Abstract
A recent breakthrough showing that direct trans-differentiation of chondrocytes into bone cells commonly occurs during endochondral bone formation in the growth plate, articular cartilage, and mandibular condylar cartilage suggests that chondrogenesis and osteogenesis are likely one continuous biological process instead of two separate processes. Yet, gene regulation of this cell transformation is largely unclear. Here, we employed cartilage-specific β-catenin loss-of-function (β-catenin fx/fx) and gain-of-function (β-catenin fx(exon3)/ fx(exon3)) models in the R26RTomato background (for better tracing the cell fate of chondrocytes) to study the role of β-catenin in cell trans-differentiation. Using histological, immunohistochemical, and radiological methods combined with cell lineage tracing techniques, we showed that deletion of β-catenin by either Acan-CreERT2 or Col10a1-Cre resulted in greatly reduced cell trans-differentiation with a significant decrease in subchondral bone volume during mandibular condylar growth. Molecular studies demonstrated severe defects in cell proliferation and differentiation in both chondrocytes and bone cells. The gain of function studies (constitutive activation of β-catenin with Acan-CreERT2 at ages of postnatal day 7, 4-weeks and 6-months) led to more bone cell trans-differentiation of chondrocytes in the mandibular condyle due to increased proliferation and accelerated chondrocyte differentiation with incipient osteogenic changes within the cartilage matrix, resulting in an increased volume of poorly-formed immature subchondral bone. These results support the notion that chondrogenesis and osteogenesis are one continuous process, in which β-catenin signaling plays an essential role in the cell trans-differentiation of chondrocytes into bone cells during mandibular condylar development and growth.
Keywords: chondrocyte, osteoblast, cartilage, cell signaling, growth/development
Introduction
The development and growth of the temporomandibular joint (TMJ) have long been recognized as different from that of long bone joints 1, 2. However, whether these cartilages with different developmental histories and structures also differ in their genetic regulation has only begun to be investigated in the last decade 3. We have shown that deletion of Bmpr1a, a primary receptor for BMP-2 and BMP-4, in growth plate 4 and mandibular condylar cartilage (MCC) chondrocytes 5 not only leads to defects in chondrogenesis, but also in osteogenesis. These results support the new concept recently demonstrated by our lab and others that chondrocytes directly transform in considerable numbers into bone cells, thus making them the primary progenitor cells for endochondral bone formation rather than in-migrating cells from either bone marrow or periosteum 6-11. In the growth plate, Bmpr1a null mice demonstrate a virtual cessation of endochondral bone formation 4 while in MCC of the same Bmpr1a null mice the subchondral bone phenotype is completely different 5. This indicates that there may be diverse mechanisms for the same receptor in different skeletal locations (i.e., MCC vs growth plate) or that other regulators of chondrogenesis might affect MCC growth in unique ways.
Signaling via the Wnt/β-catenin pathway is a well-established regulator of skeletal development and growth including growth plate and articular cartilage 12-20. Cartilage-derived β-catenin is a central mediator for major events during endochondral bone formation, including chondrogenesis, primary and secondary ossification center development, vascularization, and perichondrial bone formation 14. Moreover, β-catenin signaling is required for determining osteoblast versus chondrocyte cell fate and promoting chondrocyte proliferation and maturation 20. In vivo mouse genetic studies using a constituently active form of β-catenin under the control of a Col2 promoter demonstrated that the growth plate in postnatal mice undergoes closure within weeks of tamoxifen (TM) activation of the transgene 21. β-catenin signaling in chondrocytes also plays a key role in the postnatal bone growth and bone remodeling likely through its regulation of osteoclast formation 22.
Most studies have focused on limb cartilages, with little attention to cartilages in the craniofacial region. Mice lacking Wnt/β-catenin or with constitutive activation of Wnt/β-catenin have been shown to exhibit disrupted growth in the cranial base synchondroses 23, but these are primary cartilages with developmental affinities to limb growth plate. The role of β-catenin signaling during TMJ development and growth has been little studied, with only two very different studies comprising our knowledge base; a developmental study showing agenesis of the MCC in mice with stabilization of β-catenin 24 and osteoarthritic changes in mice with conditional activation of β-catenin in MCC chondrocytes 25.
In this study, we employed recent advances in cell lineage tracing technology to investigate the role of β-catenin signaling in the regulation of condylar growth. Using the background of R26RTomato, we demonstrated an essential role for β-catenin in the cell transformation of chondrocytes into bone cells (osteoblasts/osteocytes) by employing chondrocyte-specific β-catenin loss-of-function models (using the β-cateninfx/fx line crossed to either the Aggrecan-CreERT2 or Col10a1-Cre line) and gain-of-function genetic mouse models (using the tamoxifen-inducible Aggrecan-CreERT2).
Materials and Methods
Breeding transgenic mice
To generate triple mice to conditionally knockout β-catenin in all chondrocytes or specifically in hypertrophic chondrocytes, β-cateninflox 26, and R26RTomato (B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, stock number: 007905 from the Jackson Laboratory) were internally crossed with Aggrecan (Acan)-CreERT2 (one-time tamoxifen injection at day 3, i.p.) 27 or Col10a1-Cre mice 28, respectively. Similarly, to generate triple mice for studies of the fate of chondrocytes with constitutively active β-catenin in chondrocytes (CA-β-cat) 29, Acan-CreERT2 (one-time tamoxifen injection at day 3 or 14, i.p.), β-catenin flox(Ex3)/flox(Ex3), and R26RTomato were internally crossed three times. Tamoxifen (Sigma, T5648) was dissolved in 90% corn oil (Sigma, C8267) and 10% ethanol. Four animals were used in either control or mutant group at each time point.
All protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Texas A&M University College of Dentistry.
