Abstract
Background: Our previous study showed that cancer-associated fibroblast MRC-5 promoted hepatocellular carcinoma progression by enhancing migration and invasion capability. However, few studies have explored the role of MRC-5 in pancreatic cancer (PC). In this study, we examined the exact role and associated mechanisms of MRC-5.
Methods: The conditioned media for MRC-5 was used to culture PC cell lines SW1990 and PANC-1. Cell proliferation was compared based on colony formation assays of PC cells in normal media and of PC cells cultured with conditioned media of MRC-5. Cell migration and invasion were assayed by transwell chambers. The expression of EMT-related proteins and apoptosis-related proteins was evaluated using Western blot. And confocal microscopy was used to further detect the expression of EMT-related proteins. qRT-PCR was used to confirm the expression changes of related genes at the mRNA level. We also used flow cytometry to examine the cell cycle, apoptotic rate, and expression of CD3, CD4, CD14, CD25, CD45, CD61, CD90, TLR1, and TLR4.
Results: MRC-5 repressed the colony formation ability of PC cells and significantly inhibited cell migration and invasion potential. MRC-5 induced S-phase cell cycle arrest but did not augment the apoptotic effects in PC cells. We hypothesized that the weakened malignant biological behavior of PC cells was correlated with MRC-5-induced altered expression of the cancer stem cell marker CD90; the immune-related cell surface molecules CD14, CD25, TLR4, and TLR1; and cell polarity complexes Par, Scribble, and Crumbs.
Conclusion: MRC-5 limits the malignant activities of PC cells by suppressing cancer stem cell expansion, remolding epithelial polarity, and blocking the protumoral cascade reaction coupled to TLR4, TLR1, CD14, and CD25.
Keywords: Cancer associated fibroblast (CAF), Cancer stem cell (CSC), Toll-like receptors (TLRs), Epithelial-to-mesenchymal transition (EMT), Cell polarity complex
Introduction
Despite advances in surgical and non-surgical treatments, pancreatic cancer (PC), one of the most common and lethal gastrointestinal cancers, has a very low 5-year survival rate of less than 7% 1. This poor prognosis is likely due to late diagnosis, the high level of biological aggressiveness, extrapancreatic metastasis, and unresponsiveness to radiation or available systemic therapies (e.g., adjuvant chemotherapy, immunotherapy) 2. Thus, a further understanding of the factors that lead to PC invasion and metastasis is important for treating this deadly disease.
Emerging evidence suggests that the tumor microenvironment (TME), composed of cellular and acellular components including cancer-associated fibroblasts (CAFs), immune cells, blood and lymphatic tumor vessels, the extracellular matrix, chemokines, cytokines, and growth factors, plays a key role in the proliferation, invasion, and metastasis of many solid cancers 3. CAFs, the predominant stromal cell type, are among the most critical components of the TME. They are characterized by highly heterogeneous cell origins (originating from fibroblast and endothelial cells) and the expression of skeletal muscle actin alpha (alpha-SMA) and play an important role in tumor invasion and metastasis 4. A dense background of desmoplastic stroma originating from CAFs is the hallmark of PC 5. Basic studies have increased our understanding of the interplay between PC cells and CAFs (especially pancreatic stellate cells, PSCs) 6-10. Doudou Li et al. showed that, by creating an advantageous microenvironment, CAF crosstalk with cancer cells stimulates tumor evolution 11. Douglas T. Fearon showed that CAFs play an important role in the adaptation of the tumor to the host by causing an immune disorder, thereby enabling cancer cells to proliferate and spread to distant metastatic sites 12. However, the precise molecular mechanisms responsible for the tumor-promoting activity of CAFs remain unclear. Additionally, once PC cells migrate to distant “hideouts,” the majority of CAFs surrounding tumor cells are probably “not indigenous to the pancreas” or are newly recruited. The lung, rich in fibroblasts, is one of the most common sites of distant metastasis in patients with PC. Therefore, it is important to characterize the specific roles of lung fibroblasts in PC.
Several lines of evidence indicate that cancer stem cells (CSCs), characterized by the ability to self-renew and give rise to heterogeneous cancer cell progeny, have a profound effect on tumorigenesis and progression 13-15. CSCs can be identified based on increased sphere formation ability and in vitro clonogenicity, enhanced in vivo tumor-initiating ability, or the expression of specific CSC markers. Well-characterized CSC markers include CD90, CD44, CD24, CD133, c-MET, aldehyde dehydrogenase (ALDH), and epithelial-specific antigen (ESA) 16-21. Recently, it has been proposed that CSCs can be generated by EMT 22, 23, whereby epithelial cells lose their epithelial properties and acquire a mesenchymal-like phenotype and stem cell-like features 24, 25. Classical EMT is a dynamic and reversible program that is often activated during tumor invasion and metastasis and is characterized by the expression of EMT-inducing transcription factors, such as snail, slug, twist-1, ZEB-1, and ZEB-2; upregulation of vimentin and N-cadherin; and downregulation of E-cadherin, alpha-catenin, and P120-catenin 26. During EMT, while front-rear polarity develops, apico-basal polarity is disrupted. And, three protein complexes (Par, Scribble, and Crumbs) which are considered as tumor suppressors, are responsible for the establishment of apico-basal polarity 27, 28. Additionally, there is a causal relationship between EMT and CSCs in PC 29-32. Nonetheless, the interplay between these two entities is complex, and it is difficult to establish whether acquisition of CSC or initiation of EMT occurs first. Interestingly, PSCs accelerated PC progression by promoting EMT 33, providing further insight into these relationships. Hence, deciphering the relationships between these essential processes can increase our understanding of the biological behavior of PC and lead to advancements in clinical management.
Previous studies suggest that mediators and cellular effectors of chronic inflammation are important ingredients of TME, and toll-like receptors (TLRs) play an important role in this context. TLRs, a family of evolutionally conserved pattern recognition receptors involved in innate and consequent adaptive immune responses, play an active role in the progression of PC 34-36. There is a strong association between immune cells and cancer cells. It is possible that cells of the immune system stimulate tumor genesis and progression, whereas, conversely, cancer cells promote a local inflammatory response through the recruitment of immune cells. Moreover, recent studies showed that cell-cell interactions between immune cells and CAFs may induce their own activation, thereby promoting tumor progression 37. Accordingly, understanding the biology of CAFs may provide novel therapeutic targets for combating PC.
Our previous study showed that MRC-5 promoted HCC cell migration and invasion by inducing EMT-like transitions. MRC-5, the human fetal lung fibroblast, plays an important role in lung fibrosis 38. MRC-5, a CAF that produces hepatocyte growth factor, plays an essential role in cancer evolution, such as breast cancer 39, pleural mesothelioma 40, and lung cancer 41. Seven years ago, the role of MRC-5 in PC cells began to be explored 42. However, few subsequent studies have been performed, and the underlying mechanisms remain unclear. In the present study, the effect of MRC-5 on PC cell proliferation, cell cycle and apoptosis, cell migration, and invasion was examined. Furthermore, to explore the associated molecular mechanisms in PC cells, we evaluated the expression profiles of cell surface markers (CD3, CD4, CD14, CD25, CD45, CD61, CD90, TLR1, and TLR4), apoptosis-associated molecules, EMT-related molecules, cell polarity complexes, matrix metalloproteases (MMPs), integrins, and other adhesion molecules. These findings will be useful in the development of therapeutic interventions for PC.
Materials and methods
Cell culture
Cancer-associated fibroblast MRC-5 was donated by Dr. Xi, Chen (Zhejiang University, China). SW1990 and PANC-1 cell lines were purchased from Shanghai Cell Bank, Chinese Academy of Sciences. MRC-5 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a 5% CO2 water-saturated environment. Conditioned medium of MRC-5 cells (MRC-5-CM) was collected as previously described 43. DMEM medium supplemented with 10% FBS serverd as the control medium. SW1990 and PANC-1 cells were respectively incubated in the MRC-5-CM for 14 days (n=3). SW1990 was subcultured once a week at a ratio of 1:3 or 1:5. MRC-5 and PANC-1 were subcultured once a week at a ratio of 1:1 or 1:2. 20ml MRC-5-CM was used when Pancreatic cancer cells were cultured in 75cm2 cell culture flasks.
Transwell Assay
The migration and invasion assays were performed using Transwell chambers with 8µm pore filters (Millipore, Billerica, MA, USA) as we previously reported 43.
