Abstract
The Odor Span Task is an incrementing non-matching-to-sample procedure that permits the study of behavior under the control of multiple stimuli. Rats are exposed to a series of odor stimuli and selection of new stimuli is reinforced. Successful performance thus requires remembering which stimuli have previously been presented during a given session. This procedure has been frequently used in neurobiological studies as a rodent model of working memory; however, only a few studies have examined the effects of drugs on performance in this task. The present experiments explored the behavioral pharmacology of a modified version of the Odor Span Task by determining the effects of stimulant drugs methylphenidate and methamphetamine, NMDA antagonist ketamine, and positive GABAA modulator flunitrazepam. All four drugs produced dose-dependent impairment of performances on the Odor Span Task, but for methylphenidate and methamphetamine, these occurred only at doses that had similar effects on performance of a simple odor discrimination. Generally, these disruptions were based on omission of responding at the effective doses. The effects of ketamine and flunitrazepam were more selective in some rats. That is, some rats tested under flunitrazepam and ketamine showed decreases in accuracy on the Odor Span Task at doses that did not affect simple discrimination performance. These selective effects indicate disruption of within-session stimulus control. Overall, these findings support the potential of the Odor Span Task as a baseline for the behavioral pharmacological analysis of remembering.
Keywords: Odor Span Task, non-matching-to-sample, NMDA antagonist, positive GABAA modulator, methamphetamine, methylphenidate, flunitrazepam, ketamine
The most widely studied procedures used in the behavioral pharmacology of remembering are the delayed-matching- and non-matching-to-sample (DMTS, DNMTS) procedures. Manipulation of the delay between the offset of the sample stimulus and the presentation of the comparison stimuli generally leads to the classic forgetting function wherein accuracy decreases as the delay interval increases. Drugs can alter the forgetting function with changes in the slope generally interpreted as a drug effect on the rate of forgetting (Galizio, 2016; White, 2013). Variations of the basic DMTS/DNMTS procedures used in behavioral pharmacology include titrating arrangements in which the delay interval is progressively increased following successful performances and decreased after errors. Titrating DMTS/DNMTS permits analysis of drug effects on the average delay at which stimulus control is lost (e.g., Kangas, Vaidya, & Branch, 2010). Collectively, these procedures are often used as models for human “short-term-” or “working-memory” processes, and certainly the forgetting functions obtained confer some degree of validity in this regard.
Human memory researchers often note that performances on short-term or working memory tasks are limited in the number of stimuli that can be remembered—termed limited capacity—as well as in the loss of accuracy after temporal delays. Extension of DMTS/DNMTS procedures to the analysis of multiple sample stimuli thus has the potential to add translational validity to the approach (Wright, 2007). One strategy is to present a list of sample stimuli to the subject followed by test trials on which comparison stimuli are available. These stimuli are drawn from the list items previously presented or are novel to the session with responses to one key or lever reinforced for list items (old) and a different response reinforced for novel (new) stimuli. Using such procedures in pigeons, monkeys and humans, accuracy has been shown to be a function of the number of stimuli to remember (list length), the interval between list presentation and test (delay), and the serial position of stimuli in the list (Wright, 2007), but only a few studies have assessed the effects of drugs on such procedures (Aigner, Walker, & Mishkin, 1991; Castro, 1995; 1997).
A different procedure used to study drug effects with multiple stimuli is the self-ordered spatial search task (SOSS), which was designed to study spatial working memory (Soto, Ator, Rallipali, Biawat, Clayton, Cook, & Weed, 2013; Soto, Dallery, Ator, & Katz, 2013). As used by Soto and colleagues, the SOSS involves presentation of two, three, or four identical objects on a touchscreen apparatus in any of 16 spatial positions. Rhesus monkeys were trained to touch each object on the screen with each nonrepeating touch producing food and the first repetition ending the trial. Touching all objects without a repetition (which requires remembering which stimuli were previously touched within the trial) was defined as a correct response. Soto, Ator et al. (2013) found that positive GABAA modulators such as triazolam, zaleplon, and zolpidem reduced SOSS accuracy. Additionally, they showed that the effective dose and the magnitude of the effect depended on the number of stimuli presented on the screen— trials with four objects were most sensitive to drug effects and those with only two objects to remember were least sensitive. Interestingly, Soto, Ator et al. also studied the effects of the same drugs on behavior under a DMTS procedure. The GABAA modulators also reduced DMTS accuracy, but the effects were independent of the delay interval—that is, they did not change the slope of the forgetting function. These data support the idea that behavioral pharmacological analysis of behavior controlled by multiple stimuli might be more sensitive to the effects of amnestic drugs than are DMTS baselines.
Additional support for that notion has been provided by another procedure used to study drug effects with multiple stimuli, the odor span task (OST). The OST is a variation of the non-matching-to-sample procedure in which rodents are exposed to a series of odors in an arena. Digging in a cup of scented sand (Dudchenko, Wood, & Eichenbaum, 2000) or removing a scented lid (MacQueen, Bullard, & Galizio, 2011) produces a food reinforcer and the odor presented also serves a sample for subsequent trials. Thus, on the next trial two cups are presented in the arena, one with the original odor and the other a new odor. In keeping with the nonmatching contingency, only responding to the new odor is reinforced. The session continues with a new odor (S+) presented on each trial along with previously presented comparison stimuli (S−). As the session continues, the number of sample stimuli to remember increases, and so the OST permits the analysis of stimulus control by a progressively incrementing number of stimuli.
In the initial research with the OST in rats, Dudchenko et al. (2000) found that accuracy decreased through the session as the number of odors to remember increased up to twelve and that the average number of trials until the first error was made (span length) was just over eight. Since then, the OST has been used as a rodent model for the study of working memory capacity in a number of neurobiological studies in both rats (Davies, Greba, & Howland, 2013; Davies, Molder, Greba, & Howland, 2013; Turchi & Sarter, 2000) and mice (Young et al., 2007). The CNTRICS (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia) group nominated the OST as a benchmark task to assess working memory capacity (Dudchenko, Talpos, Young, & Baxter, 2013).
The OST has recently been used as a baseline to study drug effects, but at present only a few drugs have been evaluated. Several studies have investigated the effects of N-methyl-D-aspartate (NMDA) receptor antagonists on OST performance. These compounds have been of interest because an NMDA receptor blockade prevents hippocampal long-term potentiation, which is linked to learning and memory, and because they have been shown to interfere with a variety of memory tasks (see Bannerman, Rawlins, & Good, 2006, for a review). Perhaps surprisingly, although NMDA antagonists interfere with DMTS accuracy, they generally do so only in a delay-independent fashion (Dix, Gilmour, Potts, Smith, & Tricklebank, 2010; Pontecorvo, Clissold, White, & Ferkany, 1991; Smith et al., 2011; Willmore, LaVecchia, & Wiley, 2001). So it is of some interest that both MacQueen et al., (2011) and Galizio, Deal, Hawkey, and April (2013) found that the non-competitive NMDA antagonist MK-801 (dizocilpine) impaired OST accuracy at doses that had no effect on a simple odor discrimination. In these studies, the effects of the NMDA antagonist depended on the number of stimuli to remember with impairment of accuracy greatest as the number of odors to remember was highest. Davies et al. (2013) found similar impairment of OST accuracy by the competitive NMDA antagonist CPP at doses that had no effect on response latency. Rushforth, Steckler, and Shoaib (2011) also demonstrated interference with OST performance after exposure to an NMDA antagonist, but in this case, subchronic administration of ketamine was shown to produce enduring reductions of span length in rats that lasted for 10 days or more after ketamine administration was discontinued.
The positive GABAA modulator chlordiazepoxide is the only additional drug that has been shown to selectively interfere with odor span. Galizio et al. (2013) found that chlordiazepoxide reduced span length at doses that did not affect either overall OST accuracy or simple discrimination performance. In contrast, potentially amnestic drugs such as MDMA, morphine, and scopolamine impaired OST performance only at doses that also produced comparable impairments in odor discrimination (Galizio et al., 2013; Hawkey, April, & Galizio, 2014). Finally, Rushforth and colleagues have shown enhancement of OST performance (increased span length) by nicotine (Rushforth, Allison, Wonnacut, & Shoaib, 2010; Rushforth et al., 2011).
The present study was designed to extend the behavioral pharmacological analysis of the OST to several additional drugs. One goal of the present study was to determine whether the enhancement of OST performance produced by nicotine could be observed with psychomotor stimulant drugs methamphetamine and methylphenidate. Another goal was to determine whether selective effects of MK-801 and chlordiazepoxide previously shown in our laboratory could be replicated with a different NMDA antagonist (ketamine) and positive GABAA modulator (flunitrazepam). Although Rushforth et al. (2011) studied the effects of subchronic ketamine, there is currently no research on the acute effects of any of these compounds on OST performance. As in previous research in our laboratory, the present experiments used a version of the OST modified to include a simple discrimination task to serve as a control for drug effects unrelated to within-session remembering. The procedure was also modified to include conditions addressing the possibility of control by odors other than the programmed stimulus odor and to separate the potential confound between the number of odors to remember and the number of comparison stimuli presented on a given trial.
Methods
Subjects
Subjects were 19 adult male Holtzman Sprague–Dawley albino rats ranging between 90–150 days old at the beginning of training. All rats were individually housed on a 12 hr light–dark cycle with free access to water. Food was restricted to maintain stable body weights of approximately 85% of free-feeding levels. Animal care and procedures were approved by the UNC Wilmington Animal Care and Use Committee and followed national guidelines.
Apparatus
The apparatus was an elevated circular arena 94 cm in diameter, bordered by a 32 cm high wall of sheet metal baffling. Eighteen holes, 5.5 cm in diameter, were placed in the arena floor in two concentric circles. Twelve evenly spaced holes formed an outer ring, 2.5 cm from the arena wall, and six evenly spaced holes formed an inner ring, 21.5 cm from the arena wall (see Galizio et al., 2013 for more details). Plastic cups (2 oz.) blocked each hole during sessions. White noise (c. 74 dB) was presented throughout the sessions and a digital video camera recorded each trial. In order to avoid cuing, the experimenter stood out of view of the rat during trials and observed the rat’s behavior on the video monitor.