Histological analysis
Mandibular condyles were fixed in 4% paraformaldehyde and decalcified at 4 °C. Samples for cell lineage tracing were dehydrated with sucrose and embedded in OCT followed by CryoJane frozen sections as previously described 30. Samples for histological staining were embedded in paraffin, sectioned, and stained with safranin O (proteoglycans) or toluidine blue stain 31. Immunostaining were proceeded as previously described 32 with the following antibodies: rabbit anti-aggrecan antibody (Abcam; 1:400), anti-collagen II mouse monoclonal antibody (Santa Cruz Biotechnology; 1:50), rabbit anti-collagen X antibody (Abcam; 1:400), rabbit anti-DMP1 antibody (provided by Dr. Chunlin Qin at Texas A&M University, 1:400), rabbit anti-Runx2 antibody (Abcam; 1:200), goat anti-sclerostin antibody (R&D; 1:400), rabbit anti-collagen I antibody (Abcam; 1:100), anti-osteopontin mouse monoclonal antibody (Santa Cruz Biotechnology; 1:100), or anti-β-catenin mouse monoclonal antibody (DSHB; 1:100). The immunohistochemistry experiments were detected with a 3, 3-diaminobenzidine kit (Vector Laboratories, Burlingame, CA). The immunofluorescent signals were detected with corresponding Alexa second antibody (Thermofisher, 1:200) at room temperature for 2 hours.
Cell proliferation
BrdU (Sigma, 10 mg/ml) or EdU (Life technique, 10mg/ml) was injected into mice (two times for BrdU: 24- and 2 hours before sacrifice, i.p.; one time for EdU, 2 hours before sacrifice, i.p.). Image J was used to quantify the BrdU+ or EdU+ cells in MCC proliferating layers 5.
Confocal microscopy
SP5 Leica confocal microscope was used to capture the fluorescent cell images. All images were obtained at wavelengths ranging from 488 (green)-561 (red) μm. Multiple stacked images were taken at 100Hz and dimension of 1024x1024 33. Red color reflected by tomato signal indicated the descendant cells with Cre-activity; green color represented the corresponding immunofluorescent staining; blue color was DAPI staining.
Radiographs
The mandibles were dissected free of muscles and were taken X-ray by using a Faxitron model MX-20 Specimen Radiography System (Faxitron X-Ray Corp., Lincolnshire, IL, USA). The mandibular length was measured from the middle of the condylar head curvature to the most anterior point on the incisal alveolar process, and quantified by Image J software (Figure S2A) 5.
Backscattered scanning electron microscopy (SEM), acid-etched SEM
The mandibles were fixed in 4% paraformaldehyde, dehydrated in ascending concentrations of ethanol (from 70% to 100%), and embedded in methyl-methacrylate (MMA, Buehler, Lake Bluff, IL) 34, which were cut and polished by using 1 μm and 0.3 μm alumina alpha micropolish II solution (Buehler). For acid etched SEM, samples were treated with 37% phosphoric acid for 2 to 10 seconds, 5% sodium hydrochloride for 5 minutes and then coated with gold (for acid-etched SEM imaging). For backscattered SEM, samples were coated with carbon. FEI/Philips XL30 field-emission environmental SEM (Hillsboro, OR, USA) was used to scan as described previously 35.
Statistical Analysis
All data were reported as mean (SD). The Kruskal-Wallis test was used to detect the significant differences among samples. The Mann-Whitney U test (post hoc test) was used to compare differences between the mutant and age-matched control groups. Significance level was defined as *p < 0.05; **p < 0.01.
Results
Deleting β-catenin in early chondrocytes led to a malformed ramus, defective chondrogenesis, and a lack of trans-differentiation of chondrocytes into bone cells
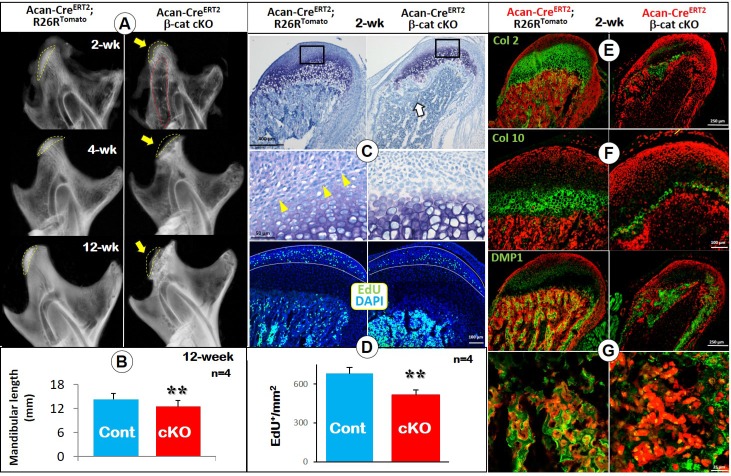
To address the impact of loss of β-catenin signaling (a cross of β-cateninfx/fx and Acan-CreERT2 mice) on MCC and ramus formation, we induced the deletion of β-catenin event in the cartilage at P3 and harvested mice at ages of 2-, 4- and 12-weeks. X-ray images displayed a radiolucent area (correlated to the calcified cartilage region) in the 2 week-old condylar head with a low mineral density in the TMJ ramus (Figure 1A, upper right). By the ages of 4- and 12-weeks, a lack of calcified cartilage in the condylar head, an expanded ramus neck and a sharply reduced ramus length were evident (Figure 1A, middle and lower right panels). Mandibular length was significantly reduced compared to controls at the age of 12-weeks (Figure 1B). Toluidine blue-stained sections of 2-week-old cKO condyles showed a reduction in the metachromatic area of the MCC, largely due to the virtual absence of the region of flattened and early chondrocytes (Figure 1C). Importantly, there was essentially no trabecular bone present except immediately deep to the MCC in cKO mice. The EdU assay showed a significant reduction in cell proliferation in the prechondroblastic zone of the cKO MCC compared to controls (Figure 1D; white arrow).
