ABSTRACT
Staphylococcus aureus is a pathogen that causes significant morbidity and mortality. Nasal carriage is a major source of transmission and of endogenous infection. Persistent carriage is detected in ∼30% of healthy individuals. While Th17 cells have been shown to play a role in S. aureus infection and clearance, the immune response to carriage is not well understood. Here, we evaluate the Th17 response and its potential inhibitors during S. aureus carriage. We recruited 25 volunteers, of whom 11 were persistent carriers. Volunteers' peripheral blood mononuclear cells (PBMCs) were stimulated with either their endogenous strain (a strain isolated from that carrier) or exogenous ones (strains not carried by that volunteer). Changes in Th17 cell frequency and numbers, interleukin-17 (IL-17) mRNA expression, and IL-17 protein abundance were measured by fluorescence-activated cell sorting, real-time PCR, and enzyme-linked immunosorbent assay. Similarly, responses of IL-17 suppressors (regulatory T cells [FOXP3], IL-10, IL-27, and IL-19) were measured. Th17 and IL-17 levels in response to stimulation with endogenous strains were significantly lower than those in response to stimulation with exogenous ones. Of the suppressive cytokines tested, only IL-19 exhibited a stronger response to endogenous than to exogenous strains. Addition of recombinant IL-19 to exogenous-strain-stimulated PBMCs caused decreased IL-17 expression, whereas addition of IL-19 antibodies to endogenous-strain-stimulated cells resulted in an increased IL-17 response. Together, our results suggest that S. aureus carriage induced a tolerogenic response to a carried strain that could be reproduced through the addition of recombinant IL-19 or prevented by the addition of IL-19 antibodies. This differential immune response may play a role in the determination of S. aureus carriage patterns.
KEYWORDS: IL-17, IL-19, Staphylococcus aureus, carriage
INTRODUCTION
Staphylococcus aureus is a common pathogen that causes severe morbidity and high mortality rates worldwide (1, 2). It is responsible for various infections, ranging from minor to major skin and soft tissue infections to life-threatening infections such as pneumonia, toxic shock syndrome, sepsis, and endocarditis. Yet, it is a ubiquitous commensal that persistently colonizes ∼30% of healthy human individuals, while others never carry it (3). S. aureus carriers are more likely to become infected than noncarriers, but they also have lower mortality rates from S. aureus infection than noncarriers do (4, 5). Persistent carriers typically harbor a single S. aureus strain for many years, while others may carry different strains for only short durations (3, 6, 7).
Studies on factors affecting S. aureus carriage are discordant. Some suggest that bacterial factors such as staphylococcal protein A may increase the likelihood of a strain being carried (8, 9), while others suggest that host factors are largely responsible for the unique interactions (10, 11). However, while much research has revealed factors that affect an individual's susceptibility to S. aureus carriage (12–14), the interaction between the host and the individually carried strain has not yet been studied.
The interaction between the host and bacterial cell is mediated by both innate and adaptive immune responses. The adaptive immune response is generally two pronged, utilizing both T cells and B cells for the detection and clearance of pathogens (15). Clearance of S. aureus carriage has been shown to be mainly T cell dependent (16, 17) and does not induce an effective antibody response (18). Recent work has determined the presence of S. aureus-specific memory T cell clones in peripheral blood that elicit a proinflammatory T cell response against S. aureus strains, specifically, T helper 17 (Th17) cells (19). Th17 cells produce interleukin-17 (IL-17), which is essential for protection against extracellular pathogens at mucosal surfaces (20–23), and they have previously been shown to play a major clinical role in protective immunity against S. aureus in both humans and mice by driving neutrophil influx. Indeed, patients with IL-17 deficiencies such as autosomal hyper-IgE syndrome suffer from recurrent S. aureus infections (24–26). Clearance of S. aureus carriage has also been shown to be IL-17 dependent in mice (16), but the link between IL-17 and human S. aureus carriage has not been extensively studied. Nurjadi et al. tested cytokine expression levels in persistent S. aureus carriers and noncarriers. They observed no difference in IL-17 mRNA expression between carriers and noncarriers but did observe a difference in gamma interferon (IFN-γ) expression between carriers and noncarriers and concluded that the ratio of IFN-γ to IL-17 could be used to predict carriage (27).
Regulation of these inflammatory immune responses is vital, not only during and following infection but also as a mechanism of tolerance toward commensal or carried bacteria. Regulatory T cells (Tregs) are largely considered to be the suppressive cells that play the most integral role in commensal tolerance (28), but commensal bacteria have also been shown to induce hyporesponsiveness in T cells in a Treg-independent manner (29).
Other cytokines that have been previously shown to suppress Th17 in a Treg-independent manner are IL-19 and IL-27. These cytokines are secreted by both macrophages and dendritic cells and have been shown to orchestrate multiple suppressive pathways. IL-19 belongs to the IL-10 family of cytokines. Although it was first reported in 2000, its function is still largely unclear (30, 31). In an S. aureus mouse infection model, IL-19, along with IL-20 and IL-24, was shown to downregulate IL-17, thereby decreasing the clearance of S. aureus (32).
The cellular arm of immunity, specifically, Th17 cells, clearly plays an important role in S. aureus infection. However, its role in healthy carriage remains relatively unstudied. Here, we aim to understand the immune mechanisms involved in S. aureus carriage and to determine whether this host-bacterial cross talk plays a role in an individual's carriage status.
RESULTS
Study population and S. aureus strains.
Of the 25 healthy individuals recruited, 11 were defined as persistent carriers and 1 was defined as a transient carrier. A strain that was carried by an individual was termed that individual's “endogenous strain,” whereas a strain not carried by the individual was termed an “exogenous strain.” To obtain a larger pool of exogenous strains, we also used strains from our precharacterized S. aureus strain collection of clinical and carried isolates (33, 34) (Table 1).
TABLE 1.