Stimuli
Odorants were presented on plastic lids that were stored in covered plastic containers containing a number of household spices and scented oils: allspice, almond, anise, banana, bay, bubble gum, caraway, carob, celery, cherry, cinnamon, clove, coriander, cumin, dill, fennel, fenugreek, garlic, ginger, grape, marjoram, mustard, nutmeg, onion, oregano, paprika, peach, pineapple, rosemary, sage, savory, spinach, strawberry, sumac, thyme, turmeric, Worcestershire, and vanilla (most purchased from Great American Spice Co). These scented lids were placed over plastic cups filled with approximately 1 cm sand and inserted into the arena to serve as odor stimuli.
Procedure
Shaping
In an initial session, rats were habituated to the arena with each of the 18 stimulus cups open and baited with a 45 mg sugar pellet (BioServe). This procedure was repeated until rats were reliably consuming pellets from each cup location. A shaping procedure was then used to train removal of unscented lids from the stimulus cups. Initially, trials consisted of a single stimulus cup placed in a random location with a lid partially covering the opening of the cup. On successive shaping trials, the lid was positioned to more fully cover the opening of the cup until rats consistently removed lids that completely covered the cups (see Table 1 for summary of training procedures).
Table 1.
Training procedure.
| Phase | Description | Trials per Session | Criterion to Advance to Next Phase |
|---|---|---|---|
| Shaping |
|
24 | Remove lid to obtain reinforcement on all trials in session |
| OST Initial Training |
|
24 | 10 consecutive correct responses within a session or two sessions with 5 consecutive correct responses |
| Baseline Training-OST Only |
|
24 | 70% accuracy for two consecutive sessions |
| Addition of SDC Trials to Baseline |
|
30 | Two consecutive days at 100% accuracy on SDC trials |
| Final Baseline Procedure |
|
30 | Stability |
Initial OST training
OST training began on the session after shaping was complete. Throughout the study the stimulus odors and locations were assigned randomly and the definition of a response was the displacement of a stimulus lid from the cup using the paws or snout. A correction procedure was used such that the trial continued until the correct lid was removed. On the first trial of each session, the arena contained one stimulus cup covered with an odorant lid (Odor A) and baited with a sugar pellet. The subject was then placed in the arena until it removed the lid and consumed the sucrose pellet or until 2 min passed, whichever came first. The rat was then removed from the arena and remained in a holding cage during an ITI of approximately 1 min. On the second trial, two odorized lids were placed over cups with one scented by Odor A placed in a different spatial position, and the other covered by a differently scented lid (Odor B). To prevent scent marking to serve as a possible cue, lids were only used once per session. As the OST is an incrementing non-matching-to-sample procedure, reinforcement was available only for removal of the lid scented with the new odor (B). Similarly, on Trial 3, three lids covered cups in the arena and responses to a new odor (C), but not A or B, were reinforced. Again, the spatial locations of cups on each trial were assigned randomly. This incrementing procedure continued with each trial including one new stimulus (reinforced) and all of the previously presented stimuli (nonreinforced) until an error was made—that is, until one of the S− comparison lids was selected. On the first trial after an error, only a single new odor was presented and the incrementing procedure then continued. Session duration was 24 trials, thus exposing the rat to 24 different odors. This training procedure continued until the animal met a criterion of 10 consecutive correct responses within a session or two sessions with 5 consecutive correct responses.
Baseline OST training
The baseline OST procedure, outlined in Table 1, was different from that of initial training in three ways. First, the number of comparison stimuli no longer reset to one following an error. Second, while the number of odors to remember continued to increment up to 24 during each session, the number of comparison stimuli presented in the arena was permitted to increment to only five. Thus, the procedure was identical to initial training until Trial 6. From Trials 6 through 24, the S+ comparison odor new to the session was presented along with four previously encountered comparison S− odors. These four were randomly selected on each trial from the set of previously presented stimuli. In this way, the size of the array of comparison stimuli was held constant at five (from Trial 5 on) while the number of stimuli to remember continued to increment up to 23, thus removing the confound between the number of stimuli to remember and the number of comparison stimuli which is present in many OST studies.
The third difference between initial and baseline training was the addition of a performance control: a simple odor discrimination using five scents not included in the pool for OST trials. One of these was arbitrarily designated as S+ and the other four as S− such that responses to S+ were always reinforced, but responses to any of the S− stimuli were never reinforced. The simple discrimination control (SDC) was introduced after a criterion of at least 70% accuracy for two consecutive sessions was reached. Then six simple discrimination trials were introduced at the end of each OST session (integration of the SDC is shown in Table 1). When animals were responding with accuracy on both trial types, the six simple discrimination trials were interspersed with the 24 OST trials making the final baseline procedure 30 trials in duration. Training on this baseline continued until each animal met a 10-session, 15% stability criterion (Perone, 1991) on both OST and simple discrimination accuracy with five comparison stimuli before continuing on to the drug administration phase of the study. This criterion required that the difference between mean percent correct for Sessions 1–5 and 6–10 be less than 15% of the overall 10-session mean. Number of sessions required to meet this criterion are shown in Table 2.
Table 2.
Session and unbaited control data for rats in all experiments.
| Rat ID | Sessions to Criterion | % Correct Unbaited | % Correct Baited | Drug(s) |
|---|---|---|---|---|
| A11 | 40 | 76.7 | 80.8 | METH, FLZ |
| D2 | 39 | 91.7 | 92.4 | METH |
| E1 | 51 | 84.5 | 86.7 | FLZ |
| E2 | 37 | 95.8 | 89.3 | KET, MPD |
| E4 | 35 | 92.1 | 91.9 | KET |
| E5 | 25 | 84.9 | 88.0 | KET |
| E13 | 26 | 93.8 | 94.9 | KET |
| F16 | 49 | 71.3 | 78.4 | FLZ |
| W20 | 69 | 94.8 | 93.5 | METH, MPD |
| W21 | 43 | 90.8 | 90.1 | MPD |
| W22 | 42 | 93.0 | 90.1 | MPD, METH |
| W29 | 36 | -- | -- | MPD |
| X3 | 38 | -- | -- | MPD |
| Y2 | 58 | 83.2 | 80.3 | FLZ |
| Y16 | 35 | 88.4 | 89.9 | FLZ |
| Y17 | 46 | 85.8 | 86.0 | FLZ |
| Y18 | 37 | 85.3 | 83.6 | KET |
| Z3 | 39 | 83.3 | 82.8 | KET, METH |
| Z12 | 60 | 96.9 | 96.4 | METH |
| Mean | 42.4 | 87.8 | 88.0 |
Pellet detection controls
To control for possible olfactory detection of the sugar pellet during testing, unbaited control trials were conducted as well. During one baseline training session per week, six randomly selected trials were conducted without sugar pellets present in the correct stimulus cup (S+). Instead, the experimenter manually placed a pellet in the correct stimulus cup after the trial was completed, before the subject was removed from the arena. Table 2 shows percent correct on unbaited and baited trials on these sessions for 17 of the 19 rats (two rats were not tested with this procedure). Generally, accuracies on baited trials were quite close to those obtained on unbaited trials showing that behavior was controlled by the scented lids and not the odor of the pellet (see Table 2).
Drugs
Ketamine hydrochloride (Sigma), methamphetamine hydrochloride (Sigma), and methylphenidate hydrochloride (Sigma) were dissolved in 0.9% saline solution and administered in a volume of 1 ml/kg. Flunitrazepam (National Institute of Drug Abuse Research Supply Program, RTI) was dissolved in a vehicle of 20% ethanol, 40% propylene glycol and 40% saline and administered in a volume of 1 ml/kg.
Drug Protocol
Once stability criteria were met, the drug experiments began with the following testing sequence. Sessions were conducted Monday through Friday with i.p. injections administered prior to the session onset on three sessions per week—generally Tuesday, Thursday, and Friday. One injection each week was always saline or vehicle and the other two were doses of the drug to which the rat was assigned. The first few injections for each rat were saline or vehicle only, and the first determination of drug doses was administered in ascending order beginning with a dose expected to be without effect. Subsequent determinations were ordered randomly with the constraint that each dose was presented before a new cycle of determinations was begun. Doses were assigned with the goal of identifying a dose that was without effect and one that suppressed both OST and SDC accuracy for each rat. In some cases, this technique resulted in the introduction of lower doses for some rats and higher doses for others after the initial cycle of determinations. Generally two to four determinations were made of each dose, depending on how much variability was observed between determinations. If responding was completely eliminated on the first determination of a high dose, it was not repeated. Five of the rats were exposed to two of the study drugs (see Table 2). In these cases, 2 weeks of testing without injections intervened between the two drug studies.
Behavioral Measures and Interrater Reliability
The first response in each trial was used to determine accuracy on both OST and SDC trials. A failure to respond within 2 min was considered a response omission and such trials were removed from the denominator to calculate percent correct. Programmed trials were repeated following omissions and the session was terminated following four consecutive omissions. Span length was defined as the number of consecutive correct trials until the first error of the session (excluding Trial 1 on which only a single stimulus was present). The longest run of consecutive correct responses was also recorded, as this was not always identical to the span length. For example, a rat might make an error on Trial 2 producing a span length of 1. The rat could then go on to make correct responses on Trials 3–15 producing a run of 13 consecutive correct which would be recorded as the longest run of the session. Thus, the longest run is always greater than or equal to the span length.
High doses of each drug generally increased response omissions, but casual observations during the study suggested that the topography of behavior varied across drugs. To assess this possibility, observers scored videos of all injection sessions with respect to several variables. These included the number of visits to S− without responding (approaching to within 1 cm of S− lid), visits to S+ (approaching to within 1 cm of S+ lid) without responding, rearing (both front paws off the floor), grooming (touching fur with either paw or muzzle), biting cups, urinating, and defecating.
To assess interrater reliability, two observers independently scored the lid removal responses, visits to S− without lid removal, visits to S+ without lid removal, rears, and grooms for 21 video-recorded sessions. This sample was chosen pseudorandomly to include sessions after saline injections and sessions after several different drug manipulations. The proportion of trials with 100% agreement between observers was calculated for each dependent measure for each session, resulting in very strong interrater reliability for the lid removal response (M = 1.0), visits to S+ without lid removal (M = .95, SD = .12), rears (M = .92, SD = .13), grooms (M = .97, SD = .06), and visits to S− per session (M = .92, SD = .06). For the most common behavior, visits to the S− lid without lid removal, observers demonstrated satisfactory reliability for proportion of trials with 100% agreement per session (M = .82, SD = .09). Grooming, urination, defecation, and lid biting occurred so rarely that they were not analyzed further.
