SYNOPSIS
Vitamin D insufficiency and deficiency can be diagnosed with measurements of serum 25hydroxyvitamin D (25OHD). Most vitamin D is derived from sunlight (80 percent) so serum 25OHD levels are lowest in late winter and early spring. Dietary vitamin D in North America is small about 100–200 IU daily. A review of the literature up to 2016 shows many association studies relating vitamin D deficiency and insufficiency to several diseases such as type 2 diabetes, cancer, immunity bone health but there are no clinical trials yet that shows vitamin D reduces the incidence of any disease other than bone. Large randomized trials of vitamin D are underway that will soon provide answers on the dose of vitamin D and the serum 25OHD level that may be clinically effective.
Fractures incidence is higher when serum 25OHD levels are less than 20ng/ml (50nmol/l) and rates of bone loss from the hip are faster below 20ng/ml (50nmol/l). To reach a 25OHD level of 20ng/ml(50nmol/l) the Institute of Medicine (IOM) set the RDA for vitamin D at 800 IU for people over age 70 years and 600 IU for ages 50–70 years. Treatment of elderly people with vitamin D alone does not reduce fractures but when vitamin D 400–800 IU is combined with calcium 1,000mg daily there is a significant reduction in fractures of 8–12 percent.
Keywords: Vitamin D supplementation, IOM, RDA for vitamin D
Introduction: Nutritional considerations
Vitamin D deficiency, insufficiency and sufficiency are three categories representing different degrees of the nutritional status of vitamin D and they are divided according to serum 25OHD levels. These categories are not distinct and merge into each other.
The Institute of Medicine (IOM) defined vitamin D deficiency as < 10ng/ml (25nmol/l), insufficiency as 10–20ng/ml (25–50nmol/l) and sufficiency as > 20ng/ml (> 50nmol/l) (1) similar to the definition of the World Health Organization(WHO) in 2003 [2] and European Food Safety association-EFSA in 2016 (3). An Endocrine Society working group used higher levels, < 20ng/ml for deficiency, 20–30 ng/ml (75nmol/l) for insufficiency and > 30ng/ml (> 75nmol/l) for sufficiency (4) Table 1. Interest in vitamin D status has led to a twelve-fold increase in serum 25OHD tests since 2005 (5).
TABLE 1.
Categories of low vitamin D (25OHD) levels
| IOM, WHO, EFSA | Endocrine society | |||
|---|---|---|---|---|
| serum 25OHD ng/ml | serum 25OHD nmol/l | serum 25OHD ng/ml | serum 25OHD nmol/l | |
| Vitamin D deficiency | < 10 | 25 | < 20 | < 50 |
| insufficiency | 10–20 | 25–50 | 20–30 | 50–75 |
| sufficiency | > 20 | > 50 | >30 | >75 |
Vitamin D deficiency and insufficiency
A survey from the 3,871 adults in the NHANES study carried out on populations in North America shows the relative numbers of the serum 25OHD categories (6,7). Recent re-measurement of the 25OHD levels as part of a vitamin D standardization program showed the prevalence of insufficiency (12–20ng/ml) was high at 31%(8).
In most of Northern Europe the percent of the population with serum 25OHD < 20ng/ml (50nmol/l) is higher due to a combination of social and environmental factors that differ from North America, such as lack of fortification of food and milk, and less exposure to sunlight because of the Northern latitude and cloud cover (9,10). In certain ethnic groups, such as traditional Arabic women, there is an even higher risk because of cultural habits such as veiling and purdah preventing ultraviolet activation of pre-vitamin D in skin (11).
The history of the present RDA for vitamin D
In determining the RDA for vitamin D for the elderly, < age 50–69 and > 70 years, the Institutes of Medicine (IOM) in 2010 performed a comprehensive survey and analysis of literature that linked low levels of vitamin D to the prevalence of several diseases such as cardiovascular disease, cancer, asthma, bone and others. The analyses for IOM was conducted by an independent group of epidemiologists and statisticians AHRQ-Tufts in 2009 and 2011 (12,13,) and followed an earlier analysis by another AHRQ-Ottawa group in 2007. 14)). Even though there are numerous association studies that linked serum 25OHD levels to various diseases, the epidemiologists found no consistent evidence of a significant effect of vitamin D treatment on any health outcomes other than fractures and BMD. Thus, the IOM committee decided that only clinical studies related to bone health would be used to derive new Dietary reference intakes (DRI) for vitamin D. To look for a relationship between disorders of calcium metabolism and serum 25OHD levels the IOM used data on rickets, calcium absorption, fracture risk and bone health. Clinical studies were reviewed to find a dose of vitamin D that would meet the target level of a specified level of serum 25OHD.
Calcium absorption
A cross sectional study of calcium absorption and serum 25OHD in 319 patients with a mean age of 67 years shows reduced calcium absorption at mean serum 25OHD levels of 4 ng/ml (9 nmol/l) together with a decrease in serum 1,25 dihydroxyvitamin D) (15). As mean serum 25OHD increased from 4ng/ml (9nmol/l) to 7ng/ml (17nmol/l) there was an increase in serum 1,25dihydroxyvitamin D and increase in calcium absorption from 36% to 56%. The results show that calcium absorption was almost maximal at very low levels of 25OHD 4–7 ng/ml (10–17nmol/l) and although mean 25OHD increased to 14ng/ml (35nmol/l) there was no further increase in calcium absorption. In prospective studies, calcium absorption was reduced at low levels of serum 25OHD ~10ng/ml (25nmol/l). After vitamin D treatment, there was an increase in serum 25OHD from 10ng/ml to 60ng/ml (150nmol/l), however, the increase in calcium absorption from 55% to 61% was relatively small ~ 6 percent (16,17). This is not surprising since calcium absorption is controlled by 1,25dihydroxyvitamin D and not 25OHD (18,19).
