ABSTRACT
Staphylococcus aureus causes an array of serious infections resulting in high morbidity and mortality worldwide. This study evaluated naturally occurring serum anti-alpha-toxin (anti-AT) antibody levels in human subjects from various age groups, individuals with S. aureus dialysis and surgical-site infections, and S. aureus-colonized versus noncolonized subjects. Anti-AT immunoglobulin G (IgG) and neutralizing antibody (NAb) levels in infants (aged ≤1 year) were significantly lower than those in other populations. In comparison to adolescent, adult, and elderly populations, young children (aged 2 to 10 years) had equivalent anti-AT IgG levels but significantly lower anti-AT NAb levels. Therefore, the development of anti-AT NAbs appears to occur later than that of AT-specific IgG, suggesting a maturation of the immune response to AT. Anti-AT IgG levels were slightly higher in S. aureus-colonized subjects than in noncolonized subjects. The methicillin susceptibility status of colonizing isolates had no effect on anti-AT antibody levels in S. aureus-colonized subjects. The highest anti-AT IgG and NAb levels were observed in dialysis patients with acute S. aureus infection. Anti-AT IgG and NAb levels were well correlated in subjects aged >10 years, regardless of colonization or infection status. These data demonstrate that AT elicits a robust IgG humoral response in infants and young children that becomes stable prior to adolescence, matures into higher levels of NAbs in healthy adolescents, and becomes elevated during S. aureus infection. These findings may assist in identifying subjects and patient populations that could benefit from vaccination or immunoprophylaxis with anti-AT monoclonal antibodies.
KEYWORDS: alpha-toxin, human subjects, serum antibodies, Staphylococcus aureus
INTRODUCTION
Staphylococcus aureus is a Gram-positive bacterium that is part of the normal human flora on the skin and in the nasopharyngeal cavity (1). Approximately half of all individuals have permanent or transient S. aureus nasal colonization (2). S. aureus causes both community- and hospital-acquired infections, ranging from minor skin infections to life-threatening surgical-site infections, pneumonia, and sepsis. Certain patient populations have well-described risks for serious S. aureus infections, including patients undergoing cardiothoracic and orthopedic surgery, hemodialysis patients, patients with atopic dermatitis, and patients on ventilator support (3–5). These infections have become increasingly serious as the number of multidrug-resistant S. aureus isolates has increased in the last 20 years. A 2007 study estimated that methicillin-resistant S. aureus (MRSA) cost the health care system (patients and hospitals) an extra $830 million to $9.7 billion during the years 1995 to 2005 in the United States alone (6).
Despite the introduction of new antibiotics against S. aureus, the spread of drug resistance and the emergence of highly virulent strains require new approaches to address the existing and potentially expanding unmet medical need for effective prophylaxis strategies to prevent S. aureus infection and disease. Currently, there is no approved vaccine or prophylactic antibody to prevent S. aureus infection, and all previous active and passive immunization approaches have been unsuccessful. The failure to develop an effective vaccine or monoclonal antibody against S. aureus is possibly due to targeting of wrong antigens, testing in wrong patient populations, complexity of S. aureus pathogenesis, unclear correlates of protection in humans, and limitations of existing animal models (7, 8).
During infection, S. aureus releases a number of toxins designed to lyse innate immune cells, cause tissue necrosis, and evade the host immune response. These toxins include alpha-toxin (AT), other hemolysins, phenol-soluble modulins, leukocidins, and superantigens, such as enterotoxins and toxic shock syndrome toxin. AT is a secreted 33-kDa monomeric protein that binds the disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) and forms heptameric pores in cell membranes, leading to cell lysis, inflammation, immune evasion, and tissue damage (9–12). The pivotal role of AT in S. aureus pathogenesis has been demonstrated in animal models of dermonecrosis, pneumonia, sepsis, endocarditis, and mastitis (13–19). Importantly, the presence and sequence of the AT gene, hla, is highly conserved in diverse S. aureus clinical isolates from around the world (20). Recently there has been an increased interest in targeting AT for immunoprophylaxis, due to the success of AT vaccination or monoclonal antibody prophylaxis in the prevention of pneumonia, dermonecrosis, and sepsis in animal models (21–24). In addition, observational studies in humans have correlated high anti-AT antibody levels with a reduced incidence of serious S. aureus infections and improved clinical outcome (25–28). Thus, AT represents an attractive target for immunoprophylaxis to prevent S. aureus infections in high-risk patient populations. One example of such an immunoprophylactic agent is MEDI4893, a novel AT-specific monoclonal antibody currently in phase 2 development for the prevention of pneumonia in mechanically ventilated patients with S. aureus colonization of the lower respiratory tract (29–32).
To inform the development of MEDI4893 and other future vaccine- and antibody-based approaches to prevent S. aureus infections, it is important to understand the relationship between AT-specific antibody levels and S. aureus colonization and infection in diverse human populations. This study was conducted to characterize serum anti-AT antibody levels as functions of patient age, S. aureus colonization, and disease status. Previous studies have highlighted the need for monitoring the quality of antibody response to ensure optimal vaccine efficacy (33–35). For this reason, we measured neutralizing antibody (NAb) levels in addition to anti-AT immunoglobulin G (IgG) levels and determined NAb/IgG ratios to quantify functional antibodies in the study samples.
RESULTS
Anti-AT antibody levels as a function of age.
