ABSTRACT
CpxRA is an envelope stress response system found in all members of the family Enterobacteriaceae; CpxA has kinase activity for CpxR and phosphatase activity for phospho-CpxR, a transcription factor. CpxR also accepts phosphate groups from acetyl phosphate, a glucose metabolite. Activation of CpxR increases the transcription of genes encoding membrane repair and downregulates virulence determinants. We hypothesized that activation of CpxR could serve as an antimicrobial/antivirulence strategy and discovered compounds that activate CpxR in Escherichia coli by inhibiting CpxA phosphatase activity. As a prelude to testing such compounds in vivo, here we constructed cpxA (in the presence of glucose, CpxR is activated because of a lack of CpxA phosphatase) and cpxR (system absent) deletion mutants of uropathogenic E. coli (UPEC) CFT073. By RNA sequencing, few transcriptional differences were noted between the cpxR mutant and its parent, but in the cpxA mutant, several UPEC virulence determinants were downregulated, including the fim and pap operons, and it exhibited reduced mannose-sensitive hemagglutination of guinea pig red blood cells in vitro. In competition experiments with mice, both mutants were less fit than the parent in the urine, bladder, and kidney; these fitness defects were complemented in trans. Unexpectedly, in single-strain challenges, only the cpxA mutant was attenuated for virulence in the kidney but not in the bladder or urine. For the cpxA mutant, this may be due to the preferential use of amino acids over glucose as a carbon source in the bladder and urine by UPEC. These studies suggest that CpxA phosphatase inhibitors may have some utility for treating complex urinary tract infections.
KEYWORDS: Escherichia coli, UPEC, antivirulence, cpxA, cpxR, cpxRA, uropathogenic, virulence determinants
INTRODUCTION
Urinary tract infections (UTIs) are common, particularly in women under 30 years of age (1). Nearly 50% of all women develop at least one UTI in their lifetimes, and up to 30% have a recurrence within 3 months despite appropriate therapy (1, 2). Approximately 80% of uncomplicated UTIs in the United States are caused by uropathogenic Escherichia coli (UPEC) (3). Unfortunately, some common clones of UPEC, such as E. coli sequence type 131, express extended-spectrum beta-lactamases and quinolone resistance (4–6). Such infections are usually treated with carbapenems, but UPEC can acquire plasmids encoding carbapenemase, such as NDM-1, rendering them resistant to all beta-lactams (7, 8). In addition, acquisition of the mcr-1 plasmid has rendered some carbapenemase-producing strains of E. coli resistant to colistin, the last line of defense against carbapenem-resistant Enterobacteriaceae (9). These findings raise the possibility that infections caused by UPEC may become untreatable. A deeper understanding of the pathogenesis of UPEC may help to direct the identification of novel targets and strategies to combat these panresistant infections.
One attractive target for an antimicrobial/antivirulence strategy is the CpxRA envelope stress response system. CpxRA is highly conserved across members of the family Enterobacteriaceae, and CpxRA homologs are also found in pathogens of other families, including Legionella pneumophila, Neisseria gonorrhoeae, and Haemophilus ducreyi (10–15). The core of this system is composed of CpxA, an inner membrane sensor kinase/phosphatase, and its cognate response regulator, CpxR. In the absence of membrane stress, CpxP, a periplasmic chaperone, binds to CpxA and inhibits its kinase activity; in this circumstance, CpxA acts as a net phosphatase, rendering CpxR inactive. When the bacterial envelope is subjected to stress, marked by the accumulation of misfolded periplasmic proteins, CpxP binds the misfolded proteins and dissociates from CpxA, relieving the inhibition. CpxA then autophosphorylates at a conserved histidine residue and donates its phosphate group to CpxR at a conserved aspartate residue, activating the system (16). In nonpathogenic E. coli, activated (phosphorylated) CpxR regulates the transcription of ∼100 genes or operons and increases the production of membrane chaperones and proteases, which alleviate periplasmic stress (17, 18). In pathogenic E. coli, activated CpxR also downregulates the expression of secreted proteins, including multiple virulence determinants, likely in an attempt to relieve periplasmic stress (11, 19).
When E. coli is grown in peptide-based media containing an excess of rapidly metabolized carbon sources such as glucose, CpxR accepts phosphate groups from small-molecule phosphate donors such as acetyl phosphate (20–22). In such media, cpxA deletion mutants, which lack CpxR phosphatase activity, accumulate phosphorylated CpxR, resulting in activation of the system (20). cpxA* alleles usually have mutations in the sensing domain of CpxA that result in constitutive kinase activity and an even higher level of CpxR activation than cpxA deletion (20, 22).
In several pathogenic bacteria, activation of CpxR via mutations in cpxA abrogates virulence but deletion of cpxR does not. For example, 106 CFU of ΔcpxA or cpxA* mutant Salmonella enterica serotype Typhimurium given orally are unable to infect mice, whereas the same dose of the wild type or a ΔcpxR mutant causes infection (23). In a human volunteer model of H. ducreyi infection, a ΔcpxR mutant is as virulent as its parent in the ability to form skin abscesses, but a ΔcpxA mutant is unable to form abscesses and is cleared (12, 24). Deletion of H. ducreyi cpxA downregulates the expression of seven virulence factors, each of which is individually required for human infection (25). Taken together, these experiments suggest that there are sufficient carbon sources in vivo to allow the ΔcpxA mutants to make acetyl phosphate and accumulate activated CpxR and that activation of CpxR via chemical targeting of CpxA might be an attractive antivirulence strategy (22). As the amino acid sequences of E. coli CpxA and CpxR are 95 to 99% identical to homologs found in Klebsiella, Enterobacter, Salmonella, and Citrobacter, compounds that activate the system in E. coli could be effective against multiple drug-resistant pathogens (22). By high-throughput screening of E. coli K-12 grown in media containing peptides and glucose, we identified one class of compounds that activate CpxR by inhibiting CpxA phosphatase activity (22). Since such compounds chemically induce a ΔcpxA-like mutant phenotype, they could lead to activation of CpxR, diminished secretion of virulence determinants, and clearance of pathogens by the host immune system.
A potential problem with the activation strategy is that escape mutants could develop through loss of expression or mutations in cpxR. However, in N. gonorrhoeae, deletion of either cpxA or cpxR homologs abrogates virulence in a murine model of vaginal colonization (14). In the UPEC cystitis isolate UTI89, a cpxRA deletion mutant is impaired in the ability to infect the urinary bladder but activation of CpxR by deletion of cpxP has no effect on virulence (26). Similarly, deletion of cpxR in UTI89 leads to acute and chronic impairment of bladder colonization, perhaps through overexpression of the hemolysin encoded by hlyA, which causes inflammatory cell death and sloughing of infected bladder epithelial cells (27). Since cpxR is required for UTI89 to infect the bladder, resistance to CpxA phosphatase inhibitors is less likely to develop via mutations or loss of cpxR. However, UPEC strains are genetically highly heterogeneous (28–31) and whether these findings are generalizable to other UPEC strains is unknown.
As a prelude to testing the hypothesis that CpxA phosphatase inhibitors could be used to treat UPEC infections in vivo, here we tested the hypothesis that both activation of CpxR by deletion of cpxA and deletion of cpxR would dysregulate the expression of virulence factors by UPEC strain CFT073, a highly virulent pyelonephritis isolate. Toward this end, we used transcriptome sequencing (RNA-Seq) to define the genes regulated by the activation and deletion of CpxR. Additionally, we evaluated the relative fitness and virulence of CFT073 and its ΔcpxA and ΔcpxR mutants in a murine model of UTI and extended the previous studies to include recovery of the strains from the bladder to the urine and kidney.
RESULTS
Construction and characterization of CFT073 cpxA and cpxR mutants.
Loss of CpxR impairs the ability of UPEC strain UTI89 to infect the murine bladder (26, 27). Deletion of cpxA impairs the virulence of multiple pathogens in human and murine models of infection; this effect is likely due loss of CpxA phosphatase activity, subsequent accumulation of activated CpxR, and downregulation of the expression of virulence determinants (12, 14, 23). In this study, we extended our assessment of the role played by the CpxRA signal transduction pathway in virulence in UPEC infections. To do so, we constructed cpxA and cpxR insertion/deletion mutants of strain CFT073 by using a promoterless cat cassette. PCR and sequence analysis verified the expected sequence for CFT073ΔcpxA::cat and CFT073ΔcpxR::cat (here the cpxA and cpxR mutants) but showed that the cpxR deletion is polar on cpxA and thus phenocopies a cpxRA double mutant. No growth defects were observed in the mutants compared to the parent strain (see Fig. S1 in the supplemental material).
Genes regulated by the CpxRA system.
