Abstract
In today’s pharmaceutical arena, it is estimated that more than 40% of new chemical entities produced during drug discovery efforts exhibit poor solubility characteristics. However, over the last decade hot-melt extrusion (HME) has emerged as a powerful processing technology for drug delivery and has opened the door to a host of such molecules previously considered unviable as drugs. HME is considered to be an efficient technique in developing solid molecular dispersions and has been demonstrated to provide sustained, modified and targeted drug delivery resulting in improved bioavailability. This article reviews the myriad of HME applications for pharmaceutical dosage forms such as tablets, capsules, films and implants for drug delivery through oral, transdermal, transmucosal, transungual, as well as other routes of administration. Interest in HME as a pharmaceutical process continues to grow and the potential of automation and reduction of capital investment and labor costs have made this technique worthy of consideration as a drug delivery solution.
Keywords: Hot-melt extrusion, solid dispersion, melt extruded tablets, drug delivery systems, bioavailability, sustained release, controlled release, melt extruded films, bioadhesion
1. INTRODUCTION
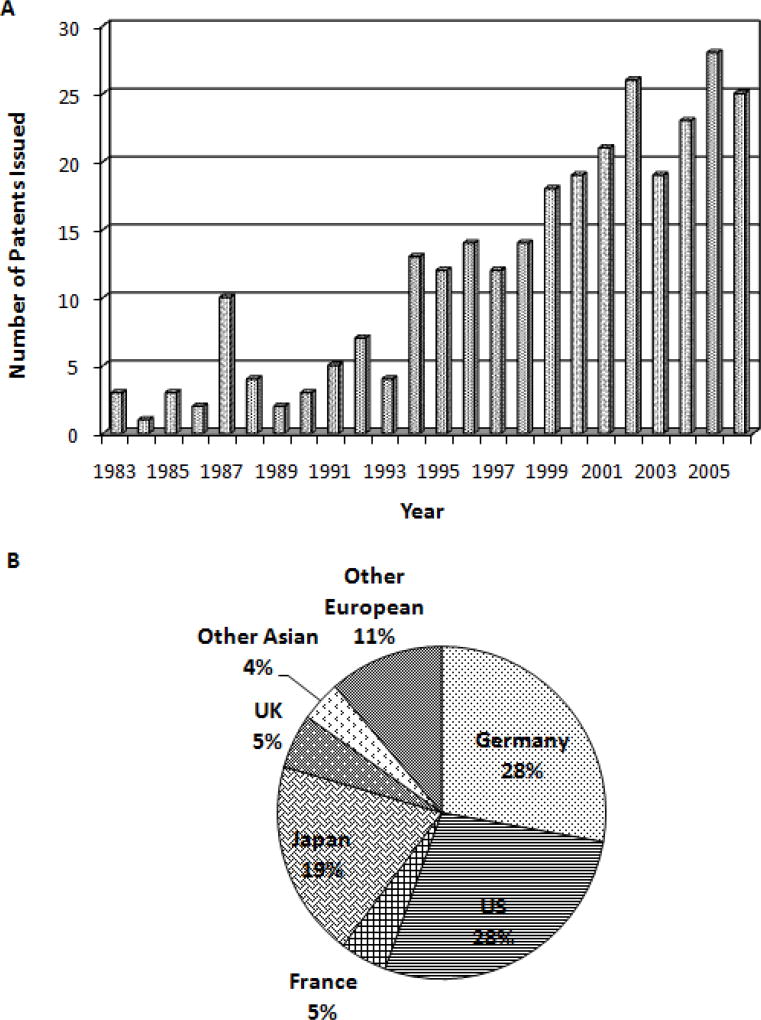
Hot-melt extrusion (HME), a widely used manufacturing process in the plastics industry for more than half of a century, has a significant potential as a “continuous process” in the pharmaceutical industry. Over the last two decades, pharma has gradually focused immensely on HME techniques for drug delivery applications, which is evident from the scientific literature. In the last 15 years, over 150 research papers and many reviews (1–5) have been published on the subject. In addition, HME patents issued world-wide for pharmaceutical systems have increased since the early 1980’s (Figure 1).
Figure 1.
The number of hot-melt extrusion patents issued for pharmaceutical applications from 1983 to 2006 (A), and percentage of hot-melt extrusion patents issued by country since 1983 for pharmaceutical applications (B).
In drug discovery, the emergence of combinatorial chemistry and high through-put screening has resulted in developing new chemical entities with poor bioavailability due to their solubility limitations (6). Such compounds would be prime candidates for HME, wherein the active component is embedded in a carrier system, usually comprised of one or more thermoplastic polymers (7–14) and/or low melting waxes (15–17). Prior to extrusion, an extensive knowledge on the choice of drugs, polymers and other excipients that undergo the process is essential in designing a pharmaceutical dosage form. The selection of such materials is based on their cohesive energies, which in turn determine the physico-chemical properties of the drugs and excipients and their interaction potential. These cohesive energies can be readily quantified using solubility parameters and thus the physical properties of the components and their potential interactions may be determined (18). Solubility parameters have been successfully utilized to predict the miscibility of drugs with polymers in solid dispersions (19, 20). The difference between the solubility parameters of two materials provides an estimation of the likelihood that they will be miscible. The use of solubility parameters has been explained by Crowley et al. wherein the miscibility of guaifenesin and ketoprofen in polyethylene oxide (PEO) was predicted by calculating the Hansen solubility parameter using the method of Hoftyzer and van Krevelen (21).
Several functional excipients such as plasticizers (21–29), fillers (10, 30–32), pH and release modifiers (33, 34), stabilizers (35, 36), surfactants (35, 37), antioxidants (12, 25) and processing aids (10, 38, 39) can also be included in the powder blend and extruded at relatively low temperatures. The extrusion process involves conversion of these raw materials into a product of uniform shape and density by forcing these materials through a die under controlled conditions. During the extrusion process, the molten polymers can function as thermal binders and act as drug release retardants upon cooling and solidification of the extrudate. Intense mixing and agitation imposed by the rotating screws cause de-aggregation of suspended drug particles in the molten polymer, resulting in a more uniform dispersion, as a solid solution, or a combination of both in the final product. The physical state of the active moiety will have a significant effect on processing, drug release properties and stability of the drug in the final extrudate.
The physical and chemical stability of hot-melt extruded dosage forms is influenced by the nature of the polymer and excipients, physical state of the drug in the final extrudate, storage and packaging conditions. Although melt-extruded dosage forms tend to demonstrate good long term stability, at times recrystallization of actives has been observed during their storage. This instability is one of the common problems observed with crystalline drugs processed via HME wherein the drug first is converted to an amorphous state during extrusion and reverts back into the crystalline state upon storage (40). To overcome such problems several crystallization inhibitors such as polycarbophil and polyvinyl pyrrolidone (PVP) K25 have been introduced into formulations as functional excipients. These additives have shown to either prevent or reduce the recrystallization of drugs in HME formulations to a significant extent (41). Stability of such extrudates can be assessed by thermo-analytical techniques such as hot stage microscopy, differential scanning calorimetry, thermogravimetric analysis as well as non-thermal techniques such as scanning electron microscopy, powder X-ray diffraction, fourier transform infrared spectroscopy, solid state nuclear magnetic resonance spectroscopy and high performance liquid chromatography (4).
HME offers several advantages over traditional processing techniques for pharmaceutical applications. The process is anhydrous, entails a continuous operation necessitating fewer processing steps, requires no compression of the actives, and improves bioavailability due to dispersion of the drug at the molecular level in the final dosage forms (11, 42–44). All of the components in the formulation must be thermally stable at the processing temperatures utilized, which may limit the extrusion of thermosensitive actives. However, advent of new techniques such as a combination of HME with nanotechnology (45), powder coating (46, 47) and complexation (48) over the last several years continuously adds ‘new’ active pharmaceutical ingredients (APIs) to the HME list of candidates. Advancements in types of extruders have also expanded the number of drugs available for processing. For example, twin-screw extruders with flexible screw designs and inclusion of injection ports at different stages within the barrel permit the pharmaceutical scientist more freedom in the development of intricate dosage forms. For a more detailed discussion on extruder types and designs, including processing, readers are referred to a recent publication on the subject (4).
Interest and utilization of HME is steadily emerging within the pharmaceutical industry. In recent years, several research groups have demonstrated HME as an innovative and viable approach to produce various pharmaceutical drug delivery systems in the field of formulation such as pellets (49, 50), granules (10, 51, 52), immediate and modified release tablets (12, 53–55), oral fast dissolving systems (56), transdermal (23, 57–59) and transmucosal delivery systems (8, 24, 35, 60–63), transungual delivery systems (64, 65) and implants (66–68). This review focuses on various HME applications in drug delivery through oral, transdermal, transmucosal, transungual, and other routes of administration.
2. Hot-Melt Extrusion in Drug Delivery
2.1. Oral Drug Delivery
Recently, HME technology has been gaining much attention, as many of its newer advantages are being investigated and applied toward the field of pharmaceutics and drug delivery. In this regard, several model drugs have been studied for various applications utilizing HME, irrespective of dosage form size, shape or design. HME technology has proved its potential in producing various solid oral dosage forms, providing the flexibility to modify drug release as required.
2.1.1. Rapid and Immediate Release
A United States patent, McGinity et al. has disclosed a novel method of preparing effervescent granules utilizing hot-melt extrusion techniques (52, 69, 70). The granules were prepared by hot-melt extruding an acidic and an alkaline agent coupled with a hot-melt extrudable binder (melting/softening point temperature less than 150°C), which was capable of forming a eutectic mixture with the acidic agent. The granules thus produced demonstrated a controllable rate of effervescence (52).
Among the various patient friendly dosage forms developed for facilitating ease of medication, orally disintegrating tablets (ODT) have been the product of choice by many pharmaceutical companies. Recently, scientists have been investigating newer applications of hot-melt extrusion and extended the technique towards developing such a product. In a US patent application by Sherry et al. ODTs of a non-steroidal anti-inflammatory drug (NSAID) and paracetamol were prepared by dry blending the drugs with sugar alcohols (xylitol, mannitol, sorbitol) and melt extruding the mixture by heating to a temperature above the melting point of the sugars (71). The extrudates obtained after cooling and solidification were milled utilizing a cone mill. The milled extrudates or granules were mixed with other formulation components and compressed into tablets. The ODTs obtained by complete melting of xylitol (low melting sugar alcohol) in the physical mixture were reported to be more robust compared to those produced by conventional dry blending processes.
Many pharmaceutical scientists have demonstrated the application of HME in formulating immediate release granules utilizing both single-screw (72) and ram (73) extruder types. Koleng and McGinity granulated acetaminophen and filler excipients with various low molecular weight poly(ethylene glycol) (PEG) using a hot-melt extrusion process (51). The granules attained were subsequently combined with disintegrants and lubricant and compressed into tablet compacts. The HME granules demonstrated superior drug release as compared to the tablets. Tablets containing 15% PEG released more than 80% of the incorporated drug after 30 minutes, which is a requirement for acetaminophen tablets in the USP 30.
Additionally, hot-melt extrusion has been utilized to enhance the dissolution rate of the actives by preparing solid dispersions for immediate and sustained release applications. Hulsmann et al utilized a HME technique to increase the solubility rate of a poorly water-soluble drug, 17-Estradiol hemihydrates (74, 75). These researchers used PEG 6000, PVP or a vinylpyrrolidone–vinylacetate copolymer within the polymer matrix with sucroester WE15 or Gelucire® 44/14 as functional excipients. The formulation mixtures were extruded as rods, milled into granules and finally, compressed into tablets. The solid dispersions thus formed exhibited a significant increase in dissolution rate compared to the pure drug or to the physical mixtures. A formulation containing 10% 17-Estradiol, 50% PVP and 40% Gelucire® 44/14 demonstrated a 32-fold increase in the dissolution rate.
