Abstract
Calcium (Ca2+) is a ubiquitous second messenger that performs significant physiological task such as neurosecretion, exocytosis, neuronal growth/differentiation, and the development and/or maintenance of neural circuits. An important regulatory aspect of neuronal Ca2+ homeostasis is store-operated Ca2+ entry (SOCE) which, in recent years, has gained much attention for influencing a variety of nerve cell responses. Essentially, activation of SOCE ensue following the activation of the plasma membrane (PM) store-operated Ca2+ channels (SOCC) triggered by the depletion of endoplasmic reticulum (ER) Ca2+ stores. In addition to the TRPC (transient receptor potential canonical) and the Orai family of ion channels, STIM (stromal interacting molecule) proteins have been baptized as key molecular regulators of SOCE. Functional significance of the TRPC channels in neurons has been elaborately studied however, information on Orai and STIM components of SOCE, although seems imminent, is currently limited. Importantly, perturbations in SOCE have been implicated in a spectrum of neuropathological conditions. Hence, understanding the precise involvement of SOCC in neurodegeneration would presumably unveil avenues for plausible therapeutic interventions. We thus review the role of SOCE-regulated neuronal Ca2+ signaling in select neurodegenerative conditions.
27.1 Introduction
27.1.1 Molecular Determents of Ca2+ Homeostasis
Calcium (Ca2+) plays an important role in influencing virtually every aspect of cell’s life (Berridge et al. 2000; Putney 2011; Berridge 2012). Most of the cellular signaling events are orchestrated by an increase in the level of cytosolic Ca2+ ([Ca2+]cyt), which under basal condition is maintained at about 100nM (Berridge et al. 2003; Clapham 2007; Meldolesi et al. 1988; Miller 1988). The elevations in [Ca2+]cyt is largely regulated by a combination of intracellular Ca2+ release and extracellular Ca2+ influx events (Putney and Bird 2008). Intracellular compartments such as the endoplasmic reticulum (ER) and mitochondria, where the level of stored Ca2+ ranges from 0.1–1.0 mM, serve as a primary source of internal Ca2+ release (Birnbaumer 2009). On the other hand, Ca2+ channels located at the plasma membrane (PM) bring about an elevation in the cytosolic Ca2+ level by transporting Ca2+ across the membrane from the extracellular milieu, where the levels of Ca2+ is generally maintained at about 1–2 mM (Berridge et al. 2003; Clapham 2007). Following an increase in [Ca2+]cyt, the Ca2+ pumps located in the PM, ER or the Golgi complex work in concert to help in attaining a basal level of [Ca2+]cyt, either by extrusion of Ca2+ to the extracellular environment or by its sequestration into the organellar lumen. Ca2+ is intracellularly compartmentalized by ATP-driven Ca2+ pumps such as sarco-endopasmic reticulum Ca2+ ATPase (SERCA) and secretory pathway Ca2+ ATPase (SPCA) localized at the ER and Golgi complex, respectively (Guerini et al. 2005; Strehler and Treiman 2004). Yet another important organelle, which helps in sequestering Ca2+ is the mitochondrion. Ca2+ readily diffuses through large pores in the mitochondrial outer membrane but crosses through channels and transporters across the inner membrane. One such highly Ca2+-selective channel identified was the MiCa (Kirichok et al. 2004). Extrusion of [Ca2+]cyt is achieved by PM-associated pumps such as PMCA and exchangers like Na+/Ca2+ (NCX) and Na+/Ca2+-K+ (NCKX) exchangers. The NCX/NCKX exchange one Ca2+ for three Na+ (NCX) or co-transport one K+ with one Ca2+ in exchange for four Na+ (NCKX) (Guerini et al. 2005; Hilgemann et al. 2006). On the other hand, Ca2+ ATPases lower intracytoplasmic Ca2+ levels by exchanging protons for two (SERCA) or one (PMCA) Ca2+ per ATP hydrolyzed. These ATPases strive to strike a balance and maintain low steady-state [Ca2+]cyt (Brini and Carafoli 2009; Gouaux and Mackinnon 2005). In essence, this regulated flux of Ca2+ in and out of the cytosol results in [Ca2+]cyt oscillations that range from about 100nM in resting to about a 1000nM in stimulated state. These oscillatory pattern of [Ca2+]cyt is physiologically vital for the regulation of numerous signaling processes (Berridge 1997; Parekh 2011). The cellular responses mediated by Ca2+ are broadly categorized as fast or short-term responses (operates in seconds to minutes) and slow or long-term responses (that take from an hour up to several days). The fast responses such as muscle contraction, neurotransmission and neurosecretion require rapid and localized Ca2+ signals with reasonably short span of Ca2+ spikes (Putney and Bird 2008), whereas long-term cellular responses such as gene regulation, proliferation, differentiation rely on relatively slow and sustained Ca2+ signals for efficient execution (Berridge et al. 2000; Gwack et al. 2007). Hence, the onset of Ca2+ signals and the regulation of associated physiological events are spatio-temporally regulated (Berridge 1997; Berridge et al. 2003; Berridge and Dupont 1994).
27.1.2 Neuronal Store-operated Ca2+ Entry (SOCE)
In general, TRP channels are defined as non-selective cation-permeable channels since, in addition to permeating Ca2+, they also allow Na+ and Mg2+ to flow through (Zhu et al. 1996). The TRPC (TRP Canonical) sub family constitutes a total of seven TRPC proteins from TRPC1 through TRPC7 (Montell 2005b; Venkatachalam and Montell 2007). Physiological activation of TRPC channels is brought about by a PLC-mediated signaling event following the activation of PM-associated G protein-coupled receptors (GPCR) or Receptor Tyrosine Kinases (RTK), which in turn lead to the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into membrane-bound diacylglycerol (DAG) and soluble IP3. IP3 binds to IP3R, which functions as ligand-gated channel and evokes Ca2+ release from the ER into the cytoplasm through IP3R expressed on the ER membrane leading to a depletion of ER Ca2+ stores. This very store depletion triggers the activation of PM-associated Ca2+ channels and hence the phenomenon is termed SOCE (as indicated in Fig. 1). Recent advances have identified that TRPC and Orai channels could promote SOCE and are thus referred to as store-operated Ca2+ channels or SOCC (Barritt 1999; Berridge 1995; Parekh and Penner 1997; Putney 2013). Activated TRPC channels mediate transmembrane fluxes of Ca2+ down their electrochemical gradients, thereby raising intracellular Ca2+ levels. A part of the Ca2+ is pumped back into the ER by SERCA pumps to maintain a steady state Ca2+ concentration of the ER and thus preventing it from exhausting all the Ca2+ stores, which partially explains the significance of SOCE. Alternatively, increase in cytoplasmic Ca2+ levels also regulate a plethora of critical cellular functions including, but not limited to, secretion, proliferation, differentiation, apoptosis and motility (Beck et al. 2008; Bollimuntha et al. 2005a; Cai et al. 2006; Fabian et al. 2008; Pani et al. 2006; Singh et al. 2001; Selvaraj et al. 2012). More recently, the ER Ca2+ sensor STIM1 (Liou et al. 2005; Roos et al. 2005), and the PM Ca2+ channel Orai1 (Feske et al. 2006; Vig et al. 2006) have been identified as molecular components of SOCE. As depicted in Fig. 1, STIM1 has also been shown to activate TRPC channels albeit in a way distinct from that of activating Orai1 channels (Huang et al. 2006; Yuan et al. 2009; Zeng et al. 2008). Interestingly, TRPC proteins have been reported to associate with STIM1 as well as Orai1 and exist as ternary complexes (Cheng et al. 2008; Liao et al. 2008; Liao et al. 2007; Ong et al. 2007). Thus, this heterotypic association of PM-SOCC and their differential activation by STIM1 reveals yet another level of diversity in the regulation of SOCE.
Fig. 27.1.