We used RNA-Seq to compare the transcriptomes of the parent strain (intact CpxRA system) and the cpxA (system activated) and cpxR (system absent) mutants grown in a peptide-based medium containing glucose to the stationary phase of growth. We chose this medium so that cpxA deletion would lead to accumulation of phosphorylated CpxR and this phase of growth because previous transcriptome analysis of CpxR-regulated genes in E. coli K-12 showed maximal activation in the stationary phase (20, 32). As described previously, we considered a 2-fold change in gene expression and a false-discovery rate of ≤0.1 to be statistically significant for differential gene expression by the strains (25).
As expected, the highest level of differential gene regulation occurred between the cpxA and cpxR mutants, a comparison of an activated state and an inactive state. There were 514 genes that were differentially regulated in the cpxA and cpxR mutants; of those, 309 were downregulated and 205 were upregulated (Fig. 1 and Table 1; see Tables S1 and S2 in the supplemental material). Comparison of the cpxA mutant and the parent strain identified 433 genes that were differentially regulated; 247 genes were downregulated, and 186 genes were upregulated (Fig. 1 and Table 1; see Tables S1 and S2). Comparison of the parent strain and the cpxR mutant revealed a very small number of differentially regulated genes; 10 genes were differentially regulated in the two strains, only 1 of which was upregulated (Fig. 1 and Table 1; see Tables S1 and S2). These results indicate that the CpxRA system in CFT073 potentially regulates a substantial number of genetic targets but that the CpxRA system is only minimally active in the parent under these experimental growth conditions. The latter finding is in contrast to previous studies with nonpathogenic E. coli, in which CpxRA is activated in stationary phase, but is consistent with findings on other pathogens such as H. ducreyi and Vibrio cholerae (25, 32, 33).
FIG 1.
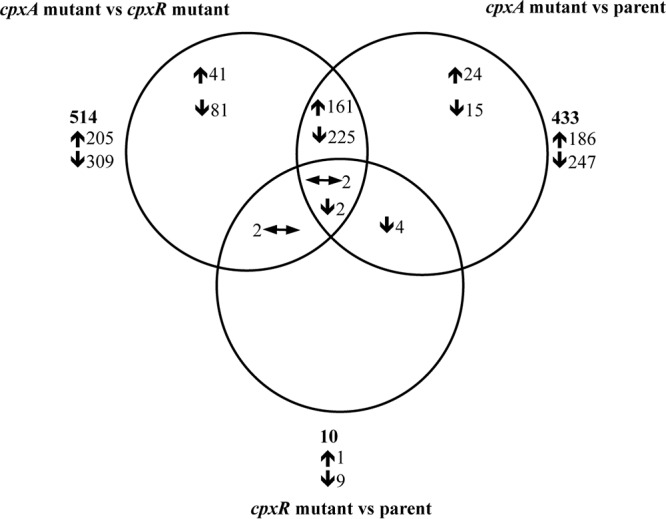
Venn diagram showing the number of genes differentially expressed in the cpxA mutant versus the cpxR mutant, the cpxA mutant versus the parent, and the cpxR mutant versus the parent. The up- and downregulated genes or operons are indicated by up (↑) and down (↓) arrows, respectively; genes that are differentially regulated in opposite directions in two or more strains are indicated by sideways arrows (↔). The total numbers of genes differentially expressed are shown in bold outside the Venn diagram.
TABLE 1.
Functional classification of genes differentially regulated in the parent strain, the cpxA mutant, and the cpxR mutant
| COG functional class or category | No. of genes |
|||||
|---|---|---|---|---|---|---|
|
cpxA vs cpxR mutant |
cpxA mutant vs parent |
cpxR mutant vs parent |
||||
| Up | Down | Up | Down | Up | Down | |
| Cellular processes and signaling | ||||||
| Cell cycle control, cell division, and chromosome partitioning | 3 | 1 | 2 | 0 | 0 | 0 |
| Cell motility and intracellular trafficking, secretion, and vesicular transport | 4 | 19 | 4 | 17 | 0 | 0 |
| Cell wall/membrane/envelope biogenesis | 7 | 19 | 7 | 19 | 0 | 1 |
| Defense mechanisms | 1 | 1 | 1 | 1 | 0 | 0 |
| Posttranslational modification, protein turnover, and chaperones | 8 | 7 | 5 | 3 | 0 | 0 |
| Signal transduction mechanisms | 1 | 13 | 1 | 10 | 0 | 0 |
| Information storage and processing | ||||||
| Replication, recombination, and repair | 4 | 6 | 2 | 2 | 0 | 0 |
| Transcription | 5 | 14 | 15 | 9 | 0 | 0 |
| Translation, ribosomal structure, and biogenesis | 11 | 0 | 0 | 0 | 0 | 0 |
| Metabolism | ||||||
| Amino acid transport and metabolism | 19 | 15 | 18 | 15 | 0 | 0 |
| Carbohydrate transport and metabolism | 4 | 7 | 4 | 4 | 0 | 1 |
| Coenzyme transport and metabolism | 7 | 2 | 5 | 1 | 0 | 0 |
| Energy production and conversion | 6 | 20 | 4 | 21 | 0 | 0 |
| Inorganic ion transport and metabolism | 34 | 25 | 30 | 22 | 0 | 1 |
| Lipid transport and metabolism | 4 | 7 | 3 | 6 | 0 | 0 |
| Nucleotide transport and metabolism | 7 | 1 | 9 | 0 | 0 | 0 |
| Secondary metabolite biosynthesis, transport, and catabolism | 4 | 2 | 5 | 2 | 0 | 0 |
| Poorly characterized | ||||||
| Function unknown | 8 | 4 | 5 | 7 | 0 | 0 |
| General function prediction only | 7 | 16 | 4 | 14 | 0 | 3 |
| Unclassified | 61 | 129 | 62 | 94 | 1 | 3 |
| Total | 205 | 309 | 186 | 247 | 1 | 9 |
Identification of differentially regulated genes preceded by a CpxR binding motif.
In E. coli, the consensus CpxR binding motif is the tandem repeat GTAAA(N5)GTAAA (17). For the cpxA-versus-cpxR mutant transcriptome comparison, there were a total of 514 differentially regulated genes contained in 348 operons. A genome-wide search using a position-specific scoring matrix derived from this tandem repeat showed that this logo was present in the upstream promoter sequences of 30 (68.2%) of the 44 downregulated genes or operons and in 29 (9.5%) of the 304 upregulated genes or operons (P < 0.001) (see Table S3 in the supplemental material). These data suggest that the majority of the downregulated targets may be directly regulated by CpxRA, while the majority of the upregulated genes or operons are indirectly regulated by activation of the system.
qRT-PCR validation of the RNA-Seq results.
To validate the RNA-Seq results, we performed quantitative reverse transcriptase PCR (qRT-PCR) on 13 representative genes that were differentially expressed in the cpxA and cpxR mutants. We selected these genes on the basis of the level of expression (high, moderate, or low), the direction of differential expression (upregulated or downregulated), and the extent of the change (−8.1- to 5.8-fold) (25) (see Table S4 in the supplemental material). The qRT-PCR data confirmed the directionality of the differentially expressed genes determined by RNA-Seq; the calculated correlation coefficient was 0.5 (Table S4).
Classification of CpxR-regulated genes into functional categories.
The genes that were differentially expressed in the parent strain and each of the cpxA and cpxR mutants were grouped into several different functional categories based on the Clusters of Orthologous Groups (COG) functional classification system. Gene annotations from the sequenced CFT073 genome were obtained from the KEGG, BioCyc, and NCBI databases. A complete list of the differentially expressed genes is provided in Tables S1 and S2. Here, we will focus on genes that have been well described in CFT073 or in nonpathogenic E. coli.
Activation of CpxR upregulates the expression of genes encoding protein folding and chaperone factors and downregulates the expression of a number of envelope proteins.
The CpxRA system regulates protein traffic across the bacterial envelope and repairs the bacterial membrane during times of stress. Comparison of the transcriptome of the cpxA mutant to that of the cpxR mutant showed that many of the differentially regulated genes were involved in envelope biogenesis and maintenance. Activation of CpxR upregulated the expression of genes encoding chaperones and protein folding factors (cpxP, htrA, ppiA, htpX, nrdH, hypB, hypC) (Table S2). On the other hand, activation of CpxR downregulated the expression of genes involved in peptidoglycan synthesis (dacC, ydhO), lipopolysaccharide biosynthesis (arnT, yfbE, wzzB), phospholipid synthesis (cfa), outer membrane protease (ompT), and the membrane porins (ompF, phoE) (Table S2). Similar to a recent report on enteropathogenic E. coli, activation of CpxR also downregulated the expression of genes encoding respiratory protein complexes (nuo and cyo operons) that reside in the bacterial cytoplasmic membrane (Table S2) (34). These data are consistent with the known functions of CpxRA in E. coli.