Western-blot Analysis
Whole Pancreatic cancer cells were lysed on ice in a lysis buffer (RIPA, Beyotime, Shanghai, China) with a protease inhibitor mixture cocktail (Roche, Switzerland) after culturing in MRC-5-CM for 14 days. Western-blot analysis was performed by established protocols 43. Anti-CTNNA1 and CTNNB1, anti-E-cadherin, anti-N-cadherin, anti-ZEB-1, ZEB-2, Snail, Slug and anti-vimentin primary antibodies were purchased from (Abcam); anti-MMP-2, 3, 13 and 21, anti-WNT-2, anti-WNT16, anti-TGFB1, anti-Src, P-Src-Y418 and P-Src-Y529 primary antibodies were purchased from (Epitomics); anti-Bcl-2, Bcl-xl, Bad, Bax, Bim, Bid, Caspase-3 and anti-β-actin were from ( Cell Signaling Technology).
Confocal immunofluorescent analysis
Confocal immunofluorescent analysis was performed and analyzed as our previously described 43.
Flow cytometry
The presence of CD3, CD4, CD14, CD25, CD45, CD61, CD90, TLR1 and TLR4 were analyzed using a flow cytometer (CYTOMICS FC 500, Beckman Coulter, Miami, FL) according to manufacturer's instruction and the data was analyzed using Flow Jo analysis software. Anti-CD14-PE-Cy7, CD25-FITC, TLR4-PE, CD61-PE, CD90-FITC, CD45-APC, CD3-FITC and anti-CD4-PE-Cy5 were purchased from (BD Biosciences) ; anti-TLR1-PE was purchased from (eBioscience).
Cell cycle analysis and apoptosis analysis were performed according to the manufacturer's protocol as previously described 43. All experiments were performed at least in triplicate.
Colony formation assay
1× 103 cells were plated in a 8 cm plate. After two weeks of culture, the colonies (>10cells) were stained with crystal violet and counted.
Quantitative reverse-transcription-PCR
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA). First strand cDNA was synthesized from total RNA (2μg) using M-MLV Reverse Transcriptase (Promega, San Luis Obispo, CA, USA) according to the manufacturer's protocol. The primers for q-RT PCR were purchased from Biosune and the SYBR Premix Dimmer Eraser kits were from TaKaRa (TaKaRa, Dalian, China). GAPDH was used as an internal control to normalize target mRNA level. qRT-PCR reactions were performed by ABI7500 (Applied Biosystems, CA). The relative quantification of gene expression was calculated by the 2-ΔΔCt method.
Statistical Analysis
Student's t-test was performed to compare the differences between 2 groups using SPSS statistical software (Version 16.0; SPSS, Chicago, IL, USA). P values <0.05 were considered significant.
Results
MRC-5 inhibited the colony formation capability of PC cells
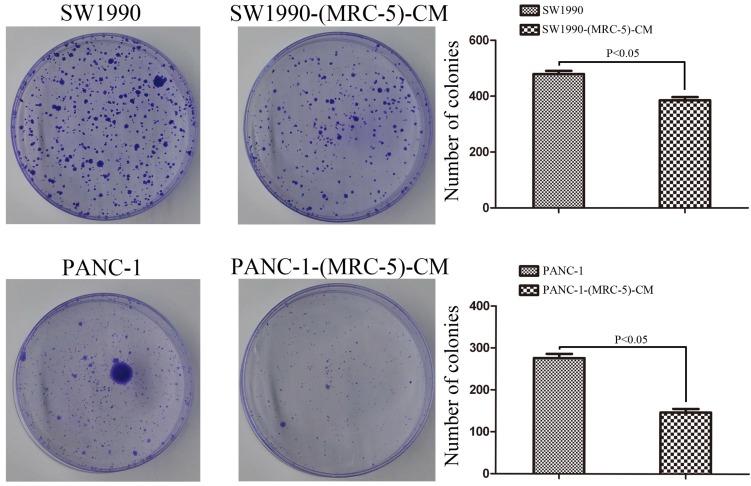
PANC-1 cells were cultured in MRC-5-CM, hereafter referred to as PANC-1-(MRC-5)-CM. SW1990 cells were cultured in MRC-5-CM, hereafter called SW1990-(MRC-5)-CM. To explore the effect of MRC-5 on cell proliferation, we performed colony formation assays. We observed a significant reduction in the colony formation capability of PANC-1-(MRC-5)-CM compared with PANC-1 cells (p<0.05; Fig. 1). Similarly, the colony formation ability of SW1990-(MRC-5)-CM cells was weakened compared with that of SW1990 cells. (p<0.05; Fig. 1).
Fig 1.
The colony formation capability of PC cells cultured in MRC-5-CM was decreased compared with that of PC cells without MRC-5-CM
Effect of MRC-5 on the cell cycle and apoptosis of PC cells
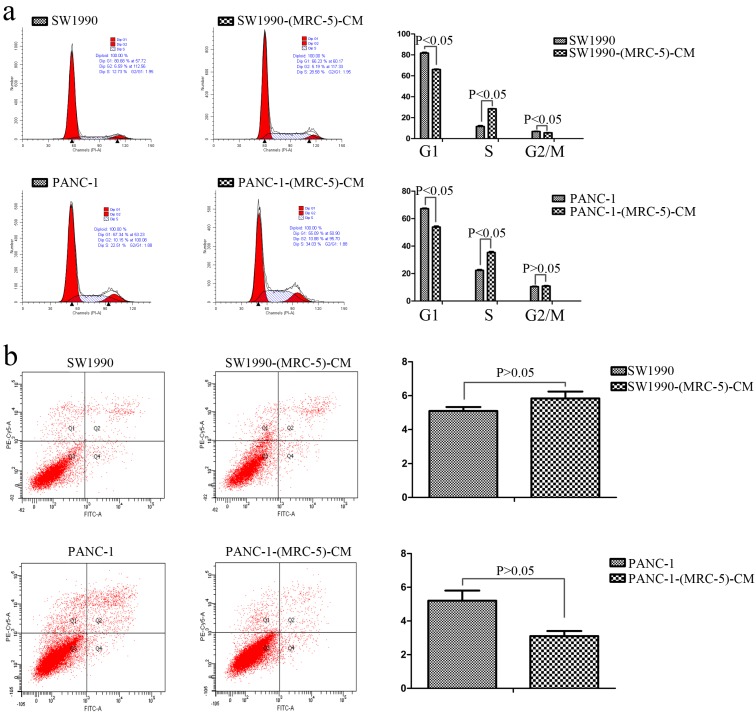
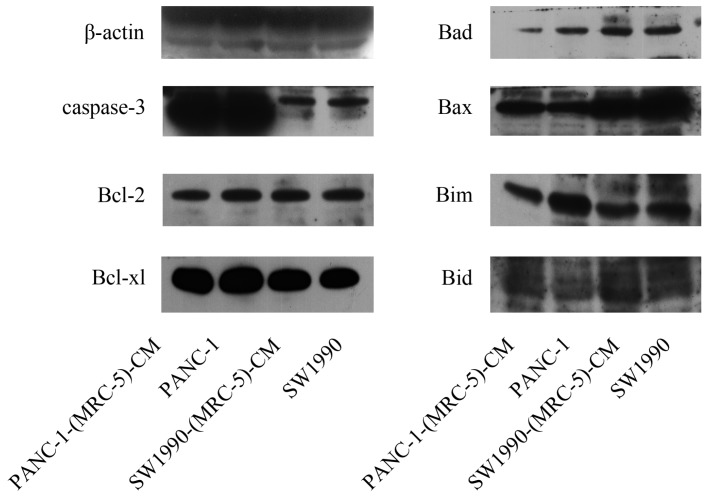
To further explore the effect of MRC-5-CM on PC cell viability, we investigated the cell cycle distribution and apoptosis rate of PC cells and PC cells cultured with MRC-5-CM. We found that MRC-5-CM induced S phase arrest in PANC-1 and SW1990 cells. The proportions of PANC-1-(MRC-5)-CM and SW1990-(MRC-5)-CM cells during G1 phase decreased significantly, whereas the proportions of PANC-1-(MRC-5)-CM and SW1990-(MRC-5)-CM cells in S phase was significantly increased compared with controls (Figure 2a). However, MRC-5-CM had no effect on cell death (Figure 2b). These results were confirmed by evaluating the expression of apoptosis-related proteins by Western blot (Figure 3). The anti-apoptotic genes Bcl-2 and Bcl-xl remained unchanged. Pro-apoptotic genes Bad and Bim were downregulated in PANC-1-(MRC-5)-CM, but they did not change significantly in SW1990-(MRC-5)-CM relative to in the control. In contrast, the pro-apoptotic gene Bid was increased moderately in PANC-1-(MRC-5)-CM and in SW1990-(MRC-5)-CM relative to in the control. The pro-apoptotic Caspase-3 and Bax did not change significantly in PANC-1-(MRC-5)-CM or in MHCC-LM3-(MRC-5)-CM cells relative to in the control (the ratio discrepancy is listed in Additional File 1: Figure S1). Based on these results, the effect of MRC-5-CM on PC cell growth was partially due to cell cycle arrest.