To understand the relationship between age and AT-specific IgG and NAb levels, we conducted a survey of 309 serum samples collected from subjects ranging between ≤1 and 75 years of age (Fig. 1). The subject-to-subject variability of antibody levels observed in infants (aged ≤1 year) and young children (aged 2 to 10 years) was higher than in the rest of the age groups. The mean IgG and NAb levels in infants were significantly lower than in the other groups. NAb and IgG levels were, respectively, 6.31-fold (P = 0.0064) and 12.92-fold (P < 0.0001) higher in young children than in infants, whereas the NAb/IgG ratio was approximately 2-fold (P = 0.1162) higher in the infants than in the young children. The NAb/IgG ratio in the young children was the lowest among the age groups. IgG levels in adolescents (11 to 20 years old) were similar to those in young children, whereas NAb levels and the NAb/IgG ratio were 5.52-fold (P = 0.0001) and 4.7-fold (P < 0.0001) higher, respectively. Among subjects aged 11 to 50 years, NAb and IgG levels and NAb/IgG ratios remained similar. The levels of NAb (ratio = 0.55, P = 0.0006) and IgG (ratio = 0.62, P = 0.0007) were lower in adults aged 51 to 60 years than in subjects aged 11 to 50 years, although the NAb/IgG ratios were not significantly different (ratio = 0.88, P = 0.2767). Levels of NAb (1.45-fold, P = 0.1107) and IgG (1.38-fold, P = 0.1272) in elderly subjects (61 to 75 years old) were slightly higher than those in adults aged 51 to 60 years.
FIG 1.
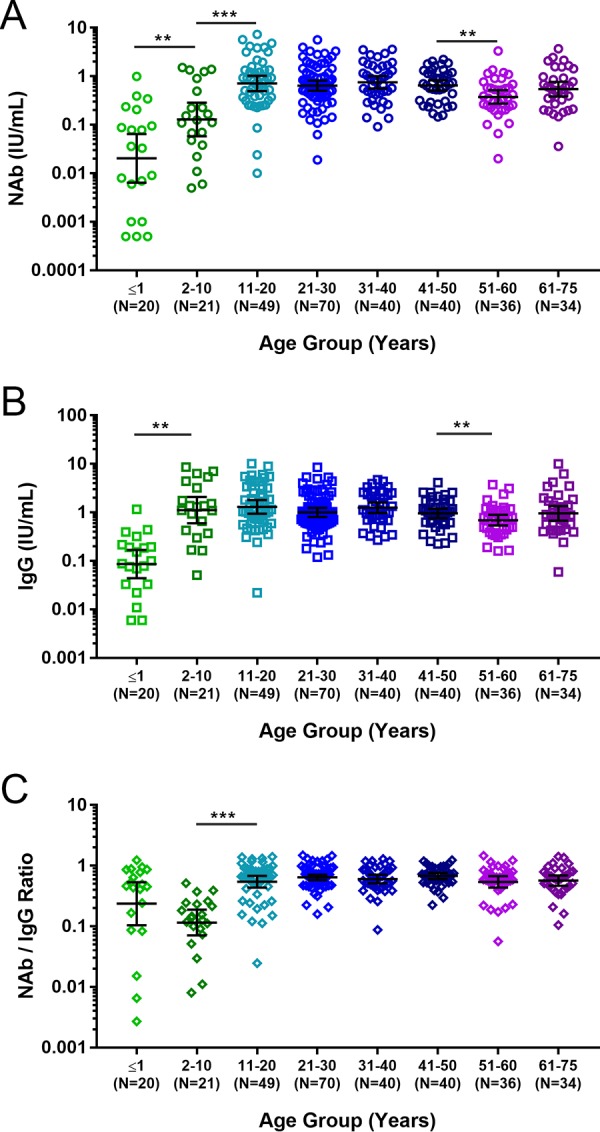
Serum anti-AT antibody levels in healthy subjects from different age groups. Shown are individual measurements, geometric mean values, and 95% confidence intervals for anti-AT NAb levels (A), anti-AT total IgG antibody levels (B), and NAb/IgG ratios (C). **, P < 0.01; ***, P < 0.001.
Anti-AT antibody levels as a function of S. aureus colonization.
A previous study suggested that higher anti-AT antibody levels in intravenous-drug users may result from recurrent intravenous exposure to S. aureus and may correlate with protection from S. aureus-induced endocarditis (27). To determine whether subjects colonized with S. aureus might have been self-immunized against AT and have higher AT-specific antibody levels, we measured serum anti-AT IgG and NAb levels in S. aureus-colonized (n = 53) and noncolonized (n = 147) subjects (Fig. 2). NAb levels in colonized subjects were 1.38-fold higher than in noncolonized subjects (P = 0.0356), whereas differences in IgG levels (1.23-fold, P = 0.1188) and NAb/IgG ratios (1.12-fold, P = 0.1602) were not significant. Among the subjects colonized with MRSA (n = 14) or methicillin-susceptible S. aureus (MSSA) (n = 39), NAb (0.82, 0.73) and IgG (1.18, 1.12) levels and NAb/IgG ratios (0.70, 0.66) were similar. These data suggest that there is a modest effect of S. aureus colonization on serum anti-AT antibody levels at a population level.
FIG 2.