Activation of CpxR downregulates the transcription of the fim, pap, and F1C fimbria operons.
To attach to host uroepithelial cells and establish infection, UPEC possesses 10 chaperone-usher gene clusters (which include type 1 fimbriae, P pili, and F1C fimbriae) (35). Comparison of the transcriptional profiles of the cpxA mutant and the parent strain demonstrated that activation of CpxR downregulates the expression of many chaperone-usher systems, including the fim operon (fimA, fimI, fimC, fimD, fimF, fimG, fimH), the Pap genes (papA, papI, papA_2), and the F1C fimbria genes (focA, focC, focD, focF, focG, focH, sfaB, sfaD) (Table 2 and Table S1). Thus, activation of CpxR may result in a reduced ability of UPEC to attach to bladder and kidney epithelial cells.
TABLE 2.
Differential regulation by the CpxRA system of known UPEC fitness or virulence genes
| Category and locus tag | Genea | Product | cpxA mutant vs cpxR mutant | Reference |
|---|---|---|---|---|
| Adhesion | ||||
| c2103 | ydiV | Anti-FlhDC factor | −2.24 | 59 |
| c5394 | fimI | Fimbrin-like protein FimI | −3.13 | 60 |
| c5395 | fimC | Chaperone protein FimC | −2.68 | 60 |
| c5396 | fimD | Outer membrane usher protein FimD | −2.12 | 60 |
| c5400 | fimH | FimH protein | −2.02 | 60 |
| c3782 | ygiA | Hypothetical protein | 3.28 | 61 |
| Autotransporters, c0426 | upaB | Autotransporter | −2.21 | 38 |
| Iron transport and metabolism | ||||
| c1717 | tonB | Transport protein TonB | 2.95 | 40 |
| c4308 | chuA | Outer membrane heme/hemoglobin receptor | 3.01 | 40 |
| Phosphate transport and metabolism | ||||
| c4648 | phoU | Transcriptional regulator PhoU | −3.25 | 60 |
| c4649 | pstB | Phosphate transporter ATP-binding protein | −2.54 | 37 |
| c4651 | pstA | Phosphate transporter permease subunit PstA | −2.23 | 37 |
| c4652 | pstC | Phosphate transporter permease subunit PstC | −2.48 | 37 |
| c4653 | pstS | Phosphate ABC transporter periplasmic substrate-binding protein PstS | −3.64 | 37 |
| Nickel transport and metabolism | ||||
| c4269 | nikA | Nickel-binding periplasmic protein | 2.14 | 30 |
| c4270 | nikB | Nickel transporter permease NikB | 2.13 | 30 |
| c4271 | nikC | Nickel transporter permease NikC | 2.22 | 30 |
| Copper transport and metabolism | ||||
| c0658 | cusC | Copper/silver efflux system outer membrane protein CusC | −5.88 | 30 |
| c0660 | cusB | Copper/silver efflux system membrane fusion protein CusB | −3.79 | 30 |
Shown are mutated genes or operons of strains that have been tested in the murine urinary tract and classified as fitness or virulence genes.
Type 1 fimbria expression in UPEC can be assessed by hemagglutination (HA) of guinea pig red blood cells (RBCs) in vitro. To test whether the downregulation of fim operon transcription leads to a biological phenotype, we compared the abilities of the parent, the cpxA mutant, and the cpxR mutant to hemagglutinate guinea pig RBCs. CFT073ON and CFT073OFF, in which fim expression is phase locked on and off, respectively, were used as positive and negative controls for fim-specific HA (36). Activation of CpxR by deletion of cpxA reduced the HA of guinea pig RBCs to a level comparable to that of the fim off mutant, whereas deletion of cpxR did not have any effect on fim-mediated HA (Fig. 2A). Complementation of the cpxA mutant with cpxRA in trans partially restored the ability of the mutant to hemagglutinate guinea pig RBCs (Fig. 2A). Mannose completely inhibited the ability of CFT073ON and the complemented cpxA mutant to hemagglutinate guinea pig RBCs (Fig. 2B). Thus, the downregulation of fim transcripts observed in the cpxA mutant correlates with the loss of Fim-related functions.
FIG 2.
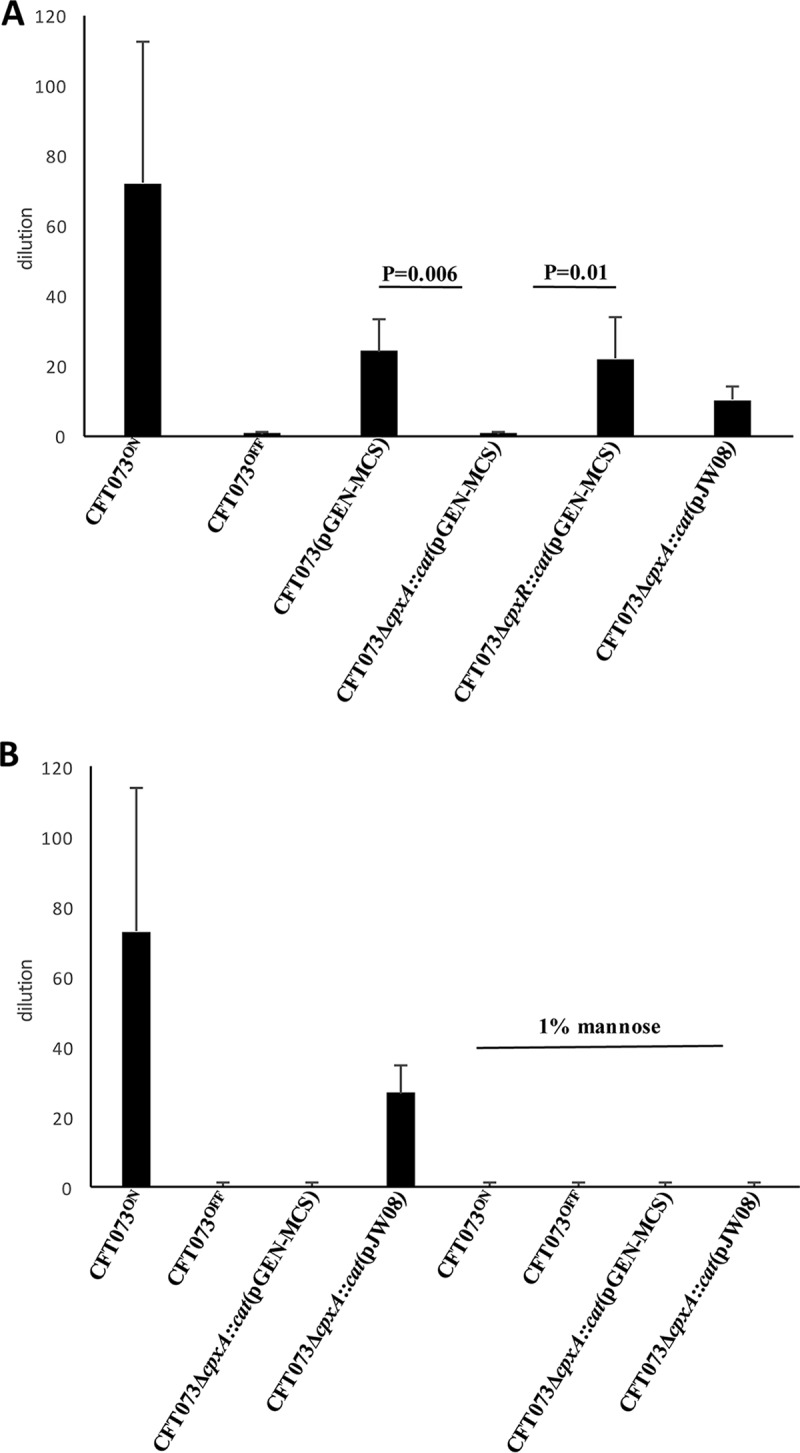
HA of 1% guinea pig RBCs by UPEC CFT073 and its derivatives (A) and by the complemented cpxA mutant and positive and negative controls in the absence and presence of 1% mannose (B). The data represent the mean and SD of the final dilution of bacteria at which agglutination occurred; the results are from four independent experiments. P values were calculated by ANOVA. Note that in the presence of mannose, no HA was observed.