Fig 2.
Effect of MRC-5 on cell viability. a) MRC-5-CM induced S-phase cell cycle arrest, and b) MRC-5-CM had no effect on apoptosis
Fig 3.
Expression of apoptosis-related proteins
MRC-5 inhibited PC cell migration and invasion
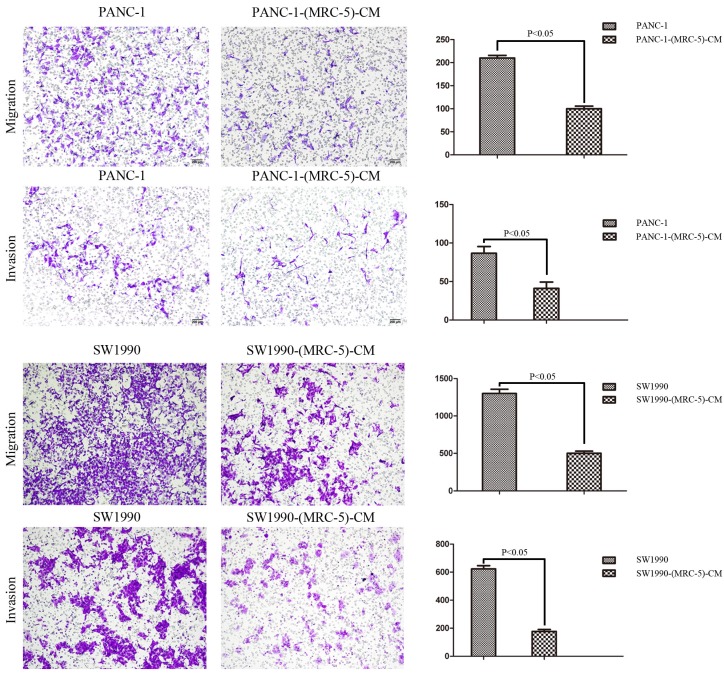
To explore the effect of MRC-5 on the cell migration and invasion potential of PC cells, we performed transwell assays. Our findings showed that the number of migrating PANC-1 cells decreased by culturing with MRC-5-CM compared with control medium (p<0.05; Fig. 4). Invasion followed the same pattern as migration; the number of invading cells was significantly lower during culturing with MRC-5-CM compared with during culturing in control medium (p<0.05; Fig. 4). Notably, consistent results were obtained with SW-1990 cells (p<0.05; Fig. 4).
Fig 4.
MRC-5-CM inhibited the cell migration/invasion potential of PC cells
Effect of MRC-5 on expression of EMT-related genes in PC cells
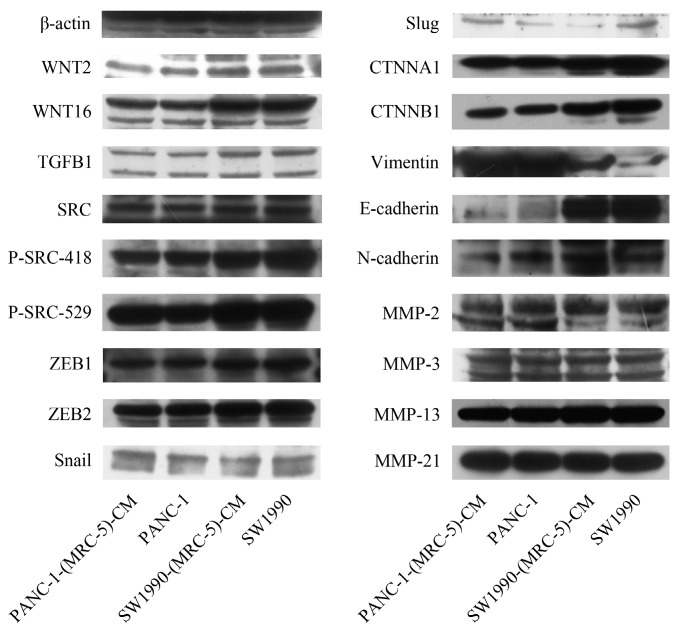
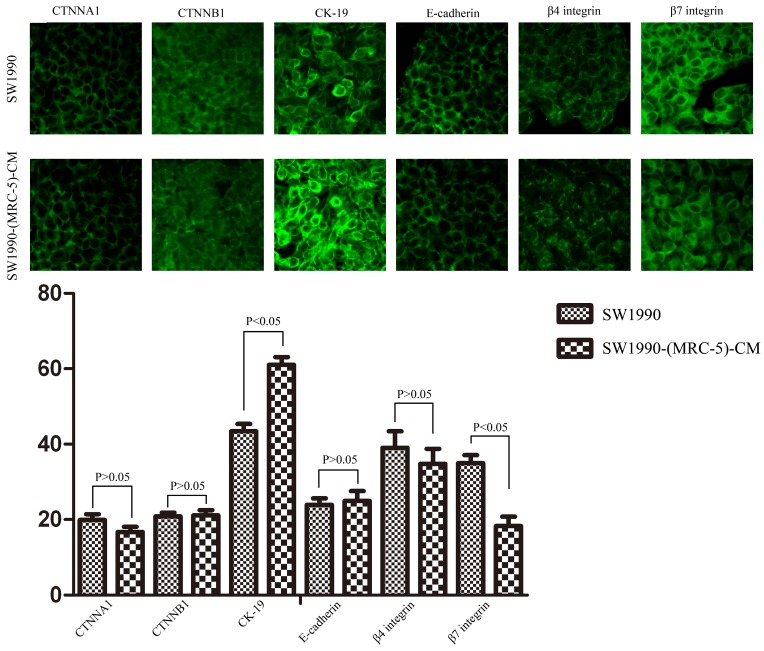
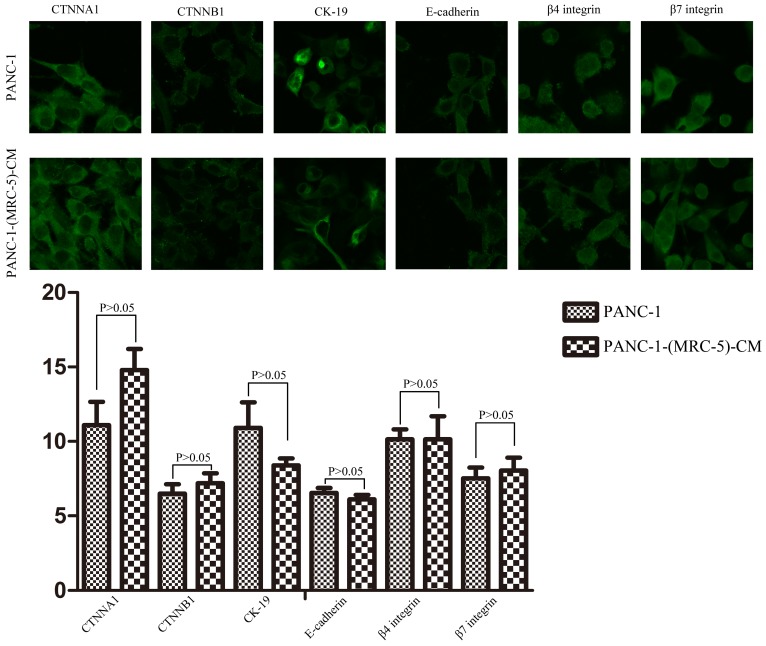
To explore the mechanism underlying the impaired migration and invasion ability of PC cells, we evaluated the expression profiles of epithelial markers (E-cadherin and CTNNA1), mesenchymal markers (N-cadherin, vimentin, and CTNNB1), EMT-related signaling molecules (WNT2, WNT16, TGFB1, SRC, P-SRC-418, and P-SRC-529), EMT-promoted transcription factors (Snail, Slug, ZEB-1, and ZEB-2), and MMPs (MMP-2, 3, 13, and 21) by Western blot (Figure 5). However, there were no significant changes in the expression of epithelial markers, mesenchymal markers (except for vimentin), MMPs, EMT-related signaling molecules, or EMT-promoted transcription factors (excluding Slug). Based on our results, Slug was downregulated in SW1990-(MRC-5)-CM cells compared with in SW1990, whereas Slug was upregulated in PANC-1-(MRC-5)-CM compared with in PANC-1. On the other hand, vimentin was upregulated in SW1990-(MRC-5)-CM cells compared with in SW1990, whereas vimentin did not differ markedly between PANC-1-(MRC-5)-CM and PANC-1 (the ratio discrepancy is listed in Additional Files 2-3: Figure S2-3). Thus, to explore whether the impaired migration and invasion potential was due to the reinforced construction of the E-cadherin/catenin complex on the cell membrane, we performed immunofluorescence analysis using confocal microscopy (Figure 6-7). Nevertheless, the E-cadherin/catenin complex was not distributed on the cell membranes of SW1990-(MRC-5)-CM or of PANC-1-(MRC-5)-CM compared to the control. Cytokeratin 19 (CK19), which has been associated with increased metastatic potential and poor outcome in pancreatic neuroendocrine tumors patients, was increased in SW1990-(MRC-5)-CM relative to in the control. β7 integrin, which is correlated with cancer progression through an interplay with MMPs, was decreased. The results also indicated that β4 integrin expression did not change significantly in SW1990-(MRC-5)-CM compared with in SW1990. Expression of CK 19, β4 integrin, and β7 integrin did not differ significantly between PANC-1-(MRC-5)-CM and PANC-1. Taken together, our results showed that MRC-5 decreased PC cell migration and invasion, did not reverse the classical EMT program, and may be related to decreased expression of β7 integrin.