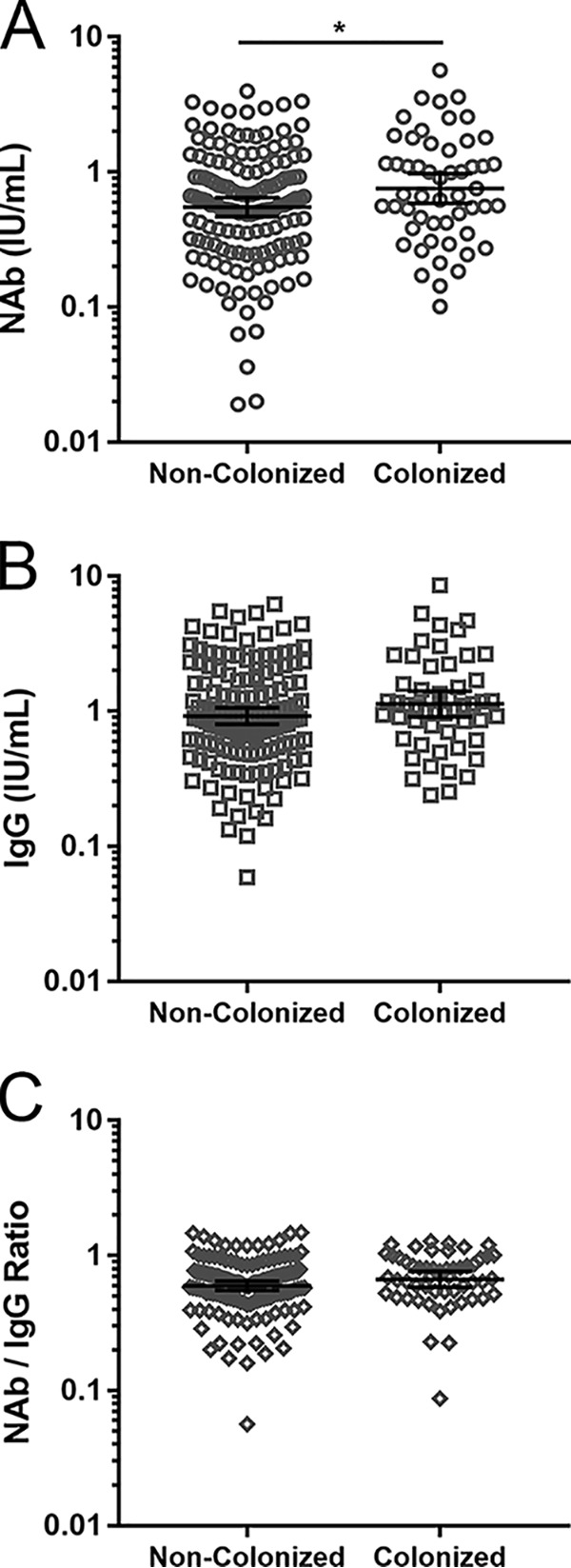
Serum anti-AT antibody levels as a function of S. aureus colonization. Shown are individual measurements, geometric mean values, and 95% confidence intervals for anti-AT NAb levels (A), anti-AT total IgG antibody levels (B), and NAb/IgG ratios (C). *, P < 0.05.
Anti-AT antibody levels as a function of S. aureus infection.
To investigate whether S. aureus infection results in increased AT-specific antibody levels, we measured anti-AT IgG and NAb levels in serum samples collected from dialysis patients (n = 100) and surgery patients (n = 100) with documented S. aureus bacteremia. The anti-AT antibody levels, S. aureus strains, AT production and sequence, and clinical outcomes in the same patients have been reported previously (36). The current study involved a larger group of healthy control subjects (n = 229) that included adolescent, adult, and elderly patients aged 20 to 75 years who were different from the healthy controls used in the previously published study. In addition, samples from dialysis patients with S. aureus bacteremia and subjects with bacteremia due to surgical-site infection were compared with those from age-matched, uninfected dialysis and elective-surgery subjects (see Table 1). As shown in Fig. 3, AT-specific antibody levels were higher in S. aureus-infected, bacteremic dialysis patients (2.14-fold for NAb, P < 0.0001; 1.97-fold for IgG, P < 0.0001) and in S. aureus-infected, bacteremic surgery patients (1.68-fold for NAb, P = 0.0032; 1.38-fold for IgG, P = 0.0493) than in healthy controls. NAb/IgG ratios were elevated in elective-surgery patients (1.52-fold, P = 0.0074) and S. aureus-infected surgery patients (1.22-fold, P = 0.0023) relative to healthy controls.
TABLE 1.
Serum samples used in the study
| Subject group | Study group | Age (yr) | No. of samples | Source |
|---|---|---|---|---|
| Healthy subjects (n = 309)a | Infants | ≤1 | 20 | BioreclamationIVT |
| Young children | 2–10 | 21 | BioreclamationIVT | |
| Adolescent | 11–20 | 49b | BioreclamationIVT | |
| Adults | 21–60 | 185b | BioreclamationIVT | |
| Elderly | 61–75 | 34b | BioreclamationIVT | |
| Uninfected patients (n = 60) | Dialysis | 27–78 | 30 | BioreclamationIVT |
| Prior to elective surgery | 20–86 | 30 | BioreclamationIVT | |
| Patients with confirmed S. aureus infection (n = 200)c | Surgical-site infection | 18–83 | 100 | Duke University |
| Dialysis infection | 23–84 | 100 | Duke University |
Nasal colonization and methicillin susceptibility status of colonizing S. aureus isolates were assessed in a subgroup of 200 healthy subjects as described in the text.
