ABSTRACT
Salmonella enterica serovars Typhi and Typhimurium cause typhoid fever and gastroenteritis, respectively. A unique feature of typhoid infection is asymptomatic carriage within the gallbladder, which is linked with S. Typhi transmission. Despite this, S. Typhi responses to bile have been poorly studied. Transcriptome sequencing (RNA-Seq) of S. Typhi Ty2 and a clinical S. Typhi isolate belonging to the globally dominant H58 lineage (strain 129-0238), as well as S. Typhimurium 14028, revealed that 249, 389, and 453 genes, respectively, were differentially expressed in the presence of 3% bile compared to control cultures lacking bile. fad genes, the actP-acs operon, and putative sialic acid uptake and metabolism genes (t1787 to t1790) were upregulated in all strains following bile exposure, which may represent adaptation to the small intestine environment. Genes within the Salmonella pathogenicity island 1 (SPI-1), those encoding a type IIII secretion system (T3SS), and motility genes were significantly upregulated in both S. Typhi strains in bile but downregulated in S. Typhimurium. Western blots of the SPI-1 proteins SipC, SipD, SopB, and SopE validated the gene expression data. Consistent with this, bile significantly increased S. Typhi HeLa cell invasion, while S. Typhimurium invasion was significantly repressed. Protein stability assays demonstrated that in S. Typhi the half-life of HilD, the dominant regulator of SPI-1, is three times longer in the presence of bile; this increase in stability was independent of the acetyltransferase Pat. Overall, we found that S. Typhi exhibits a specific response to bile, especially with regard to virulence gene expression, which could impact pathogenesis and transmission.
KEYWORDS: bile responses, cell invasion, H58 clade, RNA-Seq, SPI-1 regulation, typhoid fever
INTRODUCTION
In humans, the outcome of infection with Salmonella enterica depends primarily on the infecting serovar; while nontyphoidal, broad-host-range serovars such as Salmonella enterica serovar Typhimurium (S. Typhimurium) cause self-limiting gastroenteritis, infections with human-restricted typhoidal serovars such as Salmonella enterica serovar Typhi (S. Typhi) result in typhoid fever (1). The virulence of both serovars depends on the activity of two type III secretion systems (T3SS) carried on Salmonella pathogenicity islands 1 and 2 (SPI-1 and SPI-2), which secrete a pool of over 40 effectors to subvert host cell processes resulting in invasion, immune evasion, and intracellular growth (2). The SPI-1 T3SS is active when Salmonella is extracellular, and its activity permits Salmonella invasion of nonphagocytic cells and also promotes early adaptation to the intracellular environment (2). Expression of the SPI-1 T3SS and its associated genes (several of which are encoded outside the SPI-1 pathogenicity island) is controlled by a hierarchy of regulators (HilD, HilA, HilC, RtsA, InvF). These regulators are controlled by a variety of factors, including two-component systems, RNA binding proteins, and global regulators, which respond to a range of environmental stimuli (3, 4).
Typhoid is an acute illness characterized by high fever, malaise, and abdominal pain (5). S. Typhi causes systemic infection during which the pathogen colonizes the intestine and mesenteric lymph nodes, the liver, spleen, bone marrow, and gallbladder (5). It is estimated that there are more than 20 million typhoid fever cases per year, resulting in more than 200,000 deaths (6). Although with adequate treatment most patients recover from the acute phase of S. Typhi infection, S. Typhi can persist asymptomatically within the gallbladder following clinical recovery (7). Overall, 10% of those infected will carry S. Typhi within their gallbladder for up to 3 months, while 1 to 3% will continue to harbor S. Typhi for longer than 1 year (5, 8). Given the host restriction of S. Typhi, chronic gallbladder carriage represents a key environmental reservoir of S. Typhi bacteria, enabling typhoid transmission (7, 9).
Although the exact mechanism(s) by which S. Typhi persists within the gallbladder are debated (7), it certainly encounters high bile concentrations during carriage, as the gallbladder is where bile is stored and concentrated prior to secretion into the small intestine, where it plays a role in the emulsification and absorption of fats (10). In part due to its detergent activity, bile is also a potent antimicrobial agent (10, 11). However, enteric pathogens—including Salmonella—are intrinsically resistant to bile (12) and instead often utilize bile as a means to regulate gene expression and virulence (10, 13). In S. Typhimurium, expression of the SPI-1 and motility genes is repressed by bile exposure, resulting in a significant repression of epithelial cell invasion (14, 15).
Despite the importance of asymptomatic carriage, the behavior of S. Typhi within bile remains poorly understood (7). As the transcriptomic responses of S. Typhimurium to bile under various conditions have been well characterized (15–18), the behavior of S. Typhimurium has become an accepted model as to how Salmonella in general behaves in bile (11, 19). However, a study comparing changes in protein expression by two-dimensional (2D) gel electrophoresis within S. Typhimurium and S. Typhi following exposure to 3% bile found that there was “little overlap apparent between proteins affected by bile in S. Typhi and in S. Typhimurium” (12), suggesting that the response to bile differs between these serovars. Furthermore, a study comparing the genomes of S. Typhimurium LT2 and S. Typhi CT18 revealed that less than 90% of genes are shared between the two strains, with over 600 genes present in CT18 not found in LT2 (20); therefore, S. Typhimurium cannot be used to model the regulation of S. Typhi-specific genes, which include key virulence factors such as the Vi antigen and the CdtB and HlyE/ClyA toxins (20).
The need to better understand S. Typhi infection has been intensified by the recent spread of haplotype 58 (H58), also known as 4.3.1 (21, 22). Following its emergence around 30 years ago, S. Typhi strains belonging to haplotype H58 have clonally expanded worldwide to become the dominant cause of multidrug-resistant (MDR) typhoid within regions of endemicity (21). As yet, the reasons underlying the relative success of H58 strains remain unknown.
The aim of this study was to compare the global bile responses of S. Typhi and S. Typhimurium isolates, which in turn might explain differences in pathogenesis and reveal processes important for the carrier state.
RESULTS
Bile exposure alters global gene expression in Salmonella.
We performed transcriptome sequencing (RNA-Seq) on S. Typhimurium 14028, S. Typhi Ty2, and a clinical S. Typhi H58 isolate (129-0238) grown in LB to late exponential phase in the presence or absence of 3% bile. Given the extensive description of S. Typhimurium behavior in bile (14, 15), S. Typhimurium 14028 was considered a control. For these studies, 3% ox bile was chosen, as this concentration robustly affects gene expression in S. Typhimurium (14, 15, 23) but does not affect the growth of the investigated Salmonella strains (see Fig. S1 in the supplemental material). Overall, following growth in bile, 249 and 389 genes were differentially expressed in S. Typhi Ty2 (182 upregulated; 67 downregulated) and 129-2038 (223 upregulated; 166 downregulated) (Fig. 1), respectively, while 453 genes were differentially regulated in S. Typhimurium 14028 (293 upregulated; 179 downregulated) (Fig. 1).
FIG 1.
Comparison of pathways differentially regulated by bile between S. Typhi and S. Typhimurium. Overrepresented gene ontology (GO) terms within upregulated and downregulated genes following growth in 3% bile for each strain.
Gene ontology (GO) enrichment and KEGG pathway analysis on the pools of upregulated and downregulated genes revealed broad differences between S. Typhi and S. Typhimurium (Fig. 1). While S. Typhimurium upregulated metabolic processes and downregulated processes linked with pathogenicity, including T3SS, flagella, and chemotaxis (motility), in line with previous findings (14, 15, 17), both S. Typhi Ty2 and 129-0238 upregulated these processes, while downregulating various metabolic pathways (Fig. 1). KEGG pathway analysis also revealed that fatty acid degradation (represented by the GO term “fatty acid beta-oxidation”) and tyrosine metabolism were upregulated in all isolates, implicating these processes in the general Salmonella response to bile.
Similarities of S. Typhi and S. Typhimurium in their responses to bile.
The overlap in genes either downregulated or upregulated in bile between all strains was small; only one gene (pagP), a PhoP-PhoQ-regulated gene involved in modifying lipid A (24), was downregulated in all strains (Fig. 2). Twenty genes were upregulated in all isolates in response to bile (Fig. 2; Table 1), representing genes involved in tyrosine metabolism, in sialic acid uptake and utilization (t1787-1790) (25), and in the production of acetyl coenzyme A (acetyl-CoA) from acetate (actP-acs) and fatty acids (fad genes). Of the upregulated genes, expression of acs and fadE was validated by quantitative reverse transcription-PCR (RT-qPCR) (Table 2). Upregulation of sialic acid and acetate metabolic pathways may reflect adaptation to the small intestine, where these metabolites are abundant (26), while upregulation of fad genes is consistent with the ability of Salmonella to utilize phospholipids present in bile as a carbon/energy source (27). Interestingly, the fatty acid transporter fadL was strongly upregulated in S. Typhimurium but was not upregulated in either S. Typhi Ty2 or 129-0238, suggesting that S. Typhi may possess additional fatty acid transporters.
FIG 2.
