Abstract
Background
Literature on the effectiveness of simulation-based medical education programs for caring for acute ischemic stroke (AIS) patients is limited.
Objective
To improve coordination and door-to-needle (DTN) time for AIS care, we implemented a stroke simulation training program for neurology residents and nursing staff in a comprehensive stroke center.
Methods
Acute stroke simulation training was implemented for first-year neurology residents in July 2011. Simulations were standardized using trained live actors, who portrayed stroke vignettes in the presence of a board-certified vascular neurologist. A debriefing of each resident's performance followed the training. The hospital stroke registry was also used for retrospective analysis. The study population was defined as all patients treated with intravenous tissue plasminogen activator for AIS between October 2008 and September 2014.
Results
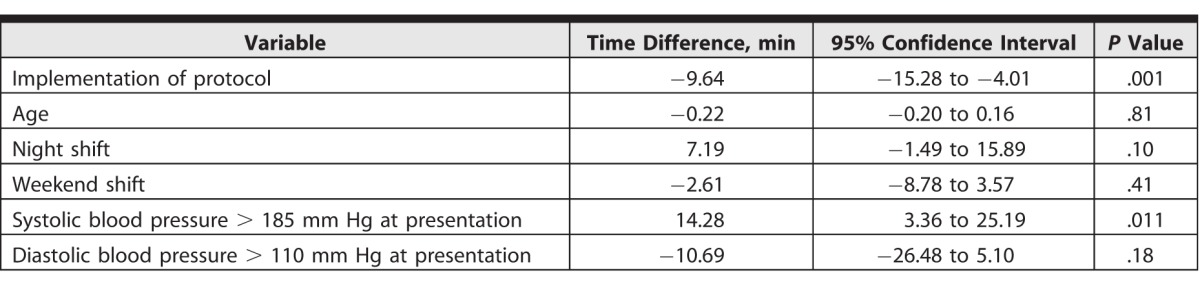
We identified 448 patients meeting inclusion criteria. Simulation training independently predicted reduction in DTN time by 9.64 minutes (95% confidence interval [CI] –15.28 to –4.01, P = .001) after controlling for age, night/day shift, work week versus weekend, and blood pressure at presentation (> 185/110). Systolic blood pressure higher than 185 was associated with a 14.28-minute increase in DTN time (95% CI 3.36–25.19, P = .011). Other covariates were not associated with any significant change in DTN time.
Conclusions
Integration of simulation based-medical education for AIS was associated with a 9.64-minute reduction in DTN time.
Introduction
Contemporary treatment of acute ischemic stroke (AIS) requires a cooperative effort from a multidisciplinary team (including a neurologist, an endovascular interventionist, and a rapid response nursing staff) in order to administer appropriate treatments in a safe and timely manner. The American Heart Association's Get With the Guidelines program mandates a door-to-needle (DTN) time within 60 minutes for intravenous tissue plasminogen activator (IV-tPA), and a door-to-puncture time within 90 minutes for endovascular therapy in AIS.1 In practice, this recommended timeline is not consistently met, possibly due to a lack of cohesive teamwork for stroke care. The interaction between residents and nursing staff is a crucial factor in efficient and appropriate patient care.2
Neurology residents are mandated during their training to show competence in the systems-based practice milestone. Simulation-based medical education has been shown to be superior to traditional clinical medical education in achieving competence for specific clinical skills.3,4 A common form of simulation-based education in medicine is deliberate practice. This technique produces expert performance contingent on 4 actions: (1) intense repetition of a skill; (2) rigorous assessment of performance; (3) specific and informative feedback; and (4) improved performance in a controlled setting.5 Research has shown that deliberate practice leads to clinical improvement in procedural performance in the operating room (eg, laparoscopy or robotic surgery).6,7 Other studies have indicated that residents trained on simulators are more likely to adhere to the advanced cardiovascular life support protocol than those who receive standard training.8,9
To streamline the process for inpatient acute stroke management, enhance cooperation between nurses and residents, and possibly improve DTN, we developed an acute stroke simulation training program for a group of first-year neurology residents (postgraduate year 2 [PGY-2]) and neurology nursing staff to become first responders to an acute stroke code.
