Abstract
The most specific autoimmunity known for rheumatoid arthritis (RA) is reflected by generation of anti-citrullinated protein antibodies (ACPA). Presence of ACPA in established RA is associated with disease severity, while generation of ACPA at early developmental phases of RA can have a strong predictive value for progressing to the full-blown disease. Hence, development of ACPA may be of crucial importance to the pathogenesis of RA. Therefore, a lot of effort has been put recently to investigate the feature of ACPA at early developmental stages of RA (before disease onset) and functional activities of these autoantibodies. Results of these studies enlarged the knowledge about the nature of ACPA, which is essential for planning the therapeutic or preventive strategies interfering with their development and pathogenic functions. In this review we describe recent evidence for a role of ACPA in the etiopathogenesis of RA and indicate key unresolved issues regarding ACPA biology that need to be clarified in the future.
Keywords: rheumatoid arthritis, pathogenesis, anti-citrullinated protein antibodies
Introduction
Rheumatoid arthritis (RA) is the most common autoimmune joint disease. The principal sites of pathology in RA are joint tissues affected by chronic synovial inflammation, synovial hyperplasia and joint cartilage, and bone destruction. Numerous extra-articular disease manifestations also occur frequently, among which cardiovascular complications are associated with an increased mortality rate in RA patients. Despite the significant improvement in therapeutic management by using biologic disease-modifying drugs, the true long-term remission rarely occurs and the disease progresses leading ultimately to joint dysfunction and disability. It was recently shown that application of aggressive inflammation-targeted therapy within the first months from the onset of symptoms is very beneficial to the patients through slowing the rate of long-term structural damage [1, 2]. These results indicate the existence of the therapeutic window of opportunity – the short period of time for initiation of efficacious therapeutic intervention, very early in the course of RA. Therefore, recent research interests in rheumatology focused on characterisation of pathogenic events occurring over the early period of RA, prior to its onset. To facilitate studies regarding this issue, the hypothetical phases of disease (from its putative initiating events to full-blown clinical manifestation) were distinguished (Fig. 1, left panel) [3].
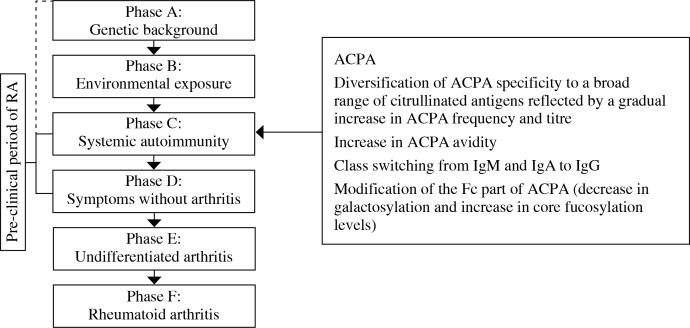
Fig. 1.
Phases of development of RA. Left panel: In prospective studies individuals are described as having genetic risk factors for RA (Phase A), environmental risk factors for RA (Phase B), systemic autoimmunity associated with RA (Phase C), non-specific symptoms such as arthralgia and morning stiffness without clinical arthritis (Phase D), undifferentiated arthritis – UA (Phase E) and, ultimately RA (Phase F). The term “arthritis” describes clinically apparent soft tissue swelling or fluid. The term “pre-clinical RA” indicates any phase up to phase D when the arthritis cannot be detected on physical examination. Time of systemic ACPA appearance is depicted. The qualitative and quantitative changes of ACPA over the period from their systemic appearance up to RA onset are listed
Accordingly, in prospective studies individuals at phases A and B have genetic predisposition and are exposed to environmental determinants associated with RA, respectively. The greatest genetic risk of RA constitutes the HLA-DRB1 alleles of the major histocompatibility complex molecules (MHC). These alleles contain a conserved sequence of amino acids in the hypervariable region of the β chains, termed “shared epitope”. Regarding environmental factors, smoking and infections are currently considered to play an important role. The third phase (C) is the last one where individuals are still asymptomatic in clinical terms but present systemic autoimmunity, reflected by the appearance of RA-associated autoantibodies in their sera. Next phases are associated with signs and symptoms of joint-centered inflammation. At phase D, non-specific manifestations such as joint pain (arthralgia) and morning stiffness can appear and subclinical inflammation of synovium and bone marrow can be detected. The last two phases are already associated with the signs and symptoms of an illness (arthritis) defined as undifferentiated arthritis – UA (patients not yet fulfilling diagnostic criteria for RA) – phase E, and finally RA, diagnosed according to ACR criteria of 1987 or 2010 – phase F. This concept of RA development indicates that appearance of systemic autoimmunity is the first detectable immunological disturbance (phase C) that may reflect the initial pathologic processes leading ultimately to full-blown disease.
To date, the most specific autoimmunity known for RA has been expressed by generation of anti-citrullinated protein antibodies (ACPA).