Store-operated Ca2+ entry and neuronal function. This illustration depicts the current understanding of SOCE regulated by STIM1-mediated activation of PM-SOCC (TRPC/Orai channels). Agonist-induced activation of PM receptors (GPCR/RTK) results in the generation of the diffusible cellular messenger IP3 following the PLC-mediated hydrolysis of PIP2. IP3 binds to its receptor (IP3R) in the ER depleting the ER Ca2+ stores. This leads to STIM1 oligomerization and recruitment of the STIM1-clusters to ER-PM juxtaposed sites where STIM1 physically gates the TRPC and Orai1 channels to bring about Ca2+ entry. This raises the [Ca2+]cyt which not only aids in the SERCA pump-mediated ER store refilling but also promotes the regulation of several cellular functions. Additionally, diacylglycerol (DAG), the membrane-associated lipid messenger also has the ability to activate select TRPC channels independent of the ER store and presumably STIM1 as well.
SOCE has also been identified in many types of excitable cells and is important in maintaining Ca2+ homeostasis (Majewski and Kuznicki 2015; Sun et al. 2014). For a long time neuronal cells have been thought not to have a SOCE pathway (Friel and Tsien 1992), since these cells have the voltage-gated Ca2+ channels and the receptor activated Ca2+ channels such as glutamate and AMPA receptors as their prime Ca2+ influx components. However, many recent studies have provided evidence of functional SOCE in a variety of excitable cell types (Akbari et al. 2004; Emptage et al. 2001; Selvaraj et al. 2012). The impact of Ca2+ -release from neuronal ER stores, and subsequent activation of SOCE channels on physiological function has been best studied using various Drosophila mutant phenotypes (Banerjee et al. 2004; Montell 2005a; Venkiteswaran and Hasan 2009). These studies support the fundamental concept that IP3-mediated Ca2+ release, followed by Ca2+ entry is required in specific classes of neurons for phototransduction or other neuronal functions. This is further supported by a recent study which confirmed the functional importance of Type 1 IP3R function in mouse neuronal cells (van de Leemput et al. 2007). The existence of SOCE in neuronal cells was originally demonstrated in bovine adrenal chromaffin cells and in the PC12 cell line (Cheek and Thastrup 1989; Clementi et al. 1992) and later in other neuronal cells including the Aplyesia bag cell neurons (Philipp et al. 1998; Pizzo et al. 2001). Importantly, the relevance of SOCE channels in the development and plasticity of the nervous system was initially demonstrated by Mu-ming Poo’s group (Nishiyama et al. 2000). These results were further verified by Emptage and colleagues (Emptage et al. 2001) who showed that store depletion was essential in the opening of the SOCE channels in hippocampal synaptic boutons, and this entry was essential in modulating the rate of neurotransmitter release along with modulating the synaptic plasticity. This underscores SOCE as a critical regulator of neuronal (patho)physiology.
Ca2+ entry via the G protein-coupled mechanism has been implicated in the shaping of action potentials, synaptic transmission, and sensory transduction (Congar et al. 1997; Linden 1994). Additionally, changes in cytosolic Ca2+ is also known to regulate the motility of many cellular structures, including the axonal growth cones (Gomez et al. 2001) and dendritic filopodia of developing neurons (Lohmann et al. 2005). Thus, it can be anticipated that TRPC channels may have a significant role in regulating these fundamental neuronal processes. Indeed, Ca2+ entry through the TRPC channel has been shown to play a critical role in basic fibroblast growth factor (bFGF)-induced cortical neural stem cells (NSCs) proliferation (Fiorio Pla et al. 2005). It has been shown that activation of TRPC1 (C1), but not TRPC3 (C3) regulates cell proliferation in neural stem cells. In contrast, Ca2+ influx through C1 and C3 controlled the switch between proliferation and differentiation in immortalized hippocampal H19-7 cells (Wu et al. 2004). Similarly in mammals, C3 and C6, but not C1, have been shown to be associated with BDNF-mediated neuronal growth (Jia et al. 2007). These distinct physiological outcomes can be partially explained by the differences in spatial localization and functional activation of individual TRPC channels in diverse neuronal populations. Additionally, the differential distribution of membrane receptors (GPCRs/RTKs), concentration and availability of the desired agonist can further add to the complex regulation of distinct set of TRPC channels. Importantly, both Orai and STIM proteins are expressed in skeletal and brain tissues, albeit with different expression patters (Kraft 2015) and STIM2-deficient mice were protected from cerebral damage after ischemic stroke (Berna-Erro et al. 2009). However, another study showed that loss of STIM2 induced a massive neuronal loss in the hippocampal region (Sun et al. 2014), suggesting that STIM2 could have differential role on different cells.
27.2 Ca2+ and Neuropathophysiology
Neuronal cell injury is mediated via both increase and decrease of cytosolic Ca2+ concentration (Sattler and Tymianski 2000). Changes in intracellular Ca2+ concentration stimulate a number of intracellular events and could either trigger or inhibit the cell death process (Berridge et al. 2000; Putney 2003). Importantly, disturbances in Ca2+ homeostasis have been implicated in many neurodegenerative diseases such as, PD, AD, and HD (Albers and Beal 2000; Bollimuntha et al. 2005b; O’Bryant et al. 2009; Zuccato and Cattaneo 2007; Zuccato and Cattaneo 2009; Sun et al. 2014). It is not surprising that disturbances in Ca2+ signaling pathways underlie neuronal loss, since many factors involved in neuronal function are dependent on Ca2+ signaling (Berridge et al. 2000; Putney 2003). However, the cellular mechanism(s) underlying neurodegeneration, due to alterations in Ca2+ homeostasis, remains to be elucidated (Bezprozvanny and Mattson 2008). In addition, several factors including generation of free radicals, impairment of mitochondrial function, ER stress, and apoptosis have been proposed to be regulated by alterations in cytosolic Ca2+. Increased cytosolic Ca2+ leads to inappropriate activation of Ca2+-dependent processes, that stay inactive at low Ca2+ levels, causing metabolic derangements leading to neuronal death (Berridge et al. 2000; Bezprozvanny and Mattson 2008; Putney 2003). In contrast, decrease in ER Ca2+ can induce ER stress, which can activate cell death cascades (Ermak and Davies 2002) suggesting that controlled Ca2+ influx from external media is critical for neuronal function and its survival (Selvaraj et al. 2012). Since TRPCs are essential for replenishing and maintaining ER Ca2+, chronic depletion of ER Ca2+, as would occur in the absence of TRPC function, could influence ER-dependent processes such as protein folding and trafficking, the ER stress response, and apoptosis. In the following sections, we discuss the findings that link TRP channels and SOCE with the neurodegenerative condition.