Fig 5.
Expression of EMT-related proteins
Fig 6.
Immunofluorescence analysis of E-cadherin/catenin complex, CK19, β7 integrin, and β4 integrin between SW1990-(MRC-5)-CM and SW1990
Fig 7.
Immunofluorescence analysis of E-cadherin/catenin complex, CK19, β7 integrin, and β4 integrin between PANC-1-(MRC-5)-CM and PANC-1
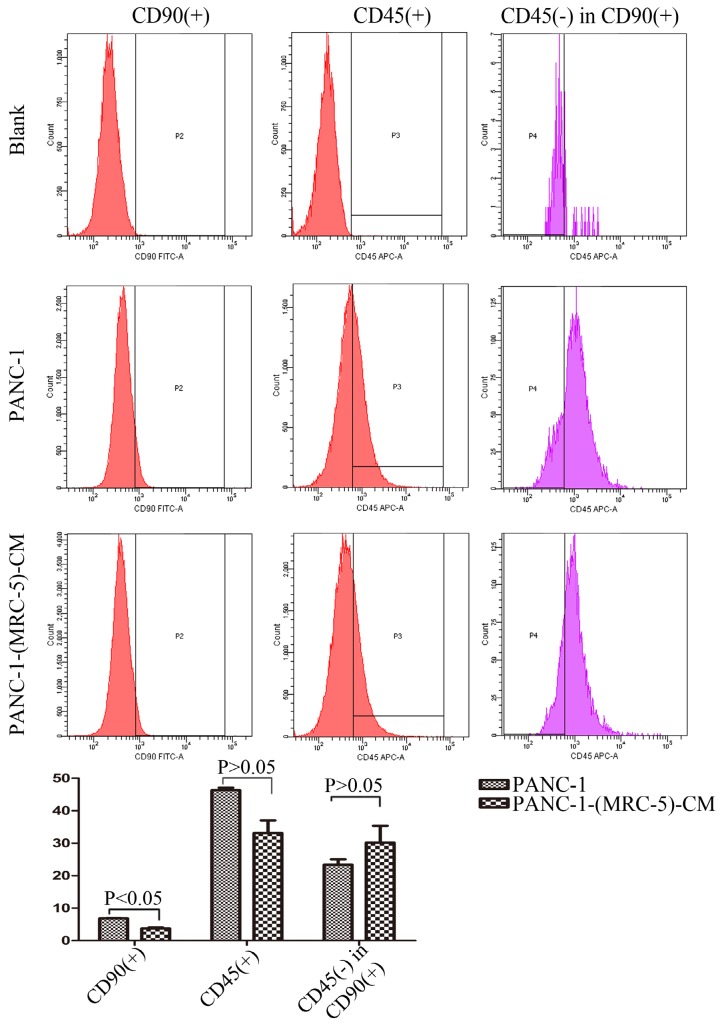
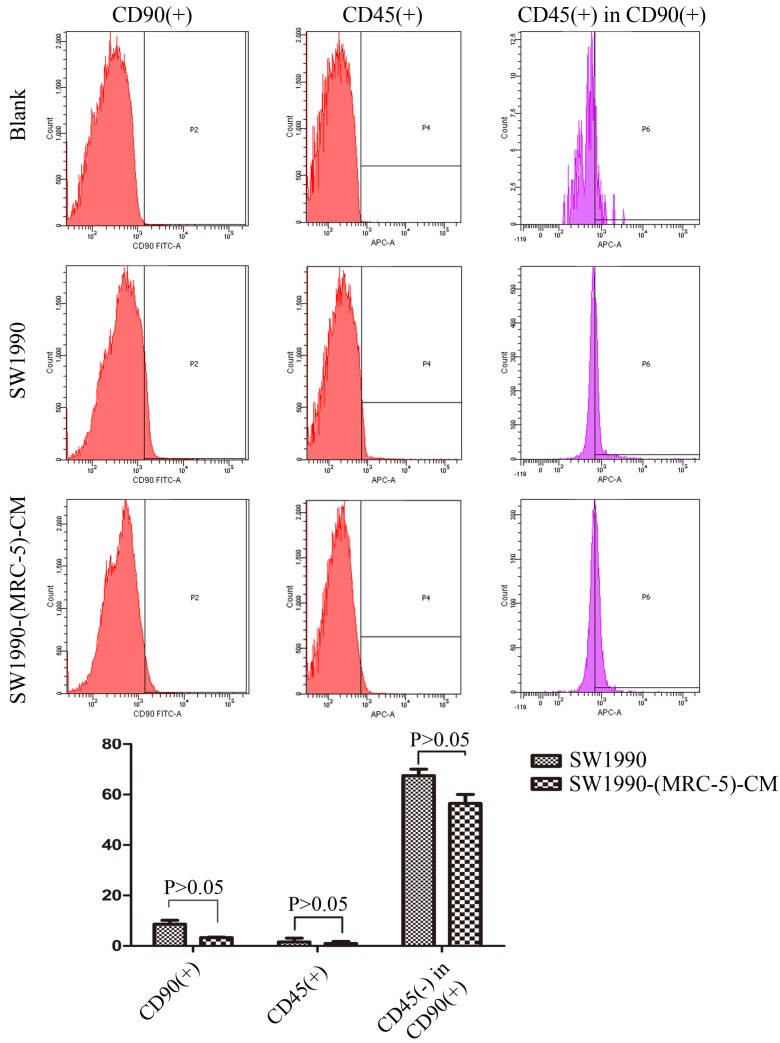
MRC-5 inhibited PC CSC expansion
It is possible that CSCs can be generated through EMT, which is regulated by CAFs. In this study, we used flow cytometry to explore the effect of MRC-5 on CSCs in PC, as shown in Figure 8-9. The quantity of CD90+ cells in PANC-1-(MRC-5)-CM decreased significantly compared with the quantity in PANC-1. However, the proportion of CD90+/CD45- cells in PANC-1-(MRC-5)-CM increased slightly. Similarly, the quantity of CD90+ cells in SW1990-(MRC-5)-CM decreased moderately compared with the quantity in SW1990, and the proportion of CD90+/CD45- cells in SW1990-(MRC-5)-CM also decreased. Our results did not agree with previous studies, indicating that indigenous CAFs that could travel with cancer cells to distant metastatic sites play an essential role in promoting cancer progression.
Fig 8.
The population of CSC was decreased in PANC-1-(MRC-5)-CM compared with PANC-1
Fig 9.