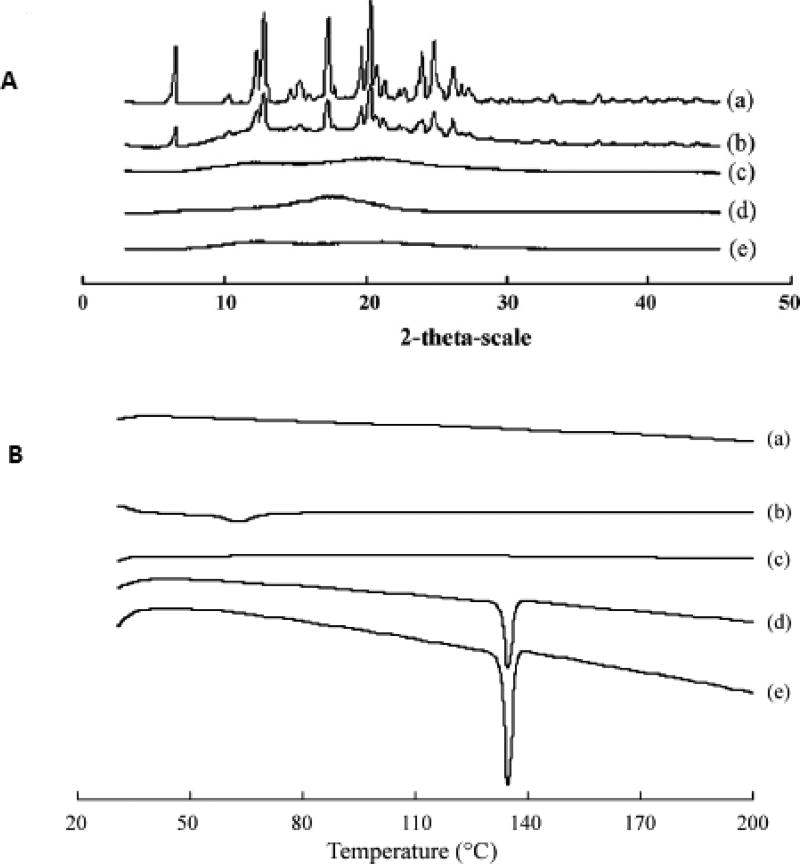
Sun Yunzhe et al. prepared a semi-solid capsule containing nimodipine solid dispersion prepared by HME technology (76). Initially, a solid dispersion of the drug was prepared incorporating Eudragit® E100 and Plasdone® S630 using a twin-screw extruder. Later, this solid dispersion was finely dispersed into a semi-solid system, prepared using PEG 400 and Plasdone®S630 as the vehicle and PEG 6000 as a suspending agent. The final product was filled into an HPMC capsule. The high pressures generated during the extrusion process resulted in extensive interactions between the drug and the carriers. This was evident from the PXRD and DSC data, wherein the loss of drug crystallinity was observed in the solid dispersion formed (Figure 2). The bioavailability study conducted in beagle dogs, with Nimotop® as a reference formulation, exhibited a similar bioavailability. However, time to reach peak concentration was much faster for the HME product than the reference formulation. In this study authors have demonstrated that the combination of a solid dispersion technique and semi-solid filling into the capsule not only produced a rapid and pH-independent release of the drug, but also prevented recrystallization of the drug in the matrix. The above examples illustrate the fact that the choice of excipients is of utmost importance in designing HME dosage forms with fast or immediate release characteristics.
Figure 2.
Physical characterization of nimodipine solid dispersion (SD) (50% nimodipine, 40% Eudragit® E100, 10% Plasdone® S630).
PXRD patterns (A): (a) pure NMD; (b) physical mixture; (c) SD; (d) Eudragit® E100; (e) Plasdone® S630, and DSC thermograms (B): (a) Plasdone® S630; (b) Eudragit® E100; (c) SD; (d) physical mixture; (e) pure NMD.
"Reprinted with kind permission from Elsevier, Ref. (76)"
2.1.2. Sustained Release
It was the researchers’ goal to prepare a dosage form in a simple and continuous manner. Mank and co-workers reported the extrusion of numerous thermoplastic polymers to produce sustained release pellets around 1990 (72, 77). Later, Follonier and co-workers in 1994 investigated the production of a relatively stable and freely soluble drug, diltiazem hydrochloride sustained release pellets utilizing a continuous process, HME technology (49). Four Polymers were considered for investigation in the study. The drug release characteristics of diltiazem were biphasic, with the cellulose acetate butyrate (CAB) and poly (ethylene-co-vinyl acetate; EVAC) pellets giving the slowest release rate. These researchers also reported that the type and amount of plasticizer used, drying time of the polymers, extrusion temperatures, and plasticization times varied with each formulation. The stability of Eudragit® RSPM was also found to be adequate when extruded at a temperature of 130°C.
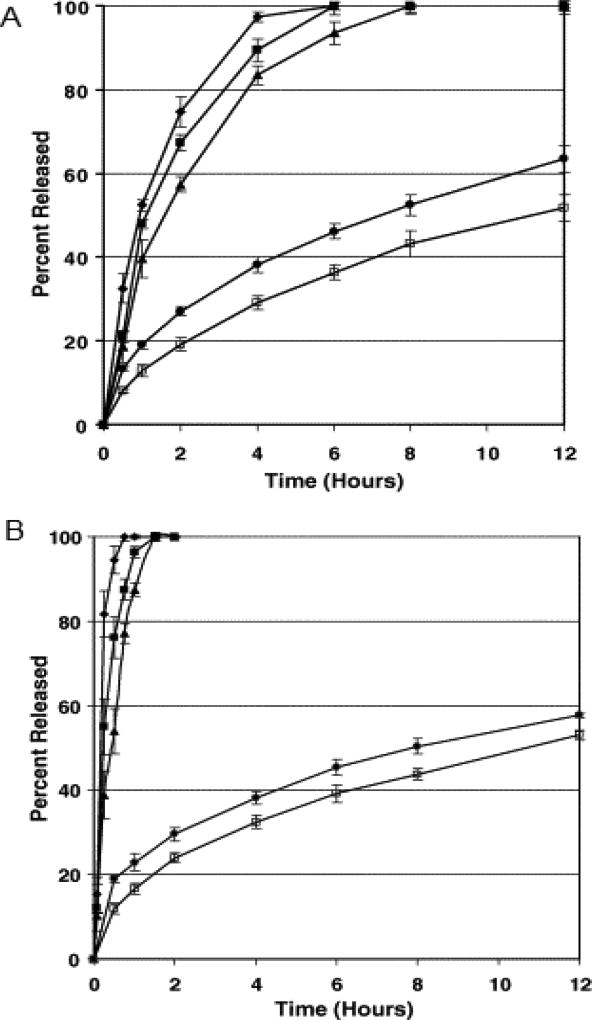
Crowley and co-workers investigated the physicochemical properties and drug release mechanism from matrix tablets containing binary mixtures of a water soluble drug, guaifenesin and a water insoluble polymer, ethyl cellulose, prepared by both hot-melt extrusion and direct compression (78). Ethyl cellulose was separated into “fine or coarse”, corresponding to 325–80 and 80–30 mesh particles, respectively. Hot-melt extruded tablets containing 30% guaifenesin were processed at temperatures of 80–90°C and 90–110°C and the directly compressed tablets were prepared at 10, 30 or 50KN compaction forces. From the drug release data obtained (Figure 3), the release rate of guaifenesin appeared to be slower from directly compressed tablets prepared with the “fine” ethyl cellulose particle size as compared to that of the “coarse”. However, difference in drug release rate was not observed between hot-melt extruded tablets prepared with ethyl cellulose of different particle sizes. In addition, tablets prepared by HME demonstrated a sustained drug release relative to those prepared by direct compression. Release profiles of the hot-melt extruded tablets were found to be consistent with the Higuchi diffusion model. The directly compressed tablets using “coarse” ethyl cellulose, however, were reported to release guaifenesin by both diffusion and erosion mechanisms. The overall results of this study demonstrated that the guaifenesin release rate was dependent upon the particle size of ethyl cellulose for directly compressed tablets and the processing conditions employed were found to influence the surface morphology of the melt extruded tablets. It is evident from the above study that HME techniques, irrespective of the polymer particle size, may exhibit a more controlled drug release profile.
Figure 3.
Influence of ethyl cellulose particle size, compaction force and extrusion temperature on guaifenesin release from matrix tablets prepared by direct compression and hot-melt extrusion containing 30% guaifenesin and 70% ethyl cellulose using USP Method II at 37 °C and 50 rpm in 900 ml of purified water. Each point represents the mean ±standard deviation, n=6.
(A) Matrix tablets prepared using “fine” ethyl cellulose (325–80 mesh) and (B) matrix tablets prepared using “coarse” ethyl cellulose (80–30 mesh). (◆) Direct compression, 10 kN; (▪) direct compression, 30 kN; (▲) direct compression, 50 kN; (•) hot-melt extrusion, 80, 85, 85, 90 °C; (□) hot-melt extrusion, 90, 105, 105, 110 °C.
Brabender et al. investigated the bioavailability of ibuprofen from hot-melt extrudates based on ethyl cellulose and a hydrophilic excipient, xanthum gum, which the authors termed “mini-matrices” (79). During in vivo evaluation an oral dose of 300 mg ibuprofen was administered in hard gelatin capsules to healthy volunteers (n=9) in a randomized cross-over study and was compared to the sustained release product, Ibu-slow®. One mini-matrix formulation (F-1) consisted of 30% ibuprofen, 35% ethyl cellulose and 35% hydroxypropyl methylcellulose, while the second formulation (F-2) contained 60% ibuprofen, 20% ethyl cellulose and 20% xanthum gum. Both formulations demonstrated sustained release, in vivo with an HVDt50% Cmax value (time during which the plasma concentration is at least 50% of the Cmax value) of 7.6 and 12hr for formulations F-1 and F-2, respectively. In contrast, a value of 5.2h was observed for Ibu-slow® (Table 1). Although the two mini-matrix formulations exhibited significantly lower Cmax and AUC0–24 h values than those of the commercial formulation, relative bioavailability of both experimental formulations achieved approximately 80%. The study concluded that the mini-matrices formulated with ethyl cellulose in combination with either xanthum gum or hydroxypropyl methylcellulose can be used to prepare sustained release dosage forms.
Table 1.
Mean pharmacokinetic parameters (± S.D.) after oral administration of 300 mg ibuprofen to healthy volunteers (n=9): Ibu-slow® 600 (1/2 tablet), mini-matrix F-1 containing HPMC and mini-matrix F-2 containing xanthan gum.
"Reprinted with kind permission from Elsevier, Ref. (79)"
| Ibu-slow® | F-1 | F-2 | |
|---|---|---|---|
| tmax (h) | 2.7 ± 0.8 | 4.1 ± 0.9 | 6.4 ± 3.8 |
| Cmax (µg/ml) | 14.1 ± 3.4 | 7.8 ± 2.7a | 6.1 ± 1.1a |
| AUC0–24h (µg h/ml) | 104.1 ± 34.0 | 79.0 ± 24.5a | 80.9 ± 24.1a |
| Frel(%) | - | 76.9 ± 13.3 | 79.7 ± 17.7 |
| HVDt50%Cmax (h) | 5.2 ± 2.0 | 7.6 ± 3.3 | 12.0 ± 6.3a |
| RΔ | 2.8 ± 1.1 | 4.2 ± 1.8 | 6.5 ± 3.5 |
| C24h/Cmax (%) | 5.9 ± 3.6 | 26.4 ± 17.3a | 51.0 ± 31.0a |
RΔ: ratio between HVDt50%Cmax of the test formulation and HVDt50%Cmax of an immediate release reference formulation (1.8 h for an ibuprofen suspension
Significantly different from Ibu-slow® according to a two-way analysis of variance (P < 0.05).
Zhang and McGinity described a novel method to prepare sustained release matrix tablets directly from a single-screw hot-melt extruder (53, 54). These researchers studied the properties of PEO as a carrier and assessed the release mechanism of chlorpheniramine maleate (CPM) from the matrix tablets produced. 4.5mm diameter rods were extruded and subsequently cut into the tablets. A plasticizer, PEG 3350, was incorporated to facilitate processing. The stability of the matrix polymer, PEO, was determined as a function of processing temperature. The type of polymer, extrusion temperatures, and residence time in the extruder were found to influence PEO stability. The researchers demonstrated that additional mixing of the active and other additives occurred in the barrel of the extruder. Content uniformity of the extruded tablets attained was 99.0% to 101.0% of the theoretical content. The release of CPM from the extruded matrix tablets was found to increase with an increase in the plasticizer concentration. The rate of hydration and rate of dissolution of the entire matrix system was thereafter accelerated due to the presence of the plasticizer, PEG 3350. The drug release rate was not significantly affected by changes in drug concentration until drug loading was ≥ 20%.
HME was also demonstrated to be a viable option in producing sustained release co-extrudates, in a single step. Quintavalle et al. have demonstrated applicability of HME for the production of theophylline sustained release concentric co-extrudates composed of two concentric extruded matrices, where the inner layer was based on hydrophilic polyethylene glycol and outer layer was based on a lipophilic microcrystalline wax (80). The extrusion was carried out using a laboratory scale vertical ram extruder, with a modified head, which consisted of two concentric chambers. An in vivo study conducted in healthy male human volunteers with the final co-extrudate, which was selected based on in vitro dissolution profiles, exhibited sustained release characteristics of the dosage form. These authors also developed a mathematical model for predicting drug plasma concentration profile following oral administration of co-extrudates. The reliability of this mathematical model was also reported (80).