Systemic ACPA appearance as an early hallmark of RA
The unquestioned success of the last years in an aspect of RA diagnosis was characterisation of ACPA. ACPA are a collection of autoantibodies with different isotypes usage (i.e.: IgG, IgA, IgM) that recognize the nonessential amino acid – citrulline in proteins. Citrulline is formed as a result of posttranslational modification (citrullination/deamination) of arginine, which is catalysed by intracellular enzymes – peptidylarginine deiminases (PADs). Importantly, citrullination may alter the properties of self-peptides leading to their immunogenicity [4]. Detection of ACPA in body fluids rely on their immunoreactivity with several cyclic citrullinated peptide (CCP) fragments of natural human proteins (the composition of peptides is unknown as patent-protected), which is assessed by the enzyme-linked immunosorbent assay (ELISA). Using commercially available ELISAs, anti-CCP antibodies could be detected in the sera of 60-80% of RA patients with a specificity of 85-99% [5]. The results of comparative studies suggest that ACPA assays have an advantage over the rheumatoid factor – RF (including analyses of RF high titre and combined RF isotypes levels) in terms of specificity [5]. It was also revealed that the presence of ACPA is associated with RA irrespective of RF status, while the association of RF with disease rely on its interaction with ACPA [6]. Recently, the high diagnostic accuracy of ACPA for RA was also demonstrated in the general population [7]. Thus, ACPA alone appear to have enough predictive power to effectively distinguish high-risk individuals from the background population. Importantly, adding ACPA results to the 1987 criteria increased sensitivity for early RA (≥ 6 months’ disease duration) diagnosis from 25 to 44% and have excellent discriminative ability to assess progression from UA to RA [8].
Studies regarding humoral anti-citrulline response in the pre-clinical period of RA were realisable by testing stored blood serum samples derived from blood donors, who subsequently developed RA. Analysis of these serum samples showed that systemic appearance of RF and anti-CCP antibodies predate the onset of RA by years (in the case of anti-CCP antibodies, even up to two decades) [9-12] with gradually increasing prevalence up to disease onset and increased sensitivity and specificity for RA, in comparison with RF [9, 10, 13, 14]. Some studies revealed that ACPA appear significantly earlier than RF and their presence has the highest predictive value for development of RA [9, 15]. However, the concomitant presence of RF could increase the risk of development of RA [9, 13, 16]. A strong association between anti-CCP antibody-positivity status and development of arthritis was further confirmed in different patients being at phases C-E of disease development (Fig. 1, left panel), who subsequently progressed to RA, i.e. patients with UA [17], patients with arthralgia [16] or patients with non-specific musculoskeletal symptoms (tenderness, early morning stiffness) [18].
Thus, the generation of ACPA is a highly specific phenomenon for RA and starts even years before disease onset, and their presence has a high positive predictive value for future development of RA.
ACPA evolution before RA onset
A high predictive value of ACPA for development of RA prompted the researchers to study the autoimmune humoral response development before RA onset. First findings concerned the fine specificity of ACPA. The reactivity of ACPA to CCPs is commonly used in commercial diagnostic assays. However, the naturally occurring epitopes recognised by these autoantibodies are not precisely characterised. Several studies aimed to profile the fine specificity of ACPA in patients with established RA. Intriguingly, sera from RA patients were shown to react with various different citrullinated epitopes of endogenous proteins (including fibrin, fibrinogen, vimentin, type II collagen, α-enolase, histones, immunoglobulin binding protein-BIP, tenascin-C), as well as microbial components (VCP1 and VCP2 derived from Epstein-Barr virus nuclear antigen 1 and α-enolase from Porphyromonas gingivalis) [6, 19-22]. In addition, ACPA are very heterogeneous in terms of fine specificity in an individual patient and also among different patients [23, 24]. This broad reactivity of ACPA could not be entirely explained by overlapping reactivities as Li et al. revealed recently that > 66% of ACPA derived from plasmablasts from RA patients are cross-reactive with different tested epitopes, while 33% were monoreactive with citrullinated fibrinogen, citrullinated enolase or citrullinated vimentin [25]. This immune response to such a broad range of citrullinated proteins was found to evolve dynamically before RA could be diagnosed, and is associated with disease development. Van der Woude et al., using a panel of 5 citrullinated ACPA targets, observed an increase in the number of epitopes recognised by ACPA in sera of blood donors over the period preceding RA onset. Moreover, at the time of disease manifestation, antibodies of patients with UA who later developed RA recognised significantly more citrullinated peptides than UA patients who did not progress to RA [26]. Similarly, among ACPA-positive arthralgia patients, recognition of higher numbers of citrullinated peptides increased the risk of developing arthritis [27]. These observations suggest that a more expanded immune response better reflects further progression towards clinical disease. In fact, the fine specificity of the ACPA response was found to be different in health and disease as ACPA-positive unaffected first-degree relatives (FDR) of RA patients have different [6] and a limited repertoire of ACPA specificities [28]. Extensive ACPA diversification may be related to epitope spreading. This term describes the situation when the autoimmune response against one epitope of particular molecule evolves and may become extended to recognise other epitopes of the same molecule (intermolecular spreading) or epitopes of other molecules (intramolecular spreading). In clinical practice, the gradual accumulation of ACPA number and reactivities over time as individuals approach the development of clinical RA appears as a raise in frequency and titre of anti-CCP antibodies [11].
Moreover, diversification of ACPA fine specificity over the preclinical period of RA is paralleled by an increase in ACPA avidity. Accordingly, ACPA derived from sera of yet asymptomatic individuals are characterised by lower avidity than ACPA from sera of individuals affected by joint symptoms [29].
