Abstract
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory disease of the lungs that usually manifests late in life. Physiologic and immunologic changes that occur in COPD often mimic changes seen in the aging lung. This has led some to characterize COPD as an “accelerated aging phenotype.” At the molecular level, COPD and aging share common mechanisms and are associated with significant dysregulation of the immune systems. Aging and COPD are characterized by increases in proinflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, which are implicated in aging-related inflammatory diseases and correlate with degree of obstruction in COPD. There is an age-dependent decline in naïve T cells with oligoclonal expansion of CD8+ CD28null T cells from chronic antigenic stimulation. The increase in CD8+ CD28 null T regulatory cells inhibits antigen-specific CD4+ T cell responses, leading to a decline in adaptive immune response. To compensate for the decline in the adaptive immune function there is a paradoxical up-regulation of innate immune system resulting in a proinflammatory state. The dysregulated adaptive immune system with activated innate immune responses seen with aging results in recruitment and retention of neutrophils, macrophages, and CD4+ and CD8+ T cells in the lungs of smokers with COPD. Once the inflammation is triggered, there is a self-perpetuating cascade of inflammation and lung parenchymal damage. This review will focus on how the aging immune system may contribute to COPD development later in life in susceptible individuals.
Keywords: aging, immune system, COPD, older adults
Chronic obstructive pulmonary disease (COPD) is among the most prevalent chronic adult diseases, affecting approximately 18 million adults in the United States and many more worldwide (1, 2). In 2002, COPD was responsible for more than six million inpatient hospital stays and more than 100,000 adult deaths, making it the fourth most common cause of death. Evidence suggests that the chronic complex interaction between the airways and noxious inhalants, most commonly cigarette smoke, leads to COPD in genetically susceptible individuals. COPD is characterized by airway and lung inflammation, mucociliary dysfunction, alveolar destruction, and airway fibrosis. The pathogenesis of COPD seems to originate from dysregulated response of the immune system to noxious agents (3–8). These changes occur over decades, and most patients express disease phenotypes well after the fourth decade of life.
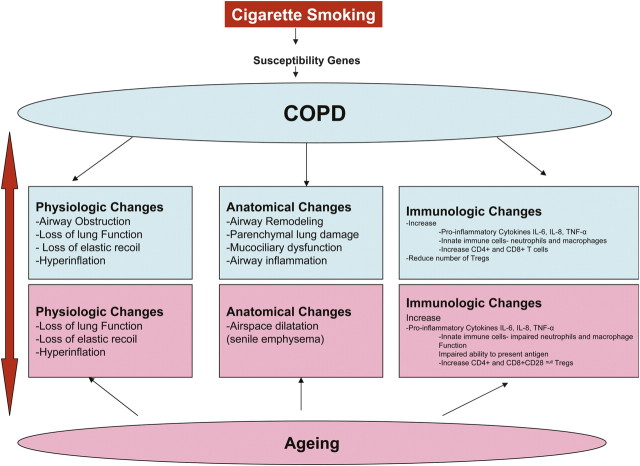
The age-dependent increase in the prevalence of COPD (9–11) suggests that changes related to aging may contribute to COPD pathogenesis (12). This association between COPD and aging is relevant and important because by 2050, approximately 22% of the U.S. population will be over 60 years old (13), and this increase will almost certainly bring about a concomitant epidemic of chronic diseases such as COPD. Physiologic changes in COPD, which include small airway obstruction, loss of elastic recoil, and dynamic hyperinflation, are responsible for the progressive impairment in exercise tolerance commonly seen in patients suffering from this disease. However, many of the anatomic and physiologic changes seen in COPD have also been described in the aging lung, even in nonsmokers, suggesting that the aging process may be a contributing factor. “Senile emphysema” characterized by airspace dilatation resulting from loss of supporting tissue without alveolar wall destruction has been described in elderly individuals without COPD (Figure 1). Furthermore, aging has been thought to be a proinflammatory condition associated with a dysregulated immune system, and aging-associated immune remodeling in the elderly is thought to play a significant role in the pathogenesis of such chronic inflammatory diseases as Alzheimer's dementia, type II diabetes mellitus, osteoporosis, and cancers. Because exaggerated systemic and tissue inflammation are important in COPD pathogenesis, immunologic changes seen in COPD may partly be secondary to the aging process. In fact, COPD has been considered an “accelerated aging phenotype” triggered by noxious stimuli like cigarette smoke (14). In this review, we will focus on the immune system changes related to aging and describe how such changes may play a role in COPD pathogenesis.
Figure 1.
Smoking- and aging-related changes to physiologic, anatomic, and immunologic parameters.
OVERVIEW OF THE IMMUNE SYSTEM
The immune system is a complex and highly regulated network of innate (natural) immunity and adaptive (acquired) immunity whose main function is to defend the host against infections.
Innate Immune System
The innate immune system provides initial defense against harmful pathogens and is composed of both noncellular and cellular constituents. Noncellular components include C-reactive protein, mannose-binding protein, serum amyloid protein, and the complement system. Cellular constituents include phagocytic cells—neutrophils, macrophages/monocytes, and natural killer (NK) cells—that recognize and engulf microbes. These cells use pattern recognition receptors (PRR) to recognize conserved molecular motifs that signal danger or infection. Toll-like receptors (TLR) are a class of PRRs which, on activation, induce downstream activation of proinflammatory cascades. Innate immune responses themselves are nonspecific and short-lived, but their subsequent downstream responses can begin antigen-specific adaptive immune responses.
Adaptive Immune System
Unlike innate immune responses, adaptive immunity uses antigen-specific receptors on B and T lymphocytes. Adaptive immunity involves humoral immune responses (mediated by B cells) and cellular responses (mediated by T cells). B cells produce immunoglobulins and are activated through T cell–dependent mechanisms or T cell–independent cross-linking of surface receptors by multi-epitope antigens.