Results
Span Length and Longest Run
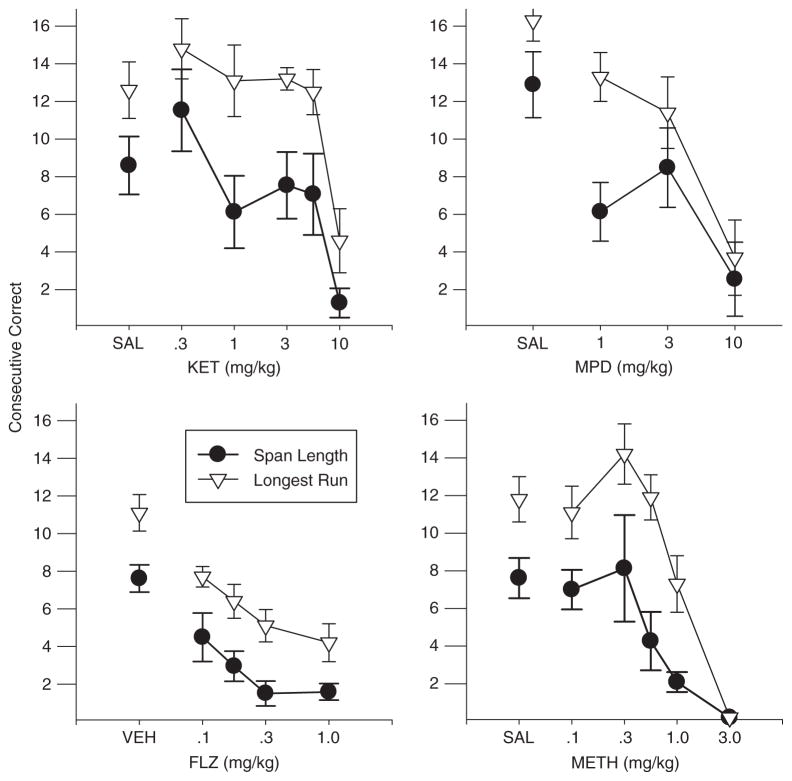
Figure 1 shows the mean span length and longest run of consecutive correct responses as a function of dose for each drug and reveals dose-dependent decreases in each case. As in previous research using the OST, mean span lengths were close to eight odors under control (saline and vehicle) conditions in three of the groups, but note the higher control span for the rats in the methylphenidate group. Note also that under control conditions and at most drug doses, the longest run was generally higher than the span length. Because the span length is defined by the first error in the session, a longest run that is greater than span length indicates that the first error of the session was not necessarily followed by inaccurate performances. In most span studies with humans, the span length (number of trials to the first error) is also the longest run of correct responses, so it is noteworthy that this was not the case with rats in the OST. Individual subject data presented in Appendices 3–6 indicate considerable variability in both measures across subjects and conditions, but longest runs were substantially greater than span in almost every case.
Fig. 1.
Mean span length and longest run plotted as a function of dose for each of the study drugs. Vertical bars indicate standard error.
The effects of all four drugs on span and longest run were generally quite similar. There was a slight increase in span length and longest run at the 0.3 mg/kg dose of ketamine and in longest run at the 0.3 mg/kg dose of methamphetamine, but otherwise each drug decreased span length relative to vehicle and the greatest declines were obtained at the highest doses when span lengths approached zero for each drug. One-way, repeated-measures ANOVAs were performed and statistically significant outcomes were obtained for each drug on both measures (flunitrazepam span F(4, 20) =11.71, longest run F(4,20) = 16.87; ketamine span F(5,25) = 4.29, longest run F(5,25) = 9.30; methamphetamine span F(5,25) = 6.16, longest run F(5,25) = 30.03; methylphenidate span F(3,15) = 8.55, longest run F(3,15) = 11.40; p < .05 in all cases). Under saline/vehicle conditions, the span lengths ranged from a low of 3.89 (Rat Z3, Appendix C) to 19.0 (Rat X3, Appendix D) and longest runs from 6.44 (Rat A11, Appendix A) to 19.7 (Rat W29, Appendix D). Inspection of the individual subject data as a function of drug dose reveals considerable variability, but the dose-dependent decrease in span length and longest run was evident in every case. Increases in span length at the individual subject level were rare, with substantial increases occurring on only a few occasions (e.g., methamphetamine, Rat Z12; ketamine, Rats E13, Z3). In contrast, ketamine and methamphetamine resulted in increased longest runs in most rats at one or more of the low doses. Another difference between the two measures is evident in the effects of methylphenidate, which produced a striking decrease in span length even at the lowest dose (1.0 mg/kg). However, longest run was much less affected, dropping only slightly below the control mean until the higher doses were reached.
Reliable reductions in span lengths and longest runs generally occurred at high doses of each drug and thus may reflect general disruptions in responding. However, even at moderate doses reduced spans might be due to multiple factors, so analyses of percent correct in OST and SDC are critical to the interpretation of the drug effects. These analyses focus on individual subject data and with separate sections for each drug condition shown in Figures 2–6 with baseline data presented in Appendix E.
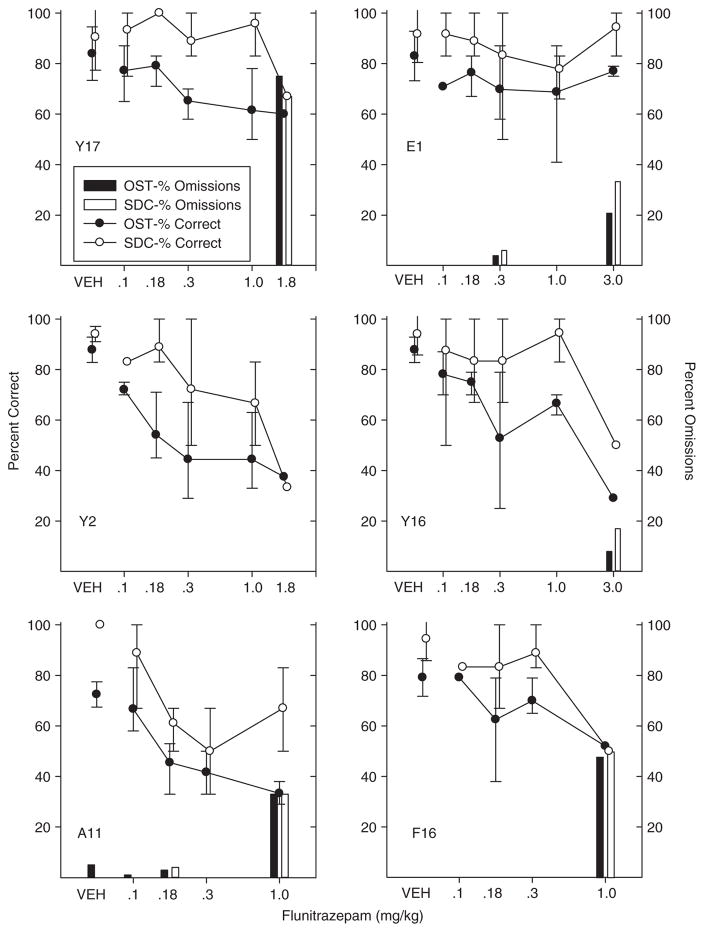
Fig. 2.
Percent correct and omissions for OST and simple discrimination for individual subjects in the flunitrazepam study. Circles show percent correct for OST (black) and SDC (white). Black bars show percent omissions for OST and white bars show SDC omissions. Vertical lines show standard deviation for vehicle (based on eight or more sessions) and indicate the range across all determinations for drug doses.
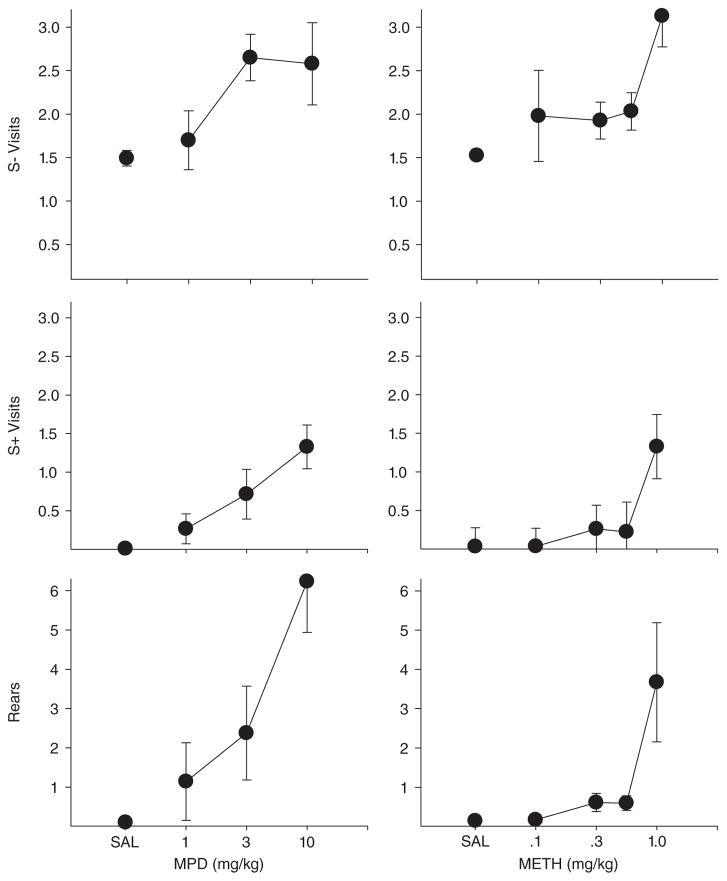
Fig. 6.
Number of visits without responding per trial to the S− comparison cups (top panels), number of visits without responding per trial to the S+ cup (middle panels), and number of rears per trial. Left panels show MPD effects and right panels show methamphetamine. Vertical lines show standard deviations.
Flunitrazepam
Figure 2 shows individual subject data for the flunitrazepam study. Under control conditions (vehicle-VEH), accuracy was high (90% correct or better) on SDC trials and only somewhat less so on OST trials (generally 75–85% correct). There was considerable variability across determinations along the flunitrazepam dose–response function. Still it is clear that at high flunitrazepam doses, both OST and SDC performances were disrupted in all rats and most showed some response omissions. The dose necessary to produce these overall performance disruptions varied across rats from 0.18 for Rat A11, 1.0 mg/kg for Rat F16, 1.8 mg/kg for Y17 and Y2, and 3.0 mg/kg for E1 and Y16.