The fim operon has not been reported to be regulated by CpxR. However, inspection of the fimA promoter sequence showed a putative CpxR binding motif ∼340 bp upstream of its transcriptional start site (see Table S3 in the supplemental material). We cloned the fimA promoter into a green fluorescent protein (GFP)-expressing reporter plasmid and assessed its Cpx dependence by transforming the plasmid into the parent, the cpxA mutant, and the cpxR mutant and measuring reporter activity by Western blotting. The expression of the fimA reporter was unaltered in the cpxA and cpxR mutants (data not shown), suggesting that regulation of the fim operon by CpxR is likely indirect, as is discussed below.
Activation of CpxR downregulates the expression of several UPEC fitness or virulence genes.
Using RNA-Seq, Subashchandrabose and colleagues defined UTI-specific genes as UPEC genes that are differentially upregulated during human UTIs compared to those in UPEC grown in uninfected human urine or laboratory medium (30). The UTI-specific genes that are required for a wild-type level of survival in a murine UTI model, such as fimH, are defined as UPEC fitness genes (30). We examined whether activation of CpxR affects the expression of additional fitness genes.
PhoBR is a two-component system that allows UPEC to respond to phosphate limitation in the environment by inducing genes in the Pho regulon (37). One member of the regulon, the pstSCAB-phoU operon, encodes a phosphate-specific transport system (37). Compared to the parent strain, activation of CpxR reduced the expression of the pstSCAB-phoU operon and also downregulated both phoB and phoR (Table 2; Table S1). Interestingly, a CFT073pstSCA deletion mutant is unable to colonize the murine bladder primarily because of favoring the off orientation of the invertible fim promoter and downregulation of type 1 fimbriae (37). Thus, activation of CpxR may interfere with the ability of UPEC to survive in environments in which phosphate is limited or impair virulence by indirectly downregulating the expression of the fim operon.
UPEC utilizes copper efflux pathways during infection to protect from copper toxicity (30). Comparison of the cpxA mutant and parent strains demonstrated that activation of CpxR downregulates the expression of some of the genes that encode copper ion channels (cusC, cusB) (Table 2; Table S1), pointing to the possibility that the cpxA mutant is not able to survive in copper-limited environments.
Although not upregulated during human UTI, the serine protease autotransporter of Enterobacteriaceae gene upaB and the oligopeptide uptake periplasmic binding protein oppA are essential for UPEC adherence to biotic surfaces and colonization of the murine urinary tract, respectively (38, 39). The expression of upaB and oppA was lower in the cpxA mutant than in the parent strain (Table 2; Table S1). Taken together, these results indicate that CpxR regulates several key fitness and virulence determinants that govern UPEC survival and adaptation in vivo.
Activation of CpxR upregulates virulence genes involved in iron and nickel transport and metabolism.
Since most iron is sequestered intracellularly within the host, UPEC has evolved mechanisms for iron acquisition that include either synthesis of iron chelators or siderophores (enterobactin and aerobactin) or utilization of iron from heme (40). Our transcriptome analysis showed that activation of CpxR upregulated the expression of iron transport genes (tonB, exbD, exbB), ferrichrome transporters (fhuA, fhuC, fhuD, fhuB), ferric enterobactin (fepA), enterobactin esterase (fes), ferric enterobactin ABC transporter complex (fepB, fepC, fepG, fepD), iron transporters (sitAD, sitC, sitB, sitA), and the outer membrane heme receptor (chuA) (Table 2; Table S2). Nickel transport genes are upregulated during human infection and required for murine infection (30); the expression of nickel transport genes (nikBCDE) was higher in the cpxA mutant than in the parent strain (Table 2; Table S2).
In summary, under growth conditions where cpxA deletion results in CpxR activation, deletion of cpxA led to a combination of downregulated and upregulated UPEC fitness and virulence determinants. Next, we assessed whether the cpxA and cpxRA mutants are attenuated for virulence in a murine UTI model.
The cpxA and cpxR mutants are less fit than the parent in the murine UTI model.
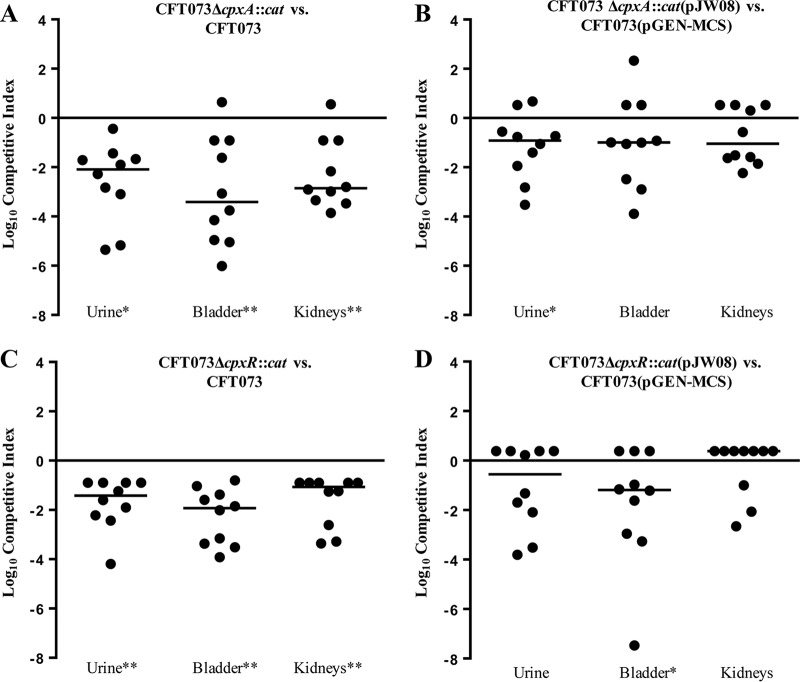
We first performed competition experiments in a mouse UTI model. CBA/J mice were transurethrally inoculated with approximately equal numbers (target dose, ∼1 × 108 CFU) of the parent strain and either the cpxA or the cpxR mutant cultured in medium containing amino acids but lacking glucose, which is usually absent from urine. The relative number of each strain recovered from the urine, bladders, and kidneys of mice was determined 48 h postinoculation. The cpxA mutant was recovered a median of 10-, 10,000-, and 1,000-fold less than the parent in the urine, bladder, and kidneys, respectively (Fig. 3A). The cpxR mutant was recovered a median of 10- to 100-fold less than the parent strain in the urine and tissues, respectively (Fig. 3C). When the cpxA and cpxR mutants were complemented with cpxRA in trans, both mutants were recovered at levels generally similar to those of the parent transformed with an empty vector (Fig. 3B and D). By quantitative real-time PCR, the complemented cpxA mutant expressed levels of cpxR and cpxA that were a mean of 363- and 258-fold higher than those of the parent transformed with the empty vector; similarly, the complemented cpxR mutant expressed levels of cpxR and cpxA that were a mean of 560- and 282-fold higher than those of the parent transformed with the empty vector. The high levels of cpxRA transcripts in the complemented mutants were likely due to their expression from a multicopy, stabilized plasmid vector (41, 42) and may explain why the complemented mutants did not fully recapitulate the infectivity of the parent in the murine model. Nevertheless, these results corroborate and extend previous findings that CpxR is required for full UPEC fitness in the bladder (26, 27) and suggest that activation of CpxR might abrogate the virulence of UPEC in the urinary tract.
FIG 3.
Competition experiments done in the murine UTI model. Shown are the log10 competitive indices of CFT073ΔcpxA::cat versus CFT073 (A), CFT073ΔcpxA::cat(pJW08) versus CFT073(pGEN-MCS) (B), CFT073ΔcpxR::cat versus CFT073 (C), and CFT073ΔcpxR::cat(pJW08) versus CFT073(pGEN-MCS) (D). Competitive indices were calculated as described in Materials and Methods; 10 mice were used for each comparison, and horizontal bars indicate the median index for each comparison. Statistical significance was determined by the Wilcoxon signed-rank test. *, P < 0.05; **, P < 0.01; all other comparisons were not significant.
The cpxA mutant is attenuated for virulence only in the kidney.
The competition experiments suggested that both cpxA and cpxR are required for virulence in the murine urinary tract. To test this hypothesis, we performed single-strain infection experiments. Groups of CBA/J mice were individually infected with approximately equal numbers (target dose, ∼1 × 108 CFU) of the parent, the cpxA mutant, and the cpxR mutant and sacrificed 48 h later. Unexpectedly, there were no differences in the CFU counts recovered from the urine and bladder among the three groups. Only the cpxA mutant was recovered at significantly lower levels than the parent in the kidney (P = 0.028) (Fig. 4). Our finding that cpxR is not required for infection of the bladder by CFT073 is not in agreement with single-strain challenges of CBA/J (26) or C3H/HeN (27) mice done with UTI89, suggesting that there are strain-specific differences in the role of cpxR in UPEC pathogenesis.
FIG 4.