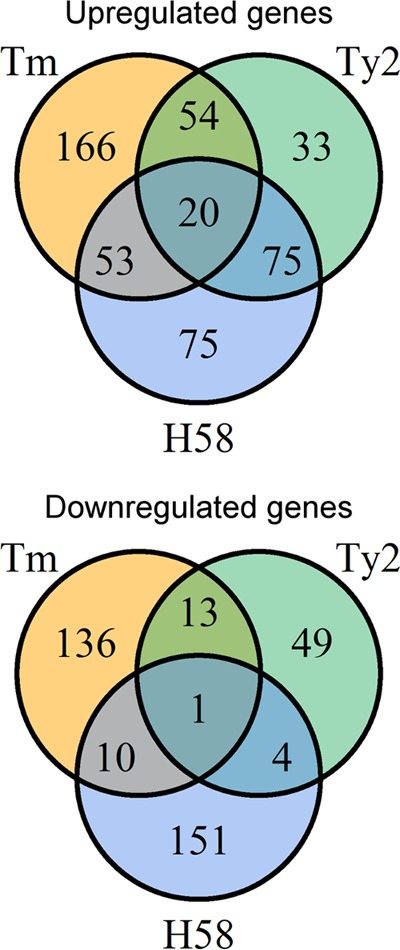
Gene expression in response to bile differs among Salmonella strains. Comparison of genes upregulated and downregulated in response to bile in S. Typhimurium (Tm), S. Typhi Ty2 (Ty2), and S. Typhi 129-0238 (H58).
TABLE 1.
Genes upregulated by bile in all strains
| Gene name | Locus tag | Product | Log2 fold change |
||
|---|---|---|---|---|---|
| Tm | Ty2 | H58 | |||
| fadI | t0475 | 3-Ketoacyl-CoA thiolase | 4.12 | 2.55 | 2.52 |
| fadJ | t0476 | Multifunctional fatty acid oxidation complex subunit alpha | 3.32 | 2.06 | 2.17 |
| fadE | t2541 | Acyl-CoA dehydrogenase | 7.44 | 4.70 | 4.18 |
| fadB | t3315 | Multifunctional fatty acid oxidation complex subunit alpha | 7.52 | 2.92 | 1.57 |
| fadA | t3316 | 3-Ketoacyl-CoA thiolase | 7.66 | 2.88 | 1.57 |
| actP | t4179 | Acetate permease | 3.41 | 1.58 | 1.27 |
| t4180 | Hypothetical protein | 3.44 | 1.72 | 1.32 | |
| acs | t4181 | Acetyl-CoA synthetase | 3.91 | 2.11 | 1.31 |
| acnA | t1625 | Aconitate hydratase | 3.18 | 1.81 | 1.59 |
| argT | t0509 | Lysine-arginine-ornithine-binding periplasmic protein | 3.36 | 2.29 | 1.33 |
| argD | t1182 | Bifunctional succinylornithine transaminase/acetylornithine transaminase | 5.61 | 2.72 | 1.20 |
| t0677 | Gentisate 1,2-dioxygenase | 2.51 | 3.91 | 3.38 | |
| t0678 | FAA-hydrolase-family protein | 2.09 | 3.21 | 2.87 | |
| t0679 | Glutathione-S-transferase-family protein | 2.09 | 2.89 | 2.49 | |
| t0680 | Salicylate hydroxylase | 1.27 | 2.09 | 2.03 | |
| t1787 | Oxidoreductase | 3.62 | 3.53 | 1.32 | |
| t1789 | Hypothetical protein | 3.17 | 4.04 | 1.44 | |
| t1790 | N-Acetylneuraminic acid mutarotase | 2.78 | 4.07 | 1.32 | |
| gabT | t2687 | 4-Aminobutyrate aminotransferase | 5.18 | 2.93 | 1.74 |
| msrA | t4462 | Methionine sulfoxide reductase A | 1.68 | 1.67 | 1.30 |
TABLE 2.
Log2 fold changes (± SD) in gene expression in strains 14028, Ty2, and 129-0238 determined by RNA-Seq and RT-qPCR
| Gene | RNA-Seq |
RT-qPCR |
||||
|---|---|---|---|---|---|---|
| 14028 | Ty2 | 129-0238 | 14028 | Ty2 | 129-0238 | |
| hilD | −4.08 | 1.23 | 3.15 | −3.48 ± 0.71 | 1.42 ± 0.25 | 2.44 ± 0.73 |
| hilA | −6.98 | 1.54 | 3.67 | −6.51 ± 0.64 | 1.71 ± 0.39 | 3.37 ± 0.39 |
| prgH | −6.36 | 1.57 | 4.02 | −6.00 ± 0.74 | 1.68 ± 0.73 | 4.00 ± 0.48 |
| sopB | −6.95 | 1.11 | 4.21 | −3.85 ± 0.44 | 1.38 ± 0.59 | 4.13 ± 0.27 |
| flhD | −1.72 | 1.05 | 1.33 | −1.25 ± 0.43 | 1.93 ± 0.38 | 2.31 ± 1.13 |
| flgA | −1.29 | 1.37 | 1.70 | −0.98 ± 0.27 | 1.99 ± 0.44 | 1.37 ± 0.84 |
| fadE | 7.44 | 4.70 | 4.18 | 3.55 ± 2.13 | 3.75 ± 0.16 | 4.75 ± 0.09 |
| acs | 3.91 | 2.11 | 1.31 | 2.03 ± 1.77 | 0.87 ± 0.67 | 2.37 ± 0.59 |
Genes implicated in stress responses were also upregulated in bile. All isolates upregulated msrA, a sulfoxide reductase upregulated in response to oxidative stress, which is required for growth within macrophages and for full virulence of S. Typhimurium in vivo (28). S. Typhimurium 14028 and S. Typhi 129-0238 also activated RpoS-mediated stress responses, with upregulation of otsAB, spoVR, yeaG, katE, sodC, poxB, ecnB, and osmY, in line with previous findings (17, 29, 30). However, upregulation of these stress-linked genes was not observed in S. Typhi Ty2, which is likely due to a frameshift mutation within rpoS in this strain (31).
Differences between S. Typhi and S. Typhimurium in their responses to bile.
Of special interest are genes that are regulated differently in response to bile in S. Typhi and S. Typhimurium. The identification of such genes was achieved by determining which genes were downregulated in S. Typhimurium in bile but upregulated in S. Typhi and vice versa. Of the 75 genes upregulated in both S. Typhi Ty2 and 129-0238 (Fig. 2), the majority (54/75) were significantly downregulated in S. Typhimurium (Table 3). As indicated by the GO and KEGG pathway analyses (Fig. 1), genes regulated in this manner predominantly encode proteins associated with the SPI-1 T3SS or motility. To validate these findings, expression of the SPI-1-associated genes hilD, hilA, prgH, and sopB, in addition to the flagellum-associated genes flhD and flgA, was confirmed by RT-qPCR (Table 2).
TABLE 3.
Genes downregulated in S. Typhimurium and upregulated in S. Typhi in bile
| Gene name | Locus tag | Product | Log2 fold change |
||
|---|---|---|---|---|---|
| Tm | Ty2 | H58 | |||
| fliO | t0899 | Flagellar biosynthesis protein FliO | −1.87 | 1.57 | 1.35 |
| fliN | t0900 | Flagellar motor switch protein FliN | −1.55 | 1.44 | 1.62 |
| fliM | t0901 | Flagellar motor switch protein FliM | −1.71 | 1.40 | 1.71 |
| fliL | t0902 | Flagellar basal body protein FliL | −1.74 | 1.41 | 1.78 |
| fliK | t0903 | Flagellar hook length control protein | −1.67 | 1.33 | 2.08 |
| fliJ | t0904 | Flagellar biosynthesis chaperone | −1.37 | 1.43 | 2.25 |
| fliI | t0905 | Flagellum-specific ATP synthase | −1.43 | 1.25 | 1.69 |
| fliH | t0906 | Flagellar assembly protein H | −1.45 | 1.41 | 1.57 |
| fliG | t0907 | Flagellar motor switch protein G | −1.44 | 1.34 | 1.53 |
| fliF | t0908 | Flagellar MS-ring protein | −1.89 | 1.32 | 1.41 |
| fliE | t0909 | Flagellar hook basal body protein FliE | −2.49 | 1.76 | 2.01 |
| flhD | t0952 | Transcriptional activator FlhD | −1.72 | 1.05 | 1.33 |
| flgJ | t1738 | Flagellar rod assembly protein/muramidase FlgJ | −1.56 | 1.30 | 1.38 |
| flgI | t1739 | Flagellar basal body P-ring biosynthesis protein FlgA | −1.69 | 1.41 | 1.39 |
| flgH | t1740 | Flagellar basal body L-ring protein | −1.71 | 1.42 | 1.68 |
| flgC | t1745 | Flagellar basal body rod protein FlgC | −1.86 | 1.39 | 1.79 |
| flgB | t1746 | Flagellar basal body rod protein FlgB | −2.05 | 1.40 | 1.73 |
| flgA | t1747 | Flagellar basal body P-ring biosynthesis protein FlgA | −1.29 | 1.37 | 1.70 |
| sprB | t2768 | AraC family transcriptional regulator | −3.76 | 1.97 | 4.11 |
| sprA | t2769 | AraC family transcriptional regulator | −3.29 | 1.97 | 3.29 |
| t2770 | Hypothetical protein | −3.69 | 1.22 | 2.11 | |
| orgA | t2771 | Oxygen-regulated invasion protein | −3.90 | 1.34 | 1.79 |
| orgA | t2772 | Oxygen-regulated invasion protein | −5.65 | 1.62 | 3.50 |
| prgJ | t2774 | Pathogenicity island 1 effector protein | −6.05 | 1.43 | 3.83 |
| prgI | t2775 | Pathogenicity island 1 effector protein | −6.15 | 1.41 | 3.89 |
| prgH | t2776 | Pathogenicity island 1 effector protein | −6.36 | 1.57 | 4.02 |
| hilA | t2778 | Invasion protein regulator | −6.98 | 1.54 | 3.67 |
| iagB | t2779 | Cell invasion protein | −6.64 | 1.35 | 3.83 |
| sicP | t2781 | Chaperone | −3.06 | 1.40 | 3.19 |
| t2782 | Hypothetical protein | −3.10 | 1.56 | 2.98 | |
| sipF or iacP | t2783 | Acyl carrier protein | −5.62 | 1.46 | 3.43 |
| sipA | t2784 | Pathogenicity island 1 effector protein | −5.84 | 1.55 | 3.60 |
| sipD | t2785 | Pathogenicity island 1 effector protein | −6.24 | 1.48 | 3.87 |
