ABSTRACT
The fusion protein DnaJ-ΔA146Ply could induce cross-protective immunity against pneumococcal infection via mucosal and subcutaneous immunization in mice in the absence of additional adjuvants. DnaJ and Ply are both Toll-like receptor 4 (TLR4) but not TLR2 ligands. However, we found that TLR2−/− mice immunized subcutaneously with DnaJ-ΔA146Ply showed significantly lower survival rates and higher bacterial loads in nasal washes than did wild-type (WT) mice after being challenged with pneumococcal strain D39 or 19F. The gamma interferon (IFN-γ) level in splenocytes decreased in TLR2−/− mice, indicating that Th1 immunity elicited by DnaJ-ΔA146Ply was impaired in these mice. We explored the mechanism of protective immunity conferred by DnaJ-ΔA146Ply and the role of TLR2 in this process. DnaJ-ΔA146Ply effectively promoted dendritic cell (DC) maturation via TLR4 but not the TLR2 signaling pathway. In a DnaJ-ΔA146Ply-treated DC and naive CD4+ T cell coculture system, the deficiency of TLR2 in DCs resulted in a significant decline of IFN-γ production and Th1 subset differentiation. The same effect was observed in adoptive-transfer experiments. In addition, TLR2−/− DCs showed remarkably lower levels of the Th1-polarizing cytokine IL-12p70 than did WT DCs, suggesting that TLR2 was indispensable for DnaJ-ΔA146Ply-induced IL-12 production and Th1 proliferation. Thus, our findings illustrate that dendritic cell expression of TLR2 is essential for optimal Th1 immune response against pneumococci in mice immunized subcutaneously with DnaJ-ΔA146Ply.
KEYWORDS: DnaJ-ΔA146Ply, dendritic cells, Toll-like receptor 2, Th1 immunity, protein vaccine
INTRODUCTION
Toll-like receptors (TLRs) have been called the “gatekeepers” of innate immunity because they can recognize microbe-associated molecular patterns (MAMP) (1, 2). Upon activation, most TLRs engage the major signal transduction adaptor MyD88, which triggers downstream signaling pathways, leading to activation of the transcription factors NF-κB and/or interferon regulatory factor 3/7 (IRF3/7) and production of proinflammatory cytokines (3–6). TLRs also regulate the activation and development of adaptive immunity. B cell intrinsic TLR signaling promotes antibody responses, B cell cytokine secretion, and memory B cell survival (7, 8). TLRs modulate T regulatory cell (Treg) function, resulting in their activation and the subsequent suppression of immune response (9–11).
Dendritic cells (DCs) have the unique ability to prime naive T cells by sensing and presenting microbial antigens (12–14). DC maturation induced by TLRs plays important roles in protective immunity conferred by protein-based vaccines (15–18). For instance, Mycobacterium tuberculosis-derived protein Rv2299c led to DC maturation through TLR4 and induced Th1 cell responses (19). A novel TLR2 agonist derived from Bordetella pertussis, lipoprotein BP1569, activated DCs via TLR2 and enhanced Th1, Th17, and IgG2a antibody responses in mice (20). The surface protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of Streptococcus pneumoniae induced DC maturation via TLR4 and TLR2 and consequently elicited protective immunity in mice (21).
The heat shock protein DnaJ and pneumolysin (Ply) are important virulence factors of Streptococcus pneumoniae (22–25). Our group constructed a novel fusion protein, DnaJ-ΔA146Ply (Ply with a single deletion of A146), and confirmed that it could evoke cross-protective immunity against pneumococcal infection via both mucosal and subcutaneous immunization routes in mice (26, 27). DnaJ and Ply are both TLR4 ligands, suggesting that TLR4 was required for the protection induced by DnaJ-ΔA146Ply. Consistently, we found that specific antibody secretion and protection induced by DnaJ-ΔA146Ply were impaired in the absence of TLR4 (27). However, after immunization with DnaJ-ΔA146Ply, TLR2 knockout mice exhibited an unexpected decline in survival compared with wild-type (WT) mice when challenged with pneumococcal strains. This indicated that TLR2 was involved in protective immunity elicited by DnaJ-ΔA146Ply, although DnaJ-ΔA146Ply is not a TLR2 ligand.
Many immune cell types, including DCs, B cells, and CD4+ T cells, express TLRs (28–33). It is possible that TLR2 modulates DC antigen presentation by altering surface molecule expression and cytokine secretion, which impacts the induction of adaptive immunity. Or TLR2 may influence directly at the level of adaptive T and B cell immune responses through regulating Th subset polarization and antibody production. We found that TLR2−/− mice vaccinated with DnaJ-ΔA146Ply showed a capacity for antigen-specific antibody production similar to that of wild-type mice, indicating that the impairment of protection in TLR2 knockout (KO) mice was not the result of an insufficient B cell immune response.
In this study, we compared TLR2 knockout and wild-type mice in their abilities for antigen presentation and T cell immune responses activation to explore the role of TLR2 in immunity mediated by DnaJ-ΔA146Ply.
RESULTS
Expression and purification of recombinant protein DnaJ-ΔA146Ply.
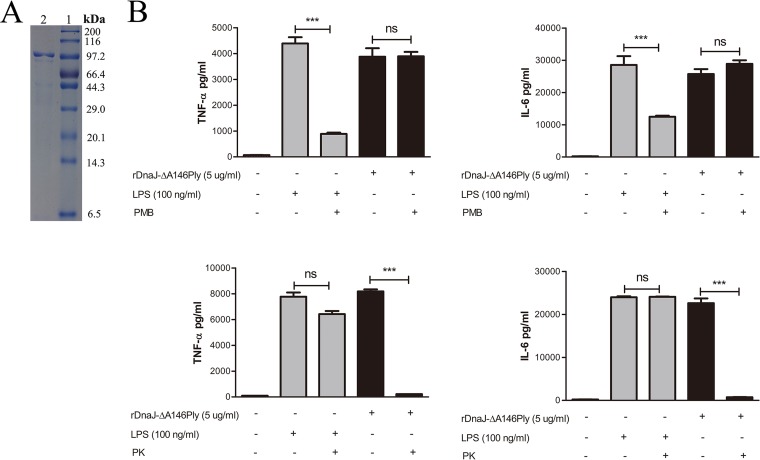
DnaJ-ΔA146Ply was expressed in Escherichia coli, and SDS-PAGE analysis showed a purity >95% (Fig. 1A). Since lipopolysaccharide (LPS) can elicit immune responses in mice, we used polymyxin B (PmB)-agarose to exclude endotoxin contamination. The residual LPS was below 0.1 endotoxin unit (EU)/ml as measured by an endpoint chromogenic assay (ECA) kit. In addition, there were no differences in tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) secretion between DnaJ-ΔA146Ply and PmB-incubated DnaJ-ΔA146Ply groups. Proteinase K treatment of the DnaJ-ΔA146Ply preparation did not activate dendritic cells (Fig. 1B). These results indicate that the recombinant fusion protein DnaJ-ΔA146Ply is reliable for mouse immunization to evaluate its protective effect.
FIG 1.
Expression and purification of recombinant protein DnaJ-ΔA146Ply. (A) Purified DnaJ-ΔA146Ply (lane 2) was analyzed by SDS-PAGE and then stained with Coomassie brilliant blue. Lane 1, molecular mass markers. (B) BMDCs were stimulated with 5 μg/ml of DnaJ-ΔA146Ply, DnaJ-ΔA146Ply digested with protease K or pretreated with polymyxin B, and LPS (100 ng/ml) for 24 h. The quantities of TNF-α and IL-6 in the culture medium were measured by ELISA. All data are expressed as means ± SDs (n = 3), and statistical significance (***, P < 0.001; ns, not significant) is indicated for treatments compared to the controls.