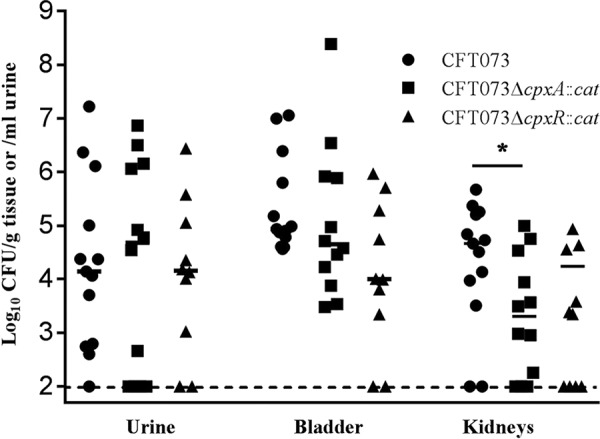
Single-strain infection experiments done in the murine UTI model. Shown are the log10 CFU counts per gram of tissue or log10 CFU counts per milliliter of urine recovered from the urine, bladders, or kidneys of mice infected with CFT073 (n = 13), CFT073ΔcpxA::cat (n = 12), or CFT073ΔcpxR::cat (n = 10); horizontal bars indicate the median recovery for each comparison. Statistical significance was determined by two-factor ANOVA. The asterisk indicates that the cpxA mutant was recovered from the kidneys at significantly lower levels than the parent strain (P = 0.028).
Deletion of cpxR in UTI89 leads to impairment of bladder colonization through overexpression of hlyA and subsequent sloughing of bladder epithelial cells (27). However, hlyA was not differentially upregulated in the cpxR mutant and CFT073, which may explain why the cpxR mutant was as virulent as CFT073 in the bladder. To verify this finding, we measured the hemolytic activity around colonies of CFT073, the cpxR mutant, and CFT073hlyA::TnphoA on blood agar plates. As expected, the zones of hemolysis around CFT073 (mean ± standard deviation [SD], 7.6 ± 1.2 mm) and the cpxR mutant (7.2 ± 1.3 mm) were similar, while CFT073hlyA::TnphoA had no hemolytic activity. We also cloned the hlyA promoter into a GFP-expressing reporter plasmid; transformed the plasmid into the parent, the cpxA mutant, and the cpxR mutant; and measured reporter activity by Western blotting. The expression of the hlyA reporter was unaltered in the cpxA and cpxR mutants (data not shown).
DISCUSSION
The rise of antibiotic-resistant Enterobacteriaceae has led to urgent calls for new antimicrobial strategies and drug targets. Antivirulence strategies that focus on targeting essential bacterial functions, rather than bacterial survival, have become an intensive area of research, because they theoretically carry a lower risk of selective pressure on bacteria (43). Our interest in CpxRA activation as a potential broad-spectrum antimicrobial strategy stems from observations that activation of this system by deletion of cpxA obliterates the virulence of several pathogens belonging to diverse bacterial families in human and murine models of infection (12, 14, 23). We identified a class of compounds that activate CpxRA in E. coli K-12 by inhibiting cpxA phosphatase activity; these compounds chemically mimic the cpxA deletion phenotype (22). In this study, we show that there are strain-specific differences in the requirement for cpxR for bladder infections due to UPEC. Our results also suggest that activation of CpxR by deletion of cpxA may only affect the ability of UPEC to infect the kidney. Taken together, our data suggest that although CpxA phosphatase inhibitors may have limited value in the treatment of uncomplicated UTIs due to UPEC, they may have value in the treatment of pyelonephritis.
An issue with using CpxA phosphatase inhibitors to treat UPEC infections is the requirement for bacterial metabolism of glucose into acetyl phosphate, which leads to the formation of phosphorylated CpxR, for compound effect (20). Except in diabetes, urine usually contains no glucose. Although the bladder epithelium utilizes glucose, the epithelium does not store glucose. As glucose is completely reabsorbed proximally in the kidney, which stores glycogen, UPEC is more likely to encounter glucose in the proximal nephron than elsewhere in the urinary tract. Even if glucose is available, UPEC preferentially uses peptides and amino acids over glucose as carbon sources during infection of the bladder epithelium and urine (44–46). Thus, the limited availability of glucose in the urinary tract and the preferential use of peptides by UPEC in this compartment may explain why the cpxA mutant was impaired for virulence only in the kidney.
Given that the cpxA mutant was attenuated in the kidney, CpxA phosphatase inhibitors could have therapeutic value in complex UTIs such as pyelonephritis. Adjunctive therapies for pyelonephritis could prevent complications associated with this infection, such as sepsis in the elderly or adverse perinatal outcomes during pregnancy. CpxA phosphatase inhibitors might also provide a strategy to prevent renal scarring in children with recurrent UTIs. Finally, phosphatase inhibitors could have efficacy against UPEC in diabetics whose UTIs occur in the setting of glycosuria or against other organisms, such as Proteus mirabilis, that contain the CpxRA system and preferentially utilize glucose as a primary carbon source during UTI (45).
Previous studies done with UTI89 showed that activation of CpxRA by deletion of cpxP had no effect on the competitive fitness of the mutant strain versus its parent in the urinary bladder (26). Deletion of cpxP relieves inhibition of CpxA kinase activity but does not cause full activation of the pathway (47); the difference in the relative fitness of the cpxA and cpxP mutants might reflect strain differences or their relative levels of CpxR activation in the mutant inocula used in each study. However, single-strain infection experiments showed that neither mutant was attenuated for virulence in the bladder, suggesting that both cpxP- and glucose (ΔcpxA)-dependent activation of the pathway is insufficient to affect virulence in this compartment.
By comparing the transcriptomes of the cpxA mutant and wild-type strains, we found that cpxA deletion downregulated the expression of multiple fitness and virulence genes, particularly those belonging to the chaperone-usher fimbriae, the type 1 fimbriae, and the P pili. Activation of CpxR downregulated the expression of the main pilus structural gene fimA and the adhesin gene fimH, as well as most of the other structural and regulatory genes in the fim operon. The decrease in transcript expression correlated with a decreased ability of the cpxA mutant to hemagglutinate guinea pig RBCs. Although motif analysis showed that the fim operon promoter contains a CpxR-binding motif, reporter assays showed that regulation of the fimA promoter does not occur through activation of CpxR. Previous studies have confirmed that the fim operon is under the control of the phosphate operon pst; deletion of pst results in bacteria that are less capable of invading bladder epithelial cells in vitro and are unable to colonize the bladder in mice by directly downregulating the expression of type 1 fimbriae (37). We found that the pst genes are also significantly downregulated upon CpxRA activation. Collectively, these results suggest that CpxRA activation indirectly inhibits type 1 fimbria operon transcription in UPEC.
While regulation of the fim operon by CpxRA in UPEC has not been previously described, P pili are regulated by the CpxRA system (48). In E. coli MC4100 containing a recombinant chromosomal pap operon, CpxRA activation in cpxA* mutants reduced the transcription of the pap pilin genes and the expression of the Pap pilus proteins, as did the overexpression of the outer membrane lipoprotein NlpE (48). In CFT073, activation of CpxR downregulated the transcription of the papA pilin gene, as well as the positive regulatory factor papI, consistent with the reduced fitness of the cpxA mutant relative to the parent in the kidney.
The cpxR mutant was attenuated for fitness in the bladder, urine, and kidney, but in single-strain infection experiments, it was as virulent as the parent in all three compartments. In contrast, a cpxR mutant of UTI89 is attenuated in the bladder. In UTI89, CpxR directly binds to the promoter of hlyA and deletion of cpxR increases the transcription of hlyA, which encodes an α-hemolysin that induces the caspase-dependent death of uroepithelial cells (27). Although the hlyA promoter region in both UTI89 and CFT073 contains putative CpxR recognition sequences in the same position (data not shown), transcriptome analysis of the CFT073 cpxR mutant did not show differential hlyA gene expression, which was confirmed in reporter and functional assays. Thus, the differential effects of CpxR deletion on hlyA expression likely explain the differences in the virulence of these strains in the bladder. If the majority of UPEC isolates are like UTI89 in the requirement for CpxR for infection within the urinary tract, then compounds that alter CpxR signaling may be useful in treatment.
The differences in infectivity between the cpxR mutants of CFT073 and UTI89 underscore the high genetic heterogeneity seen among UPEC strains. Much of this heterogeneity is due to the acquisition of pathogenicity-associated islands by horizontal gene transfer; CFT073 has 13 such islands, which make up 20% of its genome (28, 35). Consequently, a core set of virulence factors required for UPEC has not been established and the expression of virulence factors varies substantially across strains (35, 49). For example, cystitis strains express high levels of type 1 fimbriae, whereas pyelonephritis strains express low levels (50, 51). The expression of other virulence determinants such as P fimbriae and hemolysin is high in some strains and absent in others, and the set of iron chelators and their levels of expression differ across strains (49, 52). Although the CpxR-regulated transcriptome has only been determined for CFT073, the set of genes regulated by activated CpxR may differ across UPEC strains; this issue would need to be addressed before pursuing a CpxR activation/antimicrobial strategy.
