Abstract
Dengue fever has been a major cause of morbidity and mortality in subtropical and tropical countries. We report a rare case of severe dengue with spontaneous intracranial hemorrhage. A search of literature through PubMed revealed that the largest series analyzed so far only included five cases. A 47-year-old man presented with 7 days history of fever, headache, myalgia, and vomiting with hematemesis. On the day of presentation, he had reduced consciousness and an episode of generalized tonic-clonic seizure. His Glasgow Coma Scale was E1V1M3 with anisocoria. Postresuscitation computed tomography of the brain revealed a right subdural and left thalamic hemorrhage. His blood investigations revealed thrombocytopenia, dengue virus type 1 nonstructural protein antigen test was positive, dengue IgM negative, and dengue IgG positive. A right decompressive craniectomy was done. Unfortunately, the patient died soon after. Spontaneous intracranial hemorrhage in patients with dengue fever is an uncommon entity but usually carry a grave prognosis. To date, there has been no clear management guideline for such cases, as both operative and nonoperative approaches have their own inherent risks.
Keywords: Dengue encephalopathy, dengue fever, dengue hemorrhagic fever, intracranial hemorrhage
Introduction
Dengue fever is the most significant arbovirus infection in Malaysia and tropical countries with an upward trend from 44.3 cases/100,000 population in 1999 reaching 181 cases/100,000 population in 2007.[1] WHO estimates that about 2.5 billion people are at risk of dengue. The dengue virus, a member of the Flavivirus group in the family Flaviviridae, is a single-stranded enveloped RNA virus. The 1997 WHO classification divides symptomatic dengue infection into dengue fever, dengue hemorrhagic fever, and dengue shock syndrome.[2] In 2009, WHO suggested a new classification and divided dengue fever into dengue with or without warning signs and severe dengue.[3] Expanded dengue syndrome is a new category introduced by WHO recently to include unusual presentations of dengue fever with severe organ involvement including severe neurologic complications.[4] The dengue virus may rarely cause encephalopathy and encephalitis where the incidence has been found to vary between 0.5% and 6.2%.[5,6,7] There are several pathologies that may cause encephalopathy including intracranial hemorrhage, hyponatremia, cerebral hypoxia, cerebral edema, liver failure, and renal failure.[5,8] Other than the obvious coagulopathy, platelet dysfunction, and thrombocytopenia, vasculopathy may be a contributor to the intracranial hemorrhages.[5,8] Dengue fever with intracranial hemorrhage has high morbidity and mortality however studies are few.
Case Report
A 47-year-old man presented with fever, headache, and myalgia for 7 days associated with vomiting and hematemesis of three episodes. He had reduced consciousness and suffered an episode of generalized tonic-clonic seizure on the day of the presentation. On initial assessment, his Glasgow Coma Scale (GCS) was E1V1M3 with unequal pupils of the right 3 mm and left 2 mm. The patient was hemodynamically stable with a pulse rate of 82, blood pressure of 148/87 mmHg and he was febrile with a temperature of 38.7°C. He was intubated and stabilized. Following that, his computed tomography (CT) brain revealed left thalamic bleed and right frontotemporoparietal acute subdural hemorrhage 30 mm in thickness with compression of adjacent brain parenchyma and the right lateral ventricle. There was midline shift of 20 mm to the left and effacement of basal cisterns [Figure 1].
Figure 1.
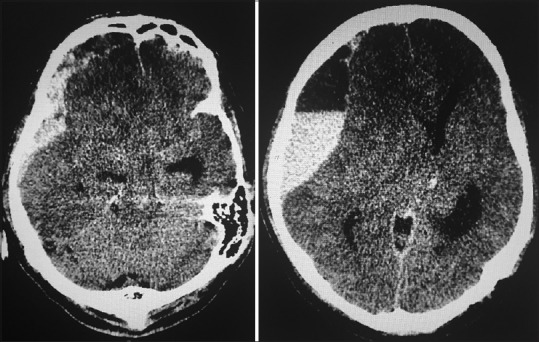
Acute right subdural hemorrhage with midline shift of 20 mm to the left and effacement of basal cisterns
The first blood tests on day 7 of fever recorded a total white cell count of 4.53 × 109/L, platelet count of 31 × 109/L, hematocrit 42.7%, and a lymphocyte count of 1.40 × 109/L. International normalized ratio (INR) was 2.63 with activated prothrombin time (APTT) 40.5 s. Nonstructural protein (NS1) antigen test was positive, dengue IgM negative and dengue IgG positive.