Serum parathyroid hormone and its relation to a serum 25OHD
Because serum PTH often increases with vitamin D deficiency and insufficiency in some but not all people, the level at which it normalizes or plateaus has often been used as a marker of vitamin D insufficiency. In the Endocrine Society paper, only 3 studies from the literature were quoted and they showed a ‘PTH plateau’ at a serum 25OHD level of 30ng/ml (75nmol/l) (4). However, there were in fact about 70 studies on serum PTH and 25OHD in the literature at that time and they showed considerable variation in a ‘plateau’ value for serum 25OHD ranging from 12 ng/ml (30nmo/l) to 40ng/ml (100nmol/l) and in 7 studies there was no plateau (19). In a recent analysis of 347,000 laboratory samples, no plateau could be easily identified, thus a serum PTH plateau is not a reliable marker of vitamin D sufficiency (20).
Bone
Increased serum PTH is inversely correlated with hip BMD (21). In an analysis of bone remodeling markers and serum 25OHD in 489 women, a plateau in serum osteocalcin was seen at a serum 25OHD of 17 ng/ml(42nmo/L), and a plateau in urine N-telopeptides at a plateau at 18ng/ml(37nmol/L) (19). Interestingly other bone data support the importance of the effect of low serum 25OHD < 20ng/ml (50nmol/L) on bone. In a study of 1279 men, bone mineral density was measured over 4.4 years and rate of bone loss divided into quartiles; rates of bone loss were increased only in those with serum 25OHD< 20ng/ml (50nmol/l) compared to >20ng/ml. [22]. In five case cohort studies of hip fracture in 6,562 patients the relative risk of hip fractures was increased significantly from 1.0 only when serum 25OHD was < 20ng/ml (50nmol/L), and these studies are summarized in a review (23). There are data also on non-vertebral fractures. In men, non-hip fractures are increased only when serum 25OHD is < 20ng/ml (50nmol/l) (24). In a case cohort study of women from the Women’s Health Initiative, Caucasian women showed a lower incidence of non-vertebral fractures when serum 25OHD was> 30ng/ml (75nmol/l) but in African American and Asian women fractures were increased when serum 25OHD was increased > 20ng/ml compared to < 20ng/ml (25).
In a recent bone biopsy study, post-mortem analysis of bone biopsies was performed in 675 people who had sudden death. They examined the occurrence of osteomalacia in the biopsies and related that to serum 25OHD levels drawn early post mortem (26). At serum 25OHD levels below 12ng/ml (30 nmol/L) more than half of the population failed to demonstrate osteomalacia. At 25OHD levels below 3 ng/ml there were ~80/687 cases with normal bone indicating that factors other than low 25OHD prevented osteomalacia, most obvious factors being that subjects had adequate intake of calcium and phosphorus before death. In the biopsy study, osteoid volume was increased in 7 of 685 (1%) cases when serum 25OHD was between 20–30ng/ml, however because there was no measurement of mineralization defect we cannot be sure that these 7 cases had osteomalacia, some might have had hyperparathyroidism.
The totality of these observations support the concept that subjects with levels of serum 25OHD < 10 ng/ml (50nmol/l) develop rickets and malabsorption of calcium and at levels < 20ng/ml(50nmol/l) there is decreased risk of bone loss and fractures. For these reasons the IOM recommended that the target figure for treatment should be a serum 25OHD of 20ng/ml (50nmol/l). A more recent analysis by the EFSA in 2016 (2) used similar reasoning and similar conclusions to those of the IOM.
Clinical studies of vitamin D
There have been 7 studies on vitamin D alone and 13 studies with vitamin D + calcium on bone. A fundamental flaw is that none of the studies had a dose response design. As a result meta analyses have been used to examine the data. (please see Ian R Reid’s article “Vitamin D Effect on Bone Mineral Density and Fractures,” in this issue.)
Regarding vitamin D only studies, there are few trials in the literature where vitamin D only was compared to placebo, and these were reviewed in two meta-analyses (27,28). On low doses of vitamin D 400–1,000IU there was no reduction in fractures (odds ratio 1.05). In two studies, one a large trial of 2256 women age 70 years who were given a single oral high dose annually of vitamin 500,000 IU there was unexpectedly a significant increase in fractures within 3 months of dosing in two separate years (relative risk (RR) 1.26, CI: 1.00 –1.59) (29). Another study of an annual injection of high dose vitamin D 300,00IU showed a significant increase in hip fractures (RR 1.82) in women but not in men (RR 1.1) (30). Thus, vitamin D alone in low doses has no effect on fractures and large bolus doses increase fractures.
In many clinical trials vitamin D was combined with calcium supplements and several different dose combinations of vitamin D and calcium have been used in trials. In a meta-analysis by Tang et al. of trials with fracture outcomes but without individual data there were 5 studies in which the active group was compared to placebo control and in 8 studies the control group was calcium (27). Their analysis showed a significant reduction in fractures of 13 percent (RR 0.87;0.77–0.97). In another meta-analysis (DiPART) the authors obtained individual person data in 7 controlled studies, 3 on vitamin D alone and 4 on vitamin D plus calcium (28). There was no significant effect of vitamin D alone on fractures (RR1.01;0.92–1.12) but a significant effect of vitamin D plus calcium on total fractures (RR 0. 92, CI: 0.86 – 0.99). Hip fractures were marginally reduced on vitamin D plus calcium (RR 0.84, CI: 0.70–1.01, p< 0.07) but not on vitamin D alone (RR1.09;0.92–1.29) (28).
In summary, vitamin D alone does not reduce fracture whereas calcium plus vitamin D supplementation reduces fractures by 8–13 percent. The results of the DIPART analysis show that vitamin D 400–800IU plus 1000mg calcium is a regimen that prevents fractures. It was not possible for the authors to define a serum 25OHD level associated with efficacy.