Strains used in this studya
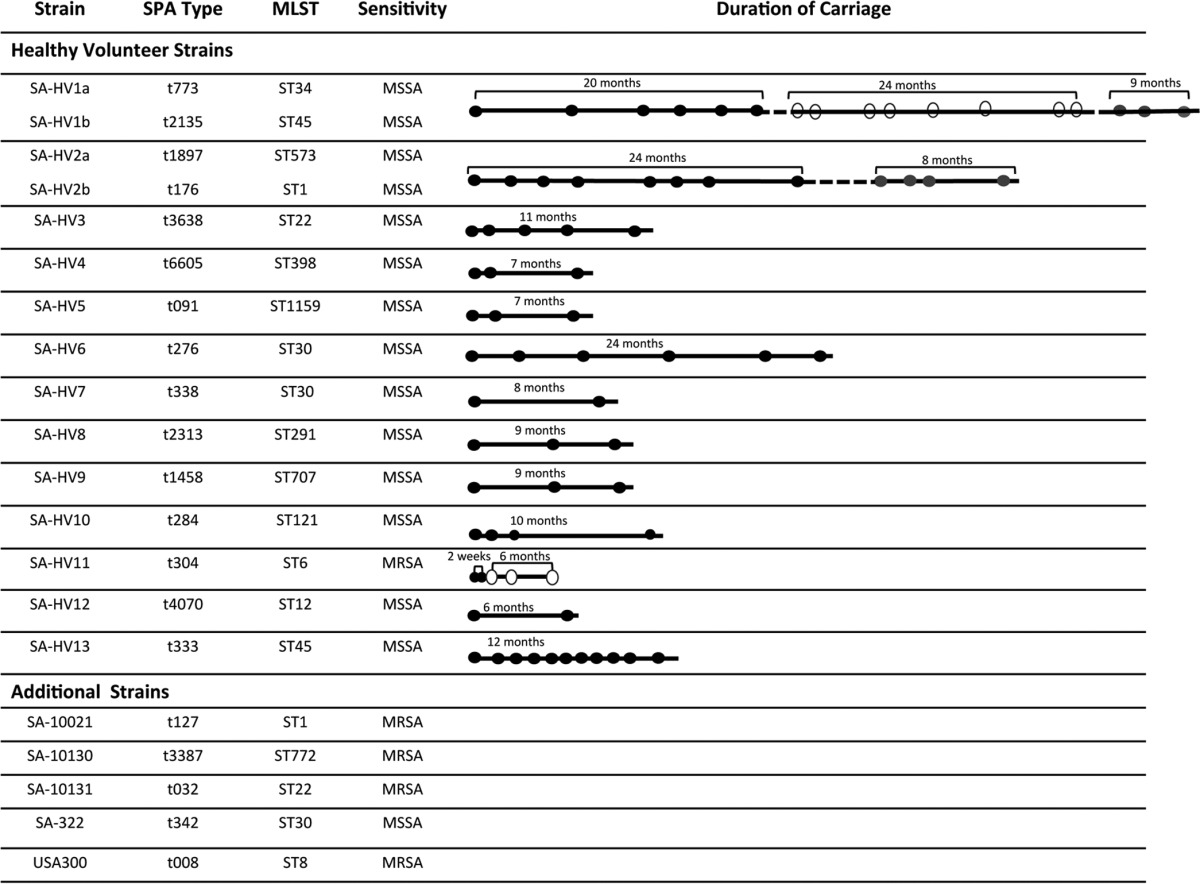
SA-HVX denotes the endogenous strain of carrier X. In cases where two strains were isolated from the same carrier, SA-HVXa and b denote the two strains. Circles denote screening results. A black circle denotes a positive result, a gray circle denotes a positive result with a second strain, and a white circle denotes a negative result. A broken line indicates a period of unknown carriage status.
Each of the carriers carried a genetically different strain with a different spa type. Two of the strains tested were of multilocus sequence type (MLST) 30, but each had a different spa type.
Two carriers who persistently carried a single strain for over a year replaced their initial endogenous strain with a genetically different strain. Healthy volunteer 1 persistently carried SA-HV1a for 20 months, followed by eight negative screening results over a 2.5-year period, and then acquired SA-HV1b. Healthy volunteer 2 replaced the initial strain, SA-HV2a, which was persistently carried for 24 months, with SA-HV2b.
Decreased IL-17, but not IFN-γ, response to endogenous strains.
To determine whether endogenous S. aureus strains stimulate host cells differently than exogenous strains, cultures of carrier peripheral blood mononuclear cells (PBMCs) were stimulated with either endogenous or exogenous whole-cell antigens (ethanol-killed strains). IL-17 and IFN-γ mRNA expression and protein secretion levels were measured.
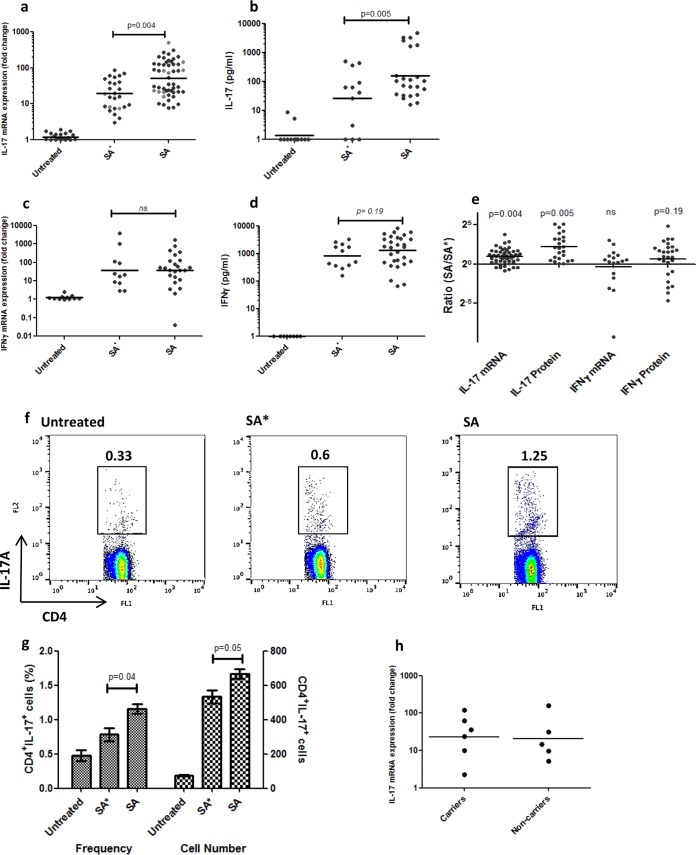
IL-17 and IFN-γ mRNA expression and protein secretion were both increased in response to stimulation with all of the strains tested compared to negative-control (untreated) samples (P < 0.01) (Fig. 1a to d). Stimulation of carrier PBMCs with that individual's endogenous strain resulted in significantly lower IL-17 mRNA expression after 48 h, compared to stimulation with all of the exogenous strains tested (P = 0.004) (Fig. 1a and e). IL-17 protein secretion after 5 days of stimulation was also decreased in response to an endogenous strain compared to stimulation with exogenous strains (P = 0.005) (Fig. 1b and e). To ensure that this effect was not due to intrinsic characteristics of carried strains but rather to a specific host response to an endogenous carried strain, we compared the responses to a specific strain by that strain's carrier to responses by other carriers to whom the strain was exogenous. This analysis was repeated for two separate strains. Indeed, IL-17 mRNA expression was significantly lower in PBMCs from that strain's carrier than in those from all other healthy volunteers (P = 0.03 and 0.04). The responses to a single strain are depicted in light gray in Fig. 1a.