Methods
Setting
Since July 2011, we have used an acute stroke simulation for all first-year neurology residents (PGY-2). Residents and a group of neurology nurses on the stroke unit function as first responders to a mock acute stroke code.
Participants
All first-year neurology (PGY-2) residents underwent this simulation training. The composition of the stroke team changed each academic year, as new incoming neurology residents entered the program. Participating nurses and the supervising vascular neurology attending physician also changed throughout the study period depending on their on-call schedules.
All PGY-2 neurology residents (5 per year) are required to successfully complete summative evaluations for the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale (mRS), and take a pretest for the web-based NIHSS/mRS certification via the National Stroke Association (or clear-3 clinical trials campus) to determine their knowledge of acute stroke code prior to the live simulation. For stroke simulations, a live actor (a neurology nurse) represented the stroke victim. The actor portraying the stroke victim was trained by the vascular neurology attending to mimic a stroke patient, and depicted focal neurological findings that correlated with the case vignette. All stroke vignettes and radiologic studies were selected by the vascular attending, and were retrieved through a secure radiology teaching case file on the hospital website.
Interventions
Each resident was evaluated by an NIHSS/mRS-certified vascular neurology attending for the accuracy of his or her NIHSS and knowledge of inclusion/exclusion criteria for acute stroke intervention. Simulations were supervised by the vascular neurology attending, who remained in the room during the mock stroke code for each simulation; participating residents were timed during their encounter (Figure 1). Computed tomography (CT) and CT angiography scans were available to the participating resident on request. The nursing staff and/or vascular neurology attending provided live responses to the resident's actions, such as pertinent lab work, vital signs, or electrocardiogram tracings. Nursing staff was educated by the stroke center coordinator on the acute stroke protocol (eg, tracking blood pressure, establishing 2 IV lines, checking glucose, international normalized ratio, and providing treatment as dictated by the physician). The vascular neurologist gave a debriefing of each resident's performance immediately after each simulation trial.
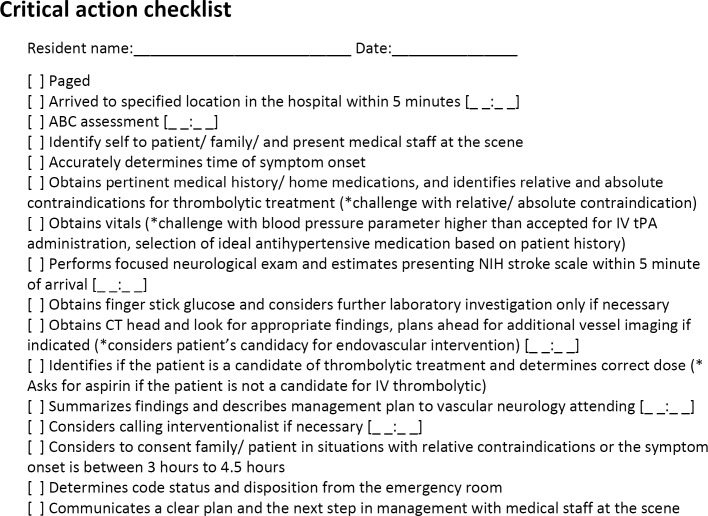
Figure 1.
Simulation Evaluation Form
A resident's performance was based on his or her adherence to the critical action checklist (Figure 1). If a critical action on the checklist was missed, the resident repeated the simulation until he or she performed it without deviating from the checklist. An exit survey was completed by each resident regarding his or her satisfaction with the simulation and recommendations for improvement (provided as online supplemental material). We debriefed all participants in the stroke simulation, using a previously tested approach that included inquiring about an individual resident's rationale on management decisions and how that can be improved based on existing guidelines for stroke care.10,11
This study was approved by the Hartford Healthcare Institutional Review Board.