Determination of the Recommended Dietary Allowance (RDA) in older persons
There are three components to Dietary reference intakes (DRI’s) that are used to determine nutrient intakes for calcium and vitamin D in North America. They are reference intakes for the healthy population and are not therapeutic guidelines for people with diseases e.g. malabsorption syndromes.
1 Recommended Dietary Allowance
(RDA) is the average daily dietary intake level estimated to meet the nutrient requirements of nearly all healthy persons in a life stage and gender, that is it meets the needs of 97.5% of individuals.
The RDA for Vitamin D3, can be descriptively defined as the dose that achieves a defined end, for example it could be the new incidence of type 2 diabetes. In this discussion, the endpoint is a serum 25OHD level of 20 ng/ml (50nmol/L) or higher in 97.5% of the subjects. This means that for a dose of vitamin D, gender and age specific, 97.5% of subjects in that dose group will have serum 25OHD of at least 20 ng/ml(50nmol/L).
Regression methodology can also be used for calculating the RDA, in that a dose response curve can be fit to the data, using linear regression model. From the linear regression model, confidence intervals and prediction intervals can be calculated. Confidence intervals are calculated for the mean or average response (serum 25OHD) at a dose. Rather than the average response of many individuals, prediction intervals apply to the response of a new individual. There is more uncertainty in predicting a serum 25OHD level for an individual than for the average of individuals, therefore prediction intervals are wider than confidence intervals. Prediction intervals must account for two levels of uncertainty, uncertainty in estimating the actual dose response curve and random error for the new individual. Without going into additional detail, linear regression models enable the estimation of 95% prediction limits for each dose level. Since the 95% prediction interval is symmetric about the predicted value (what serum 25OHD a new individual is predicted to have at that dose), 97.5% of values would be above the lower prediction limit. Therefore, the RDA can be interpreted as the dose at which a serum 25OHD of 20 ng/ml (50nmol/L) is above the lower prediction limit for a new individual. This prediction interval is a function of the standard deviation of the residuals, the sample size, the sample mean of the independent variables, and the standard deviation of the independent variables.
Prediction intervals are standard and can be calculated easily with statistical software for simple linear regression. However, more complex study designs require advanced statistical techniques for calculating prediction intervals.
In a longitudinal study design, linear mixed effects models that consider correlation within a subject over time are most appropriate. This type of model complicates the calculation of prediction intervals for estimation of the RDA. There is no standard formula for calculating prediction intervals for this type of model such as there is for simple linear regression. Widely accepted in the literature for calculating prediction intervals with linear mixed effects models is a bootstrapping technique. This is resampling methodology that can be used to calculate confidence intervals and prediction intervals when the distribution is unknown. To perform this calculation, many bootstrap samples are taken (such as 1000) at the subject level, preserving all the time points measured within a subject. The linear mixed effects model is fit for each of the bootstrapped samples and the predicted values of the random effects (called BLUPS) are found. From this the 2.5 and 97.5 percentiles are found for each dose. Again, the RDA is the dose at which the 2.5 percentile is above 20 ng/ml (50nmol/L) of serum 25OHD.
2 Estimated Average Requirement (EAR)
The average daily nutrient intake level estimated to meet the requirements of 50% of the healthy persons for each sex and age group.
The EAR is simpler to calculate than the RDA. The EAR for Vitamin D3 can be defined as the dose that achieves a serum 25OD level of 20 ng/ml(50nmol/L) or a target value of interest in 50% of the subjects. This means that at that dose 50% of subjects in that dose group will have serum 25OHD less than 20 ng/ml (50nmol/L) and 50% greater than 20 ng/ml(50nmol/L).
This is also a definition of the median. The median is defined as the middle value of a distribution, where 50% of the observations are above and 50% below that value. EAR can also be defined as the dose where the median of the serum 25OHD values meets the targeted value of 20 ng/ml. If the serum 25OHD values follow a normal distribution the mean and median should be approximately equal, so if all the assumptions from a simple linear regression model (or linear mixed effects model) are met, then the fitted line of the dose response curve corresponds to the mean response. The point where the dose response curve intersects the targeted value (20 ng/ml) is the EAR.
Estimation of the RDA by IOM
To find a total vitamin D intake derived from diet and supplements that exceeded a serum 25OHD level of 20ng/ml (50nmol/L), the IOM committee in 2011 used several studies from the literature, most studies were single dose and some used two doses (1). They specifically used studies that were performed in the winter months in Northern countries above latitude 49° degrees and from Antarctica 78 °S to minimize the effect of sun exposure. Their procedure was to use regression analysis, with a random study effect, and log distribution to estimate a dose response curve. From this they estimated the EAR of 400 IU/d exceeded a serum 25OHD of 16 ng/ml (40 nmol/L). The RDA was estimated with prediction limits from the average study values and showed that a RDA of 600 IU/d exceeded a serum 25OHD of 20 ng/ml (50 nmol/L).
It should be noted that a more precise analysis would be obtained from prediction limits had individual values been available as pointed out by Verguelers (31), although his RDA estimate of 8895 IU daily is clearly incorrect when compared to our dose response curves in the prospective ViDOS and VitaDAS trials which will be described in detail later. In another analysis, the RDA was estimated from uncontrolled vitamin D supplementation studies and arrived at a figure of 9,600 IU daily (32), an estimate that is clearly incorrect when compared to our dose response curves. The ViDOS study in older women and the VitaDAS study in younger women are dose ranging studies and were not completed at that time of the IOM report. Nevertheless, the RDA value estimated from the individual subject data in our trials are similar to those in the IOM recommendation (see later in article).