Figure 1.
Deletion of β-catenin in early chondrocytes led to malformed condylar neck, defective chondrogenesis, and reduced chondrocyte transformation. (A) X-ray images displayed a lack of calcified cartilage region (yellow dotted line) on mutant mice (arrow in upper panel) with a low mineralized condylar ramus (red dotted line) in 2-week old Acan-CreERT2; β-catenin cKO mice, and a small condylar head formation (arrows in middle and lower panel), an expanded ramus neck and a sharp reduction in the ramus length at ages of 4- and 12-weeks. (B) Quantitative data of mandibular length revealed a statistically significant reduction of the mandibular length in 12-week old cKO mice (n=4). (C) Toluidine blue staining for 2-week old cKO condyles showed a reduction in the metachromatic area of the MCC due to decreased flattened chondrocytes (yellow arrows), and a lack of subchondral bone (white arrow). (D) EdU+ cells were significantly reduced in proliferating zone of the cKO MCC (n=4). (E) Col 2 IHC combined with cell lineage tracing (Acan-CreERT2 was activated at P3, reflecting the initial cell origin in chondrocytes and its transformed bone cells) showed a sharp decrease of Col 2+ area in cKO MCC. (F) Col 10 IHC demonstrated a dramatic reduction in Col 10-expressing cells in cKO MCC, indicating a defective chondrocyte hypertrophy. (G) DMP1 IHC confirmed the dramatic reduction of subchondral bone volume, with less mature osteocytes expressing DMP1 in cKO mice (high magnification in the lower panel).
To trace the cell fate of chondrocytes lacking β-catenin signaling, we generated the above cKO mice in the background of R26RTomato (i.e., deletion of β-catenin and activation of red tomato in chondrocytes occurred at 3 days of age and traced the cell fate at 14 days). The confocal images displayed the expected presence of numerous red cells in the subchondral bone, reflecting their initial cell origin as chondrocytes. When combined with green IHC signals, Col2 (Figure 1E), Col 10 (Figure 1F) or DMP1 (Figure 1G; osteocyte marker) were located as expected in the control group (left panels). However, in cKO mice, there were few red bone cells in the area deep to the immediate subchondral bone, resulting in an open region within the ramus (Figure 1E-G). Furthermore, the extent of immunoreactivity for Col 2 and Col 10 was greatly reduced compared to controls (Figure 1E-F). DMP1 signal was greatly reduced in the trabecular bone of cKO mice (Figure 1G). In 4-week old cKO mice (induced at day 3), Col 10 expression was largely undetectable (Figure S1A). The combined red tomato and green DMP1 images revealed few transformed red bone cells in the subchondral bone area (Figure S1B).
Together, the above studies not only confirmed a critical role for β-catenin signaling in MCC chondrogenesis but also revealed a novel role for β-catenin in controlling cell trans-differentiation of chondrocytes into bone cells during MCC growth.
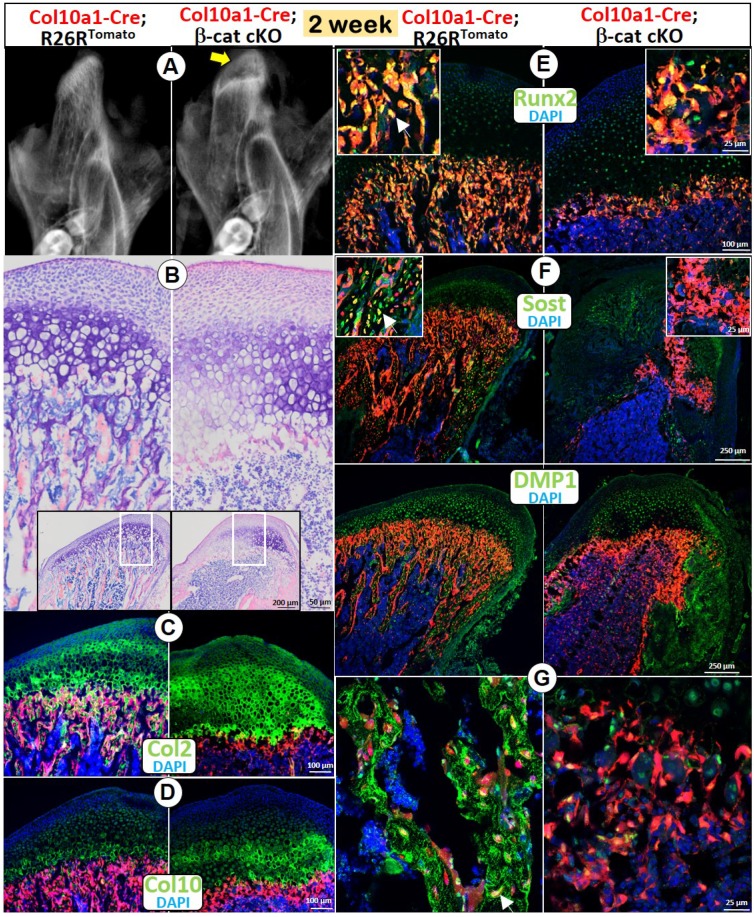
Deletion of β-catenin in hypertrophic chondrocytes (HCs) resulted in diminished endochondral bone formation
Although Acan-CreERT2 line is expressed in all chondrocytes, the deletion of β-catenin by this Cre mainly reflects the impact of β-catenin in early chondrocytes (Figure 1). To test the role of β-catenin in the cell trans-differentiation from HCs into bone cells, we generated a compound mouse line containing Col10a1-Cre, β-cateninfx/fx, and R26RTomato and harvested the mice at age of 2 weeks. X-ray images showed an expanded radiolucent area (correlated to the calcified cartilage region) in the 2 week-old condylar head with an extremely low mineral density in the MCC ramus (Figure 2A). Toluidine blue-stained sections displayed an expansion of all MCC cartilage layers with a particular increase in the cKO HCs (Figure 2B). Similar to the phenotype in Acan-CreERT2; β-catenin cKO mice, there was no trabecular bone present (Figure 2A-B). Cell lineage studies combined with IHC demonstrated a considerably thickened Col 2+ region (Figure 2C), as well as a somewhat increased thickness of Col 10+ cells (Figure 2D) in the cKO mice. In the thin layer of cKO subchondral bone, there were sharp reductions in expression of Runx2 (a marker of preosteoblasts; Figure 2E) and two markers of osteocytes: SOST (Figure 2F) and DMP1 (Figure 2G), indicating an additional role for β-catenin in the continuing bone cell maturation after the cell trans-differentiation.