27.2.1 SOCE Potentiates Alzheimer’s Disease
Alzheimer’s disease is a progressive and irreversible neurodegenerative disorder marked by neuronal atrophy, synapse degeneration, amyloid plaques and neurofibrillary tangles. This disease can be divided into two major forms, one that has an early onset – the Familial Alzheimer’s Disease (FAD) and the other that has a late onset – the Sporadic Alzheimer’s disease (SD). FAD accounts for less than 10% of all the AD, however, have provided clues to understand the molecular and cellular basis of this genetically complex disease. The pathogenesis of FAD has been attributed to missense mutations in one of the three principle genes encoding Amyloid Precursor Protein (APP), Presenilin 1 (PS1) and Presenilin 2 (PS2). APP is a plasma membrane protein which is processed in succession by β- and γ-secretase yielding Aβ (40 and 42) peptides based on the site of cleavage. Aβ40 is the soluble form and is secreted out, whereas Aβ42, formed from a missense mutation of APP, is an insoluble form and is retained within the PM. This then leads to the formation of Aβ aggregates in specific regions of the brain thereby causing neurodegeneration (De Strooper and Annaert 2000). PS1 and PS2 are integral membrane proteins localized in the ER and are implicated in the γ-secretase activity of APP. Interestingly; presenilins are also suggested to function as ER Ca2+ leak channels (Tu et al. 2006). Accumulating evidence over the last decade has shown a strong link between pathogenesis of AD and impaired Ca2+ homeostasis (Bezprozvanny and Mattson 2008). Since Ca2+ signaling depends on both the release of Ca2+ from the ER and the subsequent influx across the PM, dysregulation in any of these pathways can create an imbalance in the overall cellular Ca2+ homeostasis. Information obtained from mutant PS1 and PS2 transgenic mice as well as from primary cortical neurons expressing various presenillin mutations has shown that in AD, the basal ER Ca2+ stores are larger which results in an increased dynamic flow of Ca2+ into the cytosol (LaFerla 2002; Stutzmann et al. 2006). The proposed mechanism for this effect is reasoned due to the disruption of Ca2+ leak function of mutant presenillin creating an imbalance between Ca2+ leak and Ca2+ reuptake. Contrary to this ‘Ca2+ overload’ hypothesis, there are strong evidences showing no increase in Ca2+ stores, rather Ca2+ stores in PS1 and PS2 mutant phenotypes are reduced (Cheung et al. 2008; Giacomello et al. 2005; Lessard et al. 2005). It has also been shown that FAD PS1 and PS2 mutants interact with IP3R rendering it sensitive to lower IP3 concentrations, thus affecting the receptor’s gating and resulting in exaggerated [Ca2+]cyt (Cheung et al. 2008). These discrepancies could be attributed to the type of mutations on the presenillin and the cell type used, which would dictate the eventual phenotypic disparity. In another set of studies the increase in Ca2+ influx is also attributed to the increased expression of ryanodine receptors (RyR) in AD-affected cells (Stutzmann et al. 2006). However, irrespective of the content of Ca2+ stores, SOCE was abrogated with most of the presenilin mutation. One of the logical interpretations could be that since the size of the ER store always remains high, the threshold levels of store depletion required to trigger SOCE are not attained and hence a decrease in SOCE is observed. On the other hand, when PS1 function was abrogated by knock down or dominant negative strategy, there was a marked potentiation of SOCE suggesting that PS1 in general negatively regulate SOCE and their mutations create a gain-of-function phenotype that would further augment the inhibition. It is of note that the elevated [Ca2+]cyt acts proximal to the disease phenotype resulting in the enhanced secretase activity and thus capable of modulating Aβ peptides levels (Yoo et al. 2000). However, the addition of Aβ peptides to the cells expressing PS1 mutations did not show any significant increase in SOCE. Interestingly, a recent study showed that mouse embryonic fibroblast lacking presenilins had increased levels of STIM1 and decreased levels of STIM2 expression with a marked potentiation of SOCE. Expression of FAD mutants in these cells attenuated SOCE without altering the STIM expression (Bojarski et al. 2009). In contrast, a recent report showed that STIM2 is essential for the SOCE in hippocampal neurons and downregulation of STIM2 leads to massive loss of neurons. Consistent with this, samples of AD patients also showed a decrease in STIM2, but not STIM1 (Sun et al. 2014). However, still the molecular identity of the Ca2+ channel that could contribute to these results (Orai vs TRPC) is not yet established. In addition, STIM2 has been shown to actually inhibit SOCE in some cases and contribute to basal Ca2+ regulation, rather than agonist-mediated Ca2+ entry. Thus, in this context, the question of how STIM2 functions differently in neuronal vs non-neuronal cells needs further clarification.
27.2.2 Loss of SOCE Can Induce Parkinson’s Disease
Parkinson’s disease (PD) is the second most common progressive neurodegenerative disorder caused by the degeneration of a particular group of neurons in the substantia nigra pars compacta, which control motor regulation of the limbic system. As is the case with Alzheimer’s disease, this also have two forms – the less prevalent familial PD and the more prevalent sporadic PD. The pathological hallmarks of the disease are pronounced degeneration of nigrostriatal dopaminergic neurons and the presence of cytoplasmic inclusions called Lewy bodies (LBs) formed by the aggregation of several proteins including α-synuclein, parkin, ubiquitin and neurofilaments. Not all cases of PD exhibit LB pathogenesis and hence have to be diagnosed by clinical symptoms. Even though the actual cause or etiology of sporadic PD is not clearly understood, the most common modes of neuronal degeneration, as understood from gene mutations in familial PD, appear to be due to mitochondrial dysfunction, oxidative stress and impairment of the ubiquitin proteasome pathway. These effects are similar to those induced by 1-methyl, 4-phenyl, 1, 2, 3, 4-tetrapyridine (MPTP) neurotoxin and hence it is commonly used as toxin-based model to study PD (Dauer and Przedborski 2003). MPP+, the metabolite of MPTP, is preferentially taken up by dopaminergic neurons through dopamine transporter, which then gets accumulated in the mitochondria and inhibits complex I of the respiratory chain. As a result, there is a loss of mitochondrial membrane potential, depletion of ATP and increased free radical production creating oxidative stress within the cell (Przedborski et al. 2004). The free radicals thus released from the mitochondria disturb the intracellular Ca2+ homeostasis, possibly by compromising the function of Ca2+ signaling components of the ER and PM (Mattson 2007). With regard to ER store-depletion, the cell has its own way of replenishing the stores through the activation of SOCE channels. But following MPP+ treatment, TRPC1 expression is drastically decreased. As a result, the ER fails to maintain the normal Ca2+ levels required for its proper functioning, which eventually leads to ER stress (Bollimuntha et al. 2005b; Selvaraj et al. 2009; Selvaraj et al. 2012). The mitochondrion on the other hand takes up the excess Ca2+ present in the cytoplasm (released from the ER) and loses its membrane potential due to Ca2+ overload. This in turn exacerbates the free radical production thus creating a vicious cycle of oxidative stress, which initiates an intrinsic apoptotic cascade. Our findings also suggest that overexpression of TRPC1 combat some of the negative effects of MPP+-induced oxidative stress and mitochondrial dysfunction, thus providing the cells an opportunity to recover from the stressed state. In addition, autophagy has been also shown to work in conjunction or independently to help the cell cope with ER stress by removal of misfolded proteins, and autophagy has been shown to modulate by TRPC1 and Ca2+, suggesting that these could also contribute to the survival of these neurons (Sukumaran et al. 2016). In addition, dopamine and other neurotransmitters levels regulate mood disorders such as schizophrenia, anxiety, bipolar disorders and others, which could also be regulated by Ca2+ signaling; however, the role of Ca2+ channels in this regard is still not well defined.
27.2.3 A Functional Look at SOCE in Ataxia
Cerebellar ataxias represent a heterogeneous group of neurodegenerative disorders. To date, 26 different genetic subtypes of spinocerebellar ataxias (SCA) have been identified. Spinocerebellar ataxia type 1 (SCA1) is an inheritable autosomal dominant neurodegenerative disease caused by expansions of a CAG trinucleotide repeats encoding polyglutamine tract, within the SCA1 encoded ATXN1 (Ataxin 1) protein. The pathogenesis involves a gain-of-function mutation in ATXN1, which mainly affects the Purkinje cells of the cerebellum and brainstem neurons (Vig et al. 2001). Although the genetic diversity and affected cellular pathways of hereditary ataxias are broad, one common theme amongst these genes is their effects on maintaining Ca2+ balance primarily in the cerebellum (Mark et al. 2017). Moreover, it has also been shown that the polyglutamine mutation may affect the gene expression pattern and was found that certain neuronal genes involved in signal transduction and Ca2+ homeostasis were down regulated in a sequential pattern in SCA1 mice (Lin et al. 2000). Among other genes, this study identified IP3R, SERCA2, Inositol polyphosphate 5-phosphatase type 1 (5-phosphatase), and TRPC3 genes important for Ca2+ homeostasis. Importantly, IP3R and SERCA2 were down regulated in the first two weeks of age, whereas in the subsequent three to four weeks - phosphatase and TRPC3 were down regulated. These results are striking given the fact that IP3R, SERCA2 and TRPC3 constitute the molecular components of SOCE. This clearly infers that in SCA1 the ER Ca2+ homeostasis can be compromised under sustained stress conditions, which can lead to a variety of cellular dysfunctions. Additionally, spinocerebellar ataxia14 (SCA14), one of the sub types of spinocerebellar ataxias, is caused by mutations in the protein kinase C gamma (PKCγ) gene (Adachi et al. 2008). PKCγ has been found to negatively regulate TRPC3 activity by phosphorylating TRPC3, but in the case of SCA14 it fails to phosphorylate TRPC3 resulting in sustained Ca2+ influx following stimulation. Hence, the PKCγ kinase activity is seemingly indispensable for TRPC3 phosphorylation and feed back regulation of Ca2+ influx, which when fails, as in the case of SCA14, might cause imbalance in cellular Ca2+ homeostasis and account for disease progression. One of the intriguing features is the differences in the expression and mechanism of action of TRPC3 in two different subtypes of SCAs – rather opposing functions. Furthermore, in an attempt to identify crucial gene products implicated in cerebral ataxia, phenotype-driven dominant mutagenesis screens were performed and an ataxic mouse mutant by the name moonwalker (Mwk) mice was identified. These mice exhibited a gain-of-function mutation (T635A) of TRPC3 that rendered the channel constitutively active due to the lack of negative feedback regulation by PKCγ-mediated phosphorylation of the threonine residue (Becker et al. 2009). As a result of impaired TRPC3 channel function, diminished dendritic arborization and progressive loss of Purkinje neurons was observed. The disparity in the mode of expression and functionality of TRPC3 in various forms of SCA, which eventually results in degeneration of cerebellar neurons and ataxic phenotype can be reconciled from the fact that TRPC3 might not be the sole cause of the disease, rather a component of secondary effect in the dysfunctional signaling cascade. Importantly, to establish TRPC3-dependent mechanisms, it has been recently shown that activated Ca2+ signaling is coupled to lipid metabolism and the regulation of Purkinje cell development in the Mwk cerebellum (Dulneva et al. 2015), confirming the role of TRPC3 in ataxia.