Subjects aged 20 years (n = 10), adults (n = 185), and elderly subjects (n = 34) composed a subgroup of healthy control donors (n = 229; aged 20 to 75 years).
Described previously (36).
FIG 3.
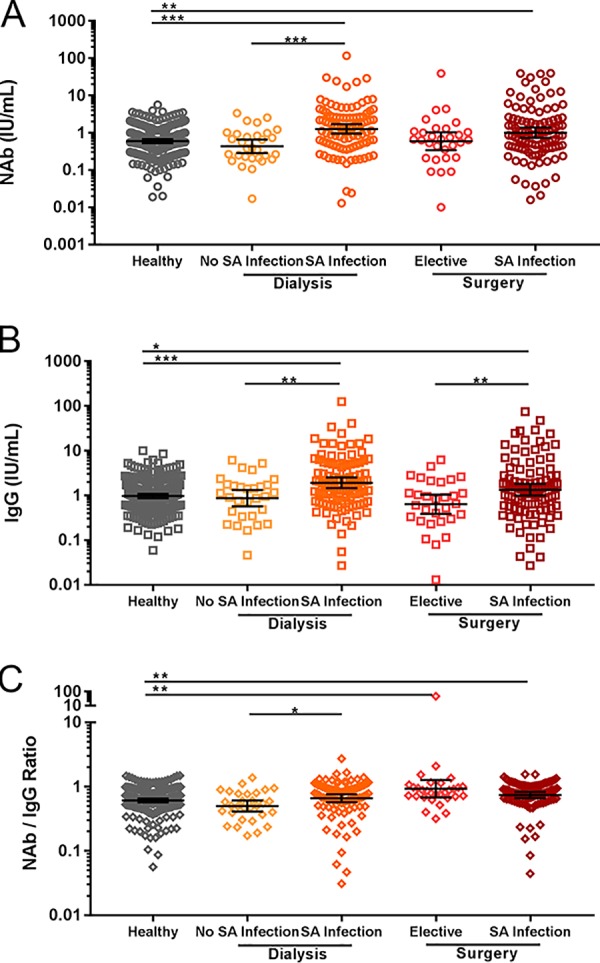
Serum anti-AT antibody levels as a function of S. aureus (SA) infection. Shown are individual measurements, geometric mean values, and 95% confidence intervals for anti-AT NAb levels (A), anti-AT total IgG antibody levels (B), and NAb/IgG ratios (C). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Within the dialysis and surgical-site infection groups, anti-AT antibody levels were generally higher in the presence of confirmed S. aureus infection. In the dialysis group, S. aureus-infected subjects had higher NAb levels (2.92-fold, P < 0.0001), IgG levels (2.20-fold, P = 0.0017), and NAb/IgG ratios (1.33-fold, P = 0.0194) than subjects without S. aureus infection. In the surgery group, S. aureus-infected subjects had higher NAb (1.69-fold, P = 0.0975) and IgG (2.11-fold, P = 0.0094) levels but slightly lower NAb/IgG ratios (0.80, P = 0.1703) than subjects with elective surgery. These data suggest that AT is expressed during bacteremia and that the host elicits a neutralizing antibody response to AT.
Correlation of AT-specific IgG and NAb levels.
To determine whether AT-specific IgG and NAb levels were correlated, we compared IgG and NAb levels among the diverse patient populations included in the current study. In general, anti-AT IgG and NAb levels were well correlated (r = 0.87, P < 0.0001) (Fig. 4A). Because of high subject-to-subject variability and lower NAb/IgG ratios observed in infants and young children, the correlation between anti-AT IgG and NAb levels was less pronounced in patients aged ≤10 years (r = 0.77, P < 0.0001) (Fig. 4B).
FIG 4.
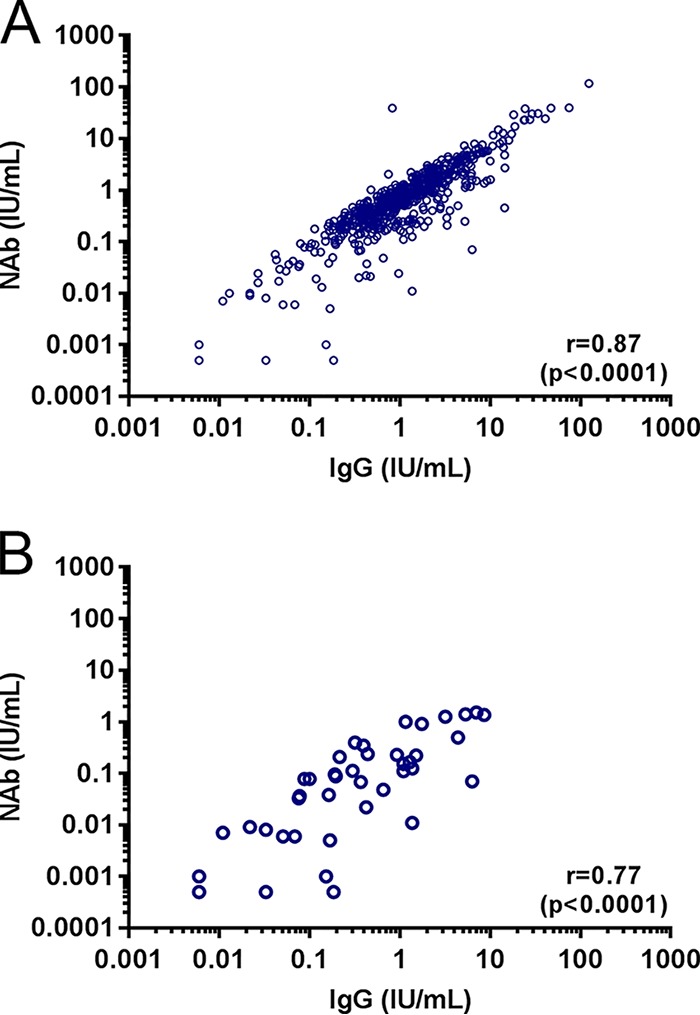
Correlation between anti-AT IgG and NAb levels. Shown are individual measurements, Spearman correlation coefficients (r), and P values for all subjects (A) and subjects aged ≤10 years (B).