Coagulopathy and thrombocytopenia were treated with transfusion of fresh frozen plasma and platelet concentrate. The patient was sedated for cerebral resuscitation. The platelet count improved to 56 × 109/L and the INR reduced to 1.2 with APTT of 34.5 s. Unfortunately, the pupils were both dilated (5 mm) and were nonreactive prior to operation.
An operation was attempted 8 h after admission. A right decompressive craniectomy, evacuation of blood clots, external ventricular drainage catheter insertion, and fascia duraplasty was done.
Postoperatively, pupils remained 5 mm and nonreactive with GCS of E1VTM1 without sedation. There was no cough or gag reflex present. A repeat CT brain postoperation showed generalized cerebral edema with loss of grey-white matter differentiation, effacement of sulci and basal cisterns, bilateral thalamic bleeds, and midline shift of 10 mm to the right. Soon after, the patient's hemodynamic status deteriorated and required inotropic support. The patient died on the 3rd day of admission.
Discussion
Lab diagnosis of dengue
Laboratory suspicion of dengue fever strongly rests upon the finding of leucopenia and thrombocytopenia.[9] Laboratory confirmatory tests include antibody detection (serology), virus isolation, detection of virus genetic materials (polymerase chain reaction), and detection of dengue virus protein through NS1 antigen.[1] Even though the IgM capture enzyme-linked immunosorbent assay is the most widely used serological test, it has been shown that in secondary dengue infections, IgM was only detected in 78% of patients after day 7 in one study.[9] Another study proved that if IgM was the only test performed, 28% of the secondary dengue infections would be undiagnosed.[10] IgG, however, was detected in 100% of patients after day 7 onset of fever.[9] NS1 antigen detection rate is better during the early phase of the disease and drops from day 4–5 of illness but there are studies in which the NS1 antigen may be detected until day 14 of illness.[11,12,13] The laboratory data for this patient where NS1 antigen test was positive, dengue IgM negative and dengue IgG positive on day 7 of fever is highly suggestive of a secondary dengue infection.
Will surgery change outcome and when should it be done?
The question of whether surgery should have been done for this patient is difficult to answer. On one hand, the bleeding tendency during surgery would be disastrous if bleeding cannot be controlled, on the other hand, the threat of a progressive increase in intracranial pressure with subsequent herniation and brainstem compression is not to be taken lightly. It is unclear whether these cases should be treated similarly to other spontaneous intracranial hemorrhages. A few cases have illustrated that surgical treatment may be superior to conservative management, but further studies on this subject will be needed.[14,15] The other question that needs to be answered is about the timing of surgery. Should patients be operated on immediately with only platelet cover despite the low platelet levels.
Recommendation for platelet transfusion
The utmost concern whether surgery is feasible or not is the platelet level in patients with dengue fever where thrombocytopenia is usually severe. However, when intracranial hemorrhage is present, there is no clear evidence as to when platelets should be given. Guidelines for platelet transfusion thresholds in thrombocytopenic surgical patients and patients undergoing invasive procedures are largely based on expert opinion and clinical experience. The current agreement for neurosurgical procedures is 100 × 109/L.[16,17] In one study, a perioperative platelet count below 100 × 109/L in patients who failed to respond to platelet transfusions had a higher risk of postoperative hematoma formation.[18] Prophylactic transfusions of platelet are not recommended as it does not change or reduce the bleeding outcome in patients with dengue hemorrhagic fever and may increase the risk of pulmonary edema.[19]
When should a computed tomography scan be done?
A headache and vomiting are among the most common manifestations of dengue infection and yet may also be the only symptom of intracranial hemorrhage in these patients. It is definitely not feasible to screen all patients with dengue infection with a brain CT. Focal neurological deficits, reduction of consciousness, and seizures would warrant further investigations but these presentations may only be present when the intracranial bleed is too severe to treat. We, therefore, suggest that fundoscopy should be done for all patients with dengue fever to detect papilledema from high intracranial pressure. Although there is evidence that papilledema is associated with increased intracranial pressure in severe head injury,[20] further studies will be needed to determine the sensitivity and specificity of this examination in detecting intracranial bleeds in severe dengue fever.
