Abstract
Background:
Nonsurgical aesthetic treatments are usually preferred by patients because their effects are visible immediately after the treatment and patients can return to their normal activities on the same day. Although many studies have indicated safety and efficacy of filler injection to improve facial appearance, it is not absolutely confirmed for nose reshaping.
Objectives:
To assess the safety and early satisfaction of 52 consecutive patients underwent nonsurgical rhinoplasty with an injection of a 20-mg/mL smooth, cohesive, and viscous hyaluronic acid (HA) filler.
Materials and Methods:
Fifty-two consecutive healthy patients, dissatisfied with the appearance of their nose, were treated with HA injections between November 2014 and November 2016. Complications and side effects were documented. Aesthetic outcomes were scored subjectively on a scale of 1–4 represented by four emoticons.
Results:
Among patients, 96.15% affirmed to be “very satisfied” at the end of the procedure (50 patients over 52 treated). No major complications and side effects occurred.
Conclusions:
Outcomes of this study, with the limitation of a non-comparative open-label study, show that surgical remodeling of the nose, with the use of a 20-mg/mL smooth, cohesive, and viscous HA filler, is a safe and predictable technique, with a high degree of satisfaction for the patients.
Keywords: Filler, hyaluronic acid, nonsurgical rhinoplasty, nose, rhinofiller, safety
INTRODUCTION
The past decade has seen an explosion of new products and techniques in aesthetic medicine due to increase in the number of patients looking for nonsurgical aesthetic procedure.[1] The American Society for Aesthetic Plastic Surgery (ASAPS) statistics, every year, confirm an ongoing request of the so-called “aesthetic medicine procedures” by patients.[2] Nonsurgical aesthetic treatments are usually preferred by patients because their effects are visible immediately after treatment and patients can return to their normal activities on the same day.[3]
Surgical rhinoplasty, “nose job”, according to ASAPS statistics (2016), is the sixth of the most requested procedures;[2] however, the so-called nonsurgical rhinoplasty with fillers in the last few years has shown to be an effective alternative for patients seeking only an aesthetic improvement of the nose.[4]
Several articles regarding Asian nose augmentation with fillers have been published;[3,4,5] however, in Western countries also, the so-called “nonsurgical rhinoplasty,” often called “rhinofiller,” is often performed although with different end points.
Differences between Western and Asian noses usually are represented by a more projected anterior nasal spine (ANS) and a more or less pronounced hump, in the first group; however, some other anesthetic features, such as drooping of the tip, lateral displacement of the nasal tip crura, and deficiency of the upper lateral cartilages (ULC), can be observed.
In this study, assessment of safety and early satisfaction of 52 consecutive patients underwent nonsurgical rhinoplasty with an injection of a 20-mg/mL smooth, cohesive, and viscous hyaluronic acid (HA) filler was performed.
MATERIALS AND METHODS
Fifty-two consecutive healthy patients (43 women and 9 men), aged 18–61 years (mean age, 29.7 years), dissatisfied with the appearance of their nose, and who, in the opinion of the injecting physician, could achieve a clinically meaningful aesthetic correction with filler injections, were treated.
Exclusion criteria included patients who were pregnant; breastfeeding; with any preexisting condition that might affect patient safety, including active inflammation, infection, cancerous or precancerous lesions, and so on; with a known hypersensitivity to lidocaine, HA, and/or gram-positive bacteria proteins; with a history of connective tissue disease or bleeding disorders; who used aspirin and/or concomitant antithrombotic therapy during the week preceding the treatment.
All patients were required to provide written informed consent, which included a photo release form.
Three patients (2 men and 1 woman), among the 52 treated, had at least one surgical rhinoplasty (in one case, two previous nose jobs were done); patients who already had surgery were treated only if at least 1 year from the operation had passed. In all the cases, a 20-mg/mL smooth, cohesive, and viscous HA filler (Juvederm Voluma; Allergan plc, Dublin, Ireland) had been used.
Injection
After a careful disinfection of the nose, without any local anesthesia injection, the “rhinofiller” was administered. The end point of injection was determined by each patient, and the injection points and directions for each additional injection were determined according to the patient’s request.
