Abstract
Background
Impaired response inhibition and poor impulse control are hallmarks of the manic phase of bipolar disorder but are also present during depressive and, to a lesser degree, euthymic periods. The neural mechanisms underlying these impairments are poorly understood, including how mechanisms are related to bipolar trait or state effects.
Methods
One-hundred four unmedicated participants with bipolar mania (BM) (n = 30), bipolar depression (BD) (n = 30), bipolar euthymia (BE) (n = 14), and healthy control subjects (n = 30) underwent functional magnetic resonance imaging during emotional and nonemotional go/no-go tasks. The go/no-go task requires participants to press a button for go stimuli, while inhibiting the response to no-go trials. In separate blocks, participants inhibited the response to happy faces, sad faces, or letters.
Results
The BE group had higher insula activity during happy face inhibition and greater activity in left inferior frontal gyrus during sad face inhibition, demonstrating bipolar trait effects. Relative to the BE group, BD and BM groups demonstrated lower insula activity during inhibition of happy faces, though the depressed sample had lower activity than manic patients. The BD and BM groups had a greater response to inhibiting sad faces in emotion processing and regulation regions, including putamen, insula, and lateral prefrontal cortex. The manic group also had higher activity in insula and putamen during neutral letter inhibition.
Conclusions
These results suggest distinct trait- and state-related neural abnormalities during response inhibition in bipolar disorder, with implications for future research and treatment.
Keywords: Bipolar disorder, depression, emotion regulation, fMRI, impulsivity, mania
Bipolar disorder is a debilitating mood disorder with unpredictable cycles of depressive (bipolar depression [BD]) and manic episodes (bipolar mania [BM]) interspersed with variable lengths of euthymia (bipolar euthymia [BE]). Individuals with bipolar disorder demonstrate a range of inhibitory deficits across mood states, including euthymia (1– 4). While impulsivity may be most pronounced during mania (1,4), depressive symptoms have also been related to impulse control deficits, particularly in the context of attention and executive functioning (3). Therefore, it is important to distinguish how brain activity during emotional inhibition is related to the disorder itself (trait effects) or to manic and depressive phases (state effects).
Impaired response inhibition to emotional stimuli could be due to exaggerated responses to emotional stimuli, an impairment of regulatory mechanisms that control emotional responses, or a combination of the two. Very few functional magnetic resonance imaging (fMRI) studies have concurrently studied all three bipolar phases, which is necessary to separate trait- and state-related neural abnormalities. In one such study, healthy individuals had higher amygdala, temporal pole, and orbitofrontal cortex (OFC) activation while viewing faces than BM, BD, and BE groups, indicating a trait-level abnormality (5). State-related increases in amygdala activity were present in depressed participants and decreases in lower right dorsolateral prefrontal cortex (PFC) activity were seen in manic patients. During cognitive tasks, blunted prefrontal activation has been reported across all mood states, relative to control subjects (6,7). In a color Stroop task, increased left ventral PFC activation was seen in BD, while lower right ventral PFC activation was present in BM. These results highlight the importance of both trait- and state-dependent modulations of brain activity and, more specifically, provide evidence that both manic and depressive states may be associated with modified top-down cortical activity during cognitive inhibition.
To examine behavioral inhibition and related neural abnormalities, the go/no-go paradigm has been extensively utilized in animal and human studies (8 –10). The go/no-go paradigm employs a continuous series of go cues to which subjects rapidly respond and no-go cues for which subjects must withhold a response. While the classic go/no-go task utilizes letters as stimuli, the emotional go/no-go paradigms aim to investigate response inhibition to emotional words or faces (11–13). In manic participants, medial prefrontal and anterior cingulate cortex activity increased during inhibition of responses to happy words (14); sad distracters were also related to higher prefrontal activity, although more laterally (14). In addition, orbitofrontal activity was greater in euthymia during inhibition of emotional faces (13), indicating a potential neural trait of bipolar disorder.
In addition to emotional inhibition, using both happy and sad faces with an emotional go/no-go paradigm provides information regarding positive and negative emotional biases (i.e., increased attention or neural activity for stimuli of a certain valence). While emotional biases in mood disorders are associated with mood-congruent processing of emotional information (10,15–21), research regarding emotional biases in phases of bipolar disorder has provided conflicting results (10,22). Murphy et al. (10) reported biases consistent with mood, with manic patients biased toward positive stimuli and depressed patients biased toward negative information (10). However, other reports have failed to replicate this finding (22) and further research is needed in this area.