Cellular immune responses mediated by T lymphocytes can be broadly classified by their cell surface markers. T cell responses defend against viruses and other intracellular pathogens and protect against future exposures by producing antigen-specific memory T cells. Three specific subgroups of T cells have been described. CD8+ T cells provide host defense effector functions against viral infection or tumor cells by directly killing infected somatic or tumor cells. CD4+ T helper cells orchestrate and amplify antigen-specific immune responses. After activation, naïve T cells differentiate into specific CD4+ T cell subtypes. CD4+ Th1 T cells induce cell mediated responses by producing interleukin (IL)-2 and interferon (IFN)-γ, increasing killing effects of macrophages and stimulating proliferation of CD8+ T cells. CD4+ Th2 T cells drive allergic responses by producing IL-4, -5, and -13, stimulating proliferation of B cells and inducing B cell antibody class switch. Recently, a third subpopulation of T cells called regulatory T cells (Tregs) has been described. Tregs suppress activation of the immune system and help maintain immune homeostasis and tolerance to self-antigens. Both TGF-β and IL-10 play important roles in regulatory T cell functions (15–19). Tregs play an important role in retroviral, mycobacterial, and parasitic infection (20–26).
Interaction between the Innate and Adaptive Immune Systems
Although often described as distinct arms of the immune system, innate and adaptive immunity actually work in concert to produce a functional immune response, and can activate and perpetuate overall immune responses by positive feedback signals to each other. Both noncellular components (complement system) and antigen-presenting cells (APCs) are required for T cell activation. APCs are responsible for the uptake, processing, and presentation of antigens to T cells in the context of self major histocompatability complex (MHC) proteins. Cytokines produced by activated T cells mediate adaptive immune responses and stimulate the expression of co-signaling molecules on APCs for further antigen presentation. In addition, cytokines such as IFN-γ can activate monocytes, macrophages, and NK cells.
Dendritic cells (DCs) are antigen-presenting cells in tissue responsible for initiating primary immune responses through T and B lymphocytes. Antigen uptake results in DC activation and maturation, characterized by up-regulation of a variety of co-stimulatory molecules on the cell surface, allowing for efficient presentation of the antigen as well as regulatory cytokine production. In summary, the innate and adaptive immune systems together mount appropriate and long-lasting responses to antigen stimuli to protect the host from immediate and future threats.
IMMUNE SYSTEM CHANGES WITH AGING
Evidence suggests that immunity deteriorates with age (27, 28), but immunosenescence is not an unavoidable and progressive decline of all immune functions, but rather a product of continuous remodeling of various parts of the immune system over time (29). Both branches of the immune system are affected by aging, adaptive more so than innate (Table 1).
TABLE 1.
AGING AND CHRONIC OBSTRUCTIVE PULMONARY DISEASE–RELATED CHANGES TO THE ADAPTIVE AND INNATE IMMUNE SYSTEM
|
Immune System |
Aging-related Changes |
COPD-related Changes |
||
|---|---|---|---|---|
| Adaptive Immune System | ||||
| T cells | Reduced T cell production | Increased CD4+ and CD8+ T cell in the airway and lung parenchyma | ||
| Increased number of memory cells | ||||
| Reduced T cell receptor repertoire | ||||
| Loss of CD28+ expression especially on CD8+ T cells | ||||
| Impaired ability to generate naive T cells | ||||
| Tregs | ||||
| CD4+ CD25hi | Increased | Increased in smokers with normal lung function. | ||
| CD8+CD28− | Increased | Reduced in smokers with COPD | ||
| B cells | Reduction in production of B cells | Increase B cells in the airway and lung parenchyma | ||
| Impaired activation and proliferation | ||||
| Reduced production and efficacy of antibodies | ||||
| Innate Immune System | ||||
| Neutrophils | Impaired apoptosis in presence of cytokines | Increased number of neutropils in the airways and lung parenchyma | ||
| Enhanced production of ROS | ||||
| Impaired killing | ||||
| Macrophages | Diminished production of IL-1, ROS, and IFN-γ | Increased number of macrophages in the airways and lung parenchyma | ||
| Increased level of proinflammatory cytokines TNF-α and IL-6 in peripheral blood monocytes of older adults after LPS stimulation | Impaired phagocytosis of apoptotic cells | |||
| Natural killer cells | Increased number of natural killer cells | |||
| Impaired cytotoxic activity | ||||
| Natural killer T cells | Increased number of natural killer T cells | |||
| Dendritic cells | Reduce number of plasmacytoid dendritic cells | Enhanced dendritic cell function | ||
| Decrease B-cell stimulation | ||||
| Reduced capacity to capture antigen | ||||
| Impaired capacity to phagocytose apoptotic cells | ||||
| Proinflammatory Cytokines | ||||
| Cytokines and chemokines | ||||
| IL-6 | Increased | Increased | ||
| IFN-γ | Increased | Increased | ||
| TNF-α |
Increased |
Increased |
||
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; ROS = reactive oxygen species; Tregs = T regulatory cells.
Aging-related Changes to the Adaptive Immune System
T cells are developed in the thymus, which involutes with aging, resulting in significantly fewer T cells in the peripheral blood of older adults. Also, the diversity of the naïve T cell receptor repertoire is reduced significantly (30). This may explain the decreased ability of the elderly to resist new infections, which requires recognition and proliferation against naïve antigens. Also, the presence of fewer CD4+ and CD8+ T cells may explain the reduced response to reinfection in elderly subjects. Hence, influenza vaccination is relatively ineffective in independently living older adults compared with younger adults (31). Even during years with the closest antigenic match between the vaccine and the circulating virus, the vaccine is effective in only 30 to 40% of adults older than 65 years (32). This is attributed to poor immunologic response in the elderly (33), as only a small fraction of elderly flu vaccine recipients show a fourfold rise in antibodies titer after a trivalent influenza vaccine (34). Moreover, those with a high number of CD8+ CD28null T cells were even less likely to have an appropriate antibody response (35). These results suggest that aging induces oligoclonal expansion of CD8+ T cells and loss of the CD28 receptor. This marked increase in CD8+ CD28null Tregs cells with aging inhibits antigen-specific CD4+ T cell responses, leading to a decline in adaptive immune response with aging and immunosenescence (36).