| spaS | t2789 | Surface presentation of antigens protein SpaS | −5.70 | 1.24 | 3.29 |
| spaQ | t2791 | Virulence-associated secretory protein | −7.26 | 1.40 | 3.00 |
| spaP | t2792 | Surface presentation of antigens protein SpaP | −6.87 | 1.43 | 3.25 |
| spaO | t2793 | Surface presentation of antigens protein SpaO | −6.72 | 1.60 | 3.66 |
| spaN | t2794 | Antigen presentation protein SpaN | −6.66 | 1.58 | 3.91 |
| spaM | t2795 | Virulence-associated secretory protein | −6.91 | 1.76 | 3.83 |
| spaL or invC | t2796 | ATP synthase SpaL | −6.61 | 1.53 | 3.43 |
| spaK or invB | t2797 | Virulence-associated secretory protein | −6.04 | 1.91 | 4.01 |
| invA | t2798 | Virulence-associated secretory protein | −6.50 | 1.40 | 3.34 |
| invE | t2799 | Cell invasion protein | −6.86 | 1.35 | 3.59 |
| invG | t2800 | Virulence-associated secretory protein | −7.12 | 1.37 | 3.60 |
| invF | t2801 | AraC family transcriptional regulator | −6.97 | 1.27 | 3.84 |
| invH | t2802 | Cell adherence/invasion protein | −4.54 | 1.57 | 2.97 |
| sopD | t2846 | Hypothetical protein | −3.76 | 1.05 | 4.33 |
| rtsB | t4220 | GerE family regulatory protein | −7.59 | 1.99 | 3.58 |
| rtsA | t4221 | AraC family transcriptional regulator | −7.33 | 1.80 | 3.83 |
| t0944 | Lipoprotein | −2.25 | 1.20 | 2.22 | |
| t1774 | Hypothetical protein | −2.09 | 1.46 | 2.60 | |
| lpxR | t1208 | Hypothetical protein | −7.02 | 1.19 | 3.44 |
| srfA | t1503 | Virulence effector protein | −1.75 | 1.64 | 1.81 |
| srfB | t1504 | Virulence effector protein | −1.48 | 1.58 | 1.88 |
Additional genes upregulated in S. Typhi and downregulated in S. Typhimurium include lpxR (t1208/STM14_1612), a lipid A-modifying protein that modulates the ability of lipid A to stimulate Toll-like receptor 4 (TLR4) (32) and promotes Salmonella growth inside macrophages (33), and srfA and srfB, virulence factors expressed under SPI-1-inducing conditions (34) and reported to modulate inflammatory signaling (35). Additionally, several hypothetical proteins, t0944 (STM14_2352), t1774 (STM14_1312), and t2782 (STM14_3479), were upregulated in S. Typhi but downregulated in S. Typhimurium. Given their regulation pattern, these genes may encode uncharacterized virulence factors or be involved in motility in Salmonella.
We also analyzed the expression profile of S. Typhi-specific genes. S. Typhi Ty2 carries 453 unique genes relative to S. Typhimurium, representing Ty2 homologues of the 601 S. Typhi-specific genes identified in CT18 (36), in addition to 29 Ty2-specific genes (37). Only two of these genes were significantly regulated by bile exposure in both S. Typhi Ty2 and 129-0238. Both genes, which are upregulated in bile, encode hypothetical proteins: t0349 (STY2749) encodes a GIY-YIG domain containing protein, and t1865 (STY1076) encodes a homologue of the NleG family of T3SS effectors (38, 39). Neither S. Typhi isolate demonstrated altered expression of genes encoding the Vi antigen or of the typhoid toxin in bile.
Bile influences SPI-1 expression and Salmonella invasion.
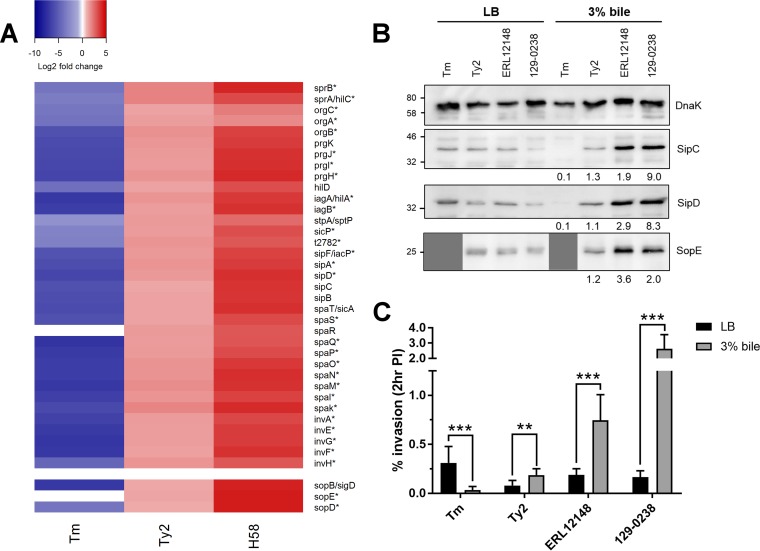
The most marked differences between S. Typhi and S. Typhimurium in response to bile was in the expression of SPI-1-associated genes. The majority of genes within the SPI-1 pathogenicity island, in addition to the SPI-1 regulators rtsA and rtsB, and effector genes carried outside SPI-1 (sopD) were significantly upregulated in S. Typhi Ty2 and 129-0238 but significantly downregulated in S. Typhimurium (Table 3; Fig. 3A). Noticeably, S. Typhi 129-0238 exhibited significantly elevated expression of SPI-1 genes relative to S. Typhi Ty2 (Table 3; Fig. 3A).
FIG 3.
Effects of bile on SPI-1 expression and activity. (A) Heatmap showing log2-fold changes in gene expression for S. Typhimurium (Tm), S. Typhi Ty2 (Ty2), and S. Typhi 129-0238 (H58) across the SPI-1 pathogenicity island and for non-SPI-1-carried effectors. Asterisks (*) indicate genes significantly affected by bile across all three strains. (B) Western blots of SipC, SipD, and SopE of S. Typhimurium 14028 (Tm), S. Typhi Ty2 (Ty2), and two H58 clinical isolates (ERL12148 and 129-0238) grown in LB with or without 3% bile; SopE panels are not shown for S. Typhimurium 14028, as this strain lacks SopE. DnaK was used as a loading control. A representative blot for two independent repeats is shown. Numbers below blots indicate fold changes in density in 3% bile compared to LB; all bands were normalized to their respective DnaK control prior to comparison. (C) Strains grown in LB or 3% bile to late exponential phase were added to HeLa cells at an MOI of 100 for 30 min. The percentages of intracellular bacteria at 2 h postinfection relative to the inoculum added are shown. n = 3; error bars show SD. Invasion rates of strains were compared by t test (**, P < 0.01; ***, P < 0.001).
To determine if changes in SPI-1 gene expression correlated with changes at the protein level, we compared the intracellular levels of the SPI-1 translocon proteins SipC and SipD and the SPI-1 effectors SopE (for S. Typhi) or SopB (for S. Typhi and S. Typhimurium) from each strain grown in the absence or presence of bile. Additional S. Typhi strains were also included to further expand and validate these findings, namely, the RpoS+ S. Typhi reference strain CT18 (37) and an additional H58 isolate, strain ERL12148, which belongs to a different sublineage of H58 from that of 129-0238 (21). All S. Typhi strains tested (Ty2, CT18, 129-0238, ERL12148) showed increased levels of SPI-1 proteins, with the H58 strains demonstrating the largest increases in SPI-1 protein expression in bile (Fig. 3B; see also Fig. S2 in the supplemental material). Conversely, S. Typhimurium 14028 showed decreased levels of SopB, SipD, and SipC following growth in bile (Fig. 3B and S2); as S. Typhimurium 14028 lacks SopE, its lanes (Tm) in the SopE blot are not shown.
Given the significant effect of bile on SPI-1 expression, we investigated the impact of bile on epithelial cell invasion. In line with previous findings (14), S. Typhimurium exposed to bile demonstrated significantly reduced invasion, achieving an invasion rate approximately 90% lower than that of S. Typhimurium grown in the absence of bile (Fig. 3C). In contrast, all S. Typhi strains tested demonstrated significantly increased invasion following bile exposure, with Ty2 and CT18 displaying an approximate 2-fold increase in the number of intracellular bacteria at 2 h postinfection and both H58 isolates demonstrating even higher increases in invasion (between 4- and 16-fold greater) (Fig. 3C and S2). An SPI-1-deficient strain of S. Typhi Ty2 (ΔinvA) did not invade HeLa cells in the presence of bile, indicating that the increased invasiveness of S. Typhi in bile is SPI-1 dependent (Fig. S2).
Transcriptional regulation of SPI-1 regulators in bile.