Outcome Analysis
A retrospective analysis of DTN for IV-tPA, from October 1, 2008, to September 31, 2014, was obtained from our hospital stroke registry. Since the simulation training was implemented in July 2011, there were 2 subgroups defined: cohort 1, patients treated with IV-tPA before simulation training (October 1, 2008–July 1, 2011); and cohort 2, patients treated with IV-tPA after simulation training was implemented (July 1, 2011–September 31, 2014). It was ascertained that there were no changes in the hospital triage system for potential IV-tPA treatment–eligible stroke patients. Multivariate linear regression analysis was used to identify whether implementation of simulation training reduced hospital DTN after controlling for potential confounders (age, night shift versus day shift, treatment during weekend versus weekday, systolic blood pressure > 185 mm Hg, and diastolic blood pressure > 110 mm Hg). Univariate analysis was used to assess change in DTN for IV-tPA.
Results
A total of 448 patients who were treated with IV-tPA met inclusion criteria: 172 patients (cohort 1) were treated with IV-tPA presimulation, and 276 patients (cohort 2) were treated after the simulation-based training started. Baseline characteristics of both groups are described in Table 1.
Table 1.
Baseline Characteristicsa
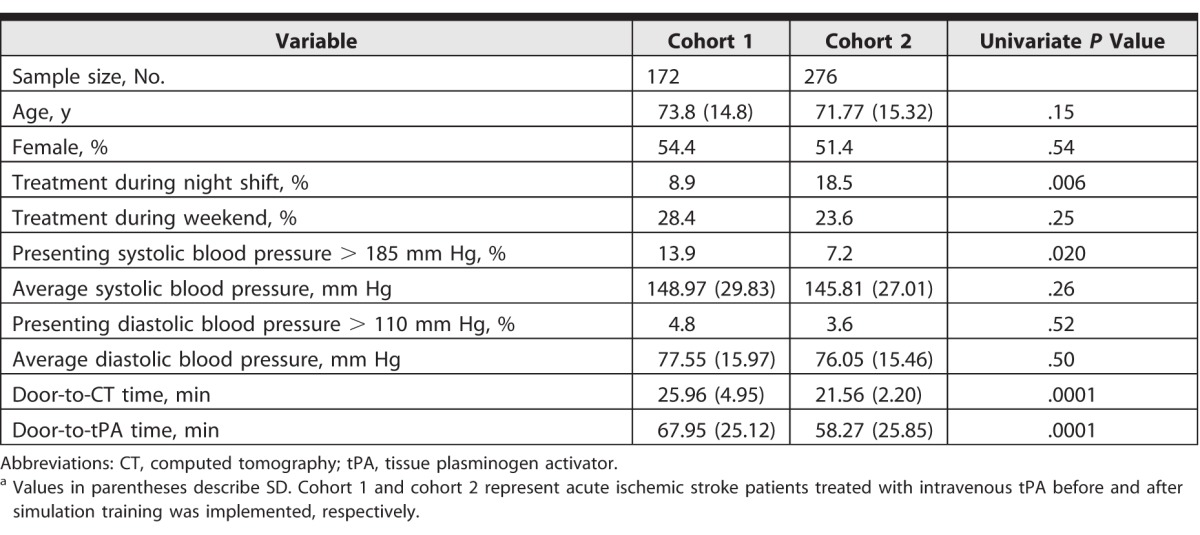
The percentage of cases treated during the night shift (8.3% versus 18.5%, P = .006) was significantly higher in cohort 2, whereas the percentage of cases presenting with systolic blood pressure higher than 185 mm Hg (13.9% versus 7.2%, P = .020) was significantly higher in cohort 1. There was no statistically significant difference in other covariates, including age, female sex, treatment during the weekend, average blood pressure, or presenting diastolic blood pressure higher than 110 mm Hg. There was a 9.86-minute reduction (P = .0001) in DTN on univariate analysis in cohort 2 (58.27 minutes, SD = 25.85) compared with cohort 1 (67.95 minutes, SD = 25.85).
Similarly, a reduction in average door-to-CT time was noted in cohort 2 (21.56 minutes, SD = 2.20) compared with cohort 1 (25.96 minutes; SD = 4.95; P < .001). Mean DTN over time is shown in Figure 2. After controlling for confounders, multivariate linear regression analysis showed that the implementation of simulation training was independently associated with a reduction in mean DTN by 9.64 minutes (95% confidence interval [CI] –15.28 to –4.01, P = .001). Presenting systolic blood pressure higher than 185 mm Hg was independently associated with a 14.28-minute increase in DTN (95% CI 3.36–25.19, P = .011). Other covariates in the model, including age, time of day (night versus day, weekday versus weekend), and presenting diastolic blood pressure higher than 110 mm Hg, were not associated with a statistically significant increase in DTN (Table 2).