Figure 2.
Deletion of β-catenin in HCs resulted in diminished endochondral bone formation. (A) The X-ray images showed an expanded radiolucent area (correlated to the calcified cartilage region, yellow arrow) with an extremely low mineralized ramus in the 2-week old Col10a1-Cre; β-catenin cKO mice. (B) Toluidine blue staining displayed an expansion of all MCC cartilage layers in cKO mice with a particular increase in HCs, but with no trabecular bone present in subchondral bone area. (C) Col 2 IHC combined with cell lineage tracing (Col10a1-Cre began activation at E14.5, in HCs and their transformed bone cells) demonstrated a considerably thickened Col2+ region in cKO mice. (D) Col10+ cells increased in cKO compared with control mice. (E-G) There was only a thin layer of cKO subchondral bone in cKO mice, with a sharp reduction in expression of Runx2 (E), SOST (F) and DMP1 (G) compared with the control mice (arrows).
Taken together, use of both Acan-CreERT2 and Col10a1-Cre loss-of-function models demonstrated that β-catenin plays a critical role in MCC proliferation and chondrogenesis and in the trans-differentiation of chondrocytes into bone cells, the major source of bone cells during MCC endochondral bone formation.
Constitutive activation of β-catenin in MCC accelerated chondrogenesis and cell trans-differentiation coupled with widespread calcification in the cartilage
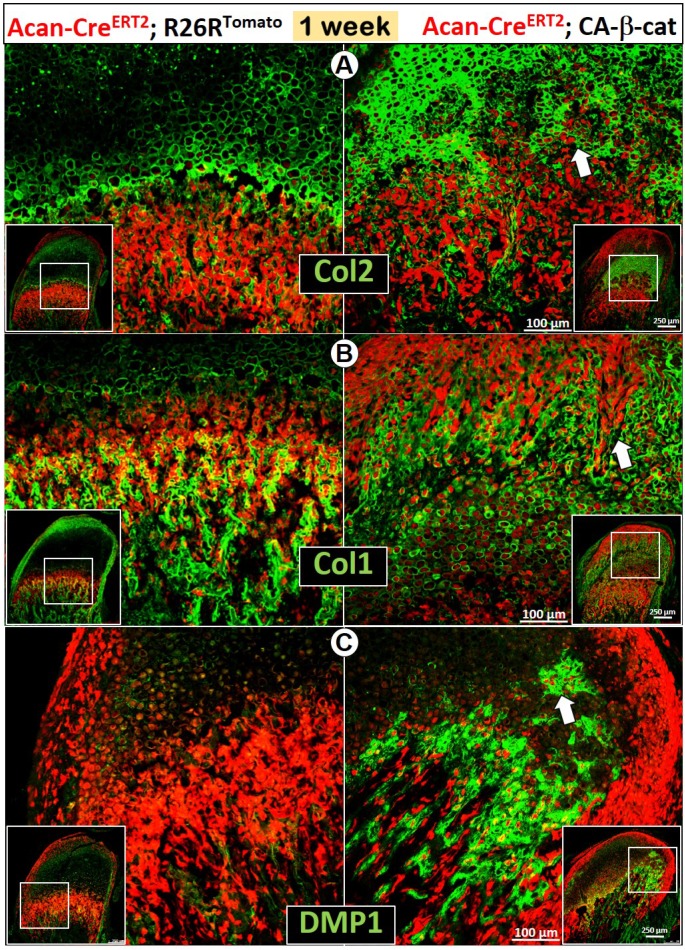
To further address the role of β-catenin in MCC growth, we generated a gain-of-function model containing Acan-CreERT2, β-cateninflox(Ex3)/flox(Ex3) and R26RTomato (CA-β-cat mice), and constitutively activated β-catenin in chondrocytes for 5 days (one-time tamoxifen injection at day 3 and harvested at day 7); 14 days (one-time tamoxifen injection at 2-week and harvested at 4-week); 5.5 months (one-time tamoxifen injection at 2-week and harvested at 6-month), respectively.