Injection sites were the following: above the ANS to project the tip of the nose and ahead of the anterior part of the medial crura to enhance columella, above the tip of the nose (by percutaneous or endonasal approach) to reshape it and create a supratip break, above the hump to ameliorate nasofrontal (NF) angle, and above ULC in case of ULC deficiency.
A needle was used for all injection procedures.
In 11 cases only the tip and/or the bridge of the nose were the injection sites; in 3 cases (only in men) only the NF angle was the injection site (ANS was not injected to avoid feminine appearance of the nose); in all other cases ANS and NF angles were the injection sites.
Volume range of the HA filler injected was between 0.2 and 1.5 mL (0.8 mL on average); only in one case more than 1 mL HA filler was used. The exact quantity of the HA injected per site is given in Table 1 [Figures 1–4]. In all the cases HA was placed deeply, over bony and cartilage tissue, just above the periosteum and/or the perichondrium; this was done to avoid vessels cannulation and related vascular problems.
Table 1.
Mean HA volume injected per anatomical site

Figure 1.
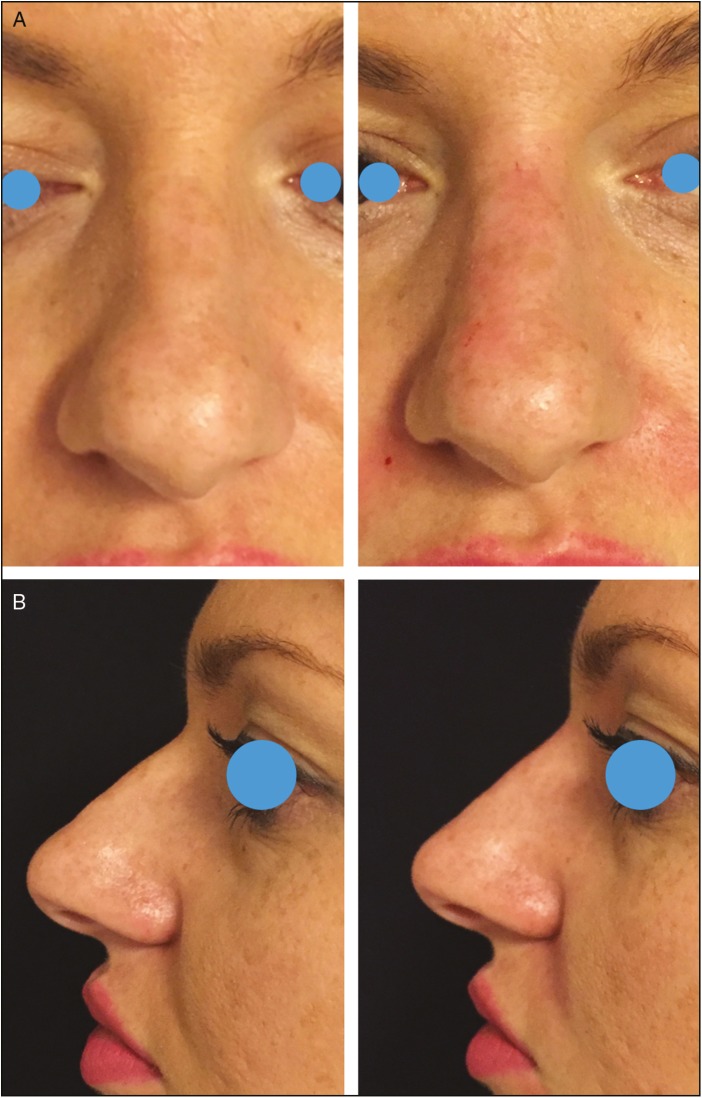
Frontal (A) and lateral views (B) of a 26-year-old woman pre-op and immediate post-op of a nonsurgical straightening of the nose. HA was injected as a spreader graft on the deviated side (0.1 mL); additional 0.1 mL was injected to improve the NF angle
Figure 4.