FIG 1.
Th17 and IFN-γ responses to S. aureus. (a) IL-17 mRNA expression (n = 27) and (b) protein secretion (n = 12) levels in response to endogenous (SA*) strains were lower than those in response to exogenous (SA) strains (P = 0.004 and 0.005, respectively). Light gray dots indicate the IL-17mRNA responses to one strain by its carriers (left column) and the carriers of other strains (right column) (P = 0.03). No difference between endogenous and exogenous strains was seen in IFN-γ mRNA expression levels (n = 12; P = 0.93) (c) and protein secretion levels (n = 14; P = 0.2) (d). Each data point represents the cytokine response of one carrier to one strain in one experiment. A horizontal line indicates the geometric mean of all of the data points in a column. (e) Ratios of cytokine responses to exogenous versus endogenous strains. (f, g) The frequency (left y axis; P = 0.04) and number (right y axis; P = 0.05) of IL-17-producing CD4+ T cells were also decreased in response to endogenous compared to exogenous strains. (h) The IL-17 mRNA response to a strain was the same in carriers of a different strain and noncarriers (P = 0.6). When the P value is >0.2, ns indicates nonsignificance.
IFN-γ mRNA expression and protein secretion levels in response to endogenous and exogenous strains, on the other hand, were not different (Fig. 1c to e) (P = 0.93 and 0.19, respectively). Fluorescence-activated cell sorter (FACS) analysis revealed that the source of the IL-17 is CD4+ T cells, and there was a significant decrease in the frequency and amounts of IL-17A-producing CD4+ T cells in PBMC cultures stimulated with endogenous strains compared to those in cultures stimulated with exogenous ones (P = 0.04 and 0.05). (Fig. 1f and g). There was no difference between the responses of carriers and noncarriers to exogenous strains (P = 0.6) (Fig. 1h).
Potential suppressors of the IL-17 response to strains.
Following the observation of a decreased IL-17 response to the endogenous strain, we sought out potential inhibitors that could mediate this tolerant response.
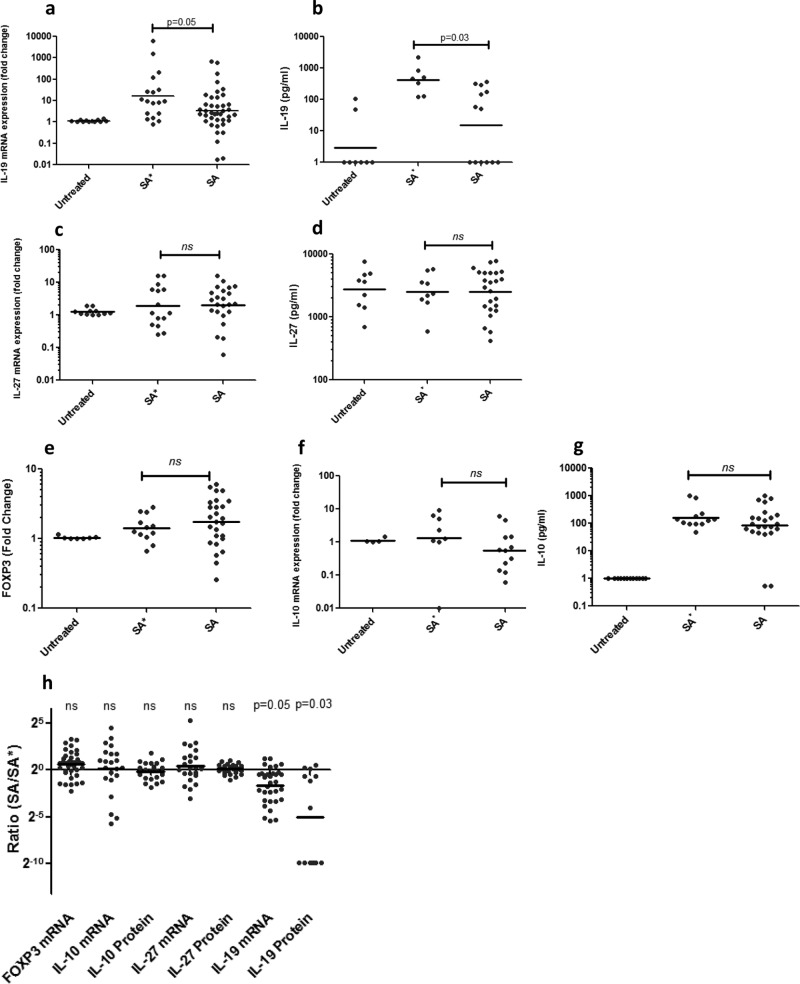
Levels of Treg-associated transcription factor FOXP3, as well as mRNA expression and protein secretion levels of suppressive cytokines IL-10, IL-27, and IL-19, were measured in PBMCs stimulated with endogenous or exogenous strains (Fig. 2). IL-19 was found to be the only cytokine tested whose expression and protein secretion levels were increased significantly in response to endogenous strains compared to exogenous strains (P = 0.05 and 0.03) (Fig. 2a, b, and h). Levels of FOXP3 (P = 0.51), IL-10 (P = 0.29; 0.95), and IL-27 (P = 0.42; 0.43) in response to stimulation with endogenous and exogenous strains were not significantly different (Fig. 2c to h).
FIG 2.
Suppressive cytokine responses to endogenous and exogenous S. aureus strains. IL-19 mRNA expression (a) and protein secretion (b) in response to endogenous strains (SA*) were increased compared to those in response to exogenous ones (SA) (n = 18 and 7; P = 0.05 and 0.03). No difference between expression levels and protein secretion levels of IL-27 (c, d) (n = 15,9; P = 0.42; 0.43) or Treg-associated factors FOXP3 (e) (n = 12; P = 0.51) and IL-10 (f, g) (n = 8,12; P = 0.29, 0.95) was seen. In each experiment, one endogenous strain was tested and compared to at least two exogenous strains. Each data point represents the FOXP3 or cytokine response of one carrier to one strain in one experiment. Lines indicate the geometric means of all of the data points in a column. (h) Ratios of cytokine responses to exogenous versus endogenous strains. When the P value is >0.2, ns indicates nonsignificance.