TLR2−/− mice immunized with DnaJ-ΔA146Ply are defective in protection against pneumococcal infection.
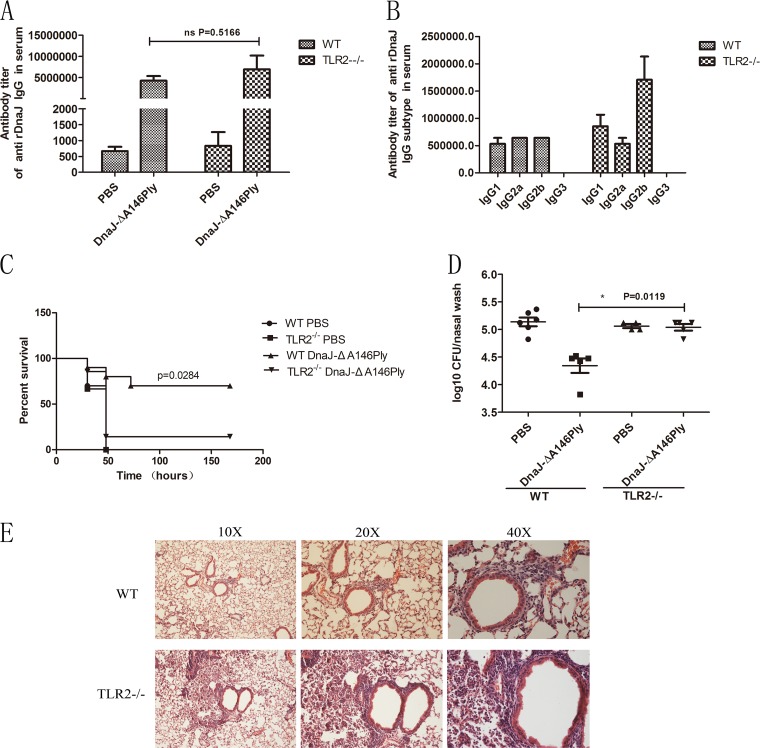
To identify whether TLR2 functions in protection induced by DnaJ-ΔA146Ply, we subcutaneously immunized C57BL/6 WT and TLR2−/− mice three times at 14-day intervals with DnaJ-ΔA146Ply or phosphate-buffered saline (PBS) as a vehicle control. The titers of anti-DnaJ antibodies and their subtypes in serum were measured 1 week after the last immunization. We found no differences in DnaJ-specific IgG antibody titers between WT and TLR2−/− mice (Fig. 2A). However, the IgG2b subtype was dominant among other subtypes in TLR2−/− mice and TLR2−/− mice produced the same amount of IgG2a as WT mice, implying that there was no defect in Th1-associated antibody production in the absence of TLR2 (Fig. 2B).
FIG 2.
Deficiency of TLR2 impairs the protection against pneumococcal infection induced by DnaJ-ΔA146Ply. WT and TLR2−/− mice were immunized subcutaneously with 18 μg of DnaJ-ΔA146Ply or PBS as a vehicle control three times at 14-day intervals. The antibody titers of anti-DnaJ IgG (A) and subtypes IgG1, IgG2a, IgG2b, and IgG3 (B) in serum were measured 1 week after the last immunization. Mice were challenged with D39 (1,000 CFU) or 19F (1 × 108 CFU) 2 weeks after the last immunization, and the survival rates were monitored (n = 10) (C) and bacterial loads in nasal wash were determined 72 h postinfection (n = 4 to 6) (D). (E) Histopathology of hematoxylin and eosin-stained lung sections from WT and TLR2−/− mice immunized with DnaJ-ΔA146Pl 72 h postinfection. *, P < 0.05 based on Mann-Whitney U or log rank test.
We next assessed the influence of TLR2 on protection against pneumococcal infection by infecting mice with a lethal dose of pneumococcal strain D39 intraperitoneally 2 weeks after the last immunization. Surprisingly, the survival rate of TLR2−/− mice immunized with DnaJ-ΔA146Ply was significantly lower than that of WT mice (Fig. 2C). These data suggest that TLR2 does participate in the immune response induced by DnaJ-ΔA146Ply. We additionally used a pneumonia model to further analyze differences in bacterial clearance between WT and TLR2−/− mice. When intranasally challenged with pneumococcal strain 19F, the immunized TLR2−/− mice showed significantly higher bacterial loads in the nasopharynx (Fig. 2D) and more severe pathological changes in the lungs, including massive inflammatory cell infiltration, thickening of the bronchial epithelium, and alveolar expansion (Fig. 2E).
Together, these findings demonstrate that TLR2 deficiency impairs DnaJ-ΔA146Ply-mediated bacterial clearance in the upper respiratory tract and aggravates lung injury. Therefore, TLR2−/− mice are defective in protection against pneumococcal infection conferred by DnaJ-ΔA146Ply.
Th1 immune response is impaired in TLR2−/− mice vaccinated with DnaJ-ΔA146Ply.
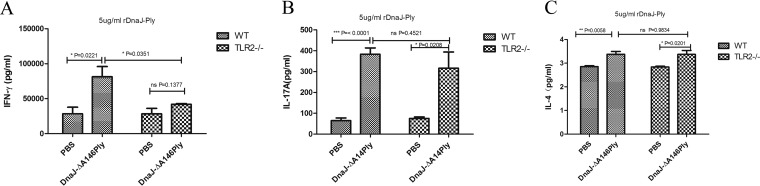
To explore the potential role of TLR2 in the induction of T cell immune responses, splenocytes were isolated from WT and TLR2−/− mice 1 week after the last immunization and cultured in vitro in the presence of DnaJ-ΔA146Ply. Production of cytokines gamma interferon (IFN-γ), IL-17A, and IL-4 in WT mice immunized with DnaJ-ΔA146Ply was significantly increased compared with that in PBS controls. TLR2−/− mice exhibited a similar capacity for activating IL-17A and IL-4 secretion, but IFN-γ secretion was dramatically impaired in TLR2−/− mice vaccinated with DnaJ-ΔA146Ply (Fig. 3). Therefore, DnaJ-ΔA146Ply can elicit strong Th1 and Th17 immune responses, and the former is partially dependent on TLR2.
FIG 3.
TLR2−/− mice exhibit deficient Th1 immune responses elicited by DnaJ-ΔA146Ply. Seven days after the last immunization, suspensions of splenocytes (1 × 105/well) were cultured and exposed to 5 μg/ml of DnaJ-ΔA146Ply for 72 h at 37°C. Levels of IFN-γ (A), IL-17A (B), and IL-4 (C) in culture medium were determined by ELISA. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DnaJ-ΔA146Ply induces DC maturation via TLR4 but not TLR2.