DISCUSSION
Previous studies have described important associations between serum anti-AT IgG levels and risks and outcomes of S. aureus infections in different patient populations, such as hospitalized adults (25), older adults with invasive S. aureus infections (26), intravenous-drug users (27), and children colonized with S. aureus or presenting with S. aureus skin and soft tissue or invasive infection (28). However, none of the prior studies have provided a comprehensive analysis of serum anti-AT IgG and NAb levels across various ages and patient groups. A recent study demonstrated a robust time-dependent anti-AT IgG response in young children after an influenza-MRSA coinfection and suggested that a low level of AT-specific NAb at the onset of infection may be responsible for increased mortality (37). To broaden our understanding of AT-specific humoral immunity in different patient populations and to identify patient populations that could benefit from vaccination or immunoprophylaxis with an anti-AT monoclonal antibody, we measured the levels of naturally occurring serum anti-AT IgG and anti-AT NAb and compared levels by various characteristics, including subject age, S. aureus colonization status, and history of S. aureus infection.
All serum samples had both AT-specific IgG and NAbs at levels ranging from 0.006 to 124.803 IU/ml and from below the lower limit of quantification (LLOQ) to 117.031 IU/ml, respectively. The lowest anti-AT antibody levels were observed in infants aged ≤1 year. The infant population appeared to have two subgroups with a >10-fold difference in mean NAb levels (mean levels of >0.01 IU/ml for one group and approximately 0.001 IU/ml for another), which was potentially due to antibodies received from either maternal transfer in utero or breastfeeding in the subgroup with the higher antibody levels. In the pediatric group aged 2 to 10 years, the AT-specific IgG levels were similar to those in adolescent, adult, and elderly populations, but NAb levels were significantly lower than those in older subjects. Consequently, the NAb/IgG ratio in the 2- to 10-year-old group was lower than in all other age groups. This observation suggests that the development of a mature anti-AT NAb immune response is an age- or exposure-dependent process. It also suggests that young children who no longer receive protective antibodies from their mothers may be at increased risk for developing S. aureus infection. Healthy subjects from the adolescent, adult, and elderly age groups had similar levels of AT-specific antibodies, suggesting that anti-AT antibody levels remain stable after maturation of anti-AT immunity.
There were no significant differences between anti-AT antibody levels in MSSA-colonized subjects versus MRSA-colonized subjects. We detected a trend for elevated anti-AT antibody levels in healthy subjects whose nasal mucosa was colonized by S. aureus compared with the antibody levels in healthy noncolonized subjects, although these differences did not reach statistical significance for IgG levels. In contrast, the highest AT-specific antibody levels were observed in surgery and dialysis patients with confirmed S. aureus infections, suggesting that the immune system had been exposed to AT in both surgical-site- and dialysis-associated infections. Previous studies have demonstrated low levels of hla gene expression in healthy subjects nasally colonized with S. aureus, whereas increased hla expression and elevated hemolytic activity by S. aureus strains correlate with progression from colonization to infection in animal models and humans (38–40). Therefore, serum AT-specific antibody levels in patients from this study may reflect AT production by colonizing or infecting S. aureus strains. Although the difference was not statistically significant, dialysis patients without S. aureus infection had the lowest level of AT-specific NAb and the lowest NAb/IgG ratio, suggesting that this patient population could benefit from vaccination or immunoprophylaxis with anti-AT NAb.
Serum anti-AT IgG and NAb levels were highly correlated in our study, indicating that anti-AT NAbs comprise the majority of AT-specific IgG antibodies. High subject-to-subject variability and lower NAb/IgG ratios contributed to a lesser correlation between NAb and IgG levels in pediatric subjects, specifically in infants and children younger than 10 years of age, which is consistent with the absence of long-term exposure to S. aureus in this patient population.
This study had several limitations. It was an observational, cross-sectional study and therefore was unable to correlate antibody levels before S. aureus exposure or infection with clinical outcome. The samples from the infected subjects (Duke University) and healthy controls (BioreclamationIVT) were collected at different locations and at different time periods, which may have resulted in some bias in the results. Nasal colonization was assessed from a one-time swabbing of the anterior nares, which did not allow a distinction between intermittent and persistent carriers of S. aureus. Because colonization data were not available for samples collected from the infants and young children, the effect of nasal colonization on serologic differences in these subject groups could not be determined.