IL-19 alone can inhibit IL-17 expression.
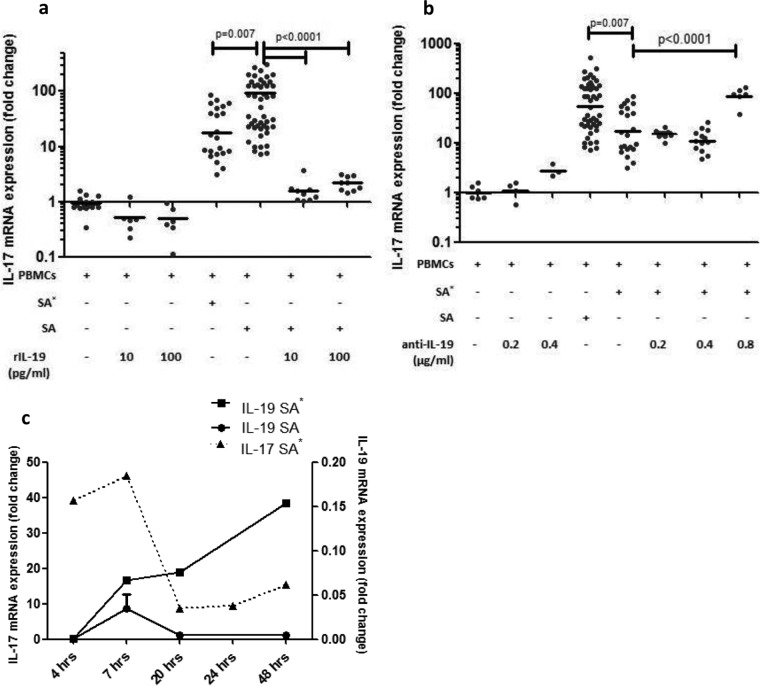
To further assess the role of IL-19 in the suppression of IL-17, we added recombinant IL-19 and IL-19 antibodies to the cell culture with and without endogenous or exogenous strains. Addition of recombinant IL-19 to PBMC cultures resulted in a slight nonsignificant decrease in IL-17 expression. Addition of recombinant IL-19 to PBMCs stimulated with exogenous S. aureus resulted in a significantly suppressed IL-17 response, compared to the exogenous-strain-stimulated sample, and IL-17 was suppressed to levels even lower than those in endogenous-strain-stimulated PBMCs (P < 0.0001) (Fig. 3a). Addition of IL-19 antibodies to PBMCs resulted in a slight nonsignificant increase in IL-17 expression. Addition of IL-19 antibodies to PBMCs stimulated with an endogenous strain resulted in an increase in IL-17 expression levels (P < 0.0001), similar to the increase resulting from exogenous strain stimulation (Fig. 3b). To suggest a causal relationship between the increase in IL-19 and suppressed IL-17, we measured the time-dependent responses of IL-19 and IL-17 expression to an endogenous strain. We observed that IL-19 mRNA expression increased steadily throughout the first 48 h, while IL-17 initially spiked but decreased by 20 h poststimulation with an endogenous strain (Fig. 3c).
FIG 3.
IL-17 expression in response to recombinant IL-19 and an anti-IL-19 antibody. (a) Addition of recombinant IL-19 to PBMCs caused a small, insignificant decrease in IL-17 mRNA expression. Addition of recombinant IL-19 at 10 or 100 pg/ml to PBMC cultures stimulated with an exogenous strain decreased IL-17 mRNA expression compared to that in exogenous-strain-stimulated cultures with no addition (P < 0.0001). (b) Addition of IL-19 antibodies to PBMCs caused an insignificant increase in IL-17 mRNA expression, and addition of IL-19 antibodies at 0.8 μg/ml to PBMC cultures stimulated with an endogenous strain resulted in an increased IL-17 mRNA response compared to that of the endogenous-strain-stimulated culture with no addition (P < 0.0001). ANOVA with Tukey's post hoc analysis was done on all repeats to determine differences in IL-17 mRNA expression. (c) mRNA expression of IL-17 and IL-19 over 48 h in PBMCs isolated from one carrier in response to an endogenous strain.
The tolerogenic response may be temporary.
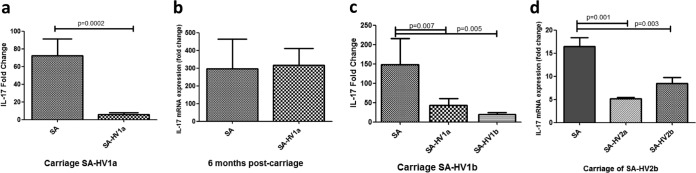
In our population, two carriers lost their original persistent strains after more than a year and acquired new, genetically different strains carried persistently for more than 6 months (Table 1). In one, the initial tolerogenic response observed could not be repeated when the endogenous strain was no longer detected; i.e., stimulation of the carrier's PBMCs with SA-HV1a resulted in an increased IL-17 response similar to that which resulted from exogenous-strain stimulation. Yet, at a later time point, when a second strain was carried persistently, both the first and second strains induced a tolerogenic response (P = 0.007 and 0.005) (Fig. 4a to c). This tolerogenic response to both strains was also observed in the second carrier, who replaced the initial strain with a second strain with no observed noncarriage period in between (P = 0.001 and 0.03) (Fig. 4d).
FIG 4.
Differential IL-17 response is transient. IL-17 mRNA expression in response to an endogenous strain (SA-HV-1a) compared to an exogenous strain during initial carriage (a), 6+ months after carriage was no longer detected (b), and during the second round of carriage, in which SA-HV1b was carried (c). Bars include data from three separate experiments done with PBMCs from carrier SA-HV1b during each of the three periods. (d) IL-17 mRNA expression in response to SA-HV2a and SA-HV2b during the SA-HV2b carriage period. ANOVA was done to determine the significance of differences between responses to endogenous and exogenous strains.
DISCUSSION
The cross talk between S. aureus and the human host is complex. While many studies have shown that S. aureus carriage can be divided into patterns of persistent carriage, transient carriage, and noncarriage, the underlying reasons and mechanisms are not fully understood. One possible reason for this is the inadequacy of current human S. aureus infection and carriage models. Mouse models of S. aureus infection and carriage have been shown to be inconsistent with human models, and this has been suggested as one of the reasons for the failure of S. aureus vaccine development (35–37). We used a novel experimental model to observe carrier immune responses to specific strains. Our model is based on that of Lu et al., which measures immune responses to pneumococcal colonization (38).