At lower doses of flunitrazepam, disruption in OST accuracy was seen at doses that did not affect SDC accuracy in most of the rats. For example, Rat Y17’s OST accuracy (Fig. 2, upper left) dropped from above 80% under control conditions almost to 60% at the 0.3 and 1.0 mg/kg doses, whereas the SDC remained at control levels. Robust selective effects were also observed in Rats Y2 and Y16 (middle panels of Fig. 2). Flunitrazepam produced a small decrease in OST accuracy in Rat Y2 at the 0.1 mg/kg dose, but at the 0.3 mg/kg dose OST accuracy dropped nearly to 50% correct with SDC performances remaining only slightly below control levels. OST accuracy continued to decline at higher levels of flunitrazepam, but these doses were accompanied by SDC effects and considerable variability as well. Unlike the other rats, Y2 did not omit responding at any dose and, although accuracy was low (below 40% correct) at the single determination of the highest dose given (1.8 mg/kg), even here it remained at above 20% (chance levels) in both tasks. Rat Y16 appeared less sensitive to flunitrazepam, but showed large effects that were selective to the OST at the 0.3 and 1.0 mg/kg doses; only at the 3.0 mg/kg doses were both OST and SDC accuracies affected.
Rats E1 and F16 showed some evidence of selective effects, but these were weaker than those noted above. Rat E1 (upper right) showed small declines in percent correct from the 0.1 mg/kg flunitrazepam dose through the 1.0 mg/kg dose, but SDC also dropped below control levels at the 0.3 mg/kg dose and there was considerable variability in responding at all the higher doses. Rat F16 (bottom right) showed some evidence of an OST impairment without SDC effects at the 0.18 and .3 mg/kg doses, but again, there was considerable variability at both doses. Finally, one rat (A11—bottom left) showed only nonselective effects of flunitrazepam. Rat A11 seemed to be highly sensitive to flunitrazepam, showing some decrease in percent correct in both OST and SDC at every dose tested.
In summary, flunitrazepam tended to produce selective effects in most rats—that is, it disrupted OST without affecting SDC accuracy. These selective effects were of fairly large magnitude in three rats (Y17, Y2 and Y16), but were weaker or less consistent in two (E1 and F16) and were absent in Rat A11. Interestingly, the doses which produced selective effects varied across rats. For example, consider that Rats Y17 and Y16 showed no effects of flunitrazepam until the 0.3 mg/kg dose was reached and although it was selective for these two rats, this same dose was high enough to disrupt both OST and SDC performances in rats E1 and Y2 (as well as A11). Given the varying sensitivity to flunitrazepam shown here, it is worth noting that analysis based on group averages would fail to detect the selective effects observed in the individual subject analyses of overall accuracy. However, group data presented for span length and longest run showed statistically significant effects even at the 0.1 mg/kg dose of flunitrazepam, a dose that had minimal effects on SDC.
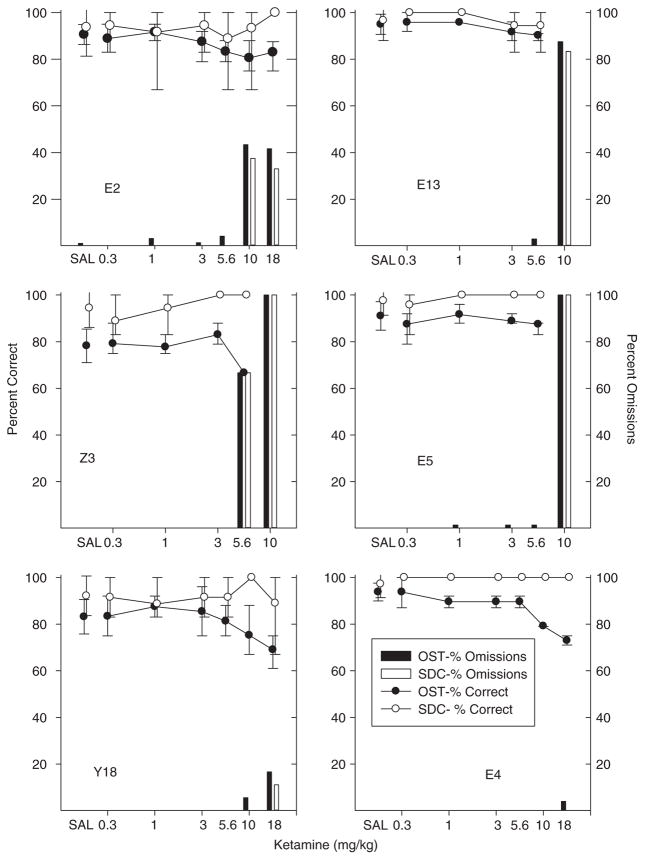
Ketamine
Figure 3 shows individual subject data from the ketamine study. Four animals (E2, E13, E5 and Z3) showed virtually no effects of ketamine until doses were reached that resulted in a substantial percentage of omissions. Rat Z3 (middle left) was unaffected until the 5.6 mg/kg dose of ketamine was reached at which point most trials were omissions. Other rats were less sensitive to ketamine. For Rat E2 (upper left) omissions were frequent at the 10.0 and 18.0 mg/kg doses, but responding remained accurate when it occurred. Rats E13 and E5 (top and middle right) also began to show effects only at the 10.0 mg/kg dose, but in both cases nearly all trials were omissions at this dose. Rat Y18 (bottom left) showed fewer omissions even at the highest (18.0 mg/kg) ketamine dose. There was a slight, albeit inconsistent, drop in OST accuracy at the 10.0 mg/kg dose without a SDC decline, and a further drop in OST accuracy at the 18.0 mg/kg dose, but this drop was accompanied by a highly variable SDC performance. Finally, Rat E4 (bottom right) showed a clearly selective effect of ketamine. Accuracy on the OST dropped below control levels at the 10.0 mg/kg and 18 mg/kg dose. Accuracy on SDC trials was not affected at either dose and omissions were rare at any dose. In sum, ketamine effects were nonselective for most rats, but a weakly selective effect was observed in Rat Y18 and a strongly selective effect for Rat E4.
Fig. 3.
Percent correct and omissions for OST and SDC for individual subjects in the ketamine study. Circles show percent correct for OST (black) and SDC (white). Black bars show percent omissions for OST and white bars show SDC omissions. Vertical lines show standard deviation for saline (based on eight or more sessions) and indicate the range across all determinations for drug doses.
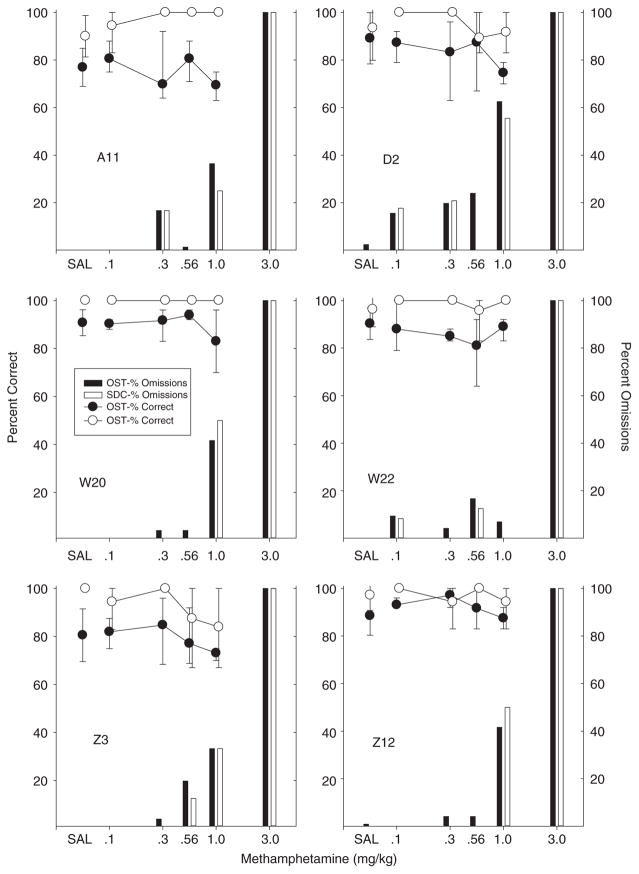
Methamphetamine
Percent correct after methamphetamine injections are shown in Figure 4 and the outcomes here were quite similar for all six rats. In each subject, methamphetamine failed to affect accuracy of responding on either OST or SDC trials until doses were reached that resulted in a high percentage of omissions. No enhancement of accuracy was observed on either OST or SDC trials at any dose; the main effect observed was disruption of responding.
Fig. 4.
Percent correct and omissions for OST and SDC for individual subjects in the methamphetamine study. Circles show percent correct for OST (black) and SDC (white). Black bars show percent omissions for OST and white bars show SDC omissions. Vertical lines show standard deviation for saline (based on eight or more sessions) and indicate the range across all determinations for drug doses.
Methamphetamine never really produced an increase in errors in most rats, and even in cases that did show a reduction in accuracy (D2, W20 at 1.0 mg/kg), both SDC and OST were affected and 40% or more of the trials were omissions at this dose. The striking feature of these data was the increase in omissions at high doses in all six rats. Also of note was that methamphetamine produced a substantial number of omissions even at moderate doses in most rats. For example, D2 failed to respond on over 10% of the trials at the 0.1 mg/kg dose and both A11 and D2 omitted responding on over 10% of the trials at the 0.3 mg/kg dose. In summary, methamphetamine did not produce selective effects at any dose in any of the six rats.