The population of CSC was decreased in SW1990-(MRC-5)-CM compared with SW1990
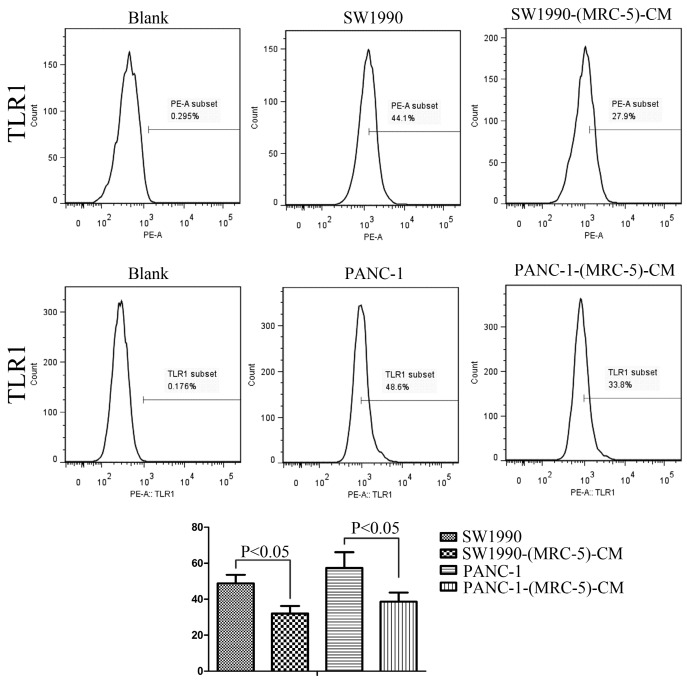
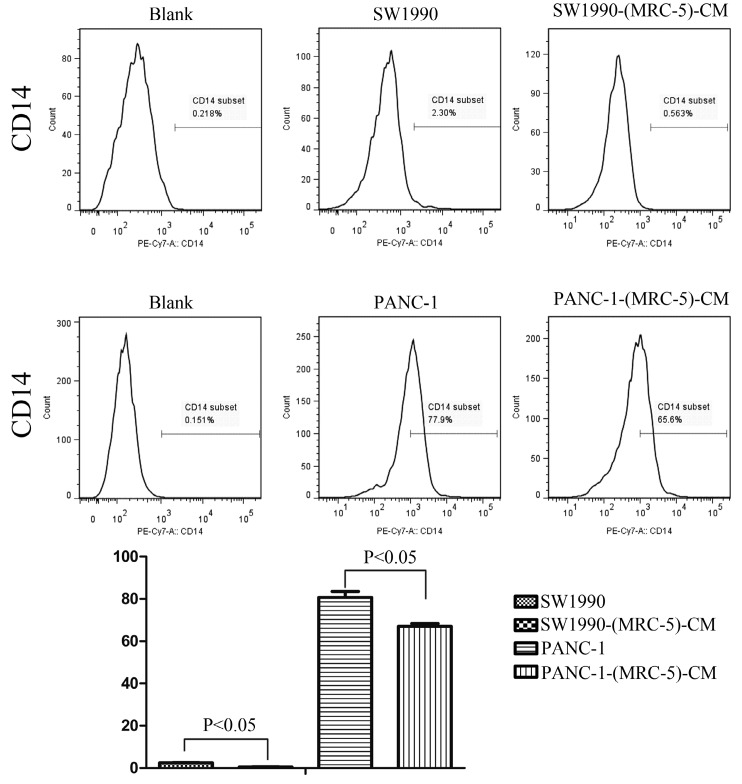
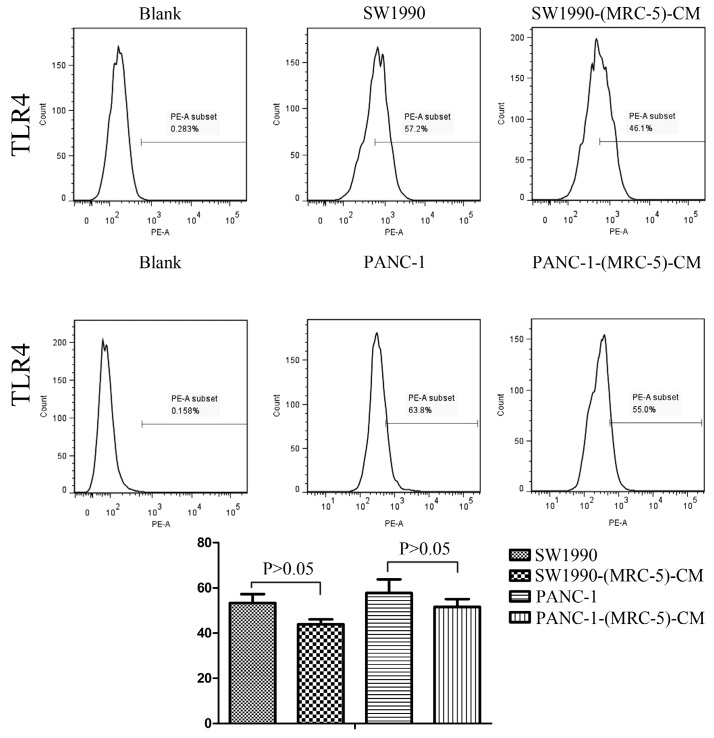
Effect of MRC-5 on expression of immune-related surface molecules
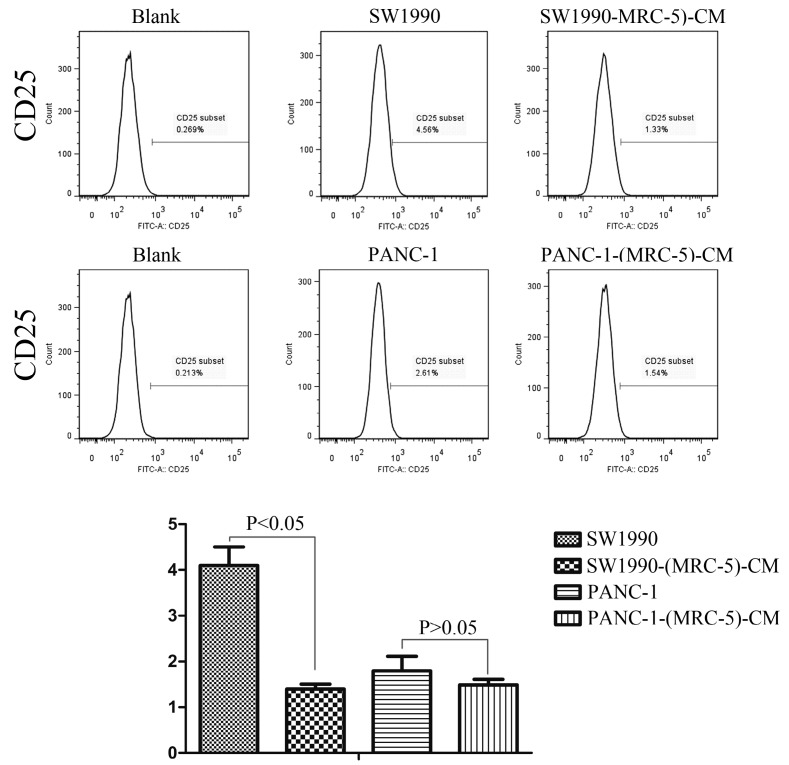
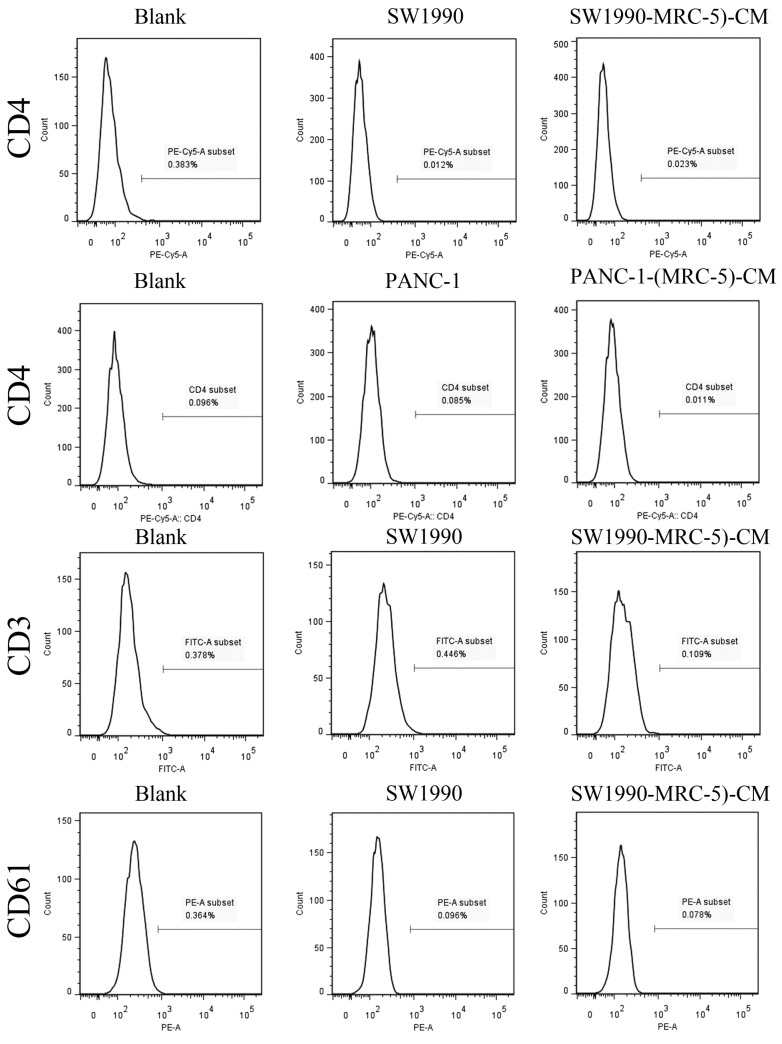
Previous studies found that local inflammation contributed to the malignant progression of cancer cells and that the cell-cell interaction between immune cells and CAFs can induce their own activation, thereby enhancing tumor advancement. Crosstalk must occur among immune cells, CAFs, and cancer cells, and this must be complicated. Additionally, the phenotype plasticity model suggested that one cell type can be transformed into another with the appropriate stimulus. Therefore, we performed flow cytometry to explore the influence of MRC-5 on the expression of immune-related surface molecules in PC cells. TLR1-positive and CD14-positive cells were decreased significantly in PANC-1-(MRC-5)-CM and SW1990-(MRC-5)-CM compared with in the control (p<0.05; Fig. 10-11). TLR4 positive cells were decreased moderately in both PANC-1-(MRC-5)-CM and SW1990-(MRC-5)-CM compared with in the control (Figure 12). CD25-positive cells were decreased significantly in SW1990-(MRC-5)-CM compared with in the control, whereas they were decreased slightly in PANC-1-(MRC-5)-CM compared with in the control (Figure 13). There was no expression of CD3, CD4, or CD61 in PC cells (Figure 14). These results indicated that the MRC-5-attenuated malignant activities of PC cells may be partly by restraining the cascade reaction coupled to TLR4, TLR1, CD14, and CD25 and that tumor cells cannot be converted into immune cells.
