Abstract
Breast cancer greatly threatens the health of women all over the word despite of several effective drugs. Targeted therapy for breast cancer is limited to human epidermal growth factor receptor 2 (HER2). Herceptin®, monoclonal antibody against HER2, is now widely used in HER2(+) breast cancer. Abraxane®, the current gold standard for paclitaxel (PTX) delivery, has shown superiority in breast cancer based on nanoparticle albumin bound technology. Despite these advances, further novel targeted therapy with more improved anti-tumor efficacy for breast cancer is still urgently needed. Here, we report the recombinant nanocomposites (NPs) composed of the above two clinical drugs of Abraxane® and Herceptin® (Abra/anti-HER2), which at first migrates to the tumor region through the unique targeting mechanism of human serum albumin (HSA) of Abraxane®, and sequentially further precisely recognize the HER2(+) breast cancer cells due to Herceptin®. The Abra/anti-HER2 NPs were fabricated by a “one-step” synthesis using EDC/NHS. In vitro analysis of cell viability, apoptosis and cell cycle revealed that Abra/anti-HER2 NPs showed more anti-tumor efficacy against HER2(+) SK-BR-3 cells than Abraxane® at equivalent PTX concentration. In addition, in HER2(+) breast cancer xenograft model, Abra/anti-HER2 NPs significantly inhibited tumor growth with less side effects. Moreover, the properties of more precise target and delayed release of PTX were proved by NIRF imaging. Thus, our results indicate that Abra/anti-HER2 NPs could represent a next-generation sequentially dual-targeting therapeutic agent for HER2(+) breast cancer.
Keywords: HER2(+) breast cancer, nanomedicine, targeted therapy
Introduction
The incidence of breast cancer is increasing with a 29% proportion of all new cancer diagnosis in women in 2016 1. There are varoius modalities of treatment based on the stages, histologies, and biomarkers. Early-stage breast cancers are usually treated with surgery, neoadjuvant chemotherapy, radiotherapy and endocrinotherapy, while therapies for recurrent or metastatic (stage IV) breast cancer with poor prognosis must be balanced in benefit of length and quality of life. Furthermore, choice of therapeutic agents depends on lymph node involvement, hormone receptor status and human epidermal growth factor receptor 2 (HER2) overexpression2, including chemotherapeutic drugs (Anthracycline, Taxane), endocrinotherapies (Aromatase Inhibitor, Estrogen Receptor Modulator), monoclonal antibodies (Trastuzumab, Pertuzumab), some derivatives and small molecule drugs (T-DM1, Afitinib, and Iniparib) 3 as well.
When referencing chemotherapy for breast cancer, taxanes and anthracyclines bear the brunt 4. Paclitaxel (PTX), a taxane derivative, is conventionally used in a wide range of tumors including breast cancer 5. For example, Taxol®, a commercial preparation of PTX, has been widely used for the treatment of breast cancer. However, the clinical application is limited by its poor aqueous solubility, P-glycoprotein (P-gp) substrate and complex syndrome caused by the used excipient (Cremophor EL) 6. Fortunately, the development of the crossover of nanotechnology and clinical medicine brings a revolutionary change to the treatment of breast cancer 7. Abraxane®, the formulation of PTX based on nanotechnology, is now widely used to overcome the limitations mentioned above for the treatment of breast cancer with reduced toxicity and increased penetration in tumor tissues 8. Abraxane® is a solvent-free suspension of PTX and human serum albumin (HSA) with an average size of 130nm. HSA is transported through the bloodstream to the endothelium of the blood vessels via a unique mechanism (gp60-caveolin-1-SPARC). At the point of conjugation of HSA and gp60 (glycoprotein 60) receptor, caveolin-1 is activated to form vesicles to migrate through the endothelial cell. When PTX and HSA are released to the extracellular matrix, SPARC (secreted protein acidic and rich in cysteine), which is highly expressed in breast cancer with high affinity for HSA, allows better delivery of PTX and more linear pharmacokinetics. In addition, passive targeting could be achieved based on EPR (enhanced permeability and retention) effect, which results from an aberrant and leaky tumor vasculature and the lack of a poorly developed lymphatic drainage system in many solid tumors. Lots of clinical trials have proved the superiority of Abraxane® in tumors, including breast cancer 9-12.
Monoclonal antibody also plays an important role in breast cancer treatment. Amplification of HER2 gene occurs in about 20-25% breast cancers with poor clinical outcome. Herceptin® (Trastuzumab), a humanized monoclonal antibody against HER2 13, has proven to inhibit growth of HER2(+) cell lines and xenografts with its high binding affinity to the extracellular domain of HER2. Multiple mechanisms through which trastuzumab effectly kills tumor cell were found, including inhibition of constitutive HER2 signaling, disruption of HER2/HER3 interactions and antibody-dependent cell mediated cytotoxicity (ADCC). Many clinical trials 14 were performed to evaluate trastuzumab efficacy and safety, which suggested a crucial role in the treatment of breast cancer. Now, it is widely utilized in the clinic for HER2(+) breast cancer. Despite the noteworthy advances in HER2 targeted therapy, patients relapse after a short time of response to trastuzumab, due to innate and acquired resistance, treatment of breast cancer seems to drop into a bottleneck. But, the effort to increase the treatment efficiencies of breast cancer never discontinue. T-DM1, a novel antibody-drug conjugate (ADC) 15 composed of cytotoxic agent DM1 and trastuzumab conjugating via a stable linker, could enhance the intracellular delivery of DM1, a derivative of maytansine, as a potent tubulin-binder to HER2(+) breast cancer cells, which resulted in more targeted inhibition of microtubule function and cell death 16 . In the randomized phase 3 EMILIA study, T-DM1 significantly prolonged median progression-free survival (mPFS) and median overall survival (mOS) with less toxicity, compared with capecitabine plus lapatinib for patients with HER2(+), locally advanced (LABC) or metastatic breast cancer (MBC) previously treated with a taxane and trastuzumab 17. But the dose-limiting neuropathy, diarrhea and fatigue prevented its clinical development 18 19.