We first aimed to determine the role of IL-17 in carriage. As expected, we observed increased IL-17, as well as IFN-γ, levels following stimulation with S. aureus strains. Yet, a differential response to endogenous versus exogenous strains was observed, wherein individuals who carried a persistent strain responded to their endogenous strain with decreased IL-17, but not IFN-γ, levels. The lack of a differential IFN-γ response is interesting, considering that Nurjadi et al. found a significant difference in IFN-γ mRNA expression between carriers and noncarriers (27). However, the model used by that group was looking at responses to one strain (essentially representing all strains) and not individual carried strains. Additionally, they were studying both carriers and noncarriers, whereas we studied carriers only.
A decreased inflammatory response to a carried strain presumably results in reduced clearance of that strain and may lead to longer carriage. These results are consistent with a recent study by Cole et al. that observed an increased inflammatory response during nasal S. aureus clearance but not carriage (9), as well as a study by Nouwen et al. (10) that reported that persistent carriers who were artificially inoculated with a mixture of S. aureus strains selected for their own strain and noncarriers artificially inoculated with S. aureus immediately purged the strain.
Evidence of tolerance toward commensal strains has been previously reported, mostly with regard to the gut microbiome (39, 40). However, until now, studies looking at induced tolerance to commensal microbiota have focused on host-bacterial interactions at the species level (41, 42). Here, we see that even within the S. aureus species, individual tolerance is strain dependent and not only species dependent. This was especially evident when we compared all of the volunteer responses to an individual strain. These results explain previous findings by us and others that have shown not only that persistent carriers maintain a single strain over long periods of time but that S. aureus carriage by those individuals is limited to their endogenous strain (7, 10).
In two cases, carriers displayed a tolerogenic response to two genetically different strains: one that they were currently carrying and one that they carried previously but was no longer detected. One possible explanation for this is that although the original strains were not detected during the second carriage periods, they persisted at levels under a detectability threshold, causing the tolerogenic response to both. This, however, is unlikely, since the tolerogenic response was not observed during carrier HV1's period of noncarriage. Another explanation could be that the tolerogenic response of PBMCs does not represent the local mucosal immune response (43). Peripheral memory Tregs have not been extensively studied (44), but studies have shown that memory T cells, including memory CD8+ T cells, are no longer found at measurable levels in the peripheral blood 6 months postinfection (45–47). Further investigation into the mucosal response may give a deeper understanding of the cells at play. Alternatively, the two strains may share an immunogenic protein that induces the tolerogenic response.
IL-19 is a relatively newly discovered cytokine, and its immune activity is still unclear. It has been shown to be upregulated in mucosal epithelium-related diseases, such as psoriasis, asthma (31, 48), and colitis (49, 50), indicating an importance in mucosal and epithelial immunity. Various studies have found a role for IL-19 in inflammation and inflammatory disease (51, 52), while others maintain that it is a suppressive cytokine (53–55), like the others in the IL-10 family. Our findings that IL-19 plays a role in the immune interaction with S. aureus during nasal carriage is consistent with its role and presence in epithelial immunity (52). Our findings of the specific interaction with IL-17 during S. aureus carriage add more proof that IL-19 acts as an inflammatory suppressor under these conditions and are consistent with previous observations of IL-19 and other IL-20 receptor-mediated cytokines as IL-17 suppressors during S. aureus infection in mice (32) and in human cells (56).
A study by Peres et al. (57) found that IL-10 and TNF-α were induced by carried S. aureus strains and that the responses varied largely between individuals. We also observed an induction of IL-10 by carried S. aureus strains and found large deviations between individuals in all reported cytokine responses, but as opposed to our findings on IL-17 and IL-19, we did not see a differential IL-10 response to endogenous and exogenous strains.
Our study has several limitations. First, we assessed immune cells in the peripheral blood, while many of the responses are probably more pronounced at the local mucosal level or in the regional lymphatic tissue. Second, although we found that IL-19 alone was able to suppress IL-17 expression, it may be important to assess other IL-19-related cytokines, such as IL-20 and IL-24, that have been shown to suppress neutrophil activity in response to S. aureus (56). Furthermore, elucidation of the in vivo interactions between these cytokines and the cellular sources of cytokine secretion is required. While our study is limited to a relatively small number of subjects, a high level of statistical significance was reached.
The results presented here suggest specific differential host-strain interactions that have not been reported previously in S. aureus or other bacterial species. Further research to determine the cellular sources of IL-19 and the specific cellular and protein interactions that induce this differential response is important and may have a role in the search of new vaccine targets for S. aureus, a species that has, until now, eluded all attempts at vaccination.
MATERIALS AND METHODS
IRB and patient consent.
Institutional Review Board (IRB) approval was given by the local committee of the Sheba Medical Center. A written informed consent form was signed by each volunteer before recruitment.
Study population.
Healthy volunteers between the ages of 21 and 50 years were recruited if they gave written informed consent. Volunteers were either newly recruited or recruited from another study in our lab (58). Volunteers were sampled periodically, every 3 to 6 months, over the course of 4 years by swabbing of both anterior nares with a cotton-tipped swab. S. aureus was identified on Staph CHROMagar (Hy Labs, Israel), and a positive staphylase test (Pastorex Staph Plus; Bio-Rad) was performed for verification. One S. aureus colony was collected and frozen at −80°C for further characterization.
Volunteers who were found to be persistent carriers, defined as carriers of the same strain for >6 months, were included as persistent carriers in this study, and carriers who carried a strain for <6 months were considered intermittent carriers and their PBMCs were not included in the experiments done with carrier blood. PBMCs from two noncarriers were included in one experiment for comparison.
Strain characterization.
Strains were characterized by sequencing of the protein A (spa) gene with the primers listed in Table 2. Where possible, the MLST was determined on the basis of reports in the literature. In cases where MLST was not identifiable in the literature or several MLSTs could correlate with the identified spa type, the MLST was determined. This was done by using the MLST genes with the primers described by Enright et al. (59). PCR products were purified (Gene JET PCR DNA purification kit; Fermentas), and Sanger sequencing of the spa gene was carried out by Hy Laboratories Ltd. (Rehovot, Israel) with the BigDye Terminator v1.1 Cycle Sequencing kit (Applied Biosystems, Inc.) on the 3730xl DNA Analyzer with DNA Sequencing Analysis Software v. 5.4. Sequences were analyzed with the Fortinbras SpaTyper (http://spatyper.fortinbras.us/) and Ridom Spa Server (60), as well as the Multi Locus Sequence Typing software (saureus.mlst.net) (61).