27.2.4 SOCE in Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is a progressive neuromuscular disease characterized by muscle degeneration. The pathology of this disease is attributed to the deficiency of dystrophin, which is located at the cytoplasmic face of the sarcolemma. In normal skeletal muscle cells, dystrophin acts as a bridge that connects the extracellular matrix protein laminin-2 to the cytoskeletal f-actin filaments. Hence, in DMD the lack of dystrophin and consequent disorganization of the cytoskeleton can make the PM vulnerable to mechanical damage and can result in functional deregulation of several PM ion channels (Gailly 2002; Vandebrouck et al. 2002). Store-operated Ca2+ channels, TRPC1 and TRPC4 in particular, are among those whose disruption can lead to increased Ca2+ influx resulting in cellular Ca2+ overload, and their knockdown resulted in a 10-fold decrease in Ca2+ influx. Hence, the imbalance in intracellular Ca2+ levels and the resulting Ca2+-dependent activation of proteases are suggested to be the probable reasons for muscle degeneration in DMD (Vandebrouck et al. 2002). In another interesting study, forced expression of minidystrophin at the plasma membrane resulted in regaining normal Ca2+ homeostasis in dystrophin-deficient Sol8 myotubes. It is suggested, that under normal conditions minidystrophin negatively regulates store-operated Ca2+ channels resulting in reduced store-dependent Ca2+ influx and hence over expression of mini dystrophin in dystrophin-lacking myotubes restored the function of store-dependent channels, resulting in less Ca2+ influx and subsequent prevention of Ca2+ mishandling (Vandebrouck et al. 2006). Importantly, it has recently been shown that Ca2+ influx across an unstable sarcolemma due to increased activity of a STIM1–Orai1 complex is a disease determinant in muscular dystrophy, and hence, SOCE represents a potential therapeutic target (Goonasekera et al. 2014).
27.2.5 SOCE Induces HIV Associated Dementia
Human immunodeficiency virus (HIV) infection-induced cognitive impairment has been suggested to precipitate in memory loss and dementia. Thus, HIV-associated dementia (HAD) forms a neuropathological manifestation of the virus infection (Masliah et al. 1996; Mattson et al. 2005). Activation of immune cells by HIV results in the secretion of soluble factors that destabilize neuronal Ca2+ homeostasis, encourage oxidative stress and result in neural damage, which is thought to underlie the cognitive-motor dysfunction that develops in many HIV-infected patients. The HIV-1 transactivator protein (Tat) has been reported to be involved in cognitive impairment (Li et al. 2004) and promotes neuronal death by inducing mitochondrial dysfunction and oxidative stress (Norman et al. 2007; Self et al. 2004). Additionally, evidence also suggests a role of HIV-1 Tat in altering cellular Ca2+ homeostasis (Bonavia et al. 2001; Self et al. 2004), presumably interfering with molecular components of Ca2+ stores (Haughey et al. 1999; Norman et al. 2008). Tat, via Ca2+ dysregulation, has been shown to promote calpain-1 cleavage of p35 to p25, which hyper-activates CDK5 resulting in abnormal phosphorylation of downstream targets such as Tau, collapsin response mediator protein-2 (CRMP2), doublecortin (DCX) and MEF2. Thus, it is conceivable that SOCE might affect Tat-toxicity in a way analogous to the protective role of TRPC1 in MPP+-induced PD (Bollimuntha et al. 2005b; Selvaraj et al. 2009). Indeed, two recent interesting studies from the same group have implicated the involvement of TRPC channels in Tat-toxicity and HAD (Yao et al. 2009a; Yao et al. 2009b). The first study reports that the HIV-1 Tat induces significant cell death in rat midbrain neurons. Treatments of the neurons with the chemokine CCL2, a member of the C-C subfamily of chemokines, alternatively known as monocyte chemoattractant protein-1 (MCP-1), substantially alleviates Tat toxicity and improves neuronal survival. The premise that CCL2 engages PLCβ-mediated IP3 production and that IP3-mediated store-depletion can activate TRPC channels, thus contributing to neuroprotection prompted this group to further investigate the involvement of TRPC channels. Interestingly, SKF-96365 and 2-APB, the non-specific SOCE blockers, significantly blocked the CCL2-induced neuroprotection against Tat toxicity. Further, using RNAi the authors found that TRPC1 and TRPC5 channels were involved in mediating the protective effects of CCL2, preferably via an ERK/CREB signaling axis (Yao et al. 2009b). In the similar context of HIV-1 Tat-induced neurotoxicity, this group demonstrated the involvement of TRPC channels in platelet-derived growth factor (PDGF)-mediated protection of the rat midbrain neurons. In addition to the neuroprotective effects of PDGF in vitro, its pretreatment in vivo also rescued loss of dopaminergic neurons following Tat exposure. This study revealed a prominent role of TRPC5 and TRPC6 channels in engaging a Pyk2/ERK/CREB pathway to elicit PDGF-mediated neuroprotection (Yao et al. 2009b). In another study, pretreatment of cells with either the PI3K inhibitor or a TRPC blocker resulted in the failure of PDGF to inactivate GSK3β, thereby suggesting the intersection of PI3K and TRPC signaling at GSK3β (Peng et al. 2012).
27.2.6 SOCE and Oxidative Stress
The steady-state generation and proper compartmentalization of free radicals to specific cellular microdomains transduce physiological redox-signals to carryout anabolic activities such as proliferation, migration, transcription and neurotransmission. However, in specific pathological condition these oxidants can leave their spatial confinements thereby leading to oxidative stress (Durackova 2010; Kamata and Hirata 1999). Oxidant-induced toxicity and cognitive impairment has been reported in a variety of neurodegenerative diseases including, AD, PD, ALS, HD, stroke and prion pathogenesis (Bezprozvanny and Hayden 2004; Cassarino et al. 1997; Dauer and Przedborski 2003; Mattson 2007; Patten et al. 2010). A prime commonality in free radical production and associated neurodegeneration is mitochondrial dysfunction and there appears to be a positive correlation between them in regulating disease progression (Lin and Beal 2006). Changes in the levels of cytosolic Ca2+ influence numerous physiological processes (Berridge 1998; Berridge et al. 2000). Free radicals can influence the physiological activity of a cell by altering cytosolic Ca2+, either by mobilizing Ca2+ from internal stores or by modulating Ca2+ influx across the PM. Interestingly, perturbation in Ca2+ homeostasis has also been described to result in the generation of free radicals and leading to oxidant-induced neuronal programmed cell death (Bezprozvanny and Mattson 2008; Chakraborti et al. 1999; Ermak and Davies 2002; Mattson 2007). Thus, it is conceivable that the components of neuronal SOCE will have a prominent influence on regulating the redox status of a cell. Alternatively, there also lies the possibility that the SOCE channels can themselves be regulated by cellular redox fluctuations. From a neuronal perspective, evidence on oxidative stress-regulated SOCE is limited; however, in non-excitable cells the same has been more elaborately studied.