In view of these researches, we were inspired naturally to explore the possibility of the recombinant NPs by the clinical drugs of Abraxane® and anti-HER2 antibody as sequentially dual-targeting therapeutic agent in breast cancer for improved therapeutic effects 20. To demonstrate this concept, Abraxane® and anti-HER2 antibody were conjugated via a stable linker as one drug delivery system (Abra/anti-HER2 NPs). Thus, the NPs could firstly migrate into the tumor region through the above unique targeting mechanism of HSA. Sequentially, anti-HER2 antibody of the NPs further precisely recognize the HER2(+) breast cancer cells for improved PTX delivery. The dual-targeting property and enhanced anti-tumor efficacy of Abra/anti-HER2 NPs against HER2(+) SK-BR-3 breast cancer cells were fully evaluated in vitro and in vivo in this study, which could provide a new strategy for breast cancer.
Materials and methods
Main materials
Abraxane® (Albumin Bound, Lot: 6109342) was from Celgene (LLC Melrose Park, IL 60160, USA). Taxol® (Paclitaxel Injection) was from Bristol-Myers Squibb (Corden Phama Latina S.P.A Via Del Murillo Km 2.800, Sermoneta, Latina, Italy). Anti-HER2 antibody (Lot: ab2428) was from Abcam. DMEM/High Glucose (Cat: SH30022.01), Pen Strep (Penicillin Streptomycin, Lot: 1665735), 0.25% Trypsin-EDTA, and PBS (Phosphate Buffered Saline, Lot: AAL211089) were from HyClone (GE Healthcare Life Sciences). FBS (Fetal Bovine Serum, Lot: 1698221) was from Gibco (Carlsbad, CA, USA). EDC (C8H17N3HCL, Cat: 25952-53-8), NHS (C4H5NO2, Cat: 6066-82-6) and NIR-797 isothiocyanate (C45H50N3NaO6S4, Cat: 152111-91-6) were purchased from Sigma-Aldrich (St.Louis, MO, USA). Cell Counting Kit-8 kit (CCK-8 kit) was from Dojindo Laboratories (Kumamoto, Japan). Annexin V-FITC/PI Apoptosis Detection kit was purchased from Nanjing KeyGen Biotech Co. (Nanjing, China). Hematoxylin-Eosin Staining Kit was from Beyotime Institute of Biotechnology (Shanghai, China). Balb/C nude mice (18-22 g, 5 weeks old, female) were obtained from Comparative Medicine Centre, Yangzhou University (Yangzhou, China). All the experiments were conducted according to the manufacturer's protocols. All reagents were of analytical grade. This study was approved by the Research Ethics Board of Zhongda Hospital affiliated to Southeast University.
Synthesis and characterization of Abra/anti-HER2 NPs
Abra/anti-HER2 NPs were synthesized using EDC/NHS by surface activation method. Briefly, 500 μL of Abraxane® was dissolved in 1mL of PBS followed by the addition of 100 μL NHS (5.75×10-7 g/mL) and 100 μL EDC (2.3×10-7 g/mL). After string at 10 rpm for 120 min, 20 μL of anti-HER2 antibody (0.2 mg/mL) was added in the suspension. After another string at 10 rpm for 120 min at 4 °C, it was ultracentrifuged at 10,000 rpm, 4 °C for 15 min to remove excess EDC, NHS and unconjugated anti-HER2 antibody. The process was repeated 3 times after sonication. Further, the recombinant Abra/anti-HER2 NPs were resuspended in 1mL of PBS and stored at -20 °C for use. Morphological characteristics of the Abra/anti-HER2 NPs were examined using a high resolution Transmission Electron Microscope (TEM). Dynamic light scattering (DLS) was performed to determine the hydrodynamic radius (Rh) of the Abra/anti-HER2 NPs at 25 °C using a DynaproTM plate reader (Wyatt Technology, Santa Barbara, CA).
Cell culture
HER2(+) SK-BR-3 breast cancer cells were cultured using high glucose Dulbecco's modified eagle medium (DMEM) supplemented with 10% FBS and 1% penicillin-streptomycin solution in 25 mL cell culture flask. Cells were cultivated in an incubator at 37 °C with 5% carbon dioxide.
Cytotoxicity
The cells were seeded onto 96-well plates at a density of 5 × 103 cells/well. After 24 h, cells were treated with Taxol®, Abraxane® or Abra/anti-HER2 NPs with equivalent PTX concentration. After incubation for an additional 48 h, CCK-8 (10 μL) were added to each well and incubated for an additional 3 h. Optical density (OD) at 450 nm was recorded by the multi-well spectrophotometer reader, then cell inhibition ratio within each group was expressed as a percentage of the viability of untreated control cells. The half maximal inhibitory concentration (IC50) were calculated to compare cytotoxicity of different drugs.
Cell cycle analyses
SK-BR-3 cells were seeded onto 6-well plates at a density of 4.0×105/well and exposed to Taxol®, Abraxane® or Abra/anti-HER2 NPs with equivalent PTX concentration for 48 h respectively. Then the cells were collected. After being washed with ice-cold PBS twice, cells were fixed with cold 70% ethanol for 20 min. After fixation, the cells were collected by centrifugation, washed with PBS and resuspended in PBS. Cells were treated with 100 μL RNase A for 30 min and then with 400 μL propidium iodide (PI) for 30 min. PI fluorescence was measured by Flow Cytometry. A plot of forward scatter versus PI intensity was used to eliminate cell debris and cell aggregates from the analysis. Fluorescence was measured for a sample of 5,000-10,000 cells, and histograms of cell number versus PI intensity were used to determine the percentage of cells in each phase of the cell cycle.