A recurrent difficulty in psychiatric neuroimaging research is that psychotropic medication may influence fMRI measurements. Although many studies have found no significant effect of medication (13,23,24), other studies have reported medication effects on brain activation in response to emotional stimuli (25–28) in individuals with bipolar disorder. To eliminate the potential confound of medication, this study investigated currently unmedicated participants in all three phases of bipolar disorder. This study investigated brain activity during processing of emotional and nonemotional cues using the go/no-go paradigm. We hypothesized that participants with bipolar disorder would demonstrate trait-level decreased activity in cortical emotional control regions, particularly in OFC, lateral PFC, and anterior cingulate cortex during emotional response inhibition. Furthermore, in manic and depressed groups, we expected state-specific mood-regulating difficulties to be reflected by a failure to inhibit the emotional response to mood-congruent emotional faces (e.g., BD with sad faces), resulting in a greater response in limbic regions (insula, amygdala, putamen) during the inhibition of mood-congruent faces.
Methods and Materials
Subjects
Medication-free BD, BM, and BE outpatients aged 18 to 60 were recruited from the Outpatient Psychiatry Clinic at University Hospital at the Indiana University School of Medicine and by community advertisements. Closely matched healthy subjects were recruited via advertisement. All subjects signed an informed consent form approved by the local Institutional Review Board and were paid $75 for screening and $75 for the magnetic resonance imaging scan.
All subjects underwent a detailed, structured diagnostic interview—the Diagnostic Interview for Genetic Studies (29), which generated DSM-IV diagnoses. Illness characteristics, including psychiatric history, were recorded (Table S1 in Supplement 1). Quantification of prior mood episodes was estimated from self-reported frequency and duration of episodes. Subjects were rated on the Hamilton Depression Rating Scale (HDRS) (30) and Young Mania Rating Scale (YMRS) (31) at the time of the baseline scan.
Participants were included if they met full lifetime DSM-IV criteria for bipolar disorder (type I or II; Table S1 in Supplement 1). Individuals were in the hypomanic or manic (YMRS > 12), depressed (HDRS > 15), or euthymic (YMRS < 10, HDRS < 12) phase. These scores are consistent with symptom levels present in unmedicated bipolar outpatients, who may have slightly less severe mania or depression than traditional research samples. Patients demonstrating symptoms consistent with a mixed state (high YMRS and HDRS scores) were excluded. Importantly, subjects were excluded if they had used psychotropic medication within the last 2 weeks or fluoxetine within the last 4 weeks. In addition, individuals could not meet present or lifetime DSM-IV criteria for schizophrenia, schizoaffective disorder, or an anxiety disorder (primary diagnosis); were not acutely suicidal or homicidal or requiring inpatient treatment; and did not reach DSM-IV criteria for substance abuse or dependence within the past year, except nicotine.
Healthy subjects had no self-reported personal or family history of psychiatric illness, neurologic illness, or substance abuse. All subjects denied alcohol use within a week of the scan session and did not have a positive urinary toxicology screening at baseline.
Functional Magnetic Resonance Imaging Procedure
Participants underwent a high-resolution three-dimensional magnetization prepared rapid acquisition gradient-echo scan on a 3T Tim Trio scanner (Siemens, Erlangen, Germany). This high-resolution (1.0 × 1.0 × 1.2 mm3 voxels) anatomical volume was comprised of 160 sagittal slices. During functional activation scans, the blood oxygen level-dependent (BOLD) signal was acquired using a T2*-weighted gradient-echo echo planar imaging sequence (129 measurements, repetition/echo time 2250/29 msec, 39 slices to cover whole brain, field of view 220 × 220 mm; 2.5 × 2.5 × 3.5 mm3 voxels). An integrated parallel acquisition technique reduction factor of 2 was implemented with a generalized autocalibrating partially parallel acquisition to improve spatial resolution and to reduce geometric distortion and scan time.
Task Description
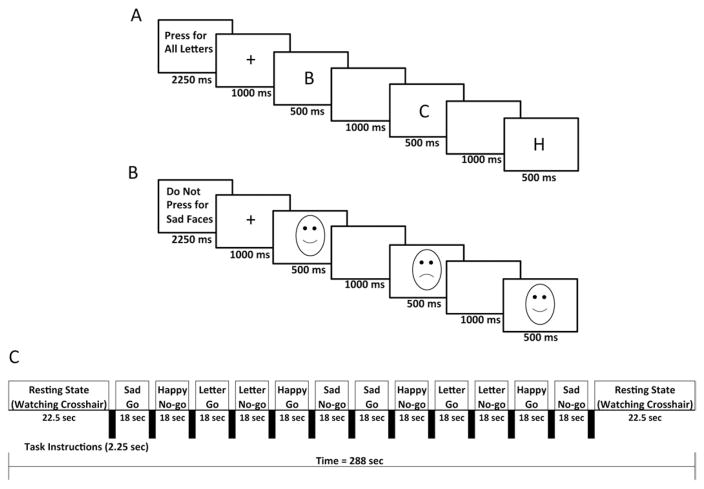
Subjects were trained on the go/no-go task before their scan. The task design was identical to that described by Shafritz et al. (32), using the Ekman and Friesen (33) Pictures of Facial Affect. This task consists of alternating go and no-go blocks between beginning and ending fixation blocks (Figure 1). There were two go and two no-go blocks each for sad faces, happy faces, and letters. During go blocks, participants were directed to press a button for each presented stimulus (face or letter), with only a single stimulus type (happy face, sad face, letter) presented. For no-go blocks, subjects were instructed to press a button for go trials (targets), while withholding a response to no-go trials (nontargets). Presented stimuli during no-go blocks were evenly divided between targets and nontargets. For the inhibition of sad faces (sad no-go), subjects were instructed to press only for happy faces and inhibit presses to sad faces. Similarly, for the happy no-go condition, instructions were to press the button only for sad faces (i.e., inhibit presses to happy faces). For the nonemotional condition (letter no-go), subjects were requested to withhold a response only for the letter X. There were 12 trials per block, with the stimulus presented for 500 milliseconds followed by a black screen for 1000 milliseconds to record the response. Before each condition, subjects were shown an instruction screen for 2.25 seconds.
