Abstract
Niemann-Pick C1-like 1 (NPC1L1) and Niemann-Pick C2 (NPC2) is a critical mediator of cholesterol absorption. The aim of the present study was to investigate the prognostic value of NPC1L1 and NPC2 in human primary hepatocellular carcinoma (HCC). The expression level of NPC1L1 and NPC2 were evaluated by Immunohistochemistry, Westen blot and Real-time Quantitative PCR. Protein expression level in tissue was represented by integral optic density (IOD). For prognosis analyses, outcome-based cut-point was calculated by X-tile software. Kaplan-Meier analysis, Cox regression analysis were used evaluate prognostic value of NPC1L1 and NPC2 and NPC1L1/NPC2 combination. Both of NPC1L1 and NPC2 were significantly decreased in HCC tissues than peritumoral liver tissues (61 pairs of tissue for Immunohistochemistry and 10 pairs of tissues for Western blot and Real-time Quantitative PCR), respectively. (n=61: p=0.0005 for NPC1L1 and p=0.0001 for NPC2; n=10: p=0.0002 for NPC1L1 and p=0.0489 for NPC2). Kaplan-Meier analyses in 265 HCC cases were showed that the low expression level of NPC1L1 and NPC2 and NPC1L1/NPC2 combination were significantly correlated with poor overall survival (OS) and shorter time to recurrence (TTR). In addition, univariate and multivariate Cox analyses showed that the expression level of NPC1L1/NPC2 combination in HCC was an independent prognostic factor for OS and TTR. Conclusion: NPC1L1 and NPC2 were lowly expressed in HCC compared with peritumoral liver tissues, and low expression of NPC1L1 and NPC2 in HCC tissues may indicate poor outcome of HCC patients after surgery. NPC1L1/NPC2 combination is an independent prognostic factor for OS and TTR in postoperative HCC patients.
Keywords: HCC: Hepatocellular Carcinoma, NPC1L1: Niemann-Pick C1-like 1, NPC2: Niemann-Pick C2, FFPE: Formalin-fixed paraffin-embedded, IHC: Immunohistochemical, OS: Overall survival, TTR: Time to recurrence.
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer related deaths in China 1. Surgical resection is most effective treatment among the numerous therapeutic methods for HCC. However, due to the high rate of recurrence or metastasis (50-70% at 5 year) after surgery 2, hinders further improvements in HCC survival 3. The identification of novel effective biomarkers to classify patients with low or high risk at survival and recurrence, would provide a guide to clinicians to select therapeutic strategies for each patients and provide personalized therapy according to the predicted risk of survival or recurrence 3-5.
Niemann-Pick C1-like 1 (NPC1L1) protein plays key rules in intestinal cholesterol absorption 6. NPC1L1 is highly expressed in the human liver and intestine 6, 7. In humans, it has been reported that ezetimibe, a cholesterol absorption inhibitor that may reduce plasma cholesterol by inhibiting NPC1L1 function in both intestine and liver, and hepatic NPC1L1 may have evolved to protect the body from excessive biliary loss of cholesterol 8. NPC2 is a small secretory protein (molecular mass is 19-23kDa) which is widely expressed in the various body cells and specifically binds un-esterified sterols, and free cholesterol 9, 10.
Yamanashi et al. has been reported that, NPC1L1 down-regulates the expression and secretion of NPC2 by inhibiting its maturation and accelerating its degradation. They also proposed that NPC2 functions as a regulator of intracellular cholesterol trafficking and biliary cholesterol secretion. Therefore, in addition to its role in cholesterol re-uptake from the bile by hepatocytes, and hepatic NPC1L1 may control cholesterol homeostasis via the down-regulation of NPC2 11.
However, the expression pattern and prognostic vale role of NPC1L1 and NPC2 in HCC remains unclear. To address this question, we used immunohistochemistry (IHC), Western blot, and Real-time Quantitative PCR to evaluated NPC1L1 and NPC2 expression features in HCC tissue and peritumoral liver tissues. Furethermore, we analyzed the association between NPC1L1 and NPC2 expression levels and clinicopathological factors or the prognosis of patients with HCC.
Materials and methods
Patients and specimens
Three hundred and twenty six HCC formalin-fixed paraffin-embedded (FFPE) specimens were selected randomly and diagnosed at the Eastern Hepatobiliary Surgery Hospital between Jan 2005 and Dec 2011, among which 61 HCC tumor cases with a paired peritumoral tissue was used as expression pattern cohort and 265 HCC tumor cases without a paired peritumoral tissue was used as prognosis cohort. Patients were followed-up until December 2014. For Western blot and Real-time Quantitative PCR analyses 10 paired HCC tissues and peritumoral liver tissues were obtained at same hospital between April 2017 and May 2017 (summarized in supplementary table S1).