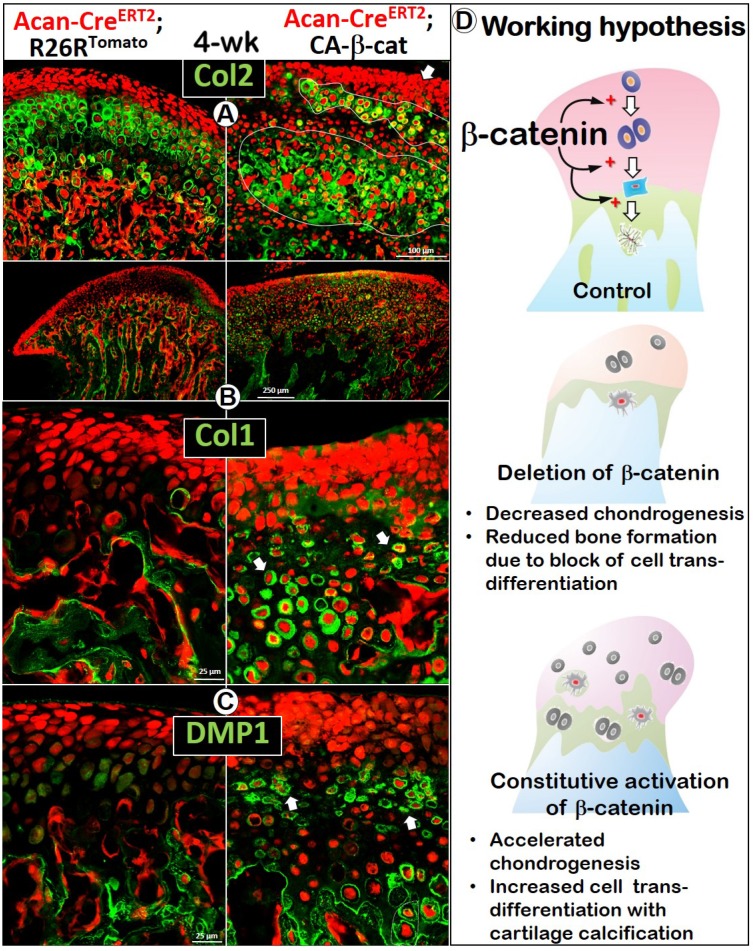
The early activation of β-catenin in chondrocytes greatly enhanced chondrogenesis reflected by sharp increases in Col 2 expression (Figure 3A), and osteogenesis revealed by increases in Col 1 (Figure 3B) and DMP1 expression (Figure 3C). Importantly, there was more accelerated trans-differentiation of chondrocytes into red bone cells in the cartilage region of CA-β-cat mice than in wild type mice (Figure 3, white arrows).
Figure 3.
Constitutive activation of β-catenin in MCC accelerated chondrogenesis and osteogenesis in early postnatal development. (A) By using Acan-CreERT2; β-cateninflox(Ex3)/flox(Ex3)mice with Cre activation at P3, we found that Col 2 IHC was more intense in CA-β-cat mice at P7 (arrow). (B-C) High level of Col 1 (B) and DMP1 (C) expression in 7-day CA-β-cat mice indicated the increased osteogenesis in MCC. In addition, there was more accelerated trans-differentiation of chondrocytes into red bone cells in the cartilage region of CA-β-cat mice (arrows).
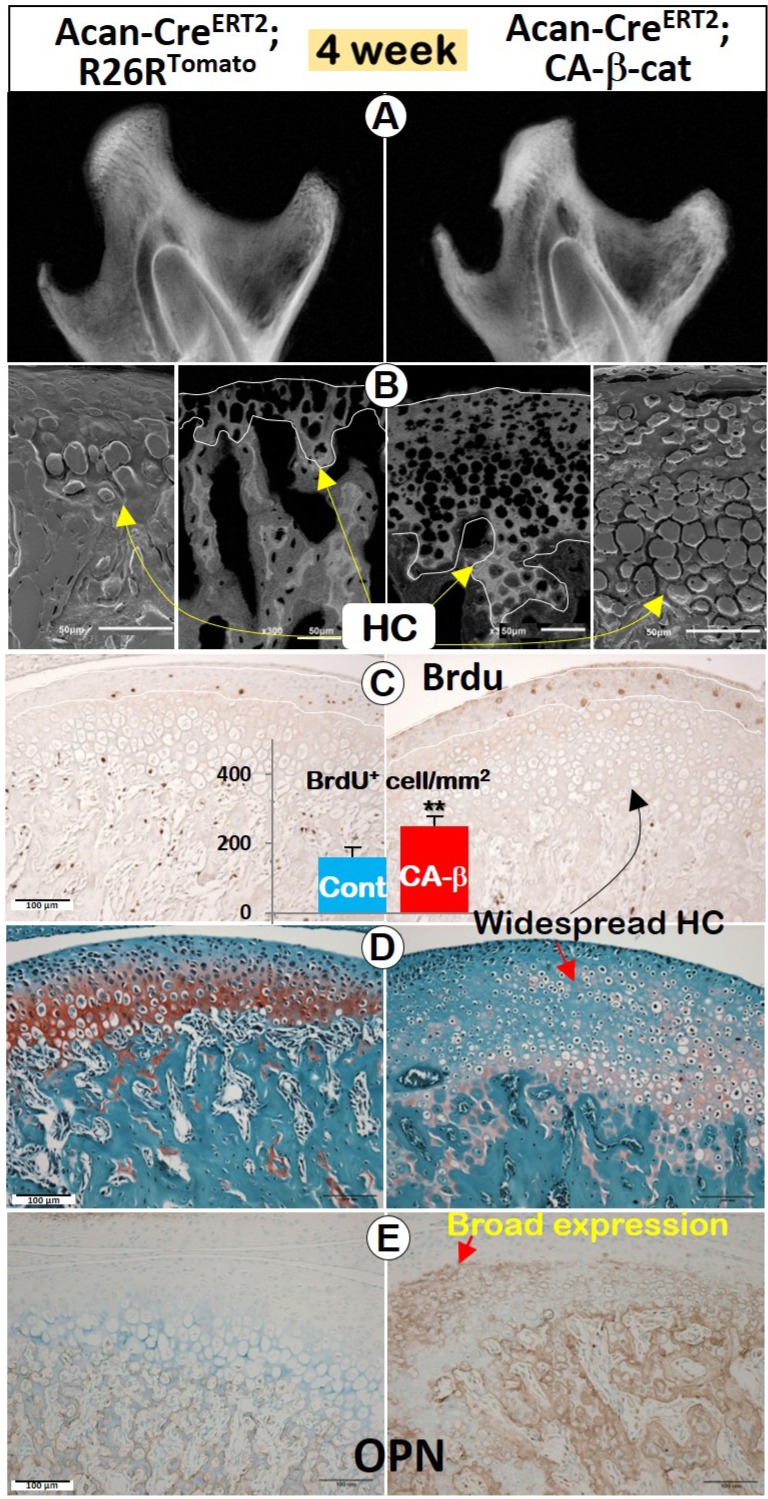
The activation of β-catenin in chondrocytes after the initial MCC pattern formation generated a similar phenotype (induced at 2 weeks, harvested at 4 weeks). In X-ray images, the mandibular condyle was more highly mineralized (Figure 4A), but the length of condylar process was statistically shorter than normal in the CA-β-cat mice (Figure S2B). Back-scattered SEM (Figure 4B, central) and acid-etched SEM (Figure 4B, lateral) demonstrated that the calcified HC layer in the mutant MCC was thicker with many more HCs than control, which was further confirmed by widespread Col 10 expression in CA-β-cat mice (Figure S2C). Goldner staining (Figure S2D) also suggested that MCC in CA-β-cat mice was more widely mineralized (indicated by green color).