Morphological characteristics of nucleus by DAPI Stain
The cells were first cultured on slides in 24-well plates at a density of 104 cells/well. After treatment with different drugs, the cells on the slides were fixed by incubation in 4% paraformaldehyde (PFA) for 30 min. After washing with PBS for three times, the cells were incubated in 1 mg/ml DAPI in methanol for 30 min in the dark. The cells were then observed under fluorescence microscope.
Western Blotting
The harvested cells, treated with different agents for 48 h, were lysed in lysis buffer for 15 min at 4 °C, and then centrifuged at 10,000 rpm for 15 min. The supernatant was then collected and protein was measured using a Bio-Rad protein assay. After the total protein being isolated, it was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. Once blocked, the membrane was incubated with primary monoclonal antibodies for either Cleaved-caspase3, Cleaved-caspase8, Cleaved-caspase9, Bax, Bcl-2 or GAPDH at 4 °C overnight and then subsequently incubated with horseradish peroxidase-conjugated IgG antibody as the secondary antibody at room temperature for 1h. The protein bands were detected using an enhanced chemiluminescence detection system. After normalization with the corresponding expression of Cleaved-caspase3, Cleaved-caspase8, Cleaved-caspase9, Bax, Bcl-2 or GAPDH, protein expression was determined using densitometry scans.
Breast cancer xenograft model in nude mice
In vivo anti-tumor efficacies of Taxol®, Abraxane® or Abra/anti-HER2 were evaluated through tumor bearing mouse model. Balb/C nude mice were kept in filter-topped cages with standard rodent chow, water available adlibitum, and a 12 h light/dark cycle. The experiment protocol was approved by the Committee on Ethical Animal Experiment at Southeast University. They were fed with sterile food in a specific pathogen-free facility. All mice were injected subcutaneously with 1×107 SK-BR-3 cells. The length (a) and width (b) of the tumor were measured every other day. When tumor volumes (V/mm3), calculated using the formula: 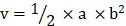 , reached approximately 60 mm3, the mice were randomly divided into four groups: saline water control group, Taxol® group, Abraxane® group and Abra/anti-HER2 group (three mice for each group, all the three groups were treated with equivalent PTX concentration at 20 mg/kg). The intravenous treatment was done twice a week for four times. The RTV (relative tumor volume) = VX/V1, where VX and V1 represent the volumes on day X and the first day of tumor treatment. The anti-tumor efficacy of tumor inhibition rate is defined as both of the tumor volume and weight inhibitory rate, which is calculated using the formula: volume inhibitory rate (%) = (1-RTVaverage experimental group / RTVaverage control group) ×100%, weight inhibitory rate (%) = (Weightaverage control group-Weightaverage experimental group/ Weightaverage control group) ×100%
, reached approximately 60 mm3, the mice were randomly divided into four groups: saline water control group, Taxol® group, Abraxane® group and Abra/anti-HER2 group (three mice for each group, all the three groups were treated with equivalent PTX concentration at 20 mg/kg). The intravenous treatment was done twice a week for four times. The RTV (relative tumor volume) = VX/V1, where VX and V1 represent the volumes on day X and the first day of tumor treatment. The anti-tumor efficacy of tumor inhibition rate is defined as both of the tumor volume and weight inhibitory rate, which is calculated using the formula: volume inhibitory rate (%) = (1-RTVaverage experimental group / RTVaverage control group) ×100%, weight inhibitory rate (%) = (Weightaverage control group-Weightaverage experimental group/ Weightaverage control group) ×100%
In vivo imaging of NIR-797 labelled NPs
Firstly, NIR-797 labelled NPs were synthesized by physical adsorption. Briefly, 1mg of NIR-797 were added to 1 mL of Taxol®, Abraxane® and Abra/anti-HER2 (5 mg/mL PTX equivalent) respectively. After stringing at 10 rpm overnight, the suspensions of Abraxane® and Abra/anti-HER2 were ultracentrifuged at 10,000 rpm, 4 °C for 15 min to remove unadsorpted dye, finally the precipitations were resuspended in 1ml of PBS for injection. For in vivo imaging, the tumor bearing mice were injected via tail vein at a single dose of NIR-797 labelled Taxol®, NIR-797 labelled Abraxane® and NIR-797 labelled Abra/anti-HER2 at 20 mg/kg PTX equivalent concentration when the tumors reached ~60 mm3. The mice were imaged in a small animal imaging system by X-ray and fluorescence at 0, 2, 4, 8, 24, 48 and 72 h after injection.
Histopathological examination
After the mice were sacrificed. Main organs and tumor tissues were quickly removed and immediately fixed in 4 % paraformaldehyde, dehydrated in a graded series of alcohol, and then embedded in paraffin. Tissue sections (4 µm) of each group were prepared, stained with hematoxylin-eosin and then observed by microscope.
Statistical analysis
The results were presented as mean±standard deviation, and analyzed with SPSS software (version 22.0; SPSS Inc, Chicago, IL, USA). The significance of differences was determined by one-way analysis of variance among multiple groups, and P<0.05 was considered statistically significant.