We conclude that CpxA phosphatase inhibitors may have some value in the treatment of kidney infections due to UPEC and may have utility against other pathogens that utilize glucose as a primary carbon source during UTI (45). Whether CpxA kinase activators, which do not need glucose for activation, could attenuate UPEC infections in the bladder is unknown. As we have yet to find kinase-activating compounds in our high-throughput screening efforts, we did not test a constitutively active cpxA* mutant in this study. However, transcriptome analysis of our activating mutant suggests that glucose-independent activation of CpxR could downregulate several key virulence factors and lead to the clearance of UPEC.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 3. Strains were maintained on Luria broth (LB) plates supplemented with ampicillin (100 μg/ml) to maintain plasmids or with chloramphenicol (20 μg/ml) to select mutants, if appropriate. Except as indicated, the UPEC strains were cultured in Tryptone broth (TB) containing 0.4% glucose at 37°C with shaking. For the animal infection experiments, the strains were grown in LB overnight with shaking with or without ampicillin.
TABLE 3.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli strains | ||
| CFT073 | Clinical urosepsis isolate, parent strain | 62 |
| CFT073ΔcpxR::cat | CFT073 cpxR insertion/deletion mutant containing a chloramphenicol resistance cassette; polar on cpxA | This study |
| CFT073ΔcpxA::cat | CFT073 cpxA in-frame insertion/deletion mutant containing a chloramphenicol resistance cassette | This study |
| CFT073ON | CFT073 fim invertible element phase locked on; constitutive type 1 fimbrial expression | 36 |
| CFT073OFF | CFT073 fim invertible element phase locked off; no type 1 fimbrial expression | 36 |
| CFT073hlyA::TnphoA | CFT073 derivative that does not express hemolysin | 62 |
| Plasmids | ||
| pKD3 | λ Red template plasmid containing a chloramphenicol resistance cassette flanked by FRT sites; Ampr | 53 |
| pKD46 | Arabinose-inducible λ Red expression plasmid; Ampr | 53 |
| pGEN-MCS | Medium-copy-number high-retention plasmid containing a promoterless multiple cloning site; Ampr | 41 |
| pJLJ42 | cpxRA sequence with native promoter from UTI89 cloned into pGEN-MCS; Ampr | 26 |
| pJW08 | pJLJ42 that was modified so that the cpxRA sequence was identical to that of CFT073; Ampr | This study |
| pRB157 | pLS88 derivative containing a BglII site for insertion of promoter sequences and a promoterless GFP cassette derived from pGreenTIR | 25 |
| pKF31 | pLS88 derivative containing fimA promoter sequences | This study |
| pKF32 | pLS88 derivative containing hlyA promoter sequences | This study |
Construction and complementation of cpxR and cpxA mutants.
We attempted to construct in-frame cpxR and cpxA insertion/deletion mutants of CFT073 by using the λ Red recombination system and a nonpolar cat cassette as previously described (53). The cat cassette was used to mark the mutants so that we could distinguish them from the parent in competition experiments and because the cassette itself does not necessarily impair the virulence of mutants in the murine model (45). In brief, the cat cassette was amplified from plasmid pDK3 (53) with primers P1 and P2 for deletion of cpxR and primers P3 and P4 for deletion of cpxA (see Table S5 in the supplemental material). Amplicons were treated with DpnI and electroporated into E. coli CFT073 containing λ Red plasmid pKD46 (53). Recombinants were selected on Luria-Bertani medium containing 20 μg/ml chloramphenicol. PCR and sequence analysis confirmed the construction of an in-frame cpxA::cat (cpxA) mutant but showed that in the cpxR::cat (cpxR) mutant, the cpxR deletion was polar on cpxA, phenocopying a cpxRA double mutant.
The cpxR and cpxA mutants were complemented with pJW09, which expresses cpxRA from its native promoter. pJW09 was modified from pJLJ42, which was kindly supplied by Matthew Mulvey of the University of Utah (26). In the initial construction of pJLJ42, cpxRA was amplified from E. coli UTI89; cpxR of UTI89 differs from that of CFT073 by one codon. Thus, QuikChange site-directed mutagenesis with primers P5 and P6 was used to replace a Thr codon (ACC) at nucleotide 446 with a Ser (AGC) codon. Additionally, QuikChange site-directed mutagenesis with P7 and P8 was used to insert a thymine at nucleotide position 1093 of cpxA to correct a frameshift mutation in pJLJ42. Sequence analysis verified the expected changes in pJW09.
RNA isolation and quality assessment.
Total RNA was extracted from four independent cultures of the three strains (parent, cpxA mutant, cpxR mutant) grown overnight with shaking and then subcultured for an additional 4 h to stationary phase (optical density at 600 nm [OD600] of 1.0) with TRIzol reagent (Invitrogen, Carlsbad, CA) in accordance with the manufacturer's protocol. RNA samples were treated twice with TURBO DNA-free DNase (Ambion-Thermo Fisher Scientific, Waltham, MA). The purity of the RNA was confirmed by agarose gel electrophoresis of the RT-PCR product of the housekeeping gene gapA with the QuantiTect SYBR green RT-PCR kit (Qiagen, Germantown, MD). The integrity of the RNA was confirmed with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA), and the RNA concentration was measured with a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA). The removal of rRNA and preparation of RNA-Seq libraries were performed with the ScriptSeq Complete kit (Illumina, San Diego, CA) in accordance with the manufacturer's protocol. mRNA libraries were sequenced on the Illumina NextSeq platform for paired-end sequencing with read lengths of 75 bases at the Center for Genomics and Bioinformatics (Bloomington, IN). The sequenced reads were mapped to the CFT073 genome (GenBank accession number NC_004431.1), and quantification of transcript levels and identification of differentially expressed genes were performed as described previously (25). Cutoffs of a 2-fold change and a false-discovery rate of ≤0.1 were chosen to define differential gene expression in the strains (25). Gene classification was performed by using the annotated CFT073 genome and the COG database (54).
qRT-PCR.
qRT-PCR was performed with the QuantiTect SYBR green RT-PCR kit (Qiagen, Germantown, MD) in an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). Gene fragments were amplified with primer pairs P9 to P40, which are listed in Table S5. All primer pairs had >95% efficiency. The expression levels of the genes were normalized to that of gapA. The fold change in expression was calculated by using the following equation: Ratio = (Etarget)ΔCT target (ΔcpxA-ΔcpxR)/(Ereference)ΔCT reference (ΔcpxA-ΔcpxR), where E is the amplification efficiency = 10−1/slope and ΔCT is the change in the cycle threshold.
Identification of differentially regulated genes with a CpxR-binding motif.
The 450-bp upstream promoter sequences of genes in the differentially regulated operons (both upregulated and downregulated) were extracted from the reference sequence of E. coli CFT073. A position-specific scoring matrix was generated on the basis of the known CpxR motif sequences (17). Find Individual Motif Occurrences software from the Multiple EM for Motif Elicitation algorithm (version 4.11.1) (55) package was used to search for the CpxR binding motif in the extracted promoter sequences.
HA assays.
HA assays were conducted by a modification of the method of Hultgren et al. (56). Briefly, UPEC strains were grown statically in TB supplemented with 0.4% glucose for 24 h (OD600 of 1.0.) and subcultured every 24 h in fresh medium for a total of 72 h. One milliliter of culture (5 × 108 to 1 × 109 CFU) was pelleted and suspended in 0.1 ml of phosphate-buffered saline (PBS) supplemented with 0.4% glucose. The cell suspension was serially diluted 2-fold in PBS-glucose in a 96-well round-bottom plate to a final volume of 25 μl/well; an equal volume of 1% guinea pig RBCs (Lonza, Walkersville, MD) was added to each well. Plates were incubated at 4°C for 4 h, and the HA endpoint was determined by the last dilution where HA visually occurred. The experiment was replicated four times, and the mean dilution and SD were calculated for each strain. The experiments were repeated in the presence of 1% mannose.
Reporter assays.