The most recent analysis in 2016 by EFSA used meta regression (3), it does not use an RDA figure but an ‘Adequate Intake’ although it is based on a 95 % prediction limits with the lower limit being 49nmo/l (19.6 ng/ml), and they conclude that vitamin D3 600 IU/d will meet a target serum 25OHD of 20ng/ml (50nmol/l) in nearly everyone (3). Other groups in Europe such as the Nordic Council support the use of 400 IU for an RDA like figure (33). The Netherlands recommend 600 IU for age 50–70 years and 800 IU for those > 70 years (34). For those younger than 70 years, the target serum 25OHD was 12ng/ml (30nmo/l). In England, they chose a serum 25OHD of 25nmol/l (10ng/ml) for all ages as protection against adverse musculoskeletal health and recommended vitamin D 400 IU to cover 97.5% of the population (35)
EAR or RDA
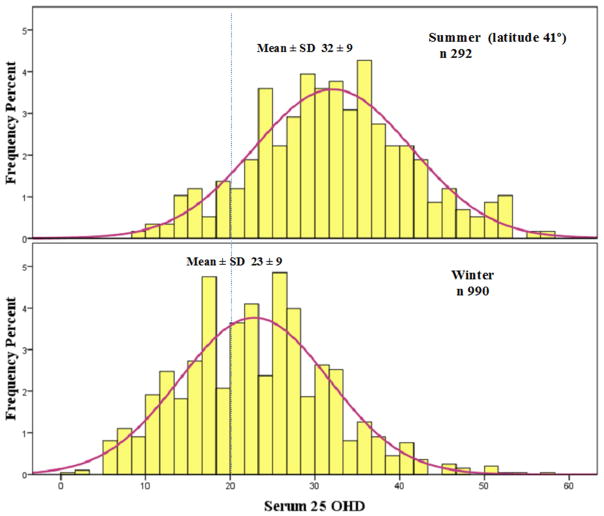
A recent argument has been that nutrient deficiency should be defined by the EAR, which is the nutrient intake for the average of the population (36). The IOM used a serum 25OHD of 16 ng/ml (40nmol/l) as the target for the EAR, and estimated that vitamin D 400 IU will meet that figure. The RDA for a serum 25OHD of 20ng/ml (50nmol/l) is based on 97.5% of the population. Another way to look at it is that 97.5% will have a requirement below that threshold, and that meeting a goal of 20ng/ml (50nmol/L) is more than necessary. In addition, the calculation for RDA was based on wintertime studies. At our latitude of 41° serum 25OHD increases from winter to summer by about 10ng/ml(25nmo/L) from 23 to 32 ng/ml (57 to 80nmol/L) (Figure 1) thus having an EAR rather that an RDA is a reasonable suggestion. Another argument that has been made is that increasing the vitamin intake to achieve an RDA target of 20 ng/ml (50nmol/L) may push some people into higher 25OHD levels associated with the Tolerable upper limit as suggested by Taylor et al in a statistical model (37).
Figure 1.
Seasonal change (cross sectional) in serum 25OHD in Omaha, latitude 41°
Tolerable Upper Level (TUL)
This is the highest average daily intake of a nutrient that does not increase the risk for adverse health effects for nearly all persons in the population. At the last IOM report the TUL was increased from 2000 to 4000 IU daily, much of the reasoning was based on lack of hypercalcemia [it is not a recommended intake]. However, as another example of an unexpected adverse result of vitamin D we found a significant increase in falls in women taking the higher doses of 4000 and 4800 IU daily, these doses were associated with serum 25OHD levels > 40–45 ng/ml(100–112nmol/L) but no hypercalcemia (38), thus the argument made by Taylor et al. is reasonable (37). In epidemiology studies increased mortality rates have been associated with higher serum 25OHD levels of 45–50ng/ml(112–125nmol/L) (39,40) and in the recent 6-year follow up of NHANES there was a reverse J-shaped curve with higher mortality below 40nmol/l (16ng/ml) and above 120nmol/l (48ng/ml) (41).
Dose response in older Caucasian and African American women
Since the IOM recommendations in 2010 we conducted a randomized clinical trial (ViDOS study) to measure the dose response of vitamin D and through this dose response curve estimate the RDA and EAR (42). The study design included randomization of women stratified by race (Caucasian or African American) into 7 dose groups and a placebo group and stratified by body mass index. Doses used were 400, 800, 1600, 2400, 3200, 4000, 4800IU, and placebo. Inclusion criteria included older women at least 7 years postmenopausal and vitamin D insufficient <20 ng/ml (50nmol/L) at baseline. Exclusion criteria were substantial comorbid conditions or medications that might affect calcium and vitamin D metabolism. A Diasorin immunoassay was used to measure serum 25OHD in this study. 163 Caucasian women were enrolled as well as 110 African American women. All 163 Caucasian women were enrolled at one site in Omaha, Nebraska, and African American women were enrolled at two sites, Nebraska and Indiana. Serum 25OHD was measured at baseline, 6 months and 12 months.
In the Caucasian women, a quadratic dose response curve was observed. Prediction intervals were calculated with bootstrap methodology for the linear mixed effects model used to fit the data. For the RDA calculation, to reach a serum 25OHD of 30 ng/ml it was determined that a vitamin D3 dose of 1600IU/day is required in postmenopausal Caucasian women. To meet a serum level of 20 ng/ml(50nmol/L) for bone health as recommended by the Institute of Medicine (IOM), a RDA dose for vitamin D3 of 800 IU/day is required. Though a dose of 600 IU/day was not studied, we can interpolate from the statistical model that a dose of 600 IU will meet a serum 25OHD level of 20ng/ml(50nmol/L). For African American women, a linear dose response curve was the best fit to the data. Prediction limits were calculated the same way as for Caucasian women and the RDA was found to be 800 IU/day to meet a serum 25OHD level of 20 ng/ml(50nmol/L), and 1600 IU/day to meet a serum 25OHD of 30ng/ml (75nmol/L) (43). To meet an average requirement for serum 25OHD of 30ng/ml (75nmol/L) the EAR was calculated as a dose between 800–1600IU/day, and to meet a serum 25OHD level of 20ng/ml (50nmol/L) a dose of 400 IU/day for Caucasian women was calculated (42). In African American women 400 and 800IU/day were determined for EAR of serum of 20ng/ml (50nmol/L) and 30ng/ml respectively (43).