Results and discussion
Characterization of Abra/anti-HER2 NPs
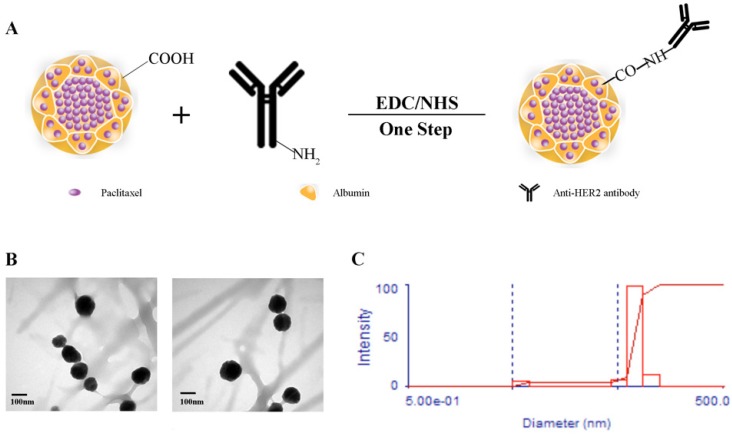
The scheme for the preparation of Abra/anti-HER2 NPs is presented in Fig.1A. Abra/anti-HER2 NPs were characterized by TEM and DLS. The general TEM images of Abraxane® (left) and Abra/anti-HER2 (right) are shown in Fig.1B. Learnt from the TEM images, the recombinant NPs have similar characteristics with the commercial Abraxane®. The average particle size of Abra/anti-HER2 was about 100 nm, as measured by DLS (Fig.1C) with a desired particle size distribution.
Figure 1.
Synthesis and characterization of Abra/anti-HER2 (A)Schematic diagram of the synthesis of Abra/anti-HER2 nanoparticles (The -COOH of Abraxane® and -NH2 of anti-HER2 antibody were combined by a “one-step” method using EDC/NHS to form a stable -CO-NH- linker) (B) Transmission electron microscopic images of Abraxane®(left) and Abra/anti-HER2(right) (C) Particle size distribution of Abra/anti-HER2.
In vitro anti-tumor efficacy
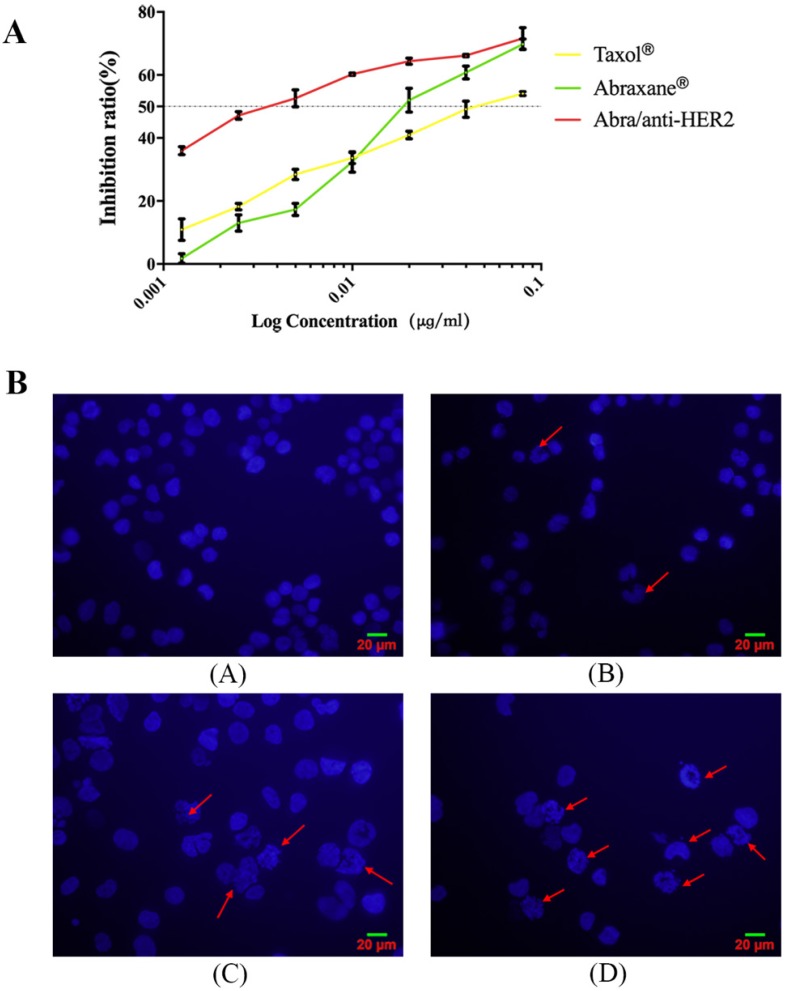
To determine the anti-tumor efficacy, we measured the cytotoxicity following treatment of HER2(+) breast cancer SK-BR-3 cells with Taxol®, Abraxane® and Abra/anti-HER2. Taxol® and Abraxane® significantly inhibited the growth of SK-BR-3 cells after 48 h of treatment, whereas Abra/anti-HER2 was found to be higher cytotoxic (Fig.2A). The half-maximal inhibitory concentration (IC50), defined as the concentration of PTX equivalent needed to kill 50% of cells, was found to be 0.04, 0.02 and 0.002 μg/ml for Taxol®, Abraxane® and Abra/anti-HER2 respectively for SK-BR-3 cells with an excellent dose-effect relationship, suggesting that the killing effects of these drugs were dose dependent. We further incubated SK-BR-3 cells with DAPI and then examined the morphology of nucleus, typical characteristic apoptotic changes, such as chromatin condensation, convoluted nucleus with cavitation's, fragmentation of the nucleus, and apoptotic bodies were more frequently observed in Abra/anti-HER2 treated SK-BR-3 cells (Fig.2B). These data clearly suggest that Abra/anti-HER2 has enhanced cytotoxic effects against SK-BR-3 cells than Taxol® and Abraxane® in vitro.