Fig 10.
TLR1+ cells were decreased
Fig 11.
CD14+ cells were decreased
Fig 12.
TLR4+ cells were decreased
Fig 13.
CD25+ cells were decreased
Fig 14.
There were no CD3+, CD4+, or CD61+ cells in PC
Expression of genes related to EMT signaling molecules, EMT-promoted transcription factors, MMPs, integrins, cell polarity molecules, cell adhesion, and apoptosis molecules in PC cells
To explore the impact of MRC-5 on the expression of genes related to EMT (JAG1, JAG2, DHH, IHH, NOTCH1, SP1, Snail, Slug, TWIST1, TGFB1, GLI1, Fox-2, WNT1, 2B, 3, 4, 5A, 5B, 7B, 10A, 10B, 16, ZEB1, ZEB2, MMP-1, 2, 3, 9, 11, 12, 13, 14, 17, and 21), cell polarity (PALS1, PALS2, PARD3, PARD3B, PARD6A, PARD6B, PARD6G, PATJ, PRKCI, PRKCZ, SCRIB, CRB-1, CRB-2, DLG1, DLG3, DLG4, DLG5, LLGL1, and LLGL2), cell adhesion molecules (CTNNA1, CTNNB1, CTNND1, JUP, E-cadherin, vimentin, vitronectin, stratifin, SERPINB5, CEACAM1, paxillin, CK19, and integrin α1, α2, α3, α4, α5, α7, α8, α9, α11, αD, αM, αE, αV, β1, β2, β3, β5, β6, β7, and β8) and apoptosis (Bad, Bax, Bcl-2, Bcl-xl, and Bid) in PC cells, we used real-time quantitative RT-PCR analysis (the results are provided in Additional Files 4-12: Figure S4-12). In agreement with the Western blot results, the Slug mRNA level was downregulated in SW1990-(MRC-5)-CM compared with in SW1990. Meanwhile, the Slug mRNA level was upregulated markedly in PANC-1-(MRC-5)-CM compared with in PANC-1. Additionally, the Bax mRNA level was consistent with the protein expression level. However, a portion of the qRT-PCR results did not agree with the Western blot results. For example, the mRNA levels of Bad, Bcl-xl, CTNNA1, CTNNB1, vimentin, CK19, MMP-3, MMP13, WNT16, and ZEB1 were upregulated in PANC-1-(MRC-5)-CM relative to in PANC-1; meanwhile, the mRNA levels of vimentin, ZEB1, MMP-2, MMP-3, MMP-13, and Bcl-2 were downregulated in SW1990-(MRC-5)-CM relative to in SW1990. It was shown that the above-mentioned gene expression was mediated by MRC-5-CM at the post-transcriptional level. Our results also showed that the mRNA levels of PALS1, PALS2, PARD6B, PARD6G, PATJ, PRKCI, PRKCZ, SCRIB, DLG5, and LLGL1 were increased both in PANC-1-(MRC-5)-CM and SW1990-(MRC-5)-CM compared with in the respective control. The mRNA levels of PARD3, PARD3B, PARD6A, DLG1, DLG3, and DLG4 were upregulated in PANC-1-(MRC-5)-CM compared with in the control and the mRNA levels of CRB-2 and LLGL2 were upregulated in SW1990-(MRC-5)-CM compared with in the control. We hypothesized that the elevated expression of cell polarity complexes Par, Scribble, and Crumbs were implicated in the impaired migration and invasion potential of PC cells. MRC-5 also affects the gene expression of stratifin, SERPINB5, paxillin, CTNND1, JUP, JAG1, IHH, SP1, WNT5A, WNT5B, WNT7B, WNT10B, and integrin α1, α2, α3, α5, α8, α9, α11, αD, αM, β1, β5, β6, and β8. The roles of these genes should be explored in future studies.
Discussion
In this study, we showed that MRC-5-CM significantly inhibited cell viability and proliferation and markedly attenuated in vitro cell migration/invasion. However, the impaired malignant activities were not ascribed to the reversal of EMT. The expression of EMT-related signaling molecules, including WNT2, WNT16, TGFB1, SRC, P-SRC-418, and P-SRC-529, of EMT-promoted transcription factors, including Snail, ZEB-1, and ZEB-2, and of mesenchymal markers, including vimentin, MMP-2, 3, 13, and 21, at the protein level was not significantly decreased in PC cells cultured in MRC-5-CM relative to in the control. Additionally, increased E-cadherin/catenin complex was not observed on the cell membrane of PC cells cultured in MRC-5-CM compared with in the control. Thus, PC cells cultured in MRC-5-CM did not undergo complete mesenchymal-to-epithelial transition or partial mesenchymal-to-epithelial transition.
We believe that MRC-5 impedes the malignant biological behavior of PC cells by suppressing CSC expansion, remolding epithelial polarity, and blocking the protumoral cascade reaction coupled to TLR4, TLR1, CD14, and CD25.
CSCs, known as cancer-propagating cells, play a role in carcinogenesis and progression 44, 45. CSCs can be identified based on the expression of various cell surface molecules, including CD90, CD105, and CD133, and on the absence of markers, such as CD45 and CD34. CD90+ has been identified as a CSC marker in PC, whereas CD90+/CD45- has not. Our results showed that the proportion of CD90+ cells decreased strongly in PACN-1-MRC-5-(CM) compared with in the control, and it decreased moderately in SW1990-(MRC-5)-CM compared with in the control. Meanwhile, CD90+/CD45- cells decreased slightly in SW1990-(MRC-5)-CM compared with in the control. However, CD90+/CD45- cells increased slightly in PACN-1-MRC-5-(CM) compared with in the control. This may be explained by the complexity and variability of CSCs. In other words, the absence of CD45 was not a CSC marker for PC cells. However, the impaired malignant behavior in PC cells was correlated with the decreased population of CD90+ cells. However, the associated mechanisms remain unclear. It is possible that MRC-5 induces CSCs into a quiescent state, leading to loss of the self-renew ability.
Recently, it has been shown that smoldering inflammation in the TME has many tumor-enhancing effects 46. TLRs play a critical role in this model. Continuous TLR stimulation by pathogen-associated molecular patterns and damage-associated molecular patterns can upregulate proinflammatory cytokines, proinflammatory chemokines, and immunosuppressive cytokines and can increase TGF-β and VEGF 47. The majority of these molecules are implicated in tumor progression. Furthermore, it has been noted that hypoxia-inducible factor-1 (HIF-1) can induce the expression of TLR4 in cancer cells under hypoxic stress 48. Additionally, upregulation of TLR4 stimulates the expression of HIF-1 by activating the nuclear factor-κB (NF-κB) signaling pathway 49. This signaling pathway forms an auto-stimulatory loop and leads to tumor cell survival, proliferation, and metastasis. Our results showed that the expression of TLR4 was downregulated moderately in both PACN-1-MRC-5-(CM) and SW1990-(MRC-5)-CM compared with in the control and that TLR1 was strongly downregulated in PACN-1-MRC-5-(CM) and SW1990-(MRC-5)-CM compared with in the control. We hypothesized that the weakened malignant biological behaviors in PC cells were associated with decreased populations of TLR4- and TLR1-positive cells, although the role of TLR1 in PC remains unclear. Thus, there may be other auto-stimulatory loops in the tumor, such as TLR4-NF-κB-HIF-1.