Figure 1.
Functional magnetic resonance imaging paradigm. Examples of presented instructions and stimuli for (A) letter go and (B) sad no-go blocks. The paradigm for the entire run is shown below (C).
Behavioral Analysis
To examine potential performance differences between groups, go and no-go accuracy scores and go reaction times were compared via one-way analysis of variance (ANOVA) with IBM SPSS Statistics, Release 19.0 (IBM, Armonk, New York). Post hoc tests on significant results were planned via Tukey’s honestly significant difference test.
Image Analysis
Imaging data were preprocessed and analyzed with standard procedures using Statistical Parametric Mapping software (SPM5, Wellcome Trust Centre for Neuroimaging, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm5). No subject included had head movement exceeding 3 mm of translation or 4° rotation in any direction. The six motion parameters were used as covariates in the analysis to account for residual motion confounds.
Each participant’s functional images were aligned and co-registered to the structural magnetization prepared rapid acquisition gradient-echo volume. After normalization to a standard Montreal Neurological Institute template with 2 mm isotropic voxels, functional data were smoothed with a 6 mm full-width at half maximum Gaussian kernel. To perform individual analyses, a delayed boxcar function was convolved with each stimulus type (fixation, instructions, and conditions) to create a general linear model to model the BOLD response. A beta coefficient was calculated for each condition of interest (happy go, happy no-go, sad go, sad no-go, letter go, letter no-go, rest) that reflected its contribution to the BOLD time series. Next, group effects were obtained using second-level analyses within SPM5, utilizing beta coefficients for each condition.
To quantify brain activity during response inhibition, no-go conditions were contrasted with go conditions within and between groups. The appropriate contrast for each emotional no-go condition was the go condition of the opposite valence (e.g., sad no-go –happy go), to best distinguish the inhibition of each particular valence. In sad no-go – happy go, for instance, both blocks require pressing a button for happy faces. However, because sad no-go has the additional requirement of monitoring for and inhibiting responses to sad faces, the contrast of sad no-go – happy go is assumed to solely reflect emotional inhibition processes. Finally, to examine potential valence-dependent differences in inhibition-related activity between diagnostic groups, the two emotional inhibition contrasts were compared to each other ([sad no-go – happy go] vs. [happy no-go – sad go]). For between-group contrasts, one-way analyses of covariance (ANCOVAs) (controlling for age, gender, reaction time, and accuracy) were conducted to test for a main effect of diagnostic group. Post hoc analyses of go only blocks were also conducted (Supplement 1), to ensure go blocks were not driving significant results in no-go – go contrasts.
From ANCOVA tests, significant clusters indicating a main effect of diagnostic group on each contrast were identified. To clarify and visualize between-group differences, mean contrast data (i.e., beta coefficients) of each subject were extracted from these clusters for the relevant contrast and analyzed within SPSS. Main effects solely from go blocks were also tested within these contrasts, via similar ANCOVA tests. Significant no-go – go contrasts were only included if go coefficients were not significant on their own. For post hoc between-group tests, Tukey’s honestly significant difference was employed to control for multiple comparisons while identifying specific group differences (e.g., BD vs. BM). Because the BE group was smaller, Levene’s test of equality of variance was performed to ensure that assumptions of equal variances were not violated.
For whole-brain analyses, Monte Carlo simulations using AFNI (National Institute for Mental Health, Bethesda, Maryland) (34) software program 3dClustSim were utilized, determining a cluster of 108 voxels corrected for multiple comparisons at p < .05, with a Z-transformed peak > 3 (uncorrected).
For a priori hypotheses, a predefined anatomical mask was created using Montreal Neurological Institute atlas-defined regions involved in regulating mood and responding to emotional stimuli: amygdala, hippocampus, cingulate cortex, insula, orbitofrontal cortex, thalamus, caudate, and putamen. Monte Carlo simulations established smaller cluster thresholds separately for each a priori region (corrected p < .05 for the smaller volumes). At least 16 contiguous voxels significant at p < .01 were required for identifying a cluster as significant within the mask to further reduce false positives. This threshold is in line with previous work (13), although in larger regions more stringent thresholds were required for appropriate corrections (cingulate: 43 voxels; OFC: 26 voxels; insula: 23 voxels).