Figure 2.
Cytotoxic effects of Taxol ®, Abraxane® and Abra/anti-HER2 on SK-BR-3 cells A: Cell viability for Taxol®, Abraxane® and Abra/anti-HER2 in SK-BR-3 cells. B: Morphologic characterizations of SK-BR-3 cells treated with different agents for 48 h under inverted fluorescence micrographs (4′,6-diamidino-2-phenylindole staining) (A) Control group; (B) 0.04 μg /mL Taxol®; (C) 0.04 μg /mL Abraxane®; (D) 0.04μg/mL Abra/anti-HER2(apoptotic cells were labelled by red arrows)
Cell cycle distributions of SK-BR-3 cells
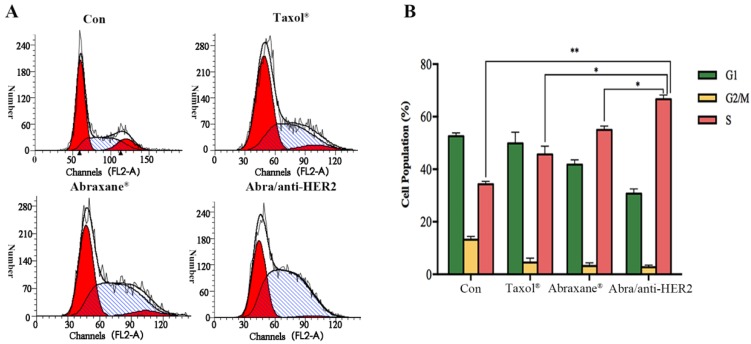
Given the fact that PTX's mode of action is disruption of microtubule dynamics, we quantified the population of SK-BR-3 cells in different stages of the cell cycle upon treatment with Taxol®, Abraxane® and Abra/anti-HER2 respectively at a concentration of 0.02 ng/ml PTX equivalent for 48 h. The percentages of cell population in each phase of the cell cycle are indicated in the histograms. Exposure of 0.02 ng/ml Abra/anti-HER2 showed significant S arrest (66.63%, P<0.05 versus free drug, Fig.3). PTX was believed to mediate G2/M cell cycle arrest in lots of cancer cells including breast cancer. In our study, significant S phase arrest was frequently observed in SK-BR-3 cells, we hypothesized that the component of anti-HER2 antibody changed the mode of disrupting microtubule dynamics which deserves further investigation.
Figure 3.
Cell cycle distributions of SK-BR-3 cells treated with different agents SK-BR-3 cells were treated with 0.02 ng/mL PTX equivalent at 37 ℃ for 48h. Samples were analysed by flow cytometry (A). Results are expressed as the percentage of each subpopulation (B) as determined by analysis of 5,000 cells per sample (*: P< 0.05, **: P<0.01)
Apoptosis-related protein expressions of SK-BR-3 cells
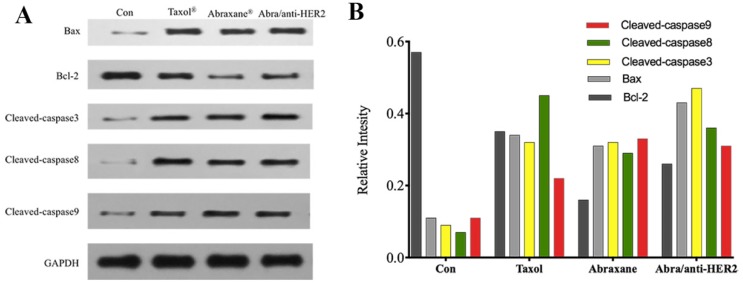
To explore the possible signaling pathways involved in apoptosis, we examined changes in the protein expression levels of apoptosis-related gene, including Cleaved-caspase3, Cleaved-caspase8, Cleaved-caspase9, Bax, Bcl-2, and GAPDH by Western blot. As shown in Fig.4A, 4B, when treated with Taxol®, Abraxane® and Abra/anti-HER2 for 48 h, the levels of Bcl-2 protein in SK-BR-3 cells were both significantly down-regulated, while those of Bax, Cleaved-caspase3, Cleaved-caspase8, Cleaved-caspase9 proteins were up-regulated as well as the ratio of Bax/Bcl-2. Caspase activation is generally considered to be the hallmark of apoptosis, and Caspase3 is the main effector caspase that is involved in apoptosis. These results indicate that Abra/anti-HER2 NPs induced anti-tumor activity in a caspase-dependent manner.
Figure 4.