It has been reported that differentiated cells can be converted back to stem-like cells under specific conditions. Thus, we also measured the frequency of CD3+, CD4+, CD14+, CD25+, and CD61+ in PC cells cultured in MRC-5-CM and in PC cells in normal media to assess whether CAFs can lead to induction. We found that CD14-positive cells decreased strongly in PACN-1-MRC-5-(CM) and SW1990-(MRC-5)-CM compared with their respective control, that CD25-positive cells were moderately decreased in PACN-1-MRC-5-(CM) compared with in the control, and that CD25-positive cells were strongly decreased in SW1990-(MRC-5)-CM compared with in the control. There were no CD3+, CD4+, or CD61+ cells in PC. Recently, several studies have shown an association between CD3, CD4, CD14, CD25, and CD61 and the progression of cancers 50-54. However, the frequency of CD3+, CD4+, CD14+, CD25+, and CD61+ in cancer cells have not been explored. CD14 is known to be produced by human hepatocytes. Our results show that CD14/CD25 can also be expressed in cancer cells, but their roles need to be further explored. Our results also indicated that cancer cells cannot switch to become immune cells.
Apico-basal polarity and planar cell polarity are characteristically a hallmarks of cells present in differentiated epithelium 55. It has been shown that the polarity complexes Par, Scribble, and Crumbs play a pivotal role in the establishment and maintenance of apico-basal polarity. The Par complex, including PRKCs, PARD3, and PARD6, is localized laterally where it interacts with tight junction proteins. The Crumbs complex, constituted by proteins CRBs, PATJ, and PALSs, is localized to the apical membrane. The Scribble complex, composed of SCRIB, LLGLs, and DLGs, is localized basolaterally. Loss of apico-basal polarity was implicated in the EMT program. Indeed, it has been shown that EMT-promoted transcription factors Snail and ZEB1 can suppress expression of the polarity complex proteins, resulting in a change in cell shape and inducing invasive behavior 56, 57. Notably, the polarity complexes can suppress the expression of EMT-promoted transcription factors and inhibit the metastatic behavior of cancer cells 58. Our qRT-PCR results showed that the mRNA levels of most cell polarity complexes were upregulated in PC cells cultured by MRC-5-CM compared with in PC cells without MRC-5-CM. We concluded that the crippled malignant behavior of PC cells was due to upregulation of the cell polarity complexes, the apico-basal polarity maintainer.
Integrins are associated with the migration and invasion of cancer cells by interacting with MMPs 59. We found that β7 integrin was decreased in SW1990-(MRC-5)-CM relative to in SW1990. However, more research is needed into the role of β7 integrin plays in PC cells. We also examined stratifin, SERPINB5, paxillin, CTNND1, JUP, JAG1, IHH, SP1, WNT5A, WNT5B, WNT7B, WNT10B, and integrin α1, α2, α3, α5, α8, α9, α11, αD, αM, β1, β5, β6, and β8 in PC cells with or without MRC-5-CM. The role of these genes should be explored in future studies.
Also, we evaluated the effect of MRC-5-CM on the growth of PC cell by flow cytometry. The results showed that MRC-5-CM induced S phase arrest in PANC-1 and SW1990 cells. The proportions of PANC-1-(MRC-5)-CM and SW1990-(MRC-5)-CM cells during G1 phase were significantly decreased compared with the proportions of the control. The mechanisms underlying the induction of PC cells to undergo cell cycle arrest remains unclear. We also found there is no effects of MRC-5-CM on cell apoptosis. However, bcl-2 family members and caspase-3 did not play a role. Based on these results, there may be other unknown mechanosensors.
Cancer metastasis is complex, and we cannot accurately determine the priming time. However, it is important to consider that only a few cancer cells can spread to distant metastatic sites and that even fewer cells can survive and grow into a solid tumor. This indicates that cancer metastasis hinges on the premetastatic milieu, including its oxygenation status, nutrient supply, and pH. Different organs provide different TME. The lung is one of the most common sites of distant metastasis in patients with PC, but PC with lung recurrence has a better prognosis than does PC with recurrence in other sites 60. This suggests that certain components of the local environment from the lung may counteract the aggressiveness of PC cells. In other words, PC cells require more time to create a protumorigenic environment by recruiting immune cells or CAFs in the lung. During this process, cancer cells negatively affect local homeostasis by secreting “saboteurs,” including inflammatory factors, growth factors, and pro-tumoral microRNAs. CAFs can travel to distant metastatic sites with cancer cells. Here, we hypothesize that cancer cells can travel with specific immune cells through cell-cell adhesion because immune cells are highly motile. PC, which is a leading cause of cancer death worldwide, causes patient and physician distress, and surgical R0 resection following systemic chemotherapies cannot halt the growth of this malignancy. Therefore, it is important to explore the mechanisms responsible for the metastatic spread and growth to ultimately combat this disease.
In a word, our results suggest that MRC-5 cells impair the malignant activities of PC cell lines PANC-1 and SW1990 by inhibiting CSCs expansion, reinforcing apico-basal polarity, and blocking the protumoral cascade reaction coupled to TLR4, TLR1, CD14, and CD25. Thus, an increased understanding of the crosstalk between MRC-5 and metastatic PC will provide novel therapeutic interventions.
Supplementary Material
Supplementary figures.
Acknowledgments
This study was supported by the project of Science and Technology Department of Zhejiang Province during the 12th Five-Year Plan period (NO. 2013T301-15).
References
- 1.Wang ZB, Liu YQ, Li RE. et al. Autologous cytokine-induced killer cell transfusion increases overall survival in advanced pancreatic cancer. Journal of Hematology & Oncology. doi: 10.1186/s13045-016-0237-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SłotwińSki R, DąbRowSkA A, Lech G. et al. Gene expression disorders of innate antibacterial signaling pathway in pancreatic cancer patients: implications for leukocyte dysfunction and tumor progression. centr eur j immunol. 2014;39:498–507. doi: 10.5114/ceji.2014.47736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feig C, Gopinathan A, Neesse A. et al. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goicoechea SM, García-Mata R, Staub J. et al. Palladin promotes invasion of pancreatic cancer cells by enhancing invadopodia formation in cancer-associated fibroblasts. Oncogene. 2014;33:1265–1273. doi: 10.1038/onc.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karnevi E, Rosendahl AH, Hilmersson KS. et al. Impact by pancreatic stellate cells on epithelial-mesenchymal transition and pancreatic cancer cell invasion: Adding a third dimension in vitro. Exp Cell Res. 2016;346:206–215. doi: 10.1016/j.yexcr.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Hwang RF, Moore T, Arumugam T. et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer research. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vonlaufen A, Joshi S, Qu C. et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer research. 2008;68:2085–2093. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z, Vonlaufen A, Phillips PA. et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. The American journal of pathology. 2010;177:2585–2596. doi: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apte MV, Wilson JS. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. Journal of gastroenterology and hepatology. 2012;27:69–74. doi: 10.1111/j.1440-1746.2011.07000.x. [DOI] [PubMed] [Google Scholar]
- 10.Erkan M, Hausmann S, Michalski CW. et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 11.Li DD, Qu C, Ning ZY. et al. Radiation promotes epithelial-to-mesenchymal transition and invasion of pancreatic cancer cell by activating carcinoma-associated fibroblasts. Am J Cancer Res. 2016;6:2192–2206. [PMC free article] [PubMed] [Google Scholar]
- 12.Fearon DT. The Carcinoma-Associated Fibroblast Expressing Fibroblast Activation Protein and Escape from Immune Surveillance. Cancer Immunol Res. 2014;2:187–193. doi: 10.1158/2326-6066.CIR-14-0002. [DOI] [PubMed] [Google Scholar]