TABLE 2.
Primers used in this study
| Gene product | Primer sequence |
|
|---|---|---|
| Forward | Reverse | |
| GAPDH | GGA AGG TGA AGG TCG GAG TC | TCA GCC TTG ACG GTG CCA TG |
| IL-17A | CGG ACT GTG ATG GTC AAC CT | GAC CAG GAT CTC TTG CTG GA |
| IFN-γ | TGA CCA GAG CAT CCA AAA GA | CTC TTC GAC CTC GAA ACA GC |
| FOXP3 | GAA ACA GCA CAT TCC AGA GTT | ATG GCC CAG CGG ATG AG |
| IL-27 p28 | GAG CAG CTC CCT GAT GTT TC | AGC TGC ATC CTC TCC ATG TT |
| IL-19 | TAC GTG GAC AGG GTG TTC AA | ATG ACT CTG GTG GCA TTG GT |
| IL-10 | AGA TCT CCG AGA TGC CCT CA | CCG TGG AGC TGA AGA AT |
| Spa | AGA CGA TCC TTC GGT GAG C | GCT TTT GCA ATG TCA TTT ACT G |
The strains used are described in Table 1.
Whole-cell antigen preparation.
Whole-cell antigens used in the stimulation were prepared from each of the S. aureus strains carried by the healthy volunteers, as well as additional previously characterized clinical and carried strains from our laboratory collections, and USA300. USA300 was used as an additional exogenous strain for regulation control in all experiments. To prepare whole-cell antigens, isolates were grown and diluted to 109 CFU/ml and killed with 70% ethanol. Killed whole-cell antigens were washed with saline and stored in Dulbecco's modified Eagle's medium (DMEM; Biological Industries, Israel) at −20°C.
PBMC isolation and stimulation.
PBMCs were isolated from the blood of healthy volunteers by Ficoll centrifugation. Cells (2.5 × 106 per well of a 24-well cell culture plate [Nunc]) were incubated with 1 ml of enriched DMEM-F12 with l-glutamine (2 mM), 10% fetal bovine serum, 1% penicillin-streptomycin, and β-mercaptoethanol (2 mM) at 37°C, 5% CO2 with or without various whole-cell antigens (multiplicity of infection, 10), recombinant protein, or antibodies for 24 h, 48 h, or 5 days. On day 3, 1 ml of medium was added.
IL-19 stimulation experiments.
Recombinant human IL-19 (R&D Systems) was reconstituted and diluted with 0.1% bovine serum albumin in accordance with the manufacturer's instructions. Recombinant IL-19 was added to the medium after 4 h to reach a final concentration of 10 or 100 pg/ml. A human IL-19 mouse IgG2B monoclonal antibody (R&D Systems) was reconstituted and diluted with sterile phosphate-buffered saline (PBS) in accordance with the manufacturer's instructions and added to medium to final concentrations of 0.2, 0.4, and 0.8 μg/ml at the beginning of the experiment.
Real-time PCR.
After 48 h of stimulation, RNA was extracted from PBMCs with the RNeasy kit (Qiagen) in accordance with the kit protocol. cDNA was produced with 4 μl of iScript supermix with random primers (Bio-Rad) with RNA at a final concentration of 1.5 ng/μl. To detect cytokine expression, cDNA was amplified with Sybr green supermix (Roche, Dyn Diagnostics) and the ABI 7000 Real-Time PCR system (Applied Biosystems) with primers described in Table 2. Cytokine expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Fold changes in gene expression were determined by calculating the relative quantification of stimulated samples (exogenous or endogenous strains) compared to that of unstimulated PBMC samples.
ELISA.
Cell cultures were centrifuged on days 2 and 5, and the cell culture supernatant was analyzed by enzyme-linked immunosorbent assay (ELISA) (DuoSet; R&D Systems) for secreted protein cytokines (IL-17, IFN-γ, IL-19, IL-10, and IL-27) in accordance with the manufacturer's instructions.
FACS analysis.
On day 4, cells were washed, fixed, permeabilized, and stained with CD4-fluorescein isothiocyanate (eBioscience) and IL-17–phycoerythrin (eBioscience) in accordance with the manufacturer's instructions. Stained cells were counted in the FACSCalibur (BD Bioscience) and analyzed with FlowJo Diagnostic software.
Statistical analysis.
To detect a difference between all cytokine responses to endogenous versus exogenous strains within individuals, we performed a linear regression with repeated measures of the log or square root transformation of responses with endogenous versus exogenous strains nested within the individual volunteer. In each experiment, the carrier response to one endogenous strain was tested and compared to the responses to at least two exogenous strains. Each observation included in the analysis is the response of one carrier to one strain and is the average of two or three biological and two or three technical replicates.
To detect the difference in IL-17 mRNA expression between carriers and noncarriers, we performed a repeated-measures linear regression with the expression of IL-17 mRNA as the dependent variable and carriage (carrier/noncarrier) as the predictor and measures nested within the subject.
To find whether the IL-17 and IFN-γ responses to both endogenous and exogenous strains were significantly different from the responses of untreated samples, we applied the same repeated-measures linear regression with the intercept as the only predictor and estimated the significance of the intercept.
In accordance with the method of measurement, we used a complete symmetry covariance matrix between the measurements of each volunteer.
For the analysis of all carrier and noncarrier responses to one strain, we used a Wilcoxon-Mann-Whitney test to compare the responses of the strain's carrier with the responses of all other healthy volunteers to that strain. For the FACS experiment and recombinant IL-19/IL-19 antibody addition experiments, analysis of variance (ANOVA) with Tukey's post hoc test was used to determine differences between treatments.
ACKNOWLEDGMENTS
We thank Itamar Goldstein and Einav Vaks for their helpful insight and technical expertise throughout the work and the writing of this paper. We thank Vladislav Litachevsky, Naim Mahroum, Anna Belkin, Nir Maaravi, and the rest of the doctors in the Infectious Diseases unit of Sheba Medical Center for their assistance in drawing blood for the experiments. Thank you to our volunteers for their participation in this study.
This research was supported by Israel Science Foundation grant 1590/09 and ISF 1658-15 (G.R.-Y.).