Results
One hundred twenty-one subjects passed screening criteria. Two participants with bipolar disorder were excluded because mood symptoms changed from screening to scan day. Four were excluded due to excessive motion or other problems in image acquisition, all from bipolar groups. Groups closely matched for age and gender were created from the remaining sample, with a total of 30 BM, 30 BD, 14 BE, and 30 healthy control (HC) participants. Groups did not significantly differ in mean age, gender, or racial makeup (Table S1 in Supplement 1). In addition, the three bipolar groups did not differ in clinical or medication history. By design, the BD group had higher HDRS scores and the BM group had higher YMRS scores (all ANOVA and post hoc t test p < .001). As expected, this unmedicated sample had more lifetime mood episodes than typically seen in research samples comprised of medicated participants. Because we recruited unmedicated outpatients, most patients in the mania subgroup (27/30) were in the hypomanic phase.
Behavioral Results
Diagnostic groups did not differ on most response time and accuracy measures (Table S2 in Supplement 1). Although a significant main effect of accuracy was found for the happy go condition and go trials during letter no-go blocks, assumptions of ANOVA tests were violated since several groups had no variance (100% accuracy). Furthermore, post hoc tests revealed no significant between-group effects.
Imaging Within-Group Results
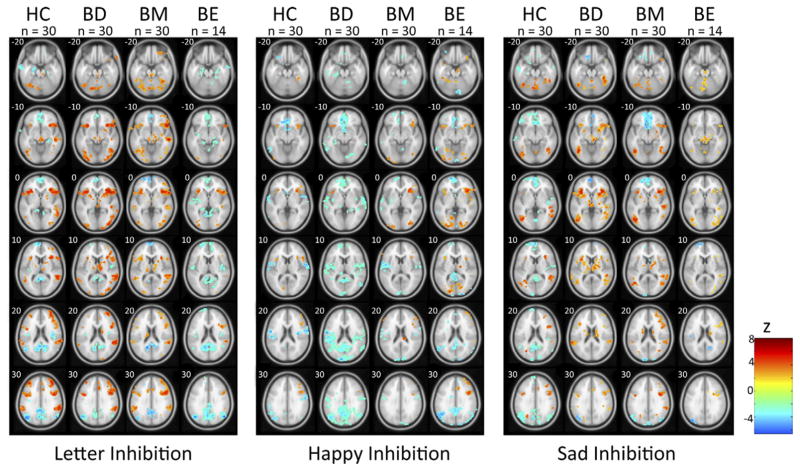
Brain activation during emotional inhibitory processing, as revealed by contrasts of no-go and go blocks, was consistent with prior research showing increased lateral prefrontal and insula activity during response inhibition (Figure 2). These regions consistently showed greater activity during no-go blocks, regardless of stimulus content.
Figure 2.
Group activation during response inhibition. Significant activity within healthy control (HC), bipolar depressed (BD), bipolar manic (BM), and bipolar euthymic (BE) groups is depicted. For HC, BD, and BM groups, voxels significant at p < .005 and within clusters of at least 76 voxels to correct for multiple comparisons (p < .05) are depicted. Due to smaller size of BE group, individual voxel threshold is lowered to p < .01 for graphical purposes.
Imaging Between-Group Results
See Table 1.
Table 1.
Bipolar State Effects During Response Inhibition (No-Go – Go) of Happy Faces, Sad Faces, or Letters
| Main Effects | Post Hoc Significant Comparisonsa | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Region | BA | MNI Peak | Z | Cluster | BE vs. HC | BD | BM |
| Happy Inhibition | |||||||
| L dorsal insula | 43 | (−54, −6, 16) | 3.48 | 112 | BE > HC | BD < BE | BM < BE |
| R sup temp gyrus/insula | 13/22 | (54, −6, 2) | 4.23 | 126 | BD < BM, BE, HC | ||
| R sup temp sulcus | 22 | (36, −40, 4) | 3.54 | 112 | BD < BE | BM < BE | |
| Sad Inhibition | |||||||
| L inf frontal gyrus | 47 | (−46, 36, −10) | 3.06 | 42 | BE > HC | BD > HC | BM > HC |
| R insula | 13 | (42, 8, −12) | 3.66 | 27 | BD > HC | BM > HC | |
| R insula | 13 | (40, −18, 4) | 4.35 | 49 | BD > HC | BM > HC | |
| R putamen | — | (30, 2, −6) | 4.47 | 104 | BD > BE, HC | BM > BE, HC | |
| Letter Inhibition | |||||||
| R insula | 13 | (44, −10, 10) | 3.27 | 24 | BM > BE | ||
| L putamen | — | (−30, −12, −10) | 3.62 | 20 | BM > BE, HC | ||
| Sad Versus Happy Inhibition | |||||||
| R medial orbitofrontal cortex | 10 | (10, 62, −8) | 3.26 | 28 | BE > HC | BD < BE | |
| L inf frontal gyrus | 47 | (−34, 36, −12) | 3.29 | 74 | BD > HC | ||
| R putamen | — | (30, 12, −2) | 3.40 | 105 | BD > BE, HC | ||
| R putamen | — | (28, 2, −6) | 3.41 | 38 | BD > BE, HC | BM > BE | |
| L putamen | — | (−30, −16, 6) | 3.12 | 24 | BD > BE | ||
| L precuneus | 30 | (−26, −50, 16) | 4.00 | 179 | BM < BD, HC | ||
Clusters of significant activity represented, with voxel of peak activation (with peak Z-score) in MNI coordinates.