Figure 2.
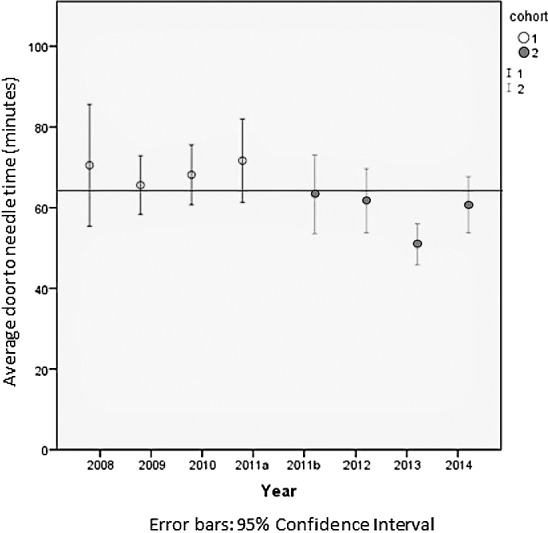
Average Door-to-Needle Time During the Study Period
Table 2.
Multivariate Linear Regression Model for Outcome of Door-to–Intravenous Tissue Plasminogen Activator Time
During the simulations, all participating residents arrived at the designated location within 5 minutes of stroke beeper activation. The average time from arrival to complete NIHSS evaluation was 4.80 minutes. As laboratory values and CT scan images were readily available on request, participating residents were able to make a decision for IV-tPA with an average time of 10.20 minutes. Individual residents received pretest results and constructive feedback from the vascular neurology attending depending on the identified weakness during their encounters. A majority of the participating residents reported that the simulation training helped identify their individual weaknesses and increase their comfort in managing AIS patients.
Discussion
In this study of simulation training to improve neurology residents' performance in AIS care in the hospital, the average DTN time improved by nearly 10 minutes, compared with historical controls.
Our curriculum used a trained live actor to portray the stroke patient, which is an important advantage for teaching acute stroke recognition. A mannequin and/or video used for simulation training would not mimic a focal neurological deficit as well as a trained live actor. Simulation has been shown to improve medical knowledge and comfort in patient management, and demonstrate improvement in performance during retesting in simulated scenarios.12,13 Simulation training for stroke and other neurological emergencies assessing trainee confidence and/or medical knowledge before and after participating in the program have been shown to be effective.14,15 Our study model evaluated objective data directly related to patient care. There is strong evidence of improved neurological outcomes with shorter IV-tPA treatment times from symptom onset in AIS patients.16
Individual feedback of each trainee's performance was generally accepted by the participating residents, and also provided an incentive to improve their skills. A majority of participating residents provided feedback that simulation training should be held in the beginning of the year for incoming neurology residents, as the program facilitated identification of weaknesses and helped improve residents' comfort in caring for stroke patients. We suspect that the gain in confidence and improved comfort in the flow of management were responsible for the improvement of DTN time.
The simulation intervention did not require any additional resources or financial costs, and the time for each simulation and feedback session was easily integrated into the participating staff and residents' work schedule. Most simulations occurred while the participating resident was already on inpatient service, and all other staff involved volunteered during active working hours. We believe other programs could readily replicate this educational intervention.
A limitation of our study is that overall temporal trends showed significant reduction in DTN time from 2003 to 2011 in the Get With The Guidelines initiative database,17 with the outcome data potentially reflecting this overall trend. It is also important to note that the time process in simulations is shorter than in real time. Another limitation may be observer bias, as only 1 observer was used to provide feedback to each resident, and we could not assess for interobserver agreement. Our study compared only door-to–IV-tPA time in terms of outcome.
The next research steps could be to include senior neurology residents (PGY-3 and PGY-4) in the simulation training, using the simulation as refresher training, and to evaluate whether DTN time further improves. Including a larger-scale implementation of a similar standardized simulation training program would allow for further data and evaluation of other patient outcomes, which is the ultimate consequence of stroke treatment.