Hematopoietic stem cell (HSC) production is another important component of immune system maintenance that slows with aging in both animals and humans. The HSC of old mice are less plastic and less able to replicate (37). Old age also seems to create an environment in which lymphocyte proliferation is reduced, partly because there are fewer T cell progenitors and less IL-7 produced by stromal cells (38–40). Several changes with aging greatly decrease the output of naïve T cells, forcing the elderly body to depend on previously existing naïve T cells rather than new T cells.
The volume of hematopoietic bone marrow tissue decreases with aging (41), resulting in reduced production of B cells. Moreover, B cells in older adults have reduced capacity to proliferate and impaired ability to be activated. The quantity and efficacy of antibodies produced in response to antigen exposure in older adults is also reduced (42). This reduction in the adaptive immune system is associated with an innate immune system stimulated and up-regulated by external or internal antigenic stimuli from oxidative stress, creating a chronic proinflammatory state with aging. In the section below, we discuss the aging-related proinflammatory changes to innate immunity.
Aging-related Changes to the Innate Immune System–associated Cells and Mediators
Neutrophils/polymorhonuclear leukocytes.
Polymorphonuclear leukocytes (PMNs) are the first responders to an infection or inflammation. The total number and phagocytic activity of neutrophils in the peripheral blood remain unchanged with age (43, 44), but their function appears to be affected. Impaired chemotaxis of the neutrophils of older adults results in reduced infiltration at an injury site (45). Even though neutrophils from older adults have impaired ability to kill infected cells, they produce more reactive oxygen species (ROS) (46). Furthermore, the exaggerated presence of proinflammatory cytokines like GM-CSF and IL-6 in elderly subjects inhibits apoptosis of neutrophils. In combination, these changes suggest that the neutrophils' ability to self-proliferate and kill infectious agents is reduced in elderly subjects. However, they have increased ROS production and impaired apoptosis that may result in sustained presence of neutrophils in either systemic circulation or local tissue sites where they may induce substantial local damage.
Macrophages/monocytes.
Even though the number and phagocytic activity of macrophages is unchanged with age, impaired antigen presentation and reduced MHC II molecule expression have been demonstrated in macrophages from aged mice (47). However, after activation by mitogen, peripheral blood mononuclear cells from older persons produce more proinflammatory cytokines such as IL-1, IL-6, and TNF-α in vitro than those from younger ones (48, 49). These results suggest that the aging process may fundamentally change the biological characteristics of macrophages and may prime them to be more profoundly activated, independent of extrinsic stimuli.
Natural killer cells.
The ability of natural killer (NK) cells to perform cytotoxic effector functions and to produce IFN-γ and IL-8 in response to IL-2 declines in vitro. The number of NK cells in circulation increases with age (50–52). This implies that aging induces decline in NK cell function, as demonstrated by defective activation and response to biological stimuli in vitro, but this decline may be offset by an increase in the absolute number of NK cells.
NKT cells.
Found throughout the lymphoid compartment, NKT cells comprise approximately 1% of total lymphocytes. NKT cells influence both APC and T cell function. Studies in both mice and humans have shown an age-related increase in their number (53). Even though longer life span, active expansion, and age-related alterations in recruitment by lymphoid tissue have been suggested, the exact mechanism for increase in their numbers with aging and their contribution to immunosenescence is not fully explored.
Dendritic cells.
Dendritic cells (DCs) play a key role in initiating an adaptive immune response. In the elderly, the number of DCs is reduced in the peripheral blood and lymphoid follicles (54, 55). In addition, their chemotaxis and phagocytosis are impaired, and therefore they may fail to stimulate naïve CD4+ T cells to generate an effective adaptive immune response to an antigen (54). This functional impairment of DCs may explain the defective adaptive immune response in elderly subjects.
Proinflammatory cytokines.
A complex interaction controls cytokine production and cellular activity of the innate immune system. A nonspecific increase in the levels of proinflammatory cytokines is seen with aging, and older adults produce high amounts of proinflammatory cytokines during inflammatory responses in vivo (56). Commonly increased levels of TNF-α, IL-1, and IL-6 in the elderly are associated with several negative effects on health (57). Healthy elderly subjects also have increased amounts of IL-6 in plasma and sera (58, 59). IL-1 and TNF-α transformed fibroblasts to a senescent phenotype in vitro, but an antioxidant supplement slowed this transformation, suggesting that inappropriate and exaggerated production of these cytokines may inhibit tissue healing (38). Recently some have suggested that decreased production of sex steroid, smoking, and underlying arthrosclerosis or obesity may contribute to the increase in low-grade inflammation seen in the elderly (60, 61). These increased levels of circulating inflammatory mediators may result from a constant, low-grade activation or persistent presence of cytokine-producing cells or a dysregulated cytokine response after stimulation (61) which is not readily damped. A prime example of such dysregulation is the increased storage of ROS and impaired apoptosis found in senescent neutrophils as described above. Studies have also suggested that persistent herpes infection, cytomegalovirus (CMV) infection, or parasite antigens may significantly contribute to the increased levels of proinflammatory factors and contribute to a number of negative clinical consequences (62–65). Together, persistent immunologic dysregulation and abnormal responses to autoantigens may help explain the increased risk of autoimmune diseases in the elderly during immunosenescence, but exactly which factors are most important and what causes these age-associated changes remains largely unclear. In the following sections we will focus on the role of persistent, underlying, low-grade inflammation in the pathogenesis of COPD in the elderly.