Dose response in younger Caucasian and African American women
We conducted a dose response in younger women (VitaDAS study). This was a randomized placebo controlled clinical trial conducted in both Caucasian and African American women, age range 25–45 years, baseline serum 25OHD less than or equal to 20 ng/ml and similar exclusion criteria to the VidOS study (44). There were 4 vitamin D dose groups, 400, 800, 1600, 2400 IU/day or placebo. The Diasorin assay was used to measure serum 25OHD. There were 119 Caucasian and 79 African American women that completed study. They were analyzed separately by race and linear mixed effects models determined a linear dose response curve that best fit the data for both races. The RDA was estimated to be 400 IU in Caucasian and 1200 IU in African American women to reach a serum 25OHD of 20ng/ml(50nmol/L). The EAR to meet 20ng/ml was determined to be 400 IU/day for both races. Because of the smaller number of dose groups and poor compliance compared to older women it was not possible to use a quadratic curve, and there is more uncertainty about the RDA and EAR.
Combining results for all ages
We attempted to combine an analysis of ViDOS and VitaDAS studies, young and old, black and white women. In a linear mixed effects model a dose response curve was fitted, determining if there were interactions between race or study (ViDOS or VitaDAS) with dose. We determined that there was no interaction between dose and study, however there was a significant interaction between race and dose. Because of the significant interaction with race, we fitted models for each race separately, but combining the studies, so we have a Caucasian dose response curve and an African American dose response curve for young and older women combined.
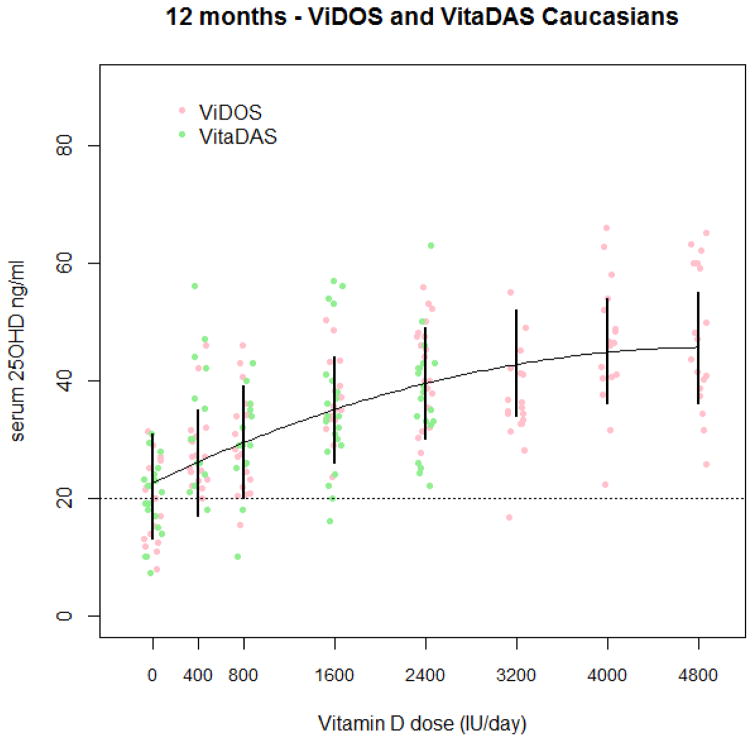
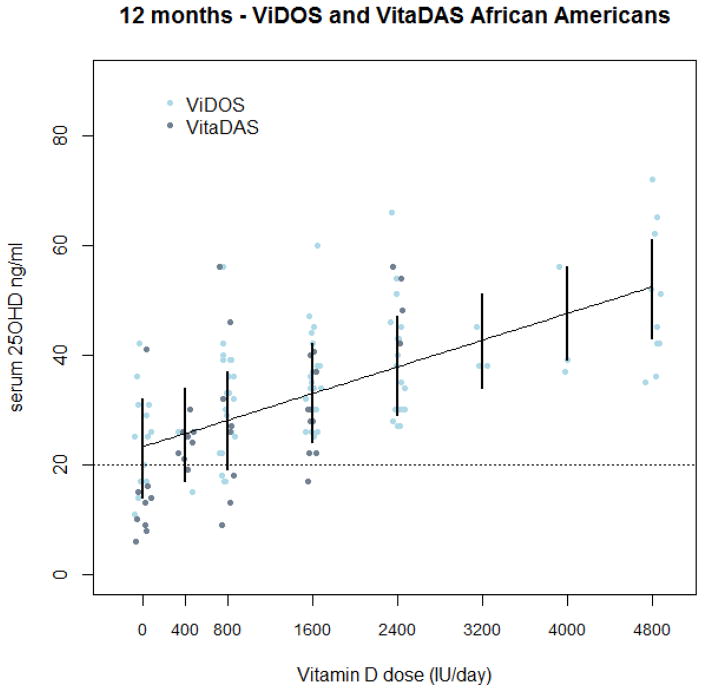
The best fitting model in Caucasians was a quadratic dose response, shown in Figure (2). 1000 bootstrapped prediction intervals were calculated for each dose, a dose of 800 IU/d was found to meet a serum 25OHD level of 20ng/ml (50nmol/L) as the RDA and 2400 IU would exceed 30 ng/ml (75nmol/L). The EAR to meet 30 ng/ml was a dose of 800 IU. By 12 months the estimated combined dose response curve had an average response above 20 ng/ml, showing that no supplementation was necessary to meet an EAR of 20 ng/ml. The best fitting model in African Americans was a linear dose response, shown in Figure (3). 1000 bootstrapped prediction intervals were calculated for each dose, and a dose of 800 IU/d was found to meet a serum 25OHD level of 20ng/ml (50nmol/L) as the RDA and 2400 IU/d is required to meet serum 25OHD of 30 ng/ml) (75nmol/L). The lower prediction limit at a dose of 800 IU/d is actually19 ng/ml. The EAR to meet 30 ng/ml(75nmol/L) was a dose of 1600 IU. By 12 months the estimated combined dose response curve had an average response above 20 ng/ml(50nmol/L), showing that no supplementation was necessary to meet an EAR of 20 ng/ml(50nmol/L). A quadratic response may be appropriate for African Americans as well, but with so few individuals at the higher doses, a quadratic curve is difficult to detect. Bootstrapped prediction limits seemed somewhat narrow in this analysis. Further investigation showed that the variability due to the average dose response curve is greater than the variability due to individuals. Therefore, the prediction intervals are being driven by the mean response and not the individual response. When estimating EAR and RDA measurements of serum 25OHD, compliance with the scheduled vitamin D intake is a consideration. Low compliance is defined as less than 80% compliance between the 9 and 12 month visits or an average compliance over the study period of less than 80%. If we exclude low compliers from the model and recalculate the bootstrap prediction intervals, they are similar to the intervals calculated with the full data. The RDA and EAR do not change if we exclude the low compliers from the estimation. These estimates for the RDA and EAR are summarized in Table 3.