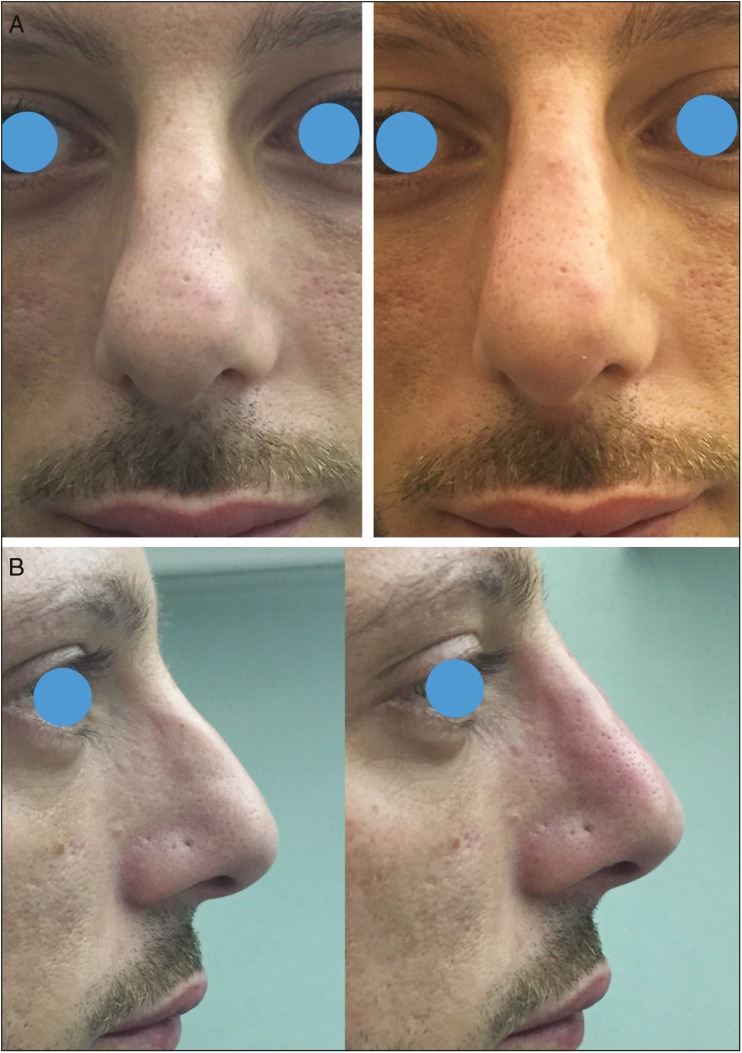
Frontal (A) and lateral views (B) of a 29-year-old man pre-op and immediate post-op of a nonsurgical rhinoplasty. HA was injected acting as a spreader graft over ULC (0.15 mL per side); 0.3 mL was injected above ANS; 0.2 mL was injected above NF angle; more 0.05 mL per side was injected at the tip of the nose
Figure 2.
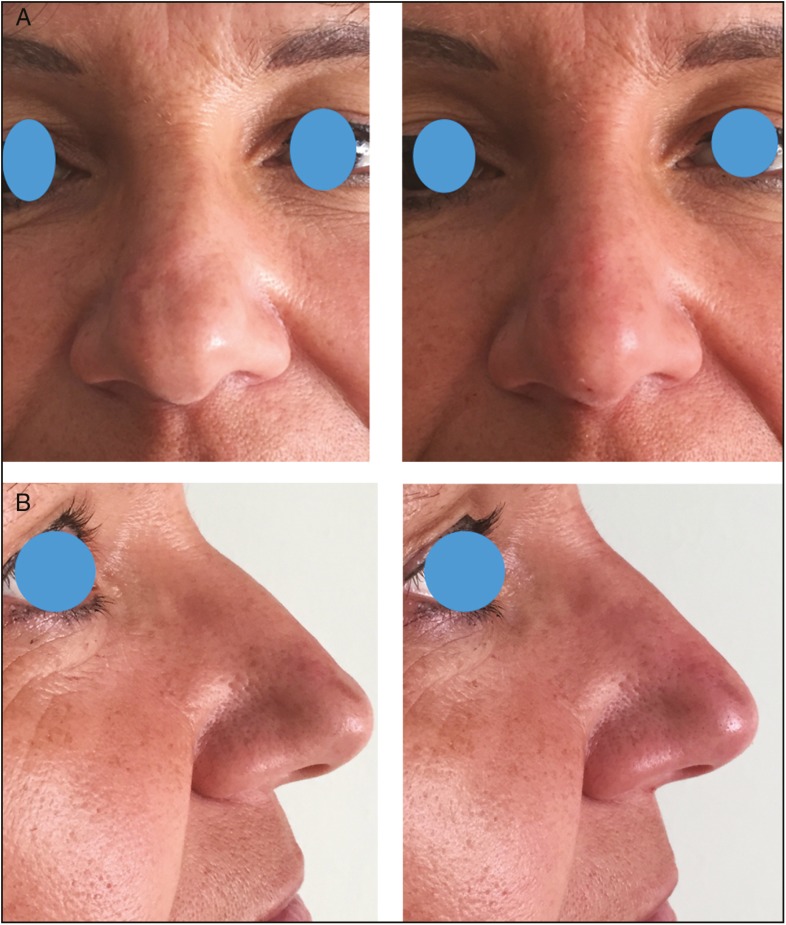
Frontal (A) and lateral views (B) of a 48-year-old woman pre-op and 1-year post-op of a nonsurgical remodeling of the tip of the nose; 0.15 mL of HA was injected acting as a tip graft to improve the appearance of laterally displacement domes
Figure 3.