Given the striking difference in SPI-1 expression between S. Typhi and S. Typhimurium in response to bile, we determined where and how SPI-1 regulation differs between the two serovars. The central regulators governing SPI-1 expression are HilA, often termed the master SPI-1 regulator, and HilD, which is the dominant regulator of HilA (3, 40). The RNA-Seq and RT-qPCR data show that the mRNA levels of these regulators significantly decrease in S. Typhimurium in response to bile but significantly increase in response to bile in the S. Typhi strains (Table 2).
In order to determine if these changes are mediated by transcriptional regulation of these genes, we constructed hilA and hilD lacZ chromosomal transcriptional reporters in S. Typhimurium 14028 and S. Typhi Ty2 (41). The reporter activity was determined by β-galactosidase assay following growth to late exponential phase in LB with or without 3% bile. In S. Typhimurium, expression of hilA was significantly reduced in the presence of bile, with expression almost 20-fold lower, while expression of hilD was unchanged (Fig. 4). In contrast, expression of hilA in S. Typhi significantly increased in bile, with expression over 3 times higher, while hilD expression was only modestly increased (Fig. 4). Taken together, these results indicate that hilA is transcriptionally regulated by bile in both S. Typhi and S. Typhimurium, while hilD is not subject to transcriptional regulation.
FIG 4.
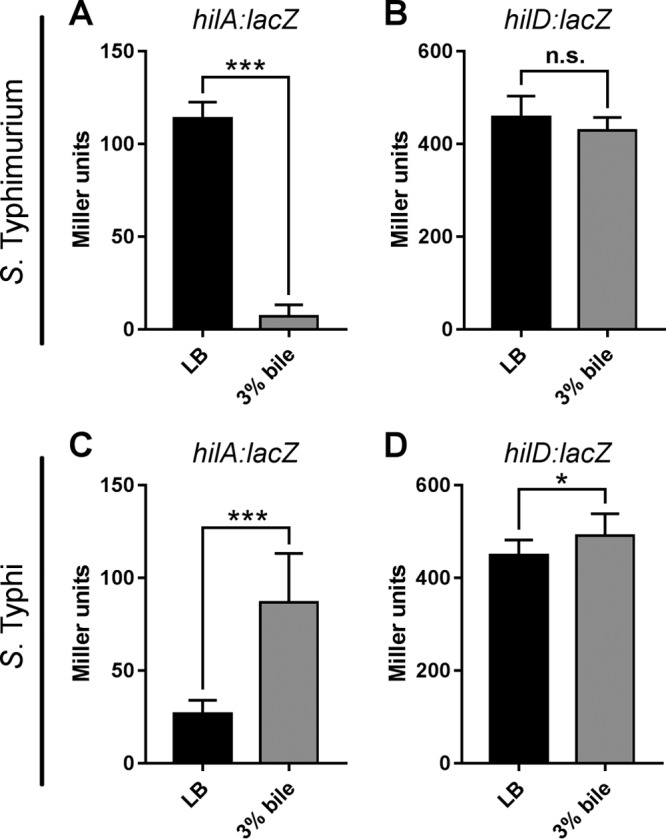
Effects of bile on hilA and hilD transcription in Salmonella. The reporter activity (β-galactosidase units) of hilA::lacZ and hilD::lacZ in S. Typhimurium 14028 (A, B) and S. Typhi Ty2 (C, D) following growth to late exponential phase in LB in the presence or absence of bile. n = 3; error bars show SD. Reporter activity between strains was compared by t test (*, P < 0.05; ***, P < 0.001).
The seeming absence of hilD transcriptional regulation in bile (Fig. 4) is at odds with the significant changes in mRNA levels observed (Table 2). One explanation is that hilD::lacZ reporter strains do not account for HilD-mediated autoregulation, as the chromosomal reporter strains were made in a ΔhilD background. HilD autoregulation has previously been reported in S. Typhimurium (42) but has not been characterized in S. Typhi. To determine if HilD autoregulation could account for transcriptional changes of hilD in bile in S. Typhi, the hilD::lacZ S. Typhi Ty2 reporter strain was transformed with a plasmid expressing HilD or an empty vector control, and reporter activity was assessed by β-galactosidase assay following growth in LB. hilD expression from the strain complemented with HilD was significantly higher than hilD expression from both the reporter strain alone and the reporter carrying the empty vector (Fig. 5), indicating that in S. Typhi HilD positively regulates its own transcription, either directly or indirectly.
FIG 5.
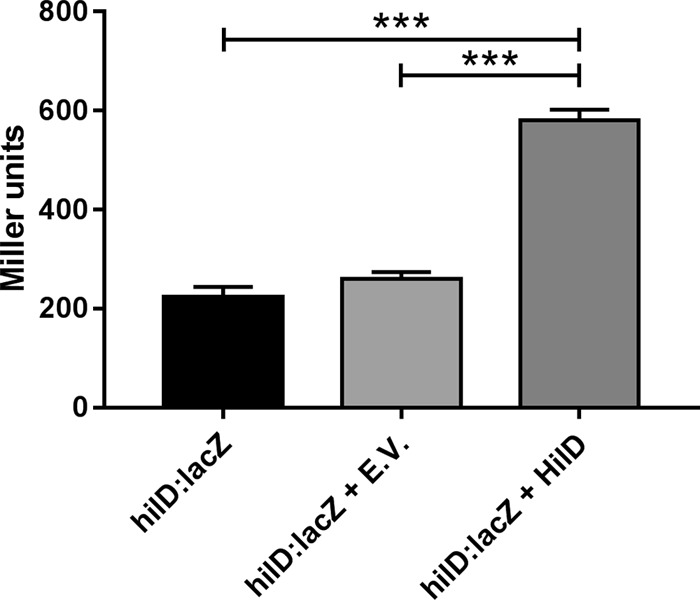
HilD autoregulation in S. Typhi. The reporter activity of an S. Typhi Ty2 hilD::lacZ chromosomal transcriptional reporter strain complemented with HilD (pWSK29-Spec HilD-4HA [HilD]) or an empty vector control (pWSK29-Spec [EV]) was determined by β-galactosidase assay following growth in LB. n = 3; error bars show SD. Reporter activity between strains was compared by one-way ANOVA (***, P < 0.001).
Bile influences HilD stability.
Given that expression of hilA, a gene directly regulated by HilD, significantly increases in bile, we investigated if HilD is posttranscriptionally regulated by bile in S. Typhi. Previous studies have shown that in S. Typhimurium, HilD stability is markedly decreased in the presence of bile, with a reported half-life almost 4 times shorter in LB supplemented with 3% bile than in LB alone (23). To determine the effect of bile on HilD stability in S. Typhi, S. Typhi Ty2 was transformed with constitutively expressed hemagglutinin (HA)-tagged HilD (from S. Typhi Ty2) and subcultured in the presence or absence of bile, and samples were taken at regular intervals following the inhibition of protein synthesis. Importantly, the HA-tagged HilD used in these studies was functional (Fig. 5), indicating that the HA tag used does not disrupt HilD structure or activity. In LB the half-life of HilD was 14 min, while in bile the half-life of HilD increased to 40 min, indicating that HilD is approximately three times more stable in the presence than in the absence of bile in S. Typhi (Fig. 6A).
FIG 6.
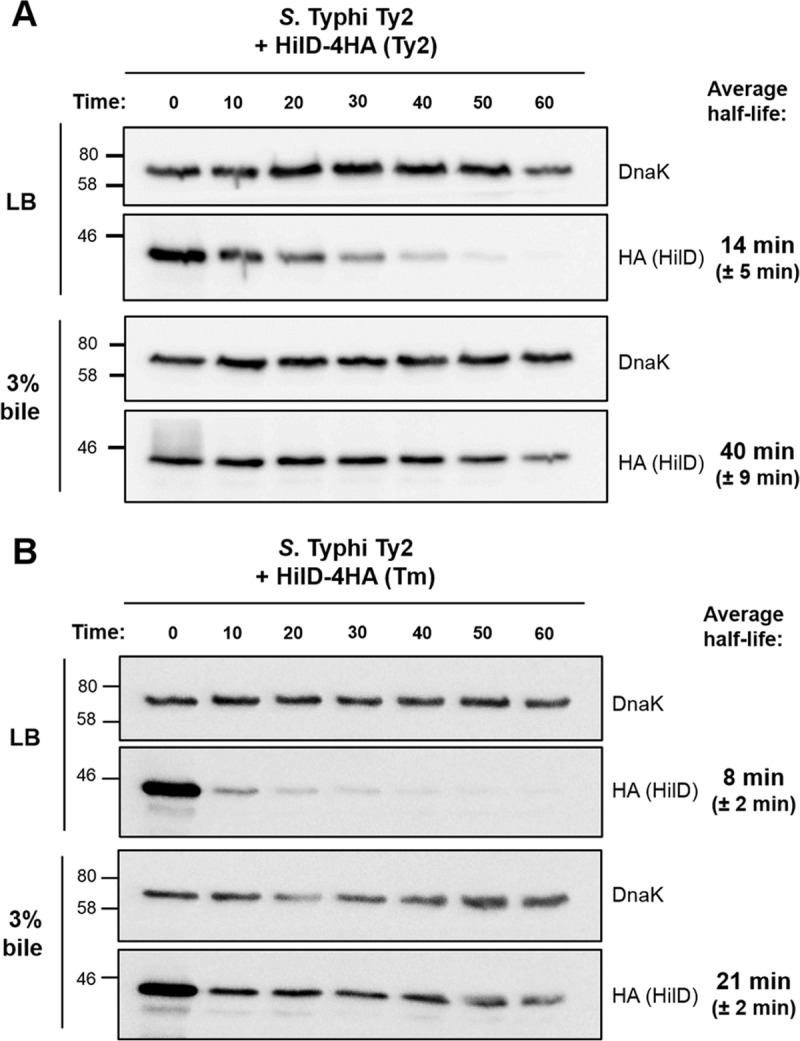
Bile promotes HilD stability in S. Typhi. WT S. Typhi Ty2 constitutively expressing C-terminally 4HA-tagged HilD from S. Typhi Ty2 (A) or S. Typhimurium 14028 (B) was grown in LB with or without bile. Thirty micrograms per milliliter chloramphenicol was added to stop protein synthesis, and samples were collected every 10 min. HilD levels were determined via Western blotting using an anti-HA antibody, and DnaK was used as a loading control. A representative blot for three independent repeats is shown. Half-life measurements are averaged from three independent repeats, and standard deviations are shown.