Reporter assays were performed as previously described (12). Approximately 485 bp of the fimA promoter region and 398 bp of the hlyA promoter region were amplified by PCR with primers P41 and P42 and primers P43 and P44 (see Table S5 in the supplemental material). The amplified PCR products were ligated to pRB157 by using the BglII restriction site preceding a promoterless gfp cassette. The orientation of the insert with respect to the gfp cassette was confirmed by PCR with a promoter-specific forward primer and the reverse primer P45, which hybridized to a region of the gfp cassette downstream of the BglII site. Final constructs were confirmed by sequencing and then electroporated into the parent, the cpxA mutant, and the cpxR mutant. Whole-cell lysates were prepared from each transformant harvested at the stationary growth phase and analyzed by Western blotting with monoclonal antibodies specific to GFP (Clontech, Mountain View, CA) and GroEL (Abcam, Cambridge, MA) (12). As a control for the Western blot assays, we used 35000HP(pKF1), which expresses GFP driven by the lspB promoter (12). Reporter activity was assessed in four independent experiments.
Hemolysin assays.
CFT073, CFT073ΔcpxR::cat, and CFT073hlyA::TnphoA were grown on blood agar plates and incubated overnight at 37°C. Zones of hemolysis around 20 individual colonies from two independent experiments were photographed and assessed with ImageJ software as previously described (27, 57).
Murine infection experiments.
Animal experiments were conducted in accordance with the guidelines of the University of Michigan Institutional Animal Care and Use Committee. In competitive fitness experiments, female CBA/J mice (Envigo, Indianapolis, IN) were injected transurethrally with 50 μl containing a target dose of ∼108 CFU of the wild type and an equal amount of either the cpxA or the cpxR mutant (equal numbers of the parent and mutant strains). The mice were euthanized after 48 h. The bacterial loads of each strain in the urine, bladders, and kidneys of mice were calculated as previously described (58). For each comparison, competitive indices were calculated by dividing the ratio of mutant to wild-type bacteria in the tissue or urine (output) by the ratio of mutant to wild-type bacteria in the inoculum (input). Competition experiments were repeated with mutant strains that were complemented with pJW09 versus the parent transformed with pGEN-MCS. Statistical significance was determined by the Wilcoxon signed-rank test.
For single-strain infection experiments, three groups of eight female CBA/J mice (Jackson Laboratories, Bar Harbor, ME) were infected with approximately equal amounts of the parent (2.8 × 108 CFU), the cpxA mutant (1.6 × 108 CFU), and the cpxR mutant (1.5 × 108 CFU) and euthanized at 48 h so that bacterial loads could be determined as described above. In the first iteration, three mice infected with the cpxR mutant and one mouse infected with the cpxA mutant died shortly after inoculation because of mechanical difficulties encountered during the inoculation procedure. As these mice were not cultured, a second iteration was done with five mice in each group from the same supplier. The doses of the parent (1.8 × 108 CFU), the cpxA mutant (1.1 × 108 CFU), and the cpxR mutant (1.1 × 108 CFU) in the second iteration were similar to those in the first iteration. Therefore, the data from the two iterations were combined for statistical analysis. After log transformation of the data, a two-factor analysis of variance (ANOVA), adjusting for iteration, was performed comparing the recovery of the three strains in each compartment (urine, bladder, and kidney). Pairwise comparisons of each strain were adjusted for multiple comparisons by the Tukey procedure.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a Project Development Team within Indiana Clinical and Translational Sciences Institute (CTSI) NIH/NCRR grant UL1TR001108 to S.M.S. and by U.S. Public Health Service grant AI059722 to H.L.T.M.
RNA sequencing was performed by the Center for Genomics and Bioinformatics at Indiana University Bloomington, a core facility of the Indiana CTSI. We thank Byron Batteiger for his careful review of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00798-17.
REFERENCES
- 1.Hooton TM. 2012. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med 366:1028–1037. doi: 10.1056/NEJMcp1104429. [DOI] [PubMed] [Google Scholar]
- 2.Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. 2012. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev 36:616–648. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sivick KE, Mobley HL. 2010. Waging war against uropathogenic Escherichia coli: winning back the urinary tract. Infect Immun 78:568–585. doi: 10.1128/IAI.01000-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. 2010. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis 51:286–294. doi: 10.1086/653932. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee R, Johnston B, Lohse C, Porter SB, Clabots C, Johnson JR. 2013. Escherichia coli sequence type 131 is a dominant, antimicrobial-resistant clonal group associated with healthcare and elderly hosts. Infect Control Hosp Epidemiol 34:361–369. doi: 10.1086/669865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, Lewis JS II, Howard WJ, Johnson LE, Polsky B, Jorgensen JH, Richter SS, Shutt KA, Paterson DL. 2013. Community-associated extended-spectrum beta-lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis 56:641–648. doi: 10.1093/cid/cis942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paterson DL, Doi Y. 2007. A step closer to extreme drug resistance (xdr) in Gram-negative bacilli. Clin Infect Dis 45:1179–1181. doi: 10.1086/522287. [DOI] [PubMed] [Google Scholar]
- 8.Bonomo RA. 2011. New Delhi metallo-β-lactamase and multidrug resistance: a global SOS? Clin Infect Dis 52:485–487. doi: 10.1093/cid/ciq179. [DOI] [PubMed] [Google Scholar]
- 9.Mediavilla JR, Patrawalla A, Chen L, Chavda KD, Mathema B, Vinnard C, Dever LL, Kreiswirth BN. 2016. Colistin- and carbapenem-resistant Escherichia coli harboring mcr-1 and blaNDM-5, causing a complicated urinary tract infection in a patient from the United States. mBio 7:e01191-16. doi: 10.1128/mBio.01191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogt SL, Raivio TL. 2012. Just scratching the surface: an expanding view of the Cpx envelope stress response. FEMS Microbiol Lett 326:2–11. doi: 10.1111/j.1574-6968.2011.02406.x. [DOI] [PubMed] [Google Scholar]
- 11.Raivio TL. 2014. Everything old is new again: an update on current research on the Cpx envelope stress response. Biochim Biophys Acta 184:1529–1541. doi: 10.1016/j.bbamcr.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Spinola SM, Fortney KR, Baker B, Janowicz DM, Zwickl B, Katz BP, Blick RJ, Munson RS Jr. 2010. Activation of the CpxRA system by deletion of cpxA impairs the ability of Haemophilus ducreyi to infect humans. Infect Immun 78:3898–3904. doi: 10.1128/IAI.00432-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y, Lenz J, Arvidson CG. 2005. Global gene expression and the role of sigma factors in Neisseria gonorrhoeae in interactions with epithelial cells. Infect Immun 73:4834–4845. doi: 10.1128/IAI.73.8.4834-4845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gangaiah D, Raterman E, Wu H, Fortney KR, Gao H, Liu Y, Jerse AE, Spinola SM. 2017. Both MisR (CpxR) and MisS (CpxA) are required for Neisseria gonorrhoeae infection in a murine model of lower genital tract infection. Infect Immun 85:e00307-17. doi: 10.1128/IAI.00307-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldheim YS, Zusman T, Speiser Y, Segal G. 2016. The Legionella pneumophila CpxRA two-component regulatory system: new insights into CpxR's function as a dual regulator and its connection to the effectors regulatory network. Mol Microbiol 99:1059–1079. doi: 10.1111/mmi.13290. [DOI] [PubMed] [Google Scholar]
- 16.Raivio TL, Silhavy TJ. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol 179:7724–7733. doi: 10.1128/jb.179.24.7724-7733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Wulf P, McGuire AM, Liu X, Lin ECC. 2002. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J Biol Chem 277:26652–26661. doi: 10.1074/jbc.M203487200. [DOI] [PubMed] [Google Scholar]