Table 3.
RDA and EAR estimates based on randomized clinical trials, 1000 bootstrapped samples from linear mixed effects model
| Study | population | EAR 20 ng/ml |
EAR 30 ng/ml |
RDA 20 ng/ml |
RDA 30 ng/ml |
|---|---|---|---|---|---|
| ViDOS | Caucasian, age>57 | 400 IU/d | 800–1600 IU/d | 800 IU/d (600 IU*) | 1600 IU/d |
| ViDOS | African American, age>57 | 0–400 IU/d | 800 IU/d | 800 IU/d | 1600 IU/d |
| VitaDAS | Caucasian, age 25–45 | 400 IU/d | 800 IU/d | 400 IU/d | 2400 IU/d |
| VitaDAS | African American, age 25–45 | 400 IU/d | 1600 IU/d | 1200* IU/d | 2400 IU/d |
| ViDOS & VitaDAS | Caucasian, young and old combined | 0 IU/d | 800 IU/d | 800 IU/d | 2400 IU/d |
| ViDOS & VitaDAS | African Americans, young and old combined | 0 IU/d | 1600 IU/d | 800 IU/d | 2400 IU/d |
Based on interpolation
Recently a large study of 328 African Americans age 40–60 years over 3-months compared placebo, vitamin D 1,000, 2000 and 4,000 IU daily, they estimated that a vitamin D dose of ~1600IU would exceed serum 25OHD of 20ng/ml(50nmol/L). (45). Whether 3 months is long enough to reach a steady state for serum 25OHD on lower doses of vitamin D is a possible limitation of this study.
Who should be screened for serum 25OHD?
This issue was recently addressed by the U.S. Preventive Services Task Force (USPSTF) who examined the evidence on screening for vitamin D deficiency and found insufficient evidence to assess the benefits and harms in asymptomatic people since there was no evidence at the time that treatment of asymptomatic people has benefit on cancer, diabetes, mortality or risk of fracture (46). The IOM report recommend an RDA of 600IU or an EAR of 400IU to meet the requirements of most people in the winter without any risk from taking vitamin D. Therefore, screening healthy individuals for 25OHD levels is not justified. However, screening high risk patients with fractures, malabsorption syndromes and other medical conditions related to abnormal calcium and vitamin D metabolism may be clinically beneficial.
Summary
Several groups here and in Europe have arrived at a consensus that an RDA for vitamin D of 400–600IU daily will meet the needs of the population in protecting bone health. Addition of calcium to vitamin D has a protective effect in preventing fractures. Screening for serum 25OHD is unnecessary in normal subjects and not cost effective, however, patients with clinical problems affecting calcium and vitamin d metabolism may benefit from 25OHD testing. Large ongoing trials involving up to 50,000 subjects will produce results on the effect of vitamin D on several other diseases within 3–5 years.
Figure 2.
Combined dose response curve in ViDOS and VitaDAS Caucasian subjects. Complete 12-month data are displayed by dose and study with a quadratic dose response curve. Bootstrapped 95% prediction intervals from the linear mixed effects model are shown.
Figure 3.
Combined dose response curve in ViDOS and VitaDAS African American subjects. Complete 12-month data are displayed by dose and study with a linear dose response curve. Bootstrapped 95% prediction intervals from the linear mixed effects model are shown.
TABLE 2.
Prevalence of low vitamin D levels
| Serum 25OHD | Original | Corrected values | |
|---|---|---|---|
| Vitamin D deficiency | < 12 ng/ml (< 30nmol/l ) | 4% | 6%* |
| Vitamin D insufficiency | 12–20 ng/ml (30–50nmol/l) | 22% | 31%* |
| Vitamin D sufficiency | 20–50 ng/ml (50–125nmol/L) | 55% | 71%* |
KEY POINTS.
Incidence of vitamin D deficiency and insufficiency in North America is high.
Serum 25 hydroxyvitamin D (25OHD) level of 20ng/ml(50nmol/l) is clinically important for bone health.
Clinical studies of vitamin D and vitamin D with calcium show that vitamin D 400–800 IU plus calcium 1000mg daily is a combination that significantly reduces fractures by 10–12 percent. Vitamin D alone is not effective in reducing fractures.
Ongoing trials with vitamin D are expected to show the risk-benefit of vitamin D on other clinical diseases within 5 years.
RDA (Recommended Dietary Allowance) for vitamin D is as follows: 800 IU needed to reach 25OHD level of 20ng/ml (50nmol/l) and vitamin D 1600IU to reach a serum 25OHD level 30ng/ml(75nmol/L). The EAR (Estimated average requirement) is 400 IU for serum 25OHD level 20ng/ml(50nmol/L) and 800 IU for serum level of 30ng/ml (75nmol/L).