The enrollment criteria for all patients used in the study were: (1) diagnosis was consistent with histological diagnostic criteria of WHO; (2) pathological diagnosis of hepatocellular lesions; (3) patient had not undergone pre-operative anti-cancer treatment and there was no evidence of extrahepatic metastases; and (4) complete follow-up data was available for prognosis cohort of 265 cases. Institutional review board approval and written informed consent from all patients were obtained. The OS was defined as the interval between surgery and death or the last observation taken. The time-to-recurrence (TTR) was defined as from the date of tumor resection until the detection of tumor recurrence, death or last observation. Patient follow-up was performed every 3 months during the first year after surgery and every 6 months thereafter until December 2014. Follow-up were performed by two physicians unaware of the study. All patients were monitored by abdomen ultrasonography, chest X-ray, and serum alpha fetoprotein (AFP) concentration 1-6 month during the first year after surgery and every 3-6 months thereafter. Computed tomography scanning or magnetic resonance imaging of the abdomen was performed every 6 months or immediately after a recurrence was suspected. The diagnosis criteria for recurrence were equal to that for the preoperative diagnosis. Hematoxylin and eosin (HE) stained slides were made from each FFPE tissue and were reviewed by two experienced hepatopathologists 12.
Construction of tissue microarrays and immunohistochemistry (IHC)
Tissue microarrays construction, IHC and integrated optical density (IOD) measurement performed as the method previously reported 12-14. All patients of HE-stained slides were reviewed and identified by two experienced pathologists and the representative cores were pre-marked in the paraffin blocks. Tissue cylinders with a diameter of 1.0 mm were punched from the marked areas of each block and incorporated into a recipient paraffin block. Sections of 4 μm-thick were placed on slides coated with 3-aminopropyltriethoxysilane.
Paraffin sections were deparaffinized in xylene and rehydrated through decreasing concentrations of ethanol (100%, 95%, and 85% for 5 min each). Antigens were unmasked by microwave irradiation for 3 min in pH 6.0 citric buffer and cooled at room temperature for 60 min. Endogenous peroxidase activity was blocked by incubation of the slides in 3% H2O2/phosphate-buffered saline, and non-specific binding sites were blocked with goat serum.
Primary antibody was as follows: Rabbit polyclonal to NPC1L1-HRP (ab201773; Abcam, USA; 1:100 dilution, cytoplasmic or/and membrane staining), Rabbit polyclonal to NPC2 (A5413; Abclonal, USA; 1:2000 dilution, cytoplasmic or/and membrane staining). An EnVision Detection kit (GK500705: Gene Tech, Shanghai, China) was used to visualize NPC2 and NPC1L1 was directly used DAB as the chromogen. Tissue sections were counterstained with hematoxylin for 5 min. Negative control slides omitting primary antibody were created for all assays. For measurement IOD, the image system comprised a Leica CCD camera DFC420 connected to a Leica DM IRE2 microscope (Leica Microsystems Imaging Solutions Ltd, Cambridge, United Kingdom). Photographs of representative fields were captured under high-power magnification (×200) using Leica QWin Plus v3 software. The IODs of in each image were counted and measured using Image-Pro Plus v6.0 software (Media Cybernetics Inc, Bethesda, MD, USA).
Protein extraction and western blotting
Western blot was performed according to previous study 15. Briefly, tissue samples were homogenated in a RIPA buffer (Qiagen, China) with a cocktail of proteinase inhibitors (Roche Applied Science, Switzerland) and a cocktail of phosphatase inhibitors (Roche Applied Science). The protein concentrations were determined using the Bicinchoninic Acid (BCA) Kit (Pierce). Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The membranes were incubated with primary NPC2 antibodies (A5413; Abclonal, USA) at 1:1500, or NPC1L1-HRP (ab201773; Abcam, USA): 1:2000) overnight at 4 °C. After washing the membrane, NPC1L1 was visualised using ECL development solution. For detection of NPC2, secondary antibodies incubated at room temperature for 1~2 h and NPC2 was visualised using ECL development solution.