2.1.3. Enteric and Sustained Release
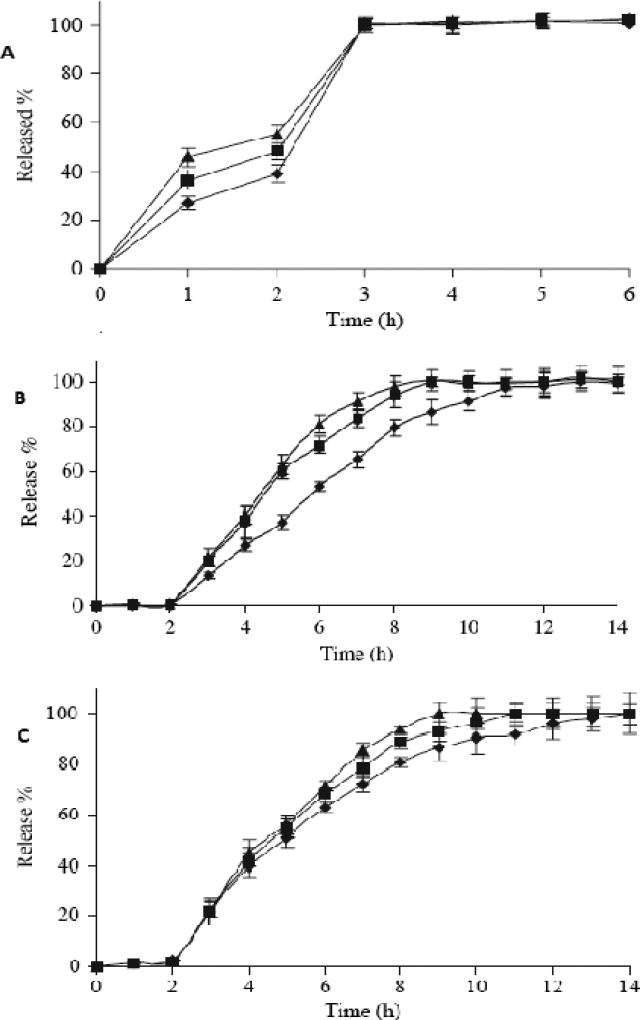
In a study performed by Rui Yang et al. ketoprofen tablets with enteric and sustained release properties was developed involving a HME technique (81). The authors used Eudragit® L100 and diethyl phthalate (DEP) as the carrier and plasticizer, respectively. Three types of tablets were prepared; firstly, one with a physical mixture of the components; secondly, one by direct cutting of the extrudates; and third one in which the extrudates were pulverized and then compressed into tablets. Dissolution testing of these formulations revealed that tablets prepared from the physical mixture were not resistant to acidic media (i.e. more than 10% of the drug was released in 0.1N HCl in 2h). However, the other two formulations were demonstrated to be resistant to acidic media and exhibited a sustained release profile in basic media (Figure 4). However, their mechanism of drug release was different. The researchers reported that the release mechanism exhibited only an erosion mechanism for the tablets obtained directly from cutting the extrudates, whereas both erosion and diffusion mechanisms were attributed to the tablets prepared from the pulverized extrudates.
Figure 4.
Dissolution profiles of ketoprofen-Eudragit® L100 physical mixture tablets (A), ketoprofen-Eudragit® L100 tablets from extrudates (B), and ketoprofen-Eudragit® L100 tablets using pulverized extrudates (C) in 0.1 M HCl for the first 2 hours, then in pH 6.8 Phosphate buffer for subsequent hours (M ± SD, n = 6).
Formulation 1(▲: drug/polymer ratio 1:1); Formulation 2 (
 drug/polymer ratio 1:1.5); Formulation 3(◆: drug/polymer ratio 1:2).
drug/polymer ratio 1:1.5); Formulation 3(◆: drug/polymer ratio 1:2).
Similarly, Andrews et al. evaluated the suitability of HME as a continuous process for the production of enteric tablets with sustained release properties, exhibiting significant advantages over traditional coating technology (82). In this study, Eudragit® L100-55 pre-plasticized with triethyl citrate (TEC; a liquid plasticizer) and citric acid (17% w/w as a solid-state plasticizer) was used as the enteric polymer, 5-amino salicylic acid (5-ASA) as the model drug and PVP K30 or Carbopol® 971P as an optional gelling agent. The powder blends were extruded as cylinders, cut into tablets and were characterized by PXRD, DSC and also studied for drug release. The enteric tablets thus formed demonstrated excellent gastric resistance, releasing less than 10% w/w of the API in acidic media, unlike the compressed tablets prepared from milled extrudates which released a significant amount of drug. This was attributed to the poor plastic deformation of the milled extrudates during compression, resulting in formation of tablets with lower strength and faster disintegration times. Increase in the TEC concentrations decreased the integrity of the polymer matrix network, leading to the ingression of water molecules inside the tablet cores, which resulted in increased drug diffusion/dissolution. Although the addition of a gelling agent significantly lowered tablet erosion, enteric properties of the matrix could not be retained to control the release of the drug in acidic media due to formation of porous channels within the extruded tablets. Also, the drug release was higher in pH 6.8 buffer as expected due to polymer matrix neutralization in the neutral media. Hence, the drug release from HME enteric tablets produced was dependent upon the concentration of plasticizer, presence of citric acid or Carbopol® 971P in the matrix and pH of the dissolution media.
The solid state properties of two solid dispersion formulations prepared by HME and co-precipitation (CP) processes, were evaluated by Dong et al. (83). Hypromellose acetate succinate (HPMC-AS), an enteric coating material for enteric or sustained release properties was used in this study. Both of the processes produced amorphous solid dispersions of the model drug and spectroscopic properties, powder X-ray diffraction, true density and water vapor sorption/desorption behavior were similar. However, specific surface area was higher for the product prepared by CP than HME, which was attributed to highest porosity and a rougher particle surface. Because of its high surface area, the dissolution rate was faster for the CP product, but the intrinsic dissolution rate was higher for the HME product. In addition, the HME dispersion exhibited superior physical stability over that prepared by CP. These studies demonstrate the feasibility of utilizing the HME technique in tailoring drug release and its potential superiority in eclipsing performance over conventional processing techniques.
2.1.4. Enteric Release
In 1969, Rippie and Johnson prepared pellets containing cellulose acetate phthalate using a ram extruder to assess the dissolution rates based upon pellet geometry (84). Young and co-workers also successfully prepared active-loaded spherical pellets by utilizing a combination of hot-melt extrusion and spheronization processes (50, 85–87). Recently, Young et al. investigated the film coating of melt-extruded beads containing guaifenesin within the matrix polymer Eudragit® L30 D-55, producing a melt extruded pellet system with pH-dependent drug release characteristics (87). The powder blends of guaifenesin, PEO and functional excipients were extruded as thin, symmetrical composite rods using melt-extrusion, cut into pellets and processed in a traditional spheronizer to produce spherical pellets. Subsequently, the formed pellets were subjected to film coating in a fluidized bed apparatus. Despite the initial miscibility of the drug/polymer blends, pellets obtained were reported to be morphologically unstable. This result was attributed to the recrystallization of both the drug and the polymer during storage. However, addition of ethyl cellulose to the extrudate and film coating with Eudragit® L30 D-55 stabilized the drug release of the thermally processed pellets. Despite the low melting point of the thermoplastic polymer matrix, film polymer coating was shown to be an effective process for producing melt-extruded beads with stable, pH-dependent drug release properties.
An alternative technique for enteric delivery was developed by Vervaet and co-workers utilizing hot-melt extrusion (88).The enteric polymers polyvinyl acetate phthalate (PVAP) and HPMC-AS were premixed with triacetin, a plasticizer, and extruded into hollow cylinders utilizing a co-rotating twin-screw extruder. The hollow “pipes” obtained were filled with a model drug (hydralazine) and both ends of the cylinders were sealed to yield hot-melt extruded enteric capsules which demonstrated excellent resistance to the gastric environment.
Later, Sauer et al. utilized a novel coating technology with HME and demonstrated a process to study the prevention of movement of highly water soluble and ionizable compounds into the polymeric film during the coating process (47). In this study, chlorpheniramine maleate (CPM) was utilized as a highly soluble model drug and Eudragit® L 100-55, pre-plasticized with different concentrations of TEC utilizing hot-melt extrusion, was used as a coating polymer. The extruded plasticized polymer was cut into pellets and cryogenically ground in to a fine powder. Also, due to reported plasticizing effects, PEG 3350 was incorporated as a primer in the coating powder to enhance the adhesion of the polymer onto the tablet cores and to improve the film formation and coalescence of polymeric particles. Tackiness of the coating polymer was reduced by addition of talc to the powder mixture after curing. The coating process was performed on a modified laboratory spheronizer and the effect of processing and formulation factors on release characteristics of CPM loaded tablets, powder coated with Eudragit® L 100-55 was studied. Drug release was found to be dependent on curing time, coating level and on the plasticizer content. Higher plasticizer levels, although decreasing drug release, reduced the polymer weight gain necessary to control CPM release in acidic medium. However, the Eudragit® L100-55 powder coated CPM tablets stored at 25°C/60% RH, over a period of 3 months demonstrated excellent stability without demonstrating any detectable differences in their drug release profiles.
2.1.5. Controlled Release
In 1996 and 1997, Miyagawa, Sato, and co-workers utilized a twin-screw compounding extruder to prepare controlled release granules composed of carnauba wax, diclofenac as a model drug, and other rate controlling agents by a hot-melt extrusion technique (15, 17). The authors demonstrated that a wax matrix with significant mechanical strength could be achieved even when the formulation was processed below the wax’s melting point. Drug release from the wax matrix granules was strongly influenced by the formulation components. Hydroxypropylcellulose, methacrylic acid copolymer (Eudragit® L-100), and sodium chloride were studied as dissolution rate controlling agents. In the process of preparing wax matrix tablets, these researchers also emphasized the advantages of utilizing the twin-screw extruder. These included lower processing temperatures, more efficient mixing and dispersing ability, and a shorter residence time. In their latter study, the authors observed that the selection of rate controlling materials based upon solubility and swelling characteristics significantly impacted drug release from the extruded wax matrix granules (16).
Recently, Fukuda, Peppas and McGinity investigated the influence of sodium bicarbonate on the physicochemical properties of controlled release HME tablets containing Eudragit® RS PO and/or Eudragit® E PO (89). In this study, acetohydroxamic acid and chlorpheniramine maleate were used as the model drugs. The authors studied the drug release properties and buoyancy in the dissolution media for both HME and directly compressed (DC) tablets by incorporating sodium bicarbonate into the tablet. The HME tablets prepared from the powder blend containing both Eudragit® RS PO and sodium bicarbonate demonstrated sustained release properties. In addition, the tablets floated on the surface of the dissolution media for up to 24h. The cross-sectional morphology of the hot-melt extruded tablets exhibited a porous structure, reportedly due to carbon dioxide gas generation resulting from thermal decomposition of sodium bicarbonate in the softened acrylic polymers at elevated temperature during processing. On the contrary, the DC tablets exhibited rapid drug release in the dissolution media and did not demonstrate any buoyancy. The drug release rate from floating HME tablets was controlled by both the incorporation of Eudragit® E PO into the matrix tablet and the diameter of the die utilized in the extrusion equipment.
2.1.6. Targeted Release
HME technology has been extended to explore novel applications such as targeted drug delivery by the virtue of flexibility in the selection of suitable polymers and excipients. Improved antifungal therapy was achieved by targeted drug delivery to the small intestine (90). The authors produced amorphous solid dispersions of itraconazole (ITZ) in Eudragit® L100-55 utilizing hot-melt extrusion technology. Carbopol® 974P (20% and 40% w/w of polymer) was incorporated in the formulation as a stabilizing agent to produce supersaturated levels of the amorphous drug which would significantly increase the oral absorption of ITZ in the intestine. The extrudates were characterized for their amorphous content and drug homogeneity utilizing a DSC and a qualitative energy dispersive X-ray spectroscopy, respectively. The addition of Carbopol® 974P in the Eudragit® L100-55 matrix slowed the drug release in acidic pH and retained its enteric properties. Moreover, it also retarded the drug release following pH transition from acidic to neutral media and substantially improved the duration of supersaturation. Oral dosing studies of these formulations were performed in male Sprague-Dawley rats and the pharmacokinetic data generated exhibited a statistically significant improvement in Cmax and T1/2 for the 20% Carbopol® 974P formulation compared to the formulation containing 40% (Table 2). These in vivo results also demonstrated a substantial reduction in the variability of drug absorption, which was attributed to the prolonged exposure of supersaturated ITZ hot-melt extruded dispersions to the intestinal membrane. The AUC value achieved with a 20% Carbopol® 974P formulation exhibited significantly higher (5-fold increase) oral absorption over these researcher’s previously reported ITZ particulate dispersions which limited supersaturation primarily to the stomach.
Table 2.