BA, Brodmann area; BD, bipolar depression; BE, bipolar euthymia; BM, bipolar mania; HC, healthy control subjects; inf, inferior; L, left; MNI, Montreal Neurological Institute; R, right; sup, superior; temp, temporal. Contrasts are defined as Happy Inhibition (Happy No-go – Sad Go); Sad Inhibition (Sad No-go –Happy Go); Letter Inhibition (Letter No-go – Letter Go); and Sad Versus Happy Inhibition ([Sad No-go – Happy Go] – [Happy No-go – Sad Go]).
Post hoc comparisons were performed on mean activity within significant main effect clusters, with a Tukey honestly significant difference correction for p < .05, to clarify the nature of group differences.
Happy Face Inhibition
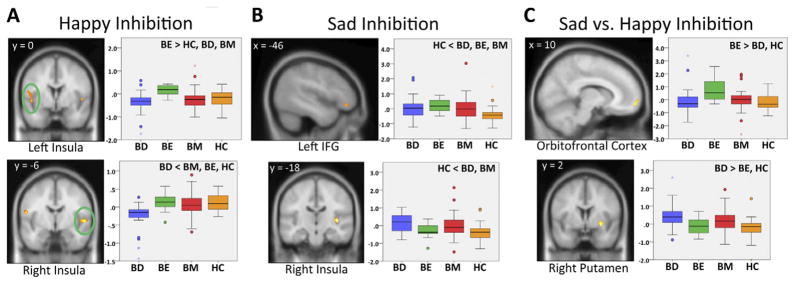
During the happy no-go condition, a main effect for diagnosis was present in bilateral insula (Figure 3A) and right temporal cortex. Post hoc tests revealed increased left insula activation in the BE group compared with the HC group. The BD group had lower neural activation than all other groups in a cluster encompassing right insula and superior temporal cortex. Both the BM and BD groups showed lower activity than the BE group in additional insula and temporal clusters.
Figure 3.
Group activation differences during inhibition of happy faces (happy no-go – sad go) (A), inhibition of sad faces (sad no-go – happy go) (B), and sad inhibition vs/happy inhibition ([sad no-go – happy go] – [happy no-go – sad go]) (C). Depicted voxels demonstrate a main effect of diagnosis at p < .01, with cluster-size thresholds correcting for multiple comparisons (p < .05). Boxplots represent mean activation of all voxels within the cluster. BD, bipolar depressed; BE, bipolar euthymic; BM, bipolar manic; HC, healthy control group; IFG, inferior frontal gyrus.
Sad Face Inhibition
A main effect of diagnostic group was demonstrated in bilateral insula and right putamen among a priori regions (Figure 3B). In addition, a significant effect was found in left inferior frontal gyrus (IFG). Post hoc results showed increased left IFG activation in the BE group compared with the HC group. Both the BD and BM groups exhibited increased activation in right putamen, right insula, and left IFG compared with the HC group and increased activation of right putamen compared with the BE group.
Letter Inhibition
During inhibition of letters, a group main effect was seen in the right insula and left putamen in the a priori network. The BM group exhibited higher activation compared with the BE group in these regions and greater activation of left putamen only compared with the HC group.
Sad Face Inhibition Versus Happy Face Inhibition
In this contrast, greater activity indicates a greater neural response to inhibiting sad faces relative to the response during happy face inhibition. Among a priori regions, a significant diagnostic group effect was seen in right medial orbitofrontal cortex and bilateral putamen. The left IFG and left precuneus also demonstrated an effect of diagnosis. Post hoc tests revealed that the BE group demonstrated greater activity during sad face inhibition (relative to happy) in medial OFC compared with HC and BD groups (Figure 3C). In general, the BD group demonstrated greater activity while inhibiting sad faces, with a higher such response in lateral prefrontal, insula, and putamen compared with BE and HC groups. Relative to BM patients, the BD group had higher sad inhibition-related activity in the left precuneus. The BM group showed increased right putamen activity compared with the BE group and less activation of the left precuneus relative to the HC group.