RNA isolation and real-time RT-PCR
Real-time PCR was performed according to previous study 15. Briefly, total RNA was extracted from tissues using TRIzol reagent (Invitrogen, Carlsbad, CA), and reverse transcription was performed using PrimeScript™ RT Reagent Kit (Takara, Dalian, China) according to the manufacturer's instructions. The real-time polymerase chain reaction (PCR) was subsequently performed with SYBR Premix Ex Tag (Takara, Dalian, China). The PCR reaction conditions were as follows: 95°C for 15s followed by 40 cycles of 95°C for 5s and 60°C for 30s. The expression levels were normalized against those of the internal reference gene β-actin, the relative expression levels were determined by the following equation: 2-ΔCt (ΔCt=Ct target - Ct β-actin). The related primers were listed as following:
NPC1L1: Forward 5'-CCAAGTCGACTGGAAGGACC-3'; Reverse 5'-AGGGCCTCTGCCTCAGAATA-3';
NPC2: Forward 5'-CCAGTACAGATCGTTTCTCATCTCT-3'; Reverse 5'-CCGGAGCTCAGTTTCTGCTA-3';
beta-actin: Forward 5'-TTGTTACAGGAAGTCCCTTGCC3'; Reverse 5'-ATGCTATCACCTCCCCTGTGTG-3'.
X-tile analysis
X-tile plots were used for assessment of NPC1L1 and NPC2 expression which was represented as IOD and optimization of cut-points based on outcome 16. Statistical significance was assessed using the cut-off score derived from 265 cases by a standard log-rank method, with P values obtained from a lookup table.
Statistical analysis
Statistical analyses were carried out with SPSS statistical software package (SPSS Standard version 13.0; SPSS, Chicago, IL, USA) and GraphPad Prism 5.01. Optimal cut-point which used for survival analyses for NPC1L1 and NPC2 expression was obtained using X-tile software version 3.6.1 (Yale University School of Medicine, New Haven, CT, USA) 16. Statistical significance of the correlation between NPC1L1 and NPC2 expression and patient survival was estimated by the Mantel Cox log-rank test. Correlations between variables, univariate survival analysis and multiple Cox proportional hazards regression were performed using the SPSS statistical software package (SPSS Standard version 13.0; SPSS, Chicago, IL, USA). A significant difference was considered if the P value from a two-tailed test was less than 0.05.
Results
Features of NPC1L1 and NPC2 expressions in HCC tissues and Peritumoral tissues
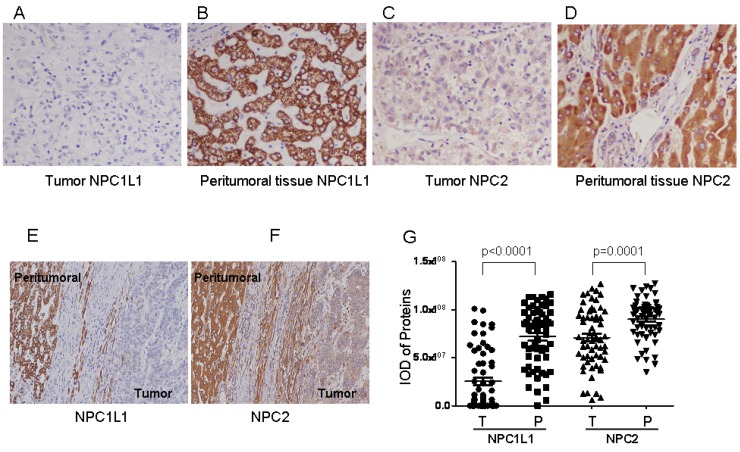
Staining feature of NPC1L1 and NPC2 in HCC showed cytoplasmic or/and membrane immunoreactivity for both HCC tissues and peritumoral tissues, respectively (Figure 1 A-F). To compare the expression levels of NPC1L1 and NPC2 in HCC tumor tissues and peritumoral tissue in expression pattern cohort (61 cases), IOD which represent the expression level from 61 HCC tumors and paired peritumoral tissue were submitted to GraphPad Prism software. As shown in Figure 1 G, expression of NPC1L1 and NPC2 were frequently lower in HCC tumor tissues than that in peritumoral tissue (p<0.0001 for NPC1L1; p=0.0001 for NPC2).
Figure 1.
NPC1L1 and NPC2 expression in peritumoral tissue and paired HCC tissues by immunohistochemistry. Expression features of NPC1L1 in (A) paired peritumoral tissue and (B) HCC tissue (×200). Expression of NPC2 in (C) paired peritumoral tissue and (D) HCC tissue (×200). Picture contains both HCC and peritumoral tissue for (E) NPC1L1 and (F) NPC2 (×100). (G) Scatter plot showing the mean staining intensity of paired peritumoral tissue (P) and HCC tumor tissue (T) (p<0.0001 for NPC1L1, p=0.0001 for NPC2).