Pharmacokinetic Data from the In vivo Absorption Study with the 20% Carbopol® 974P Additive Formulation and the 40% Carbopol® 974P Additive Formulation (n=4 per group)
"Reprinted with kind permission from Springer Science and Business Media, Ref (90)"
| Formulation | Cmax (ng/ml) | Tmax (h) | AUC (ng·h/ml) | T1/2 (h) |
|---|---|---|---|---|
| 20% Carbopol Additive | 1,198±584 | 4.4±1.6 | 11,107±3,579 | 7.94±3.7 |
| 40% Carbopol Additive | 663±186 | 5.6±0.5 | 5,830±1,943 | 4.7±1.9 |
| p value (two tail) | 0.13 | 0.19 | 0.04 | 0.18 |
In recent years, an insight into hot-melt extrusion techniques has revealed another novel application of targeted drug delivery to the colon (91). The feasibility of hot-melt extruded tablets of 5-ASA in an Eudragit® S100 polymeric matrix for colon targeting was demonstrated by Bruce et al. Citric acid was utilized as a solid state plasticizer and TEC was used as a liquid plasticizer in this study. Researchers observed that a preplasticization step was necessary when incorporating TEC into the formulation in order to achieve uniform mixing of the polymer and the plasticizer. This step effectively reduced the polymer glass transition temperature (Tg), and lowered processing temperatures. The concentration of TEC in the extrudates not only influenced the processing temperature, but also influenced the drug release rates from the extruded tablets due to leaching of the plasticizer during release testing. Although drug/polymer blends containing citric acid lowered glass transition temperatures, a heat induced dehydration reaction between the drug and citric acid was observed. This was attributed to chemical bonding between the ammonium group of 5-ASA and the carboxylate groups of citric acid representing an amide bond formation and the loss of two water molecules during hot-melt processing. This interaction resulted in lower recovery of drug from tablets containing citric acid as compared with other HME formulations. However, there was no evidence of a binding interaction between the drug and the polymeric carrier.
2.1.7. HME and Other Advanced Technologies
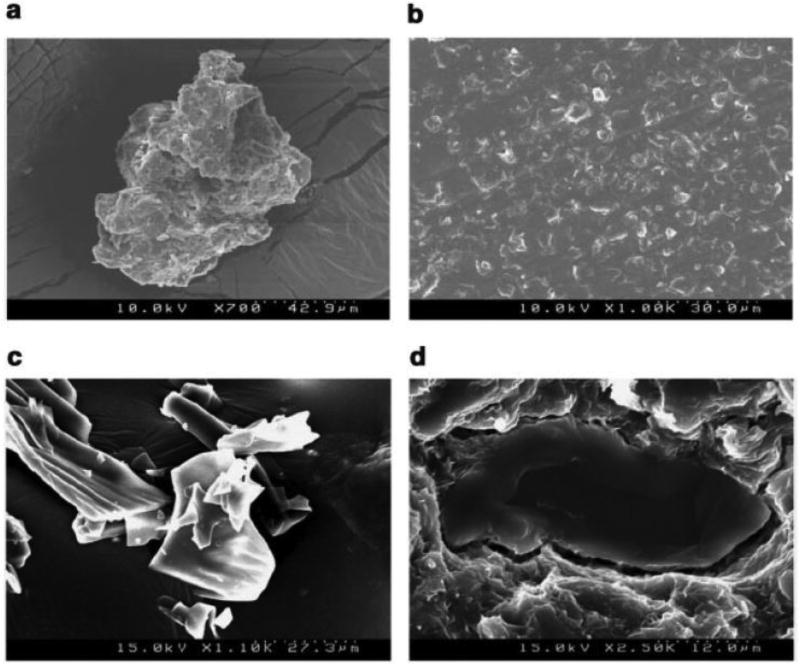
In the recent decade, drug particle engineering, utilizing nanotechnology, has gained much interest to improve the solubility limitations of poorly soluble drug compounds. However, it is associated with several limitations such as particle aggregation, morphological instability, and poor wettability. In this regard, Miller and co-workers (45) demonstrated the suitability of HME as a novel and viable approach for nanoparticle engineering by overcoming the above mentioned limitations. The focus of this investigation was to elucidate whether the shear generated during extrusion would deaggregate and disperse the micronized particles into a hydrophilic polymer matrix, without solubilizing or altering the properties of the drug particles. The concept was evaluated by developing itraconazole (ITZ)-PVP and ITZ-HPMC microparticles, subsequently dispersed in a polymer carrier system composed of Poloxamer® 407 and PEO 200M (7:3 ratio) utilizing a melt extrusion technique. From the SEM imaging, authors have provided visual confirmation and demonstrated that HME did not alter the morphology of the engineered particles and that they were homogeneously dispersed within the polymer carrier system (Figure 5). Moreover, drug release studies performed revealed that the dissolution rate of the micronized particles was improved by HME due to particle deaggregation and enhanced wetting. However, the selection of a carrier system and optimization of operating conditions during the extrusion process were considered the critical steps utilizing this methodology. It was determined that the two extruded formulations performed similarly in vivo rat model, per oral dosing as confirmed by their statistically similar AUC values. Based on the data from supersaturation dissolution testing, these co-workers concluded that rapid precipitation of ITZ resulted when entering a more neutral pH environment of the small intestine, hence providing only a small window of opportunity for absorption. This report suggested that perhaps the optimum formulation approach for ITZ was to control drug release such that precipitation is slowed as the pH is increased, thus extending the absorption window in the small intestine. Thus, HME technology may also be considered as a ‘gentle’ technique, in that it was successful in achieving a homogeneous dispersion without altering the morphological nature of the microparticles.
Figure 5.
SEM images of (a) ITZ-HPMC micronized particles (b) a typical cross section of the ITZ-HPMC micronized particle extrudates (c) ITZ-PVP micronized particles (d) close-up view of a typical ITZ-PVP micronized particle within the polymer matrix of the extrudate.
A novel solvent-free dry powder coating process was developed by pharmaceutical scientists utilizing HME technology for various applications (46, 47). In order to develop a sustained release formulation, Zheng and co-workers studied the physicochemical and dissolution properties of immediate release theophylline tablets coated utilizing a novel powder coating process(46). The initial pre-plasticization of ammonio methacrylate copolymers, Eudragit® RS PO and Eudragit® RL PO (95:5), with a plasticizer and thermal lubricant utilizing hot-melt extrusion was performed. The extrudates produced were then cryogenically ground into a fine powder and used in the coating process. The powder coating process mainly involved three steps: priming, powder layering and curing. At 8% w/w total weight gain, immediate release theophylline tablets that were powder coated with the pre-plasticized polymer matrix demonstrated a 12h sustained release profile. The authors reported a significant influence of formulation and processing parameters such as plasticizer concentration, curing temperature, coating level and particle size of the coating powder on drug release from the powder-coated tablets. The drug release from the coated tablets decreased significantly with an increase in the plasticizer concentration, the curing temperature, and the coating level. Theophylline tablets powder-coated with larger particles exhibited a faster drug release compared to those coated with smaller particles. Eudragit® RS PO/RL PO powder-coated tablets demonstrated a stable drug release profile after 3-month storage at 25°C/60% RH and 40°C/75% RH. This novel powder coating process utilized in conjunction with HME was shown to be an efficient coating method to produce stable, sustained release dosage forms.
HME has gained wide recognition because of its myriad of applications and researchers are now also focusing on novel polymeric carrier systems to suit a wide variety of drug molecules, cyclodextrins being one of them. These, however, have already been proven to improve the solubility and dissolution behavior of several poorly soluble drugs. Fukuda et al. have used sulfobutyl ether β-cyclodextrin (Captisol®) as a novel carrier system and investigated the suitability of HME for production of a ketoprofen - Captisol® complex (48). Dissolution behavior of a complex prepared by HME was compared with that of other general methods such as physical mixing, co-grinding, freeze drying and heat treatment. Extrusion temperatures were set close to the melting of point of drug (100°C) and below the melting point of Captisol® (approximately 235°C). An increase in dissolution rate in 0.1N HCl was observed for extrudates than from samples of physical mixing and pure ketoprofen. Moreover, drug release rate was equal to that of other processing techniques like freeze drying, heat treatment. Thus, intimate mixing and extent of complexation achieved with HME is equal to that of other methods. In addition, dissolution rate was unaltered for extrudates when samples were kept at 40°C/75%RH for 7 days, where as a decrease in dissolution rate was observed for samples from other methods. The observed phenomenon is due to less moisture uptake of extrudates (4.6–4.8%) compared to others, such as the heat-treated sample (6.5–6.7%), the co-ground sample (8.1–8.4%), the freeze-dried sample (10.4–10.7%), and the physical mixture (11.6–12.2%). Thus, this study demonstrated that improved mixing and increased dissolution of ketoprofen by HME is on par or even superior to that of co-grinding and freeze drying. One may now confidently conclude that HME technology is at the forefront of enabling existing excipients for newer and expanded applications.
2.2. Trans-Drug Delivery Systems
At present, HME has received considerable attention regarding the development of film/patch formulations for applications in transdermal, transmucosal, and transungual delivery. However, the most utilized methods for the production of these films are still based on casting techniques, using either aqueous or organic solvents (58). However, aqueous based film production is not suitable for many drug molecules and complete removal of the organic solvent from the films produced is challenging, to say the least. In addition, long processing times, batch waste, high costs and environmental toxicity are other prime concerns associated with these techniques (58). Moreover, other problems such as a loss of plasticity over time of acrylic films produced by solvent-based film casting was demonstrated by Gutierrez-Rocca et al. (92). This mechanical instability observed within these films was attributed to relaxation of the polymer chains as they moved toward a state of equilibrium. In this study, authors showed that a minimum of 2 months was required to achieve films with stable mechanical properties, depending on the type and level of plasticizer and storage conditions.
2.2.1. Transdermal Drug Delivery
The practicability of hot-melt extrusion technology for the production of thin, flexible acrylic films for topical delivery was investigated by Aitken-Nichol et al. in 1996 (58). In this study, lidocaine hydrochloride, a model drug, and Eudragit® E100, a thermoplastic polymer, were utilized to extrude films via HME. The authors compared the extruded films with the cast films and reported that the drug under study had plasticized the acrylic films and the differences in dissolution rate and ductile properties between films produced by both of the techniques were due to the amount of drug dissolved in the polymer. Moreover, the anhydrous HME process was not restricted by solvent concerns. The authors concluded that hot-melt extrusion was a feasible technique for the production of free films of this acrylic resin.
Repka et al. developed thin, flexible and opaque hydroxypropyl cellulose (HPC) films containing several hydrophobic (TEC, acetyltributyl citrate) or hydrophilic (PEG 400, PEG 8000) plasticizers and two model drugs (chlorpheniramine maleate and hydrocortisone) using a Killion extruder (Model: KLB-100) (23). Influence of these drugs and plasticizers on the physicochemical properties of the films was investigated. The authors recommended the inclusion of a plasticizer in the extruded HPC films since they observed generation of unwarranted torque levels on the instrument when no plasticizer was utilized. All of the plasticizers, except PEG 400, were found to be stable at the processing conditions employed in the study. Interestingly, chlorpheniramine maleate was found to be an excellent plasticizer for HPC films and the drug was completely solubilized within the films up to 10% w/w. Hydrocortisone was also reported to be a good plasticizer, however its chemical stability was found to be a function of processing temperature and residence time within the extruder.
In the same study, authors noted an interesting phenomenon between two mechanical properties, tensile strength and percent elongation, of the film. These two exhibited an inverse relationship, when testing for these parameters was performed in the direction of flow of the films versus perpendicular to flow. A minimum 4-fold decrease in tensile strength and a significant increase in percentage elongation were observed when testing was performed perpendicular to flow compared to in the direction of flow. Aitken-Nichol et al. (58) observed a similar relationship for tensile strength; however in contrast, a decrease in percentage elongation was reported. The incongruity between these two studies could be explained by the structure of cellulose and later cross-linking by added plasticizers and drugs in contrast to the structure of high density polyethylene/Eudragit® E100 used in the Aitken-Nichol study. Nevertheless, these studies exemplify how “flow orientation” can impact the mechanical properties of the hot-melt extruded matrices, which in turn can effect drug release.
Transdermal films containing guaifenesin (GFN) and ketoprofen (KTP) were prepared by Crowley et al. (21) Films were extruded using PEO with a drug loading of up to 30% GFN and 15% KTP. Both of the drugs were stable during the extrusion process and were found to decrease the drive load, increase the PEO stability and plasticize the polymer during extrusion. SEM and XRD studies revealed crystallization of GFN on the surface of the films; however, SEM studies did not reveal KTP crystallization until it reached the 15% loading level. The Hansen solubility parameters predicted good miscibility between PEO and KTP but poor miscibility between PEO and GFN. The percentage elongation decreased with increasing GFN concentrations and significantly increased with increasing levels of KTP. Increasing concentrations of both GFN and KTP decreased the tensile strength of the extruded films tested.