Discussion
This investigation is the first to study neural activity during emotional response inhibition across all three phases of bipolar disorder (i.e., mania, depression, euthymia). Because clinically significant impairments central to all phases of bipolar disorder relate to difficulties in controlling emotional responses, it is important to understand the neural mechanisms driving these emotion regulation abnormalities. By examining brain activation associated with emotional response inhibition, one aspect of emotion regulation, this study attempted to differentiate neural functioning in bipolar disorder as a trait and across all bipolar mood states.
Trait Effects
Euthymic participants had abnormal insula, prefrontal, and orbitofrontal activity during inhibition of emotional faces relative to healthy control subjects, indicating trait effects of bipolar disorder separate from any active mood symptoms. Notably, these increases in top-down cortical regions were present while inhibiting emotional information but not while inhibiting cognitive stimuli, indicating that abnormal inhibitory neural processes in bipolar disorder are dependent on emotional content. To our knowledge, no prior reports implicate these prefrontal cortical regions as trait-related neural markers of emotional inhibition in bipolar disorder; thus, replication of these findings is needed.
A similar task has shown higher OFC activity in euthymic patients during inhibition of both happy and sad faces combined (13), although happy and sad inhibition were not separately contrasted in the prior study. Other studies, however, have reported lower orbitofrontal activity in bipolar disorder across mood states (5,35–37) during a variety of cognitive and emotional tasks. Here, we found that bipolar trait-related modifications in OFC activity were dependent on facial valence. This array of findings indicates that future research needs to delineate the precise role of orbitofrontal regions in regulating emotional activity in bipolar disorder.
State Effects
In general, the BD and BM groups demonstrated similar activity during emotional face inhibition, particularly for sad faces. Both samples generally had lower activity than the BE group throughout mood-regulating regions during happy inhibition and greater activity than HC or BE groups during sad face inhibition. These similarities reflect some consistency in neural activation patterns during emotional inhibition between manic and depressed phases.
However, several notable differences were present between BD and BM groups. During happy face inhibition, the depressed sample demonstrated lower activity than manic patients in right insula, which has a known role in processing emotional information (38–40), potentially playing a role in integrating subcortical and cortical emotional processes. During depressed states, individuals may demonstrate mood-congruent biases toward negative information (10,41). Thus, inhibiting positive information from happy faces is consistent with prior literature on biases in emotional processing in depression. Decreased insula activation for the BD group may drive impairments in recognizing or responding to positive facial cues. In addition, when contrasting sad versus happy inhibition (Table 1), the BD group demonstrated more consistent and widespread activation differences from the BE and HC samples than did the BM group, although significant differences directly between BD and BM samples were limited. Therefore, in BD, neural responses during emotional inhibition may depend more on the emotional valence of cues, which would indicate a greater potential for an affective bias during BD compared with BM or BE.
The BM group, however, did not demonstrate brain responses as closely aligned with potential affective biases, instead responding similarly to the BD group, albeit with less consistent decreases in happy inhibition activity. However, BM brain activity was increased during letter response inhibition in emotional processing regions (insula and putamen). These data may reflect the overall inhibitory deficits of manic patients (10,42,43), which increase brain activity in emotion generating regions even during nonemotional tasks (44).
In similar samples, facial recognition tasks have elicited effects of bipolar phase in OFC, amygdala, anterior cingulate, and insula (5,45), underscoring the relevance of corticolimbic mood-regulating circuitry to state and trait characteristics, during both emotional inhibition/control and recognition.
The lack of differences in task performance may be due to the limited number of trials in the paradigm, as previous research has revealed performance biases in both manic and depressed patients (10,41,46). However, other studies have found no such effects on emotional attention (22,47,48), meaning that these neuropsychological measures may not be sufficiently sensitive to detect subtle biases in bipolar disorder (49), which may be better revealed by neuroimaging methods (41,46). Conversely, these discrepancies may represent a fundamental difference between how environmental input is perceived and how it is regulated.
Although group contrasts corrected for cluster size within each a priori region of interest (ROI), the use of a large number of ROIs may have impacted the significance of presented results. Given the need to balance issues of type I and type II error concerns (50), the strength of the present results is best confirmed via independent replication, with a particular focus on prefrontal cortex, insula, and putamen.
One limitation of the study is that while we can statistically define state and trait effects by how bipolar groups differ from each other or from control subjects, in reality the distinction is murkier. Because state and trait effects are additive, nonsignificant differences of both state and trait effects may combine to produce a significant effect that is solely associated with the manic or depressed state.