Another aspect of ACPA heterogeneity is related to isotype usage. Various isotypes of ACPA were detected in RA (i.e. IgG, IgA and IgM), as well as in their FDR. However, the relative distribution of IgA and IgM was higher than IgG in FDR, whereas the IgG isotype dominated in patients with RA [30]. Moreover, the frequencies and concentrations of all isotypes of anti-CCP antibodies increased up to the onset of RA symptoms with an earlier and higher increase in IgG and IgA compared with IgM [13, 30].
The unique feature of ACPA (beside they specificity to citrullinated peptides), is also associated with a decreased galactosylation and sialylation profile and increased core fucosylation of their constant fragment (Fc fragment), in comparison to the pool of serum antibodies [31-33]. Moreover, extensive glycosylation of ACPA-IgG variable domains of Fab fragments was recently revealed [34]. Such modification of ACPA can influence functional activities of these antibodies. It was demonstrated that sialylation levels control the arthritogenicity of anti-type II collagen antibodies, including ACPA [33], and the presence of N-glycans in their variable domains modulate binding avidity of ACPA to citrullinated antigens [33, 34]. Intriguingly, modifications of Fc fragment (decrease in galactosylation and increase in core fucosylation) of serum ACPA IgG1, were detected shortly before the onset of RA and preceded by months comparable changes in total serum IgG1 [22].
Although during the early phases of RA, the humoral immune response to citrullinated antigens expands, it is relatively stable after the disease onset in regard to the number of recognised peptides, frequencies of different isotypes usage and anti-CCP antibodies positivity [11, 13, 26, 27, 29, 35]. Possible explanation for this is the influence of medication applied to the patients once the diagnosis of RA has been established. The effect of anti-inflammatory treatment may be related to the reduction in the citrullination level [36].
Intriguingly, both the inflammatory markers and ACPA often appear in serum years before the first symptoms of RA and these phenomena seem to be closely connected in time. Nielen et al. found that concentrations of anti-CCP antibodies and IgM-RF are significantly associated with concentrations of secretory phospholipase A2, C-reactive protein, and combination of both these acute response markers at the same time point in the pre-clinical period of RA [10]. Also cytokines and cytokine-related markers (IL-1α, IL-1β, IL-1 receptor antagonist, IL-4, IL-10, TNF-α and soluble TNF receptor I) could be detected in serum during the 5-year interval immediately before the diagnosis of RA [12]. However, presence of anti-CCP antibodies as well as IgM-RF and IgA-RF can be generated earlier over the period of RA development. In some sera, anti-CCP antibodies could be found from up to 18 years before disease onset [12]. Thus, it seems that generation of ACPA precedes the signs of subclinical inflammation in RA. Further it was revealed that rise in serum cytokines (TNF-α, IL-6, IL-12p70, and IFN-γ) correlates with ACPA diversification in the pre-clinical period of RA. This phenomenon can be of particular relevance to RA, because it predicts the imminent onset of clinical arthritis [11]. Interestingly, an increase in systemic inflammation markers before RA onset, coincide also with changes of ACPA structure (Fc galactosylation and core fucosylation) that can enhance pathogenic activities of antibodies [32]. Recently other immunologic perturbations detected before RA onset were found to be associated with presence of ACPA. They involve the dysregulation of T-cell subsets in ACPA-positive individuals with non-specific musculoskeletal symptoms, which predicts the risk and faster progression to arthritis [37].
Collectively, accumulating evidence indicates the evolution of anti-citrullinated humoral immune response over the pre-clinical period of RA, up to the disease onset. This is reflected by qualitative and quantitative changes of ACPA associated with diversification of specificity, expansion of isotypes usage, enhancement of avidity and acquisition of specific agalactosylation and core fucosylation profiles (Fig. 1, right panel). All these changes can affect the pathogenicity of ACPA (see section “Pathogenic functions of ACPA”) and coincide with systemic appearance of inflammatory markers.