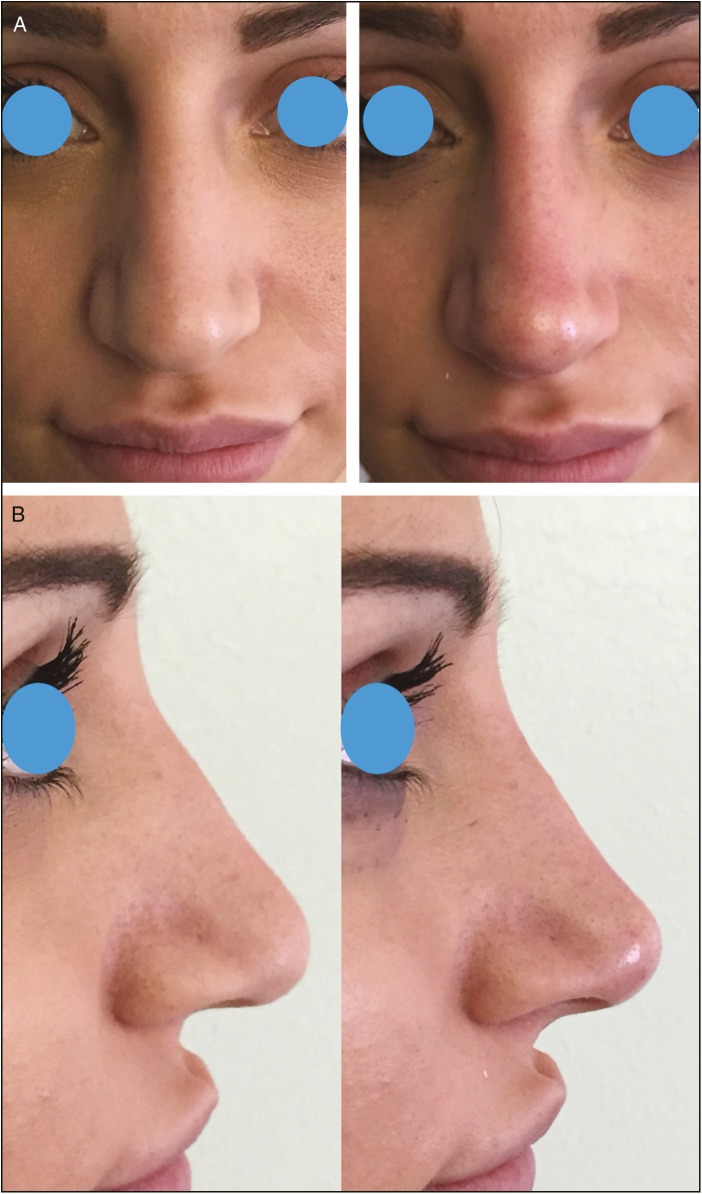
Frontal (A) and lateral views (B) of a 23-year-old woman pre-op and immediate post-op of a nonsurgical rhinoplasty. HA was injected acting as a spreader graft over ULC (0.05 mL per side); 0.3 mL was injected above ANS to upturn the tip of the nose; additional 0.05 mL per side was injected to create the suvratip break
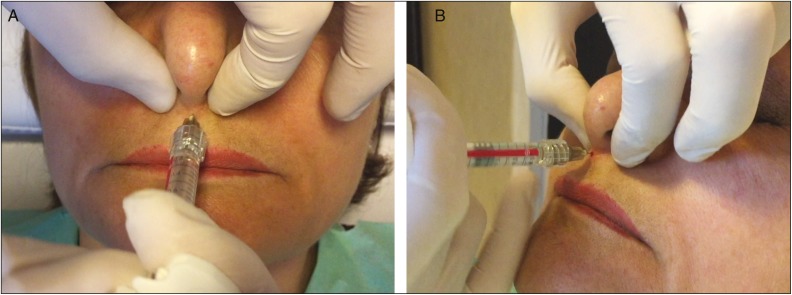
In all the cases, injections were first administered from the ANS, then the hump was remodeled, and at the end, if required, the ULC and the tip were injected. ANS injection was always administered by pinching columella to reduce pain and discomfort for the patient [Figure 5a and b].