HilD is highly conserved between S. Typhi and S. Typhimurium (>99% identity; 2 amino acid changes). Since HilD has previously been shown to be less stable in bile in S. Typhimurium (23), we next determined if this difference in stability was due to intrinsic differences between HilD between the serovars or rather due to differences in factors that act on HilD and influence its stability. To investigate this, we determined the stability of HA-tagged HilD from S. Typhimurium 14028 expressed in S. Typhi Ty2. As was observed for S. Typhi HilD, S. Typhimurium HilD was three times more stable in bile, with a recorded half-life increasing from 8 min in LB to 21 min (Fig. 6B).
Although several factors have been reported to posttranscriptionally regulate HilD (e.g., HilE, CsrA, GreE/GreB, FliZ, Hfq, RNase E [3, 43, 44]), only two have been described to directly influence HilD protein stability: the protease Lon, which degrades HilD (45), and the acetyltransferase Pat, which acetylates HilD to increase stability while decreasing DNA binding (46). To determine if these factors were involved in mediating HilD stability in bile in S. Typhi Ty2, deletions were constructed and HilD stability was determined as described previously. Unfortunately, a Δlon Ty2 strain had severe growth defects and could not be tested. Although HilD stability was decreased in a Δpat Ty2 strain, in line with previous findings in S. Typhimurium (46, 47), it was still increased in the presence of bile, increasing from 4 min in LB to 13 min in the presence of bile (see Fig. S3 in the supplemental material), indicating that Pat-mediated acetylation of HilD is not responsible for the increased stability in bile. Overall, our data suggest that factors responsible for governing the stability of HilD in response to bile (other than Pat) differ between S. Typhi and S. Typhimurium.
DISCUSSION
Transcriptomic analysis of S. Typhimurium and S. Typhi strains grown in LB or 3% bile permitted the identification of similarities and differences in each serovar's response to bile. Significant differences were observed in the regulation of the invasion-associated SPI-1 T3SS and in motility genes between nontyphoidal and typhoidal serovars. S. Typhi strains significantly upregulated these processes and displayed a significant increase in T3SS-dependent invasion in bile, a response akin to that of other enteric pathogens (13), including Vibrio parahaemolyticus (48), Vibrio cholerae (49, 50), and Shigella (51, 52). All S. Typhi strains tested (Ty2, CT18, and two H58 clinical isolates) demonstrated significantly increased invasion in bile, strongly suggesting that this is a common response of S. Typhi to bile.
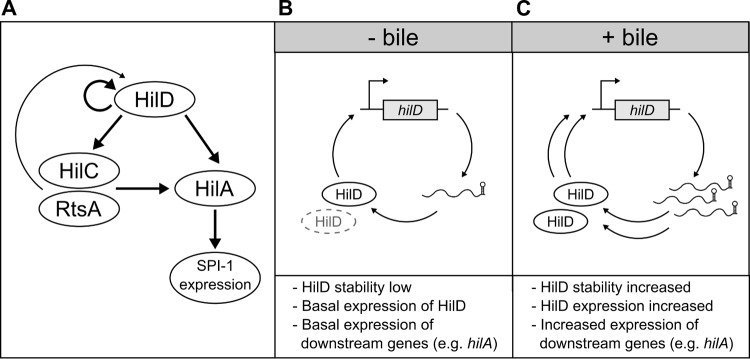
It is interesting to consider why S. Typhi and S. Typhimurium have such disparate responses to bile. During infection, Salmonella encounters bile within the small intestine and, in the case of S. Typhi, within the gallbladder. Following the observation that S. Typhimurium invasion was significantly repressed in the presence of bile (14), a model was proposed that S. Typhimurium uses bile concentration as a means to sense proximity to the intestinal epithelium; in the lumen, where bile concentration is highest, SPI-1 expression would be repressed, and as the bacteria get closer to the intestinal cells, bile concentration would decrease, leading to SPI-1 expression and invasion (14). Within the context of this model, however, S. Typhi would be less invasive when in close contact with the intestinal epithelium, which is consistent with the limited intestinal inflammatory responses induced by S. Typhi (1). Moreover, S. Typhi has a unique site of infection, the gallbladder (7, 9). One of the mechanisms by which S. Typhi has been proposed to persist within the gallbladder is via direct invasion of gallbladder epithelial cells (53, 54); bile-induced increases in SPI-1 expression and invasiveness may therefore promote S. Typhi invasion and colonization of the gallbladder epithelium. Alternatively, as S. Typhi carriage is closely associated with the presence of gallstones, it is believed that S. Typhi forms biofilms on gallstone surfaces (7, 55). Biofilm formation on gallstones depends on several factors, including the presence of flagellar filaments (56); thus, increased flagellar expression may therefore also promote biofilm formation. As such, increases in expression of SPI-1- and motility-associated genes in bile may promote S. Typhi colonization of the gallbladder and therefore reflect adaptation to this environment.
In terms of understanding how S. Typhi and S. Typhimurium differ with regard to SPI-1 expression in bile, our results, in combination with previous findings (23), demonstrate that HilD is differentially regulated by bile at the level of protein stability (consistent with the idea that HilD is controlled largely at the posttranscriptional level [40]), resulting in significant differences in the expression of downstream genes, including the SPI-1 master regulator, hilA (Fig. 7). The factor(s) responsible for mediating changes in HilD stability in response to bile remains to be established; however, this response does not appear to rely on Lon (23) or Pat (this study). A recent transposon screen that aimed to identify factors responsible for bile-mediated SPI-1 repression in S. Typhimurium failed to identify any regulatory factor other than HilD (23). There are several reasons why such an approach may have failed, including the involvement of essential genes or redundancy. Unfortunately, attempts to further identify regulatory mechanisms in S. Typhi are confounded by the limited characterization of SPI-1-regulatory processes within S. Typhi. The overall effects of bile on differences in invasiveness between S. Typhi and S. Typhimurium may also not be entirely regulatory; for example, the translocon protein SipD has been reported to interact with bile salts (57), but SipD is one of several T3SS-associated proteins reported to be “differentially evolved” (as determined by nonsynonymous amino acid changes) between typhoidal and nontyphoidal serovars, which results in functional differences (58). Importantly, in Shigella flexneri, interaction of deoxycholate or other bile salts with the SipD homologue, IpaD, promotes the recruitment of the translocator protein, IpaB, “readying” the T3SS for secretion (59, 60).
FIG 7.
Proposed model of how bile influences SPI-1 expression in S. Typhi. (A) HilD is at the top of the SPI-1-regulatory hierarchy, where it regulates its own expression and the expression of HilA. HilD also regulates expression of the additional regulators HilC and RtsA, which also control HilA expression. (B) In the absence of bile, the turnover of HilD is high and the expression of hilD is at a basal level, and as a result the expression of hilA is low. (C) In the presence of bile, HilD is more stable, leading to enhanced expression of hilD, hilA, and thus SPI-1.
Our results also demonstrate that strains belonging to the S. Typhi H58 lineage (129-0238 and ERL12148) display significantly increased responses to bile compared to S. Typhi reference strains (Ty2 and CT18). When considering chronic carriage, such responses may be advantageous by increasing the potential of H58 strains to colonize the gallbladder, increasing bacterial burden, and subsequently increasing transmission. However, it is currently unknown if this reflects differences between recently isolated clinical strains and more-laboratory-adapted reference strains or is instead due to intrinsic difference in H58 strains compared to other S. Typhi haplotypes. H58 isolates have 44 nonsynonymous single nucleotide polymorphisms (SNPs) that are not found within the S. Typhi reference strain CT18 (21), including several SNPs within the Csr system (sirA [L63F], csrB [155G>A], csrD [A620V]), which is a known regulator of SPI-1 (61). Interestingly, significant phenotypic differences in bile were also observed between the two H58 strains investigated. Further comparisons of H58 strains would be required to determine if the phenotypic differences observed are sublineage specific or simply reflect diversity within the H58 group.
In conclusion, our results confirm that bile is a key regulator of gene expression in Salmonella, influencing the expression of almost 10% of the genome, including genes associated with virulence, motility, and metabolism. These findings add to the characterization of S. Typhi responses to bile (30, 62), which may ultimately help explain the mechanisms by which S. Typhi induces chronic carriage (13).
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmid construction.
The strains and plasmids used in this study are listed in Table 4. Salmonella cells were routinely grown in LB Lennox (Sigma-Aldrich) at 37°C/200 rpm. Ox bile (3%, wt/vol; Sigma-Aldrich/Merck-Millipore) was supplemented as indicated.
TABLE 4.