Several members of TRPs and more recently the Orai1 and STIM2 components of SOCE have been identified to be redox-responsive (Aarts and Tymianski 2005; Berna-Erro et al. 2009; Birnbaumer 2009; Bogeski et al. 2010; Miller 2006). One of the earliest evidences that oxidants have the potential to activate SOCC comes from a study demonstrating H2O2 to induce Ca2+ influx in canine endothelial cells. Interestingly, this H2O2-induced Ca2+ influx was dose-dependant and sensitive to the SOCE blocker SKF-96365 (Doan et al. 1994). Additionally, in human oocytes a similar H2O2 dose-dependent increase in Ca2+ influx was observed which was sensitive to 2-APB, yet another SOCE blocker (Martin-Romero et al. 2008). Further, in porcine aortic endothelial cells (PAECs), induction of oxidative stress activates non-selective cation entry. In the initial study, treatment of the endothelial cells with tert-butyl hydroperoxide elicited cation conductance which was suppressed by dominantly interfering with the function of TRPC3 channels (Balzer et al. 1999). In a subsequent study, the same group showed that the stress-induced cation conductance in PAECs were due to the heteromeric assembly of TRPC3/TRPC4 channels. TRPC1 in these cells weakly interacted with TRPC3; however, its involvement in heteromeric channel assembly and in redox-sensing remains to be determined (Poteser et al. 2006). Interestingly, amyloid-β aggregates have been shown to induce oxidative stress (Bezprozvanny and Mattson 2008) and since TRPC3 has a redox sensing ability, it can potentially exacerbate AD. Similarly, TRPC5 has also been demonstrated to be activated by NO via S-nitrosylation (Yoshida et al. 2006) and thus posses the ability to participate in neurodegenerative conditions. In addition to members of the TRPC sub-family, the TRPMs have also been identified as redox-sensitive. In particular, TRPM2 and TRPM7 have been associated with oxidative insult-induced cell death. TRPM2 is known to be activated by ROS including H2O2 and oxidant-induced Ca2+ influx via TRPM2 channels results in cell death (Hara et al. 2002). H2O2 and amyloid β-peptide have been shown to induce cell death in rat striatal neurons expressing endogenous TRPM2 and inhibiting the function of TRPM2 improves cell survival following stress (McNulty and Fonfria 2005). A study also describes PARP (poly ADP-ribose polymerase) as a mediator between oxidative stress and TRPM2 activation and that PARP inhibitors tend to suppress TRPM2 function and protect cells from oxidative injury (Fonfria et al. 2004; Miller 2004; Yang et al. 2006). Similarly, H2O2 also activates TRPM7 conductance and RNAi-mediated inhibition of the channel reduces oxidant-induced Ca2+ influx, suppress ROS production and protects neurons from anoxic cell death (Aarts and Tymianski 2005; Yamamoto et al. 2009). Interestingly, in a human endothelial cell line it was recently shown that DHA, a polyunsaturated fatty acid attenuates H2O2-induced oxidative stress by reducing Ca2+ influx. DHA apparently alters the lipid composition of the plasma membrane raft domains and in doing so it displaces the caveolar raft-localized TRPC1 to non-raft compartment, thus rendering the channel non-functional (Ye et al. 2010). In cultured hippocampal neurons, stress-induced exacerbation in a TRP channel-related Ca2+ influx leads to neuronal death and pre-treatments of the cells with the anti-oxidant alpha-tocopherol protects the neurons by inhibiting the TRP-mediated intracellular Ca2+ overload (Crouzin et al. 2007). Additionally, in Drosophila photoreceptors, anoxia activates TRP and TRP-like (TRPL) currents and genetic depletion of these channels is shown to protect the photoreceptor cells from anoxic-stress (Agam et al. 2000). From the findings discussed above it follows that oxidative stress activates SOCE and in a pathological situation oxidant-induced exaggeration in SOCE can potentially amplify cell injury. Thus, components of SOCE emerge as attractive targets in controlling oxidative stress-induced cell death. A reduction in SOCE in glutamate-resistant HT22 cells protected against oxidative death, suggesting that dysregulated Ca2+ entry through Orai1 mediates the detrimental Ca2+ entry in programmed cell death induced by GSH depletion (Henke et al. 2013). However, ROS has also been shown to regulate SOCE, which could be due to direct effects on the core SOCE machinery, including STIM proteins and their partner channels, or indirectly by influencing the status of ER Ca2+ stores. STIM1 was shown to be S-glutathionylated at C56 amino acid during oxidative stress induced by bacterial lipopolysaccharide (LPS) or butathionine sulfoximine-induced GSH depletion, which lowered the affinity of STIM1 for Ca2+ and facilitated oligomerization, leading to store-independent activation of STIM1 (Nunes and Demaurex 2014). Similarly, Orai1 was also recently demonstrated to act as a direct redox sensor mainly by virtue of a reactive cysteine at position C195 and exposure to H2O2 inhibited Ca2+ influx in normal Orai1, but not in Orai1 mutated at position 195 (Nunes and Demaurex 2014). These results suggest that ROS regulates the Ca2+ channels and its modulators that modify Ca2+ entry and could promote degeneration.
27.3 SOCE, Membrane Rafts and Neurodegeneration
It is being widely accepted that the PM is not just a uniform stretch of the protein-lipid bilayer, rather it is spatially organized into multiple microdomains that constitute unique signaling nodes to efficiently relay external signals into the cell’s interior. Regions of the PM that are rich in cholesterol and sphingolipids fall into a broader class of membrane microdomain termed as membrane ‘lipid rafts’ that have the ability to cluster specific signaling molecules required to bring about a specific cellular response. Involvement of membrane rafts in the progression of neurodegeneration has been described in a variety of dementing conditions including AD, PD, ALS and prion pathogenesis. In AD, the generation of amyloid β-peptide by the processing of amyloid precursor protein (APP) hallmarks disease initiation (De Strooper and Annaert 2000). Interestingly, in cultured neurons it had been shown that acute extraction of membrane cholesterol by MβCD or by chronic statin treatments to inhibit cholesterol synthesis, reduces the accumulation of amyloid β-peptide (Ehehalt et al. 2003). Similarly, molecules involved in PD pathogenesis such as parkin, PINK1, α-synuclein and LRRK2 have been shown to be associated with lipid raft microdomains (Hatano et al. 2007; Park et al. 2009; Parkin et al. 1999). Importantly, most TRPCs have been identified to be expressed in lipid rafts and Ca2+ entry was dependent on the integrity of lipid rafts (Pani and Singh 2009). However, information on the membrane raft perspective of SOCE regulation in disease progression is still lacking. Since components of the SOCC localize to membrane rafts and work in concert with multiple regulators, it would be worthwhile to investigate their physical state in various neurodegenerative conditions such as attenuation of TRPC1-SOCE in PD and exaggeration of SOCE in neuronal oxidative stress and AD. This will not only provide mechanistic insight into the altered Ca2+ homeostasis but will also presumably shed light on the onset and progression of the disease.
27.4 Concluding Remarks
SOCE is a unique aspect of neuronal Ca2+ signaling wherein the intracellular Ca2+ stores communicate with the plasma membrane channels to orchestrate cellular Ca2+ homeostasis. This process is essential for maintaining intracellular Ca2+ stores as well as influencing vital physiological processes of the excitable cells. TRPC and Orai channels have been recently identified as SOCE channels and there has been intense focus on TRPC channels as recent data from various labs indicate a possible role of TRPC channels in neuronal function. However, conclusive data regarding the exact physiological function of most of these channels in neuronal cells is still lacking. Studies using single or multiple gene knockouts will be essential to validate the diverse roles of these channels in neuronal function. Additionally, as TRPC proteins cross-talk with Orai and are activated by STIM1, studies focused on delineating the function of the ‘TRPC-Orai-STIM’ complex in neuronal context will be critical in understanding their plausible role in neuronal pathophysiology.