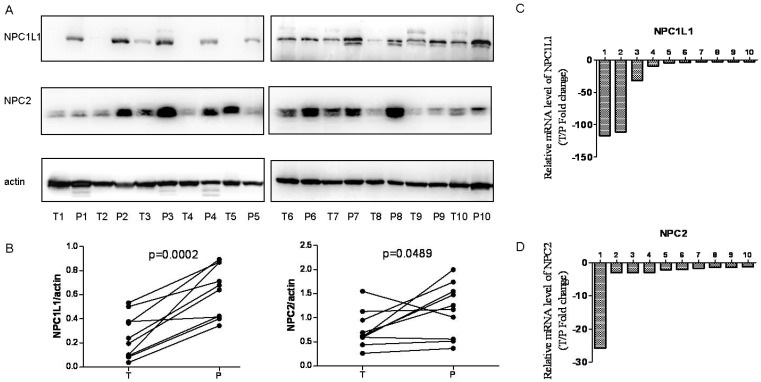
In addition, expression level of NPC1L1 and NPC2 were analyzed in 10 paired HCC and peritumoral tissue using Western blot and Real-time Quantitative PCR for further confirmation. As shown in Figure 2A and Figure 2B, NPC1L1 and NPC2 expressions were significantly reduced in HCC tissue (p=0.0002 for NPC1L1, p=0.0489 for NPC2). In parallel, the mRNA expression of NPC1L1 and NPC2 expression levels also reduced in HCC tissue compared to peritumoral tissue.
Figure 2.
NPC1L1 and NPC2 expression in peritumoral tissue and paired HCC tissues by Wetern-blot and Real-time Quantitative PCR. (A) NPC1L1 and NPC2 expression was detected by Western blot analyses in 10 paired HCC tissue (T) and peritumoral tissue (P). (B) Quantitation of proteins from Western blot analyses shows that both NPC1L1 and NPC2 expressions significantly reduced in HCC tissue (T) (p=0.0002 for NPC1L1, p=0.0489 for NPC2). The mRNA expression of NPC1L1 and NPC2 was detected by Real-time Quantitative PCR. Results clearly shows that mRNA expression levels also reduced in HCC tissue (T) compared to peritumoral tissue (P) for both NPC1L1 (C) and NPC2 (D).
Cut-off point based on outcome and Kaplan-Meier analysis
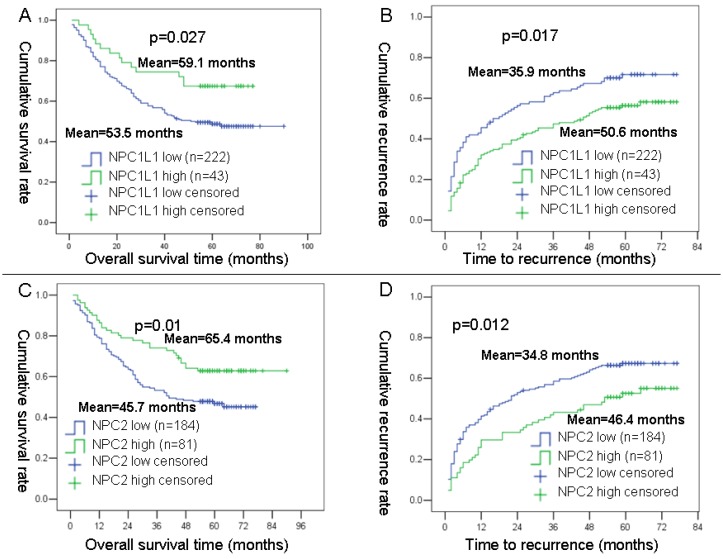
To obtain the best cut-off point of NPC1L1 and NPC2 expression level for survival analysis in 265 HCC cases of prognosis cohort, the X-tile program was used. Using a standard log-rank method, best cut-off points were obtained with minimum p values from a lookup tables for OS and TTR.