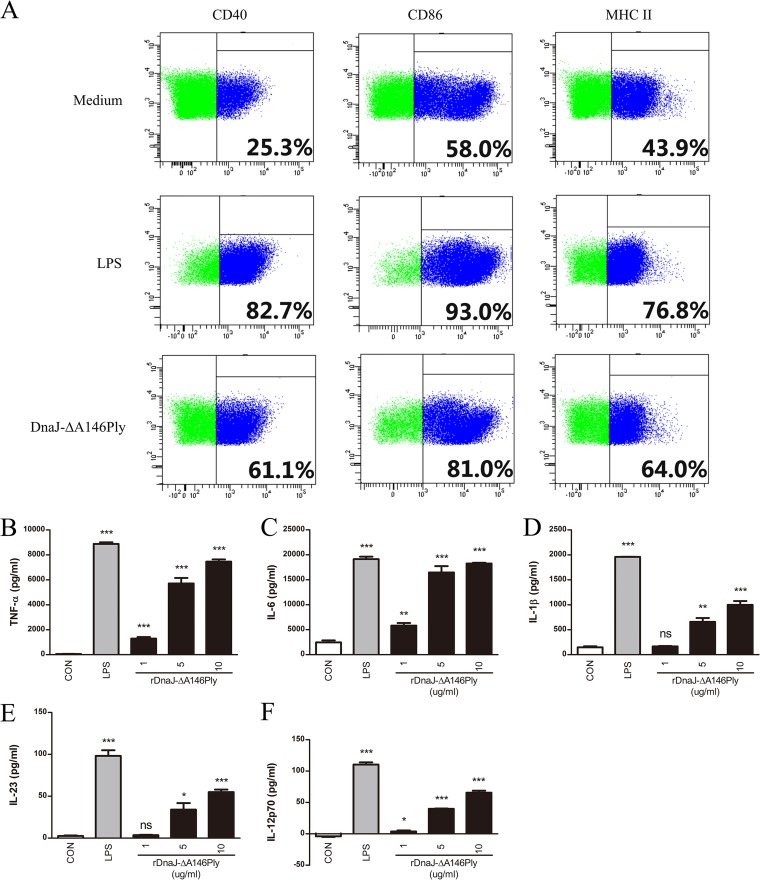
Since dendritic cells are classical antigen-presenting cells that bridge innate and adaptive immunity (34), we tested the activation and maturation of DCs induced by DnaJ-ΔA146Ply. Immature bone marrow-derived dendritic cells (BMDCs) were isolated from murine bone marrow and cultured for 6 days in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 and then stimulated with DnaJ-ΔA146Ply for 24 h (LPS served as a positive control). DCs treated with DnaJ-ΔA146Ply exhibited enhanced expression of CD40, CD86, and major histocompatibility complex class II (MHC-II) (Fig. 4A). Simultaneously, DnaJ-ΔA146Ply induced significantly higher levels of TNF-α, IL-6, and IL-1β than did blank controls (Fig. 4B, C, and D). Moreover, the dose-dependent increases of cytokines IL-23 and IL-12p70 indicated the proliferation of Th17 and Th1 immunity, respectively (Fig. 4E and F). These data suggest that DnaJ-ΔA146Ply can effectively induce the phenotypic and functional maturation of DCs and that this process has the potential to modulate Th1 and Th17 immunity.
FIG 4.
DnaJ-ΔA146Ply induces phenotypic and functional maturation of DCs. (A) Immature BMDCs (1 × 106/well) were treated with 5 μg/ml of DnaJ-ΔA146Ply or 100 ng/ml of LPS for 24 h and then stained with FITC-conjugated anti-CD11c and phycoerythrin (PE)-conjugated anti-CD40, -CD86, and MHC-II. The percentage of positive cells was analyzed by flow cytometry. Immature BMDCs (1 × 105/well) were stimulated with LPS or 1, 5, or 10 μg/ml of DnaJ-ΔA146Ply for 24 h. Levels of TNF-α (B), IL-6 (C), IL-1β (D), IL-23 (E), and IL-12p70 (F) in culture medium were assayed by ELISA. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for LPS or DnaJ-ΔA146Ply groups compared with medium control).
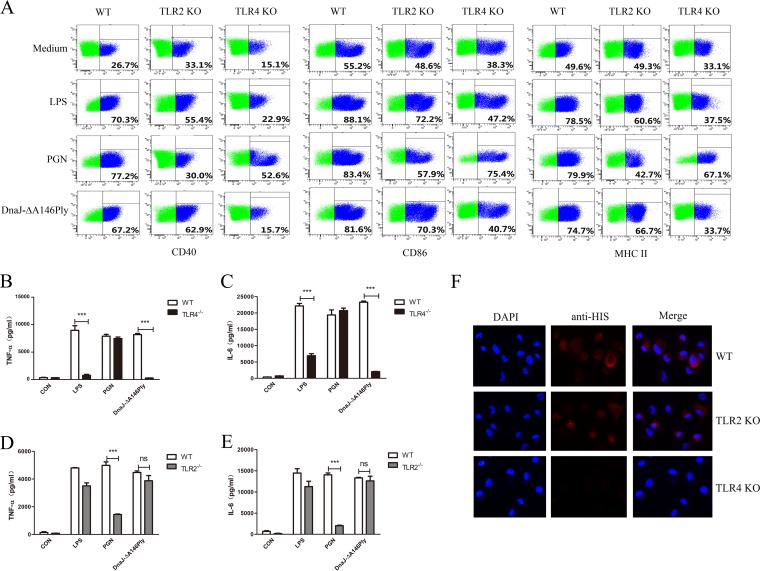
To investigate the interaction of TLR2/4 with DnaJ-ΔA146Ply, BMDCs from WT, TLR2−/−, and TLR4−/− mice were treated with peptidoglycan (PGN) (a TLR2 agonist), LPS (a TLR4 agonist), and DnaJ-ΔA146Ply. The levels of the costimulatory molecules and cytokines were measured. The expression of CD40, CD86, and MHC-II and the production of cytokines TNF-α and IL-6 declined sharply in TLR4−/− BMDCs treated with DnaJ-ΔA146Ply (Fig. 5A, B, and C). To our surprise, there was no difference in either surface molecule expression or cytokine secretion between TLR2−/− and WT BMDCs, suggesting that TLR2 was not involved in the activation and maturation of DCs induced by DnaJ-ΔA146Ply (Fig. 5A, D, and E). Further immunofluorescence revealed that DnaJ-ΔA146Ply bound preferentially to the surface of WT and TLR2−/− DCs but not to TLR4−/− DCs, indicating that DnaJ-ΔA146Ply interacted with TLR4 to induce DC maturation (Fig. 5F).
FIG 5.
DnaJ-ΔA146Ply induces DC maturation via TLR4 but not TLR2. BMDCs from WT, TLR2−/−, and TLR4−/− mice were stimulated with 100 ng/ml of LPS, 10 μg/ml of PGN, or 5 μg/ml of DnaJ-ΔA146Ply for 24 h. (A) Surface costimulatory molecules CD40, CD86, and MHC-II were analyzed by flow cytometry. (B to E) Cytokines TNF-α and IL-6 in supernatants were measured with ELISA. (F) Fluorescence intensity of the anti-His antibody bound to DnaJ-ΔA146Ply-treated DCs. DCs derived from WT, TLR2−/−, and TLR4−/− mice were treated with DnaJ-ΔA146Ply for 30 min, fixed, and stained with DAPI and anti-His antibody. The data are shown as means ± SDs. ***, P < 0.001.
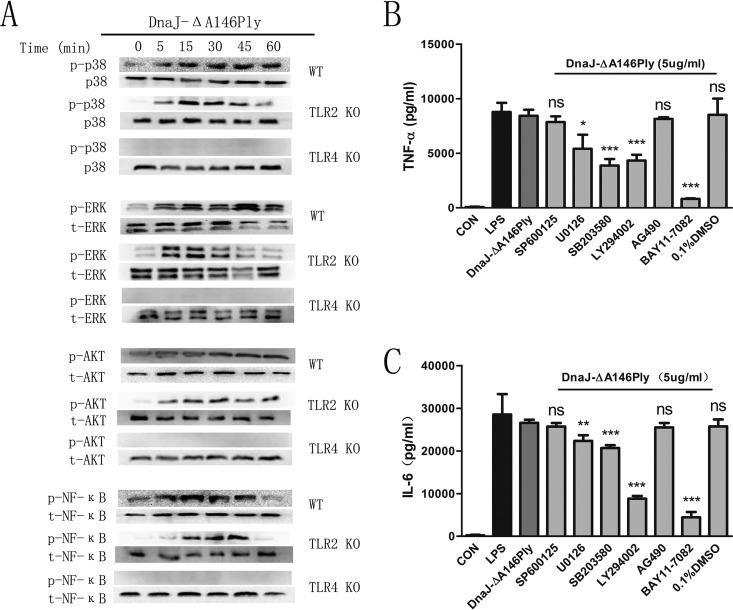
Mitogen-activated protein kinases (MAPKs), NF-κB, and phosphatidylinositol 3-kinase (PI3K)-Akt are important signaling pathways for DCs to secrete proinflammatory cytokines, so we investigated the phosphorylation of molecules in these pathways using Western blotting. As shown in Fig. 6A, DnaJ-ΔA146Ply triggered strong phosphorylation of MAPKs (including p38 and extracellular signal-regulated kinase 1/2 [ERK1/2]), PI3K-Akt, and NF-κB in DCs through TLR4 but not TLR2. To elucidate the functional roles of these kinases in DnaJ-ΔA146Ply-induced activation, BMDCs were pretreated with specific pharmacological inhibitors for 1 h prior to stimulation with DnaJ-ΔA146Ply and cytokines of supernatants were quantified with enzyme-linked immunosorbent assay (ELISA). We found that the production of TNF-α and IL-6 was partially abrogated with the use of MAPK, PI3K-Akt, and NF-κB inhibitors (Fig. 6B and C).