Pathogenic functions of ACPA
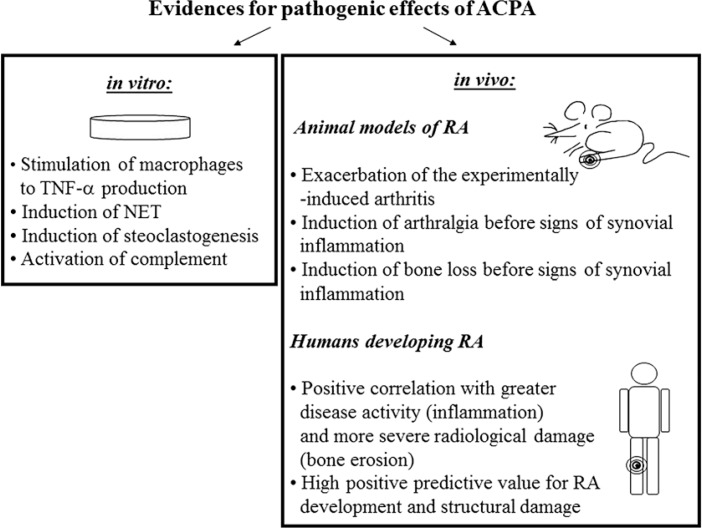
The presence of ACPA is associated with more severe RA and structural destruction [38, 39]. This suggests the involvement of ACPA in the pathogenesis of RA. Results from animal studies demonstrated arthritogenicity of some types of ACPA. Anti-citrullinated fibrinogen antibodies and anti-citrullinated collagen antibodies bound targets within the inflamed synovium and enhance tissue injury in murine experimental arthritis [40, 41]. In vitro studies revealed that ACPA exert their biological functions by binding to the Fc receptors, expressed particularly by immune cells of the myeloid lineage, and activating the complement system via the classical and alternative pathways [42, 43]. Sokolove et al. demonstrated that complexes consisted of citrullinated fibrinogen and ACPA (CitFibr-ACPA) present in RA synovium can stimulate macrophages (the important producers of inflammatory mediators in rheumatoid joint) via dual engagement of TLR-4 and FcγR. Such stimulation led to induction of TNF-α production by human macrophages [42]. Induction of TNF-α could be further amplified by IgM-RF and extended to the secretion of other proinflammatory cytokines (IL-1β, IL-6 and IL-8) that activate RA synoviocytes [44]. Observed synergistic effects of ACPA and RF can, at least partially, explain the link between concomitance of these autoantibodies and more severe RA. In addition, it was reported that presence of IgM-RF or IgA-RF increased the capacity of CitFibr-ACPA to activate the complement cascade [45]. Furthermore, it is speculated that ACPA (due to the agalactosylated profile of Fc) help in the generation of RF with high affinity for agalactosylated IgG, that is repeatedly identified in RA patients and is associated with disease pathogenesis [31]. Hypothetically, the lack of galactose residues leads to a conformational change in IgG Fc structure, which could favour high-affinity RF binding and even reveal novel epitopes that promote the generation of high-affinity RF.
Recently another ACPA-mediated mechanism of TNF-α induction that may operate in RA has been described. Through binding to surface, over-expressed citrullinated glucose-regulated protein 78 on RA peripheral blood mononuclear cells, ACPAs selectively activate ERK1/2 and JNK signalling pathways to enhance IKK-α phosphorylation, which leads to the activation of NF-κB and the production of TNF-α [46].
Pathogenic activity of ACPA in RA is also associated with induction of NETosis, a specific type of cellular death that consists in the extrusion of the intracellular material (DNA, histones, granular proteins and cytoplasmic proteins) resulting in creation of neutrophil cellular trap – NET by neutrophils. Anti-citrullinated vimentin antibodies were shown to potently induce NET formation [47]. Presence of NET augments further the activities of synovial fibroblasts, which secrete proinflammatory cytokines, chemokines and upregulate adhesion molecules. Proinflammatory cytokines are in turn the stimulus for NET formation. Furthermore, accelerated NETosis in RA is a source of citrullinated autoantigens (including vimentin and histones), and PAD enzymes that when released from intracellular compartment can citrullinate extracellular proteins [47, 48], further fuelling ACPA production. Hence, stimulation of NET formation by ACPA may perpetuate the inflammation and autoimmunization processes in RA.
In vitro and in vivo studies showed also that ACPA contribute to bone destruction. ACPA with specificity to mutated citrullinated vimentin purified from sera of RA patients bound to the surface of osteoclasts and osteoclast precursor cells and induced their differentiation as well as activated bone-resorptive activity [49]. These effects of ACPA were dependent on active citrullination in osteoclast precursor cells. Transfer of ACPA derived from RA patients into mice, caused arthralgia and systemic bone loss before signs of joint inflammation appeared [49-51]. Stimulation of osteclastogenesis by ACPA relied on inducible autocrine secretion of proinflammatory cytokines (TNF-α, IL-8) by osteoclast precursor cells.
Biological activities of ACPA include the stimulation of proinflammatory cytokines production, induction of osteoclastogenesis and promotion of autoantigens release from neutrophils (Fig. 2). All these ACPA-mediated processes can be involved in the development and/or perpetuation of RA.
Fig. 2.
Evidence for pathogenic effects of ACPA gathering during in vitro and in vivo studies. The in vivo studies concern the investigation of animal models of RA as well as clinical observations of humans developing RA. A detailed description is given in the text. NET – neutrophil intracellular trap
Directions for future investigation
A lot of data regarding ACPA biology has been gathered. They are of crucial importance to understanding the pathogenesis of RA and planning the future treatment/preventive strategies for this disease. However, many issues regarding ACPA development and functional activities still remain to be explained. They concern the following:
What protein/epitope is the primary trigger of anti-citrullinated protein response? The existence of such primary antigen is supported by signs of active selection of ACPA-producing B cells and T cells in RA patients. This is reflected by the increased mutation ratios of the immunoglobulin genes encoding ACPA in plasmablasts [25], introduced by somatic hypermutation N-glycosylation sites in ACPA variable domains of Fab fragment [34], and specifically restricted repertoire of T cells in ACPA-positive RA patients [52]. A proper genetic background may be of importance to this phenomenon. Especially the presence of HLA-DRB1 alleles containing the shared epitope associates strongly with appearance of ACPA with reactivities to selected citrullinated proteins [53, 54] (see paragraph 4). Unfortunately, because of marked differences in specificities, avidity and isotypes usage of ACPAs among RA patients, no triggering citrullinated antigen described so far is common to all patients with RA. Furthermore, no dominant epitope spreading hierarchy could also be identified [21]. In addition, recent works indicate that the ACPA profile in patients with recent-onset arthritis did not reveal associations with disease activity and progression scores [23]. These results argue against the concept of significance of ACPA specificity for development of RA. However, it should be pointed out that experimental settings and commercial anti-CCP antibody assays used so far allow for detection of limited ACPA specificities, and thus may not precisely reflect the situation in vivo. Hence, other ACPA reactivities (to autoantigens that may be more relevant to RA), could be missed. In fact, accumulation of ACPA specificities targeting citrullinated histones, fibrinogen, biglycan, and clusterin preceded anti-CCP reactivity in a subset of patients at pre-clinical phases of RA [11]. Therefore, further prospective studies to assess the specificity of anti-citrullinated response using a broader panel of ACPA targets, especially in early pre-clinical phases of RA (even in phase A and/or B, Fig. 1, left panel), may bring us closer to characterise factors responsible for triggering the anti-citrullinated protein response in RA.