As results, 222 cases were divided into low NPC1L1 expression group and 43 cases were divided into high NPC1L1 expression cases. For, NPC2, 184 cases were divided into low NPC2 expression group and 81 cases were divided into high NPC2 expression cases. After then, the OS and TTR curves were depicted by Kaplan-Meier analysis using SPSS 13 software. Results showed that mean OS time for HCC patients expressing low levels of NPC1L1 was 53.5 months, compared with 59.1 months for those expressing high levels of NPC1L1 (P=0.027, log-rank test; Figure 3 A). The mean TTR for HCC patients expressing low levels of NPC1L1 was 35.9 months, compared with those expressing high levels of NPC1L1 was 50.6 months (P=0.017, log-rank test; Figure 3 B). For NPC2, the mean OS time for HCC patients expressing low levels of NPC2 was 45.7 months, compared with 65.4 months for those expressing high levels of NPC2 (P=0.01, log-rank test; Figure 3 C). The mean TTR for HCC patients expressing low levels of NPC2 was 34.8 months, compared with those expressing high levels of NPC2 was 46.4 months (P=0.012, log-rank test; Figure 3 D).
Figure 3.
Kaplan-Meier survival analyses of NPC1L1 and NPC2 in HCC patients. (A) Probability of survival of postoperative HCC patients: low expression of NPC1L1 (n=222, mean=53.5 months), high expression of NPC1L1 (n=43, mean=59.1 months). (B) Probability of recurrence of postoperative HCC patients: low expression of NPC1L1 (n=222, mean=35.9 months), high expression of NPC1L1 (n=43, mean=50.6 months). (C) Probability of survival of postoperative HCC patients: low expression of NPC2 (n=184, mean=45.7 months), high expression of NPC1L1 (n=81, mean=65.4 months). (D) Probability of recurrence of postoperative HCC patients: low expression of NPC2 (n=184, mean=34.8 months), high expression of NPC1L1 (n=81, mean=46.4 months).
Association NPC1L1 and NPC2 combination with HCC patient's OS and TTR
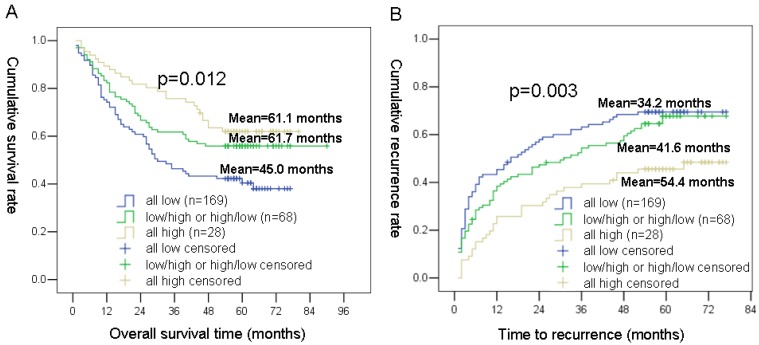
Based on the NPC1L1 and NPC2 expression levels, we classified the 265 HCC patients (prognosis cohort) into 3 groups: group 1 defined as patients with low NPC1L1 expression and low NPC2 expression; group 2 defined as patients with low NPC1L1 expression and high NPC2 expression, or high NPC1L1 and low NPC2 expression; group 3 defined as patients with high expressions for both NPC1L1 and NPC2.
Using the Kaplan-Meier method, patients with the low NPC1L1/low NPC2 expression (group 1) had the shortest OS (mean 45.0 months) and TTR (mean 34.2 months), whereas patients with the high NPC1L1/high NPC2 expression (group 3) had the longest OS (mean 61.1 months) and TTR (mean 54.4 months). While, patients in group 2 (NPC1L1 and High NPC2 expression, high NPC1L1 and low NPC2 expression) had moderate OS (mean 61.7 months) and TTR (mean 41.6 months) (Figure 4).
Figure 4.
Kaplan-Meier survival analyses of NPC1L1/ NPC2 combination in HCC patients. (A) Probability of survival of postoperative HCC patients: all low expression (n=169, mean=45.0 months), NPC1L1 low/NPC2 high or NPC1L1 high/NPC2 low expression (n=68, mean=61.7 months), all high expression (n=28, mean=61.1 months) (B) Probability of recurrence of postoperative HCC patients: all low expression (n=169, mean=34.2 months), NPC1L1 low/NPC2 high or NPC1L1 high/NPC2 low expression (n=68, mean=41.6 months), all high expression (n=28, mean=54.4 months)
Association of NPC1L1 and NPC2 expression with clinicopathological features of HCC patients
Next we investigated the association between NPC1L1 and NPC2 expression which according to the cut-off point determined by outcome based classification, and clinicopathological factors in 265 HCC patients. Result revealed that NPC1L1 expression was associated with serum AFP (p=0.007), TNM (p=0.015), tumor differentiation (p=0.003), vascular invasion (p=0.004) and did not associated with other clinicopathological factors (Table 1). In addition, NPC2 expression were associated with serum AFP (p=0.0001), tumor differentiation (p=0.012), vascular invasion (p=0.042), and did not associated with other clinicopathological factors (Table 1).