Figure 4.
Stabilization of β-catenin in 4-week old mice enhanced chondrogenesis, promoted chondrocyte proliferation, and affected condylar ramus formation. (A) X-ray images showed that the CA-β-cat mandibular condyle was highly mineralized with a significantly shorter mandibular length (Cre was activated at 2 weeks, n=4). (B) Back-scattered SEM (central) and acid-etched SEM (lateral) demonstrated that the calcified HC layer in the mutant MCC was thicker with many more hypertrophic chondrocytes than control (yellow arrows). (C) BrdU+ cells in the proliferating zone of mutant mice were significantly increased, indicating higher cell proliferation (n=4). (D) Thickness of Safranin O staining was greater in CA-β-cat MCC than in controls, but with disruption of the normal cell layering. (E) OPN expression was dramatically expanded in the CA-β-cat MCC, indicating that the ossification of chondrocytes was accelerated.
BrdU positive cells in MCC were significantly increased in CA-β-cat mice (Figure 4C). The total thickness of the CA-β-cat MCC was greater than in controls, but with disruptions in the normal cell layering such that HCs were present throughout the depth of the tissue but with much fainter Safranin O staining (Figure 4D). IHC against osteopontin (OPN, a marker for early osteogenic cells) demonstrated dramatically expanded OPN expression in the CA-β-cat MCC (Figure 4E), indicating a transition to a more osteogenic phenotype.
To further investigate the cellular trans-differentiation, we performed cell lineage tracing, which revealed more and enlarged early chondrocytes in the superficial aspect of the CA-β-cat MCC, indicating an acceleration of chondrogenesis starting from the early stage (Figure 5). Co-IHC staining with Col 2 (Figure 5A) and Col 10 (Figure S2C) demonstrated more discontinuous and patchy immunoreactivity in the CA-β-cat MCC. Strikingly, the flattened chondrocytes and HCs pericellularly expressed high levels of bone markers of Col 1 (Figure 5B) and DMP1 (Figure 5C), supporting the accelerated trans-differentiation. Interestingly, there were many red cells, which varied in size, that did not express either cartilage or bone markers (Figure S3; arrowheads), indicating a lack of cell activity.
Figure 5.
Constitutive activation of β-catenin led to accelerated cell-transformation from chondrocytes into bone cells, giving rise to osteogenesis in the cartilage. (A) Co-IHC staining with Col 2 in the CA-β-cat MCC displayed a more widespread, denser, but patchy area of chondrocytes, with many more red prechondroblastic cells (arrow) near the MCC surface. (B) Col 1 expression was much higher (arrows) in CA-β-cat MCC (high magnification in lower panel). (C) Many chondrocytes also expressed high level of DMP1, reflecting the accelerated trans-differentiation from chondrocytes into bone cells in CA-β-cat MCC (arrows). (D) Using both conditional deletion and constitutive activation of β-catenin, our study demonstrates the critical role of β-catenin in the direct trans-differentiation of chondrocytes to bone cells during MCC growth. The lack of β-catenin led to a decrease of chondrogenesis and chondrocyte-derived bone cells, accompanied by reduction of subchondral bone volume; the stabilization of β-catenin gave rise to more chondrogenesis and cell trans-differentiation, along with cartilage calcification in MCC.
By the age of 6 months, the CA-β-cat mice displayed a similar but more severe phenotype. X-ray images showed a short but hypermineralized mandibular condyle in the CA-β-cat mice (Figure S4A), and the length of mandible was significantly shorter in the mutant mice (Figure S4B). The back scattered SEM image showed a wider condyle with uneven mineralization in the CA-β-cat condylar neck (Figure S4C), Both toluidine blue staining (Figure S4D) and Col 2 IHC (Figure S4E) revealed expanded hypertrophic chondrocytes present throughout the mutant MCC.
In summary, our studies at three different ages demonstrate that constitutive activation of β-catenin promotes acceleration of chondrogenesis, osteogenesis and cell trans-differentiation during MCC growth.