Do ACPA have a role in transition from the asymptomatic autoimmune state to clinically apparent arthritis? Because ACPA can be present in asymptomatic individuals over the years before the onset of arthritis, it suggests the existence of a time point over the pre-clinical period of RA, which is critical for this transition. The quantitative and qualitative changes of ACPA occurring before RA onset, may be of essential importance to this process. In fact, it was recently revealed that by enhancing the sialylation levels of ACPA it is possible to attenuate their arthritogenic activity. Such modification of ACPA resulted in suppression of development of experimentally-induced arthritis in the antibody-infused mice [33]. Acquisition of particular RA-associated fine specificity and/or exceeding the ACPA titre threshold may also contribute to emergence of subclinical inflammation and further RA development.
Which tissue location/organ is the initiation site of anti-citrullinated humoral response in RA? The presence of ACPA is well detected before any clinical imaging or histological signs of joint pathology could be demonstrated. Hence, the source of these autoantibodies may be tissues other than joints. The plausible candidates are lungs and periodontium. These two locations are directly exposed to tobacco smoke and/or oral pathogenic bacteria such as Porphyromonas gingivalis causing protein citrullination in affected tissues [55, 56]. Both of these environmental factors are suspected to induce ACPA generation in lungs and periodontal tissue that may be associated with future development of arthritis [57].
Why citrullinated antigens are immunogenic in RA? Citrullination of proteins is an inflammation-associated process detected in inflammatory tissues in RA as well as in other diseases [58], but the induction of humoral response to citrullinated antigens is characteristic for RA. Induction of ACPA in RA may be facilitated by the increased binding of some RA-associated citrullinated antigens to HLA-DRB1 alleles containing the shared epitope and their effective presentation to T cells [59-61]. However, the ability of these alleles to present citrullinated antigens is not restricted to RA patients as it was also noted in ACPA-negative RA patients and ACPA-negative healthy individuals [62]. It suggests the involvement of an additional factor that can promote/augment the anti-citrullinated protein autoimmunity in RA. Infection with P. gingivalis may have a role in this process. This bacterium produces its own citrullinated enolase that resembles in terms of amino acid sequence and overlapping citrullination sites, the human enolase [25]. Therefore, an original immune response to P. gingivalis may redirect the immune response to human citrullinated proteins (as a consequence of molecular mimicry) and help the selection of ACPA-producing B cells [25].
Why development of anti-citrullinated protein response is associated with joint-centered pathology? Recently published data indicate that the primary target of ACPA in the pre-clinical period of RA is osteoclasts and osteoclast precursor cells residing in bone marrow [50]. Furthermore, ACPA-mediated osteoclastogenesis is dependent on TNF-α and IL-8 production by differentiating osteoclast precursor cells. Based on these observations it was hypothesised that in the pre-clinical period of disease (before signs of joint inflammation), ACPA can initiate bone destruction via induction of proinflammatory cytokines that spread subsequently to the adjacent joint and cause synovial inflammation. This supports the view that bone marrow is an important compartment in RA [63-65] and changes in bone marrow are not a secondary phenomenon to joint inflammation, and may reflect the initiation of inflammation and bone destruction in RA. Intriguingly, anti-citrullinated vimentin antibodies appear early over the pre-clinical period of RA, and their presence is associated with development of disease as the reactivity to citrullinated vimentin could be detected in sera from RA patients, while not from unaffected relatives of RA patients [6]. The ACPA expansion and systemic appearance of inflammatory markers, coordinated in time, at early phases of RA also supports this concept. Importantly, the above scenario might explain why ACPA specifically contribute to the development of the inflammatory process in the joint. In clinical terms, the direct effect of ACPA on osteoclasts differentiation and bone destruction could be associated with the progression of radiological damage in ACPA-positive patients despite achieving clinical remission [66]. It might also explain the evidence of structural bone damage in APCA-positive healthy individuals [67].
Conclusions
The majority of RA patients generate ACPA. Thus, early in the natural history of RA initial break in tolerance to citrullinated antigens is followed by expansion of anti-citrullinated humoral response that is strongly linked with the development of inflammation and ultimately arthritis. Exceeding the threshold of ACPA titre or/and achieving the pathogenic properties by ACPA in the pre-clinical period of RA, would promote the transition from asymptomatic autoimmunity to clinically apparent arthritis and full-blown RA. ACPA-mediated processes described here lead to RA-associated production of proinflammatory cytokines and differentiation of osteoclasts. Intriguingly, recent findings have indicated that in the preclinical period of RA ACPA can target osteoclasts residing in bone marrow. This observation underlines the role of bone marrow in early phases of RA development.