Strains and plasmids used in this study
| Strain or plasmid (identifier) | Relevant genotype or comments | Source and/or reference |
|---|---|---|
| Strains | ||
| S. Typhimurium | ||
| 14028 (ICC797) | WT | 64 |
| 14028 (ICC1765) | ΔhilA::lacZ Kanr | This study |
| 14028 (ICC1764) | ΔhilD::lacZ Kanr | This study |
| S. Typhi | ||
| Ty2 (ICC1500) | WT | G. Dougan |
| Ty2 (ICC1630) | ΔhilA::lacZ Kanr | This study |
| Ty2 (ICC1762) | ΔhilD::lacZ Kanr | This study |
| Ty2 (ICC1556) | ΔinvA Kanr | 64 |
| Ty2 (ICC1756) | Δpat Kanr | This study |
| CT18 (ICC1502) | WT | G. Dougan |
| 129-0238 (ICC1503) | WT, H58 isolate | G. Dougan (21) |
| ERL12148 (ICC1504) | WT, H58 isolate | G. Dougan (21) |
| Plasmids | ||
| pKD4 (pICC893) | Kanamycin cassette template plasmid | 63 |
| p3138 (pICC2515) | LacZ and kanamycin cassette template plasmid | 41 |
| pKD46 (pICC1298) | Lambda red recombinase plasmid | 63 |
| pWSK29-Spec E.V. (pICC2489) | Empty vector, spectinomycinr | 64 |
| pWSK29-Spec HilD-4HA Ty2 | S. Typhi Ty2 HilD-4HA, constitutive promoter | This study |
| pWSK29-Spec HilD 4HA Tm | S. Typhimurium 14028 HilD-4HA, constitutive promoter | This study |
All oligonucleotides used in this study are listed in Table S1 in the supplemental material. The S. Typhi Ty2 ΔinvA and Δpat deletion strains were constructed via lambda red, as previously described (63, 64). Strains with chromosomal integration of the lacZ gene were also constructed via lambda red recombination as described previously (41). Correct integration of introduced cassettes was validated by PCR.
To create HA-tagged HilD, pWSK29-Spec-4HA (64) was amplified with a reverse primer containing a PacI digestion site, and HilD was amplified from both S. Typhimurium and S. Typhi with primers containing NotI and PacI restriction sites. Both products were digested, and HilD was cloned into the existing NotI site and the introduced PacI site of pWSK29-Spec-4HA, resulting in constitutively expressed C-terminally tagged HilD-4HA. Plasmid construction was validated by sequencing.
Cell culture and HeLa invasion assays.
HeLa cells (ATCC) were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich) in 5% CO2 at 37°C. The cells were authenticated via short tandem repeat profiling in February 2016 (Microsynth).
Invasiveness of strains was determined by gentamicin protection assays, as previously described (64). Briefly, Salmonella strains were cultured overnight at 37°C/200 rpm in LB or LB supplemented with 3% bile before subculturing 1:33 in LB or LB–3% bile until late exponential phase (optical density at 600 nm [OD600], ∼1.8), when SPI-1 expression is induced (18) (data not shown). To prevent bile-mediated cell lysis, bacteria were washed twice in LB before addition to cells at a multiplicity of infection (MOI) of 100:1. As S. Typhi is less invasive than S. Typhimurium (65), S. Typhi infections were performed for 1 h and S. Typhimurium infections for 15 min, prior to the addition of gentamicin, unless otherwise indicated. At indicated time points, cells were lysed, serially diluted, and plated to enumerate intracellular CFU.
RNA extraction.
Salmonella was cultured overnight in LB or LB supplemented with 3% bile (wt/vol) before subculturing 1:33 until late exponential phase (OD600, ∼1.8). Bacteria (6 × 108) were incubated in RNAprotect (Qiagen) at room temperature (RT) for 5 min. Bacteria were digested with lysozyme (15 mg/ml) and proteinase K for 20 min at RT, and RNA was extracted using the RNeasy minikit (Qiagen) as per the manufacturer's instructions. RNA extractions for transcriptome sequencing (RNA-Seq) were performed in duplicate, and then the RNAs samples were pooled over three biological repeats. RNA extractions for quantitative reverse transcription-PCR (RT-qPCR) were performed in triplicate over three biological repeats. RNA samples for RNA-Seq and RT-qPCR were extracted independently of each other.
RNA sequencing and data analysis.
For RNA sequencing, mRNA libraries were multiplexed and prepared by utilization of the Illumina TruSeq protocol followed by sequencing via paired-end methodology on the Illumina HiSeq version 4 platform. Each lane of Illumina sequence was assessed for quality on the basis of adapter contamination, average base read quality, and any unusual G-C bias using FastQC. The median Phred score for all samples was >34. To permit comparison between strains, sequenced reads for each strain were mapped to the Ty2 genome (NC_004631) using the Rockhopper tool (66) with default parameters (see Data Sets S1 to S3 in the supplemental material). The read alignment coverage for each sample can be found in Table S2 in the supplemental material. The threshold for differentially expressed genes was gated as those displaying >2-fold change in expression in 3% bile compared to LB alone and with an adjusted P value (q value) of <0.05.
GO term enrichment for differentially regulated genes was performed with Panther (67) using the S. Typhimurium GO annotation, while KEGG pathway analysis was performed with the GAGE R package (R 3.3.1) (68), using the S. Typhi (stt) KEGG annotation. The VennDiagram (69) and gplots R packages were used for data visualization.
RT-qPCR.
For RT-qPCR, 2 μg of RNA was treated with DNase (Promega) prior to reverse transcription with Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega) according to the manufacturer's recommendations. The Fast SYBR green master mix (Applied Biosystems) was used for qPCRs alongside the Applied Biosystems StepOnePlus system. Twenty nanograms of cDNA was used per reaction mixture, and forward and reverse primers (Table S1) were used at a final concentration of 0.2 μM. Samples without reverse transcription were included as negative controls. The housekeeping gene ftsZ was used as the reference gene, as it was determined to be the least-variable gene between strains and between culture conditions of LB with and without 3% bile. qPCRs were performed in duplicate on triplicate samples over three biological replicates.
SPI-1 protein expression and stability assays.
To determine expression of SPI-1 proteins, Salmonella was subcultured in the absence or presence of 3% ox bile to late exponential phase. One milliliter of culture was pelleted and resuspended in 2× SDS loading buffer (1 M Tris [pH 6.8], 2% SDS, 20% glycerol, 5% β-mercaptoethanol, bromophenol blue) in proportion to OD600. To determine HilD stability, Salmonella strains previously transformed with 4HA-tagged constructs were subcultured in 10 ml LB with or without the addition of 3% ox bile until late exponential phase. The OD600 was recorded, and chloramphenicol (30 μg/ml) was added to inhibit protein synthesis. Bacteria (1 ml) were pelleted and resuspended in 2× SDS loading buffer in proportion to OD600. The cultures were incubated at 37°C and 200 rpm, and 1-ml samples were taken at required time points. Samples were heated at 95°C for 10 min. Whole-cell samples were subjected to Western blotting, using an anti-HA antibody to detect the protein of interest and DnaK as a loading control. Following imaging, band density was quantified using ImageJ, and half-life (in minutes) was calculated using the equation [t × ln(2)]/[ln(N0/Nf)], where t is the time elapsed between measurements (in minutes), N0 is the initial amount, and Nf is the final amount (23). To determine changes in SPI-1 proteins in bile, band density was quantified using ImageJ, levels of SPI-1 proteins were normalized to the corresponding DnaK value, and fold changes in bile relative to LB were calculated.
SDS-PAGE and Western blotting.
Proteins were separated on 12% acrylamide gels followed by semidry transfer onto polyvinylidene difluoride (PVDF) membranes (GE Healthcare). Membranes were blocked in 5% milk in phosphate-buffered saline (PBS)–0.05% Tween 20 (Sigma-Aldrich) and probed with either anti-DnaK 8E2/2 (1:10,000; Enzo Life Sciences catalog number ADI-SPA-880), anti-HA HA-7 (1:1,000; Sigma catalog number H3663), anti-SipC, anti-SipD, anti-SopB, or anti-SopE (1:5,000; V. Koronakis, University of Cambridge) primary antibodies, followed by horseradish peroxidase (HRP)-conjugated secondary antibody (1:10,000; Jackson ImmunoResearch). Chemiluminescence following the addition of EZ-ECL reagent (Geneflow) was detected using the LAS-3000 imager (Fuji).
β-Galactosidase assays.
β-Galactosidase assays were performed as previously described (70). Salmonella strains were grown under SPI-1-inducing conditions with or without the addition of 3% ox bile. The OD600 was recorded, and 1 ml of culture was pelleted and resuspended in 1 ml Z buffer (0.06 M Na2HPO4, 0.04 M NaH2PO4, 0.01 M KCl, 0.001 M MgSO4, and 0.05 M β-mercaptoethanol, pH 7). Wild-type (WT) strains were used as negative controls. Samples were permeabilized with the addition of 0.1% SDS and chloroform and vortexed for 2 min. Twenty microliters of prepared sample was added to 180 μl Z buffer in a 96-well microplate, and 2-nitrophenyl β-d-galactopyranoside (ONPG) substrate (4 mg/ml in Z buffer) was added. Plates were incubated at RT, and then the reaction was stopped with the addition of 1 M Na2CO3. The absorbance of the samples was measured at 405 nm and 540 nm using a FLUOStar Omega plate reader (BMG Labtech).
Statistical analysis.