IMMUNE SYSTEM CHANGES IN COPD
Considering the commonality of COPD and aging, intriguing links between COPD and aging can be demonstrated at the molecular level. Both aging and COPD are associated with increased oxidative stress, NF-κB activation, reduced ability to repair damaged DNA, and telomere shortening from repeated cell division (12).
Recent advances in basic science have established a fundamental role for dysregulated immune and inflammatory responses in mediating all stages of COPD, from initiation to permanent lung damage suggesting COPD as an autoimmune disease (8, 66–67). Elevated markers of inflammation predict outcomes for patients with obstructive airway diseases, and are closely related to exposure to cigarette smoke (68–72). In addition, low-grade chronic inflammation has been demonstrated to prospectively define risk of developing COPD-related complications. Dysregulated biosynthesis of several inflammatory markers such as IL-13, leukotrienes, and TGF-β have been associated with higher risk of developing COPD in both human and murine models (73–75).
An initiating event of COPD pathogenesis may likely be exposure to noxious inhalants such as cigarette smoke, which subsequently induces proinflammatory responses and in turn recruits inflammatory cells. The most common inflammatory cells in the airways of patients with COPD are macrophages, CD8+ T cells, and neutrophils. Macrophages, the most abundant cells in the bronchoalveolar lavage (BAL) fluid of patients with COPD, correlate with disease severity. Macrophages are a source of the proinflammatory cytokines TNF-α, LTB4, ROS, and IL-8. They produce elevated levels of cathepsins and matrix metalloproteases (MMPs), which cause lung parenchymal destruction. In addition, the phagocystosis of apoptotic cells by airway macrophages is impaired in patients with COPD, and the resulting decreased clearance of already recruited inflammatory cells results in persistent antigenic stimuli and inflammation (76). Neutrophils, potent mediators of proteases and ROS, are found in abundance in the sputum and BAL fluid of patients with COPD, suggesting ongoing inflammation of the airways.
Adaptive immunity also plays a role in COPD pathogenesis. Recent findings suggest that a crucial component of COPD is inappropriate T cell-mediated immune responses in time of onset, intensity, and target (77–82). Direct evidence for the critical role of T cells, both Th1 and Th2, is provided by mice with transgenic knock-in of IFN-γ and IL-13, in which emphysematous changes were predictably and massively induced (83). CD8+ T cells are increased in the airways of patients with COPD. These cells correlate with severity of airway obstruction. Lung parenchymal destruction by CD8+ T cells is mediated via the release of perforin, granzyme, and TNF-α. In addition, CD8+ T cells induce apoptosis and necrosis of airway epithelial and endothelial cells. CD4+ T cells are also found in abundance in the airways and lung parenchyma (84). Chemotaxis of innate (macrophages, neutrophils, and eosinophils) and adaptive (T and B cells) immune cells is mediated via the Th-1 response of CD4+ T cells, and is thought to be regulated by tissue-specific chemokine receptors such as CXCR3, CCR5, and CCR6 in the lungs of smokers with COPD. Moreover, airway epithelial and endothelial cells express the ligands for CXCR3 and correlate with COPD disease severity (85).
Once recruited, these leukocytes in turn activate a biological cascade of airway remodeling (86, 87) and pulmonary parenchymal destruction tilted toward protease rather than antiprotease (88, 89) activity and induce early, self-perpetuating cellular death (86, 90, 91). Each of these processes has been shown independently to induce changes similar to the emphysema in murine lungs. Later, as inflammatory mediators increase and local pulmonary damage occurs; a cycle of ongoing self-perpetuating inflammation and pulmonary damage sets in.
Less than half of smokers develop clinically significant COPD, a fact that strongly suggests the relevance of significant gene–environment interaction in the pathogenesis of this disease. This population-based observation led to a number of studies addressing the hypothesis that allelic variations in genes of innate immunity may increase disease risk (92–94). Differences in genetic regulation of inflammatory processes may explain why some people, but not others, develop the disease or develop a greater inflammatory response. Susceptible cigarette-smoking individuals with COPD have reduced levels of CD4+CD25+FOXP3 and γδ CD8+ regulatory T cells in the airways, compared with smokers with normal lung function (95, 96). These findings suggest that impaired immune ability to suppress the proinflammatory cascade may be a risk factor for developing COPD. Accordingly, common gene polymorphisms that control production of inflammatory molecules have been associated with COPD (97–100). Gene polymorphisms associated with good control of inflammation are protective against COPD development (101–102).
AGING AND COPD
COPD is characterized by progressive, poorly reversible airflow obstruction associated with an abnormal inflammatory response to smoke exposure. We believe that aging is accelerated in patients with COPD, a fact that is an important contributor to the physiologic, anatomic, and immunologic changes seen in this disease. This accelerated aging is triggered by cigarette smoke exposure in susceptible individuals.
Furthermore, aging may increase risk of developing COPD. Immunoscenescence is the complicated remodeling process of the immune system in which progressive, persistent proinflammatory adaptation occurs with loss of robust, plastic immune responses to antigens. The aging immune system seems to maintain a persistently activated innate immune response to compensate for the loss of appropriate adaptive immune response. This may create a biological environment in which the lung parenchyma becomes much more vulnerable to cigarette smoke. For example, increased levels of IL-1β, IL-6, IL-8, IL-18, and TNF-α were found in elderly subjects' plasma, serum, and peripheral white blood cells, indicating that such changes may lead to persistent presence of neutrophils in lungs, which in turn may cause emphysematous destruction via exaggerated release of neutrophil elastase (103). In this setting, cigarette smoke may further contribute to epithelial injury by inducing recruitment of neutrophils and macrophages to the lungs, and such cells in the elderly seem to be activated in uncontrolled and pathological ways, releasing more proinflammatory cytokines and amplifying a cascade of ongoing inflammation and parenchymal lung damage. Even though most studies have demonstrated that aging resulted in loss of appropriate innate immune response, recent studies by Aoshiba and coworkers and by Lambers and colleagues report intriguing possible roles for CD4+, CD8+, and CD28null in COPD pathogenesis. They demonstrated that repetitive, chronic antigen exposure induces loss of CD28 expression with aging and that CD4+ CD28null and CD8+ CD28null cells of the adaptive immune system may contribute to COPD pathogenesis (80, 104). Whether the appearance of CD28null T cells in the lungs of patients with COPD represents an epiphenomenon or true pathogenesis remains unknown.