Although the recent studies of ACPA biology provided substantial knowledge about the early phases of RA development, there are still fundamental questions that need to be answered. This is of particular importance for the identification of “at risk” individuals, who should benefit from early proper clinical care, as well as for the development of new treatment and preventive strategies for RA.
Footnotes
This work was supported by the Polish National Science Centre (grant no. 2012/06/A/NZ5/00059) and Polish Ministry of Science and Higher Education (core grant S/17).
The authors declare no conflict of interest.
References
- 1.Nell VP, Machold KP, Eberl G, et al. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford) 2004;43:906–914. doi: 10.1093/rheumatology/keh199. [DOI] [PubMed] [Google Scholar]
- 2.Landewé RB, Boers M, Verhoeven AC, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46:347–356. doi: 10.1002/art.10083. [DOI] [PubMed] [Google Scholar]
- 3.Gerlag DM, Raza K, van Baarsen LG, et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann Rheum Dis. 2012;71:638–641. doi: 10.1136/annrheumdis-2011-200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S1, Wang Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim Biophys Acta. 2013;1829:1126–1135. doi: 10.1016/j.bbagrm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal R, Liao K, Nair R, et al. Anti-citrullinated peptide antibody assays and their role in the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2009;61:1472–1483. doi: 10.1002/art.24827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ioan-Facsinay A, Willemze A, Robinson DB, et al. Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum. 2008;58:3000–3008. doi: 10.1002/art.23763. [DOI] [PubMed] [Google Scholar]
- 7.Hensvold AH, Frisell T, Magnusson PK, et al. How well do ACPA discriminate and predict RA in the general population: a study based on 12 590 population-representative Swedish twins. Ann Rheum Dis. 2017;76:119–125. doi: 10.1136/annrheumdis-2015-208980. [DOI] [PubMed] [Google Scholar]
- 8.van der Helm-van Mil AH, Detert J, le Cessie S, et al. Validation of a prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: moving toward individualized treatment decision-making. Arthritis Rheum. 2008;58:2241–2247. doi: 10.1002/art.23681. [DOI] [PubMed] [Google Scholar]
- 9.Rantapää-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 10.Nielen MM, van Schaardenburg D, Reesink HW, et al. Simultaneous development of acute phase response and autoantibodies in preclinical rheumatoid arthritis. Ann Rheum Dis. 2006;65:535–537. doi: 10.1136/ard.2005.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorgensen KT, Wiik A, Pedersen M, et al. Cytokines, autoantibodies and viral antibodies in premorbid and postdiagnostic sera from patients with rheumatoid arthritis: case-control study nested in a cohort of Norwegian blood donors. Ann Rheum Dis. 2008;67:860–866. doi: 10.1136/ard.2007.073825. [DOI] [PubMed] [Google Scholar]
- 13.Kokkonen H, Mullazehi M, Berglin E, et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther. 2011;13:R13. doi: 10.1186/ar3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chibnik LB, Mandl LA, Costenbader KH, et al. Comparison of threshold cutpoints and continuous measures of anti-cyclic citrullinated peptide antibodies in predicting future rheumatoid arthritis. J Rheumatol. 2009;36:706–711. doi: 10.3899/jrheum.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Stadt LA, de Koning MH, van de Stadt RJ, et al. Development of the anti-citrullinated protein antibody repertoire prior to the onset of rheumatoid arthritis. Arthritis Rheum. 2011;63:3226–3233. doi: 10.1002/art.30537. [DOI] [PubMed] [Google Scholar]
- 16.Bos WH, Wolbink GJ, Boers M, et al. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis. 2010;69:490–494. doi: 10.1136/ard.2008.105759. [DOI] [PubMed] [Google Scholar]
- 17.van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum. 2004;50:709–715. doi: 10.1002/art.20044. [DOI] [PubMed] [Google Scholar]
- 18.Rakieh C, Nam JL, Hunt L, et al. Predicting the development of clinical arthritis in anti-CCP positive individuals with non-specific musculoskeletal symptoms: a prospective observational cohort study. Ann Rheum Dis. 2015;74:1659–1666. doi: 10.1136/annrheumdis-2014-205227. [DOI] [PubMed] [Google Scholar]
- 19.Snir O, Widhe M, von Spee C, et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: association with HLA-DRB1 alleles. Ann Rheum Dis. 2009;68:736–743. doi: 10.1136/ard.2008.091355. [DOI] [PubMed] [Google Scholar]