A pioneering technique for the determination of bioadhesion of transdermal films to the human epidermis was demonstrated by Repka and McGinity (8). Bioadhesion testing of the HME films, containing HPC with various formulation additives, was carried out using a Chatillon testing apparatus. Effect of storage conditions, temperature and relative humidity (RH), on the bioadhesive properties was also investigated. It was found that force of adhesion, elongation at adhesive failure, and modulus of adhesion were a function of the type of additive. The in vivo testing revealed that the HPC films containing 5% polycarbophil exhibited the highest force of adhesion and elongation at adhesive failure than other films tested. This study also demonstrated that a bioadhesive film with a single layer could be prepared by HME, without a separate adhesive layer.
2.2.2. Transmucosal Drug Delivery
Transmucosal delivery is particularly advantageous for poorly-water soluble drugs and those which undergo extensive first-pass metabolism. Repka and co-workers extensively investigated the hot-melt extrusion technique as a trenchant tool for developing numerous formulations containing various drug molecules for delivery via the buccal route (35, 40, 60, 61, 63, 93, 94).
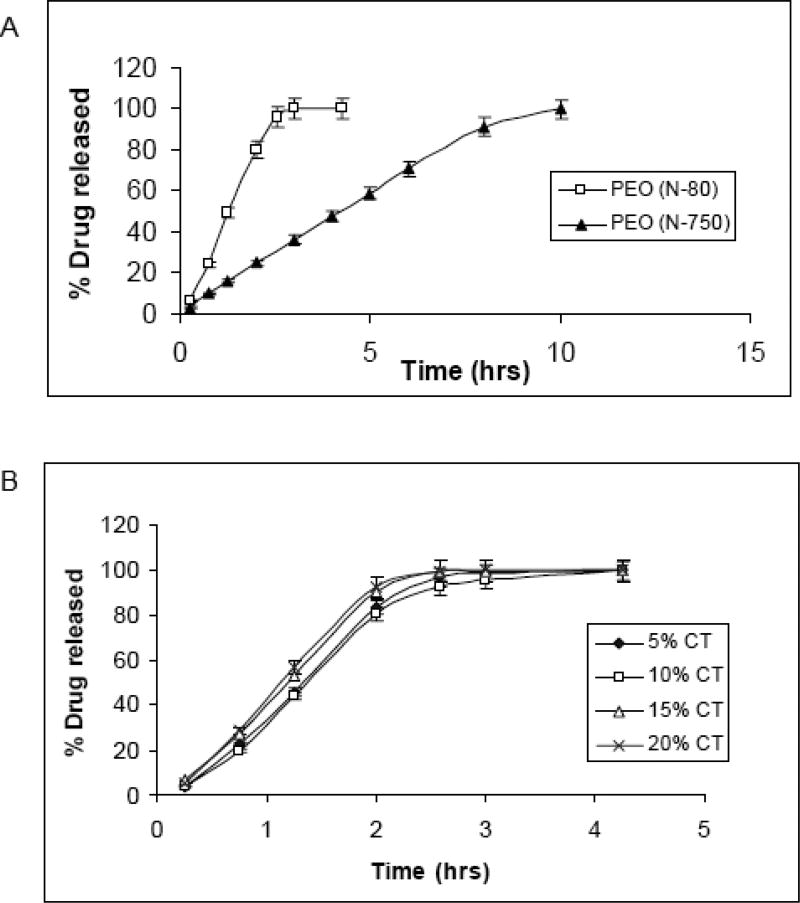
Polymeric films intended for oral or transmucosal delivery should be flexible, elastic and soft, yet sufficiently bioadhesive to withstand the mechanical stress of the oral cavity. Prodduturi et al. developed clotrimazole (CT) polymeric films utilizing hydroxylpropyl cellulose (Klucel® JF, GF and MF) (40) and PEO (PolyOx® N-80 and PolyOx® N-750) (61). From these studies, it was found that both polymeric systems exhibited zero order drug release and release rate was dependent on the molecular weight of the polymer (Figure 6). In addition, it was reported that drug release rate constant and release mechanism were independent of % of drug loading. Thus, size of the dosage form and/or release of the drug from extruded film could be tailored by altering the drug load without affecting release mechanism. These data are obviously germane for the development of controlled release dosage forms. The tested formulations were determined to possess sufficient bioadhesive strength for the oral environment. HPC and PEO formulations were found to be stable for 3 months; however recrystallization of CT was observed, in HPC films stored at 40°C/75% RH and PEO films stored at 25°C/60%RH, after 3 months. Optimum moisture content for HPC films, that increased film plasticity without compromising dissolution rate and stability, was reported to be 2–5%. These studies also indicated that the PEO films exposed to higher RH 75% demonstrated a decrease in Young’s modulus and tensile strength due to increased plasticization induced by the adsorbed water (Table 3). Surprisingly, % elongation was also found to decrease with increased plasticization when stored at a higher RH. This was thought to be due to recrystallization of clotrimazole in the PEO films (or possibly the polymer itself). Crystallized drug induces discontinuities within the film which enhance the likelihood of rupture and failure in the film structure leading to a decrease in % elongation. Therefore the mechanical properties of the PEO films, when exposed to higher RHs, are influenced by the combined effect of adsorbed moisture and recrystallization of the drug and/or polymer.
Figure 6.
Release profile of clotrimazole from hot-melt extruded PEO films as a function of polymer molecular weight in 1% SLS (50rpm, n=3) (A); effect of drug loading on the release of CT from hot-melt extruded PEO N-80 films (B)
Table 3.
Mechanical properties of hot-melt extruded PEO® N-80 (A), and PEO® N-750 (B) films stored at 25°C and different relative humidities (Mean ±SD) (n=6)
| A | |||
|---|---|---|---|
|
| |||
| Relative Humidity | Tensile Strength (MPa) |
% Elongation at Break (%) |
Elastic Modulus (MPa) |
| (A) 0% RH | 11.58 ± 0.26 | 53.73 ± 3.2 | 3.30 ± 0.10 |
| (B) 50%RH | 11.62 ± 0.34 | 50.48 ± 2.1 | 3.42 ± 0.18 |
| (C) 75% RH | 9.61 ± 0.28 | 27.41 ± 1.5 | 2.06 ± 0.12 |
| Statistical significance | p < 0.05 | p < 0.05 | p < 0.05 |
| A B C | A B C | A B C | |
| B | |||
|---|---|---|---|
|
| |||
| Relative Humidity | Tensile Strength (MPa) |
% Elongation at Break (%) |
Elastic Modulus (MPa) |
| (A) 0% RH | 13.98 ± 0.39 | 687.09 ± 5.2 | 3.76 ± 0.12 |
| (B) 50% RH | 13.56 ± 0.24 | 657.08 ± 9.5 | 3.67 ± 0.13 |
| (C) 75% RH | 11.58 ± 0.31 | 427.42 ± 5.8 | 2.51 ± 0.02 |
| Statistical significance | p < 0.05 | p < 0.05 | p < 0.05 |
| A B C | A B C | A B C | |
__, joined underlines indicate no significant difference
Repka et al. extruded lidocaine utilizing HPC and a combination of HPC and HPMC (80:20) as polymeric carriers into films, with no significant drug degradation (60). Thermal analysis confirmed the formation of a solid solution of lidocaine in the carrier system and both formulations exhibited sustained release characteristics. However, inclusion of HPMC retarded the drug release from the matrix, in addition to improving the bioadhesive properties. The mechanism of drug release was predominantly diffusion through the polymeric matrices for both of the formulations.
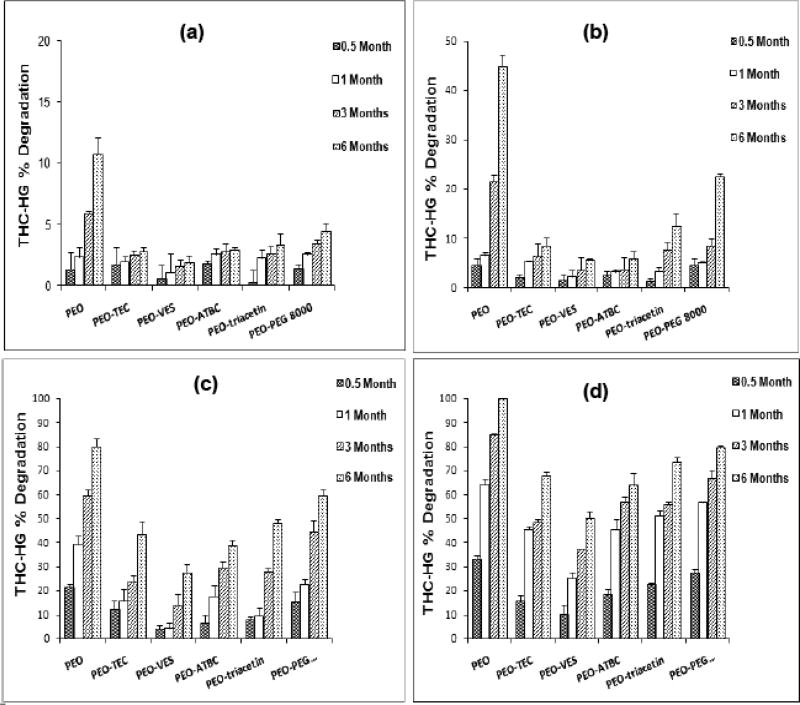
The viability of hot-melt techniques for the preparation of films containing thermolabile drugs was investigated by Munjal, Repka and co-workers. Formulations containing either Δ9-tetrahydrocannabinol (THC) (62, 63, 94) or its prodrug, THC-hemiglutarate (THC-HG) (29, 35) were produced and evaluated for mechanical and release properties, as well as stability kinetics. THC exhibits extremely poor solubility and undergoes significant first-pass metabolism, which leads to erratic pharmacokinetic profiles and low oral bioavailability. In lieu of these limitations, these researchers prepared transmucosal matrix systems utilizing various processing aids. The chemical stability of the drug in polymeric matrices was investigated with respect to processing temperature and time, formulation additives, and storage conditions. Results of the study suggested that at processing temperatures of 160°C and 200°C, THC degradation was linear as a function of time. However, in the case of THC-HG, film processing at 100°C for 7 min was found favorable, with a post-processing drug content of 95%. The degradation of the prodrug was considerably decreased at all of the temperatures studied in the presence of plasticizers. Vitamin E succinate (VES) was found to be the most effective among all of those tested (Figure 7). In addition, stability of the prodrug was further improved when citric acid was incorporated into the PEO-plasticizer matrices. This was attributed to a decrease in the micro environmental pH by addition of citric acid.
Figure 7.
Effect of plasticizers on the chemical stability of THC-HG in PEO polymeric matrices (n = 3) stored at 4 different temperatures: (a) −18° C, (b) 4° C, (c) 25° C and (d) 40° C. The films were fabricated at 110 ° C for 7 min.
Influence of physico-mechanical properties of vitamin E TPGS, an amphiphilic molecule, as a formulation additive on the properties of hydrophilic films was studied by Repka and McGinity (24). TPGS functioned as a plasticizer since a linear decrease in glass transition temperature of the films, containing either a 50:50 or 80:20 ratio of HPC to PEO, with increasing concentrations of Vitamin E TPGS (1, 3, and 5%) was observed. The reported effect was comparable to other conventional plasticizers. For example, films containing 3% Vitamin E TPGS had similar mechanical properties to films containing 3% PEG 400. In addition, TPGS was found to be an excellent processing aid, by decreasing barrel pressure, drive amps, and torque of the extruder.
Currently, a marketed denture adhesive is prepared by hot-melt extrusion using thermoplastic polymers that adhere to the mucous membrane when wetted (95). A bioadhesive film has the benefit of simplifying dosage form design and reducing preparation costs, due to the elimination of the adhesive layer in the system. The film has the desirable adhesive strength such that retention of the denture in the oral cavity is achieved.
2.2.3. Transungual Drug Delivery
One of the major challenges for transungual drug delivery systems is the adherence of the dosage form to the infected or diseased nail. Lacquers have been developed for treatment of nail fungal infections but have had little success due to their failure to withstand the physical damage, or possibly more importantly to allow a sustained release of the API via this system. In order to overcome the shortcomings of the existing delivery systems, Repka et al. developed formulations and processes for topical delivery of ketoconazole from polyethylene oxide films prepared by hot-melt extrusion technology for the treatment of onychomycosis in fingernails or toenails (96). The extruded films demonstrated excellent content uniformity and post-processing drug content. In vitro permeability profiles demonstrated that nail samples treated with an ‘etchant’ had a significant increase in drug permeability compared to the control. Differential scanning calorimeter thermograms indicated formation of a solid solution between the drug and polymer within the extruded films resulting in increased drug permeability.