CONCLUSIONS
Aging and COPD have several things in common. Changes in the lung that occur with aging often mimic those seen in COPD. Furthermore, both are associated with significant immune dysregulation. Therefore, one may reasonably speculate on the possible causal relationship between aging and COPD. COPD may be considered an “accelerated aging” phenotype, but at the same time aging may also contribute to the changes seen in COPD. Immune system changes seen in aging may be a key to consolidate this relationship. Immunoscenescence is strongly associated with persistently activated innate immunity and with possibly uncontrolled adaptive immunity as suggested by changes in neutrophils, macrophages, and regulatory T cells. In order for a genetically susceptible smoker to develop COPD, appropriate immune remodeling with aging has to be present. However, even though significant molecular similarities and associations have been demonstrated between aging and COPD, it is difficult to extrapolate or conclude a causal relationship without clear epidemiologic data characterizing the patterns of COPD prevalence in the elderly population. Further studies are necessary to better understand the relationship between aging-related immunologic alterations and COPD pathogenesis.
Acknowledgments
The authors thank William J. Calhoun, M.D. and Meera Gupta, M.D. for helpful comments, and Sarah Toombs Smith, Ph.D. for assistance with manuscript preparation. We are indebted to the anonymous reviewers for their insightful comments.
Conflict of Interest Statement: G.S. received lecture fees from Pfizer and AstraZeneca (AZ) ($1,001–$5,000). N.A.H. served as a consultant for GlaxoSmithKline (GSK), Dey, and Novartis ($5,001–$10,000). He received lecture fees from GSK, AZ ($10,001–$50,000), and Genentech ($1,001–$5,000), and received grant support from GSK ($50,001–$100,000), Novartis ($10,001–$50,000), Boehringer Ingelheim ($50,001–$100,000), and Dey ($10,001–$50,000). Y.M.S. received grant support from Merck ($50,001–$100,000), the American Lung Association ($50,001–$100,000), the Jeffress Foundation ($10,001–$50,000), and FAMRI ($100,001 or more).
References
- 1.Centers for Disease Control/National Center for Health Statistics. FastStats: chronic obstructive pulmonary disease (COPD). Includes: chronic bronchitis and emphysema [Internet]. Hyattsville, MD: NCHS (accessed 2009 Mar 1). Available from: http://www.cdc.gov/nchs/fastats/copd.htm
- 2.Centers for Disease Control. Chronic obstructive pulmonary disease (COPD) [Internet]. Atlanta, GA: CDC (accessed 2009 Mar 1). Available from: http://www.cdc.gov/copd/index.htm
- 3.Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J 2008;31:1334–1356. [DOI] [PubMed] [Google Scholar]
- 4.Cowburn AS, Condliffe AM, Farahi N, Summers C, Chilvers ER. Advances in neutrophil biology: clinical implications. Chest 2008;134:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin T, Xu X, Hereld D. Chemotaxis, chemokine receptors and human disease. Cytokine 2008;44:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabroe I, Parker LC, Calverley PM, Dower SK, Whyte MK. Pathological networking: a new approach to understanding COPD. Postgrad Med J 2008;84:259–264. [DOI] [PubMed] [Google Scholar]
- 7.Winkler AR, Nocka KH, Sulahian TH, Kobzik L, Williams CM. In vitro modeling of human alveolar macrophage smoke exposure: enhanced inflammation and impaired function. Exp Lung Res 2008;34:599–629. [DOI] [PubMed] [Google Scholar]
- 8.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med 2009;360:2445–2454. [DOI] [PubMed] [Google Scholar]
- 9.Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest 2005;128:1239–1244. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, Wang C, Yao W, Chen P, Kang J, Huang S, Chen B, Wang C, Ni D, Wang X, et al. COPD in Chinese nonsmokers. Eur Respir J 2009;33:509–518. [DOI] [PubMed] [Google Scholar]
- 11.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB, Jensen RL, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007;370:741–750. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest 2009;135:173–180. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO statistical dataset [Internet]. Geneva: the Organization; 2005. (accessed 2009 Mar 1). Available from: http://www.who.int/whosis/en
- 14.Barnes PJ. Future treatments for chronic obstructive pulmonary disease and its comorbidities. Proc Am Thorac Soc 2008;5:857–864. [DOI] [PubMed] [Google Scholar]
- 15.Awasthi A, Kuchroo VK. Th17 cells: from precursors to players in inflammation and infection. Int Immunol 2009;21:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol 2009;182:6121–6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haringer B, Lozza L, Steckel B, Geginat J. Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. J Exp Med 2009;206:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawrylowicz CM. Regulatory T cells and IL-10 in allergic inflammation. J Exp Med 2005;202:1459–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol 2008;9:632–640. [DOI] [PubMed] [Google Scholar]
- 20.Belkaid Y. The role of CD4(+)CD25(+) regulatory T cells in Leishmania infection. Expert Opin Biol Ther 2003;3:875–885. [DOI] [PubMed] [Google Scholar]