Table 1.
Association of NPC1L1 and NPC2 expression with clinicopathological features in 265 cases of HCC patient.
| Variable | NPC1L1 | NPC2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Low | High | p | Low | High | p | |||
| sex | 0.738 | 0.783 | ||||||
| Male | 192 | 38 | 159 | 71 | ||||
| Female | 30 | 5 | 25 | 10 | ||||
| age | 0.975 | 0.072 | ||||||
| ≤ 50 | 109 | 21 | 97 | 33 | ||||
| >50 | 113 | 22 | 87 | 48 | ||||
| HBsAg | 0.123 | 0.083 | ||||||
| negative | 31 | 10 | 21 | 16 | ||||
| positive | 191 | 33 | 159 | 65 | ||||
| serum AFP | 0.007 | <0.0001 | ||||||
| ≤ 20 ng/ml | 71 | 23 | 50 | 41 | ||||
| >20ng/ml | 151 | 20 | 131 | 40 | ||||
| liver cirrhosis | 0.666 | 0.779 | ||||||
| no | 70 | 15 | 60 | 25 | ||||
| yes | 152 | 28 | 124 | 56 | ||||
| TNM | 0.015 | 0.121 | ||||||
| I | 61 | 21 | 51 | 31 | ||||
| II | 124 | 19 | 101 | 42 | ||||
| III-IV | 37 | 3 | 32 | 8 | ||||
| Child-pugh class | 0.305 | 0.351 | ||||||
| A | 201 | 41 | 170 | 72 | ||||
| B | 21 | 2 | 14 | 9 | ||||
| tumor size | 0.682 | 0.617 | ||||||
| ≤5 cm | 106 | 22 | 87 | 41 | ||||
| >5 cm | 116 | 21 | 97 | 40 | ||||
| tumor number | 0.417 | 0.988 | ||||||
| single | 168 | 35 | 141 | 62 | ||||
| multiple | 54 | 8 | 43 | 19 | ||||
| tumor differentiation | 0.003 | 0.012 | ||||||
| well | 15 | 10 | . | 11 | 14 | |||
| moderate | 205 | 33 | 172 | 66 | ||||
| Poor | 2 | 0 | 1 | 1 | ||||
| vascular invasion | 0.004 | 0.042 | ||||||
| no | 73 | 24 | 60 | 37 | ||||
| yes | 149 | 19 | 124 | 44 | ||||
Univariate and multivariate survival analyses
Univariate analysis showed that serum AFP, liver cirrhosis, TNM stage, tumor size, tumor number, tumor differentiation, vascular invasion, NPC1L1, NPC2, and NPC1L1/NPC2 combination was associated significantly with OS and TTR, respectively (p value for OS were <0.0001, 0.036, <0.0001, <0.0001, <0.0001, 0.014, 0.010, 0.030, 0.011, and 0.004; p value for TTR were 0.001, 0.004, <0.0001, <0.0001, <0.0001, 0.009, 0.023, 0.020, 0.014, and 0.004) (Table 2).
Table 2.
Univariate and multivariate analyses of factors associated with OS and TTR in 265 cases of HCC patient.
| Factors | OS | TTR | ||||||
|---|---|---|---|---|---|---|---|---|
| univariate p | multivariate | univariate p | multivariate | |||||
| HR | 95% Cl | p | HR | 95% Cl | p | |||
| sex: Male vs Female | 0.493 | 0.985 | ||||||
| age: ≤50 vs >50 | 0.716 | 0.787 | ||||||
| HBsAg: positive vs negative | 0.091 | 0.069 | ||||||
| serum AFP (ng/ml): ≤20 vs >20 | <0.0001 | 1.527 | 1.008-2.315 | 0.046 | 0.001 | |||
| liver cirrhosis: yes vs no | 0.036 | 1.747 | 1.165-2.620 | 0.007 | 0.004 | 1.832 | 1.282-2.620 | 0.001 |
| TNM: I vs II vs III-IV | <0.0001 | <0.0001 | ||||||
| Child-pugh: A vs B | 0.135 | 0.174 | ||||||
| tumor size: ≤5 vs >5 | <0.0001 | 2.897 | 1.956-4.292 | <0.0001 | <0.0001 | 2.214 | 1.603-3.057 | <0.0001 |
| tumor number: single vs multiple | <0.0001 | 1.735 | 1.192-2.527 | 0.004 | <0.0001 | 2.077 | 1.483-2.910 | <0.0001 |
| tumor differentiation: well vs moderate vs poor |
0.014 | 0.009 | ||||||
| vascular invasion: no vs yes | 0.010 | 0.023 | ||||||
| NPC1L1 expression | 0.030 | 0.020 | ||||||
| NPC2 expression | 0.011 | 0.014 | ||||||
| NPC1L1/NPC2 combination: low vs High |
0.004 | 0.705 | 0.507-0.957 | 0.024 | 0.004 | 0.701 | 0.547-0.898 | 0.005 |
Multivariate analysis was performed using the Cox multivariate proportional hazard regression model in a stepwise manner (forward, conditional likelihood ratio). Factors that indicated significance in the univariate analysis were entered into a Cox multivariate proportional hazard regression analysis. Results showed that serum AFP, liver cirrhosis, tumor size, tumor number, and NPC1L1/NPC2 combination were the independent prognostic factors for OS and liver cirrhosis, tumor size, tumor number, and NPC1L1/NPC2 combination were the independent prognostic factors for TTR (Table 2).