- 20.Schwenzer A, Jiang X, Mikuls TR, et al. Identification of an immunodominant peptide from citrullinated tenascin-C as a major target for autoantibodies in rheumatoid arthritis. Ann Rheum Dis. 2016;75:1876–1883. doi: 10.1136/annrheumdis-2015-208495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson L, Pratesi F, Brink M, et al. Antibodies directed against endogenous and exogenous citrullinated antigens pre-date the onset of rheumatoid arthritis. Arthritis Res Ther. 2016;18:127. doi: 10.1186/s13075-016-1031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoda H, Fujio K, Shibuya M, et al. Detection of autoantibodies to citrullinated BiP in rheumatoid arthritis patients and pro-inflammatory role of citrullinated BiP in collagen-induced arthritis. Arthritis Res Ther. 2011;13:R191. doi: 10.1186/ar3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Beers JJ, Willemze A, Jansen JJ, et al. ACPA fine-specificity profiles in early rheumatoid arthritis patients do not correlate with clinical features at baseline or with disease progression. Arthritis Res Ther. 2013;15:R140. doi: 10.1186/ar4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Too CL, Murad S, Hansson M, et al. Differences in the Spectrum of Anti-Citrullinated Protein Antibody Fine Specificities Between Malaysian and Swedish Patients With Rheumatoid Arthritis: Implications for Disease Pathogenesis. Arthritis Rheum. 2017;69:58–69. doi: 10.1002/art.39827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Yu Y, Yue Y, et al. Autoantibodies From Single Circulating Plasmablasts React With Citrullinated Antigens and Porphyromonas gingivalis in Rheumatoid Arthritis. Arthritis Rheum. 2016;68:614–626. doi: 10.1002/art.39455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Woude D, Rantapää-Dahlqvist S, Ioan-Facsinay A, et al. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis. 2010;69:1554–1561. doi: 10.1136/ard.2009.124537. [DOI] [PubMed] [Google Scholar]
- 27.van de Stadt LA, van der Horst AR, de Koning MH, et al. The extent of the anti-citrullinated protein antibody repertoire is associated with arthritis development in patients with seropositive arthralgia. Ann Rheum Dis. 2011;70:128–133. doi: 10.1136/ard.2010.132662. [DOI] [PubMed] [Google Scholar]
- 28.Barra L, Scinocca M, Saunders S, et al. Anti-citrullinated protein antibodies in unaffected first-degree relatives of rheumatoid arthritis patients. Arthritis Rheum. 2013;65:1439–1447. doi: 10.1002/art.37911. [DOI] [PubMed] [Google Scholar]
- 29.Suwannalai P, van de Stadt LA, Radner H, et al. Avidity maturation of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum. 2012;64:1323–1328. doi: 10.1002/art.33489. [DOI] [PubMed] [Google Scholar]
- 30.Ärlestig L, Mullazehi M, Kokkonen H, et al. Antibodies against cyclic citrullinated peptides of IgG, IgA and IgM isotype and rheumatoid factor of IgM and IgA isotype are increased in unaffected members of multicase rheumatoid arthritis families from northern Sweden. Ann Rheum Dis. 2012;71:825–829. doi: 10.1136/annrheumdis-2011-200668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scherer HU, van der Woude D, Ioan-Facsinay A, et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62:1620–1629. doi: 10.1002/art.27414. [DOI] [PubMed] [Google Scholar]
- 32.Rombouts Y, Ewing E, van de Stadt LA, et al. Anti- citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis. 2015;74:234–241. doi: 10.1136/annrheumdis-2013-203565. [DOI] [PubMed] [Google Scholar]
- 33.Ohmi Y, Ise W, Harazono A, et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat Commun. 2016;7:11205. doi: 10.1038/ncomms11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rombouts Y, Willemze A, van Beers JJ, et al. Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann Rheum Dis. 2016;75:578–585. doi: 10.1136/annrheumdis-2014-206598. [DOI] [PubMed] [Google Scholar]
- 35.Verpoort KN, Jol-van der Zijde CM, Papendrecht-van der Voort EA, et al. Isotype distribution of anti-cyclic citrullinated peptide antibodies in undifferentiated arthritis and rheumatoid arthritis reflects an ongoing immune response. Arthritis Rheum. 2006;54:3799–3808. doi: 10.1002/art.22279. [DOI] [PubMed] [Google Scholar]
- 36.Makrygiannakis D, Revu S, Engström M, et al. Local administration of glucocorticoids decreases synovial citrullination in rheumatoid arthritis. Arthritis Res Ther. 2012;14:R20. doi: 10.1186/ar3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunt L, Hensor EM, Nam J, et al. T cell subsets: an immunological biomarker to predict progression to clinical arthritis in ACPA-positive individuals. Ann Rheum Dis. 2016;75:1884–1889. doi: 10.1136/annrheumdis-2015-207991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.del Val del Amo N, Ibanez Bosch R, Fito Manteca C, et al. Anti-cyclic citrullinated peptide antibody in rheumatoid arthritis: relation with disease aggressiveness. Clin Exp Rheumatol. 2006;24:281–286. [PubMed] [Google Scholar]
- 39.Meyer O, Labarre C, Dougados M, et al. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis. 2003;62:120–126. doi: 10.1136/ard.62.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhn KA, Kulik L, Tomooka B, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116:961–973. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uysal H, Bockermann R, Nandakumar KS, et al. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J Exp Med. 2009;206:449–462. doi: 10.1084/jem.20081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sokolove J, Zhao X, Chandra PE, et al. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum. 2011;63:53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trouw LA, Haisma EM, Levarht EW, et al. Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum. 2009;60:1923–1931. doi: 10.1002/art.24622. [DOI] [PubMed] [Google Scholar]
- 44.Laurent L, Anquetil F, Clavel C, et al. IgM rheumatoid factor amplifies the inflammatory response of macrophages induced by the rheumatoid arthritis-specific immune complexes containing anticitrullinated protein antibodies. Ann Rheum Dis. 2015;74:1425–1431. doi: 10.1136/annrheumdis-2013-204543. [DOI] [PubMed] [Google Scholar]
