91–4. doi: 10.1111/exd.12521. [DOI] [PubMed] [Google Scholar]
- 19.Sgonc R, Gruber J. Age-related aspects of cutaneous wound healing: A mini-review. Gerontology. 2013;59:159–64. doi: 10.1159/000342344. [DOI] [PubMed] [Google Scholar]