Figure 5.
Frontal (A) and lateral (B) pictures showing the “pinching technique” to inject HA filler above ANS and increase nasal tip projection. The “pinching technique” is useful to reduce pain and discomfort for the patient
Injections started always from ANS to achieve nasal tip projection and rotation, this let to inject a smaller quantity of HA over the dorsum; in fact, in cases of a drooping nasal tip secondary to atrophy of the underlining bone support, it produce a relatively prominent dorsal hump.
In all the cases where the bridge of the nose was injected, patients were asked to avoid unnecessary external compression of the nose, which could cause filler displacement, indentation, or depression of the dorsal surface; to this end, patients were advised to avoid wearing goggles and sun or reading glasses for 15 days.
HA Filler
In all the cases, a 20-mg/mL smooth, cohesive, and viscous HA filler (Juvederm Voluma) was used. This HA filler is highly cohesive and highly viscous, which gives a great projection capacity, ideally suited for contouring.[6] HA is derived from the bacterial fermentation by Streptococcus equi. Although most HA fillers are derived from high-molecular-weight (HMW) HA (1 ≥ MDa), the 20-mg/mL smooth, highly cohesive, high-viscous gel uses a mix of a low-molecular-weight (LMW; <1 MDa) and HMW HA polymer chains as its raw material source. The addition of LMW HA significantly improved the cross-linking efficiency of the product and produced an end result of high viscosity coupled with a relatively high cohesiveness, a combination not observed with most other HA gels.[6] The high efficiency of cross-linking, through the use of LMW HA, allows the use of less cross-linking agent—1,4-butanediol diglycidyl ether—to achieve a high linear viscosity (G′ is 330 Pa at 5 Hz) while maintaining a relatively high cohesiveness. Through the coupling of high viscosity and high cohesiveness, it is able to retain its structure without migration from deep injection sites.[7] The use of LMW HA requires minimal amount of uncross-linked (nonmodified HA chains and lightly cross-linked chains and fragments in soluble form) HA (a lubricant) within the end product, which has a positive effect on extrusion properties.
Patient Assessments of the Result
The assessment of the result was done subjectively by the patients using a questionnaire, in which the patients were asked to rate their degree of satisfaction in terms of result and treatment convenience based on a four-point scale characterized by four emoticons (the angry one = worse, the sad one = little satisfaction or not satisfied, the happy one = satisfied, the one with heart eyes = very satisfied [Figure 6]). The questionnaire was given to the patients at the end of the treatment, and 15 days later, they were asked to fill it again and return by e-mail. All the patients were recalled after 6 months to know if they would like to retouch their nose.
Figure 6.
Patients were asked to rate the nonsurgical rhinoplasty result with a four-point scale characterized by four emoticons (the angry one = worse, the sad one = little satisfaction or not satisfied, the happy one = satisfied, and the one with heart eyes = very satisfied). It was given to the patient immediately at the end of the procedure and they were asked to fill it again 15 days later (sent by e-mail)
RESULTS
In a period between November 2014 and November 2016, total 52 consecutive patients were treated with HA injection to shape their nose, and 3 of them received nose surgery at least 1 year before the nonsurgical treatment. Mean HA injected was 0.8 mL; in two cases, 3–6 weeks later, a retouch was required by the patients (an injection ranging from 0.05 to 0.2 mL was administered).