Conclusion
A single institution study found a reduction in the mean door-to–IV-tPA time by 9.64 minutes for acute ischemic stroke treatment after implementation of a simulation-based training program for first-year neurology residents.
Supplementary Material
Footnotes
Editor's Note: The online version of this article contains the exit survey questions used in the study.
Funding: The authors report no external funding source for this study.
Conflict of interest: The authors declare they have no competing interests.
The study abstract was presented as a poster at the International Stroke Conference, Houston, Texas, February 21–24, 2017.
What was known and gap
Timely and effective care for patients with acute ischemic stroke is critical; to date, few educational interventions have sought to improve first-year neurology residents' ability to care for this population.
What is new
Simulation-based training improved timeliness of initiating treatment and resident self-reported comfort with providing care.
Limitations
Beneficial outcomes may be due to a national trend in improving stroke care, not the intervention.
Bottom line
The simulation was feasible with existing time and resources.
References
- 1. . American Heart Association. Stroke fact sheet. http://www.heart.org/idc/groups/ahaecc-public/@wcm/@gwtg/documents/downloadable/ucm_491528.pdf. Accessed November 8, 2017.
- 2. . Muller-Juge V, Cullati S, Blondon KS, et al. Interprofessional collaboration between residents and nurses in general internal medicine: a qualitative study on behaviours enhancing teamwork quality. PloS One. 2014; 9 4: e96160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. . McGaghie WC, Issenberg SB, Cohen ER, et al. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011; 86 6: 706– 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. . Ericsson KA, Krampe RT, Tesch-Romer C. . The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993; 100 3: 363– 406. [Google Scholar]
- 5. . Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002; 336 4: 458– 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. . Sedlack RE, Kolars JC. . Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study. Am J Gastroenterol. 2004; 99 1: 33– 37. [DOI] [PubMed] [Google Scholar]
- 7. . Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008; 133 1: 56– 61. [DOI] [PubMed] [Google Scholar]
- 8. . Wayne DB, Butter J, Siddall VJ, et al. Mastery learning of advanced cardiac life support skills by internal medicine residents using simulation technology and deliberate practice. J Gen Intern Med. 2006; 21 3: 251– 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. . Holcomb JB, Dumire RD, Crommett JW, et al. Evaluation of trauma team performance using an advanced human patient simulator for resuscitation training. J Trauma. 2002; 52 6: 1078– 1086. [DOI] [PubMed] [Google Scholar]
- 10. . Rudolph JW, Simon R, Rivard P, et al. Debriefing with good judgment: combining rigorous feedback with genuine inquiry. Anesthesiol Clin. 2007; 25 2: 361– 376. [DOI] [PubMed] [Google Scholar]
- 11. . Cheng A, Hunt EA, Donoghue A, et al. Examining pediatric resuscitation education using simulation and scripted debriefing: a multicenter randomized trial. JAMA Pediatrics. 2013; 167 6: 528– 536. [DOI] [PubMed] [Google Scholar]
- 12. . Holcomb JB, Dumire RD, Crommett JW, et al. Evaluation of trauma team performance using an advanced human patient simulator for resuscitation training. J Trauma. 2002; 52 6: 1078– 1086. [DOI] [PubMed] [Google Scholar]
- 13. . Sedlack RE, Kolars JC, Alexander JA. . Computer simulation training enhances patient comfort during endoscopy. Clin Gastroenterol Hepatol. 2004; 2 4: 348– 352. [DOI] [PubMed] [Google Scholar]
- 14. . Braksick S, Kashani K, Hocker S. . Neurology education for critical care fellows using high fidelity simulation. Neurocrit Care. 2017; 26 1: 96– 102. [DOI] [PubMed] [Google Scholar]
- 15. . Richard S, Minone G, Varoqui C, et al. Simulation training for emergency teams to manage acute ischemic stroke by telemedicine. Medicine. 2016; 95 24: e3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. . Saver JL. . Time is brain—quantified. Stroke. 2006; 37 1: 263– 266. [DOI] [PubMed] [Google Scholar]
- 17. . Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines–Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013; 6 5: 543– 549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.