Statistical tests were performed using GraphPad Prism (version 7.00) for Windows (GraphPad Software, San Diego, CA, USA). All data are expressed as means ± standard deviations (SD). Significance (P < 0.05) was determined by unpaired t test or analysis of variance (ANOVA), with correction for multiple comparisons when required.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Gordon Dougan (Sanger Institute) for providing the S. Typhi strains used in this study, to Michael Hensel for providing the p3138 template plasmid for construction of reporter strains via lambda red, and to Vassilis Koronakis (University of Cambridge) for providing the anti-SipC, anti-SipD, anti-SopB, and anti-SopE antibodies.
R.J. is supported by MRC Centre for Molecular Bacteriology and Infection grant MR/J006874/1. M.R. is funded by the Biotechnology and Biological Sciences Research Council (grant number BB/J014567/1). G.F. is supported by a Wellcome Trust Investigator grant.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00490-17.
REFERENCES
- 1.Dougan G, Baker S. 2014. Salmonella enterica serovar Typhi and the pathogenesis of typhoid fever. Annu Rev Microbiol 68:317–336. doi: 10.1146/annurev-micro-091313-103739. [DOI] [PubMed] [Google Scholar]
- 2.McGhie EJ, Brawn LC, Hume PJ, Humphreys D, Koronakis V. 2009. Salmonella takes control: effector-driven manipulation of the host. Curr Opin Microbiol 12:117–124. doi: 10.1016/j.mib.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fàbrega A, Vila J. 2013. Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev 26:308–341. doi: 10.1128/CMR.00066-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altier C. 2005. Genetic and environmental control of Salmonella invasion. J Microbiol 43:85–92. [PubMed] [Google Scholar]
- 5.Parry C, Dougan G. 2002. Typhoid fever. N Engl J Med 347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 6.Crump JA, Luby SP, Mintz ED. 2004. The global burden of typhoid fever. Bull World Health Organ 82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 7.Gunn JS, Marshall JM, Baker S, Dongol S, Charles RC, Ryan ET. 2014. Salmonella chronic carriage: epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol 22:648–655. doi: 10.1016/j.tim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gal-Mor O, Boyle EC, Grassl GA. 2014. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol 5:391. doi: 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Escobedo G, Marshall JM, Gunn JS. 2011. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat Rev Microbiol 9:9–14. doi: 10.1038/nrmicro2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Begley M, Gahan C, Hill C. 2005. The interaction between bacteria and bile. FEMS Microbiol Rev 29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Gunn JS. 2000. Mechanisms of bacterial resistance and response to bile. Microbes Infect 2:907–913. doi: 10.1016/S1286-4579(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 12.van Velkinburgh JC, Gunn JS. 1999. PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect Immun 67:1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sistrunk JR, Nickerson KP, Chanin RB, Rasko DA, Faherty CS. 2016. Survival of the fittest: how bacterial pathogens utilize bile to enhance infection. Clin Microbiol Rev 29:819–836. doi: 10.1128/CMR.00031-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prouty AM, Gunn JS. 2000. Salmonella enterica serovar Typhimurium invasion is repressed in the presence of bile. Infect Immun 68:6763–6769. doi: 10.1128/IAI.68.12.6763-6769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prouty AM, Brodsky IE, Manos J, Belas R, Falkow S, Gunn JS. 2004. Transcriptional regulation of Salmonella enterica serovar Typhimurium genes by bile. FEMS Immunol Med Microbiol 41:177–185. doi: 10.1016/j.femsim.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Antunes LCM, Wang M, Andersen SK, Ferreira RBR, Kappelhoff R, Han J, Borchers CH, Finlay BB. 2012. Repression of Salmonella enterica phoP expression by small molecules from physiological bile. J Bacteriol 194:2286–2296. doi: 10.1128/JB.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández SB, Cota I, Ducret A, Aussel L, Casadesús J. 2012. Adaptation and preadaptation of Salmonella enterica to bile. PLoS Genet 8:e1002459. doi: 10.1371/journal.pgen.1002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kröger C, Colgan A, Srikumar S, Händler K, Sivasankaran SK, Hammarlöf DL, Canals R, Grissom JE, Conway T, Hokamp K, Hinton JCD. 2013. An infection-relevant transcriptomic compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe 14:683–695. doi: 10.1016/j.chom.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Sengupta C, Ray S, Chowdhury R. 2014. Fine tuning of virulence regulatory pathways in enteric bacteria in response to varying bile and oxygen concentrations in the gastrointestinal tract. Gut Pathog 6:38. doi: 10.1186/s13099-014-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabbagh SC, Forest CG, Lepage C, Leclerc J-M, Daigle F. 2010. So similar, yet so different: uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol Lett 305:1–13. doi: 10.1111/j.1574-6968.2010.01904.x. [DOI] [PubMed] [Google Scholar]
- 21.Wong V, Baker S, Pickard D, Parkhill J, Page A, Feasey N, Kingsley R, Thomson N, Keane J, Weill F-X, Edwards D, Hawkey J, Harris SR, Mather AE, Cain AK, Hadfield J, Hart PJ, Thieu NTV, Klemm EJ, Glinos DA, Breiman RF, Watson CH, Kariuki S, Gordon MA, Heyderman RS, Okoro C, Jacobs J, Lunguya O, Edmunds WJ, Msefula C, Chabalgoity JA, Kama M, Jenkins K, Dutta S, Marks F, Campos J, Thompson C, Obaro S, MacLennan CA, Dolecek C, Keddy KH, Smith AM, Parry CM, Karkey A, Mulholland EK, Campbell JI, Dongol S, Basnyat B, Dufour M, Bandaranayake D, Naseri TT, Singh SP, Hatta M, Newton P, Onsare RS, Isaia L, Dance D, Davong V, Thwaites G, Wijedoru L, Crump JA, De Pinna E, Nair S, Nilles EJ, Thanh DP, Turner P, Soeng S, Valcanis M, Powling J, Dimovski K, Hogg G, Farrar J, Holt KE, Dougan G. 2015. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet 47:632–639. doi: 10.1038/ng.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong V, Baker S, Connor T, Pickard D, Page A, Dave J, Murphy N, Holliman R, Sefton A, Millar M, Dyson ZA, Dougan G, Holt K, International Typhoid Consortium. 2016. An extended genotyping framework for Salmonella enterica serovar Typhi, the cause of human typhoid. Nat Commun 7:12827. doi: 10.1038/ncomms12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eade CR, Hung C-C, Bullard B, Gonzalez-Escobedo G, Gunn JS, Altier C. 2016. Bile acids function synergistically to repress invasion gene expression in Salmonella by destabilizing the invasion regulator HilD. Infect Immun 84:2198–2208. doi: 10.1128/IAI.00177-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bishop RE. 2005. The lipid A palmitoyltransferase PagP: molecular mechanisms and role in bacterial pathogenesis. Mol Microbiol 57:900–912. doi: 10.1111/j.1365-2958.2005.04711.x. [DOI] [PubMed] [Google Scholar]
- 25.Perkins TT, Davies MR, Klemm EJ, Rowley G, Wileman T, James K, Keane T, Maskell D, Hinton JCD, Dougan G, Kingsley RA. 2013. ChIP-seq and transcriptome analysis of the OmpR regulon of Salmonella enterica serovars Typhi and Typhimurium reveals accessory genes implicated in host colonization. Mol Microbiol 87:526–538. doi: 10.1111/mmi.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawhon SD, Maurer R, Suyemoto M, Altier C. 2002. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol Microbiol 46:1451–1464. doi: 10.1046/j.1365-2958.2002.03268.x. [DOI] [PubMed] [Google Scholar]
- 27.Antunes LCM, Andersen SK, Menendez A, Arena ET, Han J, Ferreira RBR, Borchers CH, Finlay BB. 2011. Metabolomics reveals phospholipids as important nutrient sources during Salmonella growth in bile in vitro and in vivo. J Bacteriol 193:4719–4725. doi: 10.1128/JB.05132-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denkel LA, Horst SA, Rouf SF, Kitowski V, Böhm OM, Rhen M, Jäger T, Bange F-C. 2011. Methionine sulfoxide reductases are essential for virulence of Salmonella Typhimurium. PLoS One 6:e26974. doi: 10.1371/journal.pone.0026974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibanez-Ruiz M, Robbe-Saule V, Hermant D, Labrude S, Norel F. 2000. Identification of RpoS (sigma(S))-regulated genes in Salmonella enterica serovar typhimurium. J Bacteriol 182:5749–5756. doi: 10.1128/JB.182.20.5749-5756.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walawalkar YD, Vaidya Y, Nayak V. 2016. Response of Salmonella Typhi to bile generated oxidative stress: implication of quorum sensing and persister cell populations. Pathog Dis 74(8):ftw090. doi: 10.1093/femspd/ftw090. [DOI] [PubMed] [Google Scholar]
- 31.Robbe-Saule V, Coynault C, Norel F. 1995. The live oral typhoid vaccine Ty21a is a rpoS mutant and is susceptible to various environmental stresses. FEMS Microbiol Lett 126:171–176. doi: 10.1111/j.1574-6968.1995.tb07412.x. [DOI] [PubMed] [Google Scholar]
- 32.Kawasaki K, Teramoto M, Tatsui R, Amamoto S. 2012. Lipid A 3′-O-deacylation by Salmonella outer membrane enzyme LpxR modulates the ability of lipid A to stimulate Toll-like receptor 4. Biochem Biophys Res Commun. [DOI] [PubMed] [Google Scholar]