Major complications, such as skin necrosis and vascular problems, were never recorded. A small swelling at the injection sites was recorded in almost all the cases; however, it resolved by itself within 48 h without any therapy. One case, the patient who received two rhinoplasties before the treatment, developed swelling of the upper lip, which resolved by itself within 3 days; in two more cases, transient blanching of the skin at the tip of the nose was noted at the end of the procedure; however, it resolved by itself in less than 5 min.
Of 52 patients, 51 rated the result as “very satisfied,” the remaining patient scored “satisfied” (this patient required a touch-up injection of 0.2 mL after 3 weeks, and refilling the questionnaire, he stated to be “very satisfied”). Satisfaction assessment performed by e-mail 15 days after the procedure (considering also the one who requested the touch-up 3 weeks later) revealed 50 “very satisfied” and 2 “satisfied” responses. Among the “satisfied” patients, except the one requiring the touch-up, another 24-year-old patient complained of an “excessive” nasal tip upward rotation; however, she refused hyaluronidase injection. After 6 months from the injections, all the patients were recalled to ask to repeat the procedure and in all the cases, expect one, it was observed that no changes were noted since the HA injection, so repeating the injections was not required. In all the cases, a selfie was requested but only 14 female patients sent it. Twelve patients were followed up for 14 months because they were referred for some other procedures (lip enhancement, botulinum toxin injections, etc.), and their nose results were found to be stable over time.
DISCUSSION
Facial rejuvenation in the last few years has shifted toward less invasive and even nonsurgical procedures that have shorter recovery time and cause less pain.[8,9,10] Soft-tissue augmentation with various soft-tissue filler materials has become one of the most popular aesthetic procedures available to the patients who desire nonsurgical facial rejuvenation.[9] Although many studies have indicated safety and efficacy of filler injection to improve facial appearance, it is not absolutely confirmed for nose reshaping.[11] One of the most devastating complications described after nonsurgical reshaping of the nose with filler is blindness.[12] In a recent review by Li et al.,[13] 75 cases of blindness secondary to facial injections were recorded; 25% of the cases were secondary to nasal dorsum injections. The hypothesis proposed by the authors is based on the presence of an anastomosis of the nasal area, consisting of a dorsal nasal artery from the ophthalmic artery, an angular artery, and a lateral nasal artery from the facial artery. Li et al.[13] concluded that injection into nasal dorsum may accidentally break into the anastomosis, resulting in retrograde embolism of the ophthalmic and clinical blindness for the patient. Liew et al. also underlined the importance of a good knowledge of standard vascular and its variant is essential to avoid vascular complications not only represented by blindness but also by nasal skin necrosis. The dorsal and external nasal arteries are also branches of the ophthalmic artery, which also provide collateral flow to the nasal tip. Isolated reports of tip necrosis have been published in the literature following the use of fillers of all types, and it has been documented as a rare complication of surgical rhinoplasty.[4] The mechanism behind this is assumed to be compression, occlusion, and/or embolization of these vessels. These events are clearly not unique to the nasal vasculature, with similar reports seen following administration of fillers in the forehead, glabellar, temple, and the nasolabial region.[14,15,16]
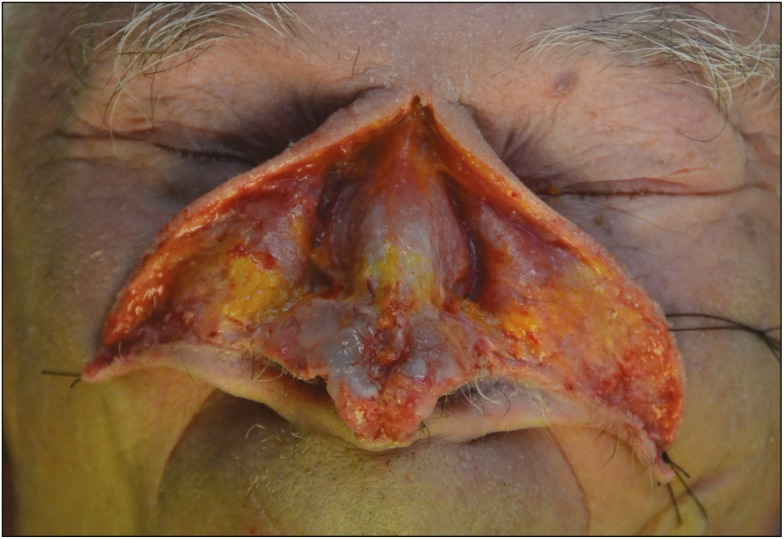
In this study, we did not record any case of skin necrosis and/or blindness, or transitory visual disturbances in postsurgical cases. It is believed that this is related to the deep placement of HA [Figure 7], just above the periosteum and the perichondrium, as recently stated by Scheuer et al.[17]
Figure 7.