- 13.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 14.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 15.Jayachandran A, Dhungel B, Steel JC. Epithelial-to-mesenchymal plasticity of cancer stem cells: therapeutic targets in hepatocellular carcinoma. Journal of Hematology & Oncology. doi: 10.1186/s13045-016-0307-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu JH, Thakolwiboon S, Liu XH. et al. Overexpression of CD90 (Thy-1) in Pancreatic Adenocarcinoma Present in the Tumor Microenvironment. PLoS ONE. doi: 10.1371/journal.pone.0115507. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Heidt DG, Dalerba P. et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 18.Hermann PC, Huber SL, Herrler T. et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, He J, Liu Y. et al. Identification of glycoprotein markers for pancreatic cancer CD24+CD44+ stem-like cells using nano-LC-MS/MS and tissue microarray. J. Proteome Res. 2012;11:2272–2281. doi: 10.1021/pr201059g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MP, Fleming JB, Wang H. et al. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. doi: 10.1371/journal.pone.0020636. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hindriksen S, Bijlsma MF. Cancer Stem Cells, EMT, and Developmental Pathway Activation in Pancreatic Tumors. Cancers. 2012;4:989–1035. doi: 10.3390/cancers4040989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaffer CL, Brueckmann I, Scheel C. et al. Normal and neoplastic nonstemcells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mani SA, Guo W, Liao MJ. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma HY, Liu XZ, Liang CM. Inflammatory microenvironment contributes to epithelial-mesenchymal transition in gastric cancer. World J Gastroenterol. 2016;22:6619–6628. doi: 10.3748/wjg.v22.i29.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhim AD, Mirek ET, Aiello NM. et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palen K, Weber J, Dwinell MB. et al. E-cadherin re-expression shows in vivo evidence for mesenchymal to epithelial transition in clonal metastatic breast tumor cells. Oncotarget. 2016;7:43363–43375. doi: 10.18632/oncotarget.9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baas AF, Smit L, Clevers H. LKB1 tumor suppressor protein: PAR taker in cell polarity. Trends Cell Biol. 2004;14:312–319. doi: 10.1016/j.tcb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Karp CM, Tan TT, Mathew R. et al. Role of the polarity determinant crumbs in suppressing mammalian epithelial tumor progression. Cancer Res. 2008;68:4105–4115. doi: 10.1158/0008-5472.CAN-07-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasheed ZA, Yang J, Wang Q. et al. Prognostic Significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin T, Wei H, Gou S. et al. Cancer stem-like cells enriched in panc-1 spheres possess increased migration ability and resistance to gemcitabine. Int J Mol Sci. 2011;12:1595–1604. doi: 10.3390/ijms12031595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Q, Yoshimitsu M, Kuwahata T. et al. Establishment of a highly migratory subclone reveals that CD133 contributes to migration and invasion through epithelial-mesenchymal transition in pancreatic cancer. Hum Cell. 2012;25:1–8. doi: 10.1007/s13577-011-0037-9. [DOI] [PubMed] [Google Scholar]
- 32.Shah AN, Summy JM, Zhang J. et al. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 33.Kikuta K, Masamune A, Watanabe T. et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403:380–384. doi: 10.1016/j.bbrc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 34.Liu CY, Xu JY, Shi XY. et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013;93:844–854. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 35.Sun YL, Wu CS, Ma JX. et al. Toll-like receptor 4 promotes angiogenesis in pancreatic cancer via PI3K/AKT signaling. Exp Cell Res. 2016;347:274–282. doi: 10.1016/j.yexcr.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Vaz J, Andersson R. Intervention on toll-like receptors in pancreatic cancer. World J Gastroenterol. 2014;20:5808–5817. doi: 10.3748/wjg.v20.i19.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komohara Y, Takeya M. CAFs and TAMs: Maestros of the tumour microenvironment. J Pathol. 2017;241:313–315. doi: 10.1002/path.4824. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Tang S, Tang XD. Thymic Stromal Lymphopoietin Promotes Fibrosis and Activates Mitogen-Activated Protein Kinases in MRC-5 Cells. Med Sci Monit. 2016;22:2357–2362. doi: 10.12659/MSM.896390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heylen N, Baurain R, Remacle C. et al. Effect of MRC-5 fibroblast conditioned medium on breast cancer cell motility and invasion in vitro. Clin Exp Metastasis. 1998;16:193–203. doi: 10.1023/a:1006532523152. [DOI] [PubMed] [Google Scholar]
- 40.Li Q, Wang W, Yamada T. et al. Pleural Mesothelioma Instigates Tumor-Associated Fibroblasts To Promote Progression via a Malignant Cytokine Network. Am J Pathol. 2011;179:1483–1493. doi: 10.1016/j.ajpath.2011.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Li Q, Takeuchi S. et al. Met kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor-induced tyrosine kinase inhibitor resistance in EGFR mutant lung cancer. Clin Cancer Res. 2012;18:1663–1671. doi: 10.1158/1078-0432.CCR-11-1171. [DOI] [PubMed] [Google Scholar]
- 42.Froeling FE, Mirza TA, Feakins RM. et al. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, beta-catenin, and Ezrin expression in tumor cells. Am J Pathol. 2009;175:636–648. doi: 10.2353/ajpath.2009.090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Songming Ding, Guoliang Chen, Wu Zhang. et al. MRC-5 fibroblast-conditioned medium influences multiple pathways regulating invasion, migration, proliferation, and apoptosis in hepatocellular carcinoma. J Transl Med. 2015;13:237. doi: 10.1186/s12967-015-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rycaj K, Tang DG. Cell-of-origin of cancer versus cancer stem cells: assays and interpretations. Cancer Research. 2015;75:4003–4011. doi: 10.1158/0008-5472.CAN-15-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mladinich M, Ruan D, Chan CH. Tackling Cancer Stem Cells via Inhibition of EMT Transcription Factors. Stem Cells Int. doi: 10.1155/2016/5285892. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mantovani A, Allavena P, Sica A. et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 47.Goto Y, Arigami T, Kitago M. et al. Activation of Toll-like receptors 2, 3, and 4 on human melanoma cells induces inflammatory factors. Mol Cancer Ther. 2008;7:3642–3653. doi: 10.1158/1535-7163.MCT-08-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan P, Zhang JJ, Wang B. et al. Hypoxia-inducible factor-1 up-regulates the expression of Toll-like receptor 4 in pancreatic cancer cells under hypoxic conditions. Pancreatology. 2012;12:170–178. doi: 10.1016/j.pan.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Han SW, Xu WG, Wang ZY. et al. Crosstalk between the HIF-1 and Toll-like receptor/nuclear factor-κB pathways in the oral squamous cell carcinoma. Microenvironment. Oncotarget. 2016;7:37773–37789. doi: 10.18632/oncotarget.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwak Y, Koh J, Kim DW. et al. Immunoscore encompassing CD3+ and CD8+ T cell densities in distant metastasis is a robust prognostic marker for advanced colorectal cancer. Oncotarget. 2016;7:81778–81790. doi: 10.18632/oncotarget.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goebel L, Grage-Griebenow E, Gorys A. et al. CD4+ T cells potently induce epithelial-mesenchymal-transition in premalignant and malignant pancreatic ductal epithelial cells-novel implications of CD4+ T cells in pancreatic cancer development. Oncoimmunology. doi: 10.1080/2162402X.2014.1000083. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kyrgidis A, Yavropoulou MP, Lagoudaki R. et al. Increased CD14+ and decreased CD14- populations of monocytes 48 h after zolendronic acid infusion in breast cancer patients. Osteoporos Int. 2017;28:991–999. doi: 10.1007/s00198-016-3807-0. [DOI] [PubMed] [Google Scholar]
- 53.Nakase K, Kita K, Kyo T. et al. High expression of interleukin-2 receptor α-chain (CD25) in myelodysplastic syndrome preceding acute myeloid leukemia and chronic myeloid leukemia in myeloid blast crisis. Leuk Lymphoma. 2016;13:1–3. doi: 10.1080/10428194.2016.1236377. [DOI] [PubMed] [Google Scholar]
- 54.Chang QS, Chen BL, Thakur C. et al. Arsenic-induced sub-lethal stress reprograms human bronchial epithelial cells to CD61- cancer stem cells. Oncotarget. 2014;5:1290–1303. doi: 10.18632/oncotarget.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gandalovičová A, Vomastek T, Rosel D. et al. Cell polarity signaling in the plasticity of cancer cell invasiveness. Oncotarget. 2016;7:5022–25049. doi: 10.18632/oncotarget.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kashyap A, Zimmerman T, Ergül N. et al. The human Lgl polarity gene, Hugl-2, induces MET and suppresses Snail tumorigenesis. Oncogene. 2013;32:1396–1407. doi: 10.1038/onc.2012.162. [DOI] [PubMed] [Google Scholar]
- 57.Aigner K, Dampier B, Descovich L. et al. The transcription factor ZEB1 (δEF1 ) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2010;26:6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodwin JM, Svensson RU, Lou HJ. et al. An AMPK-independent signaling pathway downstream of the LKB1 tumor suppressor controls Snail1 and metastatic potential. Mol Cell. 2014;55:436–450. doi: 10.1016/j.molcel.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiao Y, Feng X, Zhan Y. et al. Matrix metalloproteinase-2 promotes αvβ3 integrin-mediated adhesion and migration of human melanoma cells by cleaving fibronectin. PLoS One. doi: 10.1371/journal.pone.0041591. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wangjam T, Zhang Z, Zhou XC. et al. Resected pancreatic ductal adenocarcinomas with recurrence limited in lung have a significantly better prognosis than those with other recurrence patterns. Oncotarget. 2015;6:36903–36910. doi: 10.18632/oncotarget.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.