- 18.Price NL, Raivio TL. 2009. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J Bacteriol 191:1798–1815. doi: 10.1128/JB.00798-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raivio TL, Leblanc SK, Price NL. 2013. The Escherichia coli Cpx envelope stress response regulates genes of diverse function that impact antibiotic resistance and membrane integrity. J Bacteriol 195:2755–2767. doi: 10.1128/JB.00105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfe AJ, Parikh N, Lima BP, Zemaitaitis B. 2008. Signal integration by the two-component signal transduction response regulator CpxR. J Bacteriol 190:2314–2322. doi: 10.1128/JB.01906-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lima BP, Antelmann H, Gronau K, Chi BK, Becher D, Brinsmade SR, Wolfe AJ. 2011. Involvement of protein acetylation in glucose-induced transcription of a stress-responsive promoter. Mol Microbiol 81:1190–1204. doi: 10.1111/j.1365-2958.2011.07742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Rensburg JJ, Fortney KR, Chen L, Krieger AJ, Lima BP, Wolfe AJ, Katz BP, Zhang ZY, Spinola SM. 2015. Development and validation of a high-throughput cell-based screen to identify activators of a bacterial two-component signal transduction system. Antimicrob Agents Chemother 59:3789–3799. doi: 10.1128/AAC.00236-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humphreys S, Rowley G, Stevenson A, Anjum MF, Woodward MJ, Gilbert S, Kormanec J, Roberts M. 2004. Role of the two-component regulator CpxAR in the virulence of Salmonella enterica serotype Typhimurium. Infect Immun 72:4654–4661. doi: 10.1128/IAI.72.8.4654-4661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labandeira-Rey M, Dodd D, Fortney KR, Zwickl B, Katz BP, Janowicz DM, Spinola SM, Hansen EJ. 2011. A Haemophilus ducreyi cpxR deletion mutant is virulent in human volunteers. J Infect Dis 203:1859–1865. doi: 10.1093/infdis/jir190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gangaiah D, Zhang X, Fortney KR, Baker B, Liu Y, Munson RS Jr, Spinola SM. 2013. Activation of CpxRA in Haemophilus ducreyi primarily inhibits the expression of its targets, including major virulence determinants. J Bacteriol 195:3486–3502. doi: 10.1128/JB.00372-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debnath I, Norton JP, Barber AE, Ott EM, Dhakal BK, Kulesus RR, Mulvey MA. 2013. The Cpx stress response system potentiates the fitness and virulence of uropathogenic Escherichia coli. Infect Immun 81:1450–1459. doi: 10.1128/IAI.01213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagamatsu K, Hannan TJ, Guest RL, Kostakioti M, Hadjifrangiskou M, Binkley J, Dodson K, Raivio TL, Hultgren SJ. 2015. Dysregulation of Escherichia coli α-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc Natl Acad Sci U S A 112:E871–E880. doi: 10.1073/pnas.1500374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welch RA, Burland V, Plunkett G 3rd, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DC, Perna NT, Mobley HL, Donnenberg MS, Blattner FR. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A 99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen SL, Hung CS, Xu J, Reigstad CS, Magrini V, Sabo A, Blasiar D, Bieri T, Meyer RR, Ozersky P, Armstrong JR, Fulton RS, Latreille JP, Spieth J, Hooton TM, Mardis ER, Hultgren SJ, Gordon JI. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc Natl Acad Sci U S A 103:5977–5982. doi: 10.1073/pnas.0600938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subashchandrabose S, Hazen TH, Brumbaugh AR, Himpsl SD, Smith SN, Ernst RD, Rasko DA, Mobley HL. 2014. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc Natl Acad Sci U S A 111:18327–18332. doi: 10.1073/pnas.1415959112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salipante SJ, Roach DJ, Kitzman JO, Snyder MW, Stackhouse B, Butler-Wu SM, Lee C, Cookson BT, Shendure J. 2015. Large-scale genomic sequencing of extraintestinal pathogenic Escherichia coli strains. Genome Res 25:119–128. doi: 10.1101/gr.180190.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Wulf P, Kwon O, Lin EC. 1999. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J Bacteriol 181:6772–6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slamti L, Waldor MK. 2009. Genetic analysis of activation of the Vibrio cholerae Cpx pathway. J Bacteriol 191:5044–5056. doi: 10.1128/JB.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guest RL, Wang J, Wong JL, Raivio TL. 2017. A bacterial stress response regulates respiratory protein complexes to control envelope stress adaptation. J Bacteriol 199:e00153-17. doi: 10.1128/JB.00153-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subashchandrabose S, Mobley HL. 2015. Virulence and fitness determinants of uropathogenic Escherichia coli. Microbiol Spectr 3:1–32. doi: 10.1128/microbiolspec.UTI-0015-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunther NW IV, Snyder JA, Lockatell V, Blomfield I, Johnson DE, Mobley HL. 2002. Assessment of virulence of uropathogenic Escherichia coli type 1 fimbrial mutants in which the invertible element is phase-locked on or off. Infect Immun 70:3344–3354. doi: 10.1128/IAI.70.7.3344-3354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crépin S, Houle S, Charbonneau ME, Mourez M, Harel J, Dozois CM. 2012. Decreased expression of type 1 fimbriae by a pst mutant of uropathogenic Escherichia coli reduces urinary tract infection. Infect Immun 80:2802–2815. doi: 10.1128/IAI.00162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allsopp LP, Beloin C, Ulett GC, Valle J, Totsika M, Sherlock O, Ghigo JM, Schembri MA. 2012. Molecular characterization of UpaB and UpaC, two new autotransporter proteins of uropathogenic Escherichia coli CFT073. Infect Immun 80:321–332. doi: 10.1128/IAI.05322-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subashchandrabose S, Smith SN, Spurbeck RR, Kole MM, Mobley HL. 2013. Genome-wide detection of fitness genes in uropathogenic Escherichia coli during systemic infection. PLoS Pathog 9:e1003788. doi: 10.1371/journal.ppat.1003788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres AG, Redford P, Welch RA, Payne SM. 2001. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect Immun 69:6179–6185. doi: 10.1128/IAI.69.10.6179-6185.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane MC, Alteri CJ, Smith SN, Mobley HL. 2007. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc Natl Acad Sci U S A 104:16669–16674. doi: 10.1073/pnas.0607898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galen JE, Nair J, Wang JY, Wasserman SS, Tanner MK, Sztein MB, Levine MM. 1999. Optimization of plasmid maintenance in the attenuated live vector vaccine strain Salmonella typhi CVD 908-htrA. Infect Immun 67:6424–6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasko DA, Sperandio V. 2010. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov 9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 44.Alteri CJ, Smith SN, Mobley HL. 2009. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog 5:e1000448. doi: 10.1371/journal.ppat.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alteri CJ, Himpsl SD, Mobley HL. 2015. Preferential use of central metabolism in vivo reveals a nutritional basis for polymicrobial infection. PLoS Pathog 11:e1004601. doi: 10.1371/journal.ppat.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conover MS, Hadjifrangiskou M, Palermo JJ, Hibbing ME, Dodson KW, Hultgren SJ. 2016. Metabolic requirements of Escherichia coli in intracellular bacterial communities during urinary tract infection pathogenesis. mBio 7:e00104-16. doi: 10.1128/mBio.00104-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raivio TL, Popkin DL, Silhavy TJ. 1999. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J Bacteriol 181:5263–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernday AD, Braaten BA, Broitman-Maduro G, Engelberts P, ALD. 2004. Regulation of the Pap epigenetic switch by CpxAR: phosphorylated CpxR inhibits transition to the phase ON state by competition with Lrp. Mol Cell 16:537–547. doi: 10.1016/j.molcel.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 49.Brumbaugh AR, Mobley HL. 2012. Preventing urinary tract infection: progress toward an effective Escherichia coli vaccine. Expert Rev Vaccines 11:663–676. doi: 10.1586/erv.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snyder JA, Lloyd AL, Lockatell CV, Johnson DE, Mobley HL. 2006. Role of phase variation of type 1 fimbriae in a uropathogenic Escherichia coli cystitis isolate during urinary tract infection. Infect Immun 74:1387–1393. doi: 10.1128/IAI.74.2.1387-1393.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gunther NW IV, Lockatell V, Johnson DE, Mobley HL. 2001. In vivo dynamics of type 1 fimbria regulation in uropathogenic Escherichia coli during experimental urinary tract infection. Infect Immun 69:2838–2846. doi: 10.1128/IAI.69.5.2838-2846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagan EC, Lloyd AL, Rasko DA, Faerber GJ, Mobley HL. 2010. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog 6:e1001187. doi: 10.1371/journal.ppat.1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galperin MY, Makarova KS, Wolf YI, Koonin EV. 2015. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res 43:D261–D269. doi: 10.1093/nar/gku1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hultgren SJ, Schwan WR, Schaeffer AJ, Duncan JL. 1986. Regulation of production of type 1 pili among urinary tract isolates of Escherichia coli. Infect Immun 54:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alteri CJ, Hagan EC, Sivick KE, Smith SN, Mobley HL. 2009. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog 5:e1000586. doi: 10.1371/journal.ppat.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spurbeck RR, Alteri CJ, Himpsl SD, Mobley HL. 2013. The multifunctional protein YdiV represses P fimbria-mediated adherence in uropathogenic Escherichia coli. J Bacteriol 195:3156–3164. doi: 10.1128/JB.02254-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bahrani-Mougeot FK, Buckles EL, Lockatell CV, Hebel JR, Johnson DE, Tang CM, Donnenberg MS. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol Microbiol 45:1079–1093. doi: 10.1046/j.1365-2958.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- 61.Spurbeck RR, Stapleton AE, Johnson JR, Walk ST, Hooton TM, Mobley HL. 2011. Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: contribution of Ygi and Yad fimbriae. Infect Immun 79:4753–4763. doi: 10.1128/IAI.05621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, Jones BD, Warren JW. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun 58:1281–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.