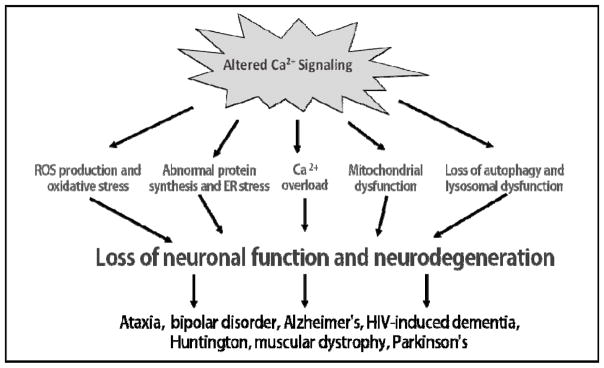
Fig. 27.2.
Consequences of abnormal Ca2+ signaling. This illustration depicts the current understanding of the consequences of abnormal Ca2+ signaling. Altered Ca2+ signaling leads to issues such as oxidative stress, ROS production, Ca2+ overload, unfolded protein response and ER stress, mitochondrial and lysosomal dysfunction and inhibition of autophagy. This leads to loss of neuronal function and induction of neuronal diseases.
Acknowledgments
We duly acknowledge the grant support from the National Institutes of Health (DE017102; DE024300; GM113123).
References
- Aarts MM, Tymianski M. TRPMs and neuronal cell death. Pflugers Arch. 2005;451:243–249. doi: 10.1007/s00424-005-1439-x. [DOI] [PubMed] [Google Scholar]
- Adachi N, Kobayashi T, Takahashi H, Kawasaki T, Shirai Y, Ueyama T, Matsuda T, Seki T, Sakai N, Saito N. Enzymological analysis of mutant protein kinase Cgamma causing spinocerebellar ataxia type 14 and dysfunction in Ca2+ homeostasis. J Biol Chem. 2008;283:19854–19863. doi: 10.1074/jbc.M801492200. [DOI] [PubMed] [Google Scholar]
- Agam K, von Campenhausen M, Levy S, Ben-Ami HC, Cook B, Kirschfeld K, Minke B. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J Neurosci. 2000;20:5748–5755. doi: 10.1523/JNEUROSCI.20-15-05748.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari Y, Hitt BD, Murphy MP, Dagher NN, Tseng BP, Green KN, Golde TE, LaFerla FM. Presenilin regulates capacitative calcium entry dependently and independently of gamma-secretase activity. Biochem Biophys Res Commun. 2004;322:1145–1152. doi: 10.1016/j.bbrc.2004.07.136. [DOI] [PubMed] [Google Scholar]
- Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl. 2000;59:133–154. doi: 10.1007/978-3-7091-6781-6_16. [DOI] [PubMed] [Google Scholar]
- Balzer M, Lintschinger B, Groschner K. Evidence for a role of Trp proteins in the oxidative stress-induced membrane conductances of porcine aortic endothelial cells. Cardiovasc Res. 1999;42:543–549. doi: 10.1016/s0008-6363(99)00025-5. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Lee J, Venkatesh K, Wu CF, Hasan G. Loss of flight and associated neuronal rhythmicity in inositol 1,4,5-trisphosphate receptor mutants of Drosophila. J Neurosci. 2004;24:7869–7878. doi: 10.1523/JNEUROSCI.0656-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barritt GJ. Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem J. 1999;337(Pt 2):153–169. [PMC free article] [PubMed] [Google Scholar]
- Beck B, Lehen’kyi V, Roudbaraki M, Flourakis M, Charveron M, Bordat P, Polakowska R, Prevarskaya N, Skryma R. TRPC channels determine human keratinocyte differentiation: new insight into basal cell carcinoma. Cell Calcium. 2008;43:492–505. doi: 10.1016/j.ceca.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Becker EB, Oliver PK, Glitsch MD, Banks GT, Achilli F, Hardy A, Nolan PM, Fisher EM, Davies KE. A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proc Natl Acad Sci U S A. 2009;106:6706–6711. doi: 10.1073/pnas.0810599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna-Erro A, Braun A, R Kraft, Kleinschnitz C, Schuhmann MK, Stegner D, Wultsch T, Eilers J, Meuth SG, Stoll G, Nieswandt B. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal. 2009;2(93):ra67. doi: 10.1126/scisignal.2000522. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Capacitative calcium entry. Biochem J. 1995;312(Pt 1):1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. The AM and FM of calcium signalling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium signalling remodelling and disease. Biochem Soc Trans. 2012;40:297–309. doi: 10.1042/BST20110766. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodeling. Nat Rev Mol Cell Biol. 2003;4:517–729. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Dupont G. Spatial and temporal signalling by calcium. Curr Opin Cell Biol. 1994;6:267–274. doi: 10.1016/0955-0674(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Hayden MR. Deranged neuronal calcium signaling and Huntington disease. Biochem Biophys Res Commun. 2004;322:1310–1317. doi: 10.1016/j.bbrc.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2+) concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- Bogeski I, Kummerow C, Al-Ansary D, Schwarz EC, Koehler R, Kozai D, Takahashi N, Peinelt C, Griesemer D, Bozem M, Mori Y, Hoth M, Niemeyer BA. Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Sci Signal. 2010;3(115):ra24. doi: 10.1126/scisignal.2000672. [DOI] [PubMed] [Google Scholar]
- Bojarski L, Pomorski P, Szybinska A, Drab M, Skibinska-Kijek A, Gruszczynska-Biegala J, Kuznicki Presenilin-dependent expression of STIM proteins and dysregulation of capacitative Ca2+ entry in familial Alzheimer’s disease. Biochim Biophys Acta. 2009;1793:1050–1057. doi: 10.1016/j.bbamcr.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Bollimuntha S, Cornatzer E, Singh BB. Plasma membrane localization and function of TRPC1 is dependent on its interaction with beta-tubulin in retinal epithelium cells. Vis Neurosci. 2005a;22:163–170. doi: 10.1017/S0952523805222058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimuntha S, Singh BB, Shavali S, Sharma SK, Ebadi M. TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. J Biol Chem. 2005b;280:2132–2140. doi: 10.1074/jbc.M407384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavia R, Bajetto A, Barbero S, Albini A, Noonan DM, Schettini G. HIV-1 Tat causes apoptotic death and calcium homeostasis alterations in rat neurons. Biochem Biophys Res Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- Cai S, Fatherazi S, Presland RB, Belton CM, Roberts FA, Goodwin PC, Schubert MM, Izutsu KT. Evidence that TRPC1 contributes to calcium-induced differentiation of human keratinocytes. Pflugers Arch. 2006;452:43–52. doi: 10.1007/s00424-005-0001-1. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Fall CP, Swerdlow RH, Smith TS, Halvorsen EM, Miller SW, Parks JP, Parker WD, Jr, Bennett JP., Jr Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson’s disease. Biochim Biophys Acta. 1997;1362:77–86. doi: 10.1016/s0925-4439(97)00070-7. [DOI] [PubMed] [Google Scholar]
- Chakraborti T, Das S, Mondal M, Roychoudhury S, Chakraborti S. Oxidant, mitochondria and calcium: an overview. Cell Signal. 1999;11:77–85. doi: 10.1016/s0898-6568(98)00025-4. [DOI] [PubMed] [Google Scholar]
- Cheek TR, Thastrup O. Internal Ca2+ mobilization and secretion in bovine adrenal chromaffin cells. Cell Calcium. 1989;10:213–221. doi: 10.1016/0143-4160(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem. 2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Clementi E, Scheer H, Zacchetti D, Fasolato C, Pozzan T, Meldolesi J. Receptor-activated Ca2+ influx. Two independently regulated mechanisms of influx stimulation coexist in neurosecretory PC12 cells. J Biol Chem. 1992;267:2164–2172. [PubMed] [Google Scholar]