REFERENCES
- 1.Boucher HW, Corey GR. 2008. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 46:S344–S349. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 2.de Kraker ME, Davey PG, Grundmann H, BURDEN Study Group. 2011. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med 8:e1001104. doi: 10.1371/journal.pmed.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.VandenBergh MFQ, Yzerman EPF, van Belkum A, Boelens HAM, Sijmons M, Verbrugh HA. 1999. Follow-up of Staphylococcus aureus nasal carriage after 8 years: redefining the persistent carrier state. J Clin Microbiol 37:3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 5.Schweizer M, Bossen A, McDanel J, Dennis L. 2012. Staphylococcus aureus colonization before infection is not associated with mortality among S. aureus-infected patients: a meta-analysis. Infect Control Hosp Epidemiol 33:796. doi: 10.1086/666628. [DOI] [PubMed] [Google Scholar]
- 6.Sakwinska O, Blanc DS, Lazor-Blanchet C, Moreillon M, Giddey M, Moreillon P. 2010. Ecological temporal stability of Staphylococcus aureus nasal carriage. J Clin Microbiol 48:2724–2728. doi: 10.1128/JCM.02091-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muthukrishnan G, Lamers RP, Ellis A, Paramanandam V, Persaud AB, Tafur S, Parkinson CL, Cole AM. 2013. Longitudinal genetic analyses of Staphylococcus aureus nasal carriage dynamics in a diverse population. BMC Infect Dis 13:221. doi: 10.1186/1471-2334-13-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthukrishnan G, Quinn GA, Lamers RP, Diaz C, Cole AL, Chen S, Cole AM. 2011. Exoproteome of Staphylococcus aureus reveals putative determinants of nasal carriage. J Proteome Res 10:2064–2078. doi: 10.1021/pr200029r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole AL, Muthukrishnan G, Chong C, Beavis A, Eade CR, Wood MP, Deichen MG, Cole AM. 2016. Host innate inflammatory factors and staphylococcal protein A influence the duration of human Staphylococcus aureus nasal carriage. Mucosal Immunol 9:1537–1548. doi: 10.1038/mi.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nouwen J, Boelens H, Van Belkum A, Verbrugh H. 2004. Human factor in Staphylococcus aureus nasal carriage. Infect Immun 72:6685–6688. doi: 10.1128/IAI.72.11.6685-6688.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole AM, Tahk S, Oren A, Yoshioka D, Kim Y-H, Park A, Ganz T. 2001. Determinants of Staphylococcus aureus nasal carriage. Clin Diagn Lab Immunol 8:1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Belkum A, Eriksen NHR, Sijmons M, Van Leeuwen W, Van Den Bergh M, Kluytmans J, Espersen F, Verbrugh H. 1997. Coagulase and protein A polymorphisms do not contribute to persistence of nasal colonisation by Staphylococcus aureus. J Med Microbiol 46:222–232. doi: 10.1099/00222615-46-3-222. [DOI] [PubMed] [Google Scholar]
- 13.Peacock SJ, Justice A, Griffiths D, de Silva GDI, Kantzanou MN, Crook D, Sleeman K, Day NPJ. 2003. Determinants of acquisition and carriage of Staphylococcus aureus in infancy. J Clin Microbiol 41:5718–5725. doi: 10.1128/JCM.41.12.5718-5725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Akker ELT, Nouwen JL, Melles DC, Rossum EFCv Koper JW, Uitterlinden AG, Hofman A, Verbrugh HA, Pols HA, Lamberts SWJ, van Belkum A. 2006. Staphylococcus aureus nasal carriage is associated with glucocorticoid receptor gene polymorphisms. J Infect Dis 194:814–818. doi: 10.1086/506367. [DOI] [PubMed] [Google Scholar]
- 15.Mitchison N. 2004. T-cell–B-cell cooperation. Nat Rev Immunol 4:308–312. doi: 10.1038/nri1334. [DOI] [PubMed] [Google Scholar]
- 16.Archer NK, Harro JM, Shirtliff ME. 2013. Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx. Infect Immun 81:2070–2075. doi: 10.1128/IAI.00084-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bröker BM, Mrochen D, Péton V. 2016. The T cell response to Staphylococcus aureus. Pathogens 5:E31. doi: 10.3390/pathogens5010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtfreter S, Nguyen TTH, Wertheim H, Steil L, Kusch H, Truong QP, Engelmann S, Hecker M, Völker U, van Belkum A, Bröker BM. 2009. Human immune proteome in experimental colonization with Staphylococcus aureus. Clin Vaccine Immunol 16:1607–1614. doi: 10.1128/CVI.00263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolata JB, Kühbandner I, Link C, Normann N, Vu CH, Steil L, Weidenmaier C, Bröker BM. 2015. The fall of a dogma? Unexpected high T-cell memory response to Staphylococcus aureus in humans. J Infect Dis 212:830–838. doi: 10.1093/infdis/jiv128. [DOI] [PubMed] [Google Scholar]
- 20.Curtis MM, Way SS. 2009. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 126:177–185. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang W, Na L, Fidel PL, Schwarzenberger P. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 22.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. 2001. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol 25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Clarke TB, Weiser JN. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML. 2008. Impaired Th17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minegishi Y, Karasuyama H. 2009. Defects in Jak–STAT-mediated cytokine signals cause hyper-IgE syndrome: lessons from a primary immunodeficiency. Int Immunol 21:105–112. doi: 10.1093/intimm/dxn134. [DOI] [PubMed] [Google Scholar]
- 26.Ochs HD, Oukka M, Torgerson TR. 2009. Th17 cells and regulatory T cells in primary immunodeficiency diseases. J Allergy Clin Immunol 123:977–983. doi: 10.1016/j.jaci.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nurjadi D, Kain M, Marcinek P, Gaile M, Heeg K, Zanger P. 2016. Ratio of T-helper type 1 (Th1) to Th17 cytokines in whole blood is associated with human β-defensin 3 expression in skin and persistent Staphylococcus aureus nasal carriage. J Infect Dis 214:1744–1751. doi: 10.1093/infdis/jiw440. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Baba N, Samson S, Bourdet-Sicard R, Rubio M, Sarfati M. 2008. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J Leukoc Biol 84:468–476. doi: 10.1189/jlb.0108017. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher G. 2010. Interleukin-19: multiple roles in immune regulation and disease. Cytokine Growth Factor Rev 21:345–352. doi: 10.1016/j.cytogfr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Witte E, Kokolakis G, Witte K, Philipp S, Doecke W-D, Babel N, Wittig BM, Warszawska K, Kurek A, Erdmann-Keding M, Kunz S, Asadullah K, Kadin ME, Volk H-D, Sterry W, Wolk K, Sabat R. 2014. IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J Invest Dermatol 134:2757–2767. doi: 10.1038/jid.2014.308. [DOI] [PubMed] [Google Scholar]