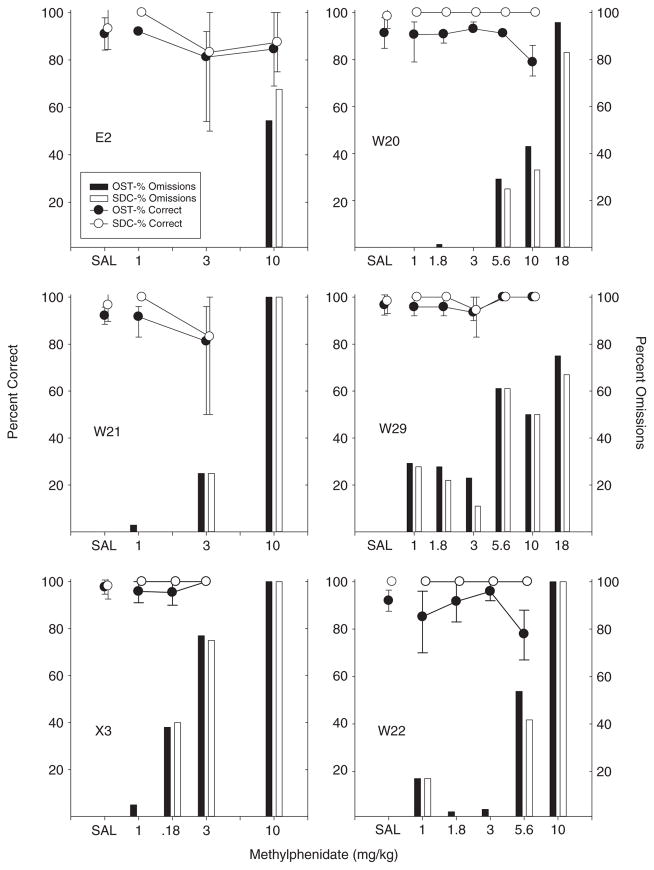
Methylphenidate
The effects of methylphenidate were very similar to those obtained with methamphetamine as is evident from Figure 5. Although rats differed considerably in sensitivity to methylphenidate, the general pattern was that accuracy was affected only slightly and non-selectively or not at all up through doses that resulted in a high percentage of omissions. No enhancement of either OST or SDC accuracy was obtained in any rat. Some decreases in both OST and SDC accuracy were observed, but generally these were either quite variable (e.g., E2, W21 at 3.0 mg/kg) or occurred only when doses that produced substantial omissions were reached. Rats X3 and W22 were relatively sensitive to the disruptive effects of MPD with X3 failing to respond on over 75% of the trials at the 3.0 mg/kg dose and W22 over 40% omissions at the 5.6 mg/kg dose. Finally, rats W20 and W22 did show some selective impairment of OST accuracy (at the 5.6 mg/kg dose for W20 and the 10.0 mg/kg dose for W22), but unlike the selective effects noted with flunitrazepam, both rats also omitted responses on more than 30% of the trials at these doses. As was noted with methamphetamine, omitted responses were observed even after low doses of methylphenidate in several rats—particularly X3 and W29. In sum, methylphenidate impaired OST responding only at doses that also disrupted SDC responding or (in the case of Rats W20 and W22) at doses that also produced frequent response failures.
Fig. 5.
Percent correct and omissions for OST and SDC for individual subjects in the methylphenidate study. Circles show percent correct for OST (black) and SDC (white). Black bars show percent omissions for OST and white bars show SDC omissions. Vertical lines show standard deviation for saline (based on eight or more sessions) and indicate the range across all determinations for drug doses.
Observational Analyses
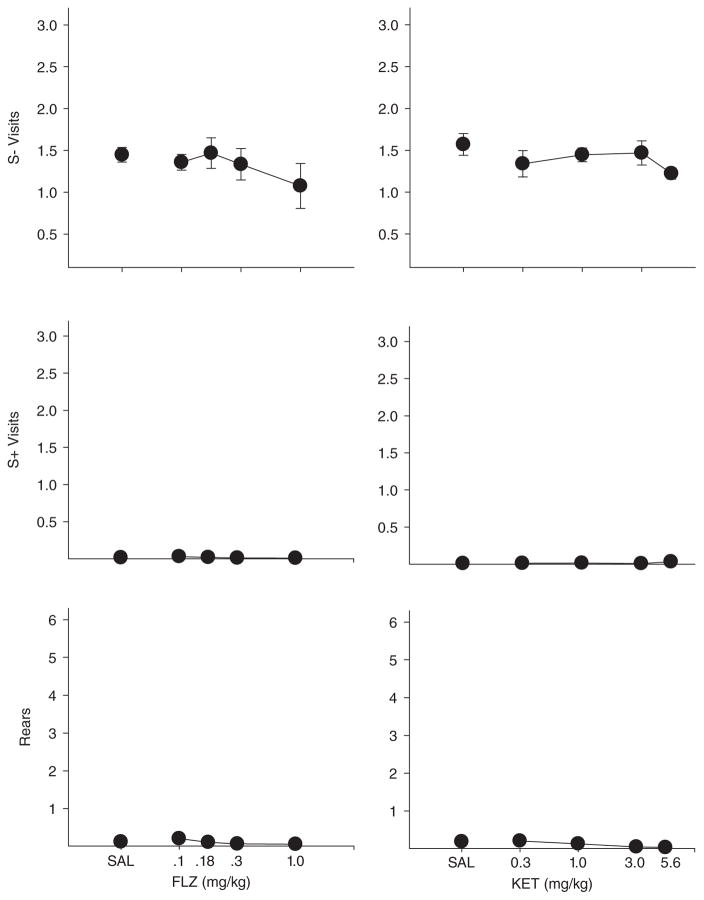
Although each drug produced a large number of omissions at moderate to high doses, it was clear to the experimenters that the topography of behavior on omission trials differed as a function of drug. Video analysis was used to develop a richer understanding of how each drug was altering behavior. Some of the behaviors scored occurred at such a low rate that data are not presented here (grooming, urination, defecation, and biting the cups). The main outcomes are shown in Appendices A–D and Figures 6 and 7. Under vehicle conditions, rearing and visits to S+ without responding were quite rare across all groups whereas visits without responding to S− were relatively more frequent. As Figure 6 clearly shows, methylphenidate and methamphetamine increased OST visits (both to S+ and S− lids) and rearing in a dose-dependent fashion whereas these measures were largely unaffected by ketamine and flunitrazepam (Fig. 7). Inspection of Appendices A–D confirms that the mean outcomes shown in Figures 6 and 7 were generally representative of individual subject data. One of the few exceptions was Rat W20 in the methamphetamine study that showed an increase in S− visits, but not rearing or S+ visits. The other exception was Rat E2 that showed some increase in S+ visits at the 10.0 mg/kg ketamine dose. It also should be noted in Appendices A and B that at high doses of both flunitrazepam and ketamine most rats showed a decline in the number of S − visits. This effect is not apparent in Figure 7 because the decreases in S− visits occurred in some rats at doses not shown in the figures (only doses to which all rats were exposed are presented in the figures). Inspection of Appendices A and B reveal that as higher doses of ketamine and flunitrazepam were reached, rats simply became inactive and visits to all cups decreased. In sum, while all four drugs in the present study produced response failures at intermediate to high doses, the topographical effects of the drugs were different. Methamphetamine and methylphenidate increased interim activities such as rearing and visiting cups without lid removal, but ketamine and flunitrazepam either had no effect or reduced these activities.
Fig. 7.
Number of visits without responding per trial to the S− comparison cups (top panels), number of visits without responding per trial to the S+ cup (middle panels), and number of rears per trial. Left panels show flunitrazepam effects and right panels show ketamine. Vertical lines show standard deviations.
Discussion
The present study demonstrated the feasibility of the OST as a baseline of interest in the study of complex stimulus control. Rats were trained to respond in this incrementing non-match-to-sample procedure such that behavior was under the control of a progressively increasing number of odor stimuli during each session. Relatively high levels of accuracy were obtained with up to 23 stimuli to remember in the present study. Importantly, a second task involving a simple discrimination control (SDC) with five odors not used in the OST was included within each session without loss of accuracy on either task. OST and SDC trials were intermixed in the baseline and rats were able to discriminate between the stimuli associated with each task and behave appropriately by responding to the new odor on OST trials and to the familiar S+ on SDC trials. Further, the SDC condition provided an important comparison with which to evaluate the nature of drug effects on behavior under complex stimulus control. Stable baseline levels of accuracy on both tasks were obtained and maintained across well over 100 sessions in most animals, making this procedure one that is suitable for behavioral pharmacological analysis.
All four drugs produced dose-dependent reductions in span length, longest run, and overall accuracy, but the nature of the effects was different across drugs. Methamphetamine and methylphenidate decreased span length and longest run at moderate to high doses, but it would be a mistake to conclude that these effects were related to within-session remembering as they were not selective to the OST. Rather, these same doses tended to result in response omissions on both SDC and OST trials and when an increase in errors was noted, this was generally nonselective with both OST and SDC accuracy impaired. This point is of some importance because many OST studies use only span length as the dependent measure of interest and do not include an SDC or comparable control condition (e.g., Rushforth et al., 2010; 2011). Without such controls drug effects may be misattributed to within-session remembering (working memory). There was little evidence for selective impairment of within-session stimulus control by methamphetamine or methylphenidate in the present study.
The results from the ketamine study were more complex, and provided evidence for a selective effect on remembering only in two rats. Although mean span length and longest run declined sharply at the 10 mg/kg ketamine dose, three of the six rats (E5, E13 & Z3) virtually stopped responding at that dose and another (Rat E2) failed to respond on over 1/3 of the trials. However, Rats E4 and Y18 did not show impaired SDC accuracy or frequent omissions at the 10 mg/kg ketamine dose, and OST performance was impaired in both. In the case of Rat E4, OST accuracy was consistently below control levels at both 10 and 18 mg/kg doses. For Rat Y18, however, the effect was less consistently selective.
In contrast, flunitrazepam produced selective impairments of OST performance in several rats. Flunitrazepam reduced OST accuracy without increasing omissions in all six rats and robust effects were obtained at doses with little or no effect on the SDC in three rats, with less consistent selective effects observed in two others. Also consistent with the claim that flunitrazepam selectively affected OST performance was the finding that both span length and longest run were disrupted even at the lowest flunitrazepam doses that had little or no effect on accuracy on SDC trials (with the exception of Rat A11). It may be of importance that the rat which failed to show selective effects of flunitrazepam (Rat A11) on any measure also showed the least accurate OST baseline levels of any rat studied here. Perhaps the weak control under baseline conditions was the basis of this rat’s heightened, but nonselective, sensitivity to flunitrazepam.