The expression of apoptosis-related proteins in the SK-BR-3 cells by Western blot after treatment of 0.04 μg/ml PTX equivalent for 48 hours (A) Representative western blot showing the expression of Bax, Bcl-2, cleaved-caspase 3, cleaved-caspase 8 and cleaved-caspase 9 protein (B) Relative intensity of related proteins
In vivo anti-tumor efficacy
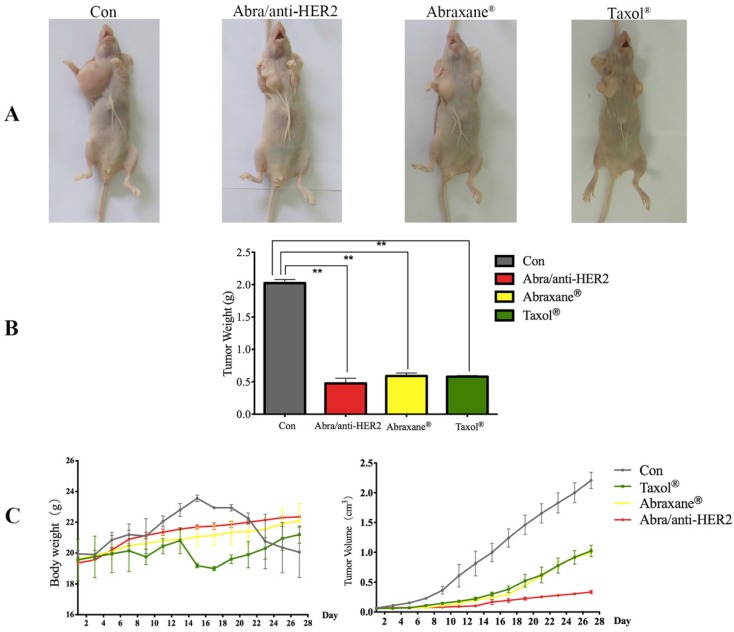
Abra/anti-HER2 NPs were evaluated for the in vivo anti-tumor efficacy in a SK-BR-3 xenograft model compared with Taxol® and Abraxane® at a 20 mg/kg PTX equivalent dose. During the experiment period, all the mice were weighed and tumor volumes were measured every other day. After treatment, all mice were sacrificed. Then mice were pictured (Fig.5A) and tumor weight was recorded (Fig.5B). Significant tumor regression with less body weight loss was observed in mice treated with Abra/anti-HER2 NPs compared with Taxol® and Abraxane®. At 4 weeks after treatment, Abra/anti-HER2 NPs treated mice had a mean tumor volume of 336 ± 27 mm3 versus 996 ± 61 mm3 for Abraxane®, 1023 ± 94 mm3 for Taxol® and 2210 ± 138 mm3 for saline-treated controls. Clearly, the Abra/anti-HER2 NPs outperform Abraxane® and Taxol® in reducing tumor volume with less side effects, which correlated with a body weight increase (Fig.5C).
Figure 5.
Photographs showing the appearance of tumor body in tumor-bearing nude mice (A); Tumor weight at the end of treatment (B); Tumor volume and body weight after the treatment. (C) (*: P<0.05, **: P<0.01)
Histopathological examination of tumor tissue
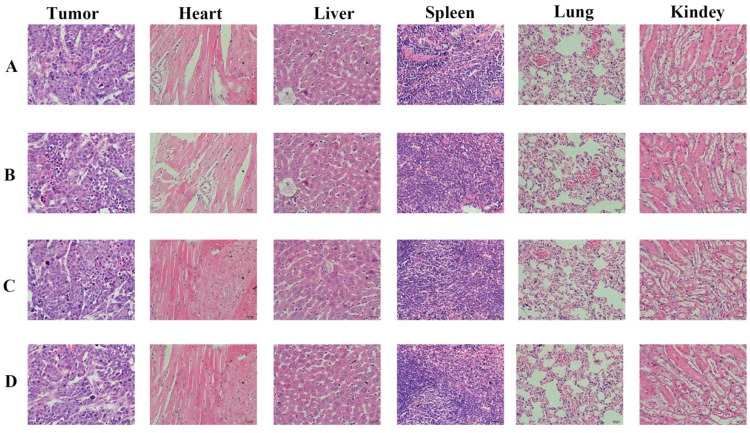
We carried out histological analysis of organs to evaluate the potential side effects of Abra/anti-HER2 NPs on the main organs of mice in vivo. There were no apparent histopathologic changes in the tissues, including heart, liver, spleen, lung, kidney (Fig.6).
Figure 6.
Histopathological examination of organs and tumor tissues in SK-BR-3 xenograft model after the treatment (hematoxylin-eosin staining, ×200). (A) Control group; (B) Taxol®; (C) Abraxane®; (D) Abra/anti-HER2
In vivo NIRF imaging and biodistribution study of NIR-797-labelled drugs in tumor-bearing mice
Inspired by the success of albumin-bound formulation for PTX, various new and innovative drug formulations based on nano-drug delivery system has kept on developing 21, after passive targeted delivery of anti-tumor drugs based on EPR effect, deliberate modifications of ligand or antibody to the surface of nanoparticles were conducted to achieve specific targeting to the corresponding receptor on tumor cell. Furthermore, trastuzumab was proved to enhance the cytotoxic effects of PTX towards HER2(+) breast cancer cells in vitro and in vivo. The conjugation of PTX and trastuzumab was thought to be promising and worthy. Although clinical results with ADCs prepared utilizing conventional anti-tumor drugs had failed to demonstrate therapeutic benefit with shortcomings of poor in vitro potency, modest in vivo activity and localization in human tumors. On this basis, we can not help to figuring out “next-generation nanocarriers” with the expectation for more efficient and targeted therapy. Maybe a new formulation of PTX would overcome the shortcomings mentioned above. Inspiration arises spontaneously, it arises a curiosity of what if we conjugated Abraxane® with anti-HER2 antibody which are both commercially approved.