Mididoddi et al. demonstrated the use of tartaric acid (TTA) as an effective plasticizer in hot-melt extruded HPC films containing polymeric additives (64). Hot-melt extruded films were prepared employing two different molecular weights of Klucel® (EF, MW: 80,000 and LF MW: 95, 000) containing ketoconazole (one batch of each MW, with and without TTA 4%) using a Killion single-screw extruder. TTA functioned as an effective plasticizer and the HPC films demonstrated an increase in percent elongation and decrease in tensile strength. In this study, TTA was reported to be a potential candidate for trans-nail devices prepared by HME technology.
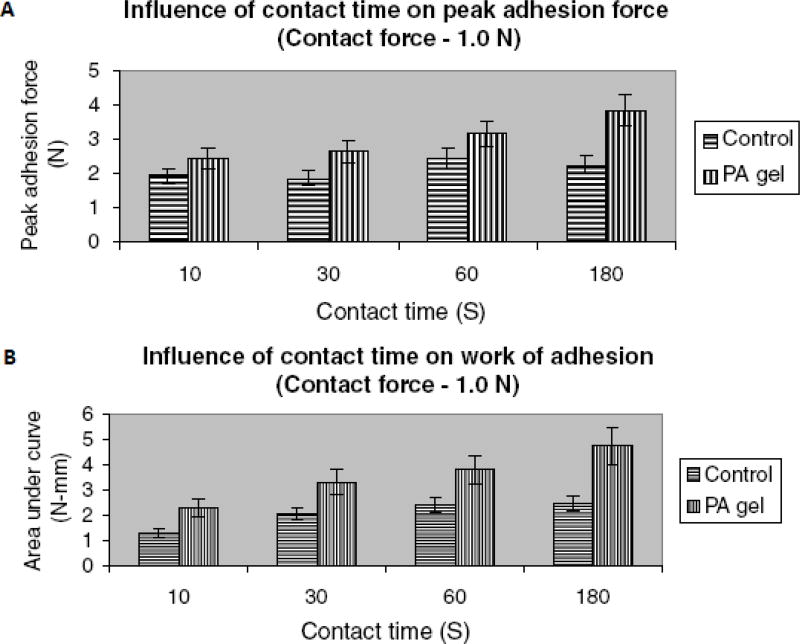
In a later study, Mididoddi et al. prepared drug loaded films by HME, containing up to 20% ketaconazole, as a novel dosage form for the treatment of onychomycosis and other nail diseases (65). The drug and the polymer mixtures were extruded as films and studied for their physico-chemical and bioadhesive properties and stability (25°C/60% RH). The drug was found to be relatively stable during the extrusion process. The authors demonstrated that the drug in the melt-cooled mixtures was miscible in the polymer and remained in an amorphous form over time. The miscibility of the drug and the polymer were predicted using solubility parameters and the results were corroborated by DSC, XRD and SEM results. Influence of varying contact times at a specific contact force on bioadhesive parameters was also investigated. The results of ketoconazole-HPC films on etched human nails treated with phosphoric acid (PA) gel demonstrated significantly higher peak adhesive force and work of adhesion compared to the control human nails at varying contact times (Figure 8). HME has been identified as a viable approach in producing stable films with superior bioadhesion and better permeation characteristics for sustained delivery of drugs across the infected nail surface.
Figure 8.
Effect of contact time on peak adhesion force (A), and work of adhesion (B) of HME film containing HPC and ketoconazole on human nail.
Control: untreated human nails; PA gel: phosphoric acid treated human nails (etched).
2.3. Implants
Rothen-Weinhold et al. prepared long-acting poly(lactic acid) implants containing vapreotide, a somatostatin analogue, by hot-melt extrusion (97). The authors reported degradation of the peptide during processing which led to the formation of an impurity, lactoyl lactyl-vapreotide conjugate. The authors concluded that the presence of residual lactide in the polylactic acid significantly influenced the formation of the peptide impurity, illustrating that carrier purity impacts the quality of the dosage form.
The release of melanotan-I (MT-I) from biodegradable implants of poly (D,L lactide-co-glycolide) (PLGA, 50:50 molar ratio of lactic/glycolic acid) copolymer produced by hot-melt extrusion have been reported by Bharadwaj and Blanchard (67, 68). The in vitro release of MT-I exhibited a triphasic profile with an initial rapid release (less than 5% of the drug load) followed by a secondary phase of slow release. It was also observed that a tertiary phase of rapid release commenced after about 3 weeks, due to erosion of the polymer. The polymer erosion and degradation were considered as the factors influencing the drug release and were controlled by the physical properties of the polymer such as molecular weight and viscosity.
Witt and co-workers investigated the implants composed of ABA triblock copolymers, consisting PLGA, poly (lactide-co-glycolide) A-block; poly (oxyethylene) B-block; and PLGA, poly (lactide-co-glycolide) A-block. The authors studied the degradation of this block copolymer with respect to swelling behavior, molecular weight loss and polymer erosion (98). Implants were either extruded using a laboratory ram extruder or prepared by compression molding. Insertion of an elastic B block did not lower the processing temperature, although the entanglement of the polymer chains was significantly reduced. This study also reported that implant geometry was found to be insignificant on degradation of the drug.
In another interesting study, a sustained release contraceptive vaginal ring was designed and manufactured utilizing a combination of single-screw and twin-screw hot-melt extruders (99). The contraceptive vaginal ring consisted of two steroids (etonogestrel and ethinyl estradiol) present in the molecularly dissolved state in a coaxial fiber made up of two types of polyethylene vinyl acetate (EVA) copolymers. The coaxial fiber consisted of a core polymer (EVA 28; 28% vinyl acetate; high solubility and permeability of steroids) with the two steroids incorporated and enveloped within a thin polymer membrane (EVA 9; 9% vinyl acetate; lower solubility and permeability of the steroids). The micronized steroid drugs were blended with the core polymer (EVA 28) and processed with a twin-screw extruder at 125°C. The strands arising from the twin-screw extruder were then cooled to room temperature and granulated using a strand granulator to obtain steroid loaded pellets. For the preparation of the coaxial fibers, co-extrusion installation was used wherein the two separate single-screw extruders were used to melt the steroid-core polymer granules and membrane polymer at temperatures above 110°C. The molten polymer strands were then delivered by gear pumps to a spinneret to form a coaxial fiber. This study demonstrated the application of hot-melt extrusion in manufacturing structurally and functionally more intricate dosage forms like the vaginal ring, incorporated with more than one API.
3. Conclusions and Scope
Hot-melt extrusion technology is an increasingly attractive process for the manufacture of various drug delivery systems. The literature published in this field so far reveals many interesting aspects such as fast dispersing systems, floating systems, complex formation within the melt, and nanoparticle engineering combined with melt extrusion technology. Many of the pharmaceutical products prepared utilizing this technique have been approved in the United States, Europe, and Asia. The drug being embedded in the carrier matrices as a solid dispersion/solution may allow for sustained or controlled release applications and dissolution rate improvement. Moreover, improved bioavailability has been achieved which demonstrates the value of HME as a powerful drug delivery tool. The primary challenges with this technique are the high temperatures employed and shear forces generated during processing, which may lead to either drug or polymer degradation. However, these issues may be overcome by formulation variables, equipment design, and engineering approaches. Although several researchers have been successful in employing HME to formulate many thermolabile molecules, exploitation of novel approaches continues for the development of efficient drug delivery systems.
4. Expert opinion
Most industries, corporations, entities, and even individuals are resistant to significant changes within their respective fields. Hot-melt extrusion technology for drug delivery processing and applications has been no exception. In the past, biases against this paradigm shift in the pharmaceutical industry were exhibited at national and international meetings to those presenting research on HME. A colleague of this author recounted recently a statement made to him by a senior executive of a multinational pharma about a decade ago: “Well, you will always have job security in the plastics industry with your melt extrusion training”.
However, that person, as well as hundreds more continued to explore HME as a solution for drug delivery issues. As outlined previously, there have been estimations that 40% of all new molecular entities have poor bioavailability because of low aqueous solubility. This percentage is very likely to increase due to combinatorial chemistry, as well as the emphasis and importance of lipophilic receptors. It has been the case, and still is, that compounds discovered with promising therapeutic value were abandoned due to suboptimal formulation/technology approaches. The last decade, however, has demonstrated that melt extrusion may increase bioavailability, for example, by formation of molecular dispersions.
Not too many years ago, pharmaceutical scientists’ retro-fitted extrusion equipment to meet their needs. However recently, pharmaceutical-class extruders have evolved and are technically adapted to mix an API with carriers for various solid dosage forms, including for the production of wet granulations. These advances have occurred due to the demand from the pharmaceutical industry, as well as academia and government. The HME technique has become an attractive alternative to traditional processing methods. These techniques offer many advantages as compared to other pharmaceutical processing technologies. Molten polymers during the extrusion process can function as thermal binders and act as drug depots and/or drug release retardants upon cooling and solidification. Organic solvents and water are not necessary, thus the number of processing steps and time-consuming drying steps are either minimized or eliminated. A matrix can be attained which is virtually independent of the powders’/polymers’ compression properties. HME’s efficient mixing and agitation applied by the rotating screw cause de-aggregation of suspended particles in the molten matrix polymer, which ultimately results in a more uniform dispersion of the active. The more recent introduction of twin-screw extruders with versatile screw designs allows them to be used to perform a large number of previously separate functions. In all, the process has been shown to be continuous, as well as efficient. In addition, a relatively large selection of excipients, high and low molecular weight polymers, as well as judiciously selecting and blending different polymers, provide new fields for formulation research.
As this review has illustrated, many types of drug delivery systems are possible with hot-melt techniques. The published literature discloses innovative and promising new approaches for drug delivery systems, such as effervescent granules, complex formation, and solid dispersions/solutions. Nanotechnology has been merged with HME—each solving inherent problems of the other. Novel powder coating techniques, rapid dissolving, as well as sustained release tablets and films are all viable using this emerging technology.
Although the industry was indeed hesitant to introduce a new technology, many small, as well as multi-national pharma, now embrace these techniques for a variety of reasons, from solving drug bioavailability issues to life-cycle management. Marketed products by innovative HME techniques validate the technology. One example is a new tablet formulation of Kaletra®, bringing new hope for HIV therapy. Others are Isoptin® (verapamil) and NuvaRing®, a controlled release, vaginal contraceptive system. However, these are only a few examples and this is only the beginning for HME drug delivery solutions. Indeed, research is continuing to expand the benefits of melt technology to sensitive macromolecules, such as peptides and proteins, which would shift the paradigm even further.