There is little previous research on psychostimulant drugs using the OST, but it is noteworthy that neither methylphenidate nor methamphetamine enhanced accuracy at any dose. Amphetamines and methylphenidate have a long history of use as treatments for Attention-Deficit-Hyperactivity-Disorder with the expectation that they enhance working memory and attention. Generally, animal research has not supported a role for either drug in the enhancement of remembering. For example, studies of methylphenidate and amphetamines on delayed-matching-to-sample performances have generally found only delay-independent impairment (Baron, Wright, & Wenger, 1998; Harper, Wisnewski, Hunt, & Schenk, 2005; Wright & White, 2003). These results closely parallel the present findings with multiple stimuli to remember in the OST—generally impairment that was task-independent and often occurred at relatively low doses was found. These findings were also quite similar to a previous study from our laboratory which found that MDMA also produced only nonselective impairment of responding with the OST/SDC procedure (Hawkey, April, & Galizio, 2014). Although none of the rats showed increased accuracy after either methamphetamine or methylphenidate, it must be noted that baseline levels were high in both tasks and it is possible that a ceiling effect may have obscured observation of enhancement. Supporting this possibility was the finding that methamphetamine increased span and longest run in at least one dose for four of the six rats tested (see Appendix C). Perhaps these measures of consecutive correct responses are more sensitive to the performance-enhancing properties of stimulant drugs in this procedure. However, the finding that ketamine also increased span and longest run in several rats at low doses and that methylphenidate generally failed to do so raises questions about such an interpretation.
Perhaps the most surprising finding was the failure of ketamine to consistently produce selective OST impairment, because there is considerable evidence that OST performance can be disrupted by NMDA antagonists. Using procedures similar to those of the present study, previous research has demonstrated that the uncompetitive NMDA receptor antagonist MK-801 (dizocilpine) and the competitive NMDA antagonist CPP reduced OST accuracy at doses which had no effect on SDC accuracy (Davies et al., 2013; Galizio et al., 2013; MacQueen et al., 2011). Other studies support an important role of NMDA receptor activity in OST performance. For example, central blockade of NMDA receptors in prefrontal cortex (Davies et al., 2013) and subchronic exposure to ketamine (Rushforth et al., 2011) have also been shown to impair OST performances. Although the present study is the first to look at acute ketamine effects, based on the above literature, we expected to see more consistent evidence of disruption of OST by the noncompetitive NMDA antagonist ketamine. That said, researchers using a variety of behavioral preparations have found that NMDA antagonists such as ketamine, MK-801, phencyclidine and others do not always produce common behavioral effects despite similar mechanisms of action (e.g., Dix et al., 2010; Smith et al., 2011). It is apparent that further research with various compounds active at the NMDA receptor will be important to determine its role in within-session remembering (Bannerman et al., 2006).
Flunitrazepam produced the most consistent evidence for selective effects on OST performance in the present study and this finding is consistent with previous work showing amnestic effects of benzodiazepine drugs across a variety of procedures. There is only one previous study of benzodiazepine effects using the OST and it showed that chlordiazepoxide reduced span length at doses that did not affect SDC or produce response omissions (Galizio et al., 2013). However, in that study the effects of chlordiazepoxide were relatively weak, whereas the effects of flunitrazepam were, at least in some rats, rather large. Interestingly, these results closely parallel the findings of Soto, Ator et al. (2013) in monkeys where various positive GABAA modulators impaired accuracy using the SSOS procedure that also assesses remembering of multiple stimuli. It certainly appears that benzodiazepine and related drugs warrant further exploration in the OST, as well as related tasks that can examine performance as a function of the number of stimuli to remember.
Observational analysis of behaviors during OST trials revealed some interesting features both about the OST baseline and the drug effects. Under baseline conditions, all rats showed similar patterns of behavior characterized by multiple visits to S− comparisons and virtually no visits to S+ (recall that visits were defined as approach to within 1 cm of the stimulus cup, but not removing the lid). First, the rarity of S+ visits may provide an indication of the nature of the stimulus control topography of the OST. One type of stimulus control that could develop in the OST would involve selection of the least familiar odor among the group of five comparisons. If this were the case, then presumably rats would visit each stimulus cup before responding. However, in every experiment, rats averaged about 1.5 S− cup visits per trial in control sessions. This, coupled with a virtual absence of S+ cup visits, argues against the notion that stimulus control by relative familiarity of the five comparisons was operative. Rather, it appears that the first time rats approach S+ on a given trial, they are highly likely to respond regardless of how many other cups have been visited. This seems to indicate a more absolute form of temporal control (no response if odor has been smelled during the current session, but respond if it has not). One caveat regarding this conclusion is that visits, as defined in the present analysis, may not have been necessary for detection of some odors. Given that there were five comparison cups available (at least on OST Trials 5–24), 1.5 S− cup visits are fewer than would be expected if S− cup visits were determined by chance alone. This suggests that rats are able to smell the odorants on at least some cups from a distance of greater than 1 cm. Further research and analysis of the topographical patterns of behavior in the OST is needed to better characterize the nature of the stimulus control in this procedure (see also April, Bruce, & Galizio, 2013; Branch, Galizio, & Bruce, 2014, for related discussions).
The observational analyses also revealed some interesting differences in drug effects. All four drugs tended to produce response omissions at higher doses, but the absence of operant responding was accompanied by substantial differences in other behaviors. Both psychostimulant drugs produced dose-dependent increases in both S+ and S− visits as well as in rearing behavior. Although rats were visiting more cups at high doses of methamphetamine and methylphenidate, these doses appeared to disrupt stimulus control of operant responding by cup odor and, as well, appear to have produced an increase in general activity or perhaps adjunctive behavior. In contrast, such effects were rarely produced by ketamine or flunitrazepam. Patterns of cup visits and rearing were unaffected by either drug until doses that suppressed overall activity were reached.
In summary, the OST appears to have potential value in the analysis of complex stimulus control and the behavioral pharmacology of remembering. Much remains to be learned about the stimulus control topography that is developed using this procedure and many drug classes remain to be studied. The procedure has the potential to combine interpolated delay intervals with variations in the number of stimuli to remember; such manipulations might allow analysis of the ways in which combinations of these variables determine drug effects. An obvious limitation of the OST is the manual (and labor-intensive) nature of the procedure, which requires special controls to address the problems of potential control by unauthorized odors and experimenter cuing. The OST procedure could, in principle, be automated using olfactometer technology (Prichard, Panoz-Brown, Bruce, & Galizio, 2015; Slotnick, 2001) and future research could exploit these techniques to enhance the study of remembering multiple stimuli.
Acknowledgments