Another limitation lies in the implementation and interpretation of the emotional go/no-go paradigm. While the task provides a way to examine inhibition of emotional stimuli distinct from traditional cognitive go/no-go paradigms, there are inherent difficulties to testing emotional control. First, while we attempted to isolate the effects of response inhibition by contrasting no-go blocks with go blocks, this contrast could not completely account for discrepancies in motor responses or the number of faces of a certain valence. We did exclude regions likely driven by differences in face viewing, identified as those clusters with significant effects of diagnosis for any go blocks (Table S3 in Supplement 1). However, in future studies, an event-related design may be better to specifically measure inhibitory processes. Next, the letter go/no-go task represents a cognitive task, rather than a neutral face task, so direct contrasts with emotional inhibition tasks were not appropriate. However, such a task would need to be fundamentally different (with responses focused on a nonemotional cue, such as gender) and the letter go/no-go task presented as a well-validated fMRI task for qualitative comparisons of state and trait effects.
The focus on currently unmedicated individuals may have created selection biases. There are many reasons why individuals with bipolar disorder could be off medication; yet, detailed interviews confirmed that individuals were indeed mentally ill and functionally impaired. Nonetheless, it is possible that medicated patients differ in illness degree or characteristics. Because unmedicated individuals are greatly understudied in psychiatric neuroimaging, the present results are particularly valuable. However, the generalizability to medicated patients is unclear, and findings should be integrated with neuroimaging studies of medicated bipolar patients across all three mood states.
Some euthymic subjects may have had mild subthreshold depressive symptoms, but this reflects the real life situation for unmedicated bipolar subjects. However, there was significant separation between the euthymic and depressive groups in terms of depressive symptomatology (Table 1).
In this investigation, the BE group was smaller than the BD and BM groups, due to greater difficulty in recruiting unmedicated euthymic patients. This lack of balance may have influenced between-group comparisons by introducing greater noise within the BE group, although variance did not differ between groups in ROI clusters. Future investigations should likewise attract large samples, particularly in unmedicated euthymic samples, and can include targeted regression analyses to associate symptom levels with neural activity. Future work with more participants with bipolar disorder, type I, can also address potential differences in neural activity related to bipolar subtype (I vs. II). While no significant group differences were present in this study in bipolar types I or II diagnoses, adequately powered samples are necessary to address this question.
Despite these limitations, this study provides strong evidence for the presence of both trait and state effects of bipolar disorder on neural activity during emotional response inhibition. These findings need to be replicated and investigated further in future studies.
Supplementary Material
Acknowledgments
This project was funded by the National Institute of Mental Health to AA (R01MH075025). The effort of LAH was funded by the National Institute on Drug Abuse (K12DA000357). None of the authors report any biomedical financial interests or potential conflicts of interest that are relevant to the conduct of this study.
Footnotes
Supplementary material cited in this article is available online.
The following does not pertain to the conduct of this study but is provided in the spirit of full disclosure. In the past 2 years, Dr. Anand has served on the advisory board of AstraZeneca, Pfizer pharmaceuticals, Merck Pharmaceuticals and Dey Pharmaceuticals. Dr. Anand has been a speaker for Merck pharmaceuticals. Dr. Anand has received grant support from Astra-Zeneca and Lilly Pharmaceuticals.
References
- 1.Swann AC, Moeller FG, Steinberg JL, Schneider L, Barratt ES, Dougherty DM. Manic symptoms and impulsivity during bipolar depressive episodes. Bipolar Disord. 2007;9:206–212. doi: 10.1111/j.1399-5618.2007.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG. Impulsivity and phase of illness in bipolar disorder. J Affect Disord. 2003;73:105–111. doi: 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- 3.Swann AC, Steinberg JL, Lijffijt M, Moeller FG. Impulsivity: Differential relationship to depression and mania in bipolar disorder. J Affect Disord. 2008;106:241–248. doi: 10.1016/j.jad.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strakowski SM, Fleck DE, DelBello MP, Adler CM, Shear PK, Kotwal R, Arndt S. Impulsivity across the course of bipolar disorder. Bipolar Disord. 2010;12:285–297. doi: 10.1111/j.1399-5618.2010.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Schot A, Kahn R, Ramsey N, Nolen W, Vink M. Trait and state dependent functional impairments in bipolar disorder. Psychiatry Res. 2010;184:135–142. doi: 10.1016/j.pscychresns.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, et al. A functional magnetic resonance imaging study of bipolar disorder: State- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601– 609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 7.Townsend J, Bookheimer SY, Foland-Ross LC, Sugar CA, Altshuler LL. fMRI abnormalities in dorsolateral prefrontal cortex during a working memory task in manic, euthymic and depressed bipolar subjects. Psychiatry Res. 2010;182:22–29. doi: 10.1016/j.pscychresns.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishkin M, Pribram KH. Analysis of the effects of frontal lesions in monkey. I. Variations of delayed alternation. J Comp Physiol Psychol. 1955;48:492– 495. doi: 10.1037/h0040318. [DOI] [PubMed] [Google Scholar]