Acknowledgments
J Christopher Gallagher and Lynette Smith were supported by Grant AG28168 from the National Institute on Aging and the Office of Dietary Supplements and by a grant from the Department of Defense (DOD) W81XWH-07-1-201. Lynette Smith is supported also by Great Plains IDEA-CTR Network grant 1U54GM115458-01.
Footnotes
DISCLOSURE STATEMENT
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dietary Reference Intakes for Adequacy. Calcium and Vitamin D. Institute of Medicine; Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.WHO Scientific Group on the Prevention and Management of Osteoporosis. Prevention and management of osteoporosis: report of a WHO scientific group. Geneva: World Health Organization; 2003. [Google Scholar]
- 3.Outcome of a public consultation on the Draft Scientific Opinion of the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) on Dietary Reference Values for vitamin D. Wiley; 2016. Oct, EFSA Technical report. [DOI] [Google Scholar]
- 4.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 5.Shahangian S, Alspach Todd D, MS, Rex Astles J, PhD, Yesupriya Ajay, Dettwyler William K. Trends in Laboratory Test Volumes for Medicare Part B Reimbursements, 2000–2010. Arch Pathol Lab Med. 2014 Feb;138(2):189–203. doi: 10.5858/arpa.2013-0149-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. NCHS data brief, no 59. Hyattsville, MD: National Center for Health Statistics; 2011. Vitamin D status: United States(2001–2006) [PubMed] [Google Scholar]
- 7.Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010 Apr;140(4):817–22. doi: 10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binkley N, Dawson-Hughes B, Durazo-Arvizu R, Thamm M, Tian L, Merkel JM, Jones JC, Carter GD, Sempos CT. Vitamin D measurement standardization: The way out of the chaos. J Steroid Biochem Mol Biol. 2016 Dec 12; doi: 10.1016/j.jsbmb.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Bruyere O, Decock C, Delhez M, Collette J, Reginster JV. Highest prevalence of vitamin D inadequacy in institutionalized women compared with non-institutionalized women: a case–control study. Women’s Health. 2009;5(1):49–54. doi: 10.2217/17455057.5.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Cashman KD, Muldowney S, McNulty B, Nugent A, Fitzgerald AP, Kiely M, Walton J, Gibney MJ, Flynn A. Vitamin D status of Irish adults: findings from the National Adult Nutrition Survey. Br J Nutr. 2012 Aug 10;:1–9. doi: 10.1017/S0007114512003212. [DOI] [PubMed] [Google Scholar]
- 11.Holvik K, Meyer HE, Haug E, Brunvand L. Prevalence and predictors of vitamin D deficiency in five immigrant groups living in Oslo, Norway: the Oslo Immigrant study. Eur J Clin Nutr. 2005 Jan;59(1):57–63. doi: 10.1038/sj.ejcn.1602033. [DOI] [PubMed] [Google Scholar]
- 12.Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, Lichtenstein A, Patel K, Raman G, Tatsioni A, Terasawa T, Trikalinos TA. Vitamin D and Calcium: Systematic Review of Health Outcomes. Rockville, MD: Agency for Healthcare Research and Quality; Aug, 2009. Evidence Report/Technology Assessment No. 183. (Prepared by Tufts Evidence-based Practice Center under Contract No. 290-2007-10055-I). AHRQ Publication No. 09-E015. [Google Scholar]
- 13.Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(12):827–38. doi: 10.7326/0003-4819-155-12-201112200-00005. [DOI] [PubMed] [Google Scholar]
- 14.Cranney A, Horsley T, O’Donnell S, Weiler HA, Puil L, Ooi DS, Atkinson SA, Ward LM, et al. Effectiveness and Safety of Vitamin D in Relation to Bone Health. Rockville, MD: Agency for Healthcare Research and Quality; Aug, 2007. Evidence Report/Technology Assessment No. 158 (Prepared by the University of Ottawa Evidence-based Practice Center (UO-EPC) under Contract No. 290-02-0021. AHRQ Publication No. 07-E013. [PMC free article] [PubMed] [Google Scholar]
- 15.Need AG, O’Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res. 2008;23:1859–63. doi: 10.1359/jbmr.080607. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher JC, Yalamanchili V, Smith LM. The Effect of Vitamin D on Calcium Absorption in Older Women. J Clin Endocrinol Metab. 2012;97(10):3550–6. doi: 10.1210/jc.2012-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher JC, Jindal PS, Smith LM. Vitamin d does not increase calcium absorption in young women: a randomized clinical trial. J Bone Miner Res. 2014 May;29(5):1081–7. doi: 10.1002/jbmr.2121. [DOI] [PubMed] [Google Scholar]
- 18.Aloia JF, Dhaliwal R, Shieh A, Mikhail M, Fazzari M, Ragolia L, Abrams SA. Vitamin D supplementation increases calcium absorption without a threshold effect. Am J Clin Nutr. 2014 Mar;99(3):624–31. doi: 10.3945/ajcn.113.067199. [DOI] [PubMed] [Google Scholar]
- 19.Sai AJ, Walters RW, Fang X, Gallagher JC. Relationship between vitamin D, parathyroid hormone and bone health. J Clin Endocrinol Metab. 2011;18:1101–12. doi: 10.1210/jc.2010-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valcour, Blocki F, Hawkins DM, Rao Sudhaker D. 2012 Effects of Age and Serum 25-OH-Vitamin D on Serum Parathyroid Hormone Levels JCEM. 2012;97:3989–3999. doi: 10.1210/jc.2012-2276. [DOI] [PubMed] [Google Scholar]
- 21.Krall EA, Dawson-Hughes B, Hirst K, Gallagher JC, Sherman SS, Dalsky G. Bone mineral density and biochemical markers of bone turnover in healthy elderly men and women. J Gerontol A Biol Sci Med Sci. 1997 Mar;52(2):M61–7. doi: 10.1093/gerona/52a.2.m61. [DOI] [PubMed] [Google Scholar]