A cadaveric specimen. Soft-tissue envelope was almost all removed to show the right plane of injection: injections should be carried out deep into the musculoaponeurotic layers in the preperichondrial and preperiosteal layers to avoid injury or cannulation of vessels
When injecting in the nose, injections should be administered deep into the musculoaponeurotic layers in the preperichondrial and preperiosteal layers to avoid injury or cannulation of vessels.[17] In this study, HA droplets were injected using them as a cartilage graft, especially when the dorsum and the tip of the nose were injected, releasing HA above cartilage, avoiding to inject into the soft-tissue nasal envelope.
In this study, we injected a 20-mg/mL smooth, cohesive, and viscous HA filler for nonsurgical nasal reshaping. A really high degree of satisfaction was recorded by almost all the patients except two that scored “satisfied” and not “very satisfied.” However, no adverse events and complications were recorded. The results obtained were similar to the ones already published by Liew et al.,[4] although they studied only Asian patients with detracting or deficient nose.
A high degree of satisfaction achieved can be explained by the really small time needed to get the result, absence of post-op downtime, long-lasting result, and, last but not the least, cheap price compared to a surgical rhinoplasty.
In this study, another important issue regarding rhinofiller approach emerged: when a complete nose remodeling is planned, ANS was always the first anatomical landmark to be injected, then the dorsum, and at the end, the tip of the nose. Injecting ANS first assures a control of tip projection and can reduce the perception of nasal hump. Consequently, the amount of HA to be injected to remodel the dorsum will be less; only at the end, the tip is injected to create the suvra tip break. The same issue, however regarding nose occidentalization, has been already reported by Tanaka[5] regarding Oriental nose occidentalization by augmentation of ANS. The author reported how also in aging Western patients, a drooping nasal tip can occur secondary to atrophy of the underlying bone support and, at the same time, a relative dorsal hump can be resolved only by injecting above ANS.
It is also useful to discuss the choice of the filler used—a 20-mg/mL smooth, cohesive, and viscous HA filler. It is characterized by a combination of high cohesiveness and high viscosity, which gives a great projection capacity—a really important feature especially for the injection above ANS.[4] Moreover, through the coupling of high viscosity and high cohesiveness, this filler is able to retain its structure without migration from deep injection sites. This is why our deep placement, as a cartilage graft, of the filler was safe and effective.
CONCLUSION
In this study, a 20-mg/mL smooth, cohesive, and viscous HA filler was used for nonsurgical remodeling of Western patients. The satisfaction assessment score was recorded, which was high in almost all the cases and no major complications (such as nasal skin necrosis and blindness) were recorded.
Outcomes of this study, with the limitation of a noncomparative open-label study, confirm that surgical remodeling of the nose, with the use of a 20-mg/mL smooth, cohesive, and viscous HA filler, deeply injected into the musculoaponeurotic layers in the preperichondrial and preperiosteal layers to avoid injury or cannulation of vessels, is a safe and predictable technique with a high degree of satisfaction for the patients.
Financial support and sponsorship
The authors received no financial support for the research, authorship, and publication of this article.
Conflict of interest
The authors have no potential conflict of interest with respect to the research, authorship, and publication of this article.
REFERENCES
- 1.Carruthers JD, Glogau R.G, Blitzer A. Facial Aesthetics Consensus Group Faculty. Advances in facial rejuvenation: Botulinum toxin type A, hyaluronic acid dermal fillers and combination therapies—consensus recommendations. Plast Reconstr Surg. 2008;121:5S–31S. doi: 10.1097/PRS.0b013e31816de8d0. [DOI] [PubMed] [Google Scholar]
- 2. [last accessed September 22, 2017]. Available from: http://www.surgery.org/sites/default/files/ASAPS-Stats2016.pdf .