- 21.Canaday DH, Wu M, Lu S, Aung H, Peters P, Baseke J, Mackay J, Mayanja-Kizza H, and Toossi Z. Induction of HIV type 1 expression correlates with T cell responsiveness to mycobacteria in patients coinfected with HIV type 1 and Mycobacterium tuberculosis. AIDS Res Hum Retroviruses 2009;25:213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiacchio T, Casetti R, Butera O, Vanini V, Carrara S, Girardi E, Di MD, Battistini L, Martini F, Borsellino G, et al. Characterization of regulatory T cells identified as CD4(+)CD25(high)CD39(+) in patients with active tuberculosis. Clin Exp Immunol 2009;156:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB, et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med 2007;204:2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh KP, Gerard HC, Hudson AP, Reddy TR, Boros DL. Retroviral Foxp3 gene transfer ameliorates liver granuloma pathology in Schistosoma mansoni infected mice. Immunology 2005;114:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tenorio AR, Martinson J, Pollard D, Baum L, Landay A. The relationship of T-regulatory cell subsets to disease stage, immune activation, and pathogen-specific immunity in HIV infection. J Acquir Immune Defic Syndr 2008;48:577–580. [DOI] [PubMed] [Google Scholar]
- 26.Tenorio AR, Spritzler J, Martinson J, Gichinga CN, Pollard RB, Lederman MM, Kalayjian RC, Landay AL. The effect of aging on T-regulatory cell frequency in HIV infection. Clin Immunol 2009;130:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol 2008;43:718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol 2009;9:57–62. [DOI] [PubMed] [Google Scholar]
- 29.Vallejo AN. Immune remodeling: lessons from repertoire alterations during chronological aging and in immune-mediated disease. Trends Mol Med 2007;13:94–102. [DOI] [PubMed] [Google Scholar]
- 30.Fagnoni FF, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, Casti A, Franceschi C, Passeri M, Sansoni P. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood 2000;95:2860–2868. [PubMed] [Google Scholar]
- 31.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di PC, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 2005;366:1165–1174. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda F, Bridges C. Brammer TL. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 1999;48:1–28. [PubMed] [Google Scholar]
- 33.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006;24:1159–1169. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein E, Kaye D, Abrutyn E, Gross P, Dorfman M, Murasko DM. Immune response to influenza vaccination in a large healthy elderly population. Vaccine 1999;17:82–94. [DOI] [PubMed] [Google Scholar]
- 35.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol 2001;75:12182–12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simone R, Zicca A, Saverino D. The frequency of regulatory CD3+CD8+. J Leukoc Biol 2008;84:1454–1461. [DOI] [PubMed] [Google Scholar]
- 37.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 2007;447:725–729. [DOI] [PubMed] [Google Scholar]
- 38.Zediak VP, Maillard I, Bhandoola A. Multiple prethymic defects underlie age-related loss of T progenitor competence. Blood 2007;110:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuboi I, Morimoto K, Hirabayashi Y, Li GX, Aizawa S, Mori KJ, Kanno J, Inoue T. Senescent B lymphopoiesis is balanced in suppressive homeostasis: decrease in interleukin-7 and transforming growth factor-beta levels in stromal cells of senescence-accelerated mice. Exp Biol Med (Maywood) 2004;229:494–502. [DOI] [PubMed] [Google Scholar]
- 40.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood 2008;111:5553–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogawa T, Kitagawa M, Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev 2000;117:57–68. [DOI] [PubMed] [Google Scholar]
- 42.Burns EA, Goodwin JS. Immunodeficiency of aging. Drugs Aging 1997;11:374–397. [DOI] [PubMed] [Google Scholar]
- 43.Chatta GS, Dale DC. Aging and haemopoiesis: implications for treatment with haemopoietic growth factors. Drugs Aging 1996;9:37–47. [DOI] [PubMed] [Google Scholar]
- 44.Albright JW, Albright JF. Age-associated impairment of murine natural killer activity. Proc Natl Acad Sci USA 1983;80:6371–6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fortin CF, Larbi A, Lesur O, Douziech N, Fulop T Jr. Impairment of SHP-1 down-regulation in the lipid rafts of human neutrophils under GM-CSF stimulation contributes to their age-related, altered functions. J Leukoc Biol 2006;79:1061–1072. [DOI] [PubMed] [Google Scholar]
- 46.Chan SS, Monteiro HP, Deucher GP, Abud RL, Abuchalla D, Junqueira VB. Functional activity of blood polymorphonuclear leukocytes as an oxidative stress biomarker in human subjects. Free Radic Biol Med 1998;24:1411–1418. [DOI] [PubMed] [Google Scholar]
- 47.Herrero C, Sebastian C, Marques L, Comalada M, Xaus J, Valledor AF, Lloberas J, Celada A. Immunosenescence of macrophages: reduced MHC class II gene expression. Exp Gerontol 2002;37:389–394. [DOI] [PubMed] [Google Scholar]
- 48.Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, Monti D, Franceschi C, Paganelli R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol 1993;23:2375–2378. [DOI] [PubMed] [Google Scholar]
- 49.Delpedro AD, Barjavel MJ, Mamdouh Z, Faure S, Bakouche O. Signal transduction in LPS-activated aged and young monocytes. J Interferon Cytokine Res 1998;18:429–437. [DOI] [PubMed] [Google Scholar]
- 50.Mysliwska J, Bryl E, Chodnik T, Foerster J, Mysliwski A. Level of NK cytotoxic activity in the elderly aged more than 80 years. Arch Gerontol Geriatr 1992;15:21–28. [DOI] [PubMed] [Google Scholar]