Discussion
For many years, chondrogenesis and osteogenesis were thought to be closely linked but separate processes during endochondral bone formation 36. However, the studies from other groups 8-11 and our recent report in long bone challenged this dogma and raised a new hypothesis: chondrogenesis (phase one) and osteogenesis (phase two) are not separate processes but one continuous biological process 37. In this study, our loss- and gain-of-function models of β-catenin at different developmental stages demonstrated that β-catenin is a key regulator of proliferation and chondrocyte differentiation as well as the direct trans-differentiation of chondrocytes into bone cells during postnatal MCC growth (Figure 5D). The deletion of β-catenin in aggrecan-secreting cells (Figure 1) or HCs (Figure 2) produced a similar phenotype: disruptions of chondrogenesis (especially cell differentiation), a sharp reduction in mandibular length, and a drastic reduction in trans-differentiation of chondrocytes into bone cells, as well as bone cell maturation. By contrast, constitutive activation of β-catenin in chondrocytes at three ages [P3 to P7 (Figure 3), P14 to 1-month (Figure 4-5), and P14 to 6-months (Figure S4)] greatly accelerated chondrogenesis, and the trans-differentiation of chondrocytes into bone cells, leading to a shorter but wider condylar neck.
Deletion of β-catenin by Acan-CreERT2 affects both cell proliferation and cell differentiation in all chondrocytes, whereas deletion of β-catenin by Col10a1-Cre mainly induces failure of HCs to trans-differentiate into bone cells, leading to a great accumulation of HCs and pre-hypertrophic chondrocytes. However, both cKO mice models display almost identical subchondral bone phenotypes: a sharp reduction in bone volume, a great decrease in cell trans-differentiation, and a defect in cell maturation (as reflected by bone markers). This finding argues strongly that MCC subchondral bone is mainly formed via cell trans-differentiation from HCs (instead of early chondrocytes) under regulation by β-catenin, which is in agreement with previous studies in long bone 17, 37.
It is well documented that the cKO event is not a complete gene deletion process due to the limitation of Cre efficiency 38, which explains why some red bone cells and subchondral bone are still present in cKO mice (Figure 1-2). Currently, we do not know why bone markers are largely undetected in the chondrocyte-derived bone cells in both cKO models (i.e., immature bone cells, Figures 1-2) as neither Cre line is active in bone cells. We reason that β-catenin is not only important for cell trans-differentiation but also critical for bone marker expression even before the cell trans-differentiation event, as we showed that Col 1 and DMP1 are expressed in HCs (Figure S5). In other words, osteogenic genes are already activated in HCs. In fact, bone markers expressed in HCs have been reported in the literature 39. Of note, the subchondral bone phenotype is more severe in the Col10a1 cKO mice, which is likely due to its cumulative effects beginning at E14.5 6.
Although the gain of function model supports the role of β-catenin in the cell trans-differentiation hypothesis, the mutant ramus length is shorter than controls. We reason that the following two factors are likely responsible for this unexpected phenotype: a. the prematurely formed bone cells do not have the full function of mature bone cells, and the existing cartilage matrix may interfere with bone matrix production; b. the pattern of MCC ramus is largely formed by the time that β-catenin is constitutively activated and the existing bone matrix “blockades” the newly formed bone.
The pronounced changes in MCC gene expression profiles and structure in both mutant cKO models are in agreement with previous studies showing delayed chondrocyte hypertrophy and reduced proliferation in mouse growth plates in which β-catenin was deleted in chondrocytes and perichondrial cells 18-20. The phenotype in the MCC of the CA-β-cat mice is even more striking, presenting a picture of a cartilage transitioning to bone (reduced Safranin O, immunoreactivity for Col 1, osteopontin and DMP1 in the cartilage). This phenotype is similar in some respects to the only other study of the TMJ in mice with constitutively activated β-catenin, which reported progressive decreases in Alcian blue and Col 2 staining in 1-6 month-old CA-β-cat mice as well as increased Col 10 staining and greater numbers of hypertrophic chondrocytes that they interpreted as evidence of accelerated chondrocyte hypertrophy 25. Thus, β-catenin appears to have a similar regulatory effect on both long bone and TMJ. Currently, our group is searching for other genes that regulate the unique growth of the MCC.
Supplementary Material
Supplementary figures.
Acknowledgments
This study was partially supported by National Institutes of Health: DE025659 to JQF; DE024797 to SHE; National Natural Science Foundation of China (81500876) to JJJ.