- 3.Kim P, Ahn JT. Structured nonsurgical Asian rhinoplasty. Aesthetic Plast Surg. 2012;36:698–703. doi: 10.1007/s00266-012-9869-2. [DOI] [PubMed] [Google Scholar]
- 4.Liew S, Scamp T, de Maio M, Halstead M. Efficacy and safety of a hyaluronic acid filler to correct aesthetically detracting or deficient features of the Asian nose: A prospective, open-label, long term study. Aesthetic Surg J. 2016;36:760–72. doi: 10.1093/asj/sjw079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka Y. Oriental nose occidentalization and perinasal shaping by augmentation of the underdeveloped anterior nasal spine. Plast Reconstr Surg Glob Open. 2014;2:e197. doi: 10.1097/GOX.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carruthers J, Carruthers A, Tezel A, Kraemer J, Craik L. Volumizing with a 20-mg/ml smooth, highly cohesive, viscous hyaluronic acid filler and its role in facial rejuvenation therapy. Dermatol Surg. 2010;36:1886–92. doi: 10.1111/j.1524-4725.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith K. Practical use of Juvederm: early experience. Plast Reconstr Surg. 2007;120:67S–73S. doi: 10.1097/01.prs.0000285107.16836.16. [DOI] [PubMed] [Google Scholar]
- 8.Rohrich RJ, Rios JL, Fagien S. Role of new fillers in facial rejuvenation: a cautious outlook. Plast Reconstr Surg. 2003;112:1899–902. doi: 10.1097/01.PRS.0000097307.62862.27. [DOI] [PubMed] [Google Scholar]
- 9.Rohrich RJ, Ghavami A, Crosby MA. The role of hyaluronic acid fillers (Restylane) in facial cosmetic surgery: review and technical considerations. Plast Reconstr Surg. 2007;120:41S–54S. doi: 10.1097/01.prs.0000248794.63898.0f. [DOI] [PubMed] [Google Scholar]
- 10.Monstrey SJ, Pitaru S, Hamdi M, Van Landuyt K, Blondeel P, Shiri J, et al. A two-stage phase I trial of Evolence30 collagen for soft-tissue contour correction. Plast Reconstr Surg. 2007;120:303–11. doi: 10.1097/01.prs.0000264402.97692.b6. [DOI] [PubMed] [Google Scholar]
- 11.Thomas WW, Bucky L, Friedman O. Injectables in the nose: facts and controversies. Facial Plast Surg Clin North Am. 2016;24:379–89. doi: 10.1016/j.fsc.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Cohen E, Yatziv Y, Leibovitch I, Kesler A, Cnaan RB, Klein A, et al. A case report of ophthalmic artery emboli secondary to calcium hydroxylapatite filler injection for nose augmentation—long-term outcome. BMC Ophthalmol. 2016;16:98. doi: 10.1186/s12886-016-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Du L, Lu JJ. A novel hypothesis of visual loss secondary to cosmetic facial filler injection. Ann Plast Surg. 2015;75:258–60. doi: 10.1097/SAP.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunebaum LD, Bogdan Allemann I, Dayan S, Mandy S, Baumann L. The risk of alar necrosis associated with dermal filler injection. Dermatol Surg. 2009;35:1635–40. doi: 10.1111/j.1524-4725.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 15.Lazzeri D, Agostini T, Figus M, Nardi M, Pantaloni M, Lazzeri S. Blindness following cosmetic injections of the face. Plast Reconstr Surg. 2012;129:995–1012. doi: 10.1097/PRS.0b013e3182442363. [DOI] [PubMed] [Google Scholar]
- 16.Carruthers JD, Fagien S, Rohrich RJ, Weinkle S, Carruthers A. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134:1197–201. doi: 10.1097/PRS.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 17.Scheuer JF, 3rd, Sieber DA, Pezeshk RA, Gassman AA, Campbell CF, Rohrich RJ. Facial danger zones: techniques to maximize safety during soft-tissue filler injections. Plast Reconstr Surg. 2017;139:1103–8. doi: 10.1097/PRS.0000000000003309. [DOI] [PubMed] [Google Scholar]