FIG 6.
MAPK, NF-κB, and PI3K-Akt pathways are involved in DC maturation mediated by DnaJ-ΔA146Ply. (A) BMDCs from WT, TLR2−/−, and TLR4−/− mice were treated with 5 μg/ml of DnaJ-ΔA146Ply for the indicated times and lysed in lysis buffer. The phosphorylation of corresponding molecules was analyzed by Western blotting. (B and C) BMDCs from WT mice were pretreated with specific inhibitors of p38 (SB203580), ERK1/2 (U0126), Jun N-terminal protein kinase (JNK) (SP600125), PI3K (LY294002), and NF-κB (BAY11-7082) for 1 h prior to stimulation with DnaJ-ΔA146Ply. Cytokines TNF-α and IL-6 in supernatants were quantified with ELISA kits. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
These findings confirm that DnaJ-ΔA146Ply induces DC maturation via TLR4 but not TLR2, implying that TLR2 is not involved in antigen presentation of DnaJ-ΔA146Ply.
Expression of TLR2 by DCs is essential for Th1 differentiation induced by DnaJ-ΔA146Ply in vitro.
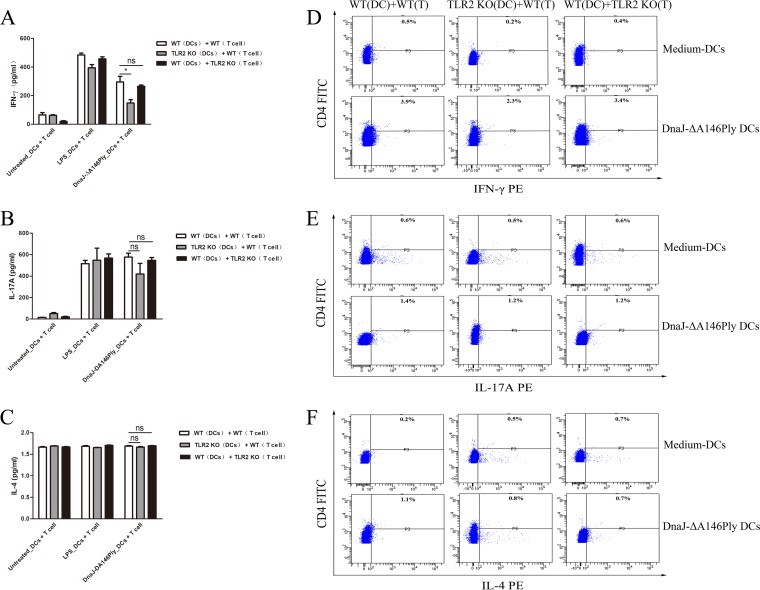
Since TLR2 does not affect DnaJ-ΔA146Ply-mediated DC maturation, it probably influences Th1 polarization induced by DnaJ-ΔA146Ply. Coculture of DCs and T cells was used to verify our hypothesis. BMDCs from WT and TLR2−/− mice were treated with DnaJ-ΔA146Ply for 24 h and then incubated with purified CD4+ T cells isolated from spleens of WT and TLR2−/− mice. After 72 h, the concentrations of IFN-γ, IL-17A, and IL-4 in culture supernatants were measured.
BMDCs activated with DnaJ-ΔA146Ply triggered significantly higher levels of IFN-γ and IL-17A in CD4+ T cells than did untreated BMDCs, indicating that DnaJ-ΔA146Ply elicited strong Th1 and Th17 immune responses. However, the IFN-γ secretion induced by DnaJ-ΔA146Ply declined remarkably in the coculture group including TLR2−/− DCs and WT CD4+ T cells, while there were no differences between the group with WT DCs plus WT CD4+ T cells and the group with WT-DCs plus TLR2−/− CD4+ T cells (Fig. 7A, B, and C). Likewise, the intracellular IFN-γ and IL-17A levels increased in CD4+ T cells cocultured with DnaJ-ΔA146Ply-activated BMDCs compared with untreated BMDCs. The differentiation of IFN-γ+ CD4+ T cells was also impaired in the coculture group including TLR2−/− DCs plus WT CD4+ T cells (Fig. 7D, E, and F). These results prove that DnaJ-ΔA146Ply directs CD4+ T cells toward Th1- and Th17-polarizing phenotypes. The expression of TLR2 by DCs is essential for IFN-γ production induced by DnaJ-ΔA146Ply.
FIG 7.
Absence of TLR2 in DCs impairs Th1 polarization in a DC and CD4+ T cell coculture system. Immature BMDCs from WT and TLR2−/− mice were treated with 5 μg/ml of DnaJ-ΔA146Ply or 100 ng/ml of LPS for 24 h and then cocultured with purified naive CD4+ T cells sorted from spleens of WT and TLR2−/− mice, respectively. (A to C) After 72 h, IFN-γ, IL-17A, and IL-4 levels in culture medium were assayed by ELISA. (D to F) Intracellular cytokines IFN-γ, IL-17A, and IL-4 were analyzed by flow cytometry. *, P < 0.05 for IFN-γ level in WT CD4+ T cells cocultured with WT BMDCs compared to TLR2−/− BMDCs.
Adoptive transfer of TLR2−/− DCs treated with DnaJ-ΔA146Ply induces an insufficient Th1 immune response in vivo.
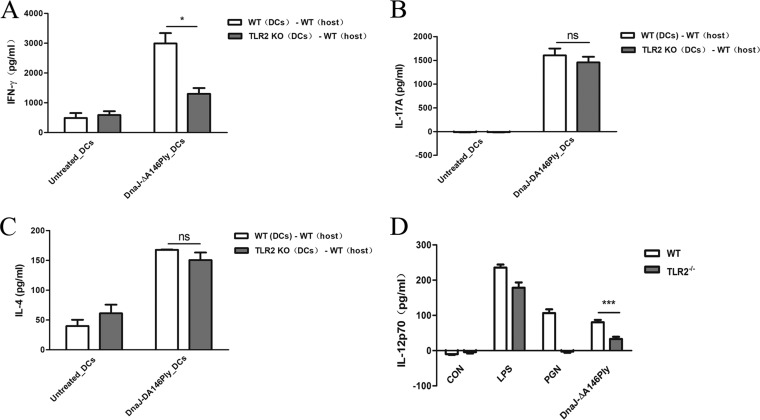
We have demonstrated that TLR2 expression by DCs is critical for Th1 immunity in vitro. We next used adoptive transfers to verify the role of TLR2 signaling in Th1 differentiation in vivo. BMDCs from WT and TLR2−/− mice were activated with DnaJ-ΔA146Ply and adoptively transferred into naive mice at days 0, 14, and 28. Seven days after the last transfer, splenocytes were isolated from the mice which had undergone transfer and restimulated with DnaJ-ΔA146Ply for 72 h. IFN-γ, IL-17A, and IL-4 levels in the culture medium were then measured. After treatment with DnaJ-ΔA146Ply, WT BMDCs induced strong Th1 and Th17 cellular immunity as well as unexpected IL-4 production when adoptively transferred into naive mice. Similarly, Th1 cellular immunity elicited by TLR2−/− BMDCs was impaired (Fig. 8A, B, and C).