Discussion
A multinational, long-term HCC survival analysis indicates the significant improvement in outcomes over the past three decades observed in both the East and the West for HCC patients after surgical resection 17. In detail, postoperative mortality and overall survival rates after major hepatectomy were improved over time with 5-year survival rates of 30 %, 40 %, and 51 % for the years 1981-1989, 1990-1999, and the most recent era of 2000-2008, respectively 17. However, from the above dada, we learn that almost half of the HCC patients still with unsatisfactory prognosis.
The prognosis of HCC patients is often poor because of the high risk of recurrence and metastasis after curative resection especially in HBV-related HCC 18. Therefore, the identification of prognostic markers to identify the risk of prognosis of HCC patients after surgery is still critical.
Recently, it was reported that NPC2 may regulates liver cancer progression via modulating ERK1/2 pathway and low NPC2 protein expression levels may predict poor OS of postoperative HCC patients in small size of patients number (n=50) 19. In addition, NPC1L1 inhibition has been shown to prevent metabolic disorders such as fatty liver disease, obesity, diabetes, and atherosclerosis 20. However, there was no report concerning about that NPC1L1 in HCC. In 2006, Jiang et al., summarized that decreased serum levels of cholesterol and apoAI may indicate a poor prognosis 21. Recently, a Taiwanese based cohort study reported that, serum cholesterol level is a potential risk factor for BMI associated surgical outcome after hepatectomy 22. Taken together, our results may be a new Insight into serum cholesterol level and NPC1L1 in HCC.
In conclusion, our study is first to demonstrate the expression pattern of NPC1L1 in HCC tumor tissue and peritumoral tissues, and was the first to demonstrate the prognostic value of NPC1L1, and NPC1L1/NPC2 combination is an independent prognostic factor for OS and TTR in postoperative HCC patients. Thus, undertaking the expression level of NPC1L1 and NPC2 in HCC patient tissues may provide a clinicians risk information about OS and TTR of postoperative HCC patient and maybe helpful to study mechanism for relationship between cholesterol and HCC.
In this present study, we found that NPC1L1 was decreased in HCC compared with paired peritumoral liver tissues, and low NPC1L1 protein expression may predict poor OS and TTR of postoperative HCC patients. We further validated that low NPC2 protein expression levels may predict poor OS of postoperative HCC patients (n=265), and found that low NPC2 protein expression levels may predict poor TTR of postoperative HCC patients. Interestingly, we further found that, NPC1L1/NPC2 combination is an independent prognostic factor for OS and TTR in postoperative HCC patients.
Supplementary Material
Supplementary table 1.
Acknowledgments
This study was supported by National Natural Science Foundation of China (81201939, 81472769).