This research was supported in part by NIH grant DA 029252 to MG. The authors would like to thank Christine Hausmann, Kevin Jacobs, and Luke Watterson for assisting with data collection and to Angela Goolsby for editorial assistance.
Appendix A. Span, longest run, visits and rears in the flunitrazepam (FLZ) study
| Rat & FLZ Dose | Span Length | Longest Run | S− Visits | S+ Visits- | OST Rears |
|---|---|---|---|---|---|
| Y16 | |||||
| VEH | 8.79 | 11.86 | 1.55 | 0.00 | 0.04 |
| 0.1 | 3.25 | 7.50 | 1.69 | 0.00 | 0.05 |
| 0.18 | 2.67 | 8.33 | 1.92 | 0.00 | 0.03 |
| 0.3 | 0.67 | 4.00 | 1.76 | 0.00 | 0.07 |
| 1 | 2.00 | 4.33 | 1.58 | 0.00 | 0.04 |
| 3 | 0.00 | 3.00 | 0.00 | 0.00 | 0.00 |
| Y17 | |||||
| VEH | 8.71 | 12.71 | 1.73 | 0.01 | 0.14 |
| 0.1 | 5.00 | 8.33 | 1.57 | 0.13 | 0.79 |
| 0.18 | 4.33 | 8.67 | 1.88 | 0.11 | 0.32 |
| 0.3 | 0.33 | 6.67 | 1.82 | 0.06 | 0.12 |
| 1 | 0.67 | 7.00 | 1.73 | 0.01 | 0.17 |
| 1.8 | 0.00 | 6.00 | 0.40 | 0.00 | 0.00 |
| Y2 | |||||
| VEH | 8.79 | 11.86 | 1.18 | 0.00 | 0.04 |
| 0.1 | 0.33 | 6.00 | 1.13 | 0.00 | 0.00 |
| 0.18 | 0.33 | 4.33 | 1.67 | 0.00 | 0.00 |
| 0.3 | 0.33 | 4.33 | 1.17 | 0.00 | 0.01 |
| 1 | 0.67 | 2.67 | 1.65 | 0.01 | 0.00 |
| 1.8 | 0.00 | 4.00 | 1.58 | 0.00 | 0.00 |
| A11 | |||||
| VEH | 4.56 | 6.44 | 1.34 | 0.01 | 0.04 |
| 0.1 | 4.33 | 7.33 | 1.19 | 0.05 | 0.05 |
| 0.18 | 1.33 | 3.67 | 0.74 | 0.01 | 0.03 |
| 0.3 | 0.50 | 2.00 | 0.65 | 0.00 | 0.07 |
| 1 | 1.67 | 2.33 | 0.19 | 0.01 | 0.04 |
| E1 | |||||
| VEH | 6.33 | 12.75 | 1.60 | 0.06 | 0.37 |
| 0.1 | 4.00 | 7.00 | 1.20 | 0.00 | 0.16 |
| 0.18 | 5.67 | 8.00 | 1.26 | 0.00 | 0.21 |
| 0.3 | 3.50 | 5.75 | 1.03 | 0.01 | 0.20 |
| 1 | 3.50 | 7.25 | 0.65 | 0.00 | 0.04 |
| 3 | 0.00 | 2.00 | 0.90 | 0.02 | 0.09 |
| F16 | |||||
| VEH | 8.50 | 11.17 | 1.28 | 0.02 | 0.11 |
| 0.1 | 10.00 | 10.00 | 1.37 | 0.01 | 0.22 |
| 0.18 | 3.33 | 5.33 | 1.34 | 0.00 | 0.09 |
| 0.3 | 3.67 | 8.00 | 1.57 | 0.00 | 0.00 |
| 1 | 1.00 | 1.50 | 0.65 | 0.00 | 0.09 |
Appendix B. Span, longest run, visits and rears in the ketamine (KET) study
| Rat & KET Dose | Span Length | Longest Run | S− Visits | S+ Visits | Rears |
|---|---|---|---|---|---|
| E13 | |||||
| SAL | 9.40 | 17.80 | 1.28 | 0.00 | 0.02 |
| 0.3 | 19.50 | 19.50 | 1.33 | 0.00 | 0.02 |
| 1 | 14.00 | 18.00 | 1.31 | 0.00 | 0.02 |
| 3 | 9.33 | 13.33 | 1.31 | 0.00 | 0.00 |
| 5.6 | 0.33 | 17.33 | 1.31 | 0.02 | 0.00 |
| 10 | 0.33 | 3.00 | * | * | * |
| E5 | |||||
| SAL | 9.43 | 13.57 | 1.32 | 0.00 | 0.02 |
| 0.3 | 9.25 | 12.50 | 0.84 | 0.02 | 0.15 |
| 1 | 1.67 | 16.67 | 1.21 | 0.00 | 0.13 |
| 3 | 0.67 | 15.00 | 0.88 | 0.00 | 0.04 |
| 5.6 | 10.00 | 13.33 | 1.17 | 0.00 | 0.00 |
| 10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Y18 | |||||
| SAL | 7.06 | 10.35 | 2.10 | 0.04 | 0.61 |
| 0.3 | 7.75 | 12.00 | 1.98 | 0.04 | 0.42 |
| 1 | 7.33 | 9.67 | 1.64 | 0.08 | 0.14 |
| 3 | 7.25 | 11.00 | 1.79 | 0.00 | 0.13 |
| 5.6 | 4.25 | 9.75 | 1.51 | 0.07 | 0.17 |
| 10 | 4.67 | 10.00 | 1.65 | 0.09 | 0.06 |
| 18 | 0.00 | 7.00 | 1.29 | 0.00 | 0.02 |
| Z3 | |||||
| SAL | 2.44 | 7.33 | 1.75 | 0.00 | 0.17 |
| 0.3 | 4.67 | 9.67 | 1.49 | 0.03 | 0.51 |
| 1 | 5.00 | 6.67 | 1.73 | 0.00 | 0.27 |
| 3 | 6.33 | 13.33 | 1.38 | 0.00 | 0.13 |
| 5.6 | 10.00 | 10.00 | 1.23 | 0.00 | 0.04 |
| 10 | 0.00 | 0.00 | 0.00 | 0.33 | 0.33 |
| E4 | |||||
| SAL | 14.00 | 14.33 | 1.36 | 0.01 | 0.00 |
| 0.3 | 14.00 | 16.5 | 1.11 | 0.00 | 0.00 |
| 1 | 7.50 | 11.00 | 1.46 | 0.00 | 0.00 |
| 3 | 14.00 | 14.00 | 1.46 | 0.00 | 0.00 |
| 5.6 | 14.50 | 14.50 | 1.06 | 0.02 | 0.00 |
| 10 | 2.50 | 7.50 | 1.19 | 0.04 | 0.00 |
| 18 | 0.50 | 7.50 | 1.34 | 0.11 | 0.00 |
| E2 | |||||
| SAL | 9.25 | 12.13 | 1.62 | 0.03 | 0.30 |
| 0.3 | 14.00 | 18.67 | 1.42 | 0.00 | 0.15 |
| 1 | 1.25 | 16.75 | 1.33 | 0.02 | 0.23 |
| 3 | 7.67 | 12.33 | 1.86 | 0.04 | 0.17 |
| 5.6 | 3.33 | 10.33 | 1.42 | 0.10 | 0.00 |
| 10 | 0.25 | 6.25 | 1.41 | 0.61 | 0.00 |
| 18 | 0.00 | 8.00 | 1.08 | 0.00 | 0.00 |
no video available
Appendix C. Span, longest run, visits and rears in the methamphetamine (METH) study
| Rat & METH Dose | Span Length | Longest Run | OST Visits | S+ Visits-OST | Rears |
|---|---|---|---|---|---|
| W22 | |||||
| SAL | 9.00 | 12.44 | 1.73 | 0.04 | 0.00 |
| 0.1 | 9.00 | 12.75 | 1.66 | 0.12 | 0.00 |
| 0.3 | 5.50 | 12.25 | 2.02 | 0.35 | 0.00 |
| 0.56 | 0.75 | 11.25 | 2.71 | 0.51 | 0.00 |
| 1 | 3.33 | 12.67 | 4.32 | 1.15 | 0.11 |
| 3 | 0.00 | 0.00 | 0.00 | 3.33 | 0.00 |
| W20 | |||||
| SAL | 9.56 | 15.22 | 1.45 | 0.04 | 0.09 |
| 0.03 | 7.00 | 16.67 | 1.28 | 0.00 | 0.03 |
| 0.1 | 10.00 | 10.67 | 1.50 | 0.00 | 0.05 |
| 0.3 | 4.67 | 16.00 | 1.39 | 0.25 | 0.48 |
| 0.56 | 8.33 | 16.00 | 1.89 | 0.22 | 0.93 |
| 1 | 1.00 | 5.67 | 2.92 | 1.15 | 9.15 |
| 3 | 0.00 | 0.00 | 2.33 | 3.00 | 17.00 |
| Z12 | |||||
| SAL | 7.00 | 13.50 | 1.52 | 0.01 | 0.04 |
| 0.03 | 15.67 | 15.67 | 1.37 | 0.00 | 0.04 |
| 0.1 | 10.33 | 17.00 | 1.37 | 0.00 | 0.04 |
| 0.3 | 20.00 | 20.00 | 1.27 | 0.00 | 0.01 |
| 0.56 | 9.33 | 13.67 | 1.58 | 0.03 | 0.00 |
| 1 | 2.67 | 11.00 | 1.70 | 0.03 | 0.01 |
| 3 | 0.00 | 1.00 | 0.80 | 0.20 | 0.00 |
| Z3 | |||||
| SAL | 3.89 | 7.67 | 1.56 | 0.05 | 0.30 |
| 0.03 | 1.50 | 8.75 | 1.65 | 0.07 | 0.33 |
| 0.1 | 5.33 | 8.67 | 1.32 | 0.00 | 0.50 |
| 0.3 | 5.75 | 15.00 | 2.22 | 0.12 | 0.73 |
| 0.56 | 0.50 | 12.00 | 2.22 | 0.15 | 0.99 |
| 1 | 0.50 | 5.50 | 3.04 | 0.93 | 5.09 |
| 3 | 0.00 | 0.00 | 2.67 | 3.00 | 5.33 |
| A11 | |||||
| SAL | 5.47 | 8.47 | 1.40 | 0.01 | 0.06 |
| 0.1 | 4.33 | 7 | 4.59 | 0.02 | 0.00 |
| 0.3 | 3.33 | 8.33 | 2.65 | 0.36 | 1.48 |
| 0.56 | 6.67 | 7.33 | 1.33 | 0.04 | 0.26 |
| 1 | 1.25 | 4.00 | 3.64 | 1.20 | 5.60 |
| 3 | 0.00 | 0.00 | * | * | * |
| D2 | |||||
| SAL | 10.78 | 13.61 | 1.49 | 0.06 | 0.28 |
| 0.03 | 3.00 | 11.00 | 1.33 | 0.00 | 0.17 |
| 0.1 | 9.50 | 10.25 | 1.43 | 0.08 | 0.34 |
| 0.3 | 0.00 | 13.50 | 2.02 | 0.50 | 0.80 |
| 0.56 | 3.75 | 11.00 | 2.46 | 0.40 | 1.02 |
| 1 | 0.00 | 5.00 | 3.15 | 3.51 | 1.39 |
| 3 | 0.00 | 0.00 | 4.30 | 2.67 | 11.30 |
no video available
Appendix D. Span, longest run, visits and rears in the methylphenidate (MPD) study
| Rat & MPD Dose | Span Length | Longest Run | S− Visits | S+ Visits | Rears |
|---|---|---|---|---|---|
| W22 | |||||
| SAL | 8.64 | 15.36 | 1.85 | 0.01 | 0.20 |
| 1 | 6.00 | 9.00 | 2.23 | 0.00 | 0.23 |
| 1.8 | 6.33 | 13.00 | 2.13 | 1.14 | 0.33 |
| 3 | 8.00 | 14.33 | 2.15 | 0.19 | 0.08 |
| 5.6 | 2.00 | 4.00 | 1.73 | 4.00 | 3.10 |
| 10 | 0.00 | 0.00 | 2.79 | 2.00 | 2.79 |
| 18 | 0.00 | 0.00 | 1.83 | 5.00 | 9.67 |
| W29 | |||||
| SAL | 15.20 | 19.70 | 1.36 | 0.00 | 0.15 |
| 1 | 15.33 | 15.67 | 2.64 | 1.22 | 6.07 |
| 1.8 | 7.00 | 12.50 | 2.32 | 0.36 | 4.18 |
| 3 | 14.33 | 14.33 | 3.19 | 1.15 | 7.04 |
| 5.6 | 8.67 | 8.67 | 2.71 | 1.37 | 11.01 |
| 10 | 12.00 | 12.00 | 2.03 | 1.59 | 10.75 |
| 18 | 3.00 | 3.00 | 2.70 | 1.04 | 12.40 |
| X3 | |||||
| SAL | 19.00 | 19.22 | 1.33 | 0.01 | 0.09 |
| 1 | 11.25 | 19.00 | 1.82 | 0.19 | 0.32 |
| 1.8 | 4.00 | 7.60 | 2.71 | 0.57 | 1.59 |
| 3 | 1.00 | 3.00 | 2.79 | 2.13 | 4.97 |
| 10 | 0.00 | 0.00 | 2.33 | 1.33 | 7.00 |
| W21 | |||||
| SAL | 7.40 | 12.80 | 1.68 | 0.01 | 0.01 |
| 1 | 5.33 | 14.00 | 0.50 | 0.00 | 0.00 |
| 3 | 7.75 | 10.50 | 3.54 | 0.19 | 0.99 |
| 10 | 0.00 | 0.00 | 1.00 | 2.00 | 8.50 |
| W20 | |||||
| SAL | 13.09 | 15.45 | 1.37 | 0.00 | 0.09 |
| 1 | 7.25 | 11.50 | 1.13 | 0.17 | 0.21 |
| 1.8 | 6.67 | 13.67 | 1.92 | 0.21 | 0.06 |
| 3 | 14.33 | 15.67 | 1.79 | 0.17 | 0.02 |
| 5.6 | 6.67 | 9.67 | 1.96 | 1.29 | 3.82 |
| 10 | 3.33 | 6.00 | 2.80 | 0.79 | 5.67 |
| 18 | 0.00 | 0.00 | 0.42 | 1.70 | 15.20 |
| E2 | |||||
| SAL | 14.00 | 15.40 | 1.36 | 0.01 | 0.08 |
| 1 | 7.50 | 10.00 | 1.40 | 0.02 | 0.02 |
| 3 | 5.50 | 10.75 | 2.43 | 0.46 | 1.15 |
| 10 | 0.00 | 4.00 | 4.52 | 0.25 | 2.70 |
Appendix E. Mean percent correct and standard deviation on baseline sessions during each of the drug studies
| Ketamine Rat # | OST | SDC |
|---|---|---|
| E2 | 89.1 (7.2) | 96.9 (6.6) |
| Y18 | 85.4 (5.3) | 97.3 (6.2) |
| Z3 | 83.9 (7.6) | 75.1 (4.4) |
| E5 | 88.0 (5.8) | 100.0 (0) |
| E13 | 97.2 (3.7) | 92.2 (10.7) |
| E4 | 88.3 (8.5) | 98.3 (5.3) |
|
| ||
| Flunitrazepam Rat # | OST | SDC |
|
| ||
| Y17 | 83.3 (8.7) | 94.7 (9.7) |
| Y16 | 89.7 (5.4) | 95.6 (7.5) |
| Y2 | 83.6 (6.6) | 89.8 (13) |
| A11 | 77.5 (11.6) | 94.1 (10.1) |
| E1 | 86.5 (5.9) | 98.8 (4.4) |
| F16 | 77.3 (8.2) | 87.8 (11.7) |
|
| ||
| Methamphetamine Rat # | OST | SDC |
|
| ||
| W22 | 88.3 (6.6) | 98.8 (4.4) |
| W20 | 92.4 (5.3) | 96.9 (6.7) |