FIG 8.
Adoptive transfer of DnaJ-ΔA146Ply-treated TLR2−/− DCs results in deficient Th1 immune responses, and TLR2 regulates IL-12p70 production. (A to C) Immature BMDCs from WT and TLR2−/− mice were treated with 5 μg/ml of DnaJ-ΔA146Ply for 24 h and then adoptively transferred intraperitoneally into naive mice at days 0, 14, and 28. One week after the last transfer, splenocytes of mice subjected to transfer were isolated and levels of IFN-γ, IL-17A, and IL-4 were measured. (D) BMDCs from WT and TLR2−/− mice were treated with 5 μg/ml of DnaJ-ΔA146Ply, 100 ng/ml of LPS, or 10 μg/ml of PGN. After 24 h, the IL-12p70 in supernatants was quantified using ELISA. *, P < 0.05 for mice subjected to adoptive transfer with WT BMDCs compared to TLR2−/− BMDCs. ***, P < 0.001 for IL-12 concentration in WT BMDCs compared to TLR2−/− BMDCs in DnaJ-ΔA146Ply treatment groups.
Together, the combined results of coculture and adoptive-transfer experiments illustrate that the expression of TLR2 by DCs is essential for Th1 polarization induced by DnaJ-ΔA146Ply in vitro and in vivo.
TLR2 modulates production of DC-derived Th1 cell-polarizing cytokine IL-12.
Since the TLR2 expressed by DCs affects Th1 polarization induced by DnaJ-ΔA146Ply, we sought to assess whether there was a difference in production of Th1 cell-polarizing cytokine IL-12 between WT and TLR2−/− BMDCs. Consistent with the above-described results, the IL-12p70 concentration decreased significantly in TLR2−/− BMDCs compared with that in WT BMDCs (Fig. 8D). Therefore, TLR2 signaling in DCs modulates IL-12p70 production and influences the subsequent Th1 immunity induced by DnaJ-ΔA146Ply. However, the precise mechanism of IL-12 regulation mediated by TLR2 needs further study.
DISCUSSION
In this study, we investigated the mechanisms underlying the defective Th1 immune response induced by DnaJ-ΔA146Ply in TLR2−/− mice. DnaJ and Ply served as TLR4 ligands, and we previously showed that DnaJ-ΔA146Ply elicited protective immunity against pneumococcal infection in a TLR4-dependent manner. However, we were intrigued by our finding that TLR2−/− mice immunized with DnaJ-ΔA146Ply showed significantly lower survival rates than did WT mice. In addition, TLR2−/− mice exhibited higher bacterial loads in the nasopharynx and more severe lung injury than WT mice when vaccinated with DnaJ-ΔA146Ply. These data indicate that the absence of TLR2 impairs the protective immunity induced by DnaJ-ΔA146Ply. TLR2 is considered the major receptor for Gram-positive bacteria in innate immunity. And TLR2−/− mice exhibit an increased or no apparent change in sensitivity to infection with S. pneumoniae, depending on the model system. However, TLR2−/− mice infected with S. pneumoniae at doses varying from nonlethal to lethal showed a modestly reduced inflammatory response in lungs and an unaltered antibacterial defense compared with those of normal WT mice (35). In addition, there were no differences between asplenic WT and TLR2−/− mice in bacterial loads in lung homogenates and blood (36). These reports indicate that TLR2 does not play a major role in host defense against pneumococcal infection. That is to say, TLR2−/− mice are not susceptible to pneumococcal infection in a pneumonia model. Finally, in this study, we used a lethal dose to test the differences in survival rates and a sublethal dose to test the differences in bacterial loads between WT and TLR2−/− mice. The results for both doses showed that host defense against pneumococcal infection was impaired in TLR2−/− mice after immunization with the fusion protein.
We found no differences in antibody production between WT and TLR2−/− mice, while IFN-γ production was obviously defective in TLR2−/− mice. In our previous study, the survival rate of IFN-γ−/− mice vaccinated with the fusion protein was only about 12% (27). So it is possible that the deficiency of Th1 immunity response that resulted in the unexpected low survival of TLR2−/− mice was induced by the fusion protein. TLR2 therefore most likely modulates T cell immune responses through antigen presentation or directly at the level of T cell differentiation.
Dendritic cells are professional antigen-presenting cells that regulate antigen-specific adaptive immune responses by modulating cell surface costimulatory-molecule expression and cytokine production. DnaJ-ΔA146Ply stimulated phenotypic maturation of DCs by upregulating the expression of costimulatory molecules CD40, CD86, and MHC-II and activated functional maturation of DCs by promoting the production of cytokines TNF-α, IL-6, and IL-1β. Additionally, the Th17 and Th1 immunity-polarizing cytokines IL-23 and IL-12p70 were elevated remarkably in DnaJ-ΔA146Ply-stimulated groups, suggesting that DnaJ-ΔA146Ply could induce strong Th1 and Th17 cellular responses. Taken together, the results show that DnaJ-ΔA146Ply can effectively induce the phenotypic and functional maturation of DCs, implying that DnaJ-ΔA146Ply is a good candidate for a protein vaccine that has the capacity to elicit Th1 and Th17 immunity.
We then tested whether TLR2 influences antigen presentation of DnaJ-ΔA146Ply. Not surprisingly, DCs from TLR4−/− mice exhibited a sharp decline not only in the expression of CD40, CD86, and MHC-II but also in the production of cytokines TNF-α and IL-6. However, DCs from TLR2−/− mice showed surface molecule expression and cytokine secretion similar to those of their WT counterparts, indicating that TLR2 neither recognized nor combined with DnaJ-ΔA146Ply. In other words, TLR2 does not affect the antigen presentation of DnaJ-ΔA146Ply. Further immunofluorescence data confirmed that DnaJ-ΔA146Ply interacted predominantly with TLR4, as there was no difference in the amount of protein combined to WT and TLR2−/− DCs, while protein was almost absent in TLR4−/− DCs. Thus, DnaJ-ΔA146Ply activates DCs via TLR4 but not TLR2.
Since TLR2 did not affect the antigen presentation of DnaJ-ΔA146Ply, it could be correlated with the regulation of adaptive immunity. To verify this hypothesis, we cocultured DCs and naive CD4+ T cells. We found that WT DCs treated with DnaJ-ΔA146Ply directed T cells toward Th1 and Th17 polarization in vitro, with noticeable secretion of the extracellular and intracellular cytokines IFN-γ and IL-17A. In addition, Th1 differentiation was obviously impaired in the TLR2−/− DCs and WT T cell coculture groups, indicating that TLR2 expression by DCs was essential for Th1 immunity mediated by DnaJ-ΔA146Ply. There were no differences in T cell differentiation between the coculture group including WT DCs plus WT T cells and the group including WT DCs plus TLR2−/− T cells, suggesting that expression of TLR2 on T cells was dispensable for their polarization induced by DnaJ-ΔA146Ply. This was further borne out by adoptive transfer in vivo. There was a significant decrease of IFN-γ production in mice subjected to transfer with TLR2−/− DCs. In short, TLR2 regulates the adaptive cellular immune response, and the expression of TLR2 by DCs is essential for Th1 immunity elicited by DnaJ-ΔA146Ply.