Despite these limitations, the study used previously validated and published assays and antibody levels calibrated to the universal World Health Organization reference standard. As such, it may provide a baseline understanding for ongoing prospective studies such as ASPIRE-ICU (41) and SAATELLITE (42), along with future epidemiology studies and clinical trials.
MATERIALS AND METHODS
Serum samples.
Human serum samples used in the study are described in Table 1. Samples from healthy subjects aged ≤1 to 75 years (n = 309), uninfected dialysis patients (n = 30), and elective-surgery patients (n = 30) were purchased from BioreclamationIVT (Westbury, NY). For analyses related to the presence or absence of S. aureus infection, a control subgroup of healthy donors (n = 229) included healthy adolescent, adult, and elderly subjects (aged 20 to 75 years), as indicated in Table 1. Samples from hemodialysis patients (n = 100) and surgery patients (n = 100) with acute S. aureus infections were obtained from Duke University Medical Center and have been described previously (36). These acute-phase S. aureus infection serum samples were collected from July 2001 to December 2009 from patients who were hospitalized at Duke University Medical Center with monomicrobial S. aureus bacteremia, had no neutropenia (absolute neutrophil count of >100 neutrophils/μl), and had either end-stage renal disease necessitating chronic hemodialysis or a surgical procedure in the preceding 30 days.
Nasal S. aureus colonization.
For analyses related to S. aureus colonization, nasal swabs and serum samples were prospectively collected from a subgroup of 200 healthy subjects (aged 18 to 71 years) who had no respiratory illness at the time of sampling. Anterior nares of both nostrils were swabbed using a Copan double transport swab and stored in Universal Transport Medium before the analysis. Nasal S. aureus colonization, along with the methicillin susceptibility status of colonizing S. aureus isolates, was assessed with the Xpert SA nasal complete PCR assay (Cepheid, Sunnyvale, CA) according to the manufacturer's instructions. Briefly, the nasal swab was put into elution reagent provided in the kit and vortexed at high speed for 10 s. The entire content of the elution reagent was then transferred to the sample chamber of the Xpert SA nasal complete assay cartridge. The cartridge was then loaded into the GeneXpert Dx instrument to perform the testing. Upon completion of the test, the presence of MRSA was determined based on simultaneous detection of the staphylococcal protein A gene (spa), the gene for methicillin resistance (mecA), and the staphylococcal cassette chromosome mec element (SCCmec). Detection of the spa gene without detection of either mecA or SCCmec indicated the presence of MSSA.
Anti-AT IgG and NAb levels in human serum.
Quantification of anti-AT IgG and NAb in serum samples was performed as described previously, using an AT-specific IgG enzyme-linked immunosorbent assay (ELISA) and a red blood cell (RBC)-based AT neutralization assay, respectively (36). The reference standard used in these assays was calibrated to the National Institute for Biological Standards and Control reference standard (43), and antibody levels for samples were reported in IU/ml.
To determine the concentrations of anti-AT neutralizing antibodies, the standard, quality control, and diluted human serum samples were mixed with an equal volume of a 10-ng/ml concentration of AT in a 96-well deep plate with shaking at 450 rpm for 2 h ± 5 min. Rabbit RBCs were washed three times with phosphate-buffered saline (PBS), and the total viable cell number was determined by using a Vi-CELL viability analyzer prior to use. The sample-AT mixtures were added to a microtiter plate and incubated with 5 × 106 rabbit RBCs with shaking at 450 rpm for 2 h ± 5 min. Following incubation, the plate was centrifuged at 500 × g for 5 min at 4°C. Intact RBCs formed a pellet in the bottom of each well. The supernatant from each well was transferred to a half-area polystyrene plate with a nonbinding surface, either manually or by using the PerkinElmer JANUS automation work station. The degree of hemolysis was determined by measuring hemoglobin absorbance at 415 nm, which is inversely related to the amount of neutralizing antibodies present in a sample. The conversion of optical density to IU per milliliter was performed by using a 4-parameter regression model without weighting (SoftMax Pro v5.4; Sunnyvale, CA), for comparison to a standard curve, assayed on each plate. The LLOQ of the assay was 0.0007 IU/ml.
To determine the concentrations of anti-AT IgG antibodies, a microtiter plate was first coated with 1.5 μg/ml native AT in carbonate/bicarbonate buffer and incubated at 4°C overnight. The plate was washed and blocked with blocking buffer (1× Dulbecco PBS with 0.1% Tween 20, 5% bovine serum albumin) at room temperature for 1 h. During this time, serum sample dilutions were prepared in dilution buffer. The blocked plate was washed, and the standard, quality control, and diluted human serum samples were added to the plate in duplicate. The plate was incubated for 1 h with shaking at 600 rpm in a 20°C incubator and washed, and then a horseradish peroxidase-conjugated secondary mouse anti-human IgG Fc fragment (HP6043; EMD Millipore, Billerica, MA) was added, after 1:7,500 dilution in dilution buffer (1× Dulbecco PBS with 0.1% Tween 20, 0.5% bovine serum albumin). The plate was incubated for 1 h with shaking at 600 rpm in a 20°C incubator. After incubation, the plate was washed and 3,3′,5,5′-tetramethylbenzidine substrate was added. The plate was incubated in the dark in a 20°C incubator without shaking. The reaction was stopped with sulfuric acid, the plate was read on a spectrophotometer at 450 nm, and data were analyzed with SoftMax Pro v5.4, using a 4-parameter logistical curve fit model without weighting. The LLOQ of the assay was 0.0001 IU/ml.