- 32.Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, Ouyang W, Datta SK. 2013. IL-20 receptor signaling inhibits cutaneous IL-1β and IL-17A production to promote methicillin-resistant Staphylococcus aureus infection. Nat Immunol 14:804. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biber A, Parizade M, Taran D, Jaber H, Berla E, Rubin C, Rahav G, Glikman D, Regev-Yochay G. 2015. Molecular epidemiology of community-onset methicillin-resistant Staphylococcus aureus infections in Israel. Eur J Clin Microbiol Infect Dis 34:1603–1613. doi: 10.1007/s10096-015-2395-9. [DOI] [PubMed] [Google Scholar]
- 34.Maayan-Metzger A, Strauss T, Rubin C, Jaber H, Dulitzky M, Reiss-Mandel A, Leshem E, Rahav G, Regev-Yochay G. 2017. Clinical evaluation of early acquisition of Staphylococcus aureus carriage by newborns. Int J Infect Dis 64:9–14. doi: 10.1016/j.ijid.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Proctor RA. 2012. Challenges for a universal Staphylococcus aureus vaccine. Clin Infect Dis 54:1179–1186. doi: 10.1093/cid/cis033. [DOI] [PubMed] [Google Scholar]
- 36.Salgado-Pabón W, Schlievert PM. 2014. Models matter: the search for an effective Staphylococcus aureus vaccine. Nat Rev Microbiol 12:585–591. doi: 10.1038/nrmicro3308. [DOI] [PubMed] [Google Scholar]
- 37.Mestas J, Hughes CCW. 2004. Of mice and not men: differences between mouse and human immunology. J Immunol 172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 38.Lu Y-J, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, Thompson CM, Harney KF, Anderson PW, Lipsitch M, Malley R. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog 4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung TC, Artis D, Sonnenberg GF. 2014. Anatomical localization of commensal bacteria in immune cell homeostasis and disease. Immunol Rev 260:35–49. doi: 10.1111/imr.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Round JL, Mazmanian SK. 2010. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR. 2005. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci U S A 102:2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galdeano CM, Perdigón G. 2006. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin Vaccine Immunol 13:219–226. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Backteman K, Andersson C, Dahlin L-G, Ernerudh J, Jonasson L. 2012. Lymphocyte subpopulations in lymph nodes and peripheral blood: a comparison between patients with stable angina and acute coronary syndrome. PLoS One 7:e32691. doi: 10.1371/journal.pone.0032691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenblum MD, Way SS, Abbas AK. 2016. Regulatory T cell memory. Nat Rev Immunol 16:90–101. doi: 10.1038/nri.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, Riberdy JM, Liu T, Tan M, Doherty PC. 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci U S A 98:6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. 2001. Protection from respiratory virus infections can be mediated by antigen-specific CD4+ T cells that persist in the lungs. J Exp Med 193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodland DL, Kohlmeier JE. 2009. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol 9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 48.Huang F, Wachi S, Thai P, Loukoianov A, Tan KH, Forteza RM, Wu R. 2008. Potentiation of IL-19 expression in airway epithelia by IL-17A and IL-4/IL-13: important implications in asthma. J Allergy Clin Immunol 121:1415–1421.e1413. doi: 10.1016/j.jaci.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fonseca-Camarillo G, Furuzawa-Carballeda J, Granados J, Yamamoto-Furusho JK. 2014. Expression of interleukin (IL)-19 and IL-24 in inflammatory bowel disease patients: a cross-sectional study. Clin Exp Immunol 177:64–75. doi: 10.1111/cei.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azuma Y-T, Matsuo Y, Kuwamura M, Yancopoulos GD, Valenzuela DM, Murphy AJ, Nakajima H, Karow M, Takeuchi T. 2010. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm Bowel Dis 16:1017–1028. doi: 10.1002/ibd.21151. [DOI] [PubMed] [Google Scholar]
- 51.Liao Y-C, Liang W-G, Chen F-W, Hsu J-H, Yang J-J, Chang M-S. 2002. IL-19 induces production of IL-6 and TNF-α and results in cell apoptosis through TNF-α. J Immunol 169:4288–4297. doi: 10.4049/jimmunol.169.8.4288. [DOI] [PubMed] [Google Scholar]
- 52.Hsing CH, Chiu CJ, Chang LY, Hsu CC, Chang MS. 2008. IL-19 is involved in the pathogenesis of endotoxic shock. Shock 29:7–15. [DOI] [PubMed] [Google Scholar]
- 53.Cooley ID, Chauhan VS, Donneyz MA, Marriott I. 2014. Astrocytes produce IL-19 in response to bacterial challenge and are sensitive to the immunosuppressive effects of this IL-10 family member. Glia 62:818–828. doi: 10.1002/glia.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallagher G, Eskdale J, Jordan W, Peat J, Campbell J, Boniotto M, Lennon GP, Dickensheets H, Donnelly RP. 2004. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int Immunopharmacol 4:615–626. doi: 10.1016/j.intimp.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Azuma Y-T, Matsuo Y, Nakajima H, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Takeuchi T. 2011. Interleukin-19 is a negative regulator of innate immunity and critical for colonic protection. J Pharmacol Sci 115:105–111. doi: 10.1254/jphs.10R02CR. [DOI] [PubMed] [Google Scholar]
- 56.Gough P, Ganesan S, Datta SK. 2017. IL-20 Signaling in activated human neutrophils inhibits neutrophil migration and function. J Immunol 198:4373–4382. doi: 10.4049/jimmunol.1700253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peres AG, Stegen C, Li J, Xu AQ, Levast B, Surette MG, Cousineau B, Desrosiers M, Madrenas J. 2015. Uncoupling of pro- and anti-inflammatory properties of Staphylococcus aureus. Infect Immun 83:1587–1597. doi: 10.1128/IAI.02832-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leshem E, Maayan-Metzger A, Rahav G, Dolitzki M, Kuint J, Roytman Y, Goral A, Novikov I, Fluss R, Keller N, Regev-Yochay G. 2012. Transmission of Staphylococcus aureus from mothers to newborns. Pediatr Infect Dis J 31:360–363 3. doi: 10.1097/INF.0b013e318244020e. [DOI] [PubMed] [Google Scholar]
- 59.Enright MC, Day NPJ, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 38:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harmsen D, Claus H, Witte W, Rothgänger J, Claus H, Turnwald D, Vogel U. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 41:5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aanensen DM, Spratt BG. 2005. The multilocus sequence typing network: mlst.net. Nucleic Acids Res 33:W728–W733. doi: 10.1093/nar/gki415. [DOI] [PMC free article] [PubMed] [Google Scholar]