- 22.Ensrud KE, Taylor BC, Paudel ML, Cauley JA, Cawthon PM, Cummings SR, Fink HA, Barrett-Connor E, Zmuda JM, Shikany JM, Orwoll ES Osteoporotic Fractures in Men Study Group. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab. 2009;94:2773–2780. doi: 10.1210/jc.2008-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallagher JC, Sai AJ. Vitamin D insufficiency, deficiency, and bone health. J Clin Endocrinol Metab. 2010;95:2630–30. doi: 10.1210/jc.2010-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cauley JA, Parimi N, Ensrud KE, Bauer DC, Cawthon PM, Cummings SR, Hoffman AR, Shikany JM, Barrett-Connor E, Orwoll E. Osteoporotic fractures in men research G. Serum 25-hydroxyvitamin D and the risk of hip and non-spine fractures in older men. J Bone Miner Res. 2012;25:545–53. doi: 10.1359/jbmr.090826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cauley JA, Danielson ME, Boudreau R, Barbour KE, Horwitz MJ, Bauer DC, Ensrud KE, Manson JE, Wactawski-Wende J, Shikany JM, Jackson RD. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: Women’s Health Initiative (WHI) J Bone Miner Res. 2011;26:2378–88. doi: 10.1002/jbmr.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C, Streichert T, Püschel K, Amling M. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25(2):305–12. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 27.Tang BM, et al. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657–66. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]
- 28.DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010 Jan 12;340:b5463. doi: 10.1136/bmj.b5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders KM, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815–22. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 30.Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women--a population-based, randomized, double-blind placebo-controlled trial. Rheumatology (Oxford) 2007 Dec;46(12):1852–7. doi: 10.1093/rheumatology/kem240. [DOI] [PubMed] [Google Scholar]
- 31.Veugelers PJ, Ekwaru JP. A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients. 2014 Oct 20;6(10):4472–5. doi: 10.3390/nu6104472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garland CF, French CB, Baggerly LL, Heaney RP. Vitamin D supplement doses and serum 25hydroxyvitamin D in the range associated with cancer prevention. Anticancer Res. Feb;31(2):607. 201. [PubMed] [Google Scholar]
- 33.Nordic Council of Ministers. Integrating nutrition and physical activity. Nordic Council of Ministers; Copenhagen, Denmark: 2014. Nordic Nutrition Recommendations 2012; p. 627. doi.org/10.6027/Nord2014-002. [Google Scholar]
- 34.Health Council of the Netherlands. Evaluation of dietary reference values for vitamin D. No 2012/15E. 2012. p. 138. [Google Scholar]
- 35.Scientific Advisory Committee on Nutrition (SACN) Vitamin D and health. Public Health England; 2016. https://www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition. [Google Scholar]
- 36.Manson JE, Brannon PM, Rosen CJ, Taylor CL. Vitamin D Deficiency - Is There Really a Pandemic? N Engl J Med. 2016 Nov 10;375(19):1817–1820. doi: 10.1056/NEJMp1608005. [DOI] [PubMed] [Google Scholar]
- 37.Taylor CL, Carriquiry AL, Bailey RL, Sempos CT, Yetley EA. Appropriateness of the probability approach with a nutrient status biomarker to assess population inadequacy: a study using vitamin D. Am J Clin Nutr. 2012 Oct 24; doi: 10.3945/ajcn.112.046094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith LM, Gallagher JC, Suiter C. Higher doses of vitamin D increase the incidence of Falls. A randomized clinical trial. J Steroid Biochem Mol Biol. 2017 doi: 10.1016/j.jsbmb.2017.03.015. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melamed ML, et al. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durup D, et al. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: the CopD study. J Clin Endocrinol Metab. 2012;97(8):2644–52. doi: 10.1210/jc.2012-1176. [DOI] [PubMed] [Google Scholar]
- 41.Sempos CT, Durazo-Arvizu RA, Dawson-Hughes B, Yetley EA, Looker AC, Schleicher RL, Cao G, Burt V, Kramer H, Bailey RL, Dwyer JT, Zhang X, Gahche J, Coates PM, Picciano MF. Is there a reverse J-shaped association between 25-hydroxyvitamin D and all-cause mortality? Results from the U.S. nationally representative NHANES. J Clin Endocrinol Metab. 2013 Jul;98(7):3001–9. doi: 10.1210/jc.2013-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallagher JC, Sai AJ, Templin TJ, Smith LM. Dose response to vitamin D supplementation in postmenopausal women 2011 A randomized clinical trial. Ann Intern Med. 2012;156:425–437. doi: 10.7326/0003-4819-156-6-201203200-00005. [DOI] [PubMed] [Google Scholar]
- 43.Gallagher JC, Peacock M, Yalamanchili V, Smith LM. Effects of Vitamin D Supplementation in Older African American Women. J Clin Endocrinol Metab. 2013 Mar;98(3):1137–46. doi: 10.1210/jc.2012-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallagher JC, Jindal PS, Smith LM. Vitamin D supplementation in young Caucasian and African American women. J Bone Miner Res. 2014 Jan;29(1):173–81. doi: 10.1002/jbmr.2010. [DOI] [PubMed] [Google Scholar]
- 45.Ng K, Scott JB, Drake BF, Chan AT, Hollis BW, Chandler PD, Bennett GG, Giovannucci EL, Gonzalez-Suarez E, Meyerhardt JA, Emmons KM, Fuchs CS. Dose response to vitamin D supplementation in African Americans: results of a 4-arm, randomized, placebo-controlled trial. Am J Clin Nutr. 2014 Mar;99(3):587–98. doi: 10.3945/ajcn.113.067777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LeFevre ML U.S. Preventive Services Task Force. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015 Jan 20;162(2):133–40. doi: 10.7326/M14-2450. [DOI] [PubMed] [Google Scholar]