The question remains as to how TLR2 expressed by DCs directs Th1 subset differentiation. IL-12p70 production from TLR2−/− DCs treated with DnaJ-ΔA146Ply was significantly decreased compared to that from WT DCs. Because IL-12p70 is a Th1 effector cytokine, impaired secretion leads to decreased polarization of Th1 cells, which is consistent with the insufficient Th1 immunity triggered by DnaJ-ΔA146Ply in TLR2−/− mice. However, the precise mechanism underlying the modulation of TLR2 in IL-12 production in DCs needs further study. We propose several hypotheses concerning the TLR2-IL-12 modulation mechanism. First, the type III histone deacetylase sirtuin 1 (SIRT1) participates in regulating immune responses in various inflammatory models (37–40). SIRT1 expressed on DCs program reciprocal Th1 and Treg differentiation by modulating the IL-12-STAT4 and transforming growth factor β1 (TGF-β1)–SMAD3 axes (41). Moreover, SIRT1 inhibited the catabolic response to IL-1β in degenerative nucleus pulposus cells through the TLR2/SIRT1/NF-κB pathway (42). Therefore, the IL-1β-TLR2-SIRT1-IL-12 axes in DCs induced by DnaJ-ΔA146Ply are possibly involved in the deficiency of Th1 immunity. Second, the activation of TLRs would induce the formation of TLR dimers with certain specificities (43, 44). TLR4 might form dimers with TLR2 in immune responses elicited by DnaJ-ΔA146Ply and lead to impairment of Th1 differentiation in the absence of TLR2. We will next explore the mechanism whereby TLR2 regulates the production of IL-12 in DCs and verify the above-mentioned hypotheses one by one.
In conclusion, our work demonstrates that TLR2 participates in the immune protection induced by DnaJ-ΔA146Ply, though it does not interact with the fusion protein. TLR2 deficiency results in decreased survival rates and increased nasopharyngeal bacterial loads in mice vaccinated with DnaJ-ΔA146Ply. The expression of TLR2 by DCs is essential for optimal Th1 immunity triggered by DnaJ-ΔA146Ply. To our knowledge, our data are the first to illustrate that TLR2 expressed by DCs influences adaptive T cell immunity, though the antigen is not a ligand for TLR2. This finding will provide potential applications for the design and development of new protein vaccines.
MATERIALS AND METHODS
Bacteria.
Escherichia coli DH5α (Invitrogen, CA) was used as the host for routing plasmid cloning. Recombinant proteins were expressed in E. coli BL21(DE3) (Novagen). Pneumococcal strain D39 (NCTC 7466, serotype 2) was purchased from the National Collection of Type Cultures (London, United Kingdom), pneumococcal strain TIGR4 (serotype 4) was purchased from the American Type Culture Collection (ATCC), and CMCC 31693 (serotype 19F) was purchased from National Center for Medical Culture Collections (CMCC; Beijing, China).
S. pneumoniae was grown on Columbia sheep blood agar or in semisynthetic casein hydrolysate medium supplemented with 0.5% yeast extract (C+Y) medium in an atmosphere of 5% CO2 at 37°C.
Mice.
Specific-pathogen-free 6- to 8-week-old female C57BL/6 wild-type (000664) and TLR2 (004650; B6.129-Tlr2tm1Kir/J) and TLR4 [029015; B6(Cg)-Tlr4tm1.2Karp/J] knockout (KO) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and raised at Chongqing Medical University, Chongqing, China. The TLR2−/− mutation was first constructed on a mixed 129SV × C57BL/6 background and then backcrossed to C57BL/6J mice for 9 generations to removal 129 background genes. The TLR2−/− homozygote and C57BL/6 mice were mated. The heterozygous littermates were used to generate experimental TLR2−/−, TLR2+/−, and TLR2+/+ littermates. TLR2−/− and TLR2+/+ mice were used in this study, and TLR4−/− mice were generated in the same way. The genotyping of wild-type, TLR2−/−, and TLR4−/− mice was tested with the use of specific PCR primers from JAX (data not shown). All animal experiments complied with the ethical and experiment regulations for animal care of Chongqing Medical University.
Expression and purification of recombinant protein DnaJ-ΔA146Ply.
DnaJ is a recombinant antigen originating from the TIGR4 strain. The ΔA146Ply (full-length wild type Ply with a deletion of A146) gene was constructed using site-directed mutagenesis by overlap extension. The DnaJ-C gene was PCR amplified using forward primer 5′-CGGCGGCCGCATGAACAATACTGAATT-3′ and reverse primer 5′-CCCTCGAGTTATTCTCCATCAAAGGCA-3′. The ΔA146Ply-N gene was PCR amplified using forward primer 5′-GGAATTCCATATGGCAAATAAAGCAGTAAAT-3′ and reverse primer 5′-CGAGCTCGTCATTTTCTACCTTATCCTCT-3′. The DnaJ-ΔA146Ply gene (yielding connection of C terminus of DnaJ with the N terminus of ΔA146Ply) was constructed as previously reported and then transformed into E. coli BL21(DE3) (26). The 6×His-tagged recombinant fusion protein DnaJ-ΔA146Ply was expressed after isopropyl-β-d-1-thiogalactopyranoside induction and then purified using Ni2+-charged column chromatography (GE Healthcare). After dialyzation with PBS (pH 8.0), the recombinant protein was incubated overnight at 4°C with polymyxin B (PmB)-agarose (Sigma) to remove endotoxin contaminates. Fractions were collected, analyzed by 10% SDS-PAGE, and stored at −80°C.
Subcutaneous immunization and pneumococcal challenge in mice.
Groups of WT and TLR2−/− C57BL/6 mice were subcutaneously immunized three times at 14-day intervals with 100 μl of PBS containing 18 μg of DnaJ-ΔA146Ply or 100 μl of PBS as a vehicle control. The serum samples were collected from the tail vein of each animal 1 week after the final immunization. The vaccinated mice were then challenged intraperitoneally with D39/NCTC7466 (1,000 CFU/mouse) after the last immunization, and the survival rates were monitored for 14 days. For the colonization model, the vaccinated mice were intranasally challenged with 19F/CMCC31693 (1 × 108 CFU/mouse). Nasal washes and lung homogenates were collected 72 h after challenge, and samples were serially diluted with sterile PBS, plated on Columbia sheep blood agar, and then incubated overnight at 37°C. After the challenge, immunized WT and TLR2−/− mice were sacrificed and the lungs were slowly removed, inflated, and fixed by instilling 4% formaldehyde intratracheally. Lung tissue sections (4 to 6 μm) were stained with hematoxylin and eosin according to routine procedures for histological analysis.
Preparations of murine bone marrow-derived DCs.
Murine bone marrow-derived dendritic cells were isolated from the femurs of WT, TLR2−/−, and TLR4−/− C57BL/6 mice. Femurs were washed with RPMI 1640 (Gibco) and passed through nylon mesh to remove tissue pieces. Then cells were lysed with red blood cell lysing buffer (Tiangen) and washed with RPMI 1640. The obtained cells were plated in 12-well plates and cultured at 37°C in the presence of 5% CO2 in RPMI 1640 supplemented with 10% heat-inactivated fetal serum (Sciencell), 1% penicillin-streptomycin (HyClone), 50 μM β-mercaptoethanol (Sigma), 10 mM HEPES, GM-CSF (R&D) at 20 ng/ml, and IL-4 (Peprotech) at 10 ng/ml. The culture medium was replaced with fresh RPMI 1640 every 2 days. On day 7, nonadherent cells were harvested and used in cell experiments.
Quantification of cytokines and antibody titers.
The levels of IL-6, TNF-α, IL-1β, IL-23, IFN-γ, IL-17A, and IL-4 (Biolegend) and IL-12p70 (R&D) in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions. For antibody titer assays, purified recombinant protein DnaJ (5 μg/ml) was used to coat 96-well plates and incubated overnight at 4°C. The plates were washed three times with PBS containing 0.05% Tween 20 and blocked with 2% bovine serum albumin for 2 h at 37°C. Serial dilutions of serum were added and the plates were incubated for 1 h at 37°C. Antibody titers were detected using peroxidase-conjugated goat anti-mouse IgG (ZSGB-Bio, China) and IgG1, IgG2a, IgG2b, and IgG3 (Santa Cruz) using the manufacturer's suggested protocols.