References
- 1.Merida-Velasco JR, Rodriguez-Vazquez JF, Merida-Velasco JA, Sanchez-Montesinos I, Espin-Ferra J, Jimenez-Collado J. Development of the human temporomandibular joint. Anat Rec. 1999;255:20–33. doi: 10.1002/(SICI)1097-0185(19990501)255:1<20::AID-AR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 2.Shibata S, Suda N, Suzuki S, Fukuoka H, Yamashita Y. An in situ hybridization study of Runx2, Osterix, and Sox9 at the onset of condylar cartilage formation in fetal mouse mandible. J Anat. 2006;208:169–77. doi: 10.1111/j.1469-7580.2006.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinton RJ. Genes that regulate morphogenesis and growth of the temporomandibular joint: a review. Dev Dyn. 2014;243:864–74. doi: 10.1002/dvdy.24130. [DOI] [PubMed] [Google Scholar]
- 4.Jing J, Ren Y, Zong Z, Liu C, Kamiya N, Mishina Y. et al. BMP receptor 1A determines the cell fate of the postnatal growth plate. Int J Biol Sci. 2013;9:895–906. doi: 10.7150/ijbs.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jing J, Hinton RJ, Mishina Y, Liu Y, Zhou X, Feng JQ. Critical role of Bmpr1a in mandibular condyle growth. Connect Tissue Res. 2014;55(Suppl 1):73–8. doi: 10.3109/03008207.2014.923858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jing Y, Zhou X, Han X, Jing J, von der Mark K, Wang J. et al. Chondrocytes Directly Transform into Bone Cells in Mandibular Condyle Growth. J Dent Res. 2015;94:1668–75. doi: 10.1177/0022034515598135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinton RJ, Jing Y, Jing J, Feng JQ. Roles of Chondrocytes in Endochondral Bone Formation and Fracture Repair. J Dent Res. 2017;96:23–30. doi: 10.1177/0022034516668321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014;10:e1004820. doi: 10.1371/journal.pgen.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang G, Zhu L, Hou N, Lan Y, Wu XM, Zhou B. et al. Osteogenic fate of hypertrophic chondrocytes. Cell Res. 2014;24:1266–9. doi: 10.1038/cr.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A. 2014;111:12097–102. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J, Gebhardt M, Golovchenko S, Perez-Branguli F, Hattori T, Hartmann C. et al. Dual pathways to endochondral osteoblasts: a novel chondrocyte-derived osteoprogenitor cell identified in hypertrophic cartilage. Biol Open. 2015;4:608–21. doi: 10.1242/bio.201411031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–59. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 13.Yasuhara R, Ohta Y, Yuasa T, Kondo N, Hoang T, Addya S. et al. Roles of beta-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Invest. 2011;91:1739–52. doi: 10.1038/labinvest.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dao DY, Jonason JH, Zhang Y, Hsu W, Chen D, Hilton MJ. et al. Cartilage-specific beta-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. J Bone Miner Res. 2012;27:1680–94. doi: 10.1002/jbmr.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golovchenko S, Hattori T, Hartmann C, Gebhardt M, Gebhard S, Hess A. et al. Deletion of beta catenin in hypertrophic growth plate chondrocytes impairs trabecular bone formation. Bone. 2013;55:102–12. doi: 10.1016/j.bone.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Usami Y, Gunawardena AT, Iwamoto M, Enomoto-Iwamoto M. Wnt signaling in cartilage development and diseases: lessons from animal studies. Lab Invest. 2016;96:186–96. doi: 10.1038/labinvest.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houben A, Kostanova-Poliakova D, Weissenbock M, Graf J, Teufel S, von der Mark K. et al. beta-catenin activity in late hypertrophic chondrocytes locally orchestrates osteoblastogenesis and osteoclastogenesis. Development. 2016;143:3826–38. doi: 10.1242/dev.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–38. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 20.Day TF, Guo XZ, Garrett-Beal L, Yang YZ. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–50. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Yuasa T, Kondo N, Yasuhara R, Shimono K, Mackem S, Pacifici M. et al. Transient activation of Wnt/{beta}-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice. Am J Pathol. 2009;175:1993–2003. doi: 10.2353/ajpath.2009.081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B, Jin H, Zhu M, Li J, Zhao L, Zhang Y. et al. Chondrocyte beta-catenin signaling regulates postnatal bone remodeling through modulation of osteoclast formation in a murine model. Arthritis Rheumatol. 2014;66:107–20. doi: 10.1002/art.38195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagayama M, Iwamoto M, Hargett A, Kamiya N, Tamamura Y, Young B. et al. Wnt/beta-catenin signaling regulates cranial base development and growth. J Dent Res. 2008;87:244–9. doi: 10.1177/154405910808700309. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Liu C, Rohr J, Liu H, He F, Yu J. et al. Tissue interaction is required for glenoid fossa development during temporomandibular joint formation. Dev Dyn. 2011;240:2466–73. doi: 10.1002/dvdy.22748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Li S, Xie W, Shen J, Im HJ, Holz JD. et al. Activation of beta-catenin signalling leads to temporomandibular joint defects. Eur Cell Mater. 2014;28:223–35. doi: 10.22203/ecm.v028a15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP. et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 27.Henry SP, Jang CW, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis. 2009;47:805–14. doi: 10.1002/dvg.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gebhard S, Hattori T, Bauer E, Schlund B, Bosl MR, de Crombrugghe B. et al. Specific expression of Cre recombinase in hypertrophic cartilage under the control of a BAC-Col10a1 promoter. Matrix Biol. 2008;27:693–9. doi: 10.1016/j.matbio.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M. et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang X, Kalajzic Z, Maye P, Braut A, Bellizzi J, Mina M. et al. Histological analysis of GFP expression in murine bone. J Histochem Cytochem. 2005;53:593–602. doi: 10.1369/jhc.4A6401.2005. [DOI] [PubMed] [Google Scholar]
- 31.Zhang R, Lu Y, Ye L, Yuan B, Yu S, Qin C. et al. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J Bone Miner Res. 2011;26:1047–56. doi: 10.1002/jbmr.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jing Y, Hinton RJ, Chan KS, Feng JQ. Co-localization of Cell Lineage Markers and the Tomato Signal. J Vis Exp; 2016. p. 10. 3791/54982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren Y, Lin S, Jing Y, Dechow PC, Feng JQ. A novel way to statistically analyze morphologic changes in Dmp1-null osteocytes. Connect Tissue Res. 2014;55(Suppl 1):129–33. doi: 10.3109/03008207.2014.923879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B. et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–5. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y, Yuan B, Qin C, Cao Z, Xie Y, Dallas SL. et al. The biological function of DMP-1 in osteocyte maturation is mediated by its 57-kDa C-terminal fragment. J Bone Miner Res. 2011;26:331–40. doi: 10.1002/jbmr.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–65. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- 37.Jing Y, Jing J, Ye L, Liu X, Harris SE, Hinton RJ. et al. Chondrogenesis and osteogenesis are one continuous developmental and lineage defined biological process. Sci Rep. 2017;7:10020. doi: 10.1038/s41598-017-10048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- 39.Gerstenfeld LC, Shapiro FD. Expression of bone-specific genes by hypertrophic chondrocytes: implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J Cell Biochem. 1996;62:1–9. doi: 10.1002/(SICI)1097-4644(199607)62:1%3C1::AID-JCB1%3E3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.