- 51.Ogata K, Yokose N, Tamura H, An E, Nakamura K, Dan K, Nomura T. Natural killer cells in the late decades of human life. Clin Immunol Immunopathol 1997;84:269–275. [DOI] [PubMed] [Google Scholar]
- 52.Mariani E, Pulsatelli L, Meneghetti A, Dolzani P, Mazzetti I, Neri S, Ravaglia G, Forti P, Facchini A. Different IL-8 production by T and NK lymphocytes in elderly subjects. Mech Ageing Dev 2001;122:1383–1395. [DOI] [PubMed] [Google Scholar]
- 53.Faunce DE, Palmer JL, Paskowicz KK, Witte PL, Kovacs EJ. CD1d-restricted NKT cells contribute to the age-associated decline of T cell immunity. J Immunol 2005;175:3102–3109. [DOI] [PubMed] [Google Scholar]
- 54.Aydar Y, Balogh P, Tew JG, Szakal AK. Follicular dendritic cells in aging, a “bottle-neck” in the humoral immune response. Ageing Res Rev 2004;3:15–29. [DOI] [PubMed] [Google Scholar]
- 55.Agrawal A, Agrawal S, Tay J, Gupta S. Biology of dendritic cells in aging. J Clin Immunol 2008;28:14–20. [DOI] [PubMed] [Google Scholar]
- 56.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol 2001;8:131–136. [DOI] [PubMed] [Google Scholar]
- 57.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007;128:92–105. [DOI] [PubMed] [Google Scholar]
- 58.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med 2000;51:245–270. [DOI] [PubMed] [Google Scholar]
- 59.Baggio G, Donazzan S, Monti D, Mari D, Martini S, Gabelli C, Dalla Vestra M, Previato L, Guido M, Pigozzo S, et al. Lipoprotein(a) and lipoprotein profile in healthy centenarians: a reappraisal of vascular risk factors. FASEB J 1998;12:433–437. [DOI] [PubMed] [Google Scholar]
- 60.Rudin E, Barzilai N. Inflammatory peptides derived from adipose tissue. Immun Ageing 2005;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol 2004;39:687–699. [DOI] [PubMed] [Google Scholar]
- 62.Xainli J, Baisor M, Kastens W, Bockarie M, Adams JH, King CL. Age-dependent cellular immune responses to Plasmodium vivax Duffy binding protein in humans. J Immunol 2002;169:3200–3207. [DOI] [PubMed] [Google Scholar]
- 63.Saino N, Ferrari RP, Romano M, Rubolini D, Moller AP. Humoral immune response in relation to senescence, sex and sexual ornamentation in the barn swallow (Hirundo rustica). J Evol Biol 2003;16:1127–1134. [DOI] [PubMed] [Google Scholar]
- 64.Luebke RW, Copeland CB, Andrews DL. Effects of aging on resistance to Trichinella spiralis infection in rodents exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicology 1999;136:15–26. [DOI] [PubMed] [Google Scholar]
- 65.Humphreys NE, Grencis RK. Effects of ageing on the immunoregulation of parasitic infection. Infect Immun 2002;70:5148–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engstrom G, Segelstorm N, Ekberg-Aronsson M, Nilsson PM, Lindgarde F, Lofdahl CG. Plasma markers of inflammation and incidence of hospitalisations for COPD: results from a population-based cohort study. Thorax 2009;64:211–215. [DOI] [PubMed] [Google Scholar]
- 67.Higashimoto Y, Iwata T, Okada M, Satoh H, Fukuda K, Tohda Y. Serum biomarkers as predictors of lung function decline in chronic obstructive pulmonary disease. Respir Med 2009;103:1231–1238. [DOI] [PubMed] [Google Scholar]
- 68.Hacker S, Lambers C, Hoetzenecker K, Pollreisz A, Aigner C, Lichtenauer M, Mangold A, Niederpold T, Zimmermann M, Taghavi S, et al. Elevated HSP27, HSP70 and HSP90 alpha in chronic obstructive pulmonary disease: markers for immune activation and tissue destruction. Clin Lab 2009;55:31–40. [PubMed] [Google Scholar]
- 69.Rovina N, Dima E, Gerassimou C, Kollintza A, Gratziou C, Roussos C. Interleukin-18 in induced sputum: association with lung function in chronic obstructive pulmonary disease. Respir Med 2009;103:1056–1062. [DOI] [PubMed] [Google Scholar]
- 70.Bartoli ML, Di FA, Vagaggini B, Houwing-Duistermaat JJ, Kotz D, Passos VL, Wouters EF. Biological markers in induced sputum of patients with different phenotypes of chronic airway obstruction. Respiration 2009;77:265–272. [DOI] [PubMed] [Google Scholar]
- 71.Piehl-Aulin K, Jones I, Lindvall B, Magnuson A, Abdel-Halim SM. Increased serum inflammatory markers in the absence of clinical and skeletal muscle inflammation in patients with chronic obstructive pulmonary disease. Respiration 2009;78:191–196. [DOI] [PubMed] [Google Scholar]
- 72.Yanbaeva DG, Dentener MA, Spruit MA, Houwing-Duistermaat JJ, Kotz D, Passos VL, Wouters EF. IL6 and CRP haplotypes are associated with COPD risk and systemic inflammation: a case-control study. BMC Med Genet 2009;10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shim YM, Zhu Z, Zheng T, Lee CG, Homer RJ, Ma B, Elias JA. Role of 5-lipoxygenase in IL-13-induced pulmonary inflammation and remodeling. J Immunol 2006;177:1918–1924. [DOI] [PubMed] [Google Scholar]
- 74.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ Jr, Chapman HA Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest 2000;106:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elias JA, Zheng T, Lee CG, Homer RJ, Chen Q, Ma B, Blackburn M, Zhu Z. Transgenic modeling of interleukin-13 in the lung. Chest 2003;123:339S–345S. [PubMed] [Google Scholar]
- 76.Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol 2003;81:289–296. [DOI] [PubMed] [Google Scholar]
- 77.Cosio MG, Majo J, Cosio MG. Inflammation of the airways and lung parenchyma in COPD: role of T cells. Chest 2002;121:160S–165S. [DOI] [PubMed] [Google Scholar]