Flow cytometry and immunofluorescence assay.
BMDCs were collected after treating with DnaJ-ΔA146Ply, LPS (Sigma), or peptidoglycan (Sigma). The cells were washed twice with PBS, preincubated with CD16/32 for 20 min at 4°C, and then stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD11c and phycoerythrin (PE)-conjugated anti-CD40, anti-CD86, and anti-I-Ab (MHC class II) (eBioscience) in the dark for 30 min at 4°C. The cells were washed three times with PBS and suspended in 500 μl of PBS. Fluorescence was analyzed by flow cytometry.
BMDCs from WT, TLR2−/−, and TLR4−/− C57BL/6 mice were plated and grown overnight on poly-l-lysine-coated glass coverslips. After treatment with DnaJ-ΔA146Ply for the desired times, cells were fixed in 4% paraformaldehyde, permeabilized in 0.1% Triton X-100 for 5 min, and then blocked with 2% bovine serum albumin for 1 h at 37°C. Cells were incubated with the primary antibody (anti-His antibody) overnight at 4°C and then with the Alexa Fluor 488-conjugated secondary antibody. After incubation in the dark for 1 h, the cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min at room temperature. The morphology and fluorescence intensity of cells were observed with a Nikon ECLIPSE 80i microscope equipped with a Nikon Intensilight C-HGFI.
Western blot analysis.
After stimulation with 5 μg/ml of DnaJ-ΔA146Ply for different times (0, 5, 15, 30, 45, and 60 min), cells were harvested by lysing in lysis buffer (radioimmunoprecipitation assay [RIPA] buffer mixed with phenylmethylsulfonyl fluoride [PMSF] and 5× SDS loading buffer). Whole-cell lysate samples were resolved on SDS-polyacrylamide gels and then transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). The membranes were blocked with 5% bovine serum albumin and incubated with primary antibodies (anti-mouse p38 MAPK and phosphor-p38 MAPK, anti-mouse ERK and phosphor-ERK, anti-mouse AKT and phosphor-AKT, anti-mouse phosphor-NF-κB and anti-mouse NF-κB, and anti-mouse β-actin antibody [Cell Signaling Technology, Danvers, MA]) overnight at 4°C. Next day, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at 37°C. Epitopes on target proteins recognized specifically by antibodies were visualized with the use of the ECL Western blotting system. BMDCs were treated with signaling pathway inhibitors, including p38 MAPK inhibitor SB203580, ERK inhibitor U0126, IκB-α phosphorylation inhibitor BAY11-7082, PI3K inhibitor LY294002, and Janus kinase inhibitor AG490, for 1 h prior to treatment with 5 μg/ml of DnaJ-ΔA146Ply for 24 h. Dimethyl sulfoxide (Sigma) was added to cultures at 0.1% (vol/vol) as a solvent control. The cytokines of supernatants were measured by ELISA.
In vitro T cell proliferation assay.
Splenocytes were isolated from spleens of WT and TLR2−/− mice, washed, and suspended in RPMI 1640. Then cells were incubated with CD4 (L3T4) MACS microbeads (Miltenyi Biotec, Germany), following the manufacturer's instructions, to sort CD4+ T cells. The purity of sorted CD4+ T cells was approximately 94% as determined by flow cytometry (data not shown). BMDCs isolated from WT and TLR2−/− mice were cultured and treated with 5 μg/ml of DnaJ-ΔA146Ply, LPS (100 ng/ml), or medium only for 24 h. Then cells were washed and cultured in 24-well plates with purified CD4+ T lymphocytes at a 1:10 ratio. After 72 h of coculture, cells were labeled with FITC-conjugated anti-CD4 monoclonal antibody (MAb) or PE-conjugated anti-IFN-γ, -IL-4, or -IL-17A MAb and analyzed by flow cytometry. The production of IFN-γ, IL-4, and IL-17A in cell culture supernatants was measured by ELISA.
Adoptive transfer of DnaJ-ΔA146Ply-stimulated BMDCs into mice.
BMDCs isolated from WT and TLR2−/− mice were treated with 5 μg/ml of DnaJ-ΔA146Ply or medium for 24 h. Cells were harvested and washed with PBS, and 5 × 105 cells were adoptively transferred intraperitoneally into naive mice three times at 14-day intervals. Spleens were removed 7 days after the last transfer for measurement of the cell-secreted cytokines IL-4, IL-17A, and IFN-γ by ELISA.
Statistical analysis.
We performed all data analysis using GraphPad Prism5 software (GraphPad Software, La Jolla, CA). The Mann-Whitney U test was used to compare cytokine levels, bacterial loads, and antibody titers. The log rank test was used to analyze survival rates. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (no. 81571622 and no. 81371778) and the Project of Chongqing Frontier and Applied Basic Research (no. cstc2015jcyjA10012).
We declare no conflict of interest.
REFERENCES
- 1.Vance RE, Isberg RR, Portnoy DA. 2009. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock KL, Latz E, Ontiveros F, Kono H. 2010. The sterile inflammatory response. Annu Rev Immunol 28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Neill LA, Bowie AG. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K. 2004. Toll-like receptor signalling. Nat Rev Immunol 4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 5.Dudek M, Puttur F, Arnold-Schrauf C, Kuhl AA, Holzmann B, Henriques-Normark B, Berod L, Sparwasser T. 2016. Lung epithelium and myeloid cells cooperate to clear acute pneumococcal infection. Mucosal Immunol 9:1288–1302. doi: 10.1038/mi.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim KH, Staudt LM. 2013. Toll-like receptor signaling. Cold Spring Harb Perspect Biol 5(1):a011247. doi: 10.1101/cshperspect.a011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hua Z, Hou B. 2013. TLR signaling in B-cell development and activation. Cell Mol Immunol 10:103–106. doi: 10.1038/cmi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchta CM, Bishop GA. 2014. Toll-like receptors and B cells: functions and mechanisms. Immunol Res 59:12–22. doi: 10.1007/s12026-014-8523-2. [DOI] [PubMed] [Google Scholar]
- 9.MacLeod H, Wetzler LM. 2007. T cell activation by TLRs: a role for TLRs in the adaptive immune response. Sci STKE 2007:pe48. doi: 10.1126/stke.4022007pe48. [DOI] [PubMed] [Google Scholar]
- 10.Majewska M, Szczepanik M. 2006. The role of Toll-like receptors (TLR) in innate and adaptive immune responses and their function in immune response regulation. Postepy Hig Med Dosw (Online) 60:52–63. (In Polish). [PubMed] [Google Scholar]
- 11.Jin B, Sun T, Yu XH, Yang YX, Yeo AE. 2012. The effects of TLR activation on T-cell development and differentiation. Clin Dev Immunol 2012:836485. doi: 10.1155/2012/836485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalod M, Chelbi R, Malissen B, Lawrence T. 2014. Dendritic cell maturation: functional specialization through signaling specificity and transcriptional programming. EMBO J 33:1104–1116. doi: 10.1002/embj.201488027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merad M, Sathe P, Helft J, Miller J, Mortha A. 2013. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou B, Reizis B, DeFranco AL. 2008. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity 29:272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan RS, Ho B, Leung BP, Ding JL. 2014. TLR cross-talk confers specificity to innate immunity. Int Rev Immunol 33:443–453. doi: 10.3109/08830185.2014.921164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Neill LA, Golenbock D, Bowie AG. 2013. The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol 13(6):453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 17.Honegr J, Soukup O, Dolezal R, Malinak D, Penhaker M, Prymula R, Kuca K. 2015. Structural properties of potential synthetic vaccine adjuvants—TLR agonists. Curr Med Chem 22:3306–3325. doi: 10.2174/0929867322666150821094634. [DOI] [PubMed] [Google Scholar]