- 9.Costantini AF, Hoving KL. The relationship of cognitive and motor response inhibition to age and IQ. J Genet Psychol. 1973;123:309–319. doi: 10.1080/00221325.1973.10532690. [DOI] [PubMed] [Google Scholar]
- 10.Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 11.Schulz KP, Fan J, Magidina O, Marks DJ, Hahn B, Halperin JM. Does the emotional go/no-go task really measure behavioral inhibition? Convergence with measures on a non-emotional analog. Arch Clin Neuropsychol. 2007;22:151–160. doi: 10.1016/j.acn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59:597– 604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- 13.Wessa M, Houenou J, Paillere-Martinot M-L, Berthoz S, Artiges E, Leboyer M, Martinot JL. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry. 2007;164:638– 646. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- 14.Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York: Guilford Press; 1979. [Google Scholar]
- 16.Elliott R, Zahn R, Deakin JFW, Anderson IM. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology. 2011;36:153–182. doi: 10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42:241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- 18.Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, et al. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- 20.Coupland NJ, Sustrik RA, Ting P, Li D, Hartfeil M, Singh AJ, Blair RJ. Positive and negative affect differentially influence identification of facial emotions. Depress Anxiety. 2004;19:31–34. doi: 10.1002/da.10136. [DOI] [PubMed] [Google Scholar]
- 21.Joormann J, Gotlib IH. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. J Abnorm Psychol. 2006;115:705–714. doi: 10.1037/0021-843X.115.4.705. [DOI] [PubMed] [Google Scholar]
- 22.Kerr N, Scott J, Phillips ML. Patterns of attentional deficits and emotional bias in bipolar and major depressive disorder. Br J Clin Psychol. 2005;44:343–56. doi: 10.1348/014466505X57755. [DOI] [PubMed] [Google Scholar]
- 23.Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: Evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry. 2001;50:651– 658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 26.Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877– 889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 27.Anand A, Li Y, Wang Y, Gardner K, Lowe MJ. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: An FMRI study. J Neuropsychiatry Clin Neurosci. 2007;19:274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal fMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- 29.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849– 859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863– 864. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56– 62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429– 435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 32.Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage. 2006;31:468– 475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 33.Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- 34.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 35.Altshuler L, Bookheimer S, Townsend J, Proenza MA, Sabb F, Mintz J, Cohen MS. Regional brain changes in bipolar I depression: A functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altshuler LL, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, et al. Blunted activation in orbitofrontal cortex during mania: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Kruger S, Alda M, Young LT, Goldapple K, Parikh S, Mayberg HS. Risk and resilience markers in bipolar disorder: Brain responses to emotional challenge in bipolar patients and their healthy siblings. Am J Psychiatry. 2006;163:257–264. doi: 10.1176/appi.ajp.163.2.257. [DOI] [PubMed] [Google Scholar]
- 38.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 40.Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, et al. The insula is not specifically involved in disgust processing: An fMRI study. Neuroreport. 2002;13:2023–2026. doi: 10.1097/00001756-200211150-00006. [DOI] [PubMed] [Google Scholar]
- 41.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 42.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strakowski SM, Adler CM, Cerullo MA, Eliassen JC, Lamy M, Fleck DE, et al. Magnetic resonance imaging brain activation in first-episode bipolar mania during a response inhibition task. Early Interv Psychiatry. 2008;2:225–233. doi: 10.1111/j.1751-7893.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleck DE, Eliassen JC, Durling M, Lamy M, Adler CM, Delbello MP, et al. Functional MRI of sustained attention in bipolar mania. Mol Psychiatry. 2012;17:325–336. doi: 10.1038/mp.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hulvershorn LA, Karne H, Gunn AD, Hartwick SL, Wang Y, Hummer TA, Anand A. Neural activation during facial emotion processing in unmedicated bipolar depression, euthymia, and mania. Biol Psychiatry. 2012;71:603– 610. doi: 10.1016/j.biopsych.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bermpohl F, Dalanay U, Kahnt T, Sajonz B, Heimann H, Ricken R, et al. A preliminary study of increased amygdala activation to positive affective stimuli in mania. Bipolar Disord. 2009;11:70–75. doi: 10.1111/j.1399-5618.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 47.Brotman MA, Rich BA, Schmajuk M, Reising M, Monk CS, Dickstein DP, et al. Attention bias to threat faces in children with bipolar disorder and comorbid lifetime anxiety disorders. Biol Psychiatry. 2007;61:819– 821. doi: 10.1016/j.biopsych.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 48.Rubinsztein JS, Michael A, Underwood BR, Tempest M, Sahakian BJ. Impaired cognition and decision-making in bipolar depression but no ’affective bias’ evident. Psychol Med. 2006;36:629– 639. doi: 10.1017/S0033291705006689. [DOI] [PubMed] [Google Scholar]
- 49.Wenzlaff RM, Rude SS, Taylor CJ, Stultz CH, Sweatt RA. Beneath the veil of thought suppression: Attentional bias and depression risk. Cogn Emot. 2001;15:435– 452. [Google Scholar]
- 50.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423– 428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.