Statistical methods.
Serum anti-AT IgG and NAb levels, in IU per milliliter, were summarized using the geometric mean by study groups. One-way analysis of variance with heterogeneous within-group variance was applied for comparison between groups. Correlation between anti-AT IgG and NAb levels was assessed by the Spearman correlation coefficient. SAS system version 9.3 was used for summary statistics, and Prism 6.05 was used to generate figures.
ACKNOWLEDGMENTS
This study was funded by MedImmune, the global research and development arm of AstraZeneca.
We thank Deborah Shuman of MedImmune for editorial assistance with the manuscript.
All authors are employees of MedImmune and own stock and/or stock interests in AstraZeneca.
REFERENCES
- 1.Sollid JU, Furberg AS, Hanssen AM, Johannessen M. 2014. Staphylococcus aureus: determinants of human carriage. Infect Genet Evol 21:531–541. doi: 10.1016/j.meegid.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 3.Kluytmans J, van Belkum A, Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10:505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chastre J, Fagon JY. 2002. Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 5.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein E, Smith DL, Laxminarayan R. 2007. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999-2005. Emerg Infect Dis 13:1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sause WE, Buckley PT, Strohl WR, Lynch AS, Torres VJ. 2016. Antibody-based biologics and their promise to combat Staphylococcus aureus infections. Trends Pharmacol Sci 37:231–241. doi: 10.1016/j.tips.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knisely JM, Liu B, Ranallo RT, Zou L. 2016. Vaccines for healthcare-associated infections: promise and challenge. Clin Infect Dis 63:657–662. doi: 10.1093/cid/ciw333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhakdi S, Tranum-Jensen J. 1991. Alpha-toxin of Staphylococcus aureus. Microbiol Rev 55:733–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walev I, Martin E, Jonas D, Mohamadzadeh M, Muller-Klieser W, Kunz L, Bhakdi S. 1993. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun 61:4972–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilke GA, Bubeck Wardenburg J. 2010. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A 107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen TS, Hilliard JJ, Jones-Nelson O, Keller AE, O'Day T, Tkaczyk C, DiGiandomenico A, Hamilton M, Pelletier M, Wang Q, Diep BA, Le VT, Cheng L, Suzich J, Stover CK, Sellman BR. 2016. Staphylococcus aureus alpha toxin potentiates opportunistic bacterial lung infections. Sci Transl Med 8:329ra31. doi: 10.1126/scitranslmed.aad9922. [DOI] [PubMed] [Google Scholar]
- 13.Bramley AJ, Patel AH, O'Reilly M, Foster R, Foster TJ. 1989. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect Immun 57:2489–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayer AS, Ramos MD, Menzies BE, Yeaman MR, Shen AJ, Cheung AL. 1997. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect Immun 65:4652–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bubeck Wardenburg J, Patel RJ, Schneewind O. 2007. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 75:1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, Bubeck Wardenburg J, Schneewind O, Otto M, Deleo FR. 2011. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis 204:937–941. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J. 2012. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis 206:352–356. doi: 10.1093/infdis/jis192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cremieux AC, Saleh-Mghir A, Danel C, Couzon F, Dumitrescu O, Lilin T, Perronne C, Etienne J, Lina G, Vandenesch F. 2014. Alpha-hemolysin, not Panton-Valentine leukocidin, impacts rabbit mortality from severe sepsis with methicillin-resistant Staphylococcus aureus osteomyelitis. J Infect Dis 209:1773–1780. doi: 10.1093/infdis/jit840. [DOI] [PubMed] [Google Scholar]
- 20.Tabor DE, Yu L, Mok H, Tkaczyk C, Sellman BR, Wu Y, Oganesyan V, Slidel T, Jafri H, McCarthy M, Bradford P, Esser MT. 2016. Staphylococcus aureus alpha-toxin is conserved among diverse hospital respiratory isolates collected from a global surveillance study and is neutralized by monoclonal antibody MEDI4893. Antimicrob Agents Chemother 60:5312–5321. doi: 10.1128/AAC.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menzies BE, Kernodle DS. 1996. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect Immun 64:1839–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bubeck Wardenburg J, Schneewind O. 2008. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tkaczyk C, Hua L, Varkey R, Shi Y, Dettinger L, Woods R, Barnes A, MacGill RS, Wilson S, Chowdhury P, Stover CK, Sellman BR. 2012. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin Vaccine Immunol 19:377–385. doi: 10.1128/CVI.05589-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adhikari RP, Ajao AO, Aman MJ, Karauzum H, Sarwar J, Lydecker AD, Johnson JK, Nguyen C, Chen WH, Roghmann MC. 2012. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis 206:915–923. doi: 10.1093/infdis/jis462. [DOI] [PubMed] [Google Scholar]