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH. et al. A decade's studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–96. doi: 10.1007/s00432-003-0511-1. [DOI] [PubMed] [Google Scholar]
- 3.Yang XR, Xu Y, Yu B, Zhou J, Li JC, Qiu SJ. et al. CD24 is a novel predictor for poor prognosis of hepatocellular carcinoma after surgery. Clin Cancer Res. 2009;15:5518–27. doi: 10.1158/1078-0432.CCR-09-0151. [DOI] [PubMed] [Google Scholar]
- 4.Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol. 2004;130:497–513. doi: 10.1007/s00432-004-0572-9. [DOI] [PubMed] [Google Scholar]
- 5.Lu YF, Zhang L, Waye MM, Fu WM, Zhang JF. MiR-218 mediates tumorigenesis and metastasis: Perspectives and implications. Experimental cell research. 2015;334:173–82. doi: 10.1016/j.yexcr.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Altmann SW, Davis HR Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G. et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 7.Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem. 2005;280:12710–20. doi: 10.1074/jbc.M409110200. [DOI] [PubMed] [Google Scholar]
- 8.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA. et al. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–78. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanier MT, Millat G. Structure and function of the NPC2 protein. Biochim Biophys Acta. 2004;1685:14–21. doi: 10.1016/j.bbalip.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Okamura N, Kiuchi S, Tamba M, Kashima T, Hiramoto S, Baba T. et al. A porcine homolog of the major secretory protein of human epididymis, HE1, specifically binds cholesterol. Biochim Biophys Acta. 1999;1438:377–87. doi: 10.1016/s1388-1981(99)00070-0. [DOI] [PubMed] [Google Scholar]
- 11.Yamanashi Y, Takada T, Shoda J, Suzuki H. Novel function of Niemann-Pick C1-like 1 as a negative regulator of Niemann-Pick C2 protein. Hepatology. 2012;55:953–64. doi: 10.1002/hep.24772. [DOI] [PubMed] [Google Scholar]
- 12.Tan N, Liu Q, Liu X, Gong Z, Zeng Y, Pan G. et al. Low expression of B-cell-associated protein 31 in human primary hepatocellular carcinoma correlates with poor prognosis. Histopathology. 2016;68:221–9. doi: 10.1111/his.12738. [DOI] [PubMed] [Google Scholar]
- 13.Jin GZ, Yu WL, Dong H, Zhou WP, Gu YJ, Yu H. et al. SUOX is a promising diagnostic and prognostic biomarker for hepatocellular carcinoma. J Hepatol. 2013;59:510–7. doi: 10.1016/j.jhep.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Jin GZ, Li Y, Cong WM, Yu H, Dong H, Shu H. et al. iTRAQ-2DLC-ESI-MS/MS based identification of a new set of immunohistochemical biomarkers for classification of dysplastic nodules and small hepatocellular carcinoma. J Proteome Res. 2011;10:3418–28. doi: 10.1021/pr200482t. [DOI] [PubMed] [Google Scholar]
- 15.Jin H, Wang C, Jin G, Ruan H, Gu D, Wei L, Regulator of Calcineurin 1 Gene Isoform 4, Downregulated in Hepatocellular Carcinoma, Prevents Proliferation, Migration, and Invasive Activity of Cancer Cells and Growth of Orthotopic Tumors by Inhibiting Nuclear Translocation of NFAT1. Gastroenterology; 2017. [DOI] [PubMed] [Google Scholar]
- 16.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 17.Andreou A, Vauthey JN, Cherqui D, Zimmitti G, Ribero D, Truty MJ. et al. Improved long-term survival after major resection for hepatocellular carcinoma: a multicenter analysis based on a new definition of major hepatectomy. J Gastrointest Surg. 2013;17:66–77. doi: 10.1007/s11605-012-2005-4. discussion p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T, Wang SK, Zhou J, Sun HC, Qiu SJ, Ye QH. et al. Positive HBcAb is associated with higher risk of early recurrence and poorer survival after curative resection of HBV-related HCC. Liver Int. 2016;36:284–92. doi: 10.1111/liv.12898. [DOI] [PubMed] [Google Scholar]
- 19.Liao YJ, Fang CC, Yen CH, Hsu SM, Wang CK, Huang SF. et al. Niemann-Pick type C2 protein regulates liver cancer progression via modulating ERK1/2 pathway: Clinicopathological correlations and therapeutical implications. Int J Cancer. 2015;137:1341–51. doi: 10.1002/ijc.29507. [DOI] [PubMed] [Google Scholar]
- 20.Park SW. Intestinal and hepatic niemann-pick c1-like 1. Diabetes Metab J. 2013;37:240–8. doi: 10.4093/dmj.2013.37.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang J, Nilsson-Ehle P, Xu N. Influence of liver cancer on lipid and lipoprotein metabolism. Lipids Health Dis. 2006;5:4. doi: 10.1186/1476-511X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YL, Li WC, Tsai TH, Chiang HY, Ting CT. Body mass index and cholesterol level predict surgical outcome in patients with hepatocellular carcinoma in Taiwan - a cohort study. Oncotarget. 2016;7:22948–59. doi: 10.18632/oncotarget.8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1.