| Z3 | 80.1 (8.4) | 96.2 (8.5) |
| Z12 | 87.9 (7.5) | 95.7 (7.4) |
| A11 | 78.8 (9.2) | 93.6 (8.4) |
| D2 | 91.5 (4.6) | 95.8 (9.2) |
|
| ||
| Methylphenidate Rat # | OST | SDC |
|
| ||
| W22 | 89.8 (5.9) | 93.8 (10.8) |
| W29 | 95.8 (4.7) | 100.0 (0) |
| X3 | 94.1 (4.9) | 100.0 (0) |
| W21 | 91.0 (6.9) | 96.7 (8.7) |
| W20 | 92.5 (6.0) | 99.1 (3.8) |
| E2 | 92.8 (6.2) | 98.6 (4.9) |
References
- Aigner TG, Walker DL, Mishkin M. Comparison of the effects of scopolamine administered before and after acquisition in a test of visual recognition memory in monkeys. Behavioral and Neural Biology. 1991;55:61–67. doi: 10.1016/0163-1047(91)80127-z. [DOI] [PubMed] [Google Scholar]
- April LB, Bruce K, Galizio M. The magic number 70 (plus or minus 20): Variables determining performance in the rodent Odor Span Task. Learning and Motivation. 2013;44:143–158. doi: 10.1016/j.lmot.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JNP, Good MA. The drugs don’t work—or do they? Pharmacological and transgenic studies of the contribution of NMDA and GluR-A containing AMPA receptors to hippocampal-dependent memory. Psychopharmacology. 2006;188:552–566. doi: 10.1007/s00213-006-0403-6. [DOI] [PubMed] [Google Scholar]
- Baron SP, Wright D, Wenger GR. Effects of drugs of abuse and scopolamine on memory in rats: Delayed spatial alternation and matching to position. Psychopharmacology. 1998;137:7–14. doi: 10.1007/s002130050587. [DOI] [PubMed] [Google Scholar]
- Branch C, Galizio M, Bruce K. What-where-when memory in the rodent odor span task. Learning and Motivation. 2014;47:18–29. doi: 10.1016/j.lmot.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro C. Primacy and recency effects in rhesus monkeys using a serial probe recognition task. I. Effects of diazepam. Psychopharmacology. 1995;119:421–427. doi: 10.1007/BF02245858. [DOI] [PubMed] [Google Scholar]
- Castro C. Primacy and recency effects in rhesus monkey using a serial probe recognition task. II. Effects of atropine sulfate. Journal of Behavioral Neuroscience. 1997;111:676–682. doi: 10.1037//0735-7044.111.4.676. [DOI] [PubMed] [Google Scholar]
- Davies DA, Greba Q, Howland JG. GluN2B-containing NMDA receptors and AMPA receptors in medial prefrontal cortex are necessary for odor span in rats. Frontiers in Behavioral Neuroscience. 2013;7:1–8. doi: 10.3389/fnbeh.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DA, Molder JJ, Greba Q, Howland JG. Inactivation of medial prefrontal cortex or acute stress impairs odor span in rats. Learning & Memory. 2013;20:665–669. doi: 10.1101/lm.032243.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix S, Gilmour G, Potts S, Smith JW, Tricklebank M. A within-subject cognitive battery in the rat: Differential effects of NMDA receptor antagonists. Psychopharmacology. 2010;212:227–242. doi: 10.1007/s00213-010-1945-1. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. Animal models of working memory: A review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neuroscience and Biobehavioral Reviews. 2013;37:2111–2124. doi: 10.1016/j.neubiorev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. The Journal of Neuroscience. 2000;20:2964–2977. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio M. Olfactory stimulus control and the behavioral pharmacology of remembering. Behavior Analysis: Research and Practice. 2016 doi: 10.1037/bar0000033. http://dx.doi.org/10.1037/bar0000033. [DOI] [PMC free article] [PubMed]
- Galizio M, Deal M, Hawkey A, April LB. Working memory in the odor span task: Effects of chlordiazepoxide, dizocilpine (MK801), morphine, and scopolamine. Psychopharmacology. 2013;225:397–406. doi: 10.1007/s00213-012-2825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper DN, Wisnewski R, Hunt M, Schenk S. (+−)3,4 Methylenedioxymethamphetamine, d-amphetamine, and cocaine impair delayed matching-to-sample performance by an increase in susceptability to proactive interference. Behavioral Neuroscience. 2005;119(2):455–463. doi: 10.1037/0735-7044.119.2.455. [DOI] [PubMed] [Google Scholar]
- Hawkey A, April LB, Galizio M. Effects of MDMA on olfactory memory and reversal learning in rats. Neurobiology of Learning and Memory. 2014;114:209–216. doi: 10.1016/j.nlm.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Vaidya M, Branch MN. Titrating-delay matching-to-sample in the pigeon. Journal of the Experimental Analysis of Behavior. 2010;94:69–82. doi: 10.1901/jeab.2010.94-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen DA, Bullard L, Galizio M. Effects of dizocilpine (MK801) on olfactory span in rats. Neurobiology of Learning and Memory. 2011;95:57–63. doi: 10.1016/j.nlm.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perone M. Experimental design in the analysis of free-operant behavior. In: Iverson IH, Lattal KA, editors. Experimental analysis of behavior: Part I. Amsterdam: Elsevier; 1991. pp. 135–172. [Google Scholar]
- Pontecorvo MJ, Clissold DB, White MF, Ferkany JW. N-methyl-D-aspartate antagonists and working memory performance: Comparison with the effects scopolamine, propanolol, diazepam and phenylisopropyladenosine. Behavioral Neuroscience. 1991;105:521–535. doi: 10.1037//0735-7044.105.4.521. [DOI] [PubMed] [Google Scholar]
- Prichard A, Panoz-Brown D, Bruce K, Galizio M. Emergent identity but not symmetry following successive olfactory discrimination training in rats. Journal of the Experimental Analysis of Behavior. 2015;104:133–145. doi: 10.1002/jeab.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushforth SL, Allison C, Wonnacut S, Shoaib M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neuroscience Letters. 2010;471:114–118. doi: 10.1016/j.neulet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Steckler T, Shoaib M. Nicotine improves working memory span capacity in rats following sub-chronic ketamine exposure. Neuropsychopharmacology. 2011;36:2774–2781. doi: 10.1038/npp.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick B. Animal cognition and the rat olfactory system. Trends in Cognitive Sciences. 2001;5:216–222. doi: 10.1016/s1364-6613(00)01625-9. [DOI] [PubMed] [Google Scholar]
- Smith JW, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, … Tricklebank M. A comparison of the effects of ketamine and phencyclidine with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology. 2011;217:255–269. doi: 10.1007/s00213-011-2277-5. [DOI] [PubMed] [Google Scholar]
- Soto PL, Ator NA, Rallapalli SK, Biawat P, Clayton T, Cook JM, Weed MR. Allosteric modulation of GABAA receptor subtypes: Effects on visual recognition and visuospatial working memory in Rhesus monkeys. Neuropsychopharmacology. 2013;38:2315–2325. doi: 10.1038/npp.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto PL, Dallery J, Ator NA, Katz BR. A critical examination of best dose analysis for determining cognitive enhancing potential of drugs: Studies with Rhesus monkeys and computer simulations. Psychopharmacology. 2013;228:611–622. doi: 10.1007/s00213-013-3070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi J, Sarter M. Cortical cholinergic inputs mediate processing capacity: Effects of 192 IgG-saporin-induced lesions on olfactory span performance. European Journal of Neuroscience. 2000;12:4505–4514. [PubMed] [Google Scholar]
- White KG. Remembering and forgetting. In: Madden GJ, editor. APA handbook of behavior analysis, Volume 1: Methods and principles. Washington DC: American Psychological Association; 2013. pp. 411–439. [Google Scholar]
- Willmore CB, LaVecchia KL, Wiley JL. NMDA antagonists produce site-selective impairment of accuracy in a delayed nonmatch-to-sample task in rats. Neuropharmacology. 2001;41:916–927. doi: 10.1016/s0028-3908(01)00143-5. [DOI] [PubMed] [Google Scholar]
- Wright AA. An experimental analysis of memory processing. Journal of the Experimental Analysis of Behavior. 2007;88:405–433. doi: 10.1901/jeab.2007.88-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright FK, White KG. Effects of methylphenidate on working memory in pigeons. Cognitive, Affective & Behavioral Neuroscience. 2003;3:300–308. doi: 10.3758/cabn.3.4.300. [DOI] [PubMed] [Google Scholar]
- Young JW, Kerr LE, Kelly JS, Marston HM, Spratt C, Finlayson K, Sharkey J. The odour span task: A novel paradigm for assessing working memory in mice. Neuropharmacology. 2007;52:634–645. doi: 10.1016/j.neuropharm.2006.09.006. [DOI] [PubMed] [Google Scholar]