- 26.Jacobsson G, Colque-Navarro P, Gustafsson E, Andersson R, Mollby R. 2010. Antibody responses in patients with invasive Staphylococcus aureus infections. Eur J Clin Microbiol Infect Dis 29:715–725. doi: 10.1007/s10096-010-0919-x. [DOI] [PubMed] [Google Scholar]
- 27.Ruotsalainen E, Karden-Lilja M, Kuusela P, Vuopio-Varkila J, Virolainen-Julkunen A, Sarna S, Valtonen V, Jarvinen A. 2008. Methicillin-sensitive Staphylococcus aureus bacteraemia and endocarditis among injection drug users and nonaddicts: host factors, microbiological and serological characteristics. J Infect 56:249–256. doi: 10.1016/j.jinf.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, Bubeck Wardenburg J, Hunstad DA. 2013. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 56:1554–1561. doi: 10.1093/cid/cit123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oganesyan V, Peng L, Damschroder MM, Cheng L, Sadowska A, Tkaczyk C, Sellman BR, Wu H, Dall'Acqua WF. 2014. Mechanisms of neutralization of a human anti-alpha toxin antibody. J Biol Chem 289:29874–29880. doi: 10.1074/jbc.M114.601328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu XQ, Robbie GJ, Wu Y, Esser MT, Jensen K, Schwartz HI, Bellamy T, Hernandez-Illas M, Jafri HS. 2017. Safety, tolerability, and pharmacokinetics of MEDI4893, an investigational, extended-half-life, anti-Staphylococcus aureus alpha-toxin human monoclonal antibody, in healthy adults. Antimicrob Agents Chemother 61:e01020-16. doi: 10.1128/AAC.01020-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francois B, Chastre J, Eggiman P, Laterre PF, Torres A, Sanchez M, Esser MT, Bishop B, Bonten M, Goosens H, Jafri HS. 2016. The SAATELLITE and EVADE clinical studies within the COMBACTE Consortium: a public-private collaborative effort in designing and performing clinical trials for novel antibacterial drugs to prevent nosocomial pneumonia. Clin Infect Dis 63(Suppl 2):S46–S51. doi: 10.1093/cid/ciw245. [DOI] [PubMed] [Google Scholar]
- 32.Francois B, Barraud O, Jafri HS. 2017. Antibody-based therapy to combat Staphylococcus aureus infections. Clin Microbiol Infect 23:219–221. doi: 10.1016/j.cmi.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 33.Rubins JB, Puri AK, Loch J, Charboneau D, MacDonald R, Opstad N, Janoff EN. 1998. Magnitude, duration, quality, and function of pneumococcal vaccine responses in elderly adults. J Infect Dis 178:431–440. doi: 10.1086/515644. [DOI] [PubMed] [Google Scholar]
- 34.Romero-Steiner S, Musher DM, Cetron MS, Pais LB, Groover JE, Fiore AE, Plikaytis BD, Carlone GM. 1999. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis 29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 35.Fattom A, Matalon A, Buerkert J, Taylor K, Damaso S, Boutriau D. 2015. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: phase III randomized study. Hum Vaccin Immunother 11:632–641. doi: 10.4161/hv.34414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma-Kuinkel BK, Wu Y, Tabor DE, Mok H, Sellman BR, Jenkins A, Yu L, Jafri HS, Rude TH, Ruffin F, Schell WA, Park LP, Yan Q, Thaden JT, Messina JA, Fowler VG Jr, Esser MT. 2015. Characterization of alpha-toxin hla gene variants, alpha-toxin expression levels, and levels of antibody to alpha-toxin in hemodialysis and postsurgical patients with Staphylococcus aureus bacteremia. J Clin Microbiol 53:227–236. doi: 10.1128/JCM.02023-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu KO, Randolph AG, Agan AA, Yip WK, Truemper EJ, Weiss SL, Ackerman KG, Schwarz AJ, Giuliano JS Jr, Hall MW, Bubeck Wardenburg J, Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) PICFlu Study Group. 2016. Staphylococcus aureus α-toxin response distinguishes respiratory virus-methicillin-resistant S. aureus coinfection in children. J Infect Dis 214:1638–1646. doi: 10.1093/infdis/jiw441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burian M, Wolz C, Goerke C. 2010. Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS One 5:e10040. doi: 10.1371/journal.pone.0010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins A, Diep BA, Mai TT, Vo NH, Warrener P, Suzich J, Stover CK, Sellman BR. 2015. Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. mBio 6:e02272-14. doi: 10.1128/mBio.02272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stulik L, Malafa S, Hudcova J, Rouha H, Henics BZ, Craven DE, Sonnevend AM, Nagy E. 2014. alpha-Hemolysin activity of methicillin-susceptible Staphylococcus aureus predicts ventilator-associated pneumonia. Am J Respir Crit Care Med 190:1139–1148. doi: 10.1164/rccm.201406-1012OC. [DOI] [PubMed] [Google Scholar]
- 41.COMBACTE. Advanced understanding of Staphylococcus aureus and Pseudomonas aeruginosa infections in Europe—intensive care units. http://www.combacte.com/COMBACTE-NET/Trials/Aspire-ICU Accessed 12 September 2017.
- 42.National Institutes of Health. Study of the efficacy and safety of MEDI4893 (SAATELLITE). https://clinicaltrials.gov/ct2/show/NCT02296320. Accessed 12 September 2017.
- 43.National Institute for Biological Standards and Controls. 2010. The 3rd international standard for staphylococcus alpha antitoxin, equine. NIBSC code: STA, vol 5.0, p 1–4. National Institute for Biological Standards and Controls, Potters Bar, Hertfordshire, England. [Google Scholar]