Conclusion
We believe that this rare case of intracranial hemorrhage in a patient with dengue fever has highlighted the deadly severity of this rare complication in dengue infected patients. Future research in needed on the best management strategy as the number of cases of severe dengue is increasing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.2nd Rev. Edition. Kuala Lumpur: Academy of Medicine Malaysia; 2010. Ministry of Health Malaysia. Clinical Practice Guidelines: Management of Dengue Infection in Adults; p. 1. [Google Scholar]
- 2.World Health Organization. Dengue Hemorrhagic Fever: Diagnosis, Treatment, Prevention, and Control. 2nd ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 3.Dengue, Geneva: Guidelines for Diagnosis, Treatment, Prevention, and Control. New Edition, World Health Organization; 2009. World Health Organization and Special Programme for Research and Training in Tropical Diseases. [Google Scholar]
- 4.New Delhi: World Health Organization South East Asia Regional Office; 2011. World Health Organization Regional Office for South East Asia. Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Hemorrhagic Fever. Revised and Expanded Edition. [Google Scholar]
- 5.Cam BV, Fonsmark L, Hue NB, Phuong NT, Poulsen A, Heegaard ED. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg. 2001;65:848–51. doi: 10.4269/ajtmh.2001.65.848. [DOI] [PubMed] [Google Scholar]
- 6.Hendarto SK, Hadinegoro SR. Dengue encephalopathy. Acta Paediatr Jpn. 1992;34:350–7. doi: 10.1111/j.1442-200x.1992.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 7.Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, Raengsakulrach B, et al. Neurological manifestations of dengue infection. Lancet. 2000;355:1053–9. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 8.Puccioni-Sohler M, Soares CN, Papaiz-Alvarenga R, Castro MJ, Faria LC, Peralta JM. Neurologic dengue manifestations associated with intrathecal specific immune response. Neurology. 2009;73:1413–7. doi: 10.1212/WNL.0b013e3181bd8258. [DOI] [PubMed] [Google Scholar]
- 9.Schilling S, Ludolfs D, Van An L, Schmitz H. Laboratory diagnosis of primary and secondary dengue infection. J Clin Virol. 2004;31:179–84. doi: 10.1016/j.jcv.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–96. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol. 2002;40:376–81. doi: 10.1128/JCM.40.2.376-381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumarasamy V, Wahab AH, Chua SK, Hassan Z, Chem YK, Mohamad M, et al. Evaluation of a commercial dengue NS1 antigen-capture ELISA for laboratory diagnosis of acute dengue virus infection. J Virol Methods. 2007;140:75–9. doi: 10.1016/j.jviromet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Wang SM, Sekaran SD. Evaluation of a commercial SD dengue virus NS1 antigen capture enzyme-linked immunosorbent assay kit for early diagnosis of dengue virus infection. J Clin Microbiol. 2010;48:2793–7. doi: 10.1128/JCM.02142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar R, Prakash O, Sharma BS. Intracranial hemorrhage in dengue fever: Management and outcome: A series of 5 cases and review of literature. Surg Neurol. 2009;72:429–33. doi: 10.1016/j.surneu.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Prakash O, Sharma BS. Dengue hemorrhagic fever: A rare presentation as atypical acute subdural hematoma. Pediatr Neurosurg. 2008;44:490–2. doi: 10.1159/000180305. [DOI] [PubMed] [Google Scholar]
- 16.College of American Pathologists. Practice parameter for the use of fresh-frozen plasma, cryoprecipitate, and platelets. JAMA. 1994;271:777–81. [PubMed] [Google Scholar]
- 17.Slichter SJ. Evidence-based platelet transfusion guidelines. Hematology Am Soc Hematol Educ Program. 2007:172–8. doi: 10.1182/asheducation-2007.1.172. [DOI] [PubMed] [Google Scholar]
- 18.Chan KH, Mann KS, Chan TK. The significance of thrombocytopenia in the development of postoperative intracranial hematoma. J Neurosurg. 1989;71:38–41. doi: 10.3171/jns.1989.71.1.0038. [DOI] [PubMed] [Google Scholar]
- 19.Lum LC, Abdel-Latif Mel-A, Goh AY, Chan PW, Lam SK. Preventive transfusion in Dengue shock syndrome-is it necessary? J Pediatr. 2003;143:682–4. doi: 10.1067/s0022-3476(03)00503-1. [DOI] [PubMed] [Google Scholar]
- 20.Joshua SP, Agrawal D, Sharma BS, Mahapatra AK. Papilloedema as a non-invasive marker for raised intra-cranial pressure following decompressive craniectomy for severe head injury. Clin Neurol Neurosurg. 2011;113:635–8. doi: 10.1016/j.clineuro.2011.05.012. [DOI] [PubMed] [Google Scholar]