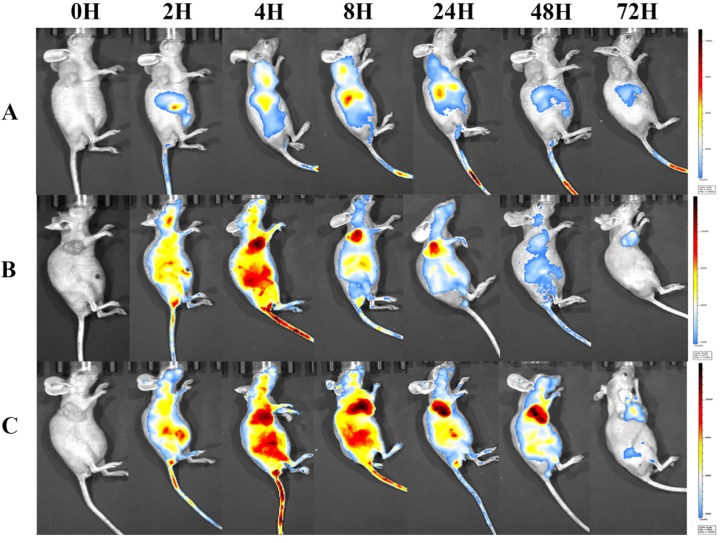
To visualize the biodistribution of Abra/anti-HER2 NPs in SK-BR-3 tumor bearing mice, a near-infrared fluorescent (NIRF) dye, NIR-797, was labelled to Taxol®, Abraxane® and Abra/anti-HER2 22. NIR fluorescence signals were clearly and dynamically observed in the mice 23. 2 h after injection, the fluorescence signals of NIR-797 labelled Taxol® was weak and mainly localized in the liver area, but it began to appear in the tumor site at 4-8 h and decreased gradually especially in the tumor site with no distribution in the brain 24. Obviously, increased accumulation of NIR fluorescence signals was observed in the tumor site of the mice injected Abraxane® and Abra/anti-HER2. Strikingly, as time increased, the fluorescence signals of tumor became stronger and on the contarary the fluorescence signals of liver began to weaken. Fluorescence signals of NIR-797 labelled Abra/anti-HER2 remained strong until 48 h after injection, at which point the fluorescence signals of NIR-797 labelled Abraxane® has already decreased. In addition, 72 h after injection, fluorescence signals could be only watched in the tumor site which indicated a better targeting and sustained release of Abra/anti-HER2 than Abraxane®(Fig.7). The unique targeting mechanism of HSA and passive targeting properties of Abraxane® along with the active targeting properties of anti-HER2 antibody may introduce sequentially dual-targeting and more efficient PTX delivery. What is interesting and exciting, fluorescence signals could be found in the head in NIR-797 labelled Abra/anti-HER2 and NIR-797 labelled Abraxane®, which may indicate the blood-brain barrier (BBB)-penetrating property of nanoparticles, further studies were needed and it may provide a promising avenue for targeting the brain tumor based on nanomedicine 25.
Figure 7.
NIRF images of major organs and part of tumors visualized through the Caliper IVIS after different treatments from 0h-72h (A) Taxol®; (B) Abraxane®; (C) Abra/anti-HER2
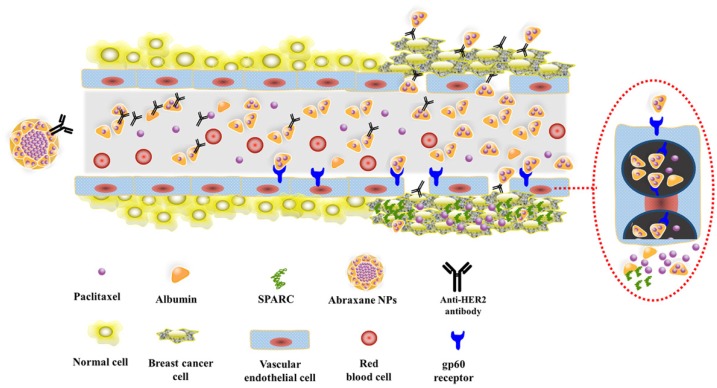
Consequently, based on the above studies, Fig.8 schematically illustrates the possible process of Abra/anti-HER2 NPs mediated sequentially dual-targeting therapy for breast cancer 26. Firstly, Abra/anti-HER2 were prepared using a “one-step” method to act as a drug delivery system, in which Abraxane plays the role of delivering PTX into tumor region through the unique targeting mechanism of HSA and passive targeting based on EPR effect, while anti-HER2 antibody plays the role of active recognition and precise targeting of HER2(+) breast cancer cells. Thereby, the increased intracellular concentration of PTX enhanced the anti-tumor efficacy of chemotherapeutic agent.
Figure 8.
Schematic diagram of the dual-targeting process
Conclusion
In summary, our research successfully synthesized a sequentially dual-targeting recombinant Abraxane®/anti-HER2 antibody nanocomposites for the treatment of HER2(+) breast cancer. In vitro and in vivo experiments demonstrated that Abra/anti-HER2 NPs outperforms Abraxane®, the current gold standard for PTX delivery with the properties of sequentially dual-targeting and more cytotoxicity to HER2(+) breast cancer. Our study may open up a new idea in such a dilemma for the treatment of breast cancer.