- 18.Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A, Buechler CR, Quinn KM, Smelkinson MG, Vanek O, Cawood R, Hills T, Vasalatiy O, Kastenmüller K, Francica JR, Stutts L, Tom JK, Ryu KA, Esser-Kahn AP, Etrych T, Fisher KD, Seymour LW, Seder RA. 2015. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol 33:1201–1210. doi: 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi H-G, Choi S, Back YW, Paik S, Park H-S, Kim WS, Kim H, Cha SB, Choi CH, Shin SJ, Kim H-J. 2017. Rv2299c, a novel dendritic cell-activating antigen of Mycobacterium tuberculosis, fused-ESAT-6 subunit vaccine confers improved and durable protection against the hypervirulent strain HN878 in mice. Oncotarget 8:19947–19967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunne A, Mielke LA, Allen AC, Sutton CE, Higgs R, Cunningham CC, Higgins SC, Mills KH. 2015. A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine. Mucosal Immunol 8:607. doi: 10.1038/mi.2014.93. [DOI] [PubMed] [Google Scholar]
- 21.Sun X, Wang J, Zhou J, Wang H, Wang X, Wu J, He Y, Yin Y, Zhang X, Xu W. 2017. Subcutaneous immunization with Streptococcus pneumoniae GAPDH confers effective protection in mice via TLR2 and TLR4. Mol Immunol 83:1–12. doi: 10.1016/j.molimm.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci U S A 100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Cui J, Zhang X, Gao S, Ma F, Yao H, Sun X, He Y, Yin Y, Xu W. 2017. Pneumococcal DnaJ modulates dendritic cell-mediated Th1 and Th17 immune responses through Toll-like receptor 4 signaling pathway. Immunobiology 222:384–393. doi: 10.1016/j.imbio.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Khan MN, Bansal A, Shukla D, Paliwal P, Sarada SKS, Mustoori SR, Banerjee PK. 2006. Immunogenicity and protective efficacy of DnaJ (hsp40) of Streptococcus pneumoniae against lethal infection in mice. Vaccine 24:6225–6231. doi: 10.1016/j.vaccine.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 25.Cui Y, Zhang X, Gong Y, Niu S, Yin N, Yao R, Xu W, Li D, Wang H, He Y, Cao J, Yin Y. 2011. Immunization with DnaJ (hsp40) could elicit protection against nasopharyngeal colonization and invasive infection caused by different strains of Streptococcus pneumoniae. Vaccine 29:1736–1744. doi: 10.1016/j.vaccine.2010.12.126. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Wang H, Zhang S, Zeng L, Xu X, Wu K, Wang W, Yin N, Song Z, Zhang X, Yin Y. 2014. Mucosal immunization with recombinant fusion protein DnaJ-ΔA146Ply enhances cross-protective immunity against Streptococcus pneumoniae infection in mice via interleukin 17A. Infect Immun 82:1666–1675. doi: 10.1128/IAI.01391-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su Y, Li D, Xing Y, Wang H, Wang J, Yuan J, Wang X, Cui F, Yin Y, Zhang X. 2017. Subcutaneous immunization with fusion protein DnaJ-DeltaA146Ply without additional adjuvants induces both humoral and cellular immunity against pneumococcal infection partially depending on TLR4. Front Immunol 8:686. doi: 10.3389/fimmu.2017.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santone M, Aprea S, Wu TYH, Cooke MP, Mbow ML, Valiante NM, Rush JS, Dougan S, Avalos A, Ploegh H, De Gregorio E, Buonsanti C, D'Oro U. 2015. A new TLR2 agonist promotes cross-presentation by mouse and human antigen presenting cells. Human Vaccin Immunother 11:2038–2050. doi: 10.1080/21645515.2015.1027467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemmi H, Akira S. 2005. TLR signalling and the function of dendritic cells. Chem Immunol Allergy 86:120–135. doi: 10.1159/000086657. [DOI] [PubMed] [Google Scholar]
- 30.Fillatreau S, Manz RA. 2006. Tolls for B cells. Eur J Immunol 36:798–801. doi: 10.1002/eji.200636040. [DOI] [PubMed] [Google Scholar]
- 31.Kabelitz D. 2007. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol 19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Kapsenberg ML. 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 33.Vasilevsky S, Chattopadhyay G, Colino J, Yeh TJ, Chen Q, Sen G, Snapper CM. 2008. B and CD4+ T-cell expression of TLR2 is critical for optimal induction of a T-cell-dependent humoral immune response to intact Streptococcus pneumoniae. Eur J Immunol 38:3316–3326. doi: 10.1002/eji.200838484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janeway CA Jr, Medzhitov R. 2002. Innate immune recognition. Annu Rev Immunol 20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 35.Knapp S, Wieland CW, van't Veer C, Takeuchi O, Akira S, Florquin S, van der Poll T. 2004. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J Immunol 172:3132–3138. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- 36.Lammers AJ1, de Porto AP, de Boer OJ, Florquin S, van der Poll T. 2012. The role of TLR2 in the host response to pneumococcal pneumonia in absence of the spleen. BMC Infect Dis 12:139. doi: 10.1186/1471-2334-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao B, Kong Q, Kemp K, Zhao Y-S, Fang D. 2012. Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2-mediated reversal of T-cell tolerance. Proc Natl Acad Sci U S A 109:899–904. doi: 10.1073/pnas.1118462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez Y, Rodríguez M, Municio C, Hugo E, Alonso S, Ibarrola N, Fernández N, Crespo MS. 2012. Sirtuin 1 is a key regulator of the interleukin-12 p70/interleukin-23 balance in human dendritic cells. J Biol Chem 287:35689–35701. doi: 10.1074/jbc.M112.391839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Lee S-M, Shannon S, Gao B, Chen W, Chen A, Divekar R, McBurney MW, Braley-Mullen H, Zaghouani H, Fang D. 2009. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clinical Invest 119:3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beier UH, Wang L, Bhatti TR, Liu Y, Han R, Ge G, Hancock WW. 2011. Sirtuin-1 targeting promotes Foxp3(+) T-regulatory cell function and prolongs allograft survival. Mol Cell Biol 31:1022–1029. doi: 10.1128/MCB.01206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu G, Bi Y, Xue L, Zhang Y, Yang H, Chen X, Lu Y, Zhang Z, Liu H, Wang X, Wang R, Chu Y, Yang R. 2015. Dendritic cell SIRT1-HIF1α axis programs the differentiation of CD4(+) T cells through IL-12 and TGF-β1. Proc Natl Acad Sci U S A 112:E957–E965. doi: 10.1073/pnas.1420419112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen J, Fang J, Hao J, Zhong X, Wang D, Ren H, Hu Z. 2016. SIRT1 inhibits the catabolic effect of IL-1β through TLR2/SIRT1/NF-κB pathway in human degenerative nucleus pulposus cells. Pain Physician 19(1):E215. [PubMed] [Google Scholar]
- 43.Botos I, Segal DM, Davies DR. 2011. The structural biology of Toll-like receptors. Structure 19:447–459. doi: 10.1016/j.str.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medzhitov R, Shevach EM, Trinchieri G, Mellor AL, Munn DH, Gordon S, Libby P, Hansson GK, Shortman K, Dong C, Gabrilovich D, Gabryšová L, Howes A, O'Garra A. 2011. Highlights of 10 years of immunology in Nature Reviews Immunology. Nat Rev Immunol 11:693–702. doi: 10.1038/nri3063. [DOI] [PMC free article] [PubMed] [Google Scholar]