- Congar P, Leinekugel X, Ben-Ari Y, Crepel V. A long-lasting calcium-activated nonselective cationic current is generated by synaptic stimulation or exogenous activation of group I metabotropic glutamate receptors in CA1 pyramidal neurons. J Neurosci. 1997;17:5366–5379. doi: 10.1523/JNEUROSCI.17-14-05366.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzin N, de Jesus Ferreira MC, Cohen-Solal C, Aimar RF, Vignes M, Guiramand J. Alpha-tocopherol-mediated long-lasting protection against oxidative damage involves an attenuation of calcium entry through TRP-like channels in cultured hippocampal neurons. Free Radic Biol Med. 2007;42:1326–1337. doi: 10.1016/j.freeradbiomed.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci. 2000;113(Pt 11):1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- Doan TN, Gentry DL, Taylor AA, Elliott SJ. Hydrogen peroxide activates agonist-sensitive Ca(2+)-flux pathways in canine venous endothelial cells. Biochem J. 1994;297(Pt 1):209–215. doi: 10.1042/bj2970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulneva A, Lee S, Oliver PL, Di Gleria K, Kessler BM, Davies KE, Becker EB. The mutant Moonwalker TRPC3 channel links calcium signaling to lipid metabolism in the developing cerebellum. Hum Mol Genet. 2015;24:4114–4125. doi: 10.1093/hmg/ddv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durackova Z. Some current insights into oxidative stress. Physiol Res. 2010;59:459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. 2002;38:713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- Fabian A, Fortmann T, Dieterich P, Riethmuller C, Schon P, Mally S, Nilius B, Schwab A. TRPC1 channels regulate directionality of migrating cells. Pflugers Arch. 2008;457:475–484. doi: 10.1007/s00424-008-0515-4. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Fiorio Pla A, Maric D, Brazer SC, Giacobini P, Liu X, Chang YH, Ambudkar IS, Barker JL. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci. 2005;25:2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfria E, Marshall IC, Benham CD, Boyfield I, Brown JD, Hill K, Hughes JP, Skaper SD, McNulty S. TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol. 2004;143:186–192. doi: 10.1038/sj.bjp.0705914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel DD, Tsien RW. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailly P. New aspects of calcium signaling in skeletal muscle cells: implications in Duchenne muscular dystrophy. Biochim Biophys Acta. 2002;1600:38–44. doi: 10.1016/s1570-9639(02)00442-9. [DOI] [PubMed] [Google Scholar]
- Giacomello M, Barbiero L, Zatti G, Squitti R, Binetti G, Pozzan T, Fasolato C, Ghidoni R, Pizzo P. Reduction of Ca2+ stores and capacitative Ca2+ entry is associated with the familial Alzheimer’s disease presenilin-2 T122R mutation and anticipates the onset of dementia. Neurobiol Dis. 2005;18:638–648. doi: 10.1016/j.nbd.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- Goonasekera SA, Davis J, Kwong JQ, Accornero F, Wei-LaPierre L, Sargent MA, Dirksen RT, Molkentin JD. Enhanced Ca2+ influx from STIM1-Orai1 induces muscle pathology in mouse models of muscular dystrophy. Hum Mol Genet. 2014;23:3706–3715. doi: 10.1093/hmg/ddu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaux E, Mackinnon R. Principles of selective ion transport in channels and pumps. Science. 2005;310:1461–1465. doi: 10.1126/science.1113666. [DOI] [PubMed] [Google Scholar]
- Guerini D, Coletto L, Carafoli E. Exporting calcium from cells. Cell Calcium. 2005;38:281–289. doi: 10.1016/j.ceca.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- Hatano T, Kubo S, Imai S, Maeda M, Ishikawa K, Mizuno Y, Hattori N. Leucine-rich repeat kinase 2 associates with lipid rafts. Hum Mol Genet. 2007;16:678–690. doi: 10.1093/hmg/ddm013. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein tat. J Neurochem. 1999;73:1363–1374. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- Henke N, Albrecht P, Bouchachia I, Ryazantseva M, Knoll K, Lewerenz J, Kaznacheyeva E, Maher P, Methner A. The plasma membrane channel ORAI1 mediates detrimental calcium influx caused by endogenous oxidative stress. Cell Death Dis. 2013;4:e470. doi: 10.1038/cddis.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW, Yaradanakul A, Wang Y, Fuster D. Molecular control of cardiac sodium homeostasis in health and disease. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S47–S56. doi: 10.1111/j.1540-8167.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci. 2007;10:559–567. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Kraft R. STIM and ORAI proteins in the nervous system. Channels (Austin) 2015;9:245–252. doi: 10.1080/19336950.2015.1071747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- Lessard CB, Lussier MP, Cayouette S, Bourque G, Boulay G. The overexpression of presenilin2 and Alzheimer’s-disease-linked presenilin2 variants influences TRPC6-enhanced Ca2+ entry into HEK293 cells. Cell Signal. 2005;17:437–445. doi: 10.1016/j.cellsig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Li ST, Matsushita M, Moriwaki A, Saheki Y, Lu YF, Tomizawa K, Wu HY, Terada H, Matsui H. HIV-1 Tat inhibits long-term potentiation and attenuates spatial learning [corrected] Ann Neurol. 2004;55:362–371. doi: 10.1002/ana.10844. [DOI] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lin X, Antalffy B, Kang D, Orr HT, Zoghbi HY. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat Neurosci. 2000;3:157–163. doi: 10.1038/72101. [DOI] [PubMed] [Google Scholar]
- Linden R. The survival of developing neurons: a review of afferent control. Neuroscience. 1994;58:671–682. doi: 10.1016/0306-4522(94)90447-2. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann C, Finski A, Bonhoeffer T. Local calcium transients regulate the spontaneous motility of dendritic filopodia. Nat Neurosci. 2005;8:305–312. doi: 10.1038/nn1406. [DOI] [PubMed] [Google Scholar]
- Majewski L, Kuznicki J. SOCE in neurons: Signaling or just refilling? Biochim Biophys Acta. 2015;1853:1940–1952. doi: 10.1016/j.bbamcr.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Martin-Romero FJ, Ortiz-de-Galisteo JR, Lara-Laranjeira J, Dominguez-Arroyo JA, Gonzalez-Carrera E, Alvarez IS. Store-operated calcium entry in human oocytes and sensitivity to oxidative stress. Biol Reprod. 2008;78:307–315. doi: 10.1095/biolreprod.107.064527. [DOI] [PubMed] [Google Scholar]
- Mark MD, Schwitalla JC, Groemmke M, Herlitze S. Keeping Our Calcium in Balance to Maintain Our Balance. Biochem Biophys Res Commun. 2017 doi: 10.1016/j.bbrc.2016.07.020. in press. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Mucke L. Pathogenesis of HIV-1 associated neurodegeneration. Crit Rev Neurobiol. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- McNulty S, Fonfria E. The role of TRPM channels in cell death. Pflugers Arch. 2005;451:235–242. doi: 10.1007/s00424-005-1440-4. [DOI] [PubMed] [Google Scholar]
- Meldolesi J, Volpe P, Pozzan T. The intracellular distribution of calcium. Trends Neurosci. 1988;11:449–452. doi: 10.1016/0166-2236(88)90197-x. [DOI] [PubMed] [Google Scholar]
- Miller BA. Inhibition of TRPM2 function by PARP inhibitors protects cells from oxidative stress-induced death. Br J Pharmacol. 2004;143:515–516. doi: 10.1038/sj.bjp.0705923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BA. The role of TRP channels in oxidative stress-induced cell death. J Membr Biol. 2006;209:31–41. doi: 10.1007/s00232-005-0839-3. [DOI] [PubMed] [Google Scholar]
- Miller RJ. Calcium signalling in neurons. Trends Neurosci. 1988;11:415–419. doi: 10.1016/0166-2236(88)90191-9. [DOI] [PubMed] [Google Scholar]
- Montell C. TRP channels in Drosophila photoreceptor cells. J Physiol. 2005a;567:45–51. doi: 10.1113/jphysiol.2005.092551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005b;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- Norman JP, Perry SW, Kasischke KA, Volsky DJ, Gelbard HA. HIV-1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. J Immunol. 2007;178:869–876. doi: 10.4049/jimmunol.178.2.869. [DOI] [PubMed] [Google Scholar]