- 33.Kawano M, Manabe T, Kawasaki K. 2010. Salmonella enterica serovar Typhimurium lipopolysaccharide deacylation enhances its intracellular growth within macrophages. FEBS Lett 584:207–212. doi: 10.1016/j.febslet.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 34.García-Calderón CB, Casadesús J, Ramos-Morales F. 2007. Rcs and PhoPQ regulatory overlap in the control of Salmonella enterica virulence. J Bacteriol 189:6635–6644. doi: 10.1128/JB.00640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lei L, Wang W, Xia C, Liu F. 2016. Salmonella virulence factor SsrAB regulated factor modulates inflammatory responses by enhancing the activation of NF-κB signaling pathway. J Immunol 196:792–802. doi: 10.4049/jimmunol.1500679. [DOI] [PubMed] [Google Scholar]
- 36.Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, Wain J, Churcher C, Mungall KL, Bentley SD, Holden MT, Sebaihia M, Baker S, Basham D, Brooks K, Chillingworth T, Connerton P, Cronin A, Davis P, Davies RM, Dowd L, White N, Farrar J, Feltwell T, Hamlin N, Haque A, Hien TT, Holroyd S, Jagels K, Krogh A, Larsen TS, Leather S, Moule S, O'Gaora P, Parry C, Quail M, Rutherford K, Simmonds M, Skelton J, Stevens K, Whitehead S, Barrell BG. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848–852. doi: 10.1038/35101607. [DOI] [PubMed] [Google Scholar]
- 37.Deng W, Liou S-R, Plunkett G, Mayhew GF, Rose DJ, Burland V, Kodoyianni V, Schwartz DC, Blattner FR. 2003. Comparative genomics of Salmonella enterica serovar Typhi strains Ty2 and CT18. J Bacteriol 185:2330–2337. doi: 10.1128/JB.185.7.2330-2337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobe T, Beatson SA, Taniguchi H, Abe H, Bailey CM, Fivian A, Younis R, Matthews S, Marches O, Frankel G, Hayashi T, Pallen MJ. 2006. An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc Natl Acad Sci U S A 103:14941–14946. doi: 10.1073/pnas.0604891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hannemann S, Galán JE, van den Beek M, Blankenberg D, Bouvier D, Čech M. 2017. Salmonella enterica serovar-specific transcriptional reprogramming of infected cells. PLoS Pathog 13:e1006532. doi: 10.1371/journal.ppat.1006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellermeier JR, Slauch JM. 2007. Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol 10:24–29. doi: 10.1016/j.mib.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Gerlach RG, Hölzer SU, Jäckel D, Hensel M. 2007. Rapid engineering of bacterial reporter gene fusions by using Red recombination. Appl Environ Microbiol 73:4234–4242. doi: 10.1128/AEM.00509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellermeier CD, Ellermeier JR, Slauch JM. 2005. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol 57:691–705. doi: 10.1111/j.1365-2958.2005.04737.x. [DOI] [PubMed] [Google Scholar]
- 43.Gaviria-Cantin T, El Mouali Y, Le Guyon S, Römling U, Balsalobre C, Rüssmann H. 2017. Gre factors-mediated control of hilD transcription is essential for the invasion of epithelial cells by Salmonella enterica serovar Typhimurium. PLoS Pathog 13:e1006312. doi: 10.1371/journal.ppat.1006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.López-Garrido J, Puerta-Fernández E, Casadesús J. 2014. A eukaryotic-like 3′ untranslated region in Salmonella enterica hilD mRNA. Nucleic Acids Res 42:5894–5906. doi: 10.1093/nar/gku222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takaya A, Kubota Y, Isogai E, Yamamoto T. 2005. Degradation of the HilC and HilD regulator proteins by ATP-dependent Lon protease leads to downregulation of Salmonella pathogenicity island 1 gene expression. Mol Microbiol 55:839–852. doi: 10.1111/j.1365-2958.2004.04425.x. [DOI] [PubMed] [Google Scholar]
- 46.Sang Y, Ren J, Qin R, Liu S, Cui Z, Cheng S, Liu X, Lu J, Tao J, Yao Y-F. 2017. Acetylation regulating protein stability and DNA-binding ability of HilD, thus modulating Salmonella Typhimurium virulence. J Infect Dis 216:1018–1026. doi: 10.1093/infdis/jix102. [DOI] [PubMed] [Google Scholar]
- 47.Sang Y, Ren J, Ni J, Tao J, Lu J, Yao Y-F. 2016. Protein acetylation is involved in Salmonella enterica Typhimurium virulence. J Infect Dis 213:1836–1845. doi: 10.1093/infdis/jiw028. [DOI] [PubMed] [Google Scholar]
- 48.Li P, Rivera-Cancel G, Kinch LN, Salomon D, Tomchick DR, Grishin NV, Orth K, Fenical W, Zhu J, Ochi S, Sasahara T, Hayashi S, Hirai Y, Sakurai J, Shinagawa H, Hattori M, Iida T. 2016. Bile salt receptor complex activates a pathogenic type III secretion system. Elife 5:e15718. doi: 10.7554/eLife.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta S, Chowdhury R. 1997. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect Immun 65:1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alam A, Tam V, Hamilton E, Dziejman M. 2010. vttRA and vttRB encode ToxR family proteins that mediate bile-induced expression of type three secretion system genes in a non-O1/non-O139 Vibrio cholerae strain. Infect Immun 78:2554–2570. doi: 10.1128/IAI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pope LM, Reed KE, Payne SM. 1995. Increased protein secretion and adherence to HeLa cells by Shigella spp. following growth in the presence of bile salts. Infect Immun 63:3642–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nickerson KP, Chanin RB, Sistrunk JR, Rasko DA, Fink PJ, Barry EM, Nataro JP, Faherty CS. 2017. Analysis of Shigella flexneri resistance, biofilm formation, and transcriptional profile in response to bile salts. Infect Immun 85:e01067-16. doi: 10.1128/IAI.01067-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Escobedo G, Gunn JS. 2013. Gallbladder epithelium as a niche for chronic Salmonella carriage. Infect Immun 81:2920–2930. doi: 10.1128/IAI.00258-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menendez A, Arena ET, Guttman JA, Thorson L, Vallance BA, Vogl W, Finlay BB. 2009. Salmonella infection of gallbladder epithelial cells drives local inflammation and injury in a model of acute typhoid fever. J Infect Dis 200:1703–1713. doi: 10.1086/646608. [DOI] [PubMed] [Google Scholar]
- 55.Crawford RW, Rosales-Reyes R, Ramírez-Aguilar Mde L, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS. 2010. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci U S A 107:4353–4358. doi: 10.1073/pnas.1000862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prouty AM, Gunn JS. 2003. Comparative analysis of Salmonella enterica serovar Typhimurium biofilm formation on gallstones and on glass. Infect Immun 71:7154–7158. doi: 10.1128/IAI.71.12.7154-7158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Nordhues BA, Zhong D, De Guzman RN. 2010. NMR characterization of the interaction of the Salmonella type iii secretion system protein SipD and bile salts. Biochemistry 49:4220–4226. doi: 10.1021/bi100335u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eswarappa SM, Janice J, Nagarajan AG, Balasundaram SV, Karnam G, Dixit NM, Chakravortty D. 2008. Differentially evolved genes of Salmonella pathogenicity islands: insights into the mechanism of host specificity in Salmonella. PLoS One 3:e3829. doi: 10.1371/journal.pone.0003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olive AJ, Kenjale R, Espina M, Moore DS, Picking WL, Picking WD. 2007. Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect Immun 75:2626–2629. doi: 10.1128/IAI.01599-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stensrud KF, Adam PR, La Mar CD, Olive AJ, Lushington GH, Sudharsan R, Shelton NL, Givens RS, Picking WL, Picking WD. 2008. Deoxycholate interacts with IpaD of Shigella flexneri in inducing the recruitment of IpaB to the type III secretion apparatus needle tip. J Biol Chem 283:18646–18654. doi: 10.1074/jbc.M802799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martínez LC, Yakhnin H, Camacho MI, Georgellis D, Babitzke P, Puente JL, Bustamante VH. 2011. Integration of a complex regulatory cascade involving the SirA/BarA and Csr global regulatory systems that controls expression of the Salmonella SPI-1 and SPI-2 virulence regulons through HilD. Mol Microbiol 80:1637–1656. doi: 10.1111/j.1365-2958.2011.07674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Langridge GC, Phan M-D, Turner DJ, Perkins TT, Parts L, Haase J, Charles I, Maskell DJ, Peters SE, Dougan G, Wain J, Parkhill J, Turner AK. 2009. Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res 19:2308–2316. doi: 10.1101/gr.097097.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson R, Byrne A, Berger CN, Klemm E, Crepin VF, Dougan G, Frankel G. 2017. The type III secretion system effector SptP of Salmonella enterica serovar Typhi. J Bacteriol 199:e00647-16. doi: 10.1128/JB.00647-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bishop A, House D, Perkins T, Baker S, Kingsley RA, Dougan G. 2008. Interaction of Salmonella enterica serovar Typhi with cultured epithelial cells: roles of surface structures in adhesion and invasion. Microbiology 154:1914–1926. doi: 10.1099/mic.0.2008/016998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McClure R, Balasubramanian D, Sun Y, Bobrovskyy M, Sumby P, Genco CA, Vanderpool CK, Tjaden B. 2013. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res 41:e140. doi: 10.1093/nar/gkt444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD. 2017. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res 45:D183–D189. doi: 10.1093/nar/gkw1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo W, Friedman MS, Shedden K, Hankenson KD, Woolf PJ. 2009. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics 10:161. doi: 10.1186/1471-2105-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen H, Boutros PC. 2011. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics 12:35. doi: 10.1186/1471-2105-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, New York, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.