Acknowledgments
This work was supported by Grant Number P20RR021929 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
BIBLIOGRAPHY
- 1.McGinity JW, Zhang F, Koleng JJ, Repka MA. Hot-Melt Extrusion as a Pharmaceutical Process. American Pharmaceutical Review. 2001;4:25–37. [Google Scholar]
- 2*.Breitenbach J. Melt extrusion: from process to drug delivery technology. Eur J Pharm Biopharm. 2002 Sep;54(2):107–17. doi: 10.1016/s0939-6411(02)00061-9. Interesting review on applications of HME in the field of pharmaceutics. [DOI] [PubMed] [Google Scholar]
- 3.Chokshi R, Hossein Z. Hot–Melt Extrusion Technique: A Review. Iran J Pharm Res. 2004;3:3–16. [Google Scholar]
- 4**.Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, et al. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev Ind Pharm. 2007 Sep;33(9):909–26. doi: 10.1080/03639040701498759. Detailed review on instrumentation, developmental aspects and characterization techniques of HME dosage forms. [DOI] [PubMed] [Google Scholar]
- 5.Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, et al. Pharmaceutical applications of hot-melt extrusion: Part II. Drug Dev Ind Pharm. 2007 Oct;33(10):1043–57. doi: 10.1080/03639040701525627. [DOI] [PubMed] [Google Scholar]
- 6.Kerns EH. High throughput physicochemical profiling for drug discovery. J Pharm Sci. 2001;90(11):1838–58. doi: 10.1002/jps.1134. [DOI] [PubMed] [Google Scholar]
- 7.Follonier N, Doelker E, Cole ET. Various ways of modulating the release of diltiazem hydrochloride from hot-melt extruded sustained release pellets prepared using polymeric materials. J Control Release. 1995;36(3):243–50. [Google Scholar]
- 8*.Repka MA, McGinity JW. Physical-mechanical, moisture absorption and bioadhesive properties of hydroxypropylcellulose hot-melt extruded films. Biomaterials. 2000 Jul;21(14):1509–17. doi: 10.1016/s0142-9612(00)00046-6. A novel approach to determine bioadhesion of films to the human epidermis is reported. [DOI] [PubMed] [Google Scholar]
- 9.Kidokoro M, Shah NH, Malick AW, Infeld MH, McGinity JW. Properties of tablets containing granulations of ibuprofen and an acrylic copolymer prepared by thermal processes. Pharm Dev Technol. 2001;6(2):263–75. doi: 10.1081/pdt-100002203. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Zhang F, McGinity JW. Properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot-melt extrusion. Eur J Pharm Biopharm. 2001 Sep;52(2):181–90. doi: 10.1016/s0939-6411(01)00162-x. [DOI] [PubMed] [Google Scholar]
- 11.Forster A, Hempenstall J, Rades T. Characterization of glass solutions of poorly water-soluble drugs produced by melt extrusion with hydrophilic amorphous polymers. J Pharm Pharmacol. 2001 Mar;53(3):303–15. doi: 10.1211/0022357011775532. [DOI] [PubMed] [Google Scholar]
- 12.Crowley MM, Zhang F, Koleng JJ, McGinity JW. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials. 2002 Nov;23(21):4241–8. doi: 10.1016/s0142-9612(02)00187-4. [DOI] [PubMed] [Google Scholar]
- 13.van Laarhoven JA, Kruft MA, Vromans H. Effect of supersaturation and crystallization phenomena on the release properties of a controlled release device based on EVA copolymer. J Control Release. 2002 Aug 21;82(2–3):309–17. doi: 10.1016/s0168-3659(02)00139-6. [DOI] [PubMed] [Google Scholar]
- 14.El-Egakey MASM, Speiser P. Hot extruded dosage forms Part-I: Technology and dissolution kinetics of polymeric matrices. Pharmaceutica Acta Helvetiae. 1971;46:31–52. [PubMed] [Google Scholar]
- 15.Miyagawa Y, Okabe T, Yamaguchi Y, Miyajima M, Sato H, Sunada H. Controlled-release of diclofenac sodium from wax matrix granule. Int J Pharm. 1996;138(2):215–24. [Google Scholar]
- 16.Sato H, Miyagawa Y, Okabe T, Miyajima M, Sunada H. Dissolution mechanism of diclofenac sodium from wax matrix granules. J Pharm Sci. 1997 Aug;86(8):929–34. doi: 10.1021/js960221w. [DOI] [PubMed] [Google Scholar]
- 17.Miyagawa Y, Sato H, Okabe T, Nishiyama T, Miyajima M, Sunada H. In vivo performance of wax matrix granules prepared by a twin-screw compounding extruder. Drug Dev Ind Pharm. 1999 Apr;25(4):429–35. doi: 10.1081/ddc-100102192. [DOI] [PubMed] [Google Scholar]
- 18*.Hancock BC, York P, Rowe RC. The use of solubility parameters in pharmaceutical dosage form design. Int J Pharm. 1997;148(1):1–21. Review of use and potential of solubility parameters for pharmaceutical dosage form design. [Google Scholar]
- 19.Forster A, Hempenstall J, Tucker I, Rades T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int J Pharm. 2001 Sep 11;226(1–2):147–61. doi: 10.1016/s0378-5173(01)00801-8. [DOI] [PubMed] [Google Scholar]
- 20.Greenhalgh DJ, Williams AC, Timmins P, York P. Solubility parameters as predictors of miscibility in solid dispersions. J Pharm Sci. 1999 Nov;88(11):1182–90. doi: 10.1021/js9900856. [DOI] [PubMed] [Google Scholar]
- 21*.Crowley MM, Fredersdorf A, Schroeder B, Kucera S, Prodduturi S, Repka MA, et al. The influence of guaifenesin and ketoprofen on the properties of hot-melt extruded polyethylene oxide films. Eur J Pharm Sci. 2004 Aug;22(5):409–18. doi: 10.1016/j.ejps.2004.04.005. Interesting discussion on recrystallization of drug during stability and its effect on mechanical properties of HME dosage forms. [DOI] [PubMed] [Google Scholar]
- 22.Wang C-C, Zhang G, H Shah N, Infeld MH, Waseem Malick A, McGinity JW. Influence of plasticizers on the mechanical properties of pellets containing Eudragit® RS 30 D. Int J Pharm. 1997;152(2):153–63. [Google Scholar]
- 23.Repka MA, Gerding TG, Repka SL, McGinity JW. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot melt extrusion. Drug Dev Ind Pharm. 1999 May;25(5):625–33. doi: 10.1081/ddc-100102218. [DOI] [PubMed] [Google Scholar]
- 24.Repka MA, McGinity JW. Influence of vitamin E TPGS on the properties of hydrophilic films produced by hot-melt extrusion. Int J Pharm. 2000 Jul 20;202(1–2):63–70. doi: 10.1016/s0378-5173(00)00418-x. [DOI] [PubMed] [Google Scholar]
- 25.Wu C, McGinity JW. Influence of methylparaben as a solid-state plasticizer on the physicochemical properties of Eudragit RS PO hot-melt extrudates. Eur J Pharm Biopharm. 2003 Jul;56(1):95–100. doi: 10.1016/s0939-6411(03)00035-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Mehta KA, McGinity JW. Influence of plasticizer level on the drug release from sustained release film coated and hot-melt extruded dosage forms. Pharm Dev Technol. 2006;11(3):285–94. doi: 10.1080/10409230600767551. [DOI] [PubMed] [Google Scholar]
- 27*.Schilling SU, Shah NH, Malick AW, Infeld MH, McGinity JW. Citric acid as a solid-state plasticizer for Eudragit RS PO. J Pharm Pharmacol. 2007 Nov;59(11):1493–500. doi: 10.1211/jpp.59.11.0005. Identification of a new application for an existing pharmaceutical excipient for the purposes of HME. [DOI] [PubMed] [Google Scholar]
- 28.Lyons JG, Hallinan M, Kennedy JE, Devine DM, Geever LM, Blackie P, et al. Preparation of monolithic matrices for oral drug delivery using a supercritical fluid assisted hot melt extrusion process. Int J Pharm. 2007 Feb 1;329(1–2):62–71. doi: 10.1016/j.ijpharm.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Thumma S, Elsohly MA, Zhang SQ, Gul W, Repka MA. Influence of plasticizers on the stability and release of a prodrug of Delta(9)-tetrahydrocannabinol incorporated in poly (ethylene oxide) matrices. Eur J Pharm Biopharm. 2008;20(2):605–14. doi: 10.1016/j.ejpb.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stepto RFT. Thermoplastic starch. Macromolecular Symposia. 2000;152(1):73–82. [Google Scholar]
- 31.De Brabander C, Vervaet C, Fiermans L, Remon JP. Matrix mini-tablets based on starch/microcrystalline wax mixtures. Int J Pharm. 2000 Apr 20;199(2):195–203. doi: 10.1016/s0378-5173(00)00383-5. [DOI] [PubMed] [Google Scholar]
- 32.Lyons JG, Devine DM, Kennedy JE, Geever LM, O'Sullivan P, Higginbotham CL. The use of agar as a novel filler for monolithic matrices produced using hot melt extrusion. Eur J Pharm Biopharm. 2006 Aug;64(1):75–81. doi: 10.1016/j.ejpb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Verhoeven E, Vervaet C, Remon JP. Xanthan gum to tailor drug release of sustained-release ethylcellulose mini-matrices prepared via hot-melt extrusion: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2006 Jul;63(3):320–30. doi: 10.1016/j.ejpb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Schilling SU, Bruce CD, Shah NH, Malick AW, McGinity JW. Citric acid monohydrate as a release-modifying agent in melt extruded matrix tablets. Int J Pharm. 2008 Sep 1;361(1–2):158–68. doi: 10.1016/j.ijpharm.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 35.Thumma S, Majumdar S, Elsohly MA, Gul W, Repka MA. Chemical stability and bioadhesive properties of an ester prodrug of Delta(9)-tetrahydrocannabinol in poly(ethylene oxide) matrices: Effect of formulation additives. Int J Pharm. 2008 Oct 1;362(1–2):126–32. doi: 10.1016/j.ijpharm.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kucera SA, Stimpel D, Shah NH, Malick AW, Infeld MH, McGinity JW. Influence of fumed silicon dioxide on the stabilization of Eudragit RS/RL 30 D film-coated theophylline pellets. Pharm Dev Technol. 2008;13(3):245–53. doi: 10.1080/10837450801949665. [DOI] [PubMed] [Google Scholar]
- 37.Ghebremeskel AN, Vemavarapu C, Lodaya M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: stability testing of selected solid dispersions. Pharm Res. 2006 Aug;23(8):1928–36. doi: 10.1007/s11095-006-9034-1. [DOI] [PubMed] [Google Scholar]
- 38.Zhou F, Vervaet C, Remon JP. Matrix pellets based on the combination of waxes, starches and maltodextrins. Int J Pharm. 1996;133(1–2):155–60. [Google Scholar]
- 39.Henrist D, Remon JP. Influence of the formulation composition on the in vitro characteristics of hot stage extrudates. Int J Pharm. 1999 Oct 15;188(1):111–9. doi: 10.1016/s0378-5173(99)00212-4. [DOI] [PubMed] [Google Scholar]
- 40.Prodduturi S, Manek RV, Kolling WM, Stodghill SP, Repka MA. Water vapor sorption of hot-melt extruded hydroxypropyl cellulose films: effect on physico-mechanical properties, release characteristics, and stability. J Pharm Sci. 2004 Dec;93(12):3047–56. doi: 10.1002/jps.20222. [DOI] [PubMed] [Google Scholar]
- 41.Bruce C, Fegely KA, Rajabi-Siahboomi AR, McGinity JW. Crystal growth formation in melt extrudates. Int J Pharm. 2007 Aug 16;341(1–2):162–72. doi: 10.1016/j.ijpharm.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Ndindayino F, Vervaet C, Van den Mooter G, Remon JP. Bioavailability of hydrochlorothiazide from isomalt-based moulded tablets. Int J Pharm. 2002 Oct 10;246(1–2):199–202. doi: 10.1016/s0378-5173(02)00354-x. [DOI] [PubMed] [Google Scholar]
- 43.Kinoshita M, Baba K, Nagayasu A, Yamabe K, Shimooka T, Takeichi Y, et al. Improvement of solubility and oral bioavailability of a poorly water-soluble drug, TAS-301, by its melt-adsorption on a porous calcium silicate. J Pharm Sci. 2002 Feb;91(2):362–70. doi: 10.1002/jps.10026. [DOI] [PubMed] [Google Scholar]
- 44.Breitenbach J, Magerlein M. In: Melt extruded molecular dispersions. Ghebre-Selassie I, Martin C, editors. Informa Healthcare; 2003. [Google Scholar]
- 45*.Miller DA, McConville JT, Yang W, Williams RO, 3rd, McGinity JW. Hot-melt extrusion for enhanced delivery of drug particles. J Pharm Sci. 2007 Feb;96(2):361–76. doi: 10.1002/jps.20806. First known published report on application of hot-melt extrusion in conjunction with nanoparticle engineering. [DOI] [PubMed] [Google Scholar]
- 46.Zheng W, Cerea M, Sauer D, McGinity JW. Properties of theophylline tablets powder-coated with methacrylate ester copolymers. STP pharma sciences. 2004;14(4):319–25. [Google Scholar]
- 47.Sauer D, Zheng W, Coots LB, McGinity JW. Influence of processing parameters and formulation factors on the drug release from tablets powder-coated with Eudragit L 100-55. Eur J Pharm Biopharm. 2007 Sep;67(2):464–75. doi: 10.1016/j.ejpb.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 48.Fukuda M, Miller DA, Peppas NA, McGinity JW. Influence of sulfobutyl ether beta-cyclodextrin (Captisol) on the dissolution properties of a poorly soluble drug from extrudates prepared by hot-melt extrusion. Int J Pharm. 2008 Feb 28;350(1–2):188–96. doi: 10.1016/j.ijpharm.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 49.Follonier N, Doelker E, Cole ET. Evaluation of hot-melt extrusion as a new technique for the production of polymer-based pellets for sustained release capsules containing high loadings of freely soluble drugs. Drug Dev Ind Pharm. 1994;20(8):1323–39. [Google Scholar]