- Norman JP, Perry SW, Reynolds HM, Kiebala M, De Mesy Bentley KL, Trejo M, Volsky DJ, Maggirwar SB, Dewhurst S, Masliah E, Gelbard HA. HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS One. 2008;3:e3731. doi: 10.1371/journal.pone.0003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes P, Demaurex N. Redox regulation of store-operated Ca2+ entry. Antioxid Redox Signal. 2014;21(6):915–932. doi: 10.1089/ars.2013.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Hobson V, Hall JR, Waring SC, Chan W, Massman P, Lacritz L, Cullum CM, Diaz-Arrastia R. Brain-derived neurotrophic factor levels in Alzheimer’s disease. J Alzheimers Dis. 2009;17:337–341. doi: 10.3233/JAD-2009-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani B, Cornatzer E, Cornatzer W, Shin DM, Pittelkow MR, Hovnanian A, Ambudkar IS, Singh BB. Up-regulation of transient receptor potential canonical 1 (TRPC1) following sarco(endo)plasmic reticulum Ca2+ ATPase 2 gene silencing promotes cell survival: a potential role for TRPC1 in Darier’s disease. Mol Biol Cell. 2006;17:4446–4458. doi: 10.1091/mbc.E06-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009;45:625–633. doi: 10.1016/j.ceca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB. Decoding cytosolic Ca(2+) oscillations. Trends Biochem Sci. 2011;36(2):78–87. doi: 10.1016/j.tibs.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Park JY, Kim KS, Lee SB, Ryu JS, Chung KC, Choo YK, Jou I, Kim J, Park SM. On the mechanism of internalization of alpha-synuclein into microglia: roles of ganglioside GM1 and lipid raft. J Neurochem. 2009;110:400–411. doi: 10.1111/j.1471-4159.2009.06150.x. [DOI] [PubMed] [Google Scholar]
- Parkin ET, Turner AJ, Hooper NM. Amyloid precursor protein, although partially detergent-insoluble in mouse cerebral cortex, behaves as an atypical lipid raft protein. Biochem J. 1999;344(Pt 1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Patten DA, Germain M, Kelly MA, Slack RS. Reactive oxygen species: stuck in the middle of neurodegeneration. J Alzheimers Dis. 2010;20(Suppl 2):S357–367. doi: 10.3233/JAD-2010-100498. [DOI] [PubMed] [Google Scholar]
- Peng F, Yao H, Akturk HK, Buch S. Platelet-derived growth factor CC-mediated neuroprotection against HIV Tat involves TRPC-mediated inactivation of GSK 3beta. PLoS One. 2012;7(10):e47572. doi: 10.1371/journal.pone.0047572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, Cavalie A, Flockerzi V. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 1998;17:4274–4282. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P, Burgo A, Pozzan T, Fasolato C. Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J Neurochem. 2001;79:98–109. doi: 10.1046/j.1471-4159.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3–TRPC4 heteromeric channels in endothelial cells. J Biol Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Tieu K, Perier C, Vila M. MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J Bioenerg Biomembr. 2004;36:375–379. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Capacitative calcium entry in the nervous system. Cell Calcium. 2003;34:339–344. doi: 10.1016/s0143-4160(03)00143-x. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr The physiological function of store-operated calcium entry. Neurochem Res. 2011;36:1157–1165. doi: 10.1007/s11064-010-0383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr Alternative forms of the store-operated calcium entry mediators, STIM1 and Orai1. Curr Top Membr. 2013;71:109–123. doi: 10.1016/B978-0-12-407870-3.00005-6. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med. 2000;78:3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- Self RL, Mulholland PJ, Nath A, Harris BR, Prendergast MA. The human immunodeficiency virus type-1 transcription factor Tat produces elevations in intracellular Ca2+ that require function of an N-methyl-D-aspartate receptor polyamine-sensitive site. Brain Res. 2004;995:39–45. doi: 10.1016/j.brainres.2003.09.052. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Watt JA, Singh BB. TRPC1 inhibits apoptotic cell degeneration induced by dopaminergic neurotoxin MPTP/MPP(+) Cell Calcium. 2009;46:209–218. doi: 10.1016/j.ceca.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Sun Y, Watt JA, Song S, Lei S, Birnbaumer L, Singh BB. Toxin-induced ER stress in mice involves down regulation of TRPC1 and inhibition of the AKT/mTOR signaling. J Clin Invest. 2012;122:1354–1367. doi: 10.1172/JCI61332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BB, Zheng C, Liu X, Lockwich T, Liao D, Zhu MX, Birnbaumer L, Ambudkar IS. Trp1-dependent enhancement of salivary gland fluid secretion: role of store-operated calcium entry. Faseb J. 2001;15:1652–1654. doi: 10.1096/fj.00-0749fje. [DOI] [PubMed] [Google Scholar]
- Strehler EE, Treiman M. Calcium pumps of plasma membrane and cell interior. Curr Mol Med. 2004;4:323–335. doi: 10.2174/1566524043360735. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran P, Schaar A, Sun Y, Singh BB. Functional role of TRP channels in modulating ER stress and Autophagy. Cell Calcium. 2016;60(2):123–132. doi: 10.1016/j.ceca.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Sukumaran P, Singh BB. Physiological function and characterization of TRPCs in non-excitable and excitable cells. Cells. 2014;3:455–475. doi: 10.3390/cells3020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Zhang H, Liu J, Popugaeva E, Xu NJ, Feske S, White CL, Bezprozvanny I. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron. 2014;82:79–93. doi: 10.1016/j.neuron.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Leemput J, Chandran J, Knight MA, Holtzclaw LA, Scholz S, Cookson MR, Houlden H, Gwinn-Hardy K, Fung HC, Lin X, Hernandez D, Simon-Sanchez J, Wood NW, Giunti P, Rafferty I, Hardy J, Storey E, Gardner RJ, Forrest SM, Fisher EM, Russell JT, Cai H, Singleton AB. Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet. 2007;3:e108. doi: 10.1371/journal.pgen.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebrouck A, Ducret T, Basset O, Sebille S, Raymond G, Ruegg U, Gailly P, Cognard C, Constantin B. Regulation of store-operated calcium entries and mitochondrial uptake by minidystrophin expression in cultured myotubes. Faseb J. 2006;20:136–138. doi: 10.1096/fj.04-3633fje. [DOI] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkiteswaran G, Hasan G. Intracellular Ca2+ signaling and store-operated Ca2+ entry are required in Drosophila neurons for flight. Proc Natl Acad Sci U S A. 2009;106:10326–10331. doi: 10.1073/pnas.0902982106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig PJ, Subramony SH, McDaniel DO. Calcium homeostasis and spinocerebellar ataxia-1 (SCA-1) Brain Res Bull. 2001;56:221–225. doi: 10.1016/s0361-9230(01)00595-0. [DOI] [PubMed] [Google Scholar]
- Wu X, Zagranichnaya TK, Gurda GT, Eves EM, Villereal ML. A TRPC1/TRPC3-mediated increase in store-operated calcium entry is required for differentiation of H19-7 hippocampal neuronal cells. J Biol Chem. 2004;279:43392–43402. doi: 10.1074/jbc.M408959200. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Shimizu S, Mori Y. Involvement of TRPM2 channel in amplification of reactive oxygen species-induced signaling and chronic inflammation. Nippon Yakurigaku Zasshi. 2009;134:122–126. doi: 10.1254/fpj.134.122. [DOI] [PubMed] [Google Scholar]
- Yang KT, Chang WL, Yang PC, Chien CL, Lai MS, Su MJ, Wu ML. Activation of the transient receptor potential M2 channel and poly(ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death. Cell Death Differ. 2006;13:1815–1826. doi: 10.1038/sj.cdd.4401813. [DOI] [PubMed] [Google Scholar]
- Yao H, Peng F, Dhillon N, Callen S, Bokhari S, Stehno-Bittel L, Ahmad SO, Wang JQ, Buch S. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009a;29:1657–1669. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Peng F, Fan Y, Zhu X, Hu G, Buch SJ. TRPC channel-mediated neuroprotection by PDGF involves Pyk2/ERK/CREB pathway. Cell Death Differ. 2009b;16:1681–1693. doi: 10.1038/cdd.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Tan L, Ma J, Shi Q, Li J. Polyunsaturated docosahexaenoic acid suppresses oxidative stress induced endothelial cell calcium influx by altering lipid composition in membrane caveolar rafts. Prostaglandins Leukot Essent Fatty Acids. 2010;83:37–43. doi: 10.1016/j.plefa.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Cheng I, Chung S, Grenfell TZ, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, Saunders AJ, Ding K, Frosch MP, Tanzi RE, Kim TW. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. Trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]