- 78.Majo J, Ghezzo H, Cosio MG. Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysema. Eur Respir J 2001;17:946–953. [DOI] [PubMed] [Google Scholar]
- 79.Harrison OJ, Foley J, Bolognese BJ, Long E III, Podolin PL, Walsh PT. Airway infiltration of CD4+ CCR6+ Th17 type cells associated with chronic cigarette smoke induced airspace enlargement. Immunol Lett 2008;121:13–21. [DOI] [PubMed] [Google Scholar]
- 80.Aoshiba K, Koinuma M, Yokohori N, Nagai A. Differences in the distribution of CD4+ and CD8+ T cells in emphysematous lungs. Respiration 2004;71:184–190. [DOI] [PubMed] [Google Scholar]
- 81.Motz GT, Eppert BL, Sun G, Wesselkamper SC, Linke MJ, Deka R, Borchers MT. Persistence of lung CD8 T cell oligoclonal expansions upon smoking cessation in a mouse model of cigarette smoke-induced emphysema. J Immunol 2008;181:8036–8043. [DOI] [PubMed] [Google Scholar]
- 82.Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T Cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol 2007;178:8090–8096. [DOI] [PubMed] [Google Scholar]
- 83.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, Chapman HA Jr, Shapiro SD, Elias JA. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med 2000;192:1587–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 85.Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med 2004;1:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rennard SI, Togo S, Holz O. Cigarette smoke inhibits alveolar repair: a mechanism for the development of emphysema. Proc Am Thorac Soc 2006;3:703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gauldie J, Kolb M, Ask K, Martin G, Bonniaud P, Warburton D. Smad3 signaling involved in pulmonary fibrosis and emphysema. Proc Am Thorac Soc 2006;3:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vogelmeier C, Buhl R. [Therapy of lung diseases with antiproteases.] Pneumologie 1994;48:57–62. In German. [PubMed] [Google Scholar]
- 89.Buhling F, Gerber A, Ansorge S, Welte T. [Cathepsin cysteine proteinases in the lung.] Pneumologie 1999;53:400–407. In German. [PubMed] [Google Scholar]
- 90.Petrache I, Fijalkowska I, Zhen L, Medler TR, Brown E, Cruz P, Choe KH, Taraseviciene-Stewart L, Scerbavicius R, Shapiro L, et al. A novel antiapoptotic role for alpha1-antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med 2006;173:1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thaikoottathil JV, Martin RJ, Zdunek J, Weinberger A, Rino JG, Chu HW. Cigarette smoke extract reduces VEGF in primary human airway epithelial cells. Eur Respir J 2009;33:835–843. [DOI] [PubMed] [Google Scholar]
- 92.Faber JP, Poller W, Olek K, Baumann U, Carlson J, Lindmark B, Eriksson S. The molecular basis of alpha 1-antichymotrypsin deficiency in a heterozygote with liver and lung disease. J Hepatol 1993;18:313–321. [DOI] [PubMed] [Google Scholar]
- 93.Busquets X, MacFarlane NG, Heine-Suñer D, Morlá M, Torres-Juan L, Iglesias A, Lladó J, Sauleda J, Agustí AG. Angiotensin-converting-enzyme gene polymorphisms, smoking and chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2007;2:329–334. [PMC free article] [PubMed] [Google Scholar]
- 94.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet 2000;66:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Girardi M, Lewis JM, Filler RB, Hayday AC, Tigelaar RE. Environmentally responsive and reversible regulation of epidermal barrier function by gammadelta T cells. J Invest Dermatol 2006;126:808–814. [DOI] [PubMed] [Google Scholar]
- 96.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol 2008;9:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He JQ, Burkett K, Connett JE, Anthonisen NR, Pare PD, Sandford AJ. Interferon gamma polymorphisms and their interaction with smoking are associated with lung function. Hum Genet 2006;119:365–375. [DOI] [PubMed] [Google Scholar]
- 98.He JQ, Foreman MG, Shumansky K, Zhang X, Akhabir L, Sin DD, Man SF, DeMeo DL, Litonjua AA, Silverman EK. Associations of IL6 polymorphisms with lung function decline and COPD. Thorax 2009;64:698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hegab AE, Sakamoto T, Saitoh W, Massoud HH, Massoud HM, Hassanein KM, Sekizawa K. Polymorphisms of IL4, IL13, and ADRB2 genes in COPD. Chest 2004;126:1832–1839. [DOI] [PubMed] [Google Scholar]
- 100.Stankovic MM, Nestorovic AR, Tomovic AM, Petrovic-Stanojevic ND, Andjelic-Jelic MS, Dopudja-Pantic VB, Nagorni-Obradovic LjM, Mitic-Milikic MM, Radojkovic DP. TNF-alpha-308 promotor polymorphism in patients with chronic obstructive pulmonary disease and lung cancer. Neoplasma 2009;56:348–352. [DOI] [PubMed] [Google Scholar]
- 101.Park JY, Chen L, Wadhwa N, Tockman MS. Polymorphisms for microsomal epoxide hydrolase and genetic susceptibility to COPD. Int J Mol Med 2005;15:443–448. [PMC free article] [PubMed] [Google Scholar]
- 102.Smith CA, Harrison DJ. Association between polymorphism in gene for microsomal epoxide hydrolase and susceptibility to emphysema. Lancet 1997;350:630–633. [DOI] [PubMed] [Google Scholar]
- 103.Meyer KC, Rosenthal NS, Soergel P, Peterson K. Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mech Ageing Dev 1998;104:169–181. [DOI] [PubMed] [Google Scholar]
- 104.Lambers C, Hacker S, Posch M, et al. T cell senescence and contraction of T cell repertoire diversity in patients with chronic obstructive pulmonary disease. Clin Exp Immunol 2009;155:466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]