- 50.Young CR, Koleng JJ, McGinity JW. Production of spherical pellets by a hot-melt extrusion and spheronization process. Int J Pharm. 2002 Aug 21;242(1–2):87–92. doi: 10.1016/s0378-5173(02)00152-7. [DOI] [PubMed] [Google Scholar]
- 51.McGinity JW, Koleng JJ. Pharmaceutical Technology Conference. Pharmaceutical technology; 1997. Preparation and Evaluation of Rapid-Release Granules using a Novel Hot-Melt Extrusion Technique; pp. 153–4. 1997. [Google Scholar]
- 52.Robinson JR, McGinity JW, inventors. Ethypharm SA, assignee. Effervescent granules and methods for their preparation. 6071539. United States patent. 2000
- 53.McGinity JZ, inventor. Hot-melt extrudable pharmaceutical formulation. 1997
- 54.Zhang F, McGinity JW. Properties of sustained-release tablets prepared by hot-melt extrusion. Pharm Dev Technol. 1999 May;4(2):241–50. doi: 10.1081/pdt-100101358. [DOI] [PubMed] [Google Scholar]
- 55.Zhang F, McGinity JW. Properties of hot-melt extruded theophylline tablets containing poly(vinyl acetate) Drug Dev Ind Pharm. 2000 Sep;26(9):931–42. doi: 10.1081/ddc-100101320. [DOI] [PubMed] [Google Scholar]
- 56.Sherry R, inventor. Reckitt Benckiser Healthcare (UK) Limited, assignee. Granules comprising paracetamol, a NSAID and a sugar alcohol made by melt extrusion patent. EP1799195. 2007
- 57.Rocca JCG. Stability and Physical-mechanical Properties of Acrylic Resin Copolymers [Dissertation] Austin: University of Texas at Austin; 1993. [Google Scholar]
- 58.Aitken-Nichol C, Zhang F, McGinity JW. Hot melt extrusion of acrylic films. Pharm Res. 1996 May;13(5):804–8. doi: 10.1023/a:1016076306279. [DOI] [PubMed] [Google Scholar]
- 59.Repka MA, McGinity JW. Influence of chlorpheniramine maleate on topical hydroxypropylcellulose films produced by hot-melt extrusion. Pharm Dev Technol. 2001 Aug;6(3):297–304. doi: 10.1081/pdt-100002610. [DOI] [PubMed] [Google Scholar]
- 60.Repka MA, Gutta K, Prodduturi S, Munjal M, Stodghill SP. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur J Pharm Biopharm. 2005 Jan;59(1):189–96. doi: 10.1016/j.ejpb.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Prodduturi S, Manek RV, Kolling WM, Stodghill SP, Repka MA. Solid-state stability and characterization of hot-melt extruded poly(ethylene oxide) films. J Pharm Sci. 2005 Oct;94(10):2232–45. doi: 10.1002/jps.20437. [DOI] [PubMed] [Google Scholar]
- 62.Munjal M, Elsohly MA, Repka MA. Polymeric systems for amorphous Delta9-tetrahydrocannabinol produced by a hot-melt method. Part II: Effect of oxidation mechanisms and chemical interactions on stability. J Pharm Sci. 2006 Nov;95(11):2473–85. doi: 10.1002/jps.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munjal M, Stodghill SP, Elsohly MA, Repka MA. Polymeric systems for amorphous Delta 9-tetrahydrocannabinol produced by a hot-melt method. Part I: chemical and thermal stability during processing. J Pharm Sci. 2006 Aug;95(8):1841–53. doi: 10.1002/jps.20667. [DOI] [PubMed] [Google Scholar]
- 64.Mididoddi PK, Prodduturi S, Repka MA. Influence of tartaric acid on the bioadhesion and mechanical properties of hot-melt extruded hydroxypropyl cellulose films for the human nail. Drug Dev Ind Pharm. 2006 Oct;32(9):1059–66. doi: 10.1080/03639040600683410. [DOI] [PubMed] [Google Scholar]
- 65.Mididoddi PK, Repka MA. Characterization of hot-melt extruded drug delivery systems for onychomycosis. Eur J Pharm Biopharm. 2007 Apr;66(1):95–105. doi: 10.1016/j.ejpb.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 66.Sam AP. Controlled release contraceptive devices: a status report. J Control Release. 1992;22:35–46. [Google Scholar]
- 67.Bhardwaj R, Blanchard J. In vitro evaluation of Poly (d, l-lactide-co-glycolide) polymer-based implants containing the alpha-melanocyte stimulating hormone analog, Melanotan-I. J Control Release. 1997;45(1):49–55. [Google Scholar]
- 68.Bhardwaj R, Blanchard J. In vitro characterization and in vivo release profile of a poly (,-lactide-co-glycolide)-based implant delivery system for the [alpha]-MSH analog, melanotan-I. Int J Pharm. 1998;170(1):109–17. [Google Scholar]
- 69.Mcginity JW, Robinson JR, inventors. inventors. Efferevescence polymeric film drug delivery system. 20010006677. United States patent. 2001
- 70.Robinson J, McGinity J, Delmas P, inventors. Ethypharm, Robinson, J. McGinity, J. Delmas, P, assignee. Effervescent granules and methods for their preparation patent. WO/2001/080,822. 2001
- 71.Sherry R, inventor. Reckitt Benckiser Healthcare (UK) Limited. Granules comprising a NSAID and a sugar alcohol made by melt extrusion. 20070254028. USA patent. 2006
- 72.Mank R, Kala H, Richter M. Preparation of extrusion pellets containing drugs on the base of thermoplastics. 2. Investigations on the improvement of the drug release on the base of thermoplastics. Pharmazie. 1990;45(8):592–3. [PubMed] [Google Scholar]
- 73.Perissutti B, Newton JM, Podczeck F, Rubessa F. Preparation of extruded carbamazepine and PEG 4000 as a potential rapid release dosage form. Eur J Pharm Biopharm. 2002 Jan;53(1):125–32. doi: 10.1016/s0939-6411(01)00209-0. [DOI] [PubMed] [Google Scholar]
- 74.Hulsmann S, Backensfeld T, Keitel S, Bodmeier R. Melt extrusion--an alternative method for enhancing the dissolution rate of 17beta-estradiol hemihydrate. Eur J Pharm Biopharm. 2000 May;49(3):237–42. doi: 10.1016/s0939-6411(00)00077-1. [DOI] [PubMed] [Google Scholar]
- 75.Hulsmann S, Backensfeld T, Bodmeier R. Stability of extruded 17 beta-estradiol solid dispersions. Pharm Dev Technol. 2001;6(2):223–9. doi: 10.1081/pdt-100002198. [DOI] [PubMed] [Google Scholar]
- 76.Sun Y, Rui Y, Wenliang Z, Tang X. Nimodipine semi-solid capsules containing solid dispersion for improving dissolution. Int J Pharm. 2008 Jul 9;359(1–2):144–9. doi: 10.1016/j.ijpharm.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 77.Mank R, Kala H, Richter M. Preparation of extrusion pellets containing drugs on the base of thermoplastics. 1. Investigation of drug release. Pharmazie. 1989;44(11):773–6. [PubMed] [Google Scholar]
- 78.Crowley MM, Schroeder B, Fredersdorf A, Obara S, Talarico M, Kucera S, et al. Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion. Int J Pharm. 2004 Jan 28;269(2):509–22. doi: 10.1016/j.ijpharm.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 79.De Brabander C, Vervaet C, Van Bortel L, Remon JP. Bioavailability of ibuprofen from hot-melt extruded mini-matrices. Int J Pharm. 2004 Mar 1;271(1–2):77–84. doi: 10.1016/j.ijpharm.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 80*.Quintavalle U, Voinovich D, Perissutti B, Serdoz F, Grassi G, Dal Col A, et al. Preparation of sustained release co-extrudates by hot-melt extrusion and mathematical modelling of in vitro/in vivo drug release profiles. Eur J Pharm Sci. 2008 Mar 3;33(3):282–93. doi: 10.1016/j.ejps.2007.12.008. Described a mathematical model for predicting a drug plasma concentration profile from in vitro data. [DOI] [PubMed] [Google Scholar]
- 81.Yang R, Wang Y, Zheng X, Meng J, Tang X, Zhang X. Preparation and evaluation of ketoprofen hot-melt extruded enteric and sustained-release tablets. Drug Dev Ind Pharm. 2008 Jan;34(1):83–9. doi: 10.1080/03639040701580572. [DOI] [PubMed] [Google Scholar]
- 82.Andrews GP, Jones DS, Diak OA, McCoy CP, Watts AB, McGinity JW. The manufacture and characterisation of hot-melt extruded enteric tablets. Eur J Pharm Biopharm. 2008;69(1):264–73. doi: 10.1016/j.ejpb.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 83.Dong Z, Chatterji A, Sandhu H, Choi DS, Chokshi H, Shah N. Evaluation of solid state properties of solid dispersions prepared by hot-melt extrusion and solvent co-precipitation. Int J Pharm. 2008 May 1;355(1–2):141–9. doi: 10.1016/j.ijpharm.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 84.Rippie EG, Johnson JR. Regulation of dissolution rate by pellet geometry. J Pharm. Sci. 1969 Apr;58(4):428–31. doi: 10.1002/jps.2600580408. [DOI] [PubMed] [Google Scholar]
- 85.Young CR, Koleng JJ, McGinity JW. Properties of drug-containing spherical pellets produced by a hot-melt extrusion and spheronization process. J Microencapsul. 2003 Sep-Oct;20(5):613–25. doi: 10.1080/0265204031000148004. [DOI] [PubMed] [Google Scholar]
- 86.Young CR, Dietzsch C, McGinity JW. Compression of controlled-release pellets produced by a hot-melt extrusion and spheronization process. Pharm Dev Technol. 2005;10(1):133–9. doi: 10.1081/pdt-49695. [DOI] [PubMed] [Google Scholar]
- 87.Young CR, Crowley M, Dietzsch C, McGinity JW. Physicochemical properties of film-coated melt-extruded pellets. J Microencapsul. 2007 Feb;24(1):57–71. doi: 10.1080/02652040601058483. [DOI] [PubMed] [Google Scholar]
- 88.Mehuys E, Remon JP, Vervaet C. Production of enteric capsules by means of hot-melt extrusion. Eur J Pharm Sci. 2005 Feb;24(2–3):207–12. doi: 10.1016/j.ejps.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 89.Fukuda M, Peppas NA, McGinity JW. Floating hot-melt extruded tablets for gastroretentive controlled drug release system. J Control Release. 2006 Oct 10;115(2):121–9. doi: 10.1016/j.jconrel.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 90.Miller DA, DiNunzio JC, Yang W, McGinity JW, Williams RO., 3rd Targeted intestinal delivery of supersaturated itraconazole for improved oral absorption. Pharm Res. 2008 Jun;25(6):1450–9. doi: 10.1007/s11095-008-9543-1. [DOI] [PubMed] [Google Scholar]
- 91.Bruce LD, Shah NH, Malick AW, Infeld MH, McGinity JW. Properties of hot-melt extruded tablet formulations for the colonic delivery of 5-aminosalicylic acid. Eur J Pharm Biopharm. 2005 Jan;59(1):85–97. doi: 10.1016/j.ejpb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 92.Gutierrez-Rocca JC, McGinity JW. Influence of Aging on the Physical-Mechanical Properties of Acrylic Resin Films Cast from Aqueous Dispersions and Organic Solutions. Drug Dev Ind Pharm. 1993;19(3):315–32. [Google Scholar]
- 93.Repka MA, ElSohly MA, Munjal M, Ross SA. Temperature stability and bioadhesive properties of delta9-tetrahydrocannabinol incorporated hydroxypropylcellulose polymer matrix systems. Drug Dev Ind Pharm. 2006 Jan;32(1):21–32. doi: 10.1080/03639040500387914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Repka MA, Prodduturi S, Stodghill SP. Production and characterization of hot-melt extruded films containing clotrimazole. Drug Dev Ind Pharm. 2003 Aug;29(7):757–65. doi: 10.1081/ddc-120021775. [DOI] [PubMed] [Google Scholar]
- 95.Repka MA, Repka SL, McGinity JW, inventors. US Patent 6,375,963, assignee. Bioadhesive hot-melt extruded film for topical and mucosal adhesion applications and drug delivery and process for preparation thereof. 2002
- 96.Repka MA, Mididoddi PK, Stodghill SP. Influence of human nail etching for the assessment of topical onychomycosis therapies. Int J Pharm. 2004 Sep 10;282(1–2):95–106. doi: 10.1016/j.ijpharm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 97.Rothen-Weinhold A, Oudry N, Schwach-Abdellaoui K, Frutiger-Hughes S, Hughes GJ, Jeannerat D, et al. Formation of peptide impurities in polyester matrices during implant manufacturing. Eur J Pharm Biopharm. 2000 May;49(3):253–7. doi: 10.1016/s0939-6411(00)00066-7. [DOI] [PubMed] [Google Scholar]
- 98.Witt C, Mader K, Kissel T. The degradation, swelling and erosion properties of biodegradable implants prepared by extrusion or compression moulding of poly(lactide-co-glycolide) and ABA triblock copolymers. Biomaterials. 2000 May;21(9):931–8. doi: 10.1016/s0142-9612(99)00262-8. [DOI] [PubMed] [Google Scholar]
- 99*.van Laarhoven JA, Kruft MA, Vromans H. In vitro release properties of etonogestrel and ethinyl estradiol from a contraceptive vaginal ring. Int J Pharm. 2002 Jan 31;232(1–2):163–73. doi: 10.1016/s0378-5173(01)00900-0. Demonstrated the application of HME in developing a structurally and functionally complicated dosage form. [DOI] [PubMed] [Google Scholar]