- 45.Anquetil F, Clavel C, Offer G, et al. IgM and IgA rheumatoid factors purified from rheumatoid arthritis sera boost the Fc receptor- and complement-dependent effector functions of the disease-specific anti-citrullinated protein autoantibodies. J Immunol. 2015;194:3664–3674. doi: 10.4049/jimmunol.1402334. [DOI] [PubMed] [Google Scholar]
- 46.Lu MC, Lai NS, Yin WY, et al. Anti-citrullinated protein antibodies activated ERK1/2 and JNK mitogen-activated protein kinases via binding to surface-expressed citrullinated GRP78 on mononuclear cells. J Clin Immunol. 2013;33:558–566. doi: 10.1007/s10875-012-9841-6. [DOI] [PubMed] [Google Scholar]
- 47.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra40.. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corsiero E, Bombardieri M, Carlotti E, et al. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann Rheum Dis. 2016;75:1866–1875. doi: 10.1136/annrheumdis-2015-208356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harre U, Georgess D, Bang Rantaää-Dahlqvist H, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122:1791–1802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krishnamurthy A, Joshua V, Haj Hensvold A, et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis. 2016;75:721–729. doi: 10.1136/annrheumdis-2015-208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wigerblad G, Bas DB, Fernades-Cerqueira C, et al. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine-dependent mechanism. Ann Rheum Dis. 2016;75:730–738. doi: 10.1136/annrheumdis-2015-208094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cantaert T, Brouard S, Thurlings RM, et al. Alterations of the synovial T cell repertoire in anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheum. 2009;60:1944–1956. doi: 10.1002/art.24635. [DOI] [PubMed] [Google Scholar]
- 53.Lundberg K, Bengtsson C, Kharlamova N, et al. Genetic and environmental determinants for disease risk in subsets of rheumatoid arthritis defined by the anticitrullinated protein/peptide antibody fine specificity profile. Ann Rheum Dis. 2013;72:652–658. doi: 10.1136/annrheumdis-2012-201484. [DOI] [PubMed] [Google Scholar]
- 54.Verpoort KN, Cheung K, Ioan-Facsinay A, et al. Fine specificity of the anti-citrullinated protein antibody response is influenced by the shared epitope alleles. Arthritis Rheum. 2007;56:3949–3952. doi: 10.1002/art.23127. [DOI] [PubMed] [Google Scholar]
- 55.Makrygiannakis D, Hermansson M, Ulfgren AK, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008;67:1488–1492. doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- 56.Harvey GP, Fitzsimmons TR, Dhamarpatni AA, et al. Expression of peptidylarginine deiminase-2 and -4, citrullinated proteins and anti-citrullinated protein antibodies in human gingiva. J Periodontal Res. 2013;48:252–261. doi: 10.1111/jre.12002. [DOI] [PubMed] [Google Scholar]
- 57.Mankia K, Emery P. Is localized autoimmunity the trigger for rheumatoid arthritis? Unravelling new targets for prevention. Discov Med. 2015;20:129–135. [PubMed] [Google Scholar]
- 58.Makrygiannakis D, af Klint E, Lundberg IE, et al. Citrullination is an inflammation-dependent process. Ann Rheum Dis. 2006;65:1219–1222. doi: 10.1136/ard.2005.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill JA, Southwood S, Sette A, et al. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538–541. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 60.James EA, Moustakas AK, Bui J, et al. HLA-DR1001 presents “altered-self” peptides derived from joint-associated proteins by accepting citrulline in three of its binding pockets. Arthritis Rheum. 2010;62:2909–2918. doi: 10.1002/art.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feitsma AL, van der Voort EI, Franken KL, et al. Identification of citrullinated vimentin peptides as T cell epitopes in HLA-DR4-positive patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:117–125. doi: 10.1002/art.25059. [DOI] [PubMed] [Google Scholar]
- 62.Law SC, Street S, Yu CH, et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res Ther. 2012;14:R118. doi: 10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudnicka W, Burakowski T, Warnawin E, et al. Functional TLR9 modulates bone marrow B cells from rheumatoid arthritis patients. Eur J Immunol. 2009;39:1211–1220. doi: 10.1002/eji.200838617. [DOI] [PubMed] [Google Scholar]
- 64.Kuca-Warnawin E, Burakowski T, Kurowska W, et al. Elevated number of recently activated T cells in bone marrow of patients with rheumatoid arthritis: a role for interleukin 15? Ann Rheum Dis. 2011;70:227–233. doi: 10.1136/ard.2009.124966. [DOI] [PubMed] [Google Scholar]
- 65.McQueen FM, Ostendorf B. What is MRI bone oedema in rheumatoid arthritis and why does it matter? Arthritis Res Ther. 2006;8:222. doi: 10.1186/ar2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Linden MP, Boja R, Klarenbeek NB, et al. Repair of joint erosions in rheumatoid arthritis: prevalence and patient characteristics in a large inception cohort. Ann Rheum Dis. 2010;69:727–729. doi: 10.1136/ard.2009.108332. [DOI] [PubMed] [Google Scholar]
- 67.Kleyer A, Finzel S, Rech J, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis. 2014;73:854–860. doi: 10.1136/annrheumdis-2012-202958. [DOI] [PubMed] [Google Scholar]