Acknowledgments
This research was supported by the Jiangsu Provincial Medical Youth Talent (QNRC2016809).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Giordano SH, Temin S, Kirshner JJ, Chandarlapaty S, Crews JR, Davidson NE. et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:2078–99. doi: 10.1200/JCO.2013.54.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabi A, Malaguti P, Vari S, Cognetti F. First-line therapy in HER2 positive metastatic breast cancer: is the mosaic fully completed or are we missing additional pieces? J Exp Clin Cancer Res. 2016;35:104. doi: 10.1186/s13046-016-0380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergh J, Jonsson PE, Glimelius B, Nygren P. A systematic overview of chemotherapy effects in breast cancer. Acta oncologica (Stockholm, Sweden) 2001;40:253–81. doi: 10.1080/02841860151116349. [DOI] [PubMed] [Google Scholar]
- 5.Spencer CM, Faulds D. Paclitaxel. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the treatment of cancer. Drugs. 1994;48:794–847. doi: 10.2165/00003495-199448050-00009. [DOI] [PubMed] [Google Scholar]
- 6.Nehate C, Jain S, Saneja A, Khare V, Alam N, Dubey RD. et al. Paclitaxel formulations: challenges and novel delivery options. Current drug delivery. 2014;11:666–86. doi: 10.2174/1567201811666140609154949. [DOI] [PubMed] [Google Scholar]
- 7.Blanco E, Ferrari M. Emerging nanotherapeutic strategies in breast cancer. Breast (Edinburgh, Scotland) 2014;23:10–8. doi: 10.1016/j.breast.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Dranitsaris G, Yu B, Wang L, Sun W, Zhou Y, King J. et al. Abraxane(R) versus Taxol(R) for patients with advanced breast cancer: A prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22:205–11. doi: 10.1177/1078155214556008. [DOI] [PubMed] [Google Scholar]
- 9.Tauchi Y, Kashiwagi S, Ishihara S, Asano Y, Sakimura C, Kurata K. et al. [Clinical experience of nab-Paclitaxel treatment in 31 patients with breast cancer] Gan to kagaku ryoho Cancer & chemotherapy. 2014;41:1948–50. [PubMed] [Google Scholar]
- 10.Gluck S. nab-Paclitaxel for the treatment of aggressive metastatic breast cancer. Clin Breast Cancer. 2014;14:221–7. doi: 10.1016/j.clbc.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Mrozek E, Layman R, Ramaswamy B, Lustberg M, Vecchione A, Knopp MV. et al. Phase II trial of neoadjuvant weekly nanoparticle albumin-bound paclitaxel, carboplatin, and biweekly bevacizumab therapy in women with clinical stage II or III HER2-negative breast cancer. Clin Breast Cancer. 2014;14:228–34. doi: 10.1016/j.clbc.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forero-Torres A, Varley KE, Abramson VG, Li Y, Vaklavas C, Lin NU. et al. TBCRC 019: A Phase II Trial of Nanoparticle Albumin-Bound Paclitaxel with or without the Anti-Death Receptor 5 Monoclonal Antibody Tigatuzumab in Patients with Triple-Negative Breast Cancer. Clin Cancer Res. 2015;21:2722–9. doi: 10.1158/1078-0432.CCR-14-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–31. [PubMed] [Google Scholar]
- 14.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Investigators B. Phase III trial comparing AC-T with AC-TH and with TCH in the adjuvant treatment of HER2 positive early breast cancer patients: second interim efficacy analysis. Breast cancer research and treatment. 2006;100:53. [Google Scholar]
- 15.Janthur WD, Cantoni N, Mamot C. Drug conjugates such as Antibody Drug Conjugates (ADCs), immunotoxins and immunoliposomes challenge daily clinical practice. International journal of molecular sciences. 2012;13:16020–45. doi: 10.3390/ijms131216020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corrigan PA, Cicci TA, Auten JJ, Lowe DK. Ado-trastuzumab emtansine: a HER2-positive targeted antibody-drug conjugate. The Annals of pharmacotherapy. 2014;48:1484–93. doi: 10.1177/1060028014545354. [DOI] [PubMed] [Google Scholar]
- 17.Welslau M, Dieras V, Sohn JH, Hurvitz SA, Lalla D, Fang L. et al. Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2-positive locally advanced or metastatic breast cancer. Cancer. 2014;120:642–51. doi: 10.1002/cncr.28465. [DOI] [PubMed] [Google Scholar]
- 18.Burris HA 3rd, Tibbitts J, Holden SN, Sliwkowski MX, Lewis Phillips GD. Trastuzumab emtansine (T-DM1): a novel agent for targeting HER2+ breast cancer. Clin Breast Cancer. 2011;11:275–82. doi: 10.1016/j.clbc.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Lambert JM, Chari RV. Ado-trastuzumab Emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. Journal of medicinal chemistry. 2014;57:6949–64. doi: 10.1021/jm500766w. [DOI] [PubMed] [Google Scholar]
- 20.Wei X, Juan ZX, Min FX, Nan C, Hua ZX, Qing FZ. et al. Recombinant immunotoxin anti-c-Met/PE38KDEL inhibits proliferation and promotes apoptosis of gastric cancer cells. J Exp Clin Cancer Res. 2011;30:67. doi: 10.1186/1756-9966-30-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keles E, Song Y, Du D, Dong WJ, Lin Y. Recent progress in nanomaterials for gene delivery applications. Biomater Sci. 2016;4:1291–309. doi: 10.1039/c6bm00441e. [DOI] [PubMed] [Google Scholar]
- 22.Tang Q, An Y, Liu D, Liu P, Zhang D. Folate/NIR 797-conjugated albumin magnetic nanospheres: synthesis, characterisation, and in vitro and in vivo targeting evaluation. PloS one. 2014;9:e106483. doi: 10.1371/journal.pone.0106483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Ruan Z, Li T, Yuan P, Yan L. Near infrared imaging-guided photodynamic therapy under an extremely low energy of light by galactose targeted amphiphilic polypeptide micelle encapsulating BODIPY-Br2. Biomater Sci. 2016;4:1638–45. doi: 10.1039/c6bm00581k. [DOI] [PubMed] [Google Scholar]
- 24.Zhang TT, Li W, Meng G, Wang P, Liao W. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater Sci. 2016;4:219–29. doi: 10.1039/c5bm00383k. [DOI] [PubMed] [Google Scholar]
- 25.Lin T, Zhao P, Jiang Y, Tang Y, Jin H, Pan Z. et al. Blood-Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano. 2016;10:9999–10012. doi: 10.1021/acsnano.6b04268. [DOI] [PubMed] [Google Scholar]
- 26.Scarano W, de Souza P, Stenzel MH. Dual-drug delivery of curcumin and platinum drugs in polymeric micelles enhances the synergistic effects: a double act for the treatment of multidrug-resistant cancer. Biomater Sci. 2015;3:163–74. doi: 10.1039/c4bm00272e. [DOI] [PubMed] [Google Scholar]