Abstract
Airway epithelial healing is defined as restoration of health or soundness; to cure. Our research indicates that two types of progenitor cells participate in this process: the tissue-specific stem cell (TSC) and the facultative basal progenitor (FBP). The TSC restores the epithelium to its normal structure and function. Thus, the TSC regenerates the epithelium. In contrast, the FBP-derived epithelium is characterized by regions of cellular hyperplasia and hypoplasia. Since the FBP-derived epithelium deviates from normal, we term the FBP-mediated process repair. Our work indicates that the TSC responds to signals from other epithelial cells, including the FBP. These signals instruct the TSC to proliferate or to select one of several differentiation pathways. We interpret these data in the context of Stephen Padget’s “seed and soil” paradigm. Therein, Padget explained that metastasis of a tumor, the seed, to a specific site, the soil, was determined by the growth and differentiation requirements of the tumor cell. By extending the seed and soil paradigm to airway epithelial healing, we suggest that proliferation and differentiation of the TSC, the seed, is determined by its interactions with other cell types, the soil. Based on this concept, we provide a set of suggestions for development of cell-based therapies that are directed toward chronic airways disease.
Keywords: tracheobronchial, basal cell, stem cell, facultative progenitor cell, β-catenin
The field of epithelial progenitor cell biology is hampered by a complex vocabulary. As a consequence, two well-meaning [pun intended] biologists may use the same word to describe different cell types and functions. To establish a common language and avoid potential misunderstandings, we will first define our terms. This glossary is an adaptation of a previously published version (1) that focuses on our cell of interest, the basal cell progenitor. Within the glossary the reader is directed to excellent reviews that present subjects that are beyond the scope of the work presented in this Aspen Lung Conference State of the Art presentation.
Airway Regions
Cartilaginous Airways
Cartilaginous airways are defined as those supported by cartilage. The human trachea and first six generations of the intrapulmonary airway meet this definition (2, 3). These airways are referred to collectively as the “tracheobronchial” airways. Human tracheobronchial architecture, including epithelial structure and cellular composition, are recapitulated by the mouse trachea (4–8). Thus, we and others have used mouse trachea to model the epithelium that lines cartilaginous airways of the human lung.
Tracheobronchial Subregions
The tracheobronchial region is further divided into subregions. The region that is defined by cartilage rings accounts for approximately two-thirds of tracheal wall mass and forms the ventral and lateral surfaces of the airway tube. The epithelium in this region is pseudostratified: each cell is in contact with the basement membrane. However, the extent of this contact varies with cell type. The other main subdivision of the tracheobronchial airway is termed the membranous region and is located on the dorsal side of the airway tube. The epithelium in this region is quite different from that overlaying the cartilaginous region. Differences include cell composition, cellular organization, and cell origin (9). Our studies have not delved into this very interesting subregion of the trachea.
Tracheobronchial Epithelial and Intraepithelial Cell Types
Multiple Epithelial Constituents
The tracheobronchial epithelium is populated by multiple cell types including basal, ciliated, secretory (mucus or nonmucus), serous cells, brush cells (also termed cholinergic chemosensory cells [10] and solitary chemosensory cells [11]), and neuroendocrine cells. Among these cell types, basal, ciliated, and secretory cells account for the majority of cells within the epithelium and are the focus of our work and that of others. These major epithelial cell types are defined below. The tracheobronchial epithelium is also populated by inflammatory cell types including dendritic cells, granulocytes, lymphocytes, and macrophages, and the frequency and function of these inflammatory cells changes with epithelial injury (12, 13). Finally, some epithelial cells are innervated (brush cells and neuroendocrine cells), while other epithelial cells interact indirectly with intraepithelial nerve processes. Herein, we focus only on the major epithelial cell types. For information on other tracheal cell types, the reader is referred to several excellent papers and reviews (14–18).
Basal Cells
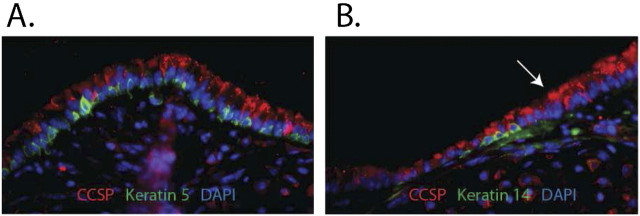
Tracheal basal cells are pyramidal cells (8). These cells have more contact with the basement membrane than other tracheal cell types. We showed that normal tracheal basal cells express keratins (K) 5 and 15 (19) (Figure 1A). All tracheal basal cells are identified by immunostaining for these pan-basal cell markers. We also showed that 20% of steady-state basal cells co-express K14 (19) (Figure 1B). The K14+ basal cells are in equilibrium with the K14− basal cell population (20). Finally, we showed that 100% of basal cells express K14 after naphthalene (see below) injury (19). Thus, genetic modifications can be induced in all basal cell subtypes using the K14-cre:erT transgene.
Figure 1.
Mouse tracheal histology. Tracheal sections from normal mice were stained using dual immunofluorescence methods. (A) The intercartilaginous region. Clara-like cells are detected as CCSP+ (red) cells. Basal cells are detected as Keratin (K) 5+ (green) cells. Note the pseudostratification in this region of the trachea. (B) The midcartilaginous region. Clara cells are stained as indicated above. The K14+ subset of basal cells is shown by K14 (green) staining. Note that this region is less stratified than the intercartilaginous region. Arrow indicates the transitional zone between the two regions.
Clara-like Cells
Tracheal nonmucus secretory cells are a subtype of Clara-type cells (21). These cells, like the bronchiolar Clara cell (22), express Clara cell secretory protein (CCSP; also known as CC10 and Scgb1a1) and cytochrome P450–2F2 (Cyp2F2). However, tracheal Clara-like cells express less CCSP and Cyp2F2 than bronchiolar Clara cells and have a distinct ultrastructure. Clara-like cells also have a distinct secretory protein repertoire: they express high levels of the CCSP-related protein Scgb3A1 (23) and the antibacterial protein PLUNC (also termed SPURT) (24, 25). In recognition of the differences between nonmucus secretory cells of the cartilaginous and bronchiolar airways, we refer to tracheal secretory cells as Clara-like cells. Clara-like cells have less contact with the basement membrane than do basal cells.
Ciliated Cells
Tracheal ciliated cells are defined by motile cilia that are detected by immunostaining for acetylated tubulin (26) or β-tubulin (27). These cells also express the transcription factor, FoxJ1 (28, 29), a nuclear marker. Selection of a ciliated cell marker depends on the other antigens that are being evaluated. Ciliated cell contact with the basement membrane is a similar to that of the Clara-like cell.
Tracheobronchial Progenitor Cell Types
Tissue-specific Stem Cell
We used classical statements to develop our definition of a tracheal tissue-specific stem cell (TSC). For instance, Becker and coworkers indicated that a TSC is “the one [cell] that is capable of self-renewal as well as differentiation to the different mature cell types that make up a specific tissue” (30). Thus, a TSC is distinguished from other progenitor cell types on the basis of proliferation and differentiation potential. The TSC has more proliferation and differentiation potential than its descendants. We define the tracheal TSC as a cell that maintains itself (it self-renews) and has the ability to generate a basal cell and a Clara-like cell and a ciliated cell (it is multipotential).
Transit-amplifying Cell
The transit-amplifying cell (TAC) is the direct descendant of the TSC. In contrast with TSC, TAC proliferate repeatedly over a short time frame. Each TAC then commits to a differentiation pathway. In the case of the tracheobronchial airways, a TAC may commit to the basal or the secretory/ciliated differentiation program. Thus, the differentiation potential of a TAC is one or two cell types and is less than that of the TSC (which is three cell types).
Facultative Progenitor Cells
The cells that populate the tracheal epithelium are long-lived but can be killed by environmental agents (biological and chemical). Injuries that result in epithelial cell death are repaired by proliferation of basal cells and/or Clara-like cells (3, 8, 19, 31–33). Since basal and Clara-like cells can proliferate, they have been referred to simply as “progenitor” cells. However, this terminology did not account for the fact that basal and Clara-like cells existed in two functional states. In the normal epithelium, basal and Clara-like cells are quiescent and contribute to homeostasis through their differentiated functions. Following injury, these cells enter the cell cycle and produce nascent differentiated cells. After repair, the progenitor cells return to their original state. In recognition of lung progenitor cell plasticity we termed these cells facultative progenitor cells (1).
Facultative Basal Brogenitor
The facultative basal progenitor cell (FBP) we will discuss briefly is analogous to the K5+ cells evaluated by others (3, 34, 35). FBP contribute to lung homeostasis via their differentiated functions: epithelial anchorage, barrier formation, and immunomodulation (8). FBP can also proliferate. We showed that FBP mitotic index is approximately 1% in the steady state and that FBP increase their mitotic index 10-fold after naphthalene injury (19). We showed that each FBP can generate a basal cell or a Clara-like cell or a ciliated cell (20, 36, 37). Since each FBP generates only one of several possible cell types, it is not a TSC. The molecular signals that regulate FBP differentiation involve direct and indirect cell–cell interactions that function through β-catenin (38, 39). A summary of these results is presented in the Vermont Stem Cell Meeting Report (manuscript in preparation).
Terminally Differentiated Cells
Cells that do not proliferate are defined as terminally differentiated cells (TDC). Following age-related attrition or injury, TDC are replaced through proliferation and differentiation of other progenitor cell types. Ciliated and mucus-secreting cells are examples of TDC within the tracheobronchial epithelium (40, 41). Although these cells do not proliferate, they can change their phenotype (42). The latter type of change is termed phenotypic plasticity (43). Although a fascinating process, we will not discuss this process herein. The reader is referred to the NHLBI workshop summary report for more information on this subject (43).
Injury Models for Analysis of Basal Progenitor Cells
Lung epithelial cells are long-lived and as a consequence proliferate infrequently (32). Thus, the pulmonary epithelium is considered to be a mitotically quiescent tissue. For instance, the steady state mitotic index of the tracheal epithelium is approximately1%, that of the bronchiolar epithelium is approximately 0.1%, and that of the alveolar epithelium is approximately 0.01%. As a consequence, studies focused on epithelial cell proliferation and differentiation employ injury to increase the mitotic index. Two of these models are discussed below.
Naphthalene Chemical Injury Model
Naphthalene is a xenobiotic chemical agent that is activated to a toxic epoxide by CyP450–2F2 (44–47). In the pulmonary epithelium, the CyP450–2F2 enzyme is expressed specifically in Clara-like and Clara cells. Thus, naphthalene-treatment is used to deplete Clara-type cells. As part of our focus on basal-type progenitor cells, we carefully evaluated naphthalene-mediated injury and repair response in the trachea (19). Adult female Fvb/n mice were exposed to 300 mg/kg naphthalene and recovered 3 to 13 days. All mice lost weight on Days 1 to 3, and 90% of treated mice gained weight between Days 3 and 13. Ten percent of naphthalene-exposed mice exhibited linear decreases in body weight after Day 3 and died between Days 6 and 9. Both Clara-like and ciliated cells were depleted in the naphthalene-injured trachea. We suspect that ciliated cell depletion was a secondary consequence of compromised cell–cell contacts that are needed to maintain the pseudostratified tracheal epithelium. On Recovery Day 13, most of the tracheal epithelial surface was normal (19), whereas approximately 20% of the epithelium showed basal and ciliated cell hyperplasia, and Clara-like hypoplasia. We reported similar results for male or female C57Bl/6 mice that were exposed to 275 mg/kg naphthalene (38). These data showed that naphthalene exposure resulted in acute injury that was rapidly resolved. The healed airway epithelium was a composite of normal and abnormal zones. We use this model to study both TSC and FBP.
Air–Liquid Interface Model
We and others have shown that air–liquid-interface (ALI) cultures of primary mouse tracheal epithelial cells can be used to evaluate the cellular and molecular mechanisms regulating FBP function (20, 29, 38, 39, 48–50). We showed that FBP initiate ALI cultures and that K5/K15/K14 triple-positive basal cells comprise 98% of the ALI culture on Culture Day 2 (38). FBP proliferate, form a polarized epithelium, and then differentiate into ciliated and Clara-like cells. The advantage of ALI cultures over in vivo analysis is that ALI cultures separate FBP proliferation from FBP differentiation and resolve FBP-to-ciliated and FBP-to-Clara-like cell differentiation into distinct waves. Thus, ALI cultures are an optimal model for analysis of the signaling pathways that regulate FBP behavior.
To study signaling in FBP, we developed a method for Ad5-cre transduction of FBP harboring floxed alleles. We showed that FBP viability and function were not altered by transduction or recombination of a marker allele (ROSA26-floxed STOP-LacZ) (39). Interestingly, Ad5-cre transduction of FBP harboring a floxed allele resulted in generation of a mosaic epithelium that was composed of wild-type and recombined cells (38, 39). This mosaicism was fortuitous, as it allowed us to study FBP behavior in genetically modified cells under identical conditions. We use these mosaic epithelia, which are a classical developmental biology technique, to evaluate the extracellular and intracellular signaling cascades that regulate FBP-mediated repair.
Identification of TSC in SITU
Label-Retention Assay
TSC are distinguished from other progenitor cell types on the basis of proliferation and differentiation potential. As indicated above, TSC have more proliferation and differentiation potential than other progenitor cell types. The labeled-nucleotide retention assay (51, 52) is used to distinguish cells that proliferate infrequently (i.e., the TSC) from other progenitor cells that proliferate repeatedly (i.e., the FBP). The label retention assay can be used to locate putative TSC on a tissue section or to evaluate the mitotic history of sorted cell populations. Interpretation of label-retention studies in the lung is challenging because differentiated cells are established early in the repair process and are long-lived (53). We address this below.
Lineage Tracing
Lineage tracing is used to determine the differentiation potential of a single cell (9, 33, 54, 55). This method, like label retention, is compatible with histologic approaches. Clones that contain “each of the different mature cell types that make up a specific tissue” are termed multipotential and are putative TSC-derived clones. We identified multipotential clones that were derived from steady and reparative K14+ cells (20, 36, 37). These data suggest that the tracheal epithelium is maintained and regenerated by a TSC. Similar results were reported for lineage-traced K5+ cells (3).
The TSC Niche
Niche Definition
The TSC is thought to reside in a protective microenvironment that is termed the stem cell niche (56–61). Initially the niche was thought to protect the stem cell from genotoxic agents. More recently the protective function of the niche has been expanded to regulation of TSC proliferation and insulation of the stem cell from differentiation signals.
Niche Location
Despite its fundamental relationship with the TSC, the niche is a poorly delineated structure. A typical description of the niche indicates that it is located at complex junctions where different cell types converge and/or at the interface with the extracellular matrix. However, the universality of these observations has not been determined.
Epithelial Healing
Age-related Changes in Lung Function
Pulmonary function, as measured by FEV1, declines as a function of age (62). Acute injury causes a further decrease in lung function that is followed by a return to the normal FEV1 versus age relationship. In contrast, the decline in lung function is accelerated in individuals with chronic lung disease. An acute exacerbation of the disease is often followed by an even more rapid decrease in lung function. In epithelial cells that are maintained by well-delineated TSC, the TSC restores the epithelium to its normal structure and function. Thus, the TSC regenerates the epithelium. In contrast, lower-ranking members of the TSC hierarchy generate an epithelium that is characterized by regions of cellular hyperplasia and hypoplasia. Since this epithelium deviates from normal, this process is termed repair. Below, we discuss the concept that a shift from TSC-mediated regeneration to FBP-mediated repair may underlie age-related decreases in lung function.
Paget’s Seed and Soil Concept
Chronic lung diseases such as chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF) are associated with increased risk for lung cancer. These carcinomas, like others, tend to metastasize to specific locations. Such secondary tumors are cause of death in many patients. The metastatic process involves detachment of a cancer cell from the primary tumor, entrée into the blood stream, attachment at the secondary site, and development of a secondary tumor. The secondary attachment site is nonrandom and often predictable. In 1889, Dr. Stephen Paget established a paradigm to explain the curious specificity of tumor metastasis. Using botany as an analogy, Paget stated: “When a plant goes to seed, its seeds are carried in all directions; but they can only live and grow if they fall on congenial soil” (63). Thus, he proposed the “seed and soil” hypothesis, which states that cancer cells (the seeds) need the proper microenvironment (the soil) for them to grow, spread, and metastasize systemically. For years the soil component was thought be the extracellular matrix and the soluble milieu. More recently the tumor microenvironment, including the vasculature and inflammatory cells, has been added to the list of soil components (64). Below, we present a modification of Paget’s seed and soil concept, which we hope will serve as the basis for future cell-based therapy of lung disease.
Stem Cell Hunting
Cell culture analysis of colony-forming cells is a typical starting point for studies that subset progenitor cells into subtypes. For instance, Barrandon and Green demonstrated that keratinocytes proliferated on tissue culture plastic and form three distinct colony types: TSC-like holoclones, TAC-like meroclones, and TDC-like paraclones (65). A clone is defined as a group of cells that are derived from a single cell. Clonality is often evaluated in vivo by analysis of colonies that were generated from differently marked cells (e.g., LacZ and alkaline phosphatase tagged cells [55, 66]). However, statistical approaches, including limiting dilution, are the preferred method for in vitro analysis (67).
Tracheal Progenitor Cell Clone Types
Tracheal cell culture.
Lung progenitor cell types require fibroblast feeders or an extracellular matrix film to form a colony or an extracellular matrix gel to form a spheroid (3, 34, 68–72). Interestingly, the functional properties of lung progenitor cells vary with culture condition (73). Many of these studies evaluated clones by limiting dilution and reported the clone-forming cell frequency (68–70, 72).
Tracheal rimmed and nonrimmed clones.
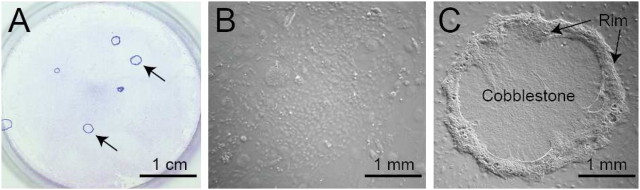
We recovered tracheal cells using dispase/collagenase/trypsin digestion (74) and cultured these cells on irradiated NIH3T3 fibroblast feeder layers in mouse tracheal epithelial culture-plus (MTEC+) medium (48) (Figure 2A). Two types of clones were detected: nonrimmed (Figure 2B) and rimmed (Figure 2C). The nonrimmed clone cells were a typical cobblestone epithelial clone type and mitotically quiescent on Culture Day 14. Nonrimmed clones could not be passaged (75). These results indicated that non-rimed clone forming cells had limited proliferation potential.
Figure 2.
Tracheal progenitor clone types. (A) Tracheal progenitor cells growing on an irradiated NIH3T3 fibroblast feeder layer. Giemsa stain. Arrows indicate rimmed clones. (B) Phase-contrast image of a non-rimmed clone. (C) Phase-contrast image of a rimmed clone. The rim domain is indicated by the arrows. The cobblestone domain is located in the center of the clone.
In contrast with nonrimmed clones, rimmed clones were highly mitotic on Culture Day 14 (75). The mitotic cells were K5+/K14+ basal cells and were spatially restricted within the clone (see below). Rimmed clones also contained a small population of BrdU label–retaining cells that were spatially restricted (Figure 3). Rimmed clones could be passaged, at clonal density, up to five times (75). These data suggested that rimmed clones were derived from the TSC. We describe additional analysis of stem cell function of rimmed clone-forming cells below.
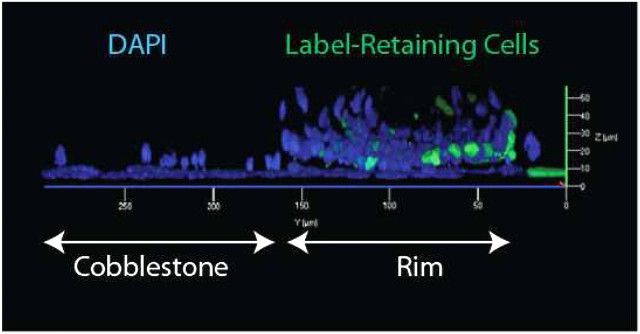
Figure 3.
The rimmed clone is a three-dimensional structure. A rimmed clone was generated from tissue stem cells (TSC) that harbored the K5-rTA and TRE-Histone 2B transgenes. The clone was cultured in doxycycline-free medium to identify label-retaining (GFP+) cells. Confocal microscopy was used to generate a three-dimensional rendering of the rimmed clone. The central cobblestone domain is to the left and the rim domain is to the right. Label-retaining cells have green nuclei. Label-diluting cells are GFP negative and are stained with DAPI (blue).
Rimmed clones are, in fact, a three-dimensional structure that is composed of two domains (Figure 3). The rim domain is approximately 4 cells wide and 10 cells tall. This raised structure is reminiscent of the intercartilaginous region of the trachea (Figure 1A). The rim domain is inhabited by K5+/14+ basal cells, and the majority of these cells are mitotically active (75). In contrast, the cobblestone domain contains a single cell layer that is a classical cobblestone epithelium (Figure 3). This flat domain is reminiscent of the midcartilaginous region of the trachea (Figure 1B). The cobblestone domain is composed of mitotically quiescent K5+/14− basal cells (75). Rimmed clones that are cultured on Transwell membranes generate an electrically tight epithelium. These data indicate that the cell layer adjacent to the membrane forms a barrier.
Purification of Basal Cell Subsets
Previous analysis of lung epithelial progenitor cell types.
Analysis of lung stem and progenitor cells has used markers that were initially identified in other tissues. Among these, Stem cell antigen-1 (Sca-1) has received a great deal of attention. This marker was used as part of an iterative sorting strategy to isolate a putative TSC that was termed the bronchoalveolar stem cell (BASC) (68). Subsequently, two groups demonstrated that Sca-1 was expressed by multiple lung cell types (70, 71). These studies illustrated the power of FLOW cytometry, which uses both positive and negative selection markers, for identification of putative lung TSC.
Markers for fractionation of the tracheal basal cell population.
The purpose of a TSC purification project is to isolate viable cells that can then be tested for TSC properties using functional assays. As a consequence, TSC are typically selected using cell surface markers and vital stains that a indicate unique biochemical property. For instance, the mouse hematopoietic TSC is frequently defined as lin-/Sca-1+/C-kit+/CD38+/CD34lo/-/Thy1.1+/lo. Other activities such as Hoechst dye efflux (76, 77) and Aldehyde dehydrogenase activity (measured by metabolism of ALDEFLUOR) have been added to (78, 79) and subtracted from (78, 80) this definition. Markers are typically “discredited” if a knockout study indicates that the gene product is unnecessary for TSC survival, proliferation, and/or differentiation. However, their utility as a component of an iterative sorting strategy is not disputed.
Selection of rimmed clone-forming tracheal basal cells.
We used rimmed clone formation as an assay to test tracheal cell subsets for stem cell characteristics. This function was tested in six tracheal epithelial cell subsets. We found that rimmed clone-forming activity was highly enriched in the CD45−/CD31−/Ter119−/CD49fbright/Sca1+/ALDH+ (CD49fbright/Sca1+/ALDH+) cell subset (75). Immunostaining and gene expression analysis verified that CD49fbright/Sca1+/ALDH+ cells were highly enriched for basal cell markers (K 5, K14, p63) and for the aldehyde dehydrogenase (ALDH) isoforms ALDH1a1 and ALDH3a1 (75). CD49fbright/Sca1+/ALDH+ did not express markers or genes associated with differentiated tracheal epithelial cell types (Clara, ciliated) or mesenchymal cells. Limiting dilution analysis demonstrated that CD49fbright/Sca1+/ALDH+ cells generated only rimmed clones.
NIH3T3 fibroblasts can be replaced by normal tracheal fibroblasts.
One concern with the clone formation system we developed for analysis of tracheal progenitor cells is that fibroblast feeder layer may alter the function of the test progenitor cell type. To determine if rimmed clone formation was a consequence of co-culture with a fibroblast cell line (irradiated NIH3T3 fibroblast cells), we tested rimmed clone formation on irradiated normal tracheal fibroblasts. This study showed that the rimmed clone formation was equivalent on normal fibroblast feeders and on irradiated NIH3T3 fibroblast feeders. In addition, we showed that rimmed clone formation required direct contact between the fibroblasts and the epithelial cells. When the feeder cells were separated from the CD49fbright/Sca1+/ALDH+ cells by a 0.6-μm pore Transwell membrane, rimmed clones were not formed. Instead, the CD49fbright/Sca1+/ALDH+ cells created an electrically tight monolayer. We also tested purified matrix components (collagen I, fibronectin, laminin) and matrigel (growth factor–containing and growth factor–reduced) for rimmed clone growth. Each of these simple and complex matrices failed to support formation of a rimmed clone. Thus, we concluded that direct cell–cell contact between the TSC and a fibroblast mediated formation of the rimmed domain. Additional studies are needed to identify the tracheal fibroblast subtype(s) that is responsible for rimmed clone formation in vitro. For more information about this critical cell type, the reader is referred to several papers that evaluate lung fibroblast subtypes and review of tracheal fibroblast subsets (43, 81–85).
Proliferation Potential of the CD49fbright/Sca1+/ALDH+ Cell
Limiting dilution analysis.
We used the iterative sorting strategy described above to select the CD49fbright/Sca1+/ALDH+ cell and tested its proliferation potential using the limiting dilution assay (67). This functional analysis demonstrated that a CD49fbright/Sca1+/ALDH+ cell generated a small number of progeny cells that retained BrdU label over 13 to 14 population doublings (75). Since a caveat to interpretation of BrdU label retention is BrdU-induced senescence, we recovered viable label-retaining CD49fbright/Sca1+ cells from clones that harbored the K5-rTA/TRE-Histone 2B:GFP transgenes (75) (Figure 3). These GFP+ label–retaining cells initiated secondary and tertiary rimmed clones and retained their GFP label. These data indicated that the rimmed clone label-retaining cells were quiescent rather than senescent and that the CD49fbright/Sca1+/ALDH+ was a self-renewing cell type.
Mitotic potential.
Each CD49fbright/Sca1+/ALDH+ cell functioned as the progenitor for 8,000 to 10,000 cells per generation (75). Seventy-five percent of CD49fbright/Sca1+/ALDH+-derived rimmed clones could be serially passaged, at clonal density, for four to five generations. These CD49fbright/Sca1+/ALDH+ cells were the precursor to approximately 40 × 106 progeny cells over five passages. These data indicate that the CD49fbright/Sca1+/ALDH+ cell has vast mitotic potential.
Differentiation Potential of CD49fbright/Sca1+/ALDH+ Cells
Differentiation of homogenous CD49fbright/Sca1+/ALDH+ cells.
We evaluated the differentiation potential of CD49fbright/Sca1+/ALDH+ cells using the ALI culture technique (75). Passage 0 rimmed clones were cultured on collagen-coated Transwell membranes in MTEC+ medium. Under these conditions, rimmed clone cells generated an polarized epithelial monolayer. These cultures were switched to differentiation conditions: air-lifting (establishment of the air–liquid interface) and removal of growth factors (switch to NuSerum medium). Under these conditions rimmed clone cell cultures were viable and polarized for at least 14 days. Surprisingly, these homogeneous cultures of CD49fbright/Sca1+/ALDH+ cells did not differentiate into ciliated or Clara-like cells. All cells remained K5+ and p63+.
Differentiation of CD49fbright/Sca1+/ALDH+ cells in mixed cell cultures.
In contrast, with the homogenous cultures, CD49fbright/Sca1+/ALDH+ cells that were co-culture of with unfractionated tracheal cells differentiated into both Clara-like and ciliated cells. These two differentiated cell types were generated at equal frequency (75). (Note that FBP-derived ALI cultures typically generate more ciliated cells than Clara-like cells [20, 38, 39].) Rimmed clone cell differentiation was also stimulated by tracheal cells that were not in contact with the rimmed clone cells. We concluded that differentiation of CD49fbright/Sca1+/ALDH+ cells required tracheal cell–derived paracrine factors.
TSC Function In Vivo
Limitations of the FLOW sorting method.
The CD49fbright/Sca1+/ALDH+ cell, as identified by FLOW cytometry, cannot be identified on a histologic section. This cell is defined in toto as a CD45−/CD31−/TER119−/CD49fbright/Sca1+/ALDH+ cell. Thus, a set of three exclusion markers (the cell is CD45−/CD31−/TER119−), an intensity difference (the cell is CD49F bright), an inclusion marker (the cell is Sca1+), and a biochemical activity (the cell metabolizes ALDEFLUOR) are used to identify the cell of interest. These parameters can be combined using a multiparametric, quantitative method such as FLOW cytometry. However, they cannot be utilized to identify the TSC in situ.
Double naphthalene injury model.
We used naphthalene injury and BrdU-label retention to identify the tracheal TSC in vivo and to determine its position relative to other anatomical markers. In the original study, FVB/n mice were treated with corn oil (vehicle) or naphthalene and labeled with BrdU on Recovery Days 1–6. BrdU-positive cells were phenotyped after a 40-day chase period. This analysis detected label-retaining basal cells as well as Clara-like cells. (A similar data set is presented in Reference 19.) This study supported the previously reported lineage relationship between basal cells and Clara-like cells (3, 20, 36, 37, 55, 86), but did not allow us to identify the tracheal TSC. Thus, we evaluated label retention in mice that were treated with naphthalene, had recovered for 40 days, and were then retreated with naphthalene. This study demonstrated that BrdU label–retaining cells proliferate in response to two injury cycles, and that the subset that retained label through both injury cycles were K5+ basal cells. These data suggested that the tracheal TSC was a K5+ basal cell.
Relationship between label-retaining and CD49fbright/Sca1+/ALDH+ cells.
To determine the relationship between the CD49fbright/Sca1+/ALDH+ cell and the tracheal label-retaining cell, we treated FVB/n mice with corn oil (vehicle) or naphthalene and pulse-labeled mitotic cells with BrdU on Recovery Days 1–6. On Recovery Day 40, TSC were recovered by FLOW cytometry and cytospun onto glass slides. In corn oil–treated mice, the frequency of label-retaining TSC was 10% (75). These data indicated that TSC participated in tracheal epithelial homeostasis. In naphthalene-treated mice, 21% CD49fbright/Sca1+/ALDH+ cells retained the BrdU label. We were able to further subset these cells into BrdU-bright and BrdU-dim subpopulations using pixel intensity analysis. This study demonstrated that 5% of CD49fbright/Sca1+/ALDH+ cells did not proliferate between Recovery Days 6 and 40. In contrast, 95% of the CD49fbright/Sca1+/ALDH+ cell population proliferated during this time period. These data indicated that TSC proliferated in response to epithelial injury and that they contributed to the regeneration process.
Summary 1
These data demonstrated that CD49fbright/Sca1+/ALDH+ cells are a rare subset of basal cells that are label-retaining, clonogenic, self-renewing, and multipotential. These cells contribute to tracheal epithelial homeostasis and regeneration after injury. Therefore, we conclude that the CD49fbright/Sca1+/ALDH+ cell is the tracheal TSC.
Niche Hunting
In Vitro TSC Niche
The in vitro niche.
We reasoned that the niche could be defined by the location of the TSC. We showed that the BrdU or GFP label–retaining cells that were derived from a single CD49fbright/Sca1+/ALDH+ cell were located in the rim domain of the rimmed clone (Figure 3) (75). Thus, we concluded that the rim domain functioned as the TSC niche. Further, the rim domain cells were the progeny of the TSC. Thus, we concluded that the TSC generated its own niche. Finally, the rim domain was composed of K5+/K14+/E-cadherin+ cells. This study indicated that the tracheal TSC niche was epithelial in character.
In vitro niche formation kinetics
To determine the kinetics of niche formation, rimmed clones were generated from cells harboring the Actin-DsRed transgene, and clone formation was followed over 14 days (Figures 4A–4D). This study showed that proliferation initiated between Days 3 and 4. By Day 5, the clones reached the 16-cell stage and a rudimentary rim was visible. Between Days 5 and 7 the rim domain developed into a distinct structure. On Days 8–9 the clones were large enough to be visualized with the (less than 40-year-old) naked eye. Rimmed clones continued to grow radially to establish the mature rimmed clone on Day 14. These observations suggest that the TSC cell population expands between Culture Days 0 and 5 and that niche formation follows this event. Confocal microscopy demonstrated that the label-retaining cells within a rimmed clone were located at the base of the rim domain (Figure 3) and that the label-retaining cells were adjacent to the fibroblast feeder layer. These data suggested that niche formation required an interaction between the TSC and the feeder layer.
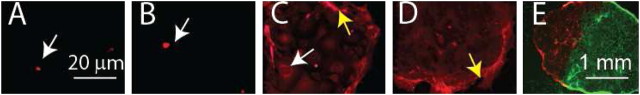
Figure 4.
Kinetics of rimmed clone formation. Rimmed clones were generated from mice harboring the actin-DsRed transgene or the Ubiquitin-GFP transgene and imaged over time. (A) Culture Day 3. Only single cells are observed (arrow). (B) Culture Day 4. A clone of 2–3 cells (arrow) is detected. C. Culture Day 5. A clone of 16–20 cells is detected. Single cells form a cobblestone epithelium (white arrow) and are the majority of clone cells. A rim (yellow arrow) is forming. (D) Culture Day 7. A rimmed clone with a well developed rim (yellow arrow) is detected. (E) Adjacent rimmed clones, one generated from a DsRed-expressing cell (red) and another from a GFP-expressing cell (green), form a conjoined clone. Note that cells from the two clones do not mix and that a rim domain does not form between the two clones.
The TSC and its niche function as an autonomous unit.
In systems in which the TSC is well defined, (e.g., the epidermis and intestine), the TSC maintains a discrete region of the epithelium (56). For instance, the bulge stem cell maintains a single hair follicle, and the intestinal stem cell maintains a single crypt-villus region. To determine if the tracheal TSC had a similarly limited “region of influence,” we evaluated the behavior of rimmed clones generated from actin-DsRed and ubiquitin-GFP transgenic mice, in vitro. Two TSC that initiated rimmed clone formation at a similar location formed a conjoined clone (Figure 4E). These clones contained two independent rim and cobblestone domains: the cells from one clone did not mix with those from another clone. Interestingly, a rim did not demarcate the intersection between the two clones. We also noted that the rimmed domain broke down if two rimmed clones came into contact with each other (not shown). After contact, clone growth decreased. These data suggested that TSC and their descendents functioned as an autonomous unit and that interactions between these units limited the region maintained by each TSC.
Niche Location In Vivo
Rationale based on in vitro studies.
We used our in vitro studies to begin to address the question, “Where is the niche in vivo?” Our in vitro studies indicated that the TSC niche was derived from the TSC, that it was epithelial in character, that it required fibroblasts to form, and that it was limited in size. Further, TSC interactions with other tracheal cells converted the niche from a promitotic environment to a differentiation environment. Thus, we surmised that the in vivo niche should be defined by a label-retaining cell, that it would be located in a region of epithelial hyperplasia, and that it would be adjacent to fibroblasts.
Label-retaining cell location.
To evaluate the location of the TSC niche, we determined the location of BrdU label–retaining cells after a double-naphthalene injury. The BrdU label–retaining cells were located exclusively in the surface epithelium of the trachea and tended to be located at the transition zone between the intercartilaginous and midcartilaginous regions (Figure 1B, arrow). This niche location is in agreement with that reported by Randell’s group (87) and with the location of multilineage clones derived from K14+ cells (20). The Randell and Gomperts (86) groups also identified label-retaining cells at the submucosal gland duct junction (87). We reported similar results (75), but did not identify multipotential clones in this region (20).
Niche plasticity.
Since TSC proliferation varied between the steady state and regenerating epithelium, we also expected that the niche would change over time. Histologic analysis of the normal and naphthalene-treated tracheal epithelium demonstrated that the boundary between the epithelial layer and the underlying fibroblasts was transiently lost on Recovery Day 2 (Figure 5). Although additional studies are needed, these preliminary results indicate that the TSC niche is indeed located at a complex junction where epithelial and fibroblasts cells converge and the basement membrane is disrupted.
Figure 5.
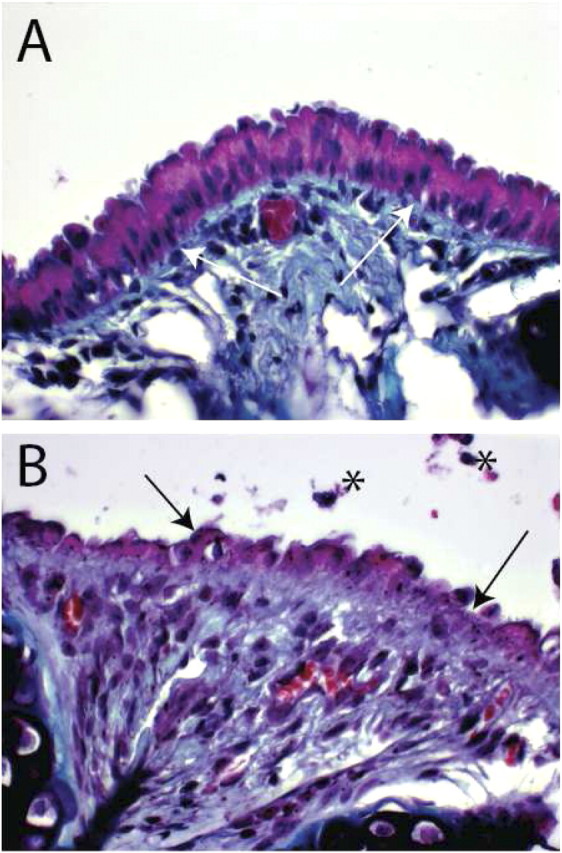
Tracheal histology. (A) An intercartilaginous region from a normal mouse. Periodic Acid Schiff stain. White arrows indicate the basement membrane. (B) An intercartilaginous region from a mouse that was treated with naphthalene and recovered for 2 days. Black arrows indicate the disrupted epithelium. Note that the boundary between the injured epithelium and the subepithelial space is distorted and that the subepithelial compartment is hyperplastic. Asterisks indicate cellular debris.
The TSC domain.
Our in vitro analysis suggested that the TSC and its niche function autonomously. A physical interaction with a second TSC-niche unit resulted in clone arrest. These observations suggested that the TSC-niche domain might be discrete in vivo. To estimate the size of this putative domain, we determined the average number of TSC per trachea (250 TSC/trachea), tracheal area (∼28 mm2), and the average number of tracheal epithelial cells (2.5 × 105 cells/trachea). These data suggest that a single tracheal TSC maintains an area of 0.1 mm2. For comparison the area maintained by an epidermal TSC is though to be 0.25 to 0.5 mm2 (88, 89). The ratio of TSC to all tracheal cells is 1 in 1,000. Thus, the TSC may be responsible for a patch of cells that is 32 × 33 cells. These data suggest that the tracheal TSC domain is similar to that of the skin.
Summary 2
These data indicated that the tracheal TSC generated its own niche. The niche is epithelial in nature, and niche formation required interactions with tracheal fibroblasts. In vivo analysis suggested that the niche was located at the junction between the epithelium overlying the intercartilaginous region of the trachea and the underlying fibroblast compartment.
Interpretation of the in VIVO and in VITRO Studies
Naphthalene-induced Injury Is a Good Model for TSC and TSC Niche Analysis
Booth and Potten summarized the issues surrounding analysis of TSC when they stated: “Unfortunately, stem cells responsible for tissue homeostasis and regeneration cannot be identified morphologically or distinguished from other cells by any recognized set of markers. Hence, interpretations of stem cell behavior are based on monitoring cohorts of cells before and after perturbation of the tissue. This approach offers direct insights into the dynamics of the stem cell population, but only limited information about stem cell behavior in the steady state” (90). This statement summarizes the issues we face in analysis of tracheobronchial TSC as well as TSC that might maintain other respiratory epithelial compartments.
We recognize that TSC behavior (and for that fact human lung disease) is far more complex than that modeled by a chemical injury such as naphthalene-exposure (19, 20, 37, 38, 47, 53, 91–94). However, the naphthalene model has several attributes that make it optimal for the analysis of the interactions that regulate TSC and FBP function. First, this injury model can be applied to all mouse strains, inbred or mixed background, after appropriate dose–response analysis (19). Second, the repair process is rapid and does not compromise alveolar structure or function (95). Consequently, survival is high (90%). Third, the TSC and FBP that repair the NA-injured airway epithelium, like their human counterparts (55), are located in the surface epithelium (36, 37). In contrast, other injury models (e.g., acid and detergent) destroy this progenitor pool (87). Fourth, the naphthalene model is relatively noninflammatory. The number of lavagable macrophage and neutrophils increases approximately twofold over control on Recovery Day 6. We have shown that the NA injury-repair response is similar in wild-type and immunocompromised (Nod/Scid) mice, indicating that lymphocytes are not necessary for epithelial repair. This attribute of the naphthalene model allows us to avoid confounding variables associated with lymphocyte-mediated injury and alteration of epithelial cell phenotype (40). We surmise that a simple model, such as naphthalene-induced injury, will lay the fundamental groundwork for analysis of TSC and their niche in disease.
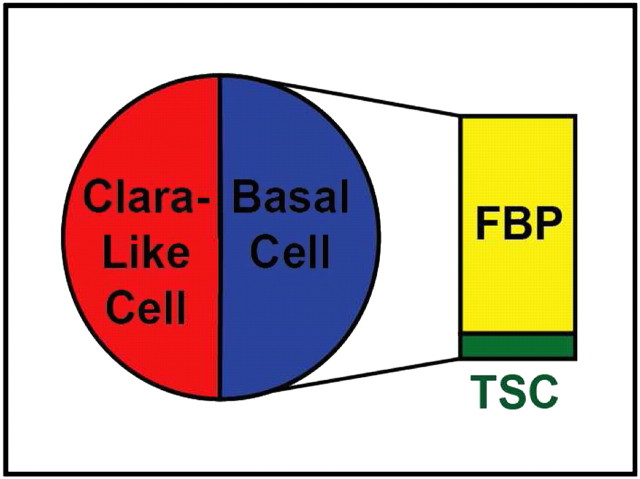
Not All Basal Cells Are Stem Cells
Our data and those of others indicate that the tracheal progenitor cell population consists of two subsets: Clara-like and basal cells (Figure 6) (19, 36, 37). Our studies also indicate that the basal cell population can be subdivided into two functionally distinct subtypes (Figure 6). Ten percent of basal cells are TSC (75) and the remainder are facultative basal progenitor (FBP) cells (75). Since we showed that the TSC was a subset of the basal cell population, we question the claim that “all basal cells are stem cells” (3, 34, 35).
Figure 6.
The tracheal progenitor cell population consists of Clara-like and basal cells. There are two subsets of basal cells. Facultative basal progenitor (FBP) cells are 90% of the basal cell pool. TSC are 10% of the basal cell pool.
Tracheal TSC Niche Function Can Be Modified
Our analysis of TSC-derived rimmed clones used an in vitro model to define three niche functions that can now be evaluated in vivo. First, we showed that the niche contains both label-retaining TSC and their label-diluting progeny. The fact that both quiescent (TSC) and actively proliferating TAC were located in the niche indicated that the niche differentially regulated the cell cycle frequency of the TSC and the TAC. Second, we showed that the TSC and TAC phenotype was distinct from that of the cobblestone domain cells. Interestingly, the phenotype of the TSC and TAC was the same as cells that repair the tracheal epithelium in vivo. In contrast, the cobblestone cells had a steady state basal cell phenotype. These data indicated that the niche inhibited differentiation of constituent TSC and TAC into a resting basal cell phenotype. Finally, we showed that rimmed clones failed to generate ciliated or Clara-like cells under standard differentiation conditions. This TSC function was stimulated through interactions with other tracheal cells. This study suggested that the niche could be modified such that its differentiation-suppression function gave way to one that was permissive for differentiation.
Comments on the Concepts that Guide Cell-based Therapy
A New Healing Concept
Our analysis of the tracheal epithelium after naphthalene exposure indicates that the TCS-mediated regeneration and FBP-mediated repair processes function synergistically to heal the epithelium. We demonstrated that TSC-mediated regeneration is dependent on FBP-mediated repair to achieve its full potential. Importantly, differentiation of TSC-derived cells is dependent on paracrine signals that are most likely derived from the FBP and its progeny. Thus, a process that depletes the FBP population or decreases production of these cells by the TSC would diminish the ability of the TSC to regenerate the epithelium. This situation could lead to alterations in epithelial health and possibly sensitization to additional injury. We suggest that epithelial healing, the process that restores the epithelium to health, requires a balance between regeneration and repair. Disruption of this interaction may lead to chronic lung diseases that involve the airways.
A Modified “Seed and Soil” Paradigm
Our data supports a corollary to Paget’s “seed and soil” paradigm. In the context of tracheal epithelial healing, we suggest that Paget’s seed could be the TSC and that Paget’s soil could be the cells that modify the TSC niche and thus regulate TSC proliferation and differentiation. Our data indicate that the TSC must interact with (at least two) additional cell types, fibroblasts and FBP, to regenerate the epithelium. Thus, we suggest that fertile ground for analysis of epithelial healing and deviations that result in chronic disease lays in a better understanding of TSC interactions with the mesenchyme and other epithelial cells.
Our immediate focus is on the TSC–fibroblast interaction. We hope to address the following questions. (1) How does the TSC initiate niche formation? (2) How does the niche maintain TSC quiescence while driving TAC proliferation? By focusing on the TSC-niche–tracheal cell interaction, we hope to determine (1) how niche function is changed from proliferation to differentiation, and (2) the signals that allow one TSC-derived cell to generate each of the differentiated cell types. Finally, by comparing the TSC interaction with fibroblasts and epithelial cells, we hope to determine (1) the differences between a mitotic and a differentiation niche, (2) how the TSC domain is determined, and (3) if the TSC domain can be reduced or expanded in health and disease.
Our long-term goal is to broaden our in vitro model to include the complexities of the intact trachea. For instance, the normal tracheal epithelium is separated from fibroblasts by a well-defined matrix that exhibits compositional and functional micro-heterogeneity (14, 15, 96–100). Similarly, the term fibroblast does not indicate a single cell type. Further work is needed to determine if the cells we termed “normal tracheal fibroblasts” are in fact a specific subtype or part of a continuum of pericohondrial/connective tissue fibroblasts that anchor the smooth muscle, pericondrium, and cartilage (to name a few). Finally, our in vivo studies concentrated on the cartilaginous region of the trachea. Thus, other putative stem cell niche that reside in the membranous region have not been evaluated and are unexplored territory that is open to the intrepid.
The Politics of Cell-based Therapy: Hope and Change
Our corollary to Paget’s Seed and Soil concept is relevant to the lung, but its broader impact will be application of the new “cellular-soil” paradigm to stem cell therapy for all organ systems. Support for our corollary will identify cellular and molecular mechanisms that can be used to enhance the success of cell-based therapy. We anticipate that purification of tracheal stem cells and demonstration that they recreate the niche in situ will overcome the key impediment to use of tracheal stem cells for cell-based therapy: generation of significant numbers of autologous cells. Our data also indicate that host conditioning protocols should focus on insuring appropriate regulation of stem cell proliferation and differentiation. Importantly, we suggest that therapeutic interventions should provide three cell types: the replacement stem cells and the cells that form the proliferation and differentiation niche.
Our seed and soil corollary also highlights considerable issues and potential dangers associated with cell-replacement therapy, particularly those that utilize the TSC exclusively. Of significant concern is the finding that TSC clones proliferate unless they encounter a physical boundary. Although tracheal TSC did not cause tumors under any of the conditions tested thus far, treatment of patients with highly mitotic cells deserves more thought.
Supplementary Material
References
- 1.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc 2008;5:328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randell SH. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 2009;106:12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M, Jetten AM. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol 2004;287:C171–C181. [DOI] [PubMed] [Google Scholar]
- 5.Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and BMPs. Differentiation 2006;74:422–437. [DOI] [PubMed] [Google Scholar]
- 6.Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for sox2 in the developing and adult mouse trachea. Development 2009;136:1899–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widdicombe JH, Chen LL, Sporer H, Choi HK, Pecson IS, Bastacky SJ. Distribution of tracheal and laryngeal mucous glands in some rodents and the rabbit. J Anat 2001;198:207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res 2001;27:401–415. [DOI] [PubMed] [Google Scholar]
- 9.Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am J Respir Cell Mol Biol 2005;33:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, et al. . Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA 2011;108:9478–9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med 2011;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller LA, Hurst SD, Coffman RL, Tyler NK, Stovall MY, Chou DL, Putney LF, Gershwin LJ, Schelegle ES, Plopper CG, et al. . Airway generation-specific differences in the spatial distribution of immune cells and cytokines in allergen-challenged rhesus monkeys. Clin Exp Allergy 2005;35:894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller LA, Plopper CG, Hyde DM, Gerriets JE, Pieczarka EM, Tyler NK, Evans MJ, Gershwin LJ, Schelegle ES, Van Winkle LS. Immune and airway effects of house dust mite aeroallergen exposures during postnatal development of the infant rhesus monkey. Clin Exp Allergy 2003;33:1686–1694. [DOI] [PubMed] [Google Scholar]
- 14.Evans MJ, Fanucchi MV, Van Winkle LS, Baker GL, Murphy AE, Nishio SJ, Sannes PL, Plopper CG. Fibroblast growth factor-2 during postnatal development of the tracheal basement membrane zone. Am J Physiol Lung Cell Mol Physiol 2002;283:L1263–L1270. [DOI] [PubMed] [Google Scholar]
- 15.Evans MJ, Van Winkle LS, Fanucchi MV, Baker GL, Murphy AE, Nishio SJ, Schelegle ES, Gershwin LJ, Sannes PL, Plopper CG. Fibroblast growth factor-2 in remodeling of the developing basement membrane zone in the trachea of infant rhesus monkeys sensitized and challenged with allergen. Lab Invest 2002;82:1747–1754. [DOI] [PubMed] [Google Scholar]
- 16.Plopper CG, Mariassy AT, Wilson DW, Alley JL, Nishio SJ, Nettesheim P. Comparison of nonciliated tracheal epithelial cells in six mammalian species: ultrastructure and population densities. Exp Lung Res 1983;5:281–294. [DOI] [PubMed] [Google Scholar]
- 17.Avadhanam KP, Plopper CG, Pinkerton KE. Mapping the distribution of neuroepithelial bodies of the rat lung: a whole-mount immunohistochemical approach. Am J Pathol 1997;150:851–859. [PMC free article] [PubMed] [Google Scholar]
- 18.Van Winkle LS, Fanucchi MV, Miller LA, Baker GL, Gershwin LJ, Schelegle ES, Hyde DM, Evans MJ, Plopper CG. Epithelial cell distribution and abundance in rhesus monkey airways during postnatal lung growth and development. J Appl Physiol 2004;97:2355–2363, discussion 2354. [DOI] [PubMed] [Google Scholar]
- 19.Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD. Tracheal basal cells: a facultative progenitor cell pool. Am J Pathol 2010;177:362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh M, Brechbuhl HM, Smith RW, Li B, Hicks DA, Titchner T, Runkle CM, Reynolds SD. Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells. Am J Respir Cell Mol Biol 2011;45:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stripp BR, Reynolds SD. Clara cells. : , Shapiro S, Laurent G, Encyclopedia of respiratory medicine. St. Louis, MO: Elsevier; 2006. pp. 471–477. [Google Scholar]
- 22.Massaro GD, Singh G, Mason R, Plopper CG, Malkinson AM, Gail DB. Biology of the Clara cell. Am J Physiol 1994;266:L101–L106. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds SD, Reynolds PR, Pryhuber GS, Finder JD, Stripp BR. Secretoglobins scgb3a1 and scgb3a2 define secretory cell subsets in mouse and human airways. Am J Respir Crit Care Med 2002;166:1498–1509. [DOI] [PubMed] [Google Scholar]
- 24.Di YP. Functional roles of splunc1 in the innate immune response against gram-negative bacteria. Biochem Soc Trans 2011;39:1051–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di YP, Harper R, Zhao Y, Pahlavan N, Finkbeiner W, Wu R. Molecular cloning and characterization of spurt, a human novel gene that is retinoic acid-inducible and encodes a secretory protein specific in upper respiratory tracts. J Biol Chem 2003;278:1165–1173. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR. Conditional clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol 2000;278:L1256–L1263. [DOI] [PubMed] [Google Scholar]
- 27.Van Winkle LS, Buckpitt AR, Nishio SJ, Isaac JM, Plopper CG. Cellular response in naphthalene-induced clara cell injury and bronchiolar epithelial repair in mice. Am J Physiol 1995;269:L800–L818. [DOI] [PubMed] [Google Scholar]
- 28.Hackett BP, Brody SL, Liang M, Zeitz ID, Bruns LA, Gitlin JD. Primary structure of hepatocyte nuclear factor/forkhead homologue 4 and characterization of gene expression in the developing respiratory and reproductive epithelium. Proc Natl Acad Sci USA 1995;92:4249–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You Y, Huang T, Richer EJ, Schmidt JE, Zabner J, Borok Z, Brody SL. Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2004;286:L650–L657. [DOI] [PubMed] [Google Scholar]
- 30.Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 1963;197:452–454. [DOI] [PubMed] [Google Scholar]
- 31.Evans MJ, Shami SG, Cabral-Anderson LJ, Dekker NP. Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to no2. Am J Pathol 1986;123:126–133. [PMC free article] [PubMed] [Google Scholar]
- 32.Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol 2008;295:L231–L234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of scgb1a1+ clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009;4:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 2010;8:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol 2004;164:577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol 2004;286:L643–L649. [DOI] [PubMed] [Google Scholar]
- 38.Brechbuhl HM, Ghosh M, Smith MK, Smith RW, Li B, Hicks DA, Cole BB, Reynolds PR, Reynolds SD. Beta-catenin dosage is a critical determinant of tracheal basal cell fate determination. Am J Pathol 2011;179:367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith RW, Hicks DA, Reynolds SD. Roles for β-catenin and doxycycline in the regulation of respiratory epithelial cell frequency and function. Am J Respir Cell Mol Biol 2012;46:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, et al. . Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol 2004;31:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larson SD, Plopper CG, Baker G, Tarkington BK, Decile KC, Pinkerton K, Mansoor JK, Hyde DM, Schelegle ES. Proximal airway mucous cells of ovalbumin-sensitized and -challenged Brown Norway rats accumulate the neuropeptide calcitonin gene-related peptide. Am J Physiol Lung Cell Mol Physiol 2004;287:L286–L295. [DOI] [PubMed] [Google Scholar]
- 42.Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, Whitsett JA. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol 2006;34:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borok Z, Whitsett JA, Bitterman PB, Thannickal VJ, Kotton DN, Reynolds SD, Krasnow MA, Bianchi DW, Morrisey EE, Hogan BL, et al. . Cell plasticity in lung injury and repair: report from an NHLBI workshop, April 19–20, 2010. Proc Am Thorac Soc 2011;8:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buckpitt A, Buonarati M, Avey LB, Chang AM, Morin D, Plopper CG. Relationship of cytochrome p450 activity to Clara cell cytotoxicity: II. Comparison of stereoselectivity of naphthalene epoxidation in lung and nasal mucosa of mouse, hamster, rat and rhesus monkey. J Pharmacol Exp Ther 1992;261:364–372. [PubMed] [Google Scholar]
- 45.Buckpitt A, Chang AM, Weir A, Van Winkle L, Duan X, Philpot R, Plopper C. Relationship of cytochrome p450 activity to Clara cell cytotoxicity: IV. Metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamsters. Mol Pharmacol 1995;47:74–81. [PubMed] [Google Scholar]
- 46.Chichester CH, Philpot RM, Weir AJ, Buckpitt AR, Plopper CG. Characterization of the cytochrome p-450 monooxygenase system in nonciliated bronchiolar epithelial (Clara) cells isolated from mouse lung. Am J Respir Cell Mol Biol 1991;4:179–186. [DOI] [PubMed] [Google Scholar]
- 47.Warren DL, Brown DL, Buckpitt AR. Evidence for cytochrome p-450 mediated metabolism in the bronchiolar damage by naphthalene. Chem Biol Interact 1982;40:287–303. [DOI] [PubMed] [Google Scholar]
- 48.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 2002;283:L1315–L1321. [DOI] [PubMed] [Google Scholar]
- 49.Huang T, You Y, Spoor MS, Richer EJ, Kudva VV, Paige RC, Seiler MP, Liebler JM, Zabner J, Plopper CG, et al. . Foxj1 is required for apical localization of ezrin in airway epithelial cells. J Cell Sci 2003;116:4935–4945. [DOI] [PubMed] [Google Scholar]
- 50.Jain R, Pan J, Driscoll JA, Wisner JW, Huang T, Gunsten SP, You Y, Brody SL. Temporal relationship between primary and motile ciliogenesis in airway epithelial cells. Am J Respir Cell Mol Biol 2010;43:731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 1989;57:201–209. [DOI] [PubMed] [Google Scholar]
- 52.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990;61:1329–1337. [DOI] [PubMed] [Google Scholar]
- 53.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 2001;24:671–681. [DOI] [PubMed] [Google Scholar]
- 54.Cepko CL, Fields-Berry S, Ryder E, Austin C, Golden J. Lineage analysis using retroviral vectors. Curr Top Dev Biol 1998;36:51–74. [DOI] [PubMed] [Google Scholar]
- 55.Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L. Progenitor cells of the adult human airway involved in submucosal gland development. Development 1995;121:2031–2046. [DOI] [PubMed] [Google Scholar]
- 56.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell 2004;116:769–778. [DOI] [PubMed] [Google Scholar]
- 57.Moore KA, Lemischka IR. Stem cells and their niches. Science 2006;311:1880–1885. [DOI] [PubMed] [Google Scholar]
- 58.Scadden DT. The stem-cell niche as an entity of action. Nature 2006;441:1075–1079. [DOI] [PubMed] [Google Scholar]
- 59.Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell 2007;1:607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature 2001;414:98–104. [DOI] [PubMed] [Google Scholar]
- 61.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science 2000;287:1427–1430. [DOI] [PubMed] [Google Scholar]
- 62.Lazaar AL, Panettieri RA. Is airway remodeling clinically relevant in asthma? Am J Med 2003;115:652–659. [DOI] [PubMed] [Google Scholar]
- 63.Paget S. The distribution of secondary growths in cancer of the breast. Lancet 1889;133:571–573. [PubMed] [Google Scholar]
- 64.Mendoza M, Khanna C. Revisiting the seed and soil in cancer metastasis. Int J Biochem Cell Biol 2009;41:1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA 1987;84:2302–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cepko C, Ryder EF, Austin CP, Walsh C, Fekete DM. Lineage analysis using retrovirus vectors. Methods Enzymol 1995;254:387–419. [DOI] [PubMed] [Google Scholar]
- 67.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol 1981;126:1614–1619. [PubMed] [Google Scholar]
- 68.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823–835. [DOI] [PubMed] [Google Scholar]
- 69.Teisanu RM, Chen H, Matsumoto K, McQualter JL, Potts E, Foster WM, Bertoncello I, Stripp BR. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am J Respir Cell Mol Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teisanu RM, Lagasse E, Whitesides JF, Stripp BR. Prospective isolation of bronchiolar stem cells based upon immunophenotypic and autofluorescence characteristics. Stem Cells 2009;27:612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K, Nilsson SK, Simmons PJ, Bertoncello I. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells 2009;27:623–633. [DOI] [PubMed] [Google Scholar]
- 72.Reynolds SD, Shen H, Reynolds P, Betsuyaku T, Pilewski J, Gambelli F, Deguiseppe M, Ortiz LA, Stripp B. Molecular and functional properties of lung side population cells. Am J Physiol Lung Cell Mol Physiol 2007;292:L972–L983. [DOI] [PubMed] [Google Scholar]
- 73.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, et al. . Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 2011;147:525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Epperly MW, Guo H, Shen H, Niu Y, Zhang X, Jefferson M, Sikora CA, Greenberger JS. Bone marrow origin of cells with capacity for homing and differentiation to esophageal squamous epithelium. Radiat Res 2004;162:233–240. [DOI] [PubMed] [Google Scholar]
- 75.Ghosh M, Helm KM, Smith RW, Giordanengo MS, Li B, Shen H, Reynolds SD. A single cell functions as a tissue-specific stem cell and the in vitro niche-forming cell. Am J Respir Cell Mol Biol 2011;45:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996;183:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of cd34 antigen exist in multiple species. Nat Med 1997;3:1337–1345. [DOI] [PubMed] [Google Scholar]
- 78.Levi BP, Yilmaz OH, Duester G, Morrison SJ. Aldehyde dehydrogenase 1a1 is dispensable for stem cell function in the mouse hematopoietic and nervous systems. Blood 2009;113:1670–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pearce DJ, Bonnet D. The combined use of hoechst efflux ability and aldehyde dehydrogenase activity to identify murine and human hematopoietic stem cells. Exp Hematol 2007;35:1437–1446. [DOI] [PubMed] [Google Scholar]
- 80.Uchida N, Leung FY, Eaves CJ. Liver and marrow of adult mdr-1a/1b(−/−) mice show normal generation, function, and multi-tissue trafficking of primitive hematopoietic cells. Exp Hematol 2002;30:862–869. [DOI] [PubMed] [Google Scholar]
- 81.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001;166:7556–7562. [DOI] [PubMed] [Google Scholar]
- 82.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 1997;124:4867–4878. [DOI] [PubMed] [Google Scholar]
- 83.Garantziotis S, Steele MP, Schwartz DA. Pulmonary fibrosis: thinking outside of the lung. J Clin Invest 2004;114:319–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol 2000;105:193–204. [DOI] [PubMed] [Google Scholar]
- 85.Knight D. Epithelium-fibroblast interactions in response to airway inflammation. Immunol Cell Biol 2001;79:160–164. [DOI] [PubMed] [Google Scholar]
- 86.Hegab AE, Ha VL, Gilbert JL, Zhang KX, Malkoski SP, Chon AT, Darmawan DO, Bisht B, Ooi AT, Pellegrini M, et al. . A novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 2001;24:662–670. [DOI] [PubMed] [Google Scholar]
- 88.Ghazizadeh S, Taichman LB. Organization of stem cells and their progeny in human epidermis. J Invest Dermatol 2005;124:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jensen UB, Lowell S, Watt FM. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: A new view based on whole-mount labelling and lineage analysis. Development 1999;126:2409–2418. [DOI] [PubMed] [Google Scholar]
- 90.Booth C, Potten CS. Gut instincts: Thoughts on intestinal epithelial stem cells. J Clin Invest 2000;105:1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Winkle LS, Johnson ZA, Nishio SJ, Brown CD, Plopper CG. Early events in naphthalene-induced acute clara cell toxicity: Comparison of membrane permeability and ultrastructure. Am J Respir Cell Mol Biol 1999;21:44–53. [DOI] [PubMed] [Google Scholar]
- 92.Stripp BR, Maxson K, Mera R, Singh G. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol 1995;269:L791–L799. [DOI] [PubMed] [Google Scholar]
- 93.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 2000;156:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mahvi D, Bank H, Harley R. Morphology of a naphthalene-induced bronchiolar lesion. Am J Pathol 1977;86:558–572. [PMC free article] [PubMed] [Google Scholar]
- 95.Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA, Mariani TJ, Yp D, Taketo MM, Stripp BR. Conditional stabilization of {beta}-catenin expands the pool of lung stem cells. Stem Cells 2008;26:1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Evans MJ, Fanucchi MV, Plopper CG, Hyde DM. Postnatal development of the lamina reticularis in primate airways. Anat Rec (Hoboken) 2010;293:947–954. [DOI] [PubMed] [Google Scholar]
- 97.Evans MJ, Fanucchi MV, Baker GL, Van Winkle LS, Pantle LM, Nishio SJ, Schelegle ES, Gershwin LJ, Miller LA, Hyde DM, et al. . The remodelled tracheal basement membrane zone of infant rhesus monkeys after 6 months of recovery. Clin Exp Allergy 2004;34:1131–1136. [DOI] [PubMed] [Google Scholar]
- 98.Evans MJ, Fanucchi MV, Baker GL, Van Winkle LS, Pantle LM, Nishio SJ, Schelegle ES, Gershwin LJ, Miller LA, Hyde DM, et al. . Atypical development of the tracheal basement membrane zone of infant rhesus monkeys exposed to ozone and allergen. Am J Physiol Lung Cell Mol Physiol 2003;285:L931–L939. [DOI] [PubMed] [Google Scholar]
- 99.Evans MJ, Fanucchi MV, Miller LA, Carlson MA, Nishio SJ, Hyde DM. Reduction of collagen vii anchoring fibrils in the airway basement membrane zone of infant rhesus monkeys exposed to house dust mite. Am J Physiol Lung Cell Mol Physiol 2010;298:L543–L547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. The attenuated fibroblast sheath of the respiratory tract epithelial-mesenchymal trophic unit. Am J Respir Cell